Biotransformative removal of cationic Red X-GRL by anaerobic activated sludge†
Bin Qiuab,
Xinzhu Xua,
Yan Danga,
Qiang Wanga,
Dezhi Sun*a,
Suying Weic and
Zhanhu Guo*b
aBeijing Key Lab for Source Control Technology of Water Pollution, Beijing Forestry University, Beijing 100083, China. E-mail: sundezhi@bjfu.edu.cn
bIntegrated Composites Laboratory (ICL), Department of Chemical and Biomolecular Engineering, University of Tennessee, Knoxville, TN 37996, USA. E-mail: zguo10@utk.edu
cDepartment of Chemistry and Biochemistry, Lamar University, Beaumont, TX 77710, USA
First published on 25th February 2015
Abstract
The decolorization of azo dyes can be easily achieved by anaerobic activated sludge, while further degradation of the aromatic amines is a challenge for treating azo dye wastewater. The anaerobic activated sludge material, which was cultured in an anaerobic unflow biological filter, was used directly for decolorizing and degrading the cationic Red X-GRL (X-GRL). The anaerobic activated sludge is mainly composed of the bacteria belonging to phyla Proteobacteria and Firmicutes, and the archaea belonging to phylum Methnomicrobiales. The cultured anaerobic sludge has been demonstrated efficient for treating X-GRL wastewater. All color and more than 95% of aromatic amines were removed with an X-GRL concentration of 200 mg L−1 and a hydraulic retention time (HRT) of 60 h. Batch assays were employed to investigate the anaerobic biotransformation of X-GRL. The sucrose added to the influent acts as an initial electron donor for the reduction of the azo bond, generating colorless aromatic amines. Aromatic amines were then completely degraded to CO2, CH4 and NH3 by anaerobic activated sludge, avoiding the secondary pollution by the dye pollutant. Finally, the transformation pathway of X-GRL under the anaerobic conditions was proposed. Therefore, the anaerobic activated sludge material is demonstrated as a sustainable material for the biotransformative removal of azo dyes from wastewater.
1. Introduction
Azo dyes are widely used in many industries such as textiles, cosmetics and paper printing. The effluents from the dye producing and dying industries are highly colored. The concentration of azo dyes in real wastewater varies from tens to hundreds of mg L−1, which is highly dependent on the efficiency during the dying process.1,2 The discharge of dye-containing wastewater causes serious environmental and health problems due to its low biodegradability and high genotoxicity, mutagenicity and carcinogenicity to plants and animals.3 Therefore, effective processes are urgently needed for treating the azo dyes-containing wastewater. Among the reported processes, anaerobic processes have attracted increasing attention due to their great ability on color removal.4–7 The reduction of azo bond (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) is considered as the first step in the process of azo dye degradation under anaerobic condition.8 During this step, the carbon sources are often added into the dye wastewater, such as the glucose,9,10 glutamine, lactate and other volatile fatty acids (VFAs).11 The reductive intermediates generated from the carbon source reduce the azo bond, leading to the decolorization of azo dye-containing wastewater and the formation of colorless aromatic amines.8,12 Unfortunately, aromatic amines are classified as environmentally hazardous and toxic substances due to their suspicious mutagens and carcinogens property.3 However, many researchers have just focused on the color removal from the dye wastewater under anaerobic condition5,6,10,12,13 rather than on the further degradation of aromatic amines. Moreover, the aromatic amines are considered to be refractory under anaerobic condition.4,14,15 The lack of easy utilized electron donors for reduction of the azo bond as well as the inhibition of toxic intermediates on bacterial activity result in the low removal efficiency for azo dyes under the anaerobic condition.16 Therefore, the aerobic processes have been usually used with following the anaerobic unit for further degradation of the toxic intermediates.8 Although the combined anaerobic/aerobic processes have been demonstrated great on both the color removal and organics degradation, the operational cost is increased by the addition of aerobic unit.17 The aromatic amines were reported to be partly mineralized by anaerobic sludge with a long hydraulic retention time.4,11 It was reported that the low recovery of aromatic amines from the reduced solution of azo dye was contributed by the transformation of aromatic amines to methane and CO2 under the anaerobic condition.4 It is of great important for the treatment of azo dye-containing wastewater if the decolorization and degradation can be achieved simultaneously under the anaerobic condition.
N–) is considered as the first step in the process of azo dye degradation under anaerobic condition.8 During this step, the carbon sources are often added into the dye wastewater, such as the glucose,9,10 glutamine, lactate and other volatile fatty acids (VFAs).11 The reductive intermediates generated from the carbon source reduce the azo bond, leading to the decolorization of azo dye-containing wastewater and the formation of colorless aromatic amines.8,12 Unfortunately, aromatic amines are classified as environmentally hazardous and toxic substances due to their suspicious mutagens and carcinogens property.3 However, many researchers have just focused on the color removal from the dye wastewater under anaerobic condition5,6,10,12,13 rather than on the further degradation of aromatic amines. Moreover, the aromatic amines are considered to be refractory under anaerobic condition.4,14,15 The lack of easy utilized electron donors for reduction of the azo bond as well as the inhibition of toxic intermediates on bacterial activity result in the low removal efficiency for azo dyes under the anaerobic condition.16 Therefore, the aerobic processes have been usually used with following the anaerobic unit for further degradation of the toxic intermediates.8 Although the combined anaerobic/aerobic processes have been demonstrated great on both the color removal and organics degradation, the operational cost is increased by the addition of aerobic unit.17 The aromatic amines were reported to be partly mineralized by anaerobic sludge with a long hydraulic retention time.4,11 It was reported that the low recovery of aromatic amines from the reduced solution of azo dye was contributed by the transformation of aromatic amines to methane and CO2 under the anaerobic condition.4 It is of great important for the treatment of azo dye-containing wastewater if the decolorization and degradation can be achieved simultaneously under the anaerobic condition.
