Ionic radius-dependent self-assembly of lanthanide organic polyhedra: structural diversities and luminescent properties†
Jing
Su
abc,
Fan
Yin
a,
Xiao-Fang
Duan
a,
Jing-Yao
Zhou
a,
Li-Peng
Zhou
 a,
Chong-Bin
Tian
a,
Chong-Bin
Tian
 *abc and
Qing-Fu
Sun
*abc and
Qing-Fu
Sun
 *abc
*abc
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China. E-mail: tianchongbin@fjirsm.ac.cn; qfsun@fjirsm.ac.cn
bCollege of Chemistry and Materials Science, Fujian Normal University, Fuzhou, 350007, China
cFujian College, University of Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China
First published on 20th February 2025
Abstract
The synthesis of nonclassical polyhedra is at the forefront of supramolecular research because of their unique anisotropic interior cavities. However, due to the difficulty in controlling the topology of Ln supramolecular systems, the preparation of nonclassical lanthanide organic polyhedrals (LOPs) remains a challenge. Herein, we explore the ionic radius-dependent self-assembly of LOPs using a rectangular tetra-tropic ligand L. Owing to the rectangular geometry of the ligand panels (rather than square), its assembly with lanthanide ions located in the middle of the Ln series afforded an irregular tetragonal antiprismatic Ln8L4 (Ln = Sm3+, Eu3+, Tb3+, Dy3+ and Ho3+) with two faces unoccupied with L ligands. Interestingly, this tetragonal antiprism possessed an oblate internal cavity that binds to four THF molecules in the solid-state structure. With an increase in radius, the larger La3+ and Nd3+ ions produced Ln4L2 with a distinct sandwich square architecture. In contrast, the smaller Er3+ and Lu3+ ions gave rise to a mixture of both Ln8L4 and Ln6L3. On adding excess Ln3+ ions, a structural transformation from Ln8L4 to Ln6L3 occurred. Structural comparisons of La4L2 and Sm8L4 revealed that the differences in architecture within these systems were governed by both the ionic radii of the lanthanides and conformational flexibility of the ligands. Photophysical investigations revealed that the ligand L exhibited a sensitizing ability toward Sm3+, Tb3+ and Dy3+ ions, displaying their characteristic luminescence emission, with a new record-setting luminescent quantum yield of 92.74% observed for Tb8L4. This work provides new insights into the effect of lanthanide size on the resulting assemblies and opens new avenues to develop nonclassical LOPs.
Introduction
Over the past two decades, preparation of self-assembled metal organic polyhedra has emerged as one of the most active research areas in supramolecular chemistry owing to their diverse potential applications, which include, but are not limited to, supramolecular catalysis,1–5 molecular recognition and separation,6–11 drug delivery12–15 and guest binding.16–22 In this context, the advancement of lanthanide organic polyhedra (LOPs) is of particular interest. This interest is driven by the intrinsic optical, catalytic and magnetic properties of Ln ions,23–27 which can be integrated into the final LOPs by the Ln center. However, research on synthesizing such LOPs remains in its infancy compared to transition coordination assemblies, and only a limited number of such supramolecular architectures have been reported.28,29 This is primarily caused by the variable coordination numbers, weak coordination abilities, diverse coordination geometries, and poor stereochemical preferences of Ln ions.Octanuclear LOPs with cubic or tetragonal prismatic architectures can be constructed by combining the C2 symmetric di-tropic tridentate or C4 symmetric tetra-tropic tridentate ligands with nine-coordinated Ln centers. They typically form cubic structures that can be represented by the formulas Ln8L12 (L = C2-symmetric ligands) or Ln8L6 (L = C4-symmetric ligands) based on the geometric matching principle between the ligand and Ln3+ ions, which was proposed by Raymond and co-workers.30 To date, several Ln8L12 or Ln8L6 compounds have been successfully developed by our group and other groups.31–36 Nevertheless, most of the reported Ln8L12 or Ln8L6 assemblies exhibit high-symmetry structures with regular Archimedean or Platonic geometries. In contrast, nonclassical Archimedean or Platonic octanuclear LOPs with missing linkers or faces have been rarely reported37,38 owing to the challenges associated with controlling the topology of Ln supramolecular systems.
One intriguing aspect of metal organic polyhedra is their dynamic and reversible metal–ligand bonds, which can be utilized to modify their structure by changing factors such as metal ions,39–42 stoichiometry,43,44 concentration,31,45,46 anions,47,48 pH49,50 and some others.51,52 Among these factors, the selection of metal ions is particularly significant in the design of metal organic polyhedra.39–42 The differences in the radii of metal centers can endow the cages with an extra degree of flexibility through the rearrangement of the coordination chelating unit, resulting in the generation of some new species. Along this line, a gradual change in the effective ionic radius across the lanthanide series, known as “lanthanide contraction”, makes Ln3+ ions ideal candidates for these studies. For example, Hooley and our team demonstrated that the Ln-selective self-assembly can occur through multivalency and cooperativity, which have potential utilization in Ln separation using a supramolecular approach.7,53 Recently, our group developed a post-synthetic transmetalation self-assembly strategy for creating near infrared emitting Yb8L6 cube, which cannot be achieved using a direct synthesis method.34 A deeper understanding of the specific role of Ln3+ ions in the self-assembly process can facilitate the rational design of LOPs and promote the discovery of novel Ln supramolecular architectures that go beyond traditional approaches. Consequently, more examples of ionic radius-dependent self-assembly of LOPs are required.
Based on the considerations mentioned above, we report the design and synthesis of a rectangular tetra-tropic tridentate ligand, L, derived from 2,6-di[pyrazol-1-yl]pyridine (bpp) chelating moieties. Its self-assembly with lanthanide ions leads to a series of distinct Ln2nLn (n = 2, 3 and 4) assemblies with the unsaturated metal sites: sandwich square Ln4L2, the triangular prism Ln6L3, and the unique twisted tetrahedron prism Ln8L4. These assemblies were characterized by a combination of NMR, mass spectrometry, and single-crystal X-ray analysis. We found that the formation of these assemblies depends on the ionic radii of the Ln series as well as the varying conformation of the ligands. Additionally, the photophysical properties of Sm8L4, Tb8L4 and Dy8L4 were investigated.
