Recent advances in glutarimide chemistry for cereblon-mediated targeted protein degradation: developments in synthesis and diversification
Xiaochu
Ba
,
Mahzad
Dehghany
,
Deborah S.
Mortensen
 and
Natalie
Holmberg-Douglas
and
Natalie
Holmberg-Douglas
 *
*
Bristol-Myers Squibb Company, San Diego, California, USA. E-mail: natalie.holmbergdouglas@bms.com
First published on 5th December 2025
Abstract
The field of targeted protein degradation has garnered significant attention over the past two decades, highlighted by the FDA approval of several therapeutics and the entry of numerous drug candidates into clinical development, the majority of which are cereblon (CRBN) based therapeutics. Synthetic strategies to access these modalities have evolved from low-yield, multi-step syntheses to more efficient methodologies emphasizing late-stage and/or one-step functionalization. In this review, we discuss 22 recently published literature studies focusing on synthetic methodologies compatible with glutarimide scaffolds, which serve as key cereblon-binding ligand for E3 ubiquitin ligase recruitment. The methodologies covered include modern one-electron transformations such as metal-catalyzed reductive couplings, decarboxylative cross-electrophile couplings, and electro/photocatalytic couplings. In addition, we highlight optimized two-electron transformations including Buchwald–Hartwig, Suzuki–Miyaura and Sonogashira couplings that tailored for glutarimide-containing substrates. Novel synthetic approaches, such as N–H insertion, click chemistry, C–H functionalization, and carbene-mediated cyclopropanation/cyclopropenation, are also discussed for their potential in enabling the rapid development of novel cereblon-mediated degraders.
Introduction
Targeted protein degradation (TPD) leverages the ubiquitin-proteasome system (UPS) to eliminate disease-associated proteins through an event-driven mechanism.1 This approach offers the potential to target proteins that are traditionally considered “undruggable” which lack traditional binding pockets or are difficult to inhibit with conventional small molecules. Within the UPS, ubiquitin-protein ligases (E3 ligases) mediate the transfer of ubiquitin molecules to substrate proteins, thereby marking them for proteasomal degradation.2 Since the identification of cereblon (CRBN) as the target of thalidomide,3 CRBN has garnered substantial attention as a key E3 ligase for therapeutic protein degradation. Small molecules that can hijack CRBN for targeted degradation primarily function through two different mechanisms. In the case of molecular glues, compounds bind to CRBN and recruit substrates with suitable degron motifs for degradation (cereblon E3 ligase modulatory drugs, CELMoDs) and heterobifunctional degraders which utilize a target binding moity to recruit a said target to the ligase complex (ligand directed degraders (LDD4) or proteolysis-targeting chimeras, PROTACs1). As of 2022, all 6 molecular glue degraders in clinical development employ CRBN as the recruiting E3 ligase, while 10 of the 11 disclosed heterobifunctional degraders in clinical trials also utilize CRBN as the E3 ligase (Fig. 1a).2 This predominance is likely attributed to the favorable physicochemical properties of the glutarimide moiety, which confers low molecular weight and more desirable drug-like characteristics compared to ligands for other E3 ligases such as Von Hippel–Lindau (VHL)5 and DCAF16.6 Over the last decade, the structural diversity of glutarimide-containing CRBN binders has expanded well beyond the canonical isoindolinone and phthalimide scaffolds, such as the indazole- or benzoimidazole-based CRBN binders (Fig. 1b).7–9 With the structural basis of thalidomide and lenalidomide binding to CRBN well estabilished,10–12 subsequent modifications have focused on expanding chemical diversity and elucidating SAR for the recruitment of known neosubstrates, notably IKZF1, IKZF3 and GSPT1. While some strategies have exploited steric clashes with neosubstrate G-loops to enhance selectivity,13–15 the increasing diversity of CRBN binders has aimed to expand the toolbox for the development of heterobifunctional and and molecular glues. Specifically, these efforts seek to achieve greater selectivity, improved potency and depth of degradation, and improved drug-like properties.7 | ||
| Fig. 1 a) Representative CRBN-based protein degraders currently in clinical development;2 b) selected examples of novel cereblon-binding scaffolds reported in recent literature. See ref. 7–9. | ||
Alongside the identification of novel non-traditional or alternative glutarimide based CRBN binders, substantial progress has also been achieved in their synthetic methodologies. This review highlights recent advances in the rapid construction and functionalization of CRBN binders, with a particular emphasis on glutarimide-containing compounds (Fig. 2). Other novel glutarimide analogues such as succinimide and oxetane derivatives are beyond the scope of this discussion. Instead, we focus on recent literature publications detailing improved synthetic strategies tailored for glutarimide-containing molecules. Commonly used terminologies (e.g., linker) related to heterobifunctional degraders are highlighted for an example compound in Fig. 1a and will be used throughout this review. For a comprehensive overview of historical synthetic approaches to CRBN binders, readers are directed to several excellent review articles.16,17
 | ||
| Fig. 2 Overview of recent advances in glutarimide chemistry. Bonds formed are colored in blue (C–N) or red (C–C). | ||
Challenges in glutarimide chemistry
The synthesis of glutarimide-containing molecules remains challenging due to several factors: (1) the inherent instability of the glutarimide ring under aqueous basic conditions, which often leads to ring-opening;18,19 (2) the susceptibility of the stereocenter on the glutamine ring to epimerization;20 and (3) the presence of an acidic proton on the nitrogen atom, which complicates traditional metal-catalyzed cross-coupling reactions and Mitsunobu-type chemistries.16 Consequently, there is a pressing need for the development of strategies enabling late-stage installation of the glutarimide ring with enantioselective control, or for functionalization methods that are compatible with the glutarimide moiety.Historical methods for glutarimide-based CRBN binders synthesis
Traditional synthetic approaches for constructing cereblon binders can be broadly classified into three main categories: (1) direct attachment of the glutarimide ring; (2) late-stage cyclization to form the glutarimide ring; and (3) masked glutarimide strategies.Direct attachment of the glutarimide ring
A direct substitution strategy is frequently employed in the synthesis of cereblon binders, wherein the glutarimide ring is linked to a heterocycle through a C–N bond. Commonly used starting materials include commercially available 3-bromopiperidine-2,6-dione or 3-aminopiperidine-2,6-dione (Scheme 1a). One representative example is the optimized industrial-scale synthesis of lenalidomide, where 3-aminopiperidine-2,6-dione was used as a nucleophile. Ponomaryov et al. established mild conditions to cyclize isoindolinone from 3-aminopiperidine-2,6-dione and ortho-toluic ester, yielding a nitro precursor of lenalidomide on a multi-hundred grams scale with an excellent yield of 89% (Scheme 1b).21 In one of the earliest examples of functionalized thalidomide-core compounds, Stewart and colleagues employed this strategy to prepare substituted thalidomide derivatives in moderate yields (49–61%) (Scheme 1c).22 More recently, novel cereblon binders with non-phthalimide and non-isoindolinone scaffolds have been reported.7,13,23 For example, Nie et al. described a benzimidazole-based cereblon binder synthesized via direct nucleophilic substitution of a functionalized benzimidazole with 3-bromopiperidine-2,6-dione in the presence of sodium hydride (Scheme 1d).13 This publication identified benzimidazole derivatives as potent, cell-permeable CRBN ligands with reduced recruitment of Ikaros/Aiolos. Similarly, Norris and co-workers reported an indazole-based cereblon binder using a comparable synthetic approach (Scheme 1e), where 3-bromopiperidine-2,6-dione was used as electrophile.7 However, this strategy often results in low yields, possibly due to side reactions involving elimination byproducts of 3-bromopiperidine-2,6-dione. Furthermore, since this approach is commonly employed early in the synthetic sequence, it presents potential drawbacks in downstream transformations. These include epimerization, ring-opening of the glutarimide, or complications arising from the presence of an acidic proton.16,18–20 The indazole based glutarimides were shown to be potent CRBN binders, while showing no degradation of Aiolos. | ||