In our previous work,18 an adapted anaerobic upflow biological filter (UBF), which combines the activated sludge and biofilm, has been established for treating the synthesized azo dye (cationic Red X-GRL (X-GRL)) wastewater for more than one year. Both the bacterial and archaeal populations play the important role in the decolorization and degradation of azo dyes.19 However, the roles of different microbial members in the X-GRL metabolic pathways have not been assigned. Moreover, the biodegradation pathway of X-GRL is unproven and remains subjects of speculation under anaerobic condition.
In this study, the anaerobic UBF was operated with an HRT of 60 h. The color removal and the degradation of COD and aromatic amine by the anaerobic UBF were investigated with increasing the X-GRL concentrations from 50 to 200 mg L−1. The bacterial and archaeal communities in the UBF were detected by clone library method, and their roles in the biotransformation of X-GRL were explored. The biotransformation of X-GRL under anaerobic condition was investigated by batch experiments. The concentrations of the intermediates and final products, including aromatic amines, methane and NH4+–N, were measured. Finally, the biotransformation pathway of X-GRL under anaerobic condition was proposed as well.
2. Materials and methods
2.1 Materials
Cationic Red X-GRL (C18H23N6Cl2Zn, λmax = 530 nm) was purchased from Zhejiang Runtu Co., Ltd. China. Sucrose, NH4Cl and KH2PO4 were purchased from Sigma Aldrich. All the chemicals were used as received without any further treatment. Activated sludge used in UBF was collected from a lab-scale UASB reactor for the treatment of azo dye wastewater.2.2 Operation of anaerobic UBF
The anaerobic UBF has been described in previous work.18 In brief, a plexiglass cylinder with a working volume of 3.0 L was seeded with 1.0 L anaerobic activated sludge (VSS: 8.5 g L−1). 1.5 L plastic biocarriers (Φ 5 × 8 mm) were then added to the reactor to facilitate the growth of biofilm on their surface. The UBF reactor was operated with different X-GRL concentrations: stage I (day 1–30), 50 mg L−1; stage II (day 31–60), 100 mg L−1; stage III (day 61–90), 150 mg L−1 and stage IV (day 91–120), 200 mg L−1. The HRT was controlled at 60 h and the temperature of the reactor was kept at 25 ± 1 °C. The influent of the UBF consisted of (per liter): X-GRL (as desired), sucrose (1000 mg), NH4Cl (96 mg), KH2PO4 (22 mg) and trace solution (1 mL), giving a COD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 200
P ratio of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The trace solution contained the following components (mg L−1): ZnCl2 (0.05), MnSO4 (0.05), CuCl2 (0.03), CoCl2·6H2O (0.05), NiCl2 (0.05), and HBO3 (0.05).
1. The trace solution contained the following components (mg L−1): ZnCl2 (0.05), MnSO4 (0.05), CuCl2 (0.03), CoCl2·6H2O (0.05), NiCl2 (0.05), and HBO3 (0.05).
2.3 X-GRL biotransformation assays
The biodegradation of X-GRL under anaerobic condition was conducted by batch experiment. The activated sludge used in the assays was collected from the anaerobic UBF operated at stage IV. It was washed and resuspended by O2-free water with a concentration of 8.65 g SS L−1 before being used for the experiments. The nutrient solution was composed of (per liter): sucrose (1000 mg), NH4Cl (96 mg), KH2PO4 (22 mg) and trace solution (1 mL). Different amounts of X-GRL solution were added into 150 mL serum bottles to get the X-GRL concentrations between 25 to 300 mg L−1 in the N2 atmosphere. 5 mL anaerobic sludge was added, and then O2-free water was supplemented to a final volume of 75 mL. A parallel control was also prepared in the same manner but without X-GRL. The color, the concentrations of VFAs and aromatic amines in the wastewater were measured at different time interval. The concentrations of the methane and CO2 in the top space were analyzed as well. All the incubations were conducted in triplicate.2.4 Clone libraries
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min and the supernatant was decanted. The remaining pellet was washed with 1 mL deionized and distilled water and centrifuged again in the same manner to ensure a maximal removal of residual medium. Then the total DNA was extracted and was purified using a Fast DNA kit for soil following the manufacturer's instructions (Q-BIOgene, CA, USA). The 16 rRNA fragments of archaea were amplified using the primer pair PRUN109F and PRUN915R, whereas the primer pair EUB8F and EUB1492R was applied to amplify the 16S rRNA fragments of eubacteria. The PCR conditions were as follows: predenaturation at 94 °C for 5 min; 25 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 1 min; and then post elongation at 72 °C for 10 min. The amplified products were separated on 1.2% (mL) agarose Tris–acetate–EDTA (TAE) gels, stained with ethidium bromide, and visualized under UV light. The bands were reclaimed and purified with a DNA purification kit (Biowatson Biotechnology, China) before ligation into vectors.