Results and discussion
Ligand design, synthesis and assembly with lanthanides
The tetra-tropic ligands are commonly employed to fabricate octanuclear metal organic polyhedra. However, the square geometry of these ligand panels results in a regular cubic configuration of octanuclear assemblies.34,54–59 To create octanuclear assemblies using the nonclassical Archimedean or Platonic solids, designing ligands with rectangular panels may be a promising strategy, as recently demonstrated by Nitschke et al. in their subcomponent self-assembled transition-based metal–organic architectures.60,61 In addition, tetra-tropic ligands contain very complex conformations, which can enhance the structural complexity and diversity after coordination with metal ions. The 2,6-di[pyrazol-1-yl]pyridine (bpp) and its derivatives are widely used tridentate coordination units in constructing transition metal complexes.62,63 However, this motif has been less explored in preparing rare earth coordination compounds.64,65 Consequently, we opted to introduce bpp tridentate chelating units into the tetra-tropic ligand, and its assembly with Ln3+ ions generally afforded octanuclear LOPs. The tetra-topic tridentate ligand L was synthesized by Suzuki coupling (the experimental details are shown in the Experimental section of the ESI†) and was characterized by NMR.Synthesis and characterization of La4L2
When 2.0 equiv. of ligand L was treated with 4.0 equiv. of La(OTf)3 in acetonitrile at 70 °C for 30 min, the turbid suspension of the ligand gradually turned into a homogeneous clear solution. The 1H NMR spectrum clearly indicates the generation of La4L2 assembly. Compared with the highly symmetric free L, the 1H NMR spectrum of La4L2 showed 2-fold desymmetrization of the L (Fig. 1B and C), in agreement with the D2 point symmetry of a sandwich square. The DOSY spectrum displays a single diffusion band with a diffusion coefficient of 10 × 10−10 m2 s−1, corresponding to a hydrodynamic radius of 12.72 Å for La4L2 calculated using the Stokes–Einstein equation (Fig. 1D). As shown in Fig. 1E, high-resolution ESI-TOF-MS clearly suggests the presence of sandwich square assembly with a molecular formula of La4(L)2(OTf)12. A set of prominent peaks was assigned according to the consecutive loss of OTf− counter ions. For example, peaks with m/z = 715.9999, 932.4878, 1292.6353 and 2013.4301 correspond to [La4(L)2(OTf)7]5+, [La4(L)2(OTf)8]4+, [La4(L)2(OTf)9]3+ and [La4(L)2(OTf)10]2+, respectively. Moreover, the isotopic pattern of each assignment was in line with the simulated one (Fig. 1E, inset).Synthesis and characterization of Sm8L4
Previous studies have demonstrated that the generation of lanthanide architectures is sensitive to the radius of lanthanide ions.34,40,53,66–69 Consequently, we hypothesize that using lanthanide ions bearing a smaller radius than La3+ ions may lead to the development of a different architecture during the self-assembly process. To our delight, when Sm(OTf)3 was employed as the metal source at the same 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
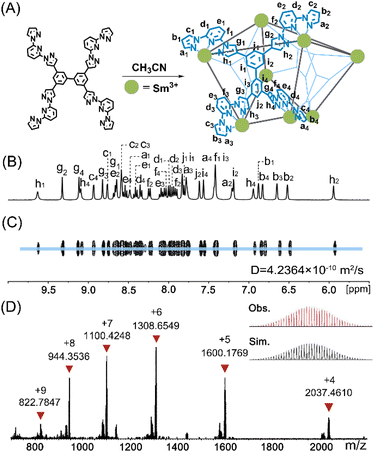
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal-to-ligand ratio as La4L2, a noticeably different 1H NMR spectrum was observed, suggesting the formation of a new species. Based on the NMR, ESI-MS, and single-crystal X-ray diffraction data (vide infra), the newly formed species is a tetragonal antiprismatic Sm8L4 LOP. Analysis of 1H NMR spectroscopy uncovered a different molecular symmetry compared to La4L2. Unlike the two sets of signals for the ligand arms found in La4L2, we detected four sets of ligand arm signals after substituting La(OTf)3 with Sm(OTf)3 (Fig. 2B). The proton signals are completely assigned based on 1H–1H COSY and NOE 2D spectra (Fig. S14 and S15†). Because the four sets of proton signals exhibit equal intensity on the Sm8L4 cage, we conclude that there is only one magnetically distinct ligand, which is in agreement with its S4 symmetry observed on the solid-state structure (vide infra). Evidence for the exclusive formation of a sole species in solution was offered by 1H diffusion ordered spectroscopy (DOSY), where all signals were located in a single band (Fig. 2C), from which the kinetic diameter of 30.03 Å could be estimated. To verify the stoichiometry of the Sm3+ assembly, the ESI-MS spectrum was recorded using acetonitrile as the solvent. The observed peaks at m/z = 822.7847, 944.3536, 1100.4248, 1308.6549, 1600.1769 and 2037.4610 belong to the fragments [Sm8(L)4(OTf)15]9+, [Sm8(L)4(OTf)16]8+, [Sm8(L)4(OTf)17]7+, [Sm8(L)4(OTf)18]6+, [Sm8(L)4(OTf)19]5+ and [Sm8(L)4(OTf)20]4+, respectively. The ESI-MS result certificates a tetragonal prism composition of Sm8L4 assembly.
1 metal-to-ligand ratio as La4L2, a noticeably different 1H NMR spectrum was observed, suggesting the formation of a new species. Based on the NMR, ESI-MS, and single-crystal X-ray diffraction data (vide infra), the newly formed species is a tetragonal antiprismatic Sm8L4 LOP. Analysis of 1H NMR spectroscopy uncovered a different molecular symmetry compared to La4L2. Unlike the two sets of signals for the ligand arms found in La4L2, we detected four sets of ligand arm signals after substituting La(OTf)3 with Sm(OTf)3 (Fig. 2B). The proton signals are completely assigned based on 1H–1H COSY and NOE 2D spectra (Fig. S14 and S15†). Because the four sets of proton signals exhibit equal intensity on the Sm8L4 cage, we conclude that there is only one magnetically distinct ligand, which is in agreement with its S4 symmetry observed on the solid-state structure (vide infra). Evidence for the exclusive formation of a sole species in solution was offered by 1H diffusion ordered spectroscopy (DOSY), where all signals were located in a single band (Fig. 2C), from which the kinetic diameter of 30.03 Å could be estimated. To verify the stoichiometry of the Sm3+ assembly, the ESI-MS spectrum was recorded using acetonitrile as the solvent. The observed peaks at m/z = 822.7847, 944.3536, 1100.4248, 1308.6549, 1600.1769 and 2037.4610 belong to the fragments [Sm8(L)4(OTf)15]9+, [Sm8(L)4(OTf)16]8+, [Sm8(L)4(OTf)17]7+, [Sm8(L)4(OTf)18]6+, [Sm8(L)4(OTf)19]5+ and [Sm8(L)4(OTf)20]4+, respectively. The ESI-MS result certificates a tetragonal prism composition of Sm8L4 assembly.
Synthesis and characterization of Lu6L3
With the above fascinating finding, we are curious to know whether a new assembly will be formed when using the even smaller Lu3+ ion. By replacing the metal source of Sm3+ with Lu3+, we observed a distinct 1H NMR pattern that showed more complicated signals compared with Sm8L4 species (Fig. S21†). It was impossible to make a detailed proton assignment because of obvious line broadening and overlapping signals. However, the ESI-MS spectrum indicated the formation of mixed Lu6L3 and Lu8L4 (Fig. S42†).It is known that an excess of metal can trigger an initial assembly transformation into a new species.68 Inspired by this, we explored the effect of metal excess on the structure. The 1H NMR titration experiment indicates that the broadening and overlapping signals evolve into a set of well-resolved peaks once the metal-to-ligand ratio reaches 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S22†), which consists of ten different signals. This simplified 1H NMR spectrum was also observed by directly assembling the Lu3+ ion with L in a metal-to-ligand ratio of 4
1 (Fig. S22†), which consists of ten different signals. This simplified 1H NMR spectrum was also observed by directly assembling the Lu3+ ion with L in a metal-to-ligand ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The ESI-MS spectrum of the above solution demonstrates the presence of the hexanuclear complex with chemical formula Lu6L3 (Fig. 3A), where the three tetra-topic tridentate ligand L may cover the rectangular faces of Lu6L3, and gives a trigonal prism structure (Scheme 1).