| Scheme 1 a) Common starting materials and syntheses of b) isoindolinone; c) phthalimide; d) benzimidazole; e) indazole-based cereblon binders by historical methods. See ref. 7, 13, 21 and 22. | ||
Late-stage glutarimide ring cyclization
In the past decade, various tandem cyclization strategies for constructing the glutarimide ring have been developed.24–26 One of the advantages of this approach is the minimization of hazardous exposure associated with the cyclized glutarimide ring, which is required for CRBN binding and brings the risk of potential teratogenicity and/or cytotoxicity depending on the substrates recruited. The synthesis of the well-characterized isoindolinone core commonly employs phthalic anhydride in dehydrative condensation reaction with either L-glutamic acid or L-glutamine (Scheme 2a).24–26 A relatively recent study by Vu and co-workers reported an improved two-step synthesis of thalidomide in multi-hundreds grams scale, achieving a high overall yield of 65%.26 In this method, phthalic anhydride is first reacted with L-glutamic acid to afford N-phthaloyl-D-glutamic acid, which is subsequently cyclized in the presence of ammonium acetate in diphenyl ether to form the glutarimide ring (Scheme 2a). Analogous cyclization strategies have been employed in the synthesis of other IMiDs® such as lenalidomide, as well as novel cereblon binders. In the most recent work by Norris et al.,7 phenyl-substituted glutarimide derivatives were synthesized via a Michael addition of substituted phenylacetonitrile to an acrylate, forming a cyano-phenylbutanoate intermediates, which was then cyclized to yield novel phenyl glutarimides (Scheme 2b). One significant advantage of the late-stage cyclization strategy lies in its ability to afford chiral final compounds. In the synthesis of Iberdomide (CC-220), as reported by Matyskiela et al. at Celgene/Bristol-Myers Squibb, an optically pure, ring-opened glutarimide intermediate was tethered to the lenalidomide core and preserved throughout the entire synthetic sequence (Scheme 2c).27 Cyclization was performed as the final step to yield enantiomerically pure CC-220. This approach retains a stable, ring-opened glutarimide until the final stage, is commonly employed to ensure enantiopurity of the target compound while mitigating issues related to glutarimide stability and reactivity during earlier steps in the synthesis. | ||
| Scheme 2 Cyclization strategy applied on a) synthesis of thalidomide; b) synthesis of functionalized phenyl glutarimides; c) synthesis of CC-220. See ref. 7, 26 and 27. | ||
Masked glutarimide strategy
One of the key challenges in glutarimide synthesis is the incompatibility of the acidic N–H proton in glutarimide with traditional metal-catalyzed cross-coupling methodologies.28 A common strategy to circumvent this issue involves the use of a bis(benzyloxy)pyridine moiety, followed by hydrogenation to generate the glutarimide core. This approach was first published by Min and co-workers during the discovery of a BRD4 (bromodomain-containing protein 4) heterobifunctional degrader.29 In their work, a phenyl glutarimide cereblon binder was synthesized via Suzuki–Miyaura cross-coupling between 2,6-bis(benzyloxy)-3-bromopyridine and (4-hydroxyphenyl)boronic acid to yield 4-(2,6-bis(benzyloxy)pyridin-3-yl)phenol (Scheme 3a). This intermediate subsequently underwent linker attachment, followed by catalytic hydrogenation (71% yield), to afford the key intermediate tert-butyl 2-(4-(2,6-dioxopiperidin-3-yl)phenoxy)acetate for final heterobifunctional degrader assembly. The authors proposed that the hydrolytic instability observed in traditional glutarimides arises from activation by the electron-withdrawing phthalimide moiety and demonstrated that replacing it with a phenyl group preserves CRBN binding affinity while significantly increasing stability. Furthermore, phenyl glutarimides offer additional advantages including reduced molecular size and TPSA, improved ligand efficiency, and greater synthetic accessibility. | ||
| Scheme 3 Synthesis of a) BRD4 heterobifunctional degrader; b) indazole-based cereblon binder. See ref. 7 and 29. | ||
Norris et al. also employed a masked glutarimide strategy to synthesize a novel indazole-based cereblon binder.7 A Suzuki–Miyaura coupling was performed between 3-bromo-1-methyl-6-nitro-1H-indazole and 2,6-bis(benzyloxy)-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, followed by palladium-catalyzed hydrogenation to obtain the corresponding indazole-based cereblon binder bearing an amine in 31% yield (Scheme 3b). This synthetic approach often suffers from low overall yields, which may be attributed to incomplete reduction of the 2,6-bis(benzyloxy)pyridine moiety due to residual ligands or catalyst poisons remaining from the prior cross-coupling step. The direct phenyl-glutarimide and indazolo- linked glutarimides reported generally demonstrated good CRBN binding affinities and reduced degradation of Aiolos and GPST1. Notably, minor structural variations, such as meta vs. para direct aryl connections, influenced GPST1 activity. An analogous strategy to the masked glutarimide approach involves the use of a nitrogen protecting group, such as 2,4-dimethoxybenzyl (DMB)7 or tert-butyloxycarbonyl (Boc)9 for glutarimides or p-methoxybenzyl (PMB)30 protection for dihydrouracils, with deprotection near the end of the synthesis to reveal the glutarimide. Deprotection of protected glutarimides has been accomplished under strongly acidic conditions at elevated temperatures31 or under oxidative conditions using ammonium cerium(IV) nitrate (CAN) at ambient temperature.7,32
Recent advances in glutarimide chemistry
Recent synthetic methodologies developed for CRBN binders can be categorized into three main strategies: Section 1. C–H functionalization for construction of glutarimides; Section 2. Structural diversification of glutarimide containing scaffolds; and Section 3. Dihydrouracil synthesis.Section 1. C–H functionalization for construction of glutarimides
The lenalidomide core has traditionally been synthesized via a nucleophilic substitution reaction between 3-aminopiperidinedione and ortho-toluic ester, as illustrated in Scheme 1b. In 2021, Liu and co-workers reported an novel intramolecular palladium-catalyzed ring-closure via γ-C(sp3)–H functionalization of a carboxylic acid–amide precursor to construct ring-opened lenalidomide-based CRBN binder precursors.33 Through systematic ligand design and optimization of reaction conditions, the authors identified ligand L (Scheme 4) as critical for promoting the desired transformation. The introduction of a C6-methyl substituent on the pyridone ligand is proposed to enhance steric hindrance, which facilitates the formation of monomeric palladium species and promotes the subsequent reductive elimination step.33 | ||
