Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Push–pull carbazole twin dyads as efficient sensitizers/co-sensitizers for DSSC application: effect of various anchoring groups on photovoltaic performance†
Kavya S.
Keremane
 ab,
Islam M.
Abdellah
ab,
Islam M.
Abdellah
 *cd,
Mohamed R.
Eletmany
de,
Praveen
Naik
*cd,
Mohamed R.
Eletmany
de,
Praveen
Naik
 f,
P.
Anees
g and
Airody Vasudeva
Adhikari
f,
P.
Anees
g and
Airody Vasudeva
Adhikari
 *ah
*ah
aOrganic Materials Laboratory, Department of Chemistry, National Institute of Technology Karnataka, Surathkal, Mangalore-575025, India
bMaterials Research Institute, The Pennsylvania State University, University Park, PA 16802, USA
cChemistry Department, Faculty of Science, Aswan University, Aswan, 81528, Egypt. E-mail: islamabdellah2@gmail.com
dTECS Department, Wilson College of Textiles, NC State University, Raleigh, 27606, USA
eChemistry Department, Faculty of Science, South Valley University, Qena 83523, Egypt
fDepartment of Chemistry, Nitte Meenakshi Institute of Technology, Yelahanka, Bengaluru, 560064, Karnataka, India
gMaterials Science Group, Indira Gandhi Centre for Atomic Research, A CI of Homi Bhabha National Institute, Kalpakkam, 603102, Tamilnadu, India
hYenepoya Research Centre, Yenepoya (Deemed to be) University, Deralakatte, Mangalore, 575018, India. E-mail: avachem@gmail.com
First published on 8th April 2025
Abstract
To investigate the effect of various anchoring groups of organic sensitizers on fundamental processes occurring inside DSSCs and their overall performance, we designed and synthesized nine new double donor–acceptor (D–A) type organic dyes DCH1–9 comprising carbazole-based twin molecules as electron donors, with a non-conjugated linear alkyl chain as an extended linker featuring multiple acceptor units. Their photophysical, thermal, electrochemical, and theoretical properties were examined to gain a deeper understanding of the structure–property relationship. Photophysical results revealed that all dyes display λabs and λemi in the range of 400–470 nm and 500–560 nm, respectively, with a bandgap in the range of 2.46–2.74 eV. The push–pull structure with extended conjugation results in strong fluorescence characteristics. Photophysical and electrochemical studies confirm their thermodynamic feasibility for electron injection, recombination, and dye regeneration in cells. Quantum chemical simulations further provided insights into their structural, electronic, and optical parameters. New DSSCs were fabricated employing dyes DCH1–9 as sensitizers/co-sensitizers. The cell sensitized with DCH1 achieved the highest power conversion efficiency (PCE) of 2.45% under standard AM 1.5 solar conditions. Furthermore, co-sensitization of DCH1–9 with the Ru-based HD-2 sensitizer resulted in an improved PCE of 8.82% for DCH2, surpassing HD-2 alone (6.79%). EIS studies were conducted to further explore their energy conversion processes. Conclusively, these investigations highlight the significant potential of dyes carrying carbazole twin molecules with different anchoring units in enhancing the overall performance of DSSCs.
Introduction
In the pursuit of clean and solar energy conversion and production from renewable energy resources, dye-sensitized solar cells (DSSCs) have emerged as an important breakthrough because of their potential low processing costs, facile fabrication, and eco-friendly devices.1,2 These devices are particularly advantageous for dim, diffuse light conversion in indoor applications. Additionally, in non-ideal atmosphere, they generate electricity directly from sunlight due to their partial optical transparency.3 DSSCs typically consist of a dye-adsorbed mesoporous semiconductor TiO2 layer deposited on a transparent conducting oxide (FTO) layer, coupled with a platinum counter electrode filled with an electrolyte containing a redox system.4 Among the four major components of DSSCs, the light-harvesting component photosensitizers (or dyes) play a pivotal role in the overall performance, since it facilitates light absorption and injects the excited electrons into the conduction band of TiO2.5,6 An ideal dye must fulfil certain features, such as (i) high photostability, (ii) superior intramolecular charge transfer, (iii) appropriate electronic energy between HOMO and LUMO levels, (iv) favourable anchoring group to firmly bind on TiO2, (v) rapid electron injection to TiO2 and reduced recombination, and (vi) broad structural diversity.7,8Over the last three decades, organic dyes featuring donor–acceptor (D–A) or donor–π–acceptor (D–π–A) motifs have been widely studied, particularly those with carbazole cores due to their excellent charge-transport properties, energy levels, and stability.9–11 Carbazole-based di-anchoring dyes, such as A–D–π–A, A–π–D–π–A configurations, have shown great promise in DSSCs due to their multi-binding ability, increased dye loading, and enhanced electron extraction, which leads to improved device performance.12–15 Dyes with double donor–acceptor units and extended alkyl chain linkers have proven to be more effective than single donor–acceptor dyes, as they suppress dark currents, increase light absorption efficiency, and improve the electron injection and lifetime.16–22 Twin molecules with non-conjugated alkyl chains have demonstrated state-of-the-art performance due to their better solubility and improved pore-filling in mesoporous TiO2.
In general, the efficiency of DSSCs largely depends on the interaction between the dye and the TiO2 semiconductor surface.14,23–27 A strong electronic overlap between the two ensures rapid charge transfer, making the anchoring group crucial for binding the dye to the TiO2 layer. Thus, the anchoring group plays a pivotal role. Different anchoring groups, such as carboxylic, cyanoacrylic, rhodamine-3-acetic acid, and phosphonic acids, have been explored to understand their impact on dye binding, electron injection, and long-term stability.28–31 Among all, cyanoacrylic acid (CAA) is widely recognized as an effective anchoring group. However, exploring dyes with alternative anchoring or electron-withdrawing groups remains essential for advancing this technology. Despite this need, very few studies have systematically compared the impacts of different anchoring groups on dye aggregation, electron injection, and recombination kinetics in dual donor–acceptor dyes with extended linkers. In this context, it is necessary to compare the effect of various anchoring groups to address the undesirable processes above in solar cells.32–35 The primary objective of our study is to systematically evaluate different anchoring groups beyond the commonly used CAA, and assess their influence on the photophysical and electrochemical properties. Our findings contribute to a broader understanding of how alternative anchoring groups, including rhodanine-3-acetic acid and barbituric acid derivatives can be optimized for DSSCs.36–38 Each new anchoring group brings the potential for unique electronic interactions with the semiconductor surface, which could optimize electron injection or reduce charge recombination in ways not achievable with traditional groups. Exploring new groups also enables tailored designs for specialized applications, such as indoor photovoltaics or building-integrated systems. This pursuit expands the versatility, efficiency, and sustainability of DSSCs, while still acknowledging the value of established moieties.
Furthermore, concerted companion (CC) dyes have emerged as a promising approach to enhance the DSSC efficiency by linking two complementary dye units to achieve broad absorption and improved photovoltaic performance.39 As an emerging class of di-branched dyes, CC dyes have been designed to enhance the DSSC efficiency by linking porphyrin and organic sub-dye units. The intramolecular engineering of CC dyes simplifies the adsorption process while enhancing the efficiency and stability. Di-branched dyes, which integrate porphyrin and organic dye subunits, have demonstrated superior light-harvesting capabilities. Notably, the incorporation of wrapped porphyrin dye units has led to the development of doubly concerted companion (DCC) effects, further improving the performance. For instance, Xie et al. reported on a series of CC dyes, where an optimized linker length improved the molecular orientation and resulted in a PCE of up to 12.4% after coadsorption with CDCA.40–42 In addition to the excellent efficiency, this strategy shows the advantages of avoiding the competitive adsorption problem and time-consuming optimization of the dye adsorption process. Additionally, double-anchored dyes exhibit stronger adsorption, enhancing cell stability, with less than 10% PCE loss after 1000 hours of light exposure, making them a superior alternative to traditional DSSC dyes. Moving forward, the co-sensitization and CC dye strategies should be further explored to achieve enhanced panchromatic absorption, particularly in the near-infrared (NIR) region.43,44
Co-sensitization is another way to optimize the performance of DSSCs.45 It is a key strategy to overcome the limitations of using a single dye in the cell, as organic dyes often have narrow absorption spectra and limited photon-harvesting capabilities.46 By combining two or more dyes, the overall light-harvesting ability can be enhanced. For example, Ru(II) dyes, which have lower absorption in the higher wavelength region, can be complemented by dyes with higher molar extinction coefficients, improving light absorption and boosting power conversion efficiency (PCE).47,48 To meet the stringent requirements of high-performance sensitizers, Nazeeruddin et al.49 successfully developed a panchromatic ruthenium complex, known as black dye, incorporating carboxylated terpyridyl and three thiocyanate ligands, achieving a power conversion efficiency (PCE) of 10.4%. Similarly, DSSCs employing the widely used N719 sensitizer, demonstrated PCEs exceeding 9.18% under AM 1.5 sunlight. Wang et al. later introduced K19, an amphiphilic ruthenium complex, which exhibited a PCE of 7.0%.50
In recent years, extensive research efforts have been directed toward the development of efficient sensitizers based on organic frameworks, zinc porphyrins, and ruthenium complexes. Notably, porphyrin-based dyes have achieved efficiencies above 13%, making them promising candidates for DSSCs.51,52 However, porphyrin dyes inherently suffer from limited absorption in the UV, green, and near-infrared (NIR) regions, which restricts their overall efficiency. For successful co-sensitization, the energy levels (HOMO and LUMO) of the dyes must align to ensure efficient electron injection into the TiO2 conduction band and smooth dye regeneration. We selected HD-2 dye for co-sensitization due to its broad absorption, stability, efficient charge transfer, and strong anchoring properties.53 The chemical stability of HD-2 and its ability to complement organic or metal-free dyes enhance the performance of DSSCs by improving light harvesting, reducing recombination, and increasing overall efficiency. Recent studies show that co-sensitization with donor–acceptor organic dyes and Ru-based complexes leads to enhanced photovoltaic performance. This improvement is attributed to better adsorption into the pores of TiO2, where bulky ruthenium dyes struggle. Understanding the role of simple co-sensitizers and optimizing interfacial charge transfer are crucial for maximizing the DSSC efficiency.54–58
Encouraged by this, in order to enhance the light-harvesting units and broaden the absorption spectra, in the present study we have designed nine novel dual D–A configured organic dyes DCH1–9, carrying twin carbazole units as an effective electron donor, and cyanoacetic acid (DCH1), rhodanine-3-acetic acid (DCH2), rhodamine (DCH3), barbituric acid (DCH4), 1,3-dimethylbarbituric acid (DCH5), 1,3-diethyl-2-thiobarbituric acid (DCH6), 2,4-thiazolidinedione (DCH7), oxindole (2-indolone) (DCH8), and 4-nitrophenyl acetonitrile (DCH9) groups were employed as an electron acceptor/anchoring units, while molecular designing. The bridged non-conjugated linear alkyl chain of double-branched dyes facilitates higher surface coverage on TiO2 films, influencing the overall performance of solar cell.