1. The ESI-MS spectrum of the above solution demonstrates the presence of the hexanuclear complex with chemical formula Lu6L3 (Fig. 3A), where the three tetra-topic tridentate ligand L may cover the rectangular faces of Lu6L3, and gives a trigonal prism structure (Scheme 1).
 | ||
| Fig. 3 ESI-MS of Lu6L3 (A) and Sm6L3 (B) complexes with the insets showing the observed and simulated isotopic patterns of the +5 peak. | ||
Crystal structural analysis of La4L2 and Sm8L4
The slow diffusion of diethyl ether into the acetonitrile of La4L2 for one week led to high-quality single crystals suitable for X-ray structural analysis. Single crystal X-ray diffraction analysis reveals that La4L2 crystallizes in the monoclinic P21/n space group with a sandwich square structure (Fig. 4A). The asymmetric unit of La4L2 consists of one complete La4L2 molecule (Fig. S50†), where all four La3+ ions are ligated by ten donor atoms and adopt a bicapped square antiprism coordination geometry (Fig. S51 and Table S3†). The dihedral angles between metal-chelating planes around the La3+ node are 44.67, 46.02, 46.20 and 45.74° for La1, La2, La3 and La4, respectively (Fig. S52†). Owing to one whole La4L2 molecule being observed in the asymmetric unit, its point symmetry in the solid-state structure is C1 rather than the solution state point symmetry D2. This behavior should be caused by the different coordinated solvents and different numbers of coordinated OTf− anions at the La centers, which reduced the symmetry of La4L2 in the solid state. Thus, if we remove the coordination solvents and coordinated OTf− anions, the point symmetry of La4L2 can be regarded as D2 with slight deviations from perfect geometry, which is consistent with the findings from 1H NMR (vide supra). The four La3+ ions form a twisted square, with La–La distances of 11.56, 12.55, 11.44 and 12.42 Å (Fig. 4B). In La4L2, the two ligands bearing conformation mode A (Fig. 4B and Table S6†) are located above and below the La4 square, resulting in the metal centers with the same Δ or Λ configuration (Fig. S53†). The distances between the two corresponding phenyl rings belonging to the panels of two ligands, which exhibited a “face-to-face” arrangement mode, were measured to be 3.99 Å and 4.02 Å (Fig. 4C, top). These values indicate the presence of an intermolecular π–π stacking interaction between the two ligands. Furthermore, the average torsion angle between the phenyl ring of the ligand's panel in La4L2 is 27.60° (Fig. 4B).The definitive confirmation of the structure for Sm8L4 assembly was provided by a single-crystal diffraction analysis. Crystallographic analysis shows that Sm8L4 crystalizes in a tetragonal space group, with one quarter of the cage molecule present in the asymmetric unit (Fig. S54†), featuring a twisted tetragonal antiprismatic structure. The asymmetric unit of Sm8L4 consists of two crystallographic independent Sm3+ ions, both with a coordination number of nine: the Sm1 atom has a tricapped trigonal prism geometry, while the Sm2 atom has a capped square antiprism geometry (Fig. S55 and Table S4†). For Sm1 and Sm2, the dihedral angles between the metal-chelating planes around the Sm3+ center are 86.47 and 88.77°, respectively (Fig. S56†). Research into the molecular structure of Sm8L4 shows that each ligand bridged four Sm3+ ions located on the side face of the twisted tetragonal antiprism. The top and bottom faces of tetragonal antiprism are empty. Moreover, the torsion angle between the phenyl ring of the ligand's panel in Sm8L4 is 40.32° (Fig. 4E), leading to the four Sm3+ centers on the side face of the tetragonal antiprism not being in the same plane. This result generates the formation of a distorted square on the side face of the tetragonal antiprism. Four such distorted squares together form the highly twisted tetragonal antiprism. The edge length of the tetragonal antiprismatic Sm8L4 is 11.89 Å, and the side lengths of the up and bottom distorted squares in the tetragonal antiprismatic Sm8L4 are 10.84 Å and 14.45 Å, respectively (Fig. 4E). As shown in Fig. S57,† each tetragonal antiprismatic Sm8L4 cage consists of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 radio of Δ-Sm3+ and Λ-Sm3+ metal vertices, where each Sm3+ center is surrounded by the nearest neighbors of the opposite chiral configuration. On each face of the tetragonal antiprism, the Δ-Sm3+ and Λ-Sm3+ centers are arranged alternately. Such a distribution of metal chiral within Sm8L4 gives rise to an overall achiral S4 molecular symmetry, in which the C2 rotation axis runs through the centers of the up and bottom faces of tetragonal antiprism (Fig. 4F). The twist angle, defined as the dihedral angle between the face constructed by the top face Sm3+, centroids of the top and bottom faces, is measured to be 22.59° (Fig. 4F). The tetragonal antiprism provides an oblate internal cavity (Fig. S60†), which is different from the pseudospherical or prolate cavities formed by the tetragonal prism.60,61,70,71 This cavity feature makes the cage suitable for binding small planar guest molecules. To our delight, we found in the crystal structure that four THF molecules occupy the oblate cavity of Sm8L4 (Fig. S61†). 1H NMR titration experiment indicates a fast-exchange binding dynamic mode, and the apparent association constants (Ka) for THF are determined to be 127 M−1 by applying the Hill function (Fig. S63 and S64†). After removing the THF molecules, the cavity volume of Sm8L4 was calculated to be 594.52 Å3 using the MoloVol program.72
1 radio of Δ-Sm3+ and Λ-Sm3+ metal vertices, where each Sm3+ center is surrounded by the nearest neighbors of the opposite chiral configuration. On each face of the tetragonal antiprism, the Δ-Sm3+ and Λ-Sm3+ centers are arranged alternately. Such a distribution of metal chiral within Sm8L4 gives rise to an overall achiral S4 molecular symmetry, in which the C2 rotation axis runs through the centers of the up and bottom faces of tetragonal antiprism (Fig. 4F). The twist angle, defined as the dihedral angle between the face constructed by the top face Sm3+, centroids of the top and bottom faces, is measured to be 22.59° (Fig. 4F). The tetragonal antiprism provides an oblate internal cavity (Fig. S60†), which is different from the pseudospherical or prolate cavities formed by the tetragonal prism.60,61,70,71 This cavity feature makes the cage suitable for binding small planar guest molecules. To our delight, we found in the crystal structure that four THF molecules occupy the oblate cavity of Sm8L4 (Fig. S61†). 1H NMR titration experiment indicates a fast-exchange binding dynamic mode, and the apparent association constants (Ka) for THF are determined to be 127 M−1 by applying the Hill function (Fig. S63 and S64†). After removing the THF molecules, the cavity volume of Sm8L4 was calculated to be 594.52 Å3 using the MoloVol program.72
Extensive attempts to crystallize the Lu6L3 were unsuccessful. This failure may be attributed to the presence of excess Lu3+ ions in the solution, which could disrupt the crystallization process by promoting the formation of amorphous or poorly ordered competing phases rather than desired Lu6L3 single crystals.