| Scheme 4 Construction of CRBN binders via C–H functionalization. See ref. 33. | ||
The increased steric bulk at the C6 position is believed to accelerate the conversion of the unreactive L2–Pd(II) complex into an active monomeric Pd–ligand complex, thereby promoting intramolecular C–N bond formation.33 Further fine-tuning of the ligand electronics revealed that incorporation of an electron-withdrawing group, such as a C4-trifluoromethyl substituent, significantly improved catalytic performance. Under the optimized conditions, the C–H functionalization proceeded with high selectivity at the target methyl C–H bond, even in the presence of other reactive sites such as allylic and benzylic C(sp3)–H bonds.33
This protocol was successfully applied to a variety of ring-opened glutarimide precursors, tolerating a broad range of common side chains, including carboxylic acid, ester, and amide functionalities (Scheme 4). A notable limitation of this methodology is the requirement for substitution (R substituent in Scheme 4) at the ortho or meta positions of the benzoic amide moiety to prevent undesired activation of the ortho-C(sp2)–H bond.33 Substrates bearing ortho substituents also observed higher yields than those with meta substitution, likely due to effective suppression of competing ortho-C(sp2)–H activation.33 This method also demonstrated good tolerance for presence of various functional groups, including bromo, nitro, trifluoromethyl, and fluoro substituents on the ring-opened glutarimide precursor (Scheme 4), enabling further diversification. To showcase the synthetic utility, the authors reported the two-step synthesis of a substituted isoindolinone-based CRBN binder (5) using this strategy, highlighting its potential in the discovery of novel substituted CRBN ligands. Importantly, the reaction was successfully scaled up to 2.0 mmol without a significant loss in yield. Overall, this methodology enables efficient access to novel CRBN binders through site-selective C–H activation. However, careful substrate design is required to ensure appropriate substitution patterns on the benzoic amide to avoid undesired ortho-C(sp2)–H activation. Notably, the applicability of this methodology to ring-closed glutarimides was not addressed in the original study. While the authors did not specifically comment on any potential impacts on biological activity, it is important to note that 6-and 7- substitution of the lenalidomide core are known to impact neosubstrate selectivity.34 Modifications at these positions lie in close proximity to the G-loop which can influence the recruitment profile of neosubstrates.
Section 2. Structural diversification of glutarimide containing scaffolds
In the functionalization section, both one-electron and two-electron coupling methodologies specifically designed for substrates bearing glutarimides are covered, with a focus on: (1) C–N bond formation and (2) C–C bond formation aimed at diversifying CRBN binders in the context of molecular glue and heterobifunctional degraders syntheses.2.1 C–N bond formation
In 2020, Hayhow et al. reported an optimized Buchwald–Hartwig amination protocol enabling the coupling of both alkyl and aryl amines to isoindolinone-based aryl bromides at all substitution positions.28 The study identified a highly sterically hindered and electron-deficient palladium precatalyst, Pd-PEPPSI-IHeptCl, as key to the reaction's success. When combined with cesium carbonate as the base and 1,4-dioxane as the solvent, this system provided the optimal balance between conversion efficiency and suppression of hydrolysis of unreacted starting material and by-product formation. The method exhibited excellent substrate scope, affording high yields with secondary amines and anilines (Scheme 5), and demonstrated good tolerance for unprotected alcohol functionalities. Moderate yields (30–35%) were also obtained with primary amines and sterically hindered anilines or amines. For substrates with initially low conversion, they have found that increasing the amine up to 3 equivalents significantly improved product formation, indicating that excess nucleophile can drive the reaction forward in challenging cases. Importantly, the authors applied this methodology to a complex target binding motif JQ1(+) ligand35 bearing an amine linker, achieving a 27% yield in the final C–N coupling step to construct the heterobifunctional degrader (Scheme 5). This approach facilitates the late-stage introduction of diverse linkers onto CRBN ligands, streamlining structure–activity relationship (SAR) studies by reducing synthetic steps and improving overall efficiency. Moreover, the strategy allows for installation of the cyclic glutarimide at a late stage in synthesis. Despite its utility, the method has limitations. For instance, the primary amine example presented (Scheme 5) resulted in only partial conversion, which is notable given the frequent incorporation of primary amine linkers in reported heterobifunctional degraders. Additionally, substrates bearing lactam, carboxylic acid, pyrazole, and imidazole failed to undergo coupling.28 While aryl bromides were shown to be effective electrophilic partners, the applicability of this protocol to other aryl halide or to alternative CRBN binders has not yet been demonstrated.
 | ||
| Scheme 5 A Buchwald–Hartwig protocol for C–N bond formation on aryl bromides bearing glutarimide moiety. See ref. 28. | ||
A similar strategy for optimizing Buchwald–Hartwig cross-coupling conditions for reactions involving unprotected glutarimides via C–N bond formation was reported by Lejava, Miseo, and co-workers.36 Through high-throughput screening of a broad range of ligands, bases, and solvents, BrettPhos was identified as the optimal ligand for coupling with primary amines (Scheme 6, method A), whereas RuPhos was most effective for secondary amines in the synthesis of alternative glutarimide-based CRBN binders (Scheme 6, method B).36 A key finding was the importance of using lithium hexamethyldisilazide (LiHMDS) as the base, when working with glutarimides. This effect was likely due to the formation of aggregated lithium species, with the glutarimide functioning as an in situ protecting group.36 Using this approach, the authors explored 8 different alternative glutarimide core structures, including indazole, phenyl, and azaindole scaffolds, coupled with both primary and secondary amines, resulting in the synthesis of 16 alternative glutarimide cores (Scheme 6). For the specific case of N-linked glutarimides, an alternative protocol was proposed involving a reduced reaction temperature of 45 °C and extended reaction time to 16 hours, which improved conversion to the desired products. Overall, this method demonstrated compatibility with unprotected glutarimide substrates, enabling the synthesis of 30 alternative glutarimide CRBN binders, featuring diverse core structures and a variety of primary and secondary amines. However, the authors have noted limitations of this methodology, particularly with substrates containing sensitive functional groups such as esters, carboxylic acids, aldehydes, ketones, and auxiliary amines (i.e., nonreactive NH groups), these groups are not tolerated under these conditions.36 Characterization of the amino substituted indazole, phenyl and azaindazole CRBN binders showed generally strong CRBN binding affinities, with the exception of 5-fluoro- or 5-methoxy 6- amino substituted indazoles. Nearly all examples showed no degradation of the neosubstrates CK1α, GSPT1 and Aiolos. A cryo-EM structure showed that the indazole CRBN binder occupies the CRBN tri-Trp pocket and maintains the hydrogen bonding and van der Waals interactions of traditional IMiDs.