These final molecules were synthesized in good yields by adopting simple synthetic protocols, viz., N-alkylation, Vilsmeier–Hack reaction, followed by Knoevenagel condensation reactions (Scheme 1), and the respective structures are shown in Fig. 1. The target molecules and intermediates were well characterized using elemental analyses and different spectroscopic techniques. The photophysical and electrochemical characterization, including energy conversion (ΔGinj, ΔGrec, and ΔGreg), were evaluated using UV-visible absorption/photoluminescence (PL) studies and cyclic voltammetric (CV) studies, respectively. Furthermore, the thermal stability of synthesized dyes was evaluated using TGA and DSC studies. Density functional theory (DFT) and time-dependent density functional theory (TD-DFT) were also used to investigate the molecular geometry, electron distributions, absorption and charge-transfer mechanism, electrostatic potential (ESP), and density of states (DOS) of the synthesized dyes theoretically using Turbomole 7.2 V and VASP software. Eventually, the dyes DCH1–9 were employed as sensitizers and co-sensitizers with Ru(II) based HD-2 dye towards the DSSC fabrication, and their photovoltaic performance data were investigated. Finally, electrochemical impedance spectroscopy (EIS) studies were carried out to understand their interfacial charge recombination and carrier transportation processes (RS, RCT, and RPt) in depth with respect to both sensitization and co-sensitization processes.
 | ||
| Fig. 1 Chemical structures of the dual donor–acceptor configured new dyes DCH1–9 and the benchmark reference dye HD-2. | ||
Results and discussion
Synthesis and characterization
The synthetic pathways of seven new metal-free organic dyes DCH1–9 are shown in Scheme 1. The requisite intermediate 1,6-di(9H-carbazole-9-yl)hexane (1) was obtained from 9H-carbazole by treating it with 1,6-dibromohexane in the presence of a strong base, i.e., sodium hydride. This was converted to the key precursor 9,9′-(hexane-1,6-diyl)bis(9H-carbazole-3-carbaldehyde) (2) through the Vilsmeier–Hack reaction protocol with good yield. In the final step, the target molecules DCH1–9 were obtained by following the Knoevenagel condensation protocol, wherein the precursor 2 was condensed with various active methylene compounds. All the synthesized compounds were further purified using the typical recrystallization and chromatographic techniques. The molecular structures of these target compounds and their intermediates were established by using different spectroscopy tools. Their spectra are displayed in S1–S39 of ESI.†Theoretical studies
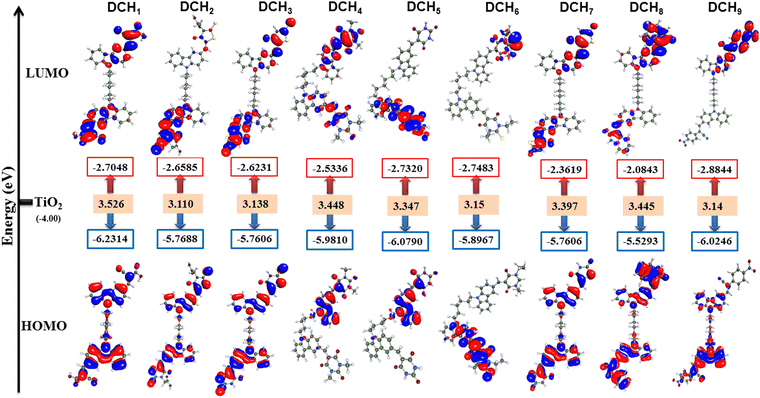
To gain deeper insight into the molecular structure, electronic distribution, and frontier molecular orbitals of DCH1–9, molecular orbital calculations were performed using the density functional theory (DFT). All the theoretical calculations were executed using the Turbomole 7.2 software package. The ground-state geometries of the synthesized dye molecules in a gas phase were optimized based on a semiempirical AM1 basis with MOPAC in Tmolex.59,60 The aforementioned geometries were further optimized using C1 point group symmetry via Becke's three-parameter hybrid functional and Lee–Yang–Parr's gradient-corrected correlation functional (B3LYP) program and basic set def-TZVPP.61 The optimized geometries of dyes DCH1–9 are given in Fig. S40 (ESI†). The geometry of the twin carbazole unit in each dye was not precisely planar, but rather slightly tilted in the middle with a butterfly shape. Therefore, the non-planar geometry of the carbazole ring can help to suppress the dye aggregation on the TiO2 surface. The electronic density distributions in the HOMO and LUMO levels of the nine different dyes DCH1–9, along with their bandgaps, are shown in Fig. 2 and the corresponding data are tabulated in Table 1. Furthermore, to understand the adsorption and charge-transfer mechanism in the presence of a supporting substrate, density functional theory (DFT) calculations were performed using the Vienna Ab initio Simulation Package (VASP). The DCH1 dye interaction with the TiO2 semiconductor is shown in Fig. S41 (details can be found in ESI†). Evaluating the FMO distribution allows for a qualitative prediction of a dye's effectiveness in DSSCs. It is equally important to analyze the molecular orbitals adjacent to the FMOs, as they significantly influence the spectral behavior of the dye molecules. A LUMO distribution localized on the acceptor moiety is particularly advantageous, as it helps the sensitizer to anchor more effectively to the TiO2 surface, facilitating intramolecular charge transfer. The bandgap energy plays a crucial role in determining the dye's photocurrent, as it governs the absorption of light at relevant wavelengths. Efficient HOMO and LUMO separation promotes effective charge separation, unlike insufficiently separated HOMO−1 and LUMO+1 distributions, ensuring better electron transfer and overall DSSC performance. As seen from Fig. 2, the electrons at the highest occupied molecular orbital (HOMO) levels are mainly populated on the carbazole donor unit, whereas the electron density distribution in the lowest unoccupied molecular orbital (LUMO) is sizably delocalized through the acceptor part of DCH1–9. The LUMO and LUMO+1 levels of the dye molecules are largely dispersed on cyanoacetic acid, while the majority of the HOMO energy levels are delocalized on the electron-donating unit. The closely overlapping HOMO–LUMO orbitals act as a channel for a quick ICT process. Thus, there is a clear electron cloud movement from the donor moiety towards the anchoring unit, facilitating an efficient photoinduced interfacial electron transfer from LUMO to the semiconductor electrode through effective intramolecular charge separation under light irradiation.| Parameters | DCH1 | DCH2 | DCH3 | DCH4 | DCH5 | DCH6 | DCH7 | DCH8 | DCH9 |
|---|---|---|---|---|---|---|---|---|---|
| a These values were obtained from DFT calculations in a vacuum. | |||||||||
| E HOMO (eV) | −6.23 | −5.76 | −5.76 | −5.98 | −6.07 | −5.89 | −5.76 | −5.52 | −6.02 |
| E LUMO (eV) | −2.70 | −2.65 | −2.62 | −2.53 | −2.73 | −2.74 | −2.36 | −2.08 | −2.88 |
| E g (eV) | 3.52 | 3.11 | 3.13 | 3.44 | 3.34 | 3.15 | 3.39 | 3.44 | 3.14 |
The theoretical HOMO energy levels obtained for dyes DCH1–9 are −6.231 (DCH1), −5.768 (DCH2), −5.760 (DCH3), −5.981 (DCH4), −6.079 (DCH5), −5.896 (DCH6), −5.760 (DCH7), −5.529 (DCH8), and −6.024 (DCH9), respectively, which are significantly lower than that of the redox potential of the I3−/I− electrolyte system (−5.2 eV), confirming that the synthesized dyes can undergo a quick ground-state regeneration process. The theoretical LUMO energy levels obtained for dyes DCH1–9 are −2.704 (DCH1), −2.658 (DCH2), −2.623 (DCH3), −2.533 (DCH4), −2.732 (DCH5), −2.748 (DCH6), −2.361 (DCH7), −2.084 (DCH8), and −2.884 (DCH9), respectively, which are significantly higher than the conduction band (CB) of TiO2 (–4.2 eV), indicating their fast electron injection. The theoretical bandgap obtained for dyes DCH1–9 are 3.52 (DCH1), 3.11 (DCH2), 3.13 (DCH3), 3.44 (DCH4), 3.34 (DCH5), 3.15 (DCH6), 3.39 (DCH7), 3.44 (DCH8), and 3.14 (DCH9), respectively, which may be due to the different anchoring abilities of the molecules. Conclusively, the well-overlapped HOMO and LUMO orbitals of the dyes DCH1–9 can guarantee a superior charge transfer and the efficient interfacial injection of electrons from the excited state of the dye molecule into the conduction band of the TiO2 semiconductor.
In addition, the electronic excited state properties of materials were studied in depth using the time-dependent density functional theory (TD-DFT) method in the presence of time-dependent perturbations. According to the adiabatic approximation, the temporal nonlocality is neglected and an assumption is made that at any point of time, the exchange–correlation (xc) functional depends exclusively on the instantaneous density.62 Based on the aforesaid approximation, time-dependent perturbations can be applied to the xc functional derived for ground-state DFT, i.e., BP (Beck–Perdew) and hybrid functional (B3LYP). In TD-DFT calculations, the accuracy of the assimilated results mainly relies on the functional and basis set employed for the calculations.63
Fig. S42 (ESI†) displays the theoretically simulated absorption spectra of the dyes DCH1–9, obtained by employing the B3LYP functional and def-TZVP basis set. The adiabatic approximation states that the TD-DFT temporal nonlocality is ignored, and that the exchange–correlation (xc) component solely depends on the instantaneous density at any given moment.64 Therefore, the hybrid functional (B3LYP) and BP (Beck–Perdew) as xc functionals were derived for ground-state DFT. The correctness of assimilation findings in TD-DFT computations is typically determined by the basic set employed for the calculations. The theoretically calculated absorption maxima (λmax) of the investigated molecules DCH1–9 are summarized in Table 1. Furthermore, the simulated spectra of DCH1–9 depict two distinct peaks in the spectrum, belonging to the mixed π–π* transition and charge-transfer process within the molecules, which help to predict the energies related to long-range charge-transfer states.