To gain insight into the structural changes related to lanthanides, we conducted detailed structural analyses of the two assemblies. Our analysis indicates that both the central lanthanide ions and the conformation of the ligands play a crucial role in the selective formation of the resulting assemblies. First, the La3+ and Sm3+ ions, which have different radii and exhibit different coordination numbers, give rise to the assemblies with different nuclearities. For example, the La3+ ion, the largest along the lanthanide series, has a coordination number of ten in La4L2. This results in a small average dihedral angle between the metal-chelating planes around the La3+ node (Avg: 45.66°), promoting the formation of a sandwich square structure. In contrast, the smaller Sm3+ ion has a coordination number of nine in Sm8L4. This reduction in the coordination number allows for enough space to increase the dihedral angle between the metal-chelating planes surrounding the Sm3+ center, which is essential for the formation of higher nuclearity assemblies. Consequently, a larger average dihedral angle was obtained around the Sm3+ node in Sm8L4 (Avg: 87.62°), leading to the generation of higher nuclearity Sm8L4. Second, the ligand L in La4L2 and Sm8L4 displays different conformations: mode A for La4L2 and mode D for Sm8L4 (Fig. 4B, E and Table S6†). Third, the average torsion angle between the phenyl ring of the ligand's panel in La4L2 and Sm8L4 is different: 27.60° in La4L2 and 40.32° in Sm8L4. Because the metal nodes in La4L2 and Sm8L4 each contain the same two coordinated bpy moieties, it can be assumed that the main reason for such structural change stems from the decreased lanthanide radius and varied ligand conformation.
Effect of Ln ions on supramolecular architecture
After characterizing the assemblies of La4L2, Sm8L4 and Lu6L3, we determine whether these assemblies were exclusively formed by their corresponding La3+, Sm3+ and Lu3+ ions, or if they could also be created with alternative lanthanide ions. Specifically, we wanted to investigate whether the structure is affected by ionic radii. To explore this, we conducted further studies using other selected lanthanides (Nd3+, Eu3+, Tb3+, Dy3+, Ho3+ and Er3+), which span the Ln series and offer a range of ionic radii. The experiments were carried out using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios of lanthanide triflates (Ln = Nd3+, Eu3+ and Er3+) and L in acetonitrile. For the Nd3+ ion, the mass spectrum analysis supported the formation of the Nd4L2 complex (Fig. S28†), similar to the results obtained with the La3+ ion. However, when the Nd3+ ion was replaced with Eu3+ ions, the mass spectrum data revealed the production of octanuclear Eu8L4 complexes (Fig. S32†), similar to those observed with Sm3+ ions. In contrast, the use of the Er3+ ion resulted in a mixture of self-assembled species, including Er8L4 and Er6L3 supramolecular architectures, as evidenced by its mass spectrum (Fig. S40†). Clearly, the structures of assemblies are influenced by the tiny difference in ionic radii across the Ln series, which is consistent with the previous reports.34,40,53,66–69 The larger lanthanide cations, such as La3+ and Nd3+, can form sandwich square structure Ln4L2, while the lanthanides positioned in the middle of the series, such as Sm3+, Eu3+, Tb3+, Dy3+ and Ho3+ (Fig. S30–S39†), can produce tetragonal antiprism Ln8L4. In contrast, the smaller lanthanide ions (Ln = Er3+ and Lu3+) yield a mixture of both Ln8L4 and Ln6L3 (Fig. S40–S43†). It is important to highlight that our observations differ from previous reports, which suggested that structural alternation was only caused by ionic radii without observing changes in ligand conformation.34,40,66–69 In our case, however, the variation in the conformation of ligands was distinctly observed (vide supra).
1 ratios of lanthanide triflates (Ln = Nd3+, Eu3+ and Er3+) and L in acetonitrile. For the Nd3+ ion, the mass spectrum analysis supported the formation of the Nd4L2 complex (Fig. S28†), similar to the results obtained with the La3+ ion. However, when the Nd3+ ion was replaced with Eu3+ ions, the mass spectrum data revealed the production of octanuclear Eu8L4 complexes (Fig. S32†), similar to those observed with Sm3+ ions. In contrast, the use of the Er3+ ion resulted in a mixture of self-assembled species, including Er8L4 and Er6L3 supramolecular architectures, as evidenced by its mass spectrum (Fig. S40†). Clearly, the structures of assemblies are influenced by the tiny difference in ionic radii across the Ln series, which is consistent with the previous reports.34,40,53,66–69 The larger lanthanide cations, such as La3+ and Nd3+, can form sandwich square structure Ln4L2, while the lanthanides positioned in the middle of the series, such as Sm3+, Eu3+, Tb3+, Dy3+ and Ho3+ (Fig. S30–S39†), can produce tetragonal antiprism Ln8L4. In contrast, the smaller lanthanide ions (Ln = Er3+ and Lu3+) yield a mixture of both Ln8L4 and Ln6L3 (Fig. S40–S43†). It is important to highlight that our observations differ from previous reports, which suggested that structural alternation was only caused by ionic radii without observing changes in ligand conformation.34,40,66–69 In our case, however, the variation in the conformation of ligands was distinctly observed (vide supra).
Inspired by the structural transformation from Lu8L4 to Lu6L3, we subsequently explore whether other lanthanide ions exhibit similar structural changes in the presence of excess Ln3+ ions. For La3+, no changes were detected in the 1H NMR spectrum after adding excess La3+ ions into the solution of La4L2 (Fig. S23†), indicating that excess La3+ ions do not trigger structural conversion. However, for Sm3+ ion, the NMR titration experiment of excess Sm3+ ions showed the emergence of new NMR peaks when the metal-to-ligand ratio reached 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. At a ratio of 4
1. At a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a set of high-symmetry 1H NMR signals was achieved (Fig. S24†). This high symmetry NMR spectrum is distinctly different from that of Sm8L4 but similar to that of Lu6L3. This observation may suggest that the excess Sm3+ ions can induce the structural transformation from Sm8L4 to Sm6L3, as the result obtained with Lu3+. The measurement of the mass spectrum demonstrates that the molecular formula of the predominant product after structural transformation was Sm6L3 (Fig. 3B). The observations presented above together with the result of the Lu3+ ion (vide supra) show that excess La3+ ions with larger radii are unable to drive structural transformation, while smaller Ln3+ ions, such as Sm3+ and Lu3+ ions, can facilitate such changes. This finding seems to go against traditional knowledge, that is, the smaller the radius of the lanthanide ions, the more stable the resulting complexes and the less likely to suffer structural transformations. Given that the ligand L adopts different conformations (mode A in La4L2 and Mode D in Sm8L4, Table S6†) and exhibits varying torsion angles between panel phenyl rings of the ligand's panel (Fig. 4B and E, 27.60° in La4L2 and 40.32° in Sm8L4) in the crystal structure of La4L2 and Sm8L4, we infer that these differences may account for why La4L2 is stable and does not undergo structural transformation, while Sm8L4 is more labile and capable of such transformation.