 | ||
| Scheme 6 An alternative Buchwald–Hartwig condition for ring-close alternative glutarimide cores with primary and secondary amines. See ref. 36. | ||
 | ||
| Scheme 7 An iridium-catalyzed N–H insertion of diazomalonates into pomalidomide, and potential post-transformations. See ref. 37. | ||
More recently, Kalinin et al. reported conditions for Rh(II)-catalyzed O–H, S–H, and N–H insertion chemistry with α-diazoglutarimide 14 which yields a diverse library of glutarimide-based CRBN ligands (Scheme 8).38,39 The optimized conditions utilize Rh2(OPiv)4 as the catalyst in dichloromethane at ambient temperature. The X–H scope tolerated alcohols, phenols, carboxylic acids, thiols, aromatic amines, and nitrogen heterocycles; however, it did not tolerate aliphatic amines. Furthermore, subsequent cycloadditions demonstrated the utility in leveraging this methodology for heterobifunctional synthesis. For example, alkynyl product 16 was subjected to a 1,3-dipolar cycloaddition reaction to afford pyrazole 17 in 88% yield. Compared to conventional multi-step synthesis methods, this method offers significant advantages in terms of step economy, yield, and substrate scope under mild conditions, but, it may be limited in scalability due to the potential explosive nature of diazo compounds. The functionalized glutarimides obtained through the X–H insertion chemistry were evaluated in a a microscale thermophoresis CRBN affinity assay, measured log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and cytotoxicity assays. The CRBN binders reported largely maintain potent binding affinity with minimal cytotoxicity.
P and cytotoxicity assays. The CRBN binders reported largely maintain potent binding affinity with minimal cytotoxicity.
 | ||
| Scheme 9 One-step construction of N-(hetero)aryl glutarimides via optimized Buchwald–Hartwig amination conditions. See ref. 41. | ||
2.2 C–C bond formation
 | ||
| Scheme 11 Direct C–C bond formation on glutarimide via Brønsted acid-assisted Ni-electrocatalytic cross-coupling. See ref. 46. | ||
The Reisman group recently reported an enantioselective nickel-catalyzed reductive coupling protocol to install enantioenriched glutarimides, a class of compounds in which the two enantiomers frequently exhibit distinct biological activities.47,48 Notably, the (S)-enantiomer of deutero thalidomide displays ∼10-fold stronger CRBN binding than the (R)-enantiomer.49 In their methodology, 4-heptylBiOX (L1, Scheme 12) was selected as the chiral ligand based on its superior enantioselectivity observed during catalysts screening. A co-solvent system of dimethylacetamide/tetrahydrofuran was employed to optimize the balance between reactivity and stereocontrol. The electronic nature of the aryl coupling partner was found to play a critical role in reaction efficiency. For electron-rich arenes, aryl iodides in combination with α-chloroimides provided superior outcomes (method A). In contrast, for electron-deficient arenes, aryl bromides coupled more effectively with α-imide mesylates (method B). In method B, the mesylate undergoes slow in situ conversion to an α-iodoimide in the presence of sodium iodide. This controlled activation ensures that the rate of oxidative addition of the aryl bromide outpaces the generation of reactive α-haloimide intermediates, thereby suppressing side reactions such as biaryl homocoupling or protodehalogenation.47 This methodology demonstrates a broad substrate scope, tolerating a wide array of sensitive functional groups, including anilines, phenols, esters, and pinacol boronate esters, yielding products in moderate to excellent yields (Scheme 12a). This functional group tolerance allows for further diversification and downstream modifications. However, ortho-substituted arenes tend to afford lower yields, due to steric hindrance. The authors also investigated coupling with heteroaryl partners. In certain cases, diminished enantioselectivity was observed, which is likely attributable to the increased tendency of the more electron-deficient heteroaryl products to undergo racemization under the reaction conditions (Scheme 12b). The methodology was further extended to novel glutarimide derivatives, including five-membered and seven-membered cyclic substrates as well as β-substituted imides (structures not shown).47 Overall, this work provides two robust and complementary reaction conditions that are broadly applicable to both electron-rich and electron-deficient aryl partners under mild conditions, enabling enantioenriched glutarimide incorporation into molecules with a variety of functional groups. The authors also reported the propensity for racemization of the α-arylglutarimide is correlated to the deprotonation energy at the α-position, providing a mechanistic basis for the rational design of hydrolytically stable α-aryl glutarimides. One limitation to this method is that the commonly available 3-bromopiperidine-2,6-dione was incompatible with the reported conditions. However, the corresponding 3-chloropiperidinedione and mesylate analogs can be readily accessed from the bromide precursor. This study highlights the potential of the methodology to support the discovery of new glutarimide-based binders through efficient C–C bond formation. Nevertheless, it remains unclear whether this protocol is applicable to the synthesis of known CRBN-binding scaffolds, such as 3-indazole-based ligands. When considered alongside the recent work by the Baran group,46 this approach represents a significant advancement in the direct functionalization of homochiral glutarimide cores through asymmetric C–C bond formation.
 | ||
| Scheme 12 C–C bond formation on glutarimide with enantioselective nickel-catalyzed reductive coupling with a) aryl halides and b) heteroaryl halide precursors. See ref. 47 and 48. | ||
Another methodology for the synthesis of enantioenriched α-aryl substituted glutarimides via nickel-catalyzed Suzuki–Miyaura cross-coupling was recently reported by Wong et al.50 This protocol employs aliphatic alcohol derivatives and aryl boronic acids as coupling partners. The optimized conditions utilize Ni(cod)2 as the catalyst and ligand L1, the same chiral ligand previously reported by the Reisman group.47 Inorganic base tripotassium phosphate and halide salt sodium iodide were found to be essential for the transformation (Scheme 13).50 Notably, sodium iodide plays a critical role by converting alkyl sulfonates to alkyl iodides in situ, which serve as key reaction intermediates.50 Regarding the α-aryl substituted glutarimide substrate scope, both electron-rich and electron-deficient (hetero)arenes were well tolerated, along with sensitive functional groups such as aryl chlorides and esters, affording products in good to high yields (Scheme 13). Although this study was not specifically focused on glutarimide chemistry, the general substrate scope demonstrated tolerance for aryl boronic acids bearing a range of functionalities, including aldehydes, nitriles, and amides.50
 | ||
| Scheme 13 Nickel-catalyzed Suzuki–Miyaura coupling on PMB protected glutarimides. See ref. 50. | ||
An additional advantage of this methodology is the conversion of alkyl alcohols to mesylates, which then undergo the desired cross-coupling in a one-pot procedure within the same reaction vessel.50 Overall, this method provides a complementary approach for the synthesis of α-aryl substituted glutarimides, alongside the studies reported by the Reisman and Baran groups. One limitation of this methodology is the apparent requirement for p-methoxybenzyl (PMB) protection on the glutarimide nitrogen atom, which adds additional steps for protection and deprotection. The authors did not report results using unprotected glutarimide, nor did they explore substrates containing acidic protons in the general substrates scope of their study.