In all of the synthesized molecules, a strong intra-dye electronic excitation occurs, owing to the HOMO → LUMO transitions. Here, the excitations at the longer wavelengths are due to the electronic transitions from the HOMO to LUMO+1 and LUMO+3. Accordingly, the lower-energy excitations are ascribed to intramolecular charge transfer (ICT) transitions from DCH1–9 to TiO2. These exactitudes of the TD-DFT results illustrate that the functional and basis set chosen for the studies are in accordance with the experimentally obtained results. From the TD-DFT results, it is clear that the functional and basis sets used for the investigations are in good agreement with the outcomes of the experimental results. The theoretically simulated IR-absorption spectra of DCH1–9 are displayed in Fig. S43 (ESI†). As expected, both the simulated and experimentally obtained results are in good agreement with each other.
To investigate the distribution of total charge density around the dye molecules, electrostatic potential (ESP) maps of DCH1–9 have been generated, as illustrated in Fig. 3. This analysis explores the ESP to predict the physicochemical properties, focusing on regions of electrophilic and nucleophilic attack and areas of electrostatic potential. The three-dimensional visualization of the ESP plot provides a better understanding of the electrostatic influence of the molecules.55 The molecular electrostatic potential, which is linked to electron density, plays a key role in understanding hydrogen bonding interactions, as well as the reactivity of electrophilic and nucleophilic sites. The cavity boundaries in the molecules resemble the density isosurface in the ESP plot, reflecting the total charge distribution, including electronegativity, dipole moment, and sites of chemical reactivity. The electrostatic potential values are color-coded in increasing order as follows: blue > green > yellow > orange > red.11 In these plots, electron-rich regions are indicated in red, while electron-deficient regions are shown in blue. This induces positive and negative charges on the cavity surface, creating local electric fields within the cavity. Green represents regions of zero electrostatic potential.
The ESP surfaces reveal both positive and negative regions: the positive regions are preferred sites for nucleophilic attacks, while the negative regions are more suitable for electrophilic attacks.63 From Fig. 3, it is clear that in dyes DCH1–2, the electron density is primarily concentrated on the cyanovinylene and carbonyl groups of cyanoacetic acid. In contrast, in DCH3, DCH7, and DCH8, the electron density is focused on the thioketone and carbonyl groups of rhodanine derivatives and oxindole. For DCH4–6, the electron density is primarily located on the carbonyl groups of barbituric acid and its derivatives. Additionally, the positive region in DCH1–9 is localized on the carbazole rings, indicating that these groups can act as efficient donors and are attractive sites for nucleophilic attacks. On the other hand, the acceptor groups, located on the opposite side of the molecule, are surrounded by regions of zero potential, suggesting vulnerability to electrophilic attacks.
Overall, the ESP plots clearly illustrate the movement of electron density from the donor moiety to the anchoring unit via spacer units, facilitating effective electron transfer in DCH1–9. This observation makes these dyes promising candidates for use as sensitizers in dye-sensitized solar cells (DSSC).
According to quantum mechanics, the density of states (DOS) of a system primarily refers to the number of available energy levels per unit energy increment. It represents the number of states available for occupation within a specific energy range.29,33 A high DOS value at a given energy level indicates that many states are available for occupation, while a zero DOS signifies that no free states are available at that energy level. In essence, DOS provides an average over time and space within the system, and describes the contribution of different orbitals across a range of energies. Moreover, DOS is crucial for understanding the charge transfer between molecules and the TiO2 semiconductor, which generates dipolar fields at the TiO2/material interface, ultimately tuning the band energy levels. A material with a smaller bandgap can facilitate faster electron transport, enhancing the driving force for rapid charge injection at the interface.32,55 The DOS plots of DCH1–9, shown in Fig. S44 (ESI†), illustrate how different orbitals contribute as the energy increases. The figure shows that as the energy rises, the contribution of p-orbitals becomes more dominant compared to s-orbitals. Additionally, the p-orbitals of oxygen and nitrogen atoms play a significant role in binding the dye to TiO2. All newly synthesized molecules exhibit similar DOS patterns, suggesting they meet the necessary criteria. Furthermore, the DOS spectra of the dyes show no significant energy gaps, but the results are consistent with existing literature. All dyes (DCH1–9) demonstrate significant charge transfer from the donor to the acceptor unit, confirming that they fulfill the basic requirements of an ideal sensitizer.
Photophysical properties
The UV-vis absorption spectra of double D–A dyes (DCH1–9) were measured in N,N-dimethyl formamide (DMF) solution at 10−5 M conc. at room temperature (Fig. 4(a)), and the corresponding spectral parameters are summarized in Table 2. All the dyes showed two distinct strong and broad absorption spectra in the ultraviolet and visible regions. The lower absorption band in the region of 280–370 nm is ascribed to the π–π* electronic excitation of conjugated molecules, whereas the higher absorption band (402–468 nm) is assigned to the intramolecular charge transfer (ICT) between the donor and acceptor unit. As shown in Fig. 4(a), the observed major λmax at 402 nm (DCH1), 422 nm (DCH2), 421 nm (DCH3), 450 nm (DCH4), 445 nm (DCH5), 468 nm (DCH6), 397 nm (DCH7), 419 nm (DCH8), and 440 nm (DCH9) are attributed to mixed intramolecular charge transfer (ICT)/π–π* transition of the chromophores, which was further confirmed by TD-DFT calculations.55 From the results, we emphasize that the λabs of organic sensitizers DCH4–6 show a bathochromic shift when compared to the other dyes. The dye DCH6 exhibits a significantly broader and more intense absorption than other dyes, likely due to its extended conjugation and enhanced light-harvesting ability, which is attributed to the presence of the strong electron-withdrawing 1,3-diethyl-2-thiobarbituric acid group. This group is a potent electron acceptor because the sulfur (S) atom in its structure increases the electron-accepting efficiency compared to the oxygen (O) atom in conventional barbituric acid. The sulfur substitution lowers the energy gap for electronic transitions, causing a redshift in the absorption peak. Additionally, the higher polarizability of sulfur improves the charge-transfer efficiency, contributing to the broader absorption profile by making the electronic transitions more accessible. | ||
| Fig. 4 (a) Normalized UV-Vis absorption and (b) fluorescence emission spectra of DCH1–9 recorded in 10−5 M DMF solution under ambient atmosphere. | ||
| Parameters | DCH1 | DCH2 | DCH3 | DCH4 | DCH5 | DCH6 | DCH7 | DCH8 | DCH9 |
|---|---|---|---|---|---|---|---|---|---|
| a Absorption and emission spectra measured in DMF (at concentration of 10−5 M) at room temperature. ε (M−1 cm−1): molar extinction coefficient. b Optical band gap E0–0 is the voltage of the intersection point between the absorption and emission spectra. c T d decomposition temperature observed from TGA 5% of weight loss at 10 °C min−1 and Tm observed from DSC at 10 °C min−1 under nitrogen atmosphere. | |||||||||
| λ abs (nm) | 402 | 422 | 421 | 450 | 445 | 468 | 397 | 419 | 440 |
| λ emi (nm) | 510 | 530 | 516 | 553 | 535 | 562 | 500 | 512 | 524 |
| Stokes shifta (cm−1) | 5275 | 4896 | 4260 | 4142 | 3782 | 3577 | 5188 | 4336 | 4080 |
| ε (M−1 cm−1) at λabsa (nm) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 393 393 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 707 707 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 930 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 468 468 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 617 617 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738 738 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 720 |
E
0–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) opt
(eV) opt
(eV) |
2.74 | 2.70 | 2.65 | 2.64 | 2.56 | 2.46 | 2.73 | 2.62 | 2.61 |
| T m (°C) | 281 | 334 | 294 | 205 | 216 | 291 | 330 | 259 | 191 |
| T d (°C) | 315 | 340 | 326 | 302 | 307 | 318 | 336 | 310 | 258 |
The fluorescence emission spectra of the dyes DCH1–9 are recorded in DMF solution at 10−5 M concentration by exciting at their respective absorption maxima. Fig. 4(b) represents the normalized emission spectra of synthesized dyes DCH1–9, and their resultant spectral parameters are tabulated in Table 2. All the dye molecules exhibit a characteristic single emission band in the region of 500–562 nm region. The molecule DCH6 displays λemi of 562 nm, which is slightly more redshifted than that of DCH1 (510 nm), DCH2 (530 nm), and DCH3 (516 nm), DCH4 (553 nm), DCH5 (535 nm), DCH7 (501 nm), DCH8 (512 nm), and DCH9 (524 nm), respectively, which may be due to the presence of strong electron-withdrawing 1,3-diethyl-2-thiobarbituric acid. The observed shift is desirable as it escalates the light-harvesting nature of the sensitizer in the visible region. Furthermore, we calculated the Stokes shift values of the dyes. The values are listed in an increasing order of DCH6 (3577 cm−1) < DCH5 (3582 cm−1) < DCH9 < (4080 cm−1) < DCH4 (3577 cm−1) < DCH3 (4260 cm−1) < DCH8 (4336 cm−1) < DCH2 (4896 cm−1) < DCH7 (5188 cm−1) < DCH1 (5275 cm−1), respectively. It is noted that the Stokes shift values of the barbituric acid derivatives are smaller than that of the cyanoacetic acid and rhodanine derivatives, which could have resulted from the higher planarity, also illustrating smaller conformational changes in the excited state.29 The dye DCH1 displayed the highest value of Stokes shift, which may be caused by large changes in the dipole moment in the excited state, as a result of charge transfer from the donor to the acceptor moiety. Additionally, the optical bandgap values of the synthesized dyes were calculated from the obtained absorption and emission spectral data. The acquired bandgaps of DCH1–9 decrease in the following order: 2.74 eV (DCH1) > 2.73 eV (DCH7) > 2.70 eV (DCH2) > 2.65 eV (DCH3) > 2.64 eV (DCH4) > 2.62 eV (DCH8) > 2.61 eV (DCH9) > 2.56 eV (DCH5) > 2.46 eV (DCH6). Furthermore, the molar absorption coefficients (ε) of the ICT bands were calculated, and are in the range of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 M−1 cm−1 to 25
720 M−1 cm−1 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 396 M−1 cm−1. Among all, the dye DCH2 showed the highest ‘ε’ value, indicating enhanced light-harvesting ability of the strong electron-withdrawing rhodanine-3-acetic acid unit in the visible region, which could produce greater short-circuit current and thus show improved performance.