1, a set of high-symmetry 1H NMR signals was achieved (Fig. S24†). This high symmetry NMR spectrum is distinctly different from that of Sm8L4 but similar to that of Lu6L3. This observation may suggest that the excess Sm3+ ions can induce the structural transformation from Sm8L4 to Sm6L3, as the result obtained with Lu3+. The measurement of the mass spectrum demonstrates that the molecular formula of the predominant product after structural transformation was Sm6L3 (Fig. 3B). The observations presented above together with the result of the Lu3+ ion (vide supra) show that excess La3+ ions with larger radii are unable to drive structural transformation, while smaller Ln3+ ions, such as Sm3+ and Lu3+ ions, can facilitate such changes. This finding seems to go against traditional knowledge, that is, the smaller the radius of the lanthanide ions, the more stable the resulting complexes and the less likely to suffer structural transformations. Given that the ligand L adopts different conformations (mode A in La4L2 and Mode D in Sm8L4, Table S6†) and exhibits varying torsion angles between panel phenyl rings of the ligand's panel (Fig. 4B and E, 27.60° in La4L2 and 40.32° in Sm8L4) in the crystal structure of La4L2 and Sm8L4, we infer that these differences may account for why La4L2 is stable and does not undergo structural transformation, while Sm8L4 is more labile and capable of such transformation.
Photophysical properties
The UV-vis absorption and luminescence spectra of L and Ln8L4 (Ln = Sm3+, Tb3+, and Dy3+) were measured in a solution at room temperature. Luminescence analysis data show that the ligand L can sensitize Sm3+, Tb3+ and Dy3+ ions (Fig. 5). Upon the excitation wavelength of 345 nm, the complex Tb8L4 exhibits characteristic line-like emission peaks at 487 nm, 543 nm, 585 nm, and 620 nm, which correspond to 5D4 → 6FJ (J = 6–3) energy level transition of Tb3+ (Fig. 5, top). The quantum yield of Tb8L4 in acetonitrile solution is up to 92.74% (Fig. S68†), which is higher than our previously reported tetrahedral Tb3+ cage with a record quantum yield of 82% in multinuclear LOPs.73 Hence, Tb8L4 represents a new record-setting quantum yield in multinuclear LOPs. The above results indicate that the ligand L has excellent sensitization efficiency towards Tb3+. In contrast, the ligand L exhibits relatively poor sensitization ability on Sm3+ and Dy3+ ions because the quantum yield for Sm8L4 and Dy8L4 is determined to be 0.88% and 2.79% (Table S7†), respectively, which are lower than that of Tb8L4. Interestingly, Dy8L4 shows single-component white-light emission when excited at 365 nm by UV light (Fig. 5, inset). Because the ligand L emits blue light (351 nm, Fig. S72†) and the Dy3+ ions mainly exhibit yellow light (572 nm, Fig. S72†), the single-component white-light emission of Dy8L4 can be ascribed to the combination of L-centered emission and Dy3+-centered emission. To elucidate the different sensitization abilities of ligand L to lanthanide ions, we measured the phosphorescence spectrum of the Gd8L4 complex at low temperatures (Fig. S75†). The triplet energy level of the ligand was calculated to be 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523 cm−1, which is closer to the excitation energy level of Tb3+ (E = 20
523 cm−1, which is closer to the excitation energy level of Tb3+ (E = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1), meaning that ligand L exerts a favorable sensitization effect on Tb3+.
500 cm−1), meaning that ligand L exerts a favorable sensitization effect on Tb3+.
Conclusions
In summary, we reported the synthesis and characterization of a series of lanthanide Ln2nLn (n = 2, 3, 4) assemblies with tetranuclear sandwich square, hexanuclear triangular prism and octanuclear tetragonal antiprism structures. The larger La3+ and Nd3+ ions favor the formation of sandwich square structure Ln4L2, while with a decrease in radius, the smaller Sm3+ and Eu3+ give rise to a different structure: the twisted tetragonal prism, Ln8L4. Meanwhile, even smaller Er3+ and Lu3+ ions lose the ability to form exclusively a single species, resulting instead in a mixture of the tetragonal prism, Ln8L4, and the trigonal prism, Ln6L3. Interestingly, the excess Ln3+ ions (Ln = Sm3+ and Lu3+) can trigger the structural transformation from Ln8L4 to Ln6L3. The luminescence investigations revealed that the tetra-topic tridentate ligand based on 2,6-di[pyrazol-1-yl]pyridine can sensitize the luminescence emissions of lanthanide ions simultaneously (Sm3+, Tb3+ and Dy3+). Moreover, a record high luminescence quantum yield (Φ = 92.74%) was achieved for Tb8L4 assembly. This work demonstrates that the ionic radii of lanthanide affect the resulting supramolecular architectures and that multidentate ligands with rectangular panels are excellent candidates for creating nonclassical Archimedean or Platonic lanthanide solids. Synthesizing additional rectangular multidentate ligands is currently being undertaken to further develop nonclassical LOPs.Author contributions
Q. F. S. and C. B. T. designed and supervised the project; J. S. completed the synthesis and performed the experiments; F. Y. solved all the crystal structures; X. F. D. and J. Y. Z. analyzed the experiment data; L. P. Z. contributed the mass experiments. S. J., C. B. T. and Q. F. S. wrote the manuscript; all the authors discussed the results and commented on the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22171264), the Science Foundation of the Fujian Province (No. 2022J01507), and the Self-deployment Project Research Program of Haixi Institutes, Chinese Academy of Sciences (CXZX-2022-GH01).References
- M. Yoshizawa, M. Tamura and M. Fujita, Diels-alder in aqueous molecular hosts: Unusual regioselectivity and efficient catalysis, Science, 2006, 312, 251–254 CrossRef CAS PubMed
.
- Q.-Q. Wang, S. Gonell, S. H. A. M. Leenders, M. Duerr, I. Ivanovic-Burmazovic and J. N. H. Reek, Self-assembled nanospheres with multiple endohedral binding sites pre-organize catalysts and substrates for highly efficient reactions, Nat. Chem., 2016, 8, 225–230 CrossRef CAS PubMed
.
- H. Takezawa, K. Shitozawa and M. Fujita, Enhanced reactivity of twisted amides inside a molecular cage, Nat. Chem., 2020, 12, 574–578 CrossRef CAS PubMed
.
- M. Morimoto, S. M. Bierschenk, K. T. Xia, R. G. Bergman, K. N. Raymond and F. D. Toste, Advances in supramolecular host-mediated reactivity, Nat. Catal., 2020, 3, 969–984 CrossRef CAS
.
- Y. Hao, Y.-L. Lu, Z. Jiao and C.-Y. Su, Photocatalysis Meets Confinement: An Emerging Opportunity for Photoinduced Organic Transformations, Angew. Chem., Int. Ed., 2024, 63, e202317808 CrossRef CAS PubMed
.