 | ||
| Scheme 14 An anhydrous and stereoretentive Suzuki–Miyaura coupling for CRBN binders. See ref. 51. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dimethoxyethane: dimethylacetamide solvent system, they achieved up to 89% yield in the model reaction (41, Scheme 15). The substrate scope included both 4- and 5-substituted isoindolinone and phthalimide scaffolds, with both primary and secondary alkyl bromides as coupling partners. Across the series, good to excellent yields (51–91%) were obtained, demonstrating broad functional group tolerance. Notably, groups such as tert-butyloxycarbonyl (Boc), polyethylene glycol (PEG) chains, tert-butyl esters, dioxolane-protected aldehydes, and even alkyl chlorides were well tolerated under the optimized conditions (Scheme 15), highlighting the potential for further downstream functionalization. The alkyne moiety was also compatible, albeit requiring an increased loading of nickel catalyst (2 mol%) and dtbbpy ligand (4 mol%), as the alkyne moiety was suspected to deactivate the nickel species.54 Despite this, high yield (76%) was maintained. However, certain limitations of the methodology were noted. Alkyl bromides bearing azide, α-bromoesters, or benzylic bromides were found to be unreactive under these conditions, failing to produce the desired coupling products.54 The scalability of the reaction was also demonstrated on 5 mmol scale. Although the reaction time increased to approximately 30 hours, no reduction in yield was observed. The authors further explored the translation of this method to flow chemistry. They noted that the organic photocatalyst 1,2,3,4-tetrakis(carbazole-9-yl)-4,6-dicyanobenzene (4CzIPN) could replace the iridium complex in batch reactions without compromising yield. However, under flow conditions, 4CzIPN gradually decomposed, resulting in diminished efficiency.54
1 dimethoxyethane: dimethylacetamide solvent system, they achieved up to 89% yield in the model reaction (41, Scheme 15). The substrate scope included both 4- and 5-substituted isoindolinone and phthalimide scaffolds, with both primary and secondary alkyl bromides as coupling partners. Across the series, good to excellent yields (51–91%) were obtained, demonstrating broad functional group tolerance. Notably, groups such as tert-butyloxycarbonyl (Boc), polyethylene glycol (PEG) chains, tert-butyl esters, dioxolane-protected aldehydes, and even alkyl chlorides were well tolerated under the optimized conditions (Scheme 15), highlighting the potential for further downstream functionalization. The alkyne moiety was also compatible, albeit requiring an increased loading of nickel catalyst (2 mol%) and dtbbpy ligand (4 mol%), as the alkyne moiety was suspected to deactivate the nickel species.54 Despite this, high yield (76%) was maintained. However, certain limitations of the methodology were noted. Alkyl bromides bearing azide, α-bromoesters, or benzylic bromides were found to be unreactive under these conditions, failing to produce the desired coupling products.54 The scalability of the reaction was also demonstrated on 5 mmol scale. Although the reaction time increased to approximately 30 hours, no reduction in yield was observed. The authors further explored the translation of this method to flow chemistry. They noted that the organic photocatalyst 1,2,3,4-tetrakis(carbazole-9-yl)-4,6-dicyanobenzene (4CzIPN) could replace the iridium complex in batch reactions without compromising yield. However, under flow conditions, 4CzIPN gradually decomposed, resulting in diminished efficiency.54
 | ||
| Scheme 15 Photoredox C(sp2)–C(sp3) cross-coupling on isoindolinone and phthalimide based CRBN binders. See ref. 52. | ||
In 2025, Lovato et al. reported a highly effective and broadly applicable nickel-catalyzed cross-electrophile coupling between alkyl tosylates and aryl bromides to construct C(sp2)–C(sp3) bonds.56 Through high-throughput experimentation (HTE), two distinct sets of optimized reaction conditions were identified: method A, employing free base of picolinamide as the ligand without any additive; and method B, utilizing 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbbpy) as the ligand in conjunction with 4-ethylpyridine as an additive (Scheme 16). In general, method A is more suitable for shorter alkyl chains (e.g., two-carbon alkyl tosylates), whereas method B performs better with longer alkyl chains (e.g., four-carbon tosylates). This methodology demonstrated broad applicability across various glutarimide bearing CRBN core scaffolds, including isoindolinone, phthalimide, and phenyl glutarimide derivatives (Scheme 16). It exhibits excellent functional group tolerance, accommodating tosylates bearing esters, Boc, PEG chains, and tert-butyldimethylsilyl (TBS) protecting groups. Both primary and secondary alkyl tosylates are compatible with the reaction, with secondary tosylates showing better reactivity under method A conditions. Importantly, the method was successfully applied to a chiral CRBN ligand (44), yielding the coupled product with complete stereoretention (Scheme 16). Leveraging the method's robust functional group tolerance, the authors employed this coupling as the final step in the synthesis of a BRD4 heterobifunctional degrader (Scheme 16), affording the target molecule in 24% overall yield. Compared to their amide counterparts, alkyl-substituted CRBN binders exhibited improved cell permeability and reduced neosubstrate activity. Molecular dynamics simulations suggested that the increased binding flexibility of the alkyl linkage, relative to the amide, may modulate off-target interactions. Overall, this general and scalable strategy enables efficient C(sp2)–C(sp3) bond formation in the presence of complex, ring-closed glutarimide scaffolds. Alkyl tosylates are readily available or can be conveniently synthesized from corresponding alcohols. The methodology is demonstrated to be scalable up to 0.75 mmol and preserves stereochemical integrity in chiral glutarimides. Its broad substrate scope, including diverse aryl bromides and both primary and secondary alkyl groups, makes it a valuable tool for constructing C(sp2)–C(sp3) bonds in CRBN binders. However, as noted by the authors, certain substrates afforded only moderate to low yields, often due to competing side reactions such as debromination and glutarimide ring-opening by-products.56 Given that only ligand identity and the presence of a single additive were varied in the HTE screening, further optimization, such as exploring alternative solvents or other additives, may enhance the desired conversion of this transformation.
 | ||
| Scheme 16 A nickel-catalyzed cross-electrophile coupling in the presence of glutarimide. aReaction run at 75 °C, b72 h reaction time. aReaction run at 75 °C, b72 h reaction time. See ref. 56. | ||
The Baran group recently reported a redox-neutral, nickel-catalyzed radical cross-coupling methodology that employs sulfonyl hydrazides as a novel class of coupling partners.57 This method enables late-stage methylation, as well as direct or protected hydroxymethylation, aminomethylation, cyanomethylation, cyclobutylation, and cyclopropylation.57 Additionally, it facilitates the installation of arylacetic acid derivatives, oxetane, azetidine, and bicyclo[1.1.1]pentane (BCP) moieties onto both electron-rich and electron-deficient hetero(arenes). The authors noted that the incorporation of these functional groups can improve drug-like properties, such as activity, solubility and metabolic stability. The sulfonyl hydrazide coupling partners can be readily synthesized via five distinct strategies: direct sulfonylation, SN2 substitution of hydrazones, Mitsunobu reactions of hydrazones, reduction of hydrazones, and amination of tosyl amines.57 While this method was not specifically developed for glutarimide scaffolds, its broad functional group tolerance renders it highly effective for both ring-opened precursors and closed glutarimides, providing good to excellent yields (Scheme 17).