396 M−1 cm−1. Among all, the dye DCH2 showed the highest ‘ε’ value, indicating enhanced light-harvesting ability of the strong electron-withdrawing rhodanine-3-acetic acid unit in the visible region, which could produce greater short-circuit current and thus show improved performance.
To further understand the light-harvesting capabilities of the synthesized dyes in DSSC fabrication, a separate experiment was conducted in which the dyes were adsorbed onto the TiO2 surface, and the corresponding UV-vis spectra were recorded. Fig. 5 shows the absorption spectra of the DCH1–9 dyes adsorbed on TiO2 films. The spectra reveal that all dyes exhibited broader absorption bands compared to their peaks in the solution state. This broadening and the observed bathochromic shift (redshift) are attributed to the interaction between the anchoring groups of the dye molecules and titanium ions on the electrode surface, along with the delocalization of the dye molecules, which lowers the overall energy of the π* orbital.55 Among all the dyes, DCH2 showed the most significant redshift over an extended region, suggesting a strong tendency to form J-aggregates on the TiO2 surface. This is largely due to the close interaction between the carboxylic anchor groups and Ti4+ ions of the semiconductor. Rhodanine-3-acetic acid contains both a carboxyl group (–COOH) and a thiazolidine ring, allowing for strong binding interactions with the TiO2 surface, particularly through stable covalent bonding between the carboxyl group and titanium atoms (Ti4+), ensuring efficient attachment of the dye to the semiconductor.33 Furthermore, the rigid structures of DCH2 and HD-2 help to maintain the dye orientation on the TiO2 surface, improving the electron injection efficiency and preventing dye desorption under prolonged illumination. Therefore, the DCH2 dye can be considered a promising candidate for light-harvesting applications due to its strong anchoring capabilities.
Thermal properties
The thermal properties of the dyes DCH1–9 were investigated by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) under nitrogen atmosphere with a heating rate of 10 °C min−1. The TGA and DSC plots of dyes DCH1–9 are shown in Fig. 6 and Fig. S45 (ESI†), respectively, and the corresponding thermal data are tabulated in Table 2. All the synthesized dyes exhibit satisfactory thermal stability with high thermal decomposition (Td) temperatures at 315 °C (DCH1), 340 °C (DCH2), 326 °C (DCH3), 302 °C (DCH4), 307 °C (DCH5), 318 °C (DCH6), 336 °C (DCH7), 310 °C (DCH8), and 258 °C (DCH9). The melting temperature ™ of dyes DCH1–9 was found to be higher than 200 °C. Among all, the dye DCH2 bear rhodaminenine-3-acetic acid as an anchoring group shows higher thermal stability, making it a more relevant material for solar cell applications. Thus, because of their promising high thermal property, their application as sensitizer/co-sensitizer in DSSC would impart enhanced stability to the fabricated devices. | ||
| Fig. 6 TGA thermograms of DCH1–9 determined at a heating rate of 10 °C min−1 under a nitrogen atmosphere. | ||
Electrochemical characterization
The feasibility of key thermodynamic processes in the solar cell, such as active electron injection from the excited state of the dye to the conduction band (CB) of the TiO2 photoanode, and dye regeneration by suppressing the charge recombination between the oxidized dye molecule and the TiO2 CB, can be effectively analyzed using cyclic voltammetry (CV). The electrochemical properties of DCH1–9 were measured using a conventional three-electrode system, where Ag/AgCl, glassy carbon, and a platinum plate served as the reference, working, and counter electrodes, respectively.32,65 Tetra n-butyl ammonium hexafluorophosphate was used as the supporting electrolyte in acetonitrile at a scan rate of 100 mV s−1. The resulting voltammograms of DCH1–9 are shown in Fig. S46 (ESI†). From the experimental data, the highest occupied molecular orbital/ground-state oxidation potential (HOMO/GSOP) and lowest unoccupied molecular orbital/excited state oxidation potential (LUMO/ESOP) values were calculated from the onset oxidation potential of the oxidation peak, and these values are presented in Table 3. The energy level diagram of DCH1–9 is depicted in Fig. 7. The GSOP and ESOP values were converted from volts (V) to electron volts (eV) relative to NHE using eqn (1) and (2).11,63| GSOP = [Eoxdonset + 4.7 eV] | (1) |
| ESOP = [GSOP − E0–0] eV | (2) |
| Compound | E OX (V vs. NHE) |
E
#OX
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (V vs. NHE)
(V vs. NHE) |
HOMOb (eV) | LUMOb (eV) | ΔGinj (eV) | ΔGrec (eV) | ΔGreg (eV) |
|---|---|---|---|---|---|---|---|
| a The E* values were formulated by, E#OX = EOX − E0–0. b All the potentials were obtained during cyclic voltammetric investigations in 0.1 M Bu4NPF6 in DMF, and platinum electrode diameter: 1 mm, sweep rate: 100 mV s−1. | |||||||
| DCH1 | 0.51 | −2.23 | −5.21 | −2.47 | 1.73 | −1.01 | −0.11 |
| DCH2 | 0.53 | −2.17 | −5.23 | −2.53 | −1.67 | −1.03 | −0.13 |
| DCH3 | 0.57 | −2.08 | −5.27 | −2.62 | −1.58 | −1.07 | −0.17 |
| DCH4 | 0.58 | −2.06 | −5.28 | −2.64 | −1.56 | −1.08 | −0.18 |
| DCH5 | 0.76 | −1.80 | −5.46 | −2.90 | −1.30 | −1.26 | −0.36 |
| DCH6 | 0.77 | −1.68 | −5.47 | 3.01 | −1.19 | −1.27 | −0.37 |
| DCH7 | 0.525 | −2.20 | −5.23 | −2.49 | −1.70 | −1.02 | −0.12 |
| DCH8 | 0.66 | −1.96 | −5.36 | −2.74 | −1.46 | −1.16 | −0.26 |
| DCH9 | 0.67 | −1.94 | −5.37 | −2.76 | −1.44 | −1.17 | −0.27 |
 | ||
| Fig. 7 Molecular energy level diagram showing the experimental HOMO, LUMO, and bandgap values of DCH1–9. | ||
The experimental GSOP/HOMO values of the dyes DCH1–9 were found to be −5.21 (DCH1), −5.23 (DCH2), −5.27 (DCH3), −5.28 (DCH4), −5.46 (DCH5), −5.47 (DCH6), −5.23 (DCH7), −5.36 (DCH8), −5.37 (DCH9) respectively, which are much lower than that of the redox potential of the I3−/I− electrolyte system (−5.2 eV), favouring quick ground-state regeneration process. Among all, the dye DCH1 has the highest positive potential, stabilizing the dye molecule in its oxidized state and facilitating effective electron injection, which is necessary for the affirmative transition of charges through the energy conversion cycle. The reason may be due to the presence of superior electron-donating carbazole twin moieties and a strong cyanoacetic acid as an anchoring unit. Cyanoacetic acid generally exhibits a higher oxidation potential due to its strong electron-withdrawing cyano (–CN) group.66 This higher potential can enhance electron injection from the dye to the TiO2 conduction band, making it favorable for applications that require strong electron transfer. Furthermore, the experimental ESOP/LUMO values of the dyes DCH1–9 are found to be −2.47 (DCH1), −2.53 (DCH2), −2.62 (DCH3), −2.64 (DCH4), −2.90 (DCH5), −3.01 (DCH6), −2.49 (DCH7), −2.74 (DCH8), −2.76 (DCH9), respectively, which are greater than the potential of the CB of the TiO2 semiconductor (−4.2 eV), facilitating enhanced electron injection phenomenon. Among all the dyes, DCH1 has ideal HOMO and LUMO levels, facilitating an adequate thermodynamic driving force that is mandatory for the affirmative transition of changes throughout the energy cycle.
To investigate the different thermodynamic pathways, such as dye injection, recombination, and regeneration processes of DCH1–9, the driving forces, viz., free energies (ΔGinj, ΔGrec, and ΔGreg) were estimated using eqn (3) through eqn (5), and the corresponding data are given in Table 3.
 | (3) |
| ΔGrec = ECB − EOX | (4) |
 | (5) |
From the calculated data, it is clear that all the free energy values are negative, describing the thermodynamical feasibility of the aforesaid processes. The ΔGinj (free energy for electron injection from LUMO to the conduction band of TiO2) values were calculated from the difference between the LUMO level of the dyes and semiconductor, and are in the following order: DCH1 (–1.73 eV) > DCH7 (–1.70 eV) > DCH2 (–1.67 eV) > DCH3 (–1.58 eV) > DCH4 (–1.56 eV) > DCH8 (–1.46 eV) > DCH9 (–1.44 eV) > DCH5 (–1.30 eV) > DCH6 (–1.19 eV). It is worth noting that the dye DCH1 has the highest value of ΔGinj, indicating efficient electron injection to the CB of TiO2. The reason may be due to the presence of the strong electron-withdrawing nature of cyanoacetic acid, along with the increased carbazole donor strength. Its D–A strength thermodynamically drags electrons from the LUMO energy level, and injects them to the CB edge of the TiO2 and HOMO energy level towards the redox electrolyte. The calculated ΔGrec values of dyes DCH1–9 are as follows: −1.01 (DCH1), −1.03 (DCH2), −1.07 (DCH3), −1.08 (DCH4), −1.26 (DCH5), −1.27 (DCH6), −1.02 (DCH7), −1.16 (DCH8), −1.17 (DCH9), respectively. Similarly, the ΔGreg values of dyes DCH1–9 are found to be: −0.11 (DCH1), −0.13 (DCH2), −0.17 (DCH3), −0.18 (DCH4), −0.36 (DCH5), −0.37 (DCH6), −0.12 (DCH7), −0.26 (DCH8), −0.27 (DCH9). From the results, it is clear that the dye DCH1 comprising two carbazole donor moieties and the strong electron acceptor cyanoacetic acid shows the highest negative free energy for the electron regeneration process to resist its recombination between electron injection and photo-oxidized dye molecules, making the ΔGrec and ΔGreg processes energetically favourable. The presence of a long linear alkyl chain functions as an insulating barrier, thereby minimizing interfacial charge recombination in the DSSCs. Conclusively, all the dyes fulfil basic requirements by providing nearly the same negative values of free energy changes, demonstrating a good balance in the thermodynamic properties of various photo-electronic processes.