000g for 10 min and the supernatant was decanted. The remaining pellet was washed with 1 mL deionized and distilled water and centrifuged again in the same manner to ensure a maximal removal of residual medium. Then the total DNA was extracted and was purified using a Fast DNA kit for soil following the manufacturer's instructions (Q-BIOgene, CA, USA). The 16 rRNA fragments of archaea were amplified using the primer pair PRUN109F and PRUN915R, whereas the primer pair EUB8F and EUB1492R was applied to amplify the 16S rRNA fragments of eubacteria. The PCR conditions were as follows: predenaturation at 94 °C for 5 min; 25 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 1 min; and then post elongation at 72 °C for 10 min. The amplified products were separated on 1.2% (mL) agarose Tris–acetate–EDTA (TAE) gels, stained with ethidium bromide, and visualized under UV light. The bands were reclaimed and purified with a DNA purification kit (Biowatson Biotechnology, China) before ligation into vectors.2.5 Analytical method
The concentration of X-GRL was measured by using a UV-vis spectrophotometer (Evolution 300, Thermo) at 530 nm. The COD and NH4+–N concentration in aqueous solution was analyzed according to the standard method of APHA. The concentration of aromatic amines was measured by the spectrophotometric method with N-(1-naphthyl)-ethylenediamine. The intermediates of the metabolites of X-GRL was detected by using GC/MS with a Finnigan/MAT (GCQ), at an ionization voltage of 70 eV in temperature programming mode on a DB-1 column. The initial column temperature was 80 °C for 2 min, increased linearly to 280 °C at 10 °C min−1 and maintained at this temperature for 7 min. The temperature of the injection port was 280 °C and the GC-MS interface was maintained at 290 °C. The helium carrier gas flow rate was 1.0 mL min−1. The concentrations of VFAs in the aqueous solutions were measured by an Agilent Gas Chromatograph (GC). Nitrogen gas was used as the carrier gas. The concentrations of methane and CO2 were analyzed by an Agilent 7890A GC equipped with an electron capture detector (ECD). Nitrogen was used as the carrier gas and the equilibrium gas was a 90% Ar/10% CH4 mixture.3. Results and discussion
3.1 Performances of UBF
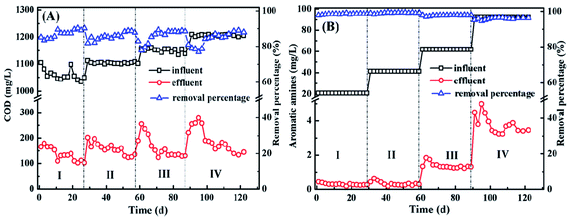
The anaerobic UBF has been demonstrated great on the decolorization and degradation of X-GRL under the anaerobic condition. All color and more than 75% COD were removed by the anaerobic UBF with an X-GRL concentration ranging from 50 to 200 mg L−1 (Fig. 1A). The colorless aromatic amines are the main intermediates during the decolorization of azo dyes under anaerobic condition. The concentration of aromatic amines was detected in order to determine the capability of anaerobic UBF for the degradation of toxic aromatic amines. As shown in Fig. 1B, ∼99% aromatic amines were removed by the UBF with ∼0.3 mg L−1 aromatic amines remained in the effluent when the X-GRL concentration in the influent was 50 mg L−1. While ∼2 and 4 mg L−1 aromatic amines were detected when the X-GRL concentration was increased to 150 and 200 mg L−1, indicating more than 95% aromatic amines were degraded. The anaerobic UBF has efficient degradation ability for the aromatic amines, leading to the decrease in the toxicity of influent. It was different from the other research, where the aromatic compounds were reported to be refractory under the anaerobic condition. For example, Wang et al.11 reported that the intermediates produced from azo dyes could be partly transformed to methane with an HRT of more than 50 h, while parts of them were refractory to be degraded by anaerobic consortium. Moreover, it is consistent with results that the azo dye and intermediates were completely degraded and transformed to methane and NH3 in an adapted methanogenic consortium with an HRT of 12 days.203.2 Microorganism communities
The microorganisms play an important role in the biodecolorization and biodegradation of azo dye under the anaerobic condition. Both fermentation and methanogenesis processes contributed to the anaerobic transformation of azo dyes,21 indicating that both bacterial and archaeal communities contribute to the decolorization and degradation of azo dyes. As shown in Fig. 2A, the dominant bacterial groups were the phyla Proteobacteria and Firmicutes. The phylogenetic tree for the bacteria detected in the activated sludge was shown in Fig. S1.† ∼45% bacteria population in the anaerobic sludge belong to phylum Proteobacteria, including β-Proteobacteria and γ-Proteobacteria, and ∼53% belong to the phylum Firmicutes, in which most were Clostridium. ∼2% Caldiserica were detected in the bacterial community as well. An obvious change was observed for the bacterial community of the anaerobic sludge in the anaerobic UBF, compared to that detected in the previous work, where the UBF was operated with an HRT of 36 h.18 The decreased diversity of bacterial community was observed in the anaerobic sludge with increasing the HRT. Compared to the bacterial community of the anaerobic sludge operated with an HRT of 36 h, the bacteria belonging to the phyla β-Proteobacteria, γ-Proteobacteria and Firmicutes were still the dominated groups, while the populations belonging to ε-Proteobacteria, δ-Proteobacteria, Actinobacteria, Spirochaetes, OP8 