 | ||
| Scheme 17 Redox-neutral nickel-catalyzed radical cross-coupling for C(sp2)–C(sp3) bond formation in glutarimide molecules for accessing a) aliphatic alkylations or b) alkoxy- and hydroxymethylations. See ref. 57. | ||
Importantly, the authors demonstrated complete stereoretention in the functionalization of a ring-opened isoindolinone-based glutarimide precursor (Scheme 17a). Tailored reaction conditions were optimized for each functional group class, involving specific combinations of ligands, bases, and temperatures. For instance, pempidine (1,2,2,6,6-pentamethylpiperidine) was employed as the base for the methylation of a small aliphatic fragment (Scheme 17a), whereas triethylamine was used for hydroxymethylation (Scheme 17b). Based on the reported substrate scope which includes thiophenes, thiazoles, pyrimidines, quinolines, and pyrazines,57 this methodology appears potentially suited for the late-stage diversification of alternative glutarimide ligands. Furthermore, its compatibility with a wide range of functional groups that including esters, protected amines, thioesters, ketones, and boron pinacolates (Bpin),57 suggests its potential utility in the construction of heterobifunctional degraders. Overall, this approach offers significant advantages, such as mild reaction conditions, operational simplicity, and the use of readily accessible and stable sulfonyl hydrazide reagents. Importantly, it avoids the need for external oxidants, reductants, or costly catalysts. The protocol also addresses traditionally challenging transformations, such as the introduction of cyanomethyl and carboxymethyl groups.57 However, certain operational details require careful consideration, for example, the necessity of aryl iodides for aminomethylation (versus aryl bromides used for other functionalization) or elevated temperatures (up to 120 °C) for CHF2 incorporation (versus mild temperatures for other functional groups functionalization). This method represents a valuable strategy for the synthesis of sp3-rich CRBN binders.
The Weix group recently reported an innovative approach for the formation of C(sp2)–C(sp3) bonds on CRBN degraders.58 This method enables hetero(aryl) carboxylic acids, that commonly used starting materials for amide bond formation, to undergo decarboxylative cross-electrophile coupling with alkyl halides or pyridinium salts, thereby constructing C(sp2)–C(sp3) bonds. During the reaction optimization, the authors identified 1,2-pyridyl esters as effective activating groups for carboxylic acids, providing improved yields while suppressing ketone by-product formation.58 The authors also developed optimized conditions for substrates bearing glutarimide moieties. Ligand identity was found to have a significant impact on reaction efficiency; ligand L2 (Scheme 18) demonstrated superior performance for glutarimide-containing compounds, whereas ligand L1 (Scheme 18) was more effective for other non-glutarimide substrates. Reaction temperature played a crucial role in minimizing side-product formation: elevated temperatures (up to 110 °C) and increased reaction headspace were necessary to release coordinated CO from the nickel complex, thereby reducing ketone side-product formation.58
 | ||
| Scheme 18 Decarboxylative cross-electrophile coupling for glutarimide containing molecules. See ref. 58. | ||
Among the electrophilic coupling partners, alkyl pyridinium salts, bromides, and iodides were most effective for generating primary alkyl products, while alkyl iodides yielded the best results for secondary alkyl products.58 The methodology exhibits broad functional group tolerance, accommodating nitriles, pinacol boronate esters, and common protecting groups such as benzyl chloroformate (Cbz), Boc, and acetals. Notably, sterically hindered aryl and (hetero)aryl carboxylic acids did not exhibit reduced reactivity or yields. Overall, this protocol provides a direct strategy for constructing C(sp2)–C(sp3) bonds from carboxylic acids, the commonly used starting materials for amide bond formation, without the need for resynthesis on hetero(aryl) glutarimide scaffolds, facilitating rapid SAR exploration of CRBN binders.
The method is compatible with both electron-rich and electron-deficient heteroarenes, as well as sterically hindered substrates and sensitive functional groups. It enables the attachment of both primary and secondary alkyl groups, offering potential utility for the synthesis of rigid and flexible linkers in heterobifunctional degrader molecules. However, limitation remains that in cases with lower yields, aryl dimers and ketones were identified as major side products.58 While the authors noted that this method can offer a route to more metabolically stable C–C linkages, compared to C–O or C–N, no supporting data was provided.
Sloane et al. recently reported a dual nickel/photoredox decarboxylative cross-coupling methodology that enables the coupling of (hetero)aryl halides with α-heteroatom carboxylic acids.59 This transformation employs an iridium-based photocatalyst, a nickel-bipyridyl complex, a phthalimide additive, and 1,1,3,3-tetramethylguanidine (TMG) as the base.59 The authors propose that the phthalimide additive is critical for achieving optimal yields, likely due to its stabilizing effect on the nickel-bipyridyl species during the decarboxylative coupling process.59 This protocol demonstrates broad substrate scope, accommodating both electron-deficient and electron-rich (hetero)aryl halides, as well as a diverse array of α-heteroatom carboxylic acids. Although the study does not specifically focus on targeted protein degradation chemistry, they have highlighted that the protocol tolerates acidic protons. By increasing the amount of TMG base to 2.5 equivalents, the method enables efficient coupling of phenyl-glutarimide (59) and isoindolinone-glutarimide (61) with α-oxy morpholine derivatives (58), achieving yields of up to 49% (Scheme 19a). Furthermore, the resulting morpholine-containing products were shown to undergo subsequent derivatization,59 suggesting potential utility in the synthesis of linkers for heterobifunctional degrader. Overall, this method provides a direct and efficient strategy for α-arylation adjacent to heteroatoms in sp3-rich precursors, facilitating the functionalization of CRBN ligands with fragments that can improve solubility, stability, and permeability. The coupled products obtained via this approach may otherwise be synthetically challenging or require multiple steps. While only two glutarimide-containing substrates were explored in the original study, the demonstrated acid tolerance suggests the method could potentially be broadly applicable to other sp3 α-heteroatom motifs, offering a valuable new tool for researchers developing CRBN-based degraders.