Photovoltaic and electrochemical impedance studies
To gain a deeper understanding of the structure–performance relationships, anchor-dependent surface chemistry, cell electrochemistry, and device photophysics, the photoelectrochemical characteristics of DSSCs sensitized with DCH1–9 were evaluated, following the procedure outlined in the ESI.† For accurate comparison, all devices were fabricated and characterized under identical conditions.55 The photocurrent density versus voltage (J–V) curves of the DSSCs, sensitized with DCH1–9 and using an I−/I3− liquid electrolyte in acetonitrile medium under standard AM 1.5G (100 mW cm−2) irradiation, are presented in Fig. 8(a), with the corresponding device performance data summarized in Table 4. The photoelectrochemical summary of dyes with similar molecular designs along with their efficiencies is presented in Table S1 (ESI†). To prevent overestimation of current due to multiple irradiations, current–voltage measurements were performed on all fabricated devices using a black metal mask with an active area of 0.18 cm2. The overall power conversion efficiency (PCE) was calculated using key parameters derived from experimental data for the DCH1–9 sensitizers, including incident light power (Pin), short-circuit photocurrent (JSC), open-circuit voltage (VOC), and fill factor (FF), as outlined in eqn (6).66,67 | (6) |
 | ||
| Fig. 8 (a) Current density–voltage plots of DSSCs sensitized with DCH1–9, (b) IPCE spectra of DSSCs sensitized with dyes DCH1–9. | ||
| Sensitizer | J SC (mA cm−2) | V OC (V) | FF (%) | PCE (%) | τ eff (ms) |
|---|---|---|---|---|---|
| DCH1 | 6.472 | 0.643 | 58.69 | 2.45 | 0.45 |
| DCH2 | 5.929 | 0.578 | 66.08 | 2.23 | 0.44 |
| DCH3 | 1.179 | 0.494 | 55.49 | 0.32 | 0.43 |
| DCH4 | 0.335 | 0.536 | 55.01 | 0.09 | 0.30 |
| DCH5 | 2.500 | 0.165 | 28.47 | 0.11 | 0.43 |
| DCH6 | 0.609 | 0.539 | 63.19 | 0.20 | 0.41 |
| DCH7 | 1.270 | 0.569 | 65.03 | 0.47 | 0.43 |
| DCH8 | 0.495 | 0.548 | 53.76 | 0.14 | 0.40 |
| DCH9 | 0.320 | 0.504 | 45.37 | 0.07 | 0.31 |
Among all the dyes, the device fabricated using dye DCH1 and DCH2 as the sensitizer exhibited the highest PCE of 2.45% (JSC = 6.47 mA cm−2, VOC = 0.64 V, and FF = 58.69%) and 2.23% (JSC = 5.92 mA cm−2, VOC = 0.57 V, and FF = 66%), respectively. Meanwhile, the dyes DCH3–9 displayed relatively lower PCE (<1%) under the same measuring conditions. The superior performance of DCH1–2 compared to the relatively lower PCE observed in others can be attributed to several key factors related to the molecular structure, charge-transfer dynamics, dye-TiO2 interactions, charge recombination, energy level mismatch, and inefficient light absorption properties.68 The molecular structures of barbituric acid (BA) and its derivatives as anchoring groups play a crucial role in determining their electronic properties, anchoring capability, and charge-transfer characteristics.69 The BA anchoring group in DCH5 contains a triketone (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) electron-withdrawing core, which promotes intramolecular charge transfer (ICT) and enables strong hydrogen bonding and coordination interactions with TiO2.70 This results in increased dye loading and better electron injection efficiency, which eventually leads to increased JSC through enhanced electron transfer into the conduction band. However, its strong interaction with the electrolyte also accelerates charge recombination at the TiO2/electrolyte interface, increasing series resistance and ultimately leading to a lower fill factor (FF). In contrast, DCH6 with 1,3-diethyl-2-thiobarbituric acid substitutes one oxygen (O) atom with sulfur (–C
O) electron-withdrawing core, which promotes intramolecular charge transfer (ICT) and enables strong hydrogen bonding and coordination interactions with TiO2.70 This results in increased dye loading and better electron injection efficiency, which eventually leads to increased JSC through enhanced electron transfer into the conduction band. However, its strong interaction with the electrolyte also accelerates charge recombination at the TiO2/electrolyte interface, increasing series resistance and ultimately leading to a lower fill factor (FF). In contrast, DCH6 with 1,3-diethyl-2-thiobarbituric acid substitutes one oxygen (O) atom with sulfur (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) and contains bulky diethyl (–C2H5) groups.69,71 It changes the electronic density and orbital overlap, induces steric hindrance, and decreases the dye surface coverage and electron injection efficiency, despite its broader light absorption. These structural changes significantly affect the overall photovoltaic performance, highlighting the critical role of the anchoring group selection in designing dye-sensitized solar cells (DSSCs).
S) and contains bulky diethyl (–C2H5) groups.69,71 It changes the electronic density and orbital overlap, induces steric hindrance, and decreases the dye surface coverage and electron injection efficiency, despite its broader light absorption. These structural changes significantly affect the overall photovoltaic performance, highlighting the critical role of the anchoring group selection in designing dye-sensitized solar cells (DSSCs).
Furthermore, BA-based dye DCH5 exhibits lower VOC due to the enhanced charge recombination at the electrolyte interface. The lowered open-circuit voltage and high effective electron lifetime (τeff) of barbituric acid, compared with rhodanine and thiazolidinedione-based dyes DCH3 and DCH7, can be attributed to the critical features of the dye-sensitized solar cell (DSSC) photophysics and interfacial charge behavior, despite these compounds having similar τeff values and different VOC. The robust dipole moment manifested by barbituric acid due to its electron-withdrawing ureido (–NHCO) groups induces a downward shift in the conduction band (CB) of TiO2. This displacement lowers VOC despite having a larger τeff. In addition, the greater dye-electrolyte interaction in BA-based devices enhances the charge recombination between the injected electrons in TiO2 and tri-iodide (I3−) in the electrolyte, leading to increased electron loss. This reaction lowers the electron density in TiO2, decreasing the quasi-Fermi level and hence lowering VOC. Although DCH5 obtains larger JSC, its lower VOC and larger recombination rates decrease the overall power conversion efficiency (PCE).72 The same pattern appears when DCH4, with more rapid electron back-transfer to I3−, leads to greater charge losses, detrimentally affecting the charge collection efficiency and further decreasing the performance of DSSCs. Conversely, thiazolidinedione and rhodamine are not affected by a substantial dipole-induced shift in the conduction band, and are thus able to retain a higher conduction band edge, resulting in an elevated VOC. Moreover, although barbituric acid can display a larger τeff caused by surface passivation effects, it also can cause decreased charge collection and further decreases VOC. Another important issue relates to the electrolyte interaction since barbituric acid also shows strong interaction with the iodide/tri-iodide redox couple, and thus causes high electron back transfer. However, rhodamine and thiazolidinedione show stronger interfacial energy barriers, thus lowering the recombination of the charge at the TiO2/electrolyte interface, which in turn helps retain a higher VOC.
The poor efficiency of DCH8 and DCH9 is mainly due to their weaker binding affinity to TiO2, leading to lower surface coverage and suboptimal electron injection efficiency. The oxindole anchoring group in DCH8, due to its rigid fused ring structure and lactam (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) functionality, has a lower driving force for electron transfer and weaker electronic coupling with TiO2, resulting in poor charge separation and higher recombination rates.73 While the oxindole group can form hydrogen bonds, it lacks the strong covalent interactions that the carboxyl-based anchoring groups in DCH1 and DCH2 provide, leading to weaker dye adsorption and higher dye desorption over time, negatively impacting the device performance. Similarly, the 4-nitrophenyl acetonitrile anchoring group in DCH9, despite containing a nitro (–NO2) electron-withdrawing group, exhibits poor energy level alignment, which can increase the charge recombination losses and reduce VOC. Overall, the weaker electron-withdrawing ability, inefficient charge injection, poor dye anchoring, and increased recombination losses contribute to the lower efficiency of DCH8 and DCH9 compared to the cyanoacetic acid and rhodanine-3-acetic acid derivatives.
O) functionality, has a lower driving force for electron transfer and weaker electronic coupling with TiO2, resulting in poor charge separation and higher recombination rates.73 While the oxindole group can form hydrogen bonds, it lacks the strong covalent interactions that the carboxyl-based anchoring groups in DCH1 and DCH2 provide, leading to weaker dye adsorption and higher dye desorption over time, negatively impacting the device performance. Similarly, the 4-nitrophenyl acetonitrile anchoring group in DCH9, despite containing a nitro (–NO2) electron-withdrawing group, exhibits poor energy level alignment, which can increase the charge recombination losses and reduce VOC. Overall, the weaker electron-withdrawing ability, inefficient charge injection, poor dye anchoring, and increased recombination losses contribute to the lower efficiency of DCH8 and DCH9 compared to the cyanoacetic acid and rhodanine-3-acetic acid derivatives.
The superior PCE values of DCH1–2 can be attributed to the efficient electron extraction from the twin carbazole donor units and strong electron injection into the TiO2 conduction band, enabled by the electron-withdrawing cyanoacetic acid and rhodanine-3-acetic acid anchoring groups. Additionally, the alkyl chain reduces the surface hydration on the semiconductor. DCH1–2 achieves the highest JSC due to the higher dye loading on the TiO2 surface, driven by the strong binding capacity of the anchoring groups, leading to improved charge collection and favorable electron injection (ΔGinj). In contrast, the lower JSC of DCH3–9 is due to the weaker adsorption of the anchoring units, impairing the light-harvesting efficiency. The higher VOC of DCH1–2 is attributed to the n-hexyl alkyl chain, which reduces dye aggregation and minimizes interfacial recombination. Most dyes exhibit FF values between 50–65%, except for DCH5, where the increased interfacial recombination, likely caused by the higher series and lower shunt resistance, reduces the FF. Overall, cyanoacetic acid and rhodanine-3-acetic acid are superior anchoring groups due to their strong electron-withdrawing properties, better TiO2 binding, enhanced light absorption, and efficient charge transfer, contributing to improved device efficiency.