and Bacteroidetes were not detected in this study. It is consistent with the results that the microbial populations belonging to Klebsiella, Escherichia, Bacillus and Clostridium were reported as the dominated populations in the anaerobic sludge treated with azo dyes.22 The bacterium belonging to Klebsiella has a high utilization capability for simple substrates under anaerobic conditions,21 e.g., sucrose, generating the reductive intermediates, such as H2 and the VFAs. The reductive intermediates can serve as electron donors for the reduction of azo bond, leading to the decolorization of azo dyes.8 The bacteria belonging to β-Proteobacteria and γ-Proteobacteria were reported to have capability on the decolorization of azo dyes under anaerobic condition as well.23,24 The bacteria belonging to Firmicutes are responsible for degrading the aromatic amines to CO2 and alkenes.25 Moreover, most bacteria belonging to the phylum Firmicutes exhibit heterotrophy, and can accelerate the generation of electron equivalents, indicating that it can improve the decolorization of azo dyes when the aromatic intermediates were degraded.26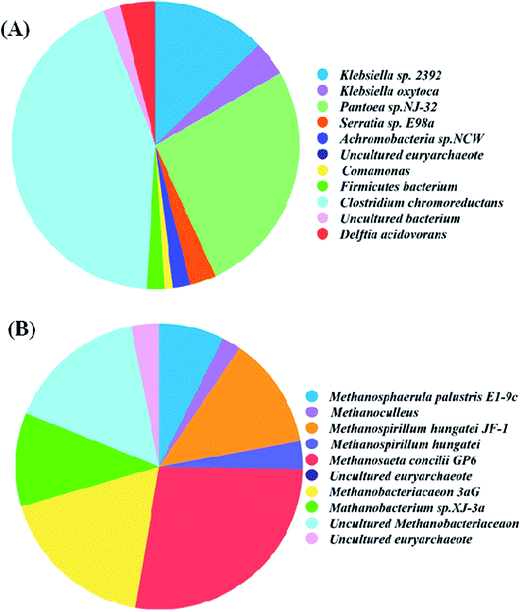 | ||
| Fig. 2 Relative abundance of (A) bacteria and (B) archaea in the activated sludge fed with 200 mg L−1 X-GRL. | ||
9 methanogenic strains were detected in the anaerobic activated sludge by colon library method (Fig. 2B). The phyla Methanomicrobiales, Methnoseataceae and Methnobacteriaceae were found as the dominant archaeal groups in the anaerobic sludge treated with X-GRL. Phylogenetic tree for the archaea detected in the activated sludge was shown in Fig. S2.† The hydrogenotrophic Methanomicrobiales (43%) and Methanosaetanceae (27%) accounted for ∼70% of the total archaea. The other methanogenic strains were acetoclastic Methnobacteriaceae, accounting for 30%. It indicates that the hydrogenotrophic methanogenesis is the main route for the methane production from the X-GRL-containing culture. Electrons donors such as H2 are needed for the decolorization of X-GRL, leading to the inhibition on hydrogenotrophic methanogenes. Therefore, the acetoclastic methanogen is supposed as the main groups in this anaerobic sludge. However, the acetoclastic methanogens accounts for only ∼30% of the total archaea, indicating the acetoclastic methanogens were highly inhibited by the toxic X-GRL and the intermediates. It is consistent with the result that the pollutants with a strong electron withdrawing property, such as the nitroaromatics and azo dyes, highly inhibited on the activity of acetoclastic methanogens.27
3.3 Biodegradation of X-GRL
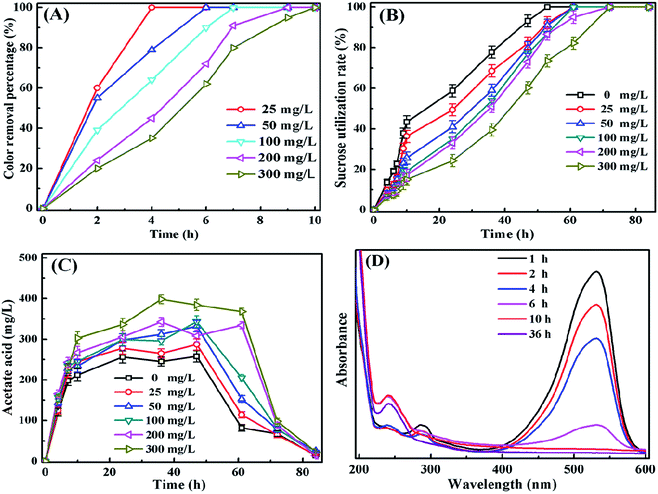
The anaerobic biotransformation pathway of the X-GRL including the decolorization and degradation processes has been investigated by batch assays in this study. 1000 mg L−1 sucrose added in the solution acts as the initiate electron donors for the reduction of azo bond (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), leading to the decolorization of X-GRL. The color removal and degradation of X-GRL were investigated with X-GRL concentration ranging from 25 to 300 mg L−1. As shown in Fig. 3A, the color of X-GRL solutions was completely removed within 10 h. The time for the complete decolorization became longer with increasing the X-GRL concentration. The more X-GRL needs more reductive electrons for the reduction of azo bond. Moreover, X-GRL with a high concentration would significantly inhibit the bacterial activity for the degradation of sucrose and the generation of reductive electron donors.14
N–), leading to the decolorization of X-GRL. The color removal and degradation of X-GRL were investigated with X-GRL concentration ranging from 25 to 300 mg L−1. As shown in Fig. 3A, the color of X-GRL solutions was completely removed within 10 h. The time for the complete decolorization became longer with increasing the X-GRL concentration. The more X-GRL needs more reductive electrons for the reduction of azo bond. Moreover, X-GRL with a high concentration would significantly inhibit the bacterial activity for the degradation of sucrose and the generation of reductive electron donors.14
In the redox reaction, the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– with a high electron withdrawing property acts as a terminal electron acceptor. Therefore, the electrons produced from the fermentation of carbon sources are important for the reduction of –N
N– with a high electron withdrawing property acts as a terminal electron acceptor. Therefore, the electrons produced from the fermentation of carbon sources are important for the reduction of –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–.3,11,13 Sucrose added in the feed water was decomposed, generating the reductive intermediates, e.g. H2 and the volatile fatty acids (VFAs). The acetic acid was detected as the main VFAs in the solution. The concentration of sucrose was decreased and the concentration of acetic acid was increased during the initial 10 h (Fig. 3B and C). The H2 generated from the fermentation of sucrose is preferred for the reduction of X-GRL, leading the accumulation of the VFAs in the solutions. The utilization rate of sucrose became slow after 10 h of incubation. More than 200 mg L−1 acetic acid was accumulated in the solutions when the X-GRL was totally decolorized. The accumulation of the acetic acid increased with increasing the X-GRL concentration, mainly due to the inhibition of microbial activity by the toxicity of aromatic compounds. After 48 h incubation, the concentration of acetate acid was decreased, generating methane by acetoclastic methanogen. Generally, the H2 produced during the acidogenic stage of the fermentation plays an important role in the reduction of –N