- X.-Q. Guo, P. Yu, L.-P. Zhou, S.-J. Hu, X.-F. Duan, L.-X. Cai, L. Bao, X. Lu and Q.-F. Sun, Low-symmetry coordination cages enable recognition specificity and selective enrichment of higher fullerene isomers, Nat. Synth., 2025 DOI:10.1038/s44160-024-00697-0
.
- X.-Z. Li, L.-P. Zhou, L.-L. Yan, Y.-M. Dong, Z.-L. Bai, X.-Q. Sun, D. Juan, W. Shuao, J.-C. Bunzli and Q.-F. Sun, A supramolecular lanthanide separation approach based on multivalent cooperative enhancement of metal ion selectivity, Nat. Commun., 2018, 9, 547 CrossRef PubMed
.
- D. Zhang, T. K. Ronson, Y.-Q. Zou and J. R. Nitschke, Metal-organic cages for molecular separations, Nat. Rev. Chem., 2021, 5, 168–182 CrossRef CAS PubMed
.
- L. J. Wang, S. Bai and Y.-F. Han, Water-Soluble Self-Assembled Cage with Triangular Metal-Metal-Bonded Units Enabling the Sequential Selective Separation of Alkanes and Isomeric Molecules, J. Am. Chem. Soc., 2022, 144, 16191–16198 CrossRef CAS PubMed
.
- P.-F. Cui, X.-R. Liu, Y.-J. Lin, Z.-H. Li and G.-X. Jin, Highly Selective Separation of Benzene and Cyclohexane in a Spatially Confined Carborane Metallacage, J. Am. Chem. Soc., 2022, 144, 6558–6565 CrossRef CAS PubMed
.
- Z. Lu, T. K. K. Ronson, A. W. W. Heard, S. Feldmann, N. Vanthuyne, A. Martinez and J. R. R. Nitschke, Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage, Nat. Chem., 2023, 15, 405–412 CrossRef CAS PubMed
.
- Y.-R. Zheng, K. Suntharalingam, T. C. Johnstone and S. J. Lippard, Encapsulation of Pt(IV) prodrugs within a Pt(II) cage for drug delivery, Chem. Sci., 2015, 6, 1189–1193 RSC
.
- J. E. M. Lewis, E. L. Gavey, S. A. Cameron and J. D. Crowley, Stimuli-responsive Pd2L4 metallosupramolecular cages: towards targeted cisplatin drug delivery, Chem. Sci., 2012, 3, 778–784 RSC
.
- Z.-E. Zhang, Y.-F. Zhang, Y.-Z. Zhang, H.-L. Li, L.-Y. Sun, L.-J. Wang and Y.-F. Han, Construction and Hierarchical Self-Assembly of Multifunctional Coordination Cages with Triangular Metal-Metal-Bonded Units, J. Am. Chem. Soc., 2023, 145, 7446–7453 CrossRef CAS PubMed
.
- Y.-H. Huang, Y.-L. Lu, Z.-M. Cao, X.-D. Zhang, C.-H. Liu, H.-S. Xu and C.-Y. Su, Multipocket Cage Enables the Binding of High-Order Bulky and Drug Guests Uncovered by MS Methodology, J. Am. Chem. Soc., 2024, 146, 21677–21688 CrossRef PubMed
.
- P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, White Phosphorus Is Air-Stable Within a Self-Assembled Tetrahedral Capsule, Science, 2009, 324, 1697–1699 CrossRef CAS PubMed
.
- Y.-H. Huang, Y.-L. Lu, J. Ruan, S.-P. Zheng, X.-D. Zhang, C.-H. Liu, Y.-H. Qin, Z.-M. Cao, Z. Jiao, H.-S. Xu and C.-Y. Su, Dynamic Metallosupramolecular Cages Containing 12 Adaptable Pockets for High-Order Guest Binding Beyond Biomimicry, J. Am. Chem. Soc., 2023, 145, 23361–23371 CrossRef CAS PubMed
.
- L. S. Lisboa, D. Preston, C. J. McAdam, L. J. Wright, C. G. Hartinger and J. D. Crowley, Heterotrimetallic Double Cavity Cages: Syntheses and Selective Guest Binding, Angew. Chem., Int. Ed., 2022, 61, e202201700 CrossRef CAS PubMed
.
- Y. Katagiri, Y. Tsuchida, Y. Matsuo and M. Yoshizawa, An Adamantane Capsule and its Efficient Uptake of Spherical Guests up to 3 nm in Water, J. Am. Chem. Soc., 2021, 143, 21492–21496 CrossRef CAS PubMed
.
- F. J. Rizzuto, L. K. S. von Krbek and J. R. Nitschke, Strategies for binding multiple guests in metal-organic cages, Nat. Rev. Chem., 2019, 3, 204–222 CrossRef
.
- S. Hasegawa, S. L. Meichsner, J. J. Holstein, A. Baksi, M. Kasanmascheff and G. H. Clever, Long-Lived C60 Radical Anion Stabilized Inside an Electron-Deficient Coordination Cage, J. Am. Chem. Soc., 2021, 143, 9718–9723 CrossRef CAS PubMed
.
- N. M. A. Speakman, A. W. Heard and J. R. Nitschke, A CuI6L4 Cage Dynamically Reconfigures to Form Suit[4] anes and Selectively Bind Fluorinated Steroids, J. Am. Chem. Soc., 2024, 146, 10234–10239 CrossRef CAS PubMed
.
- W. Thor, A. N. Carneiro Neto, R. T. Moura Jr, K.-L. Wong and P. A. Tanner, Europium(III) coordination chemistry: structure, spectra and hypersensitivity, Coord. Chem. Rev., 2024, 517, 215927 CrossRef CAS
.
- Z.-H. Pan, Z.-Z. Weng, X.-J. Kong, L.-S. Long and L.-S. Zheng, Lanthanide-containing clusters for catalytic water splitting and CO2 conversion, Coord. Chem. Rev., 2022, 457, 214419 CrossRef CAS
.
- F. Pointillart, M. Atzori and C. Train, Magneto-chiral dichroism of chiral lanthanide complexes, Inorg. Chem. Front., 2024, 11, 1313–1321 RSC
.
- N. Xu, W. Chen, Y.-S. Ding and Z. Zheng, A Cubic Tinkertoy-like Heterometallic Cluster with a Record Magnetocaloric Effect, J. Am. Chem. Soc., 2024, 146, 9506–9511 CrossRef CAS PubMed
.
- Z. Zhu, S. Hu, Z. Liu, L. Zhou, C. Tian and Q. Sun, A cationic radical lanthanide organic tetrahedron with remarkable coordination enhanced radical stability, Chin. Chem. Lett., 2025, 36, 109641 CrossRef CAS
.
- X.-Z. Li, C.-B. Tian and Q.-F. Sun, Coordination-Directed Self-Assembly of Functional Polynuclear Lanthanide Supramolecular Architectures, Chem. Rev., 2022, 122, 6374–6458 CrossRef CAS PubMed
.
- D. J. Bell, L. S. Natrajan and I. A. Riddell, Design of lanthanide based metal-organic polyhedral cages for application in catalysis, sensing, separation and magnetism, Coord. Chem. Rev., 2022, 472, 214786 CrossRef CAS
.