In general, the photovoltaic cell performance of DSSCs is notably based on the interfacial charge recombination and carrier transport processes, electron lifetime (τeff), and incident photon-to-current conversion efficiency (IPCE) of the fabricated devices.55Fig. 8(b) displays the IPCE spectra as a function of wavelength for the DSSCs based on the nine new dyes DCH1–9 as sensitizers, and the corresponding values are calculated using eqn (7).
| IPCE = LHE × ϕinj × ϕreg × ηcc | (7) |
Electrochemical impedance spectroscopy (EIS) has been performed to investigate the interfacial charge recombination, carrier transport, and their influence on the open-circuit voltage (VOC) of the fabricated devices, which directly affects the overall performance of DSSCs.74–77 The EIS spectra for DCH1–9 were recorded over a frequency range of 100–200 kHz at 298 K under AM 1.5 G illumination, with a forward bias set at VOC (bias voltage applied for DCH1–9 are −0.64 V, −0.57 V, −0.49 V, −0.53 V, −0.16 V, −0.53 V, −0.56 V, −0.54 V, −0.5 V respectively) and a 10 mV amplitude. Fig. 9(a) shows the Nyquist plots at open-circuit voltage, and the equivalent circuit for the data fitting is presented in Fig. S47 (ESI†). In these plots, the series resistance (RS) is identified by the higher frequency intercept on the real axis, while the high-frequency region represents the charge-transport resistance (RPt) and interface capacitance (CPt) at the counter electrode/redox electrolyte interface. The low-frequency region corresponds to the chemical capacitance (Cμ) and charge recombination resistance (RCT) at the TiO2/dye/electrolyte interface. For sensitized devices, Cμ and CPt are replaced by a constant phase element (CPE) in the equivalent circuit. A higher RPt value indicates a more difficult electron injection from TiO2 to the redox electrolyte, suggesting suppressed back recombination and improved VOC. The Nyquist plots display a distinct semicircle in the middle-frequency range, corresponding to charge-transfer resistance (RCT) at the TiO2/dye/electrolyte interface, which reflects electron transfer through TiO2 and back electron transfer to the I−/I3− electrolyte.55
In EIS analysis, the area of the semicircle is also equally important. Larger radii correspond to Nernst impedance, i.e., Rr (dark reaction impedance) ≫ Rd (diffusion impedance). Normally, Nernst impedance relates to the diffusion of redox species (like the I−/I3− couple) in the electrolyte. If diffusion is slow, this impedance dominates and results in a larger semicircle, particularly in the low-frequency region of the Nyquist plot.33 A larger semicircle indicates higher Nernst impedance, signifying the poor diffusion of the redox species in the electrolyte, which limits the regeneration of the dye and lowers the current generation. Gerischer impedance describes the coupling between the charge transfer at the TiO2/dye/electrolyte interface and diffusion of the redox species. Instead of a simple semicircle, Gerischer's impedance often appears as a sloped or smaller semicircle in the Nyquist plot, indicating that both charge transfer and diffusion processes are occurring simultaneously.78,79 A smaller semicircle in this region means that electron recombination is less prominent, which leads to better diffusion, allowing for faster redox reactions and more efficient dye regeneration, better charge separation, and longer electron lifetimes, improving both the short-circuit current (JSC) and VOC.66 From Fig. 11(a), it is evident that DCH3–9 follow the Nernst impedance, whereas DCH1–2 show the Gerischer impedance. In the present study, the radius of the semicircle obtained for DCH1–9 is increasing in the order, DCH1–2 < DCH3–9, indicating the electron recombination resistance decreases in the order of DCH1–2 > DCH3–9 since the type of impedance involved is Greischer impedance, which is further aligned with the VOC values of the dyes. Hence, dye DCH1 achieved the highest VOC value due to the increased electron injection, high resistance towards the recombination rate, and thus the highest PCE.
Furthermore, the Bode angle phase plots and Bode amplitude plots obtained from fabricated cells using the DCH1–9 sensitizers are depicted in Fig. 9(b). The Bode phase plots were used to calculate the effective lifetime (τeff) of the electron in CB of the semiconductor using eqn (8), and the corresponding data are summarized in Table 4.
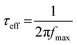 | (8) |
Anchoring studies of dyes DCH1–9 on the TiO2 surface: FTIR analysis
To investigate the anchoring behavior of dyes DCH1–9 on the TiO2 surface, FTIR spectral analysis was performed on both free and adsorbed dyes (Fig. S47, ESI†). The binding mechanisms of the dye anchoring groups were examined using Deacon–Phillips empirical rules, which relate the difference between the symmetric and asymmetric bond stretching frequencies (Δν). Three binding modes were identified: monodentate (Δνads > Δνsolid), bidentate (Δνadss < Δνsolid), and chelating (Δνads ≪ Δνsolid).55 Their FTIR spectra (Fig. S46, ESI†) indicated that DCH1 adsorbs on TiO2via a bidentate bridging mode, confirming deprotonation and involvement of the carboxylic acid group. Interestingly, the cyano group has not participated in the dye adsorption mechanism, as its peak position remains unchanged in both free and adsorbed states. Similar trends were observed for dyes DCH2–9.Co-sensitization studies
The photovoltaic properties of the DSSCs fabricated using DCH1–9 dyes as co-sensitizers, along with a ruthenium-based dye HD-2, are manifested in Fig. 10(a), and the pertaining photovoltaic parameters are listed in Table 5. The detailed fabrication procedure is given in the ESI.† Interestingly, the co-sensitizer DCH2 (i.e., DCH2 + HD-2) shows a higher PCE of 8.82% than the HD-2 dye alone (η = 6.79%). Several factors impact the overall cell performance, including the TiO2 thickness, the solvent used, and the added additives. Different solvents were tested for dyeing the TiO2 electrodes, as dyes can interact differently with solvents, affecting their interaction with the semiconductor surface. Dyeing solutions also included varying amounts of chenodeoxycholic acid (CDCA), an additive that reduces dye aggregation on TiO2, decreases recombination between TiO2 electrons and the electrolyte, and positively shifts the TiO2 surface potential, improving the VOC. Optimal results were achieved with alcoholic solutions containing 0.2 mM dye and 20 mM CDCA. The incorporation of CDCA has a positive impact in increasing VOC, suggesting a positive shift in the TiO2 vacuum level and its conduction band edge. | ||
| Fig. 10 (a) Current density–voltage plots of DSSCs sensitized with HD-2 alone and co-sensitized using DCH1–9. (b) IPCE spectra of DSSCs sensitized with HD-2 alone and co-sensitized using DCH1–9. | ||
| Sensitizer/co-sensitizer | CDCA (mM) | J SC (mA cm−2) | V OC (V) | FF (%) | PCE (%) | τ eff (ms) | |
|---|---|---|---|---|---|---|---|
| HD-2 (mM) | Dyes (0.2 mM) | ||||||
| 0.2 | — | 20 | 15.52 | 0.68 | 64.10 | 6.79 | 0.316 |
| 0.2 | DCH1 | 20 | 19.43 | 0.69 | 60.73 | 8.15 | 0.373 |
| 0.2 | DCH2 | 20 | 20.72 | 0.66 | 63.92 | 8.82 | 0.506 |
| 0.2 | DCH3 | 20 | 19.05 | 0.64 | 64.67 | 7.90 | 0.486 |
| 0.2 | DCH4 | 20 | 18.33 | 0.67 | 64.87 | 8.01 | 0.483 |
| 0.2 | DCH5 | 20 | 17.00 | 0.68 | 68.84 | 8.01 | 0.427 |
| 0.2 | DCH6 | 20 | 20.64 | 0.63 | 52.94 | 6.96 | 0.373 |
| 0.2 | DCH7 | 20 | 22.06 | 0.63 | 57.29 | 7.98 | 0.377 |
| 0.2 | DCH8 | 20 | 19.00 | 0.69 | 64.39 | 8.54 | 0.493 |
| 0.2 | DCH9 | 20 | 21.92 | 0.66 | 58.35 | 8.52 | 0.499 |
It is evident from the result that the device fabricated using DCH2 as a co-sensitizer shows excellent performance with more than a 25% increment compared to that of the device sensitized using the HD-2 dye alone. Furthermore, there is an increase in the VOC values of devices co-sensitized with dyes DCH1–9, suggesting the charge recombination of photoinjected electrons caused by ruthenium dye aggregation is significantly minimized. Specifically, the bulky ruthenium molecules of the HD-2 dye do not adsorb efficiently on the surface of the TiO2 nanoparticles, leading to larger gaps between the adsorbed dye molecules. These voids can be effectively filled by small metal-free organic molecules acting as co-sensitizers, which help create a more uniform coverage and increase the surface area for light harvesting in the visible region.45 Finally, the uniform adsorption of DCH1–9 on the TiO2 surface drastically suppresses the dye aggregation of HD-2, as well as the back-reaction of the I3−/I− on the semiconductor surface. The enhanced JSC and FF values of DCH2 may be attributed to its strong intermolecular interactions causing efficient electron injection and charge transport towards the electrodes. Furthermore, the poor device performance in the case of HD-2 is ascribed to reduced electron injection, which is because of the inhomogeneous dye loading on the TiO2 surface.
IPCE spectra of the fabricated devices using dyes DCH1–9 as co-sensitizers, along with reference dye HD-2, are shown in Fig. 10(b). The obtained spectrum of the device comprising DCH1–9 + HD-2 showed an intense external quantum efficiency (EQE) in the broad spectrum of 300–800 nm with a maximum value at 500–600 nm compared to the reference dye HD-2 (45% at 540 nm). The integrated JSC values from the IPCE spectra of DSSCs sensitized with HD-2 alone and co-sensitized with DCH1–9 are presented in Fig. S48 (ESI†). The calculated integrated JSC values of HD-2 and co-sensitized using DCH1–9 were found to be 15.02 mA cm−2 (HD-2), 19.52 mA cm−2 (DCH1), 20.12 mA cm−2 (DCH2), 19.50 mA cm−2 (DCH3), 18.23 mA cm−2 (DCH4), 17.46 mA cm−2 (DCH5), 19.96 mA cm−2 (DCH6), 21.86 mA cm−2 (DCH7), 19.02 mA cm−2 (DCH8), and 21.18 mA cm−2 (DCH9), respectively (Fig. S48, ESI†). The measured JSC values of all devices are in good agreement with the integrated current density values as estimated from the IPCE spectra of the co-sensitized devices, indicating that the emission of our solar simulator is in agreement with the spectrum of the standard air mass 1.5 illumination. The results obtained from the IPCE spectra are in agreement with the current–voltage measurements. The IPCE maximum of DCH1 is 61%, DCH2 is 65%, DCH3 is 61.5%, DCH4 is 54%, DCH5 is 52%, DCH6 is 58%, DCH7 is 64%, DCH8 is 63%, and DCH9 is 63.5% at 420 nm. Among all the co-sensitizers, barbituric acid and its derivative DCH4–5 displayed both lower IPCE and JSC values, which is due to the poor orientation of dye in between the bulky HD-2 dye molecules, as well as enhanced spatial separation of the LUMO level of the dye occupied in the excited state from the semiconductor surface. Interestingly, dye DCH2 with rhodanine-3-acetic acid as a co-sensitizer displayed a broad IPCE response in the visible range (65% at 540 nm) and an enhanced anchoring effect on the TiO2 surface compared to HD-2 alone, attributing greater flexibility in binding orientations. This improvement is attributed to the increased flexibility in binding orientations. The reorientation effect of DCH2 and its ability to wrap around the Ru center of HD-2 likely contribute to the enhanced irradiation efficiency and reduced recombination reactions.