N–.3,11,13 Sucrose added in the feed water was decomposed, generating the reductive intermediates, e.g. H2 and the volatile fatty acids (VFAs). The acetic acid was detected as the main VFAs in the solution. The concentration of sucrose was decreased and the concentration of acetic acid was increased during the initial 10 h (Fig. 3B and C). The H2 generated from the fermentation of sucrose is preferred for the reduction of X-GRL, leading the accumulation of the VFAs in the solutions. The utilization rate of sucrose became slow after 10 h of incubation. More than 200 mg L−1 acetic acid was accumulated in the solutions when the X-GRL was totally decolorized. The accumulation of the acetic acid increased with increasing the X-GRL concentration, mainly due to the inhibition of microbial activity by the toxicity of aromatic compounds. After 48 h incubation, the concentration of acetate acid was decreased, generating methane by acetoclastic methanogen. Generally, the H2 produced during the acidogenic stage of the fermentation plays an important role in the reduction of –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond of the X-GRL.3
N– bond of the X-GRL.3
3.4 Products of X-GRL biotransformation
The intermediates generated form X-GRL under the anaerobic condition were analyzed by the UV-vis spectra and GC/MS. Fig. 3D shows the UV-vis spectra of the supernatants after being treated by anaerobic sludge. The peaks at 530 and 290 nm disappeared after 10 hour incubation, indicating that the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and symmetrical –C
N– and symmetrical –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– were totally broken within 10 h. However, a new peak observed at 250 nm indicates generation of the aromatic compounds. Moreover, the peak intensity at 250 nm decreased with increasing the incubation time, indicating that the aromatic compounds were degraded by the anaerobic sludge as well. The H2 was consumed for the cleavage of the –N
N– were totally broken within 10 h. However, a new peak observed at 250 nm indicates generation of the aromatic compounds. Moreover, the peak intensity at 250 nm decreased with increasing the incubation time, indicating that the aromatic compounds were degraded by the anaerobic sludge as well. The H2 was consumed for the cleavage of the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, leading to the decolorization of X-GRL. The –C
N–, leading to the decolorization of X-GRL. The –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond in the heterocyclic compound was also reduced by the reductive H2. The GC-MS results (Table 1) show that the aromatic amines compounds e.g. the C6H7N and C7H9N, were the dominant intermediates during the decolorization of X-GRL, consistent with the results based on UV-vis spectra (Fig. 2D). The results are also consistent with previous research,4,24 which reported that the aromatic amines, such as the aniline and 1,4-diamino benzene, were the main products during the decolorization of azo dyes under the anaerobic condition. Meanwhile, the aromatic compounds can also be decomposed to alkenes and were transformed to methane and CO2.27
N– bond in the heterocyclic compound was also reduced by the reductive H2. The GC-MS results (Table 1) show that the aromatic amines compounds e.g. the C6H7N and C7H9N, were the dominant intermediates during the decolorization of X-GRL, consistent with the results based on UV-vis spectra (Fig. 2D). The results are also consistent with previous research,4,24 which reported that the aromatic amines, such as the aniline and 1,4-diamino benzene, were the main products during the decolorization of azo dyes under the anaerobic condition. Meanwhile, the aromatic compounds can also be decomposed to alkenes and were transformed to methane and CO2.27
| Retention time (min) | Formula |
|---|---|
| 4.59 | C7H8 |
| 5.75 | C6H7N |
| 5.91 | C7H9N |
| 9.5 | C15H17N2 |
3.5 Anaerobic biotransformation of X-GRL
Fig. 4A shows that the highest concentrations of aromatic amines were observed after 10 hour incubation when the X-GRL was just totally decolorized by the anaerobic sludge. The accumulation of aromatic amines during the initial period indicates that the decolorization of X-GRL is much faster than the degradation of aromatic amines. However, the concentrations of the aromatic amines decreased obviously with increasing the incubation time, indicating the degradation of the aromatic amines. No aromatic amines were detected in the solutions with 25 and 50 mg L−1 X-GRL after being treated for 72 h, indicating that X-GRL was completely degraded by the adapted anaerobic sludge. It was of importance since the aromatic amines generated from azo dyes were often considered to be recalcitrant under anaerobic condition.8,14 However, it is consistent with other results, reporting that the aromatic amines were partially or completely degraded by the anaerobic sludge.4,11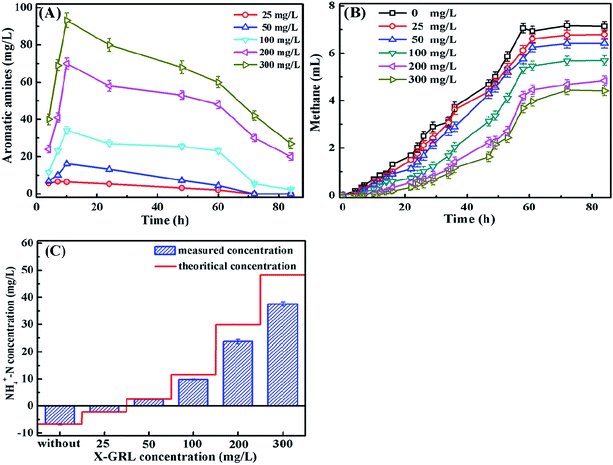 | ||