- D. L. Caulder and K. N. Raymond, Supermolecules by design, Acc. Chem. Res., 1999, 32, 975–982 CrossRef CAS
.
- X.-Z. Li, L.-P. Zhou, L.-L. Yang, D.-Q. Yuan, C.-S. Lin and Q.-F. Sun, Evolution of Luminescent Supramolecular Lanthanide M2nL3n Complexes from Helicates and Tetrahedra to Cubes, J. Am. Chem. Soc., 2017, 139, 8237–8244 CrossRef CAS PubMed
.
- Z. Wang, L. He, B. Liu, L.-P. Zhou, L.-X. Cai, S.-J. Hu, X.-Z. Li, Z. Li, T. Chen, X. Li and Q.-F. Sun, Coordination-Assembled Water-Soluble Anionic Lanthanide Organic Polyhedra for Luminescent Labeling and Magnetic Resonance Imaging, J. Am. Chem. Soc., 2020, 142, 16409–16419 CrossRef CAS PubMed
.
- X.-F. Duan, L.-P. Zhou, H.-R. Li, S.-J. Hu, W. Zheng, X. Xu, R. Zhang, X. Chen, X.-Q. Guo and Q.-F. Sun, Excited-Multimer Mediated Supramolecular Upconversion on Multicomponent Lanthanide-Organic Assemblies, J. Am. Chem. Soc., 2023, 145, 23121–23130 CrossRef CAS PubMed
.
- X.-Z. Li, L.-P. Zhou, S.-J. Hu, L.-X. Cai, X.-Q. Guo, Z. Wang and Q.-F. Sun, Metal ion adaptive self-assembly of photoactive lanthanide-based supramolecular hosts, Chem. Commun., 2020, 56, 4416–4419 RSC
.
- Y. Liu, Z. Lin, C. He, L. Zhao and C. Duan, A symmetry-controlled and face-driven approach for the assembly of cerium-based molecular polyhedra, Dalton Trans., 2010, 39, 11122–11125 RSC
.
- L. Zhao, S. Qu, C. He, R. Zhang and C. Duan, Face-driven octanuclear cerium(IV) luminescence polyhedra: synthesis and luminescent sensing natural saccharides, Chem. Commun., 2011, 47, 9387–9389 RSC
.
- X. Tang, D. Chu, W. Gong, Y. Cui and Y. Liu, Metal-Organic Cages with Missing Linker Defects, Angew. Chem., Int. Ed., 2021, 60, 9099–9105 CrossRef CAS PubMed
.
- Y. B. Tan, Y. Okayasu, S. Katao, Y. Nishikawa, F. Asanoma, M. Yamada, J. Yuasa and T. Kawai, Visible Circularly Polarized Luminescence of Octanuclear Circular Eu(III) Helicate, J. Am. Chem. Soc., 2020, 142, 17653–17661 CrossRef CAS PubMed
.
- I. A. Riddell, Y. R. Hristova, J. K. Clegg, C. S. Wood, B. Breiner and J. R. Nitschke, Five Discrete Multinuclear Metal-Organic Assemblies from One Ligand: Deciphering the Effects of Different Templates, J. Am. Chem. Soc., 2013, 135, 2723–2733 CrossRef CAS PubMed
.
- D. J. Bell, T. Zhang, N. Geue, C. J. Rogers, P. E. Barran, A. M. Bowen, L. S. Natrajan and I. A. Riddell, Hexanuclear Ln6L6 Complex Formation by Using an Unsymmetric Ligand, Chem. – Eur. J., 2023, 29, e202302497 CrossRef CAS PubMed
.
- S.-J. Hu, X.-Q. Guo, L.-P. Zhou, L.-X. Cai, C.-B. Tian and Q.-F. Sun, Ionic Radius Dependent Kinetic Behavior for the Self-Assemblyand Chiral Amplification of Lanthanide Tetrahedral Cages, Chin. J. Chem., 2023, 41, 797–804 CrossRef CAS
.
- Q. Bai, Y. Liu, T. Wu, H. Su, G. Chen, Y. Guan, M. Wang, T.-Z. Xie, Z. Zhang and P. Wang, Metal ion determined self-assembly using terpyridine building blocks, Org. Chem. Front., 2022, 9, 2343–2350 RSC
.
- Q.-F. Sun, S. Sato and M. Fujita, An M18L24 stellated cuboctahedron through post-stellation of an M12L24 core, Nat. Chem., 2012, 4, 330–333 CrossRef CAS PubMed
.
- K. Endo, H. Ube and M. Shionoya, Multi-Stimuli-Responsive Interconversion between Bowl- and Capsule-Shaped Self-Assembled Zinc(II) Complexes, J. Am. Chem. Soc., 2020, 142, 407–416 CrossRef CAS PubMed
.
- T.-Z. Xie, K. J. Endres, Z. Guo, J. M. Ludlow III, C. N. Moorefield, M. J. Saunders, C. Wesdemiotis and G. R. Newkome, Controlled Interconversion of Superposed-Bistriangle, Octahedron, and Cuboctahedron Cages Constructed Using a Single, Terpyridinyl-Based Polyligand and Zn2+, J. Am. Chem. Soc., 2016, 138, 12344–12347 CrossRef CAS PubMed
.
- J. A. Davies, T. K. Ronson and J. R. Nitschke, Twisted rectangular subunits self-assemble into a ferritin-like capsule, Chem, 2022, 8, 1099–1106 CAS
.
- X.-Q. Guo, L.-P. Zhou, S.-J. Hu, L.-X. Cai, P.-M. Cheng and Q.-F. Sun, Hexameric Lanthanide-Organic Capsules with Tertiary Structure and Emergent Functions, J. Am. Chem. Soc., 2021, 143, 6202–6210 CrossRef CAS PubMed
.
- R. Zhu, I. Regeni, J. J. Holstein, B. Dittrich, M. Simon, S. Prevost, M. Gradzielski and G. H. Clever, Catenation and Aggregation of Multi-Cavity Coordination Cages, Angew. Chem., Int. Ed., 2018, 57, 13652–13656 CrossRef CAS PubMed
.
- S. M. Jansze and K. Seyerin, Palladium-Based Metal-Ligand Assemblies: The Contrasting Behavior upon Addition of Pyridine or Acid, J. Am. Chem. Soc., 2019, 141, 815–819 CrossRef CAS PubMed
.
- L.-P. Zhou, X.-S. Feng, S.-J. Hu and Q.-F. Sun, Controlled Self-Assembly, Isomerism, and Guest Uptake/Release of Charge-Reversible Lanthanide-Organic Octahedral Cages, J. Am. Chem. Soc., 2023, 145, 17845–17855 CrossRef CAS PubMed
.
- K.-H. Yim, C.-T. Yeung, H.-Y. Wong and G.-L. Law, Structural variation of self-assembled lanthanide supramolecular complexes induced by reaction conditions, Inorg. Chem. Front., 2021, 8, 2952–2964 RSC
.
- E. Benchimol, B.-N. T. Nguyen, T. K. Ronson and J. R. Nitschke, Transformation networks of metal-organic cages controlled by chemical stimuli, Chem. Soc. Rev., 2022, 51, 5101–5135 RSC
.