To better understand the various electronic processes involved in energy conversion, EIS measurements were performed on devices co-sensitized with dyes DCH1–9, alongside a reference device sensitized with HD-2, as shown in Fig. 11 and Fig. S49 (ESI†). From Fig. 11(a), it is evident that the Nyquist plots of all devices exhibit two distinct semicircles. As reported in the literature, the smaller semicircle at the higher frequency region corresponds to the charge transfer resistance at the cathode, which is directly related to the device's fill factor (FF). In contrast, the larger semicircle at the middle-frequency region represents the recombination resistance at the TiO2/dye/electrolyte interface, which is directly linked to the open-circuit voltage (VOC). From Fig. 11(a), it is apparent that the radius of the semicircle of HD-2 is greater than that of DCH2 + HD-2, in accordance with the corresponding order of open-circuit voltage (Table 5) and is helpful in the suppression of dark current. Here, the diameter of the semicircles corresponds to the resistances (charge transfer and recombination). Co-sensitized systems with rhodamine-3-acetic acid anchoring group exhibited smaller semicircle diameters, especially at high frequencies, representing improved charge-transport dynamics.
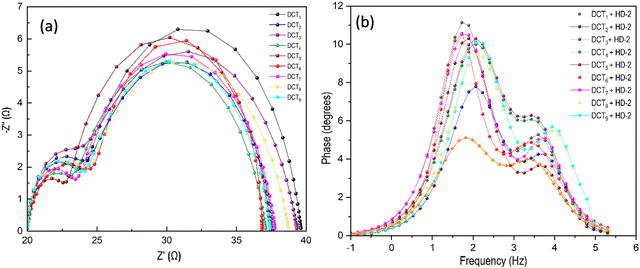 | ||
| Fig. 11 EIS spectra of devices co-sensitized with HD-2 using DCH1–9: (a) Nyquist plots, (b) Bode phase plots. | ||
Among all, dye DCH2 + HD-2 manifested enhanced series resistance (RS) of 28.46 Ω (Table S2, ESI†), which is higher than the others. The dye DCH1 + HD-2, which has a longer conjugation system, leads to a decrease in the charge transfer resistance at the TiO2/electrolyte interface. This indicates improved electron injection and a more efficient charge-transfer process due to better dye anchoring on TiO2. The RPt value of dye DCH2 + HD-2 is higher, showing the uniform filling of voids in between the Ru-based HD-2 molecules by co-sensitizer DCH2. Among dyes DCH1–9, the co-sensitizer DCH2 demonstrates enhanced JSC, VOC, and FF values, resulting in excellent device performance. Hence, it can be considered the best co-sensitizer for HD-2 dye.
Fig. 11(b) and Fig. S47 (ESI†) present the EIS Bode phase plots for co-sensitized devices using DCH1–9 and the reference dye HD-2. All devices exhibit two distinct peaks: the high-frequency peak corresponds to electron transfer at the Pt/electrolyte interface, while the low-frequency peak is associated with charge transfer at the TiO2/dye/electrolyte interface.16,64 Additionally, the electron lifetimes for all co-sensitized devices were calculated using eqn (8), with the corresponding values listed in Table 5. The electron lifetime (τeff) was derived from the low-frequency peak of the Bode phase plot, and reflects the charge recombination rate at the TiO2/dye/electrolyte interface. The results indicate that the DCH2 + HD-2 combination exhibits the longest electron lifetime. This is likely due to a reduced back reaction of injected electrons from the TiO2 conduction band with the redox electrolyte, leading to an enhanced VOC compared to other co-sensitizers and HD-2 alone. In conclusion, the DCH2 dye, when used as a co-sensitizer with the ruthenium-based dye HD-2, effectively suppresses undesirable charge recombination, thereby improving the overall performance of the device.
Reproducibility of devices
In order to investigate the reproducibility of DSSCs, data from five devices were recorded using the same procedure (as detailed in the ESI†), ensuring consistent layer thickness across all devices. Fig. 12 illustrates the statistical distribution of the key photovoltaic parameters (JSC, VOC, FF, and PCE) for the tested devices. Notably, the chromophore DCH2 carrying rhodanine-3-acetic acid anchor co-sensitized with the ruthenium-based sensitizer HD-2 demonstrated superior performance and reproducibility, as explained in previous sections.Conclusion
In summary, we designed and synthesized nine novel double donor–acceptor (D–A) organic sensitizers/co-sensitizers (DCH1–9) for dye-sensitized solar cell (DSSC) applications. These dyes feature carbazole-based twin molecules as electron donors, non-conjugated linear alkyl chains as linkers, and multiple acceptor units. The synthesis method is simple, cost-effective, and involves straightforward purification steps. We conducted comprehensive optical, thermal, electrochemical, theoretical, and photovoltaic studies to evaluate their structure–property relationship. Among the sensitizers, DCH1, containing a cyanoacetic acid acceptor, exhibited the highest PCE of 2.45%. This is attributed to the strong di-anchoring capability of the anchor on the TiO2 surface, thereby enhancing light-harvesting across the visible and near-infrared spectrum, and the favorable electron-donating and -withdrawing groups that improve the thermodynamic processes of the cell. Furthermore, co-sensitization experiments with DCH1–9 (0.2 mM) demonstrated that DCH2, carrying rhodanine-3-acetic acid as an anchoring unit, achieved the highest PCE of 8.82%, with IPCE exceeding 65%, demonstrating excellent performance with more than a 25% increment compared to that of Ru-based HD-2 dye alone (6.79%). The superior performance is due to its ability to cover voids left by the bulky molecules of HD-2, which reduces dye aggregation and enhances the surface area for light absorption. Thus, the present systematic study provides valuable insights into the impact of different anchoring groups on the performance of organic sensitizers/co-sensitizers in cells, offering a promising path for further enhancing the efficiency of DSSCs.Data availability
All data supporting the findings of this study are available within the article file and its ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are thankful to NITK, Surathkal, India, for providing the necessary laboratory facilities. The authors are also indebted to the Department of Textile Engineering, Chemistry, and Science at NCSU, Raleigh, for providing a device fabrication facility. The authors would like to express their gratitude to Dr Ahmed El-Shafei, Professor at TECS, Wilson College of Textiles, for his support and for facilitating the DSSC measurements in his laboratory.References
- B. O’Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 Search PubMed.
- D. P. Hagberg, J.-H. Yum, H. Lee, F. De Angelis, T. Marinado, K. M. Karlsson, R. Humphry-Baker, L. Sun, A. Hagfeldt, M. Grätzel and Md. K. Nazeeruddin, J. Am. Chem. Soc., 2008, 130, 6259–6266 CAS.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, Md. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CAS.
- I. M. Abdellah and A. El-Shafei, RSC Adv., 2019, 10, 610–619 RSC.
- A. Hagfeldt, U. B. Cappel, G. Boschloo, L. Sun, L. Kloo, H. Pettersson and E. A. Gibson, in McEvoy's Handbook of Photovoltaics, ed. S. A. Kalogirou, Academic Press, 3rd edn, 2018, pp. 503–565 Search PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- P. Ferdowsi, Y. Saygili, F. Jazaeri, T. Edvinsson, J. Mokhtari, S. M. Zakeeruddin, Y. Liu, M. Grätzel and A. Hagfeldt, ChemSusChem, 2020, 13, 212–220 CAS.
- Y. Farré, F. Maschietto, J. Föhlinger, M. Wykes, A. Planchat, Y. Pellegrin, E. Blart, I. Ciofini, L. Hammarström and F. Odobel, ChemSusChem, 2020, 13, 1844–1855 Search PubMed.
- P. Ferdowsi, Y. Saygili, F. Jazaeri, T. Edvinsson, J. Mokhtari, S. M. Zakeeruddin, Y. Liu, M. Grätzel and A. Hagfeldt, ChemSusChem, 2020, 13, 212–220 CrossRef CAS PubMed.
- K. S. Keremane, A. Planchat, Y. Pellegrin, D. Jacquemin, F. Odobel and A. Vasudeva Adhikari, ChemSusChem, 2022, 15, e202200520 CrossRef CAS.
- D. D. Babu, S. R. Gachumale, S. Anandan and A. V. Adhikari, Dyes Pigm., 2015, 112, 183–191 CrossRef CAS.
- G. Deogratias, O. S. Al-Qurashi, N. Wazzan, N. Seriani, T. Pogrebnaya and A. Pogrebnoi, Struct. Chem., 2020, 31, 2451–2461 CrossRef CAS.
- I. M. Abdellah, A. I. Koraiem and A. El-Shafei, Sol. Energy, 2019, 177, 642–651 CrossRef CAS.
- F. Odobel, Y. Pellegrin, E. A. Gibson, A. Hagfeldt, A. L. Smeigh and L. Hammarström, Coord. Chem. Rev., 2012, 256, 2414–2423 CrossRef CAS.
- D. D. Babu, P. Naik and K. S. Keremane, J. Mol. Liq., 2020, 310, 113189 CrossRef CAS.
- H. Wang, B. Bao, X. Hu and J.-K. Fang, Electrochim. Acta, 2017, 250, 278–284 CrossRef CAS.
- B. Xu, S. Wrede, A. Curtze, L. Tian, P. B. Pati, L. Kloo, Y. Wu and H. Tian, ChemSusChem, 2019, 12, 3243–3248 CrossRef CAS PubMed.
- W. Zhang, X. Dai, X. Liao, X. Zang, H. Zhang, X. Yin, C. Yu, C. Ke and Y. Hong, Sol. Energy, 2020, 212, 220–230 CrossRef CAS.
- M.-W. Lee, J.-Y. Kim, H.-G. Lee, H. G. Cha, D.-H. Lee and M. J. Ko, J. Energy Chem., 2021, 54, 208–216 CrossRef CAS.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, Md. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- H. Imahori, T. Umeyama and S. Ito, Acc. Chem. Res., 2009, 42, 1809–1818 CrossRef CAS PubMed.