| Fig. 4 (a) Aromatic amines concentration, (b) methane production during the X-GRL degradation, and (c) NH4+–N concentration in the solutions after 84 hour incubation. | ||
Methane is one of the terminal products of the organic pollutants under the anaerobic condition. As shown in Fig. 4B, methane production decreased with increasing the X-GRL concentration. ∼7 mL methane was produced from the control solution without X-GRL. It was noticed that little methane was detected within 10 hour incubation. The reductive hydrogen prefers to reduce the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– of X-GRL due to its strong electron withdrawing property. The competition of hydrogen by –N
N– of X-GRL due to its strong electron withdrawing property. The competition of hydrogen by –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– leads to a decreased methane production by the hydrogenotrophic methanogens. The azo dye and aromatic amines with high toxicity also inhibit the activity of both hydrogenotrophic and acetoclastic methanogenesis.15,28 During the 10–45 hour incubation, the production rate of methane from X-GRL-containing cultures became slow. Hydrogen was used for the reduction of –C
N– leads to a decreased methane production by the hydrogenotrophic methanogens. The azo dye and aromatic amines with high toxicity also inhibit the activity of both hydrogenotrophic and acetoclastic methanogenesis.15,28 During the 10–45 hour incubation, the production rate of methane from X-GRL-containing cultures became slow. Hydrogen was used for the reduction of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– rather than for the production of methane by the hydrogenotrophic methanogens. However, the methane production increased obviously after 50 hour incubation, due to the complete reduction of the –N
N– rather than for the production of methane by the hydrogenotrophic methanogens. However, the methane production increased obviously after 50 hour incubation, due to the complete reduction of the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and –C
N– and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– in the initial 50 h. The H2 would completely involve in the methane production. On the other hand, the aromatic amines can also be used as the carbon sources and transformed to the methane.27 The decreased aromatic compounds mitigates the inhibition of methane production by the toxic intermediates.
N– in the initial 50 h. The H2 would completely involve in the methane production. On the other hand, the aromatic amines can also be used as the carbon sources and transformed to the methane.27 The decreased aromatic compounds mitigates the inhibition of methane production by the toxic intermediates.
It is important to determine the N transformation of the X-GRL under the anaerobic condition. As shown in Fig. 4C, the NH4+–N concentration in the control solution without X-GRL decreased by ∼7 mg L−1 after being treated for 84 h, indicating that ∼7 mg L−1 NH4+–N has been used by the microorganisms. The final NH4+–N concentrations increased with increasing the initial X-GRL concentration, indicating that the NH4+–N was generated from the degradation of X-GRL. Assuming that all the 6 nitrogen atoms in X-GRL are completely reduced to NH3 under the anaerobic condition, the theoretical concentrations of the NH4+–N with different concentrations of X-GRL are calculated and showed in Fig. 4C. The theoretical concentrations of the NH4+–N with 25 and 50 mg L−1 X-GRL were accordance with these detected in the solutions. However, 23.1 and 37.5 mg L−1 NH4+–N were detected in the solutions with X-GRL concentration of 200 and 300 mg L−1, much lower than the theoretical values (30 and 48 mg L−1). It was mainly due to the inhibition of the microbial activity by the toxic X-GRL and aromatic amines, leading to an incomplete degradation of the intermediates.
3.6 Proposed biotransformation pathway of X-GRL
The sucrose was decomposed to VFAs and CO2, as well as H2 through the anaerobic fermentation. The –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and symmetrical –C
N– and symmetrical –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bonds were reduced by the electron donors, leading to the decolorization of X-GRL. However, the reduction of X-GRL competes H2 with the methane production by the hydrogenotrophic methanogens. It was supposed that 1 mol methane will be produced from 1 mol CO2 and 2 mol H2. The decreased methane production (M[CH4]) in the X-GRL-containing solutions was calculated as the difference of the methane production between in X-GRL-containing solutions and in the control solution. Then the ratio of 4M[CH4] to M[X-GRL] represents the electron amount for the reduction of X-GRL. As shown in Fig. 5A, the ratio is ∼4 with the X-GRL concentration lower than 50 mg L−1, indicating that 4 electrons are required for the decolorization of X-GRL. However, the ratio decreased with increasing the X-GRL concentration due to the toxicity of azo dyes and the aromatic intermediates.15 The increased toxicity inhibits the bacterial fermentation, as well as the archaeral activity, leading to a decreased methane production.