- A. M. Johnson, M. C. Young, X. Zhang, R. R. Julian and R. J. Hooley, Cooperative Thermodynamic Control of Selectivity in the Self-Assembly of Rare Earth Metal-Ligand Helices, J. Am. Chem. Soc., 2013, 135, 17723–17726 CrossRef CAS PubMed
.
- M. Otte, P. F. Kuijpers, O. Troeppner, I. Ivanovic-Burmazovic, J. N. H. Reek and B. de Bruin, Encapsulation of Metalloporphyrins in a Self-Assembled Cubic M8L6 Cage: A New Molecular Flask for Cobalt-Porphyrin-Catalysed Radical-Type Reactions, Chem. – Eur. J., 2013, 19, 10170–10178 CrossRef CAS PubMed
.
- W. Xue, T. K. Ronson, Z. Lu and J. R. Nitschke, Solvent Drives Switching between Λ and Δ Metal Center Stereochemistry of M8L6 Cubic Cages, J. Am. Chem. Soc., 2022, 144, 6136–6142 CrossRef CAS PubMed
.
- W. Meng, B. Breiner, K. Rissanen, J. D. Thoburn, J. K. Clegg and J. R. Nitschke, A Self-Assembled M8L6 Cubic Cage that Selectively Encapsulates Large Aromatic Guests, Angew. Chem., Int. Ed., 2011, 50, 3479–3483 CrossRef CAS PubMed
.
- W. Brenner, T. K. Ronson and J. R. Nitschke, Separation and Selective Formation of Fullerene Adducts within an MII8L6 Cage, J. Am. Chem. Soc., 2017, 139, 75–78 CrossRef CAS PubMed
.
- V. Mouarrawis, E. O. Bobylev, B. de Bruin and J. N. H. Reek, A Novel M8L6 Cubic Cage That Binds Tetrapyridyl Porphyrins: Cage and Solvent Effects in Cobalt-Porphyrin-Catalyzed Cyclopropanation Reactions, Chem. – Eur. J., 2021, 27, 8390–8397 CrossRef CAS PubMed
.
- L. Yang, X. Jing, C. He, Z. Chang and C. Duan, Redox-Active M8L6 Cubic Hosts with Tetraphenylethylene Faces Encapsulate Organic Dyes for Light-Driven H2 Production, Chem. – Eur. J., 2016, 22, 18107–18114 CrossRef CAS PubMed
.
- J. A. Davies, A. Tarzia, T. K. Ronson, F. Auras, K. E. Jelfs and J. R. Nitschke, Tetramine Aspect Ratio and Flexibility Determine Framework Symmetry for Zn8L6 Self-Assembled Structures, Angew. Chem., Int. Ed., 2023, 62, e202217987 CrossRef CAS PubMed
.
- J. A. Davies, T. K. Ronson and J. R. Nitschke, Triamine and Tetramine Edge-Length Matching Drives Heteroleptic Triangular and Tetragonal Prism Assembly, J. Am. Chem. Soc., 2024, 146, 5215–5223 CrossRef CAS PubMed
.
- L. J. K. Cook, R. Mohammed, G. Sherborne, T. D. Roberts, S. Alvarez and M. A. Halcrow, Spin state behavior of iron(II)/dipyrazolylpyridine complexes. New insights from crystallographic and solution measurements, Coord. Chem. Rev., 2015, 289, 2–12 CrossRef
.
- F. Yin, J. Yang, L.-P. Zhou, X. Meng, C.-B. Tian and Q.-F. Sun, 54 K Spin Transition Temperature Shift in a Fe6L4 Octahedral Cage Induced by Optimal Fitted Multiple Guests, J. Am. Chem. Soc., 2024, 146, 7811–7821 CrossRef CAS PubMed
.
- M. Feng, F. Pointillart, B. Le Guennic, B. Lefeuvre, S. Golhen, O. Cador, O. Maury and L. Ouahab, Unprecedented Sensitization of Visible and Near-Infrared Lanthanide Luminescence by Using a Tetrathiafulvalene-Based Chromophore, Chem. – Asian J., 2014, 9, 2814–2825 CrossRef CAS PubMed
.
- J. M. Stanley, X. Zhu, X. Yang and B. J. Holliday, Europium Complexes of a Novel Ethylenedioxythiophene-Derivatized Bis(pyrazolyl)pyridine Ligand Exhibiting Efficient Lanthanide Sensitization, Inorg. Chem., 2010, 49, 2035–2037 CrossRef CAS PubMed
.
- X. Hu, W. Dou, C. Xu, X. Tang, J. Zheng and W. Liu, Lanthanide radii controlled one-dimensional polymer and dinuclear complexes and their fluorescent properties, Dalton Trans., 2011, 40, 3412–3418 RSC
.
- G. Li, X. Zhao, Q. Han, L. Wang and W. Liu, Radii-dependent self-assembly of chiral lanthanide complexes: synthesis, chirality, and single-molecule magnet behavior, Dalton Trans., 2020, 49, 10120–10126 RSC
.
- A. Vuillamy, S. Zebret, C. Besnard, V. Placide, S. Petoud and J. Hamacek, Functionalized Triptycene-Derived Tripodal Ligands: Privileged Formation of Tetranuclear Cage Assemblies with Larger Ln(III), Inorg. Chem., 2017, 56, 2742–2749 CrossRef CAS PubMed
.
- H.-Y. Wong, W. T. K. Chan and G.-L. Law, Assembly of Lanthanide(III) Cubanes and Dimers with Single-Molecule Magnetism and Photoluminescence, Inorg. Chem., 2018, 57, 6893–6902 CrossRef CAS PubMed
.
- D. Chakraborty, N. Kaur, J. Sahoo, N. Hickey, M. De and P. S. Mukherjee, Host-Guest Interactions Induced Enhancement in Oxidase-Like Activity of a Benzothiadiazole Dye Inside an Aqueous Pd8L4 Barrel, J. Am. Chem. Soc., 2024, 146, 24901–24910 CrossRef CAS PubMed
.
- P. Howlader, B. Mondal, P. C. Purba, E. Zangrando and P. S. Mukherjee, Self-Assembled Pd(II) Barrels as Containers for Transient Merocyanine Form and Reverse Thermochromism of Spiropyran, J. Am. Chem. Soc., 2018, 140, 7952–7960 CrossRef CAS PubMed
.
- J. B. Maglic and R. Lavendomme, MoloVol: an easy-to-use program for analyzing cavities, volumes and surface areas of chemical structures, J. Appl. Crystallogr., 2022, 55, 1033–1044 CrossRef CAS PubMed
.
- S.-Y. Wu, X.-Q. Guo, L.-P. Zhou and Q.-F. Sun, Fine-Tuned Visible and Near-Infrared Luminescence on Self-Assembled Lanthanide-Organic Tetrahedral Cages with Triazole-Based Chelates, Inorg. Chem., 2019, 58, 7091–7098 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthesis, X-ray crystallographic data, NMR, MS, UV-Vis, FL and other physical measurements. CCDC 2413380–2413382 and 2413524. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5qi00265f |
| This journal is © the Partner Organisations 2025 |