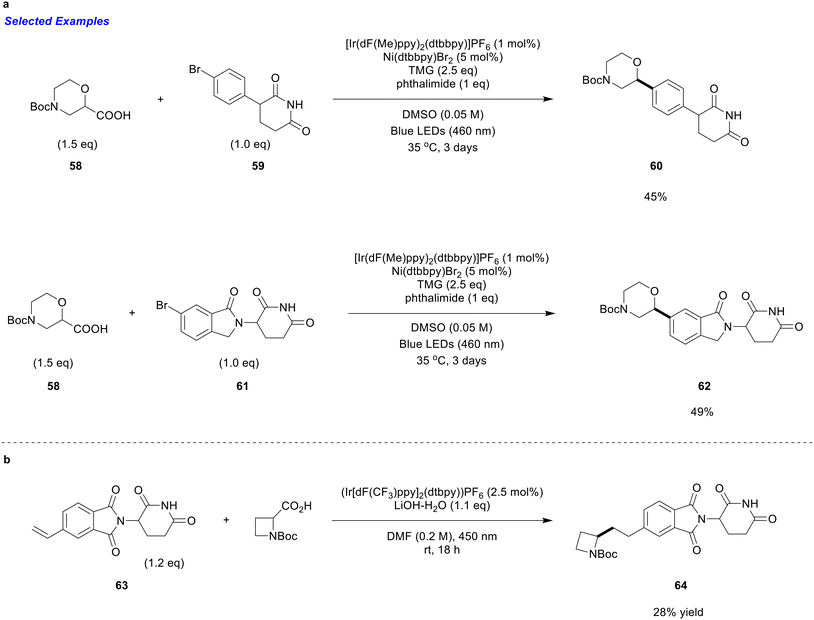 | ||
| Scheme 19 a) Dual nickel/photoredox decarboxylative cross-coupling. b) Direct azetidine functionalization toward CRBN ligands via photoredox catalysis. See ref. 59 and 60. | ||
A recent study resulting from a collaboration between scientists at Enamine and Pfizer reported a direct photochemical modification method for azetidine-2-carboxylic acids.60 Optimization of the model reaction yielded two distinct sets of conditions. One approach employed the inorganic photocatalyst Ir[dF(CF3)-ppy]2(dtbpy)PF6 with lithium hydroxide monohydrate as the base in DMF, under irradiation at 450 nm. The alternative method utilized the organic photocatalyst 4CzIPN under 365 nm irradiation, affording comparable yields and demonstrating suitability for multigram-scale synthesis via flow chemistry.60 Although the methodology does not specifically target glutarimide chemistry, one example was provided in which 5-vinyl thalidomide (63) was functionalized with an alkyl azetidine group, yielding the product in 28% (Scheme 19b). However, the authors did not discuss potential by-product formation, which may account for this relatively diminished yield compared to other non-glutarimide substrates. The reported methodology exhibits broad aryl substrate scope, including (hetero)aryl styrenes, substituted styrenes, traditional Michael acceptors, and electrophilic olefins bearing sensitive groups such as vinyl sulfones, acrylonitrile, and Bpin. This versatility suggests the potential applicability of the method to alternative or functionalized glutarimide-based CRBN binders, despite the limited exploration of glutarimide substrates in the study. Regarding the azetidine coupling partners, a variety of novel N-Boc-protected azetidine-2-carboxylic acids bearing aliphatic, ether, and quaternary substituents were well tolerated.60 Furthermore, the authors demonstrated the potential for downstream functionalization of the products, which may facilitate the synthesis of novel heterobifunctional degrader linkers.
Tracy et al. recently described the synthesis and characterization of novel CRBN binders incorporating cyclopropane and cyclopropene moieties via mild, enantioselective carbene chemistry.61 The carbene precursor 2,2,2-trichloroethyl 2-(4-bromophenyl)-2-diazoacetate (66, Scheme 20) was chosen due to its capacity to facilitate asymmetric induction and allow further derivatization.61 Initial exploratory reactions demonstrated that the chiral catalyst Rh2(p-PhTPCP)4 is effective in promoting both asymmetric cyclopropanation and cyclopropenation. This mild methodology was subsequently applied to unprotected isoindolinone and phthalimide-based CRBN binders to produce enantiomerically pure glutarimide derivatives (Scheme 20a). Generally, the approach afforded high yields and excellent diastereoselectivity, except in cases where a carbonyl group was positioned adjacent to the reactive site. In such cases, reduced yields were observed, likely due to the steric hindrance imposed by the carbonyl functionality.61 Building upon these results, the authors further demonstrated the synthetic utility of the trichloroethyl ester moiety by conducting late-stage functionalization. The ester-functionalized CRBN binder (68) was successfully reduced to the corresponding carboxylic acid and subsequently converted to an amide (Scheme 20b), yielding a structurally complex, stereochemically retained CRBN binder, structures that are challenging to access through conventional synthetic methods. Overall, this method provides a mild and efficient route for enantioselective cyclopropanation and cyclopropenation of glutarimide-based degraders, achieving high diastereoselectivity and enantioselectivity. Furthermore, the trichloroethyl ester functionality enables downstream structural diversification, facilitating access to novel and structurally complex CRBN binders. Limitations of this strategy include the current dependence on the trichloroethyl ester carbene precursor, highlighting the need for exploration of alternative carbene precursors that allow comparable reactivity and post-functionalization potential. The resulting cyclopropanated lenalidomide and thalidomide derivatives were evaluated in neosubstrate degradation assays for IKZF3, CK1α, GSPT1 and SALL4. No degradation was detected for 6- and 7-substituted lenalidomide compounds across all neosubstrates, corroborating that substitution at this position can ablate neosubstrate degadation.62 In contrast, 4-/5-substituted lenalidomide and thalidomide derivatives showed variable neosubstrate degradation, with cyclopropenes recruiting more neosubstrates than cyclopropanes. Stereochemical modifications distal to the glutarimide were also shown to influence degradation profiles. Collectively, these results highlight the nuanced nature of neosubstrate degradation and reinforce the importance of continued efforts to broaden CRBN binder diversity.
 | ||
| Scheme 20 a) Enantioselective cyclopropanation and cyclopropenation of CRBN binders under mild carbene transfer conditions. b) Subsequent diversification of the ester. aDiastereomeric ratios (d.r.) refer to the relative configurations of the two newly formed stereogenic centers, as determined by 1H NMR analysis. bD.r. reflects the degree of asymmetric induction based on the major diastereomer, as determined by supercritical fluid chromatography analysis. See ref. 61. | ||
To follow up on this study, Tracy et al. recently reported an enantioselective C–H functionalization strategy employing carbene-functionalized glutarimide precursors.63 The carbene moiety was introduced onto the glutarimide bearing core via the reaction of aryl iodides (71, Scheme 21) with 2,2,2-trichloroethyl diazoacetates. The trichloroethyl ester was selected due to its ability to enhance C–H activation and promote high levels of asymmetric induction.63 In this study, the trichloroethyl diazoacetate derivative was successfully installed at the 5-position of isoinoliniones and thalidomides bearing glutarimides (72). For substitution at the 6-position, however, ring-opened glutarimide precursor intermediates were required in certain cases, as ortho-substituted aryl iodides often present synthetic challenges under this condition.63 The ring-opened C–H functionalization product subsequently underwent acid-mediated cyclization with complete stereoretention at both the glutarimide and the newly generated stereocenters (Scheme 21b). During optimization of the C–H functionalization conditions, addition of hexafluoroisopropanol (HFIP) was observed to enhance the solubility of the diazo intermediate. Catalyst screening identified Rh2(S-tetra-p-BrPhPTTL)4 as the most effective, affording the highest levels of asymmetric induction and overall yield.63
 | ||
| Scheme 21 Dirhodium-catalyzed C–H functionalization and cyclopropanation via a) carbene-functionalized glutarimides and b) carbene functionalized ring-opened glutarimide precursors. aDiastereomeric ratio (d.r.) in red represents asymmetric induction by catalysts and d.r. in black represents the ratio for the two newly formed stereogenic centers. In black represents the ratio for the two newly formed stereogenic centers. See ref. 63. | ||
Two distinct reagent addition protocols were developed for the C–H functionalization step: A) direct addition of the catalyst to a suspension of the diazo precursor in neat alkane substrate and HFIP; or B) slow addition of the diazo and HFIP solution to a solution of the catalyst in the presence of 10 equivalents of the alkane substrate.63 Method B occasionally resulted in diminished yields due to side reactions involving carbene dimerization.63 Notably, the substrate scope revealed several important features: the method tolerates aryl bromides (75), providing a handle for downstream functionalization, and shows a preference for tertiary over primary C–H bonds (76). Furthermore, the synthetic utility of this method was demonstrated through the cyclopropanation of a protected piperazine moiety with a four-carbon linker onto the glutarimide core, affording compound 73 in 49% yield. Overall, this approach enables the efficient and enantioselective installation of stereochemically defined substituents onto both glutarimide containing and ring-opened glutarimide precursor containing scaffolds. The diazoacetate-functionalized glutarimides are competent substrates for both C–H insertion into aliphatic hydrocarbons and cyclopropanation of styrenes under dirhodium catalysis. This methodology offers a promising bearing stereochemically enriched functionalities. However, the current approach has two limitations: (1) the requirement of ring-opened glutarimide precursor intermediates for 6-substituted isoindolinone cores, and (2) reliance on the trichloroethyl ester group, which increases molecular weight and introduces additional hydrogen bond acceptors that may influence the pharmacological properties of the final CRBN-binding compounds.
2.3 Dihydrouracil synthesis
Dihydrouracil-(DHU)-based CRBN binders replace the chiral benzylic C–H with a nitrogen atom, thereby eliminating the stereocenter and the α-hydrogen implicated in hydrolytic instability.47 DHU derivatives maintain adequate CRBN binding affinity and offer a strategy to improve stability while removing a racemization prone chiral center.30,66,67 A recent study by scientists at AstraZeneca reported an N-1 selective, palladium-catalyzed cross-coupling methodology enabling the rapid synthesis of DHU-based CRBN binders.68 This method facilitates the direct coupling of unprotected DHU to (hetero)arenes, demonstrating a broad scope of aryl electrophiles, including aryl bromides, chlorides, iodides, and phenol-derived triflates (OTf) and nonaflates (ONf). Based on multiparameter screening, three complementary sets of reaction conditions were developed (Scheme 23). Each condition exhibits unique selectivity toward different aryl electrophiles. For instance, condition A demonstrates high reactivity with aryl triflates, while aryl chlorides remain unreactive. In contrast, the same aryl chlorides afford a 69% yield under condition B. Condition C, which involves the addition of a stoichiometric amount of silver oxide (Ag2O), is particularly effective for aryl iodides, which are unreactive under conditions A and B. All three conditions exhibit exclusive N-1 selectivity for DHU, with no N-3 substituted byproducts detected.68 | ||
| Scheme 23 A one-step, N-1 selective dihydrouracil-based CRBN binder synthesis. aCondition A; bcondition B acondition A; bcondition B see ref. 68. | ||
The methodology exhibits broad substrate tolerance, accommodating both electron-rich and electron-deficient (hetero)arenes. Aryl electrophiles bearing electron-donating groups generally perform well under condition A. For substrates substituted with electron-withdrawing groups at the para or meta positions, condition B typically yields improved conversions if condition A is ineffective.68 However, ortho-substituted arenes consistently fail to react under all three conditions, likely due to steric clash between the electrophile and the bulky phosphine ligands.68 Heteroarenes, including pyridines, benzothiazoles and more, are well tolerated, underscoring the potential applicability of this methodology in the synthesis of novel DHU-based CRBN binders. The authors also demonstrated the scalability of the protocol, achieving reactions on a gram scale (up to 5 grams) using only 1.25 mol% catalyst and 2.5 mol% ligand loadings.68 Overall, this method represents a significant advancement by circumventing the need for multistep syntheses and protecting group strategies traditionally employed in DHU-based CRBN binder synthesis. A key advantage is its compatibility with a variety of electrophiles, including readily available (hetero)aryl halides and phenol derivatives, thereby enabling one-step installation of DHU moieties onto CRBN binders.
Conclusions
It is our hope that this review will serve as a practical resource for medicinal chemists involved in the design, synthesis, and diversification of glutarimide-based molecular degraders for targeted protein degradation. In this review, we have covered both one-electron and two-electron coupling strategies, along with emerging methodologies for the functionalization of glutarimide containing scaffolds. These synthetic approaches not only enable the rapid construction and diversification of CRBN binders but also provide access to enantioselective routes for the synthesis of chiral glutarimides and scalable procedures amenable to multigram quantities. As readers may have observed, several methodologies offer multiple sets of conditions tailored to the electronic properties of coupling partners, such as primary versus secondary amines, various aryl halides, or differing substitution patterns. In some cases, alternative conditions are developed to be complementary, expanding the scope of substrates and reaction flexibility. We encourage practitioners to explore these parallel conditions to optimize outcomes for their specific synthetic targets. We believe that these advances in synthetic chemistry could facilitate SAR studies and promote the incorporation of C(sp3)-rich motifs, ultimately enhancing the drug-like properties of CRBN binders.Nanoscale direct-to-biology (D2B) platforms facilitate the rapid exploration of large chemical space by coupling accelerated synthesis with high-throughput phenotypic screening.69–71 However, these require synthetic methods that deliver the products in high yield and purity, as they are tested crude, and requires resynthesis and validation of any identified hits. Future directions in the TPD field include developing robust late-stage functionalization methods, improving scalability, diversifying scope and functional group tolerance, and optimizing methods to be amenable to D2B platforms.
The synthetic advancements discussed herein primarily focused on expanding the chemical diversity of CRBN binders and, in some cases, exploring neosubstrate selectivity, and impacts on cell-permeability or stability. The non-traditional CRBN binders reported predominantly maintain good CRBN binding affinity, reduce neosubstrate degradation, and in some examples, improve the desired drug-like properties. However, it is important to note, that minor structural modifications, including those remote from the CRBN binding site, were shown to significantly impact neosubstrate recruitment. The continued expansion of the known degradome and growing insights into non-canonical neosubstrates72,73 will challenge medicinal chemists to rethink established paradigms, explore novel CRBN binder structures, and develop degraders with greater specificity and therapeutic promise. In parallel, the advancements in synthetic capabilities will continue to drive this SAR exploration and deepen our understanding of the degradome.
Author contributions
X. B. wrote chapters in this review and drew schemes. M. D. and D. S. M. edited chapters and schemes. N. H.-D. supervised the project and edited chapters and schemes. All authors collected and selected literature articles for this review.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
The authors acknowledge support by Bristol Myers Squibb leadership team.References
- J. Salami and C. M. Crews, Waste disposal-An attractive strategy for cancer therapy, Science, 2017, 355, 1163–1167 CrossRef CAS PubMed.
- M. Békés, D. R. Langley and C. M. Crews, PROTAC targeted protein degraders: the past is prologue, Nat. Rev. Drug Discovery, 2022, 21, 181–200 CrossRef.
- T. Ito, H. Ando, T. Suzuki, T. Ogura, K. Hotta, Y. Imamura, Y. Yamaguchi and H. Handa, Identification of a primary target of thalidomide teratogenicity, Science, 2010, 327, 1345–1350 CrossRef CAS PubMed.
- T. Yang, E. Mukhaleva, W. Wei, D. Weiss, N. Ma, V. Shanmugasundaram and N. Vaidehi, Insights from protein frustration analysis of BRD4–cereblon degrader ternary complexes show separation of strong from weak degraders, RSC Med. Chem., 2025, 16, 1818–1828 RSC.
- D. P. Bondeson, A. Mares, I. E. D. Smith, E. Ko, S. Campos, A. H. Miah, K. E. Mulholland, N. Routly, D. L. Buckley, J. L. Gustafson, N. Zinn, P. Grandi