N– bonds were reduced by the electron donors, leading to the decolorization of X-GRL. However, the reduction of X-GRL competes H2 with the methane production by the hydrogenotrophic methanogens. It was supposed that 1 mol methane will be produced from 1 mol CO2 and 2 mol H2. The decreased methane production (M[CH4]) in the X-GRL-containing solutions was calculated as the difference of the methane production between in X-GRL-containing solutions and in the control solution. Then the ratio of 4M[CH4] to M[X-GRL] represents the electron amount for the reduction of X-GRL. As shown in Fig. 5A, the ratio is ∼4 with the X-GRL concentration lower than 50 mg L−1, indicating that 4 electrons are required for the decolorization of X-GRL. However, the ratio decreased with increasing the X-GRL concentration due to the toxicity of azo dyes and the aromatic intermediates.15 The increased toxicity inhibits the bacterial fermentation, as well as the archaeral activity, leading to a decreased methane production.
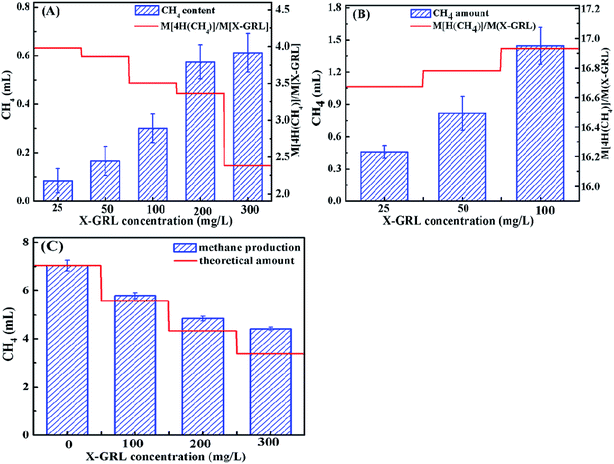 | ||
| Fig. 5 Relation between decreased methane production and amount of X-GRL after (a) decolorization and (b) 84 hour incubation; and (c) methane production after 84 hour incubation. | ||
The aromatic amines were completely degraded in 84 h with an initial X-GRL concentration lower than 100 mg L−1 (Fig. 3A). The final methane production at 84 h decreased with increasing the initial X-GRL concentration. The decreased amount of the final methane production (M[(CH4)]f) was calculated as the difference of the methane production between in X-GRL-containing solutions and in the control solution after 84 hour incubation. Therefore, the ratio of 4M[(CH4)f] to M[X-GRL] represents the electrons needed for the complete degradation of the X-GRL. As shown in Fig. 5B, the ratio was calculated as ∼16, indicating that ∼16 electrons were needed for the complete reduction of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and –N
N– and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– of X-GRL. However, the ratio increased slightly with increasing the X-GRL concentration. Assuming that 16 electrons were consumed for the reduction of X-GRL, the theoretical methane productions in the solutions with different X-GRL concentrations were calculated (Fig. 5C). More methane was produced in the X-GRL-containing solutions compared to the theoretical values. The differences between the actual detected methane productions and the theoretical values increased with increasing the X-GRL concentration, indicating that the intermediates were further degraded and transferred to methane. The aromatic amines were used as the carbon source by bacteria. The existing electron acceptors such as the nitrate and nitrite,29 iron and sulfate,30 improve the degradation of aromatic amines. In this system, both the –N
C– of X-GRL. However, the ratio increased slightly with increasing the X-GRL concentration. Assuming that 16 electrons were consumed for the reduction of X-GRL, the theoretical methane productions in the solutions with different X-GRL concentrations were calculated (Fig. 5C). More methane was produced in the X-GRL-containing solutions compared to the theoretical values. The differences between the actual detected methane productions and the theoretical values increased with increasing the X-GRL concentration, indicating that the intermediates were further degraded and transferred to methane. The aromatic amines were used as the carbon source by bacteria. The existing electron acceptors such as the nitrate and nitrite,29 iron and sulfate,30 improve the degradation of aromatic amines. In this system, both the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and the –C
N– and the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– with a strong electron withdrawing property, act as the electron acceptors, which accelerate the decomposition and transformation of the aromatic amines.
N– with a strong electron withdrawing property, act as the electron acceptors, which accelerate the decomposition and transformation of the aromatic amines.
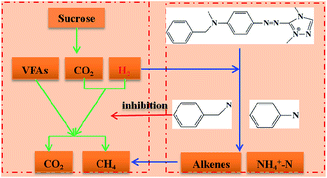
Generally, the biotransformation pathway of X-GRL under anaerobic condition was proposed. As shown in Fig. 6, the reductive VFAs and H2, produced from the sucrose by the Proteobacteria bacteria, reduce the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and –N
N– and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– of X-GRL. The X-GRL with a high toxicity inhibits the bacterial activity. The reduction of X-GRL competes with H2 to the hydrogenotrophic methanogens, leading to a decreased methane production. The generated colorless aromatic amines were further degraded by the Firmicutes bacteria and transferred to methane.
C– of X-GRL. The X-GRL with a high toxicity inhibits the bacterial activity. The reduction of X-GRL competes with H2 to the hydrogenotrophic methanogens, leading to a decreased methane production. The generated colorless aromatic amines were further degraded by the Firmicutes bacteria and transferred to methane.
4. Conclusions
Anaerobic UBF has been demonstrated effective for treating the X-GRL-containing wastewater with complete color removal and high COD removal percentage. The sucrose acts as the electron donor for the X-GRL reduction, generating colorless aromatic amines. The aromatic amines were degraded and transformed to CH4 and NH4+ under anaerobic condition. The X-GRL reduction competed with the hydrogen to the hydrogenotrophic methanogens, leading to a decreased methane production. Bacterial population belonging to phyla Proteobacteria and Firmicutes and archaea belonging to phylum Methnomicrobiales play an important role in the decolorization and degradation of X-GRL. It is great important for the treatment of the azo dye-containing wastewater because the color removal and degradation of the aromatic amines can be achieved simultaneously under an anaerobic condition.Acknowledgements
This work was supported by the Specific Programs in Graduate Science and Technology Innovation of Beijing Forestry University (no. BLYJ201311).References
- D. Rajkumar and J. G. Kim, J. Hazard. Mater., 2006, 136, 203–212 CrossRef CAS PubMed.