- M. R. Elmorsy, S. A. Badawy, E. Abdel-Latif, M. A. Assiri and T. E. Ali, Dyes Pigm., 2023, 214, 111206 CAS.
- E. Tanaka, M. S. Mikhailov, N. S. Gudim, E. A. Knyazeva, L. V. Mikhalchenko, N. Robertson and O. A. Rakitin, Mol. Syst. Des. Eng., 2021, 6, 730–738 RSC.
- J.-S. Luo, Z.-Q. Wan and C.-Y. Jia, Chin. Chem. Lett., 2016, 27, 1304–1318 CrossRef CAS.
- B.-G. Kim, K. Chung and J. Kim, Chem. – Eur. J., 2013, 19, 5220–5230 CrossRef CAS PubMed.
- K. S. Keremane and A. V. Adhikari, Electrochem. Sci. Adv., 2021, 1, e2000036 CrossRef CAS.
- Y. Hua, S. Chang, D. Huang, X. Zhou, X. Zhu, J. Zhao, T. Chen, W.-Y. Wong and W.-K. Wong, Chem. Mater., 2013, 25, 2146–2153 CrossRef CAS.
- K. S. Keremane, P. Naik and A. V. Adhikari, J. Nano-Electron. Phys., 2020, 12, 02039 CAS.
- K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara and H. Arakawa, J. Phys. Chem. B, 2003, 107, 597–606 CrossRef CAS.
- N. Robertson, Angew. Chem., Int. Ed., 2006, 45, 2338–2345 CrossRef CAS PubMed.
- P. Naik, K. S. Keremane, M. R. Elmorsy, A. El-Shafei and A. V. Adhikari, Electrochem. Sci. Adv., 2022, 2, e2100061 CrossRef CAS.
- P. Naik, I. M. Abdellah, M. Abdel-Shakour, R. Su, K. S. Keremane, A. El-Shafei and A. Vasudeva Adhikari, Sol. Energy, 2018, 174, 999–1007 CrossRef CAS.
- M. R. Elmorsy, R. Su, A. A. Fadda, H. A. Etman, E. H. Tawfik and A. El-Shafei, Dyes Pigm., 2018, 158, 121–130 CrossRef CAS.
- L. Zhang and J. M. Cole, J. Mater. Chem. A, 2017, 5, 19541–19559 RSC.
- B. Xiao, A. Tang, J. Zhang, A. Mahmood, Z. Wei and E. Zhou, Adv. Energy Mater., 2017, 7, 1602269, DOI:10.1002/aenm.201602269.
- T. Dai, Y. Meng, Z. Wang, J. Lu, Z. Zheng, M. Du, Q. Guo and E. Zhou, J. Am. Chem. Soc., 2025, 147, 4631–4642 CrossRef CAS PubMed.
- T. Dai, A. Tang, Y. Meng, C. Dong, P. Cong, J. Lu, J. Du, Y. Zhong and E. Zhou, Angew. Chem., Int. Ed., 2024, 63, e202403051, DOI:10.1002/anie.202403051.
- K. Zeng, Z. Tong, L. Ma, W.-H. Zhu, W. Wu and Y. Xie, Energy Environ. Sci., 2020, 13, 1617–1657 RSC.
- K. Zeng, Y. Chen, W.-H. Zhu, H. Tian and Y. Xie, J. Am. Chem. Soc., 2020, 142, 5154–5161 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. Zou, X. Wu, X. Gong, C. Li and Y. Xie, Chin. Chem. Lett., 2022, 33, 4313–4316 CrossRef CAS.
- Z. Li, Q. Li, C. Li and Y. Xie, Mater. Chem. Front., 2024, 8, 652–680 RSC.
- J. Luo, Q. Lu, Q. Li, Z. Li, Y. Wang, X. Wu, C. Li and Y. Xie, ACS Appl. Mater. Interfaces, 2023, 15, 41569–41579 CrossRef CAS PubMed.
- P. Naik, K. S. Keremane, M. R. Elmorsy, R. Su and A. V. Adhikari, Sol. Energy, 2018, 169, 386–391 CrossRef CAS.
- J. M. Cole, G. Pepe, O. K. Al Bahri and C. B. Cooper, Chem. Rev., 2019, 119, 7279–7327 CrossRef CAS PubMed.
- I. M. Abdellah, O. S. Zaky and M. R. Eletmany, Opt. Mater., 2023, 145, 114454 CrossRef CAS.
- C. L. Lee, W. H. Lee and C. H. Yang, Int. J. Photoenergy, 2013, 250397, DOI:10.1155/2013/250397.
- K. S. Keremane, M. R. Eletmany, I. M. Abdellah, P. Naik and A. V. Adhikari, J. Photochem. Photobiol., A, 2025, 466, 116368 CrossRef CAS.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Mueller, P. Liska, N. Vlachopoulos and M. Graetzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- P. Wang, C. Klein, R. Humphry-Baker, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 808–809 CrossRef CAS PubMed.
- A. Yella, C. L. Mai, S. M. Zakeeruddin, S. N. Chang, C. H. Hsieh, C. Y. Yeh and M. Grätzel, Angew. Chem., Int. Ed., 2014, 53, 2973–2977 CrossRef CAS PubMed.
- Y. Qin and Q. Peng, Int. J. Photoenergy, 2012, 21, 291579 Search PubMed.
- M. R. Elmorsy, R. Su, E. Abdel-Latif, S. A. Badawy, A. El-Shafei and A. A. Fadda, J. Mater. Sci.: Mater. Electron., 2020, 31, 7981–7990 CrossRef CAS.
- A. F. Buene and D. M. Almenningen, J. Mater. Chem. C, 2021, 9, 11974–11994 RSC.
- K. S. Keremane, I. M. Abdellah, P. Naik, A. El-Shafei and A. V. Adhikari, Phys. Chem. Chem. Phys., 2020, 22, 23169–23184 RSC.
- P. Naik, N. Swain, R. Naik, N. Devarajan, D. Alagarasan and H. B. Shivaprasad, Heliyon, 2024, 10(4), e25624 CrossRef CAS PubMed.
- K. A. Kumar, J. Manonmani and J. Senthilselvan, Mater. Electron., 2014, 25, 5296–5301 CrossRef.
- C.-L. Lee, W.-H. Lee and C.-H. Yang, J. Mater. Sci., 2013, 48, 3448–3453 CrossRef CAS.
- K. S. Keremane and A. V. Adhikari, J. Org. Chem. Synthesis Proc. Develop., 2021, 1, 1–9 Search PubMed.
- P. Naik, L. Elias, K. S. Keremane, D. D. Babu and I. M. Abdellah, Energy Technol., 2024, 12, 2301666 CrossRef CAS.
- D. P. Hagberg, J. H. Yum, H. Lee, A. F. De, T. Marinado, K. M. Karlsson, R. Humphry-Baker, L. Sun, A. Hagfeldt and M. Grätzel, J. Am. Chem. Soc., 2008, 130, 6259–6266 CrossRef CAS PubMed.
- K. S. Keremane, R. Rao and A. V. Adhikari, Photochem. Photobiol., 2021, 97(2), 289–300 CrossRef CAS.
- K. S. Keremane, Y. Pellegrin, A. Planchat, D. Jacquemin, F. Odobel and A. V. Adhikari, J. Phys. Chem. C, 2022, 126, 12383–12390 CrossRef CAS.
- S. Aghazada, P. Gao, A. Yella, G. Marotta, T. Moehl, J. Teuscher, J. E. Moser, A. F. De, M. Grätzel and M. K. Nazeeruddin, Inorg. Chem., 2016, 55, 6653–6659 CrossRef CAS PubMed.
- A. U. Rahman, M. B. Khan and M. Y. Rahman, ACS Omega, 2021, 6(42), 27640–27653 CrossRef CAS PubMed.
- W. C. Chen, S. Nachimuthu and J. C. Jiang, Sci. Rep., 2017, 7, 4979 CrossRef.
- I. M. Abdellah, A. I. Koraiem and A. El-Shafei, Dyes Pigm., 2019, 164, 244–256 CrossRef CAS.
- J. Wiberg, T. Marinado, D. P. Hagberg, L. Sun, A. Hagfeldt and B. Albinsson, J. Phys. Chem. C, 2009, 113, 3881–3886 CrossRef CAS.
- B. Hosseinzadeh, A. Salimi Beni, A. Najafi Chermahini, R. Ghahary and A. Teimouri, Synth. Met., 2015, 209, 1–10 CrossRef CAS.
- N. Kaur, M. Kaur, H. S. Sohal, H. Han and P. K. Bhowmik, Organics, 2024, 5, 298–345 CrossRef CAS.
- H. M. El-Shafeai, S. A. Badawy, M. A. Ismail, E. Abdel-Latif, A. A. Fadda and M. R. Elmorsy, RSC Adv., 2023, 13, 9720–9731 RSC.
- R. Brisse, C. Praveen, V. Maffeis, T. Bourgeteau, D. Tondelier, T. Berthelot, B. Geffroy, T. Gustavsson, J. M. Raimundo and B. Jousselme, Sustainable Energy Fuels, 2018, 2, 648–654 RSC.
- P. Naik, N. Pilicode, K. S. Keremane, M. Acharya and A. V. Adhikari, Opt. Mater., 2023, 142, 114002 CrossRef CAS.
- Ji Zou, Q. Yan, C. Li, Y. Lu, Z. Tong and Y. Xie, ACS Appl. Mater. Interfaces, 2020, 12(51), 57017–57024 CrossRef CAS PubMed.
- D. D. Babu, R. Su, A. El-Shafei and A. V. Adhikari, RSC Adv., 2016, 6, 30205–30216 RSC.
- L.-Y. Lin, C.-H. Tsai, K.-T. Wong, T.-W. Huang, C.-C. Wu, S.-H. Chou, F. Lin, S.-H. Chen and A.-I. Tsai, J. Mater. Chem., 2011, 21, 5950–5958 RSC.
- Q. Wang, J.-E. Moser and M. Grätzel, J. Phys. Chem. B, 2005, 109, 14945–14953 CrossRef CAS PubMed.
- A. Sacco, Renewable Sustainable Energy Rev., 2017, 79, 814–829 CrossRef CAS.
- S. Sarker, A. J. S. Ahammad, H. W. Seo and D. M. Kim, Int. J. Photoenergy, 2014, 851705, DOI:10.1155/2014/851705.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc04612a |
| This journal is © The Royal Society of Chemistry 2025 |