- S. Şen and G. N. Demirer, Water Res., 2003, 37, 1868–1878 CrossRef.
- P. I. M. Firmino, M. E. R. da Silva, F. J. Cervantes and A. B. dos Santos, Bioresour. Technol., 2010, 101, 7773–7779 CrossRef CAS PubMed.
- M. Işik and D. T. Sponza, Enzyme Microb. Technol., 2007, 40, 934–939 CrossRef PubMed.
- S. A. Ong, E. Toorisaka, M. Hirata and T. Hano, J. Environ. Sci., 2012, 24, 291–296 CrossRef CAS.
- X. Xiao, C. C. Xu, Y. M. Wu, P. J. Cai, W. W. Li, D. L. Du and H. Q. Yu, Bioresour. Technol., 2012, 110, 86–90 CrossRef CAS PubMed.
- B. Qiu, X. Cheng and D. Sun, Bioresour. Technol., 2012, 113, 102–105 CrossRef CAS PubMed.
- F. P. van der Zee and S. Villaverde, Water Res., 2005, 39, 1425–1440 CrossRef CAS PubMed.
- S. Y. Kim, J. Y. An and B. W. Kim, Dyes Pigm., 2008, 76, 256–263 CrossRef PubMed.
- A. B. dos Santos, M. P. de Madrid, F. A. M. de Bok, A. J. M. Stams, J. B. van Lier and F. J. Cervantes, Enzyme Microb. Technol., 2006, 39, 38–46 CrossRef CAS PubMed.
- X. Wang, X. Cheng, D. Sun and H. Qi, J. Environ. Sci., 2008, 20, 1218–1225 CrossRef CAS.
- K. Rasool, S. H. Woo and D. S. Lee, Chem. Eng. J., 2013, 223, 611–616 CrossRef CAS PubMed.
- T. Joshi, L. Iyengar, K. Singh and S. Garg, Bioresour. Technol., 2008, 99, 7115–7121 CrossRef CAS PubMed.
- M. C. Carvalho, C. Pereira, I. C. Gonçalves, H. M. Pinheiro, A. R. Santos, A. Lopes and M. I. Ferra, Int. Biodeterior. Biodegrad., 2008, 62, 96–103 CrossRef CAS PubMed.
- N. C. G. Tan, G. Lettinga and J. A. Field, Bioresour. Technol., 1999, 67, 35–42 CrossRef CAS.
- Y. Liu, Y. Zhang, X. Quan, J. Zhang, H. Zhao and S. Chen, Bioresour. Technol., 2011, 102, 2578–2584 CrossRef CAS PubMed.
- M. Işik and D. T. Sponza, Process Biochem., 2005, 40, 1053–1062 CrossRef PubMed.
- B. Qiu, Y. Dang, X. Cheng and D. Sun, Water Sci. Technol., 2013, 67, 976–982 CrossRef CAS PubMed.
- A. B. d. Santos, M. P. de Madrid, A. J. M. Stams, J. B. Van Lier and F. J. Cervantes, Biotechnol. Prog., 2005, 21, 1140–1145 CrossRef PubMed.
- E. Razo-Flores, M. Luijten, B. A. Donlon, G. Lettinga and J. A. Field, Environ. Sci. Technol., 1997, 31, 2098–2103 CrossRef CAS.
- H. D. Ariesyady, T. Ito and S. Okabe, Water Res., 2007, 41, 1554–1568 CrossRef CAS PubMed.
- D. Cui, G. Li, D. Zhao, X. Gu, C. Wang and M. Zhao, J. Hazard. Mater., 2012, 221, 185–192 CrossRef PubMed.
- U. U. Jadhav, V. V. Dawkar, G. S. Ghodake and S. P. Govindwar, J. Hazard. Mater., 2008, 158, 507–516 CrossRef CAS PubMed.
- B. Qiu, X. Xu, H. Guo, Y. Dang, X. Cheng and D. Sun, Int. Biodeterior. Biodegrad., 2014, 95, 102–109 CrossRef CAS PubMed.
- L. Tan, Y. Qu, J. Zhou, F. Ma and A. Li, Bioresour. Technol., 2009, 100, 3003–3009 CrossRef CAS PubMed.
- R. Srinivasan, M. N. Kathiravan and K. P. Gopinath, Bioresour. Technol., 2011, 102, 2242–2247 CrossRef CAS PubMed.
- E. a. Razo-Flores, B. Donlon, G. Lettinga and J. A. Field, FEMS Microbiol. Rev., 1997, 20, 525–538 CrossRef CAS PubMed.
- F. P. van der Zee, R. H. M. Bouwman, D. P. B. T. B. Strik, G. Lettinga and J. A. Field, Biotechnol. Bioeng., 2001, 75, 691–701 CrossRef CAS PubMed.
- R. Pereira, L. Pereira, F. P. van der Zee and M. Madalena Alves, Water Res., 2011, 45, 191–200 CrossRef CAS PubMed.
- J. Kazumi, M. M. Häggblom and L. Y. Young, Appl. Microbiol. Biotechnol., 1995, 43, 929–936 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01331c |
| This journal is © The Royal Society of Chemistry 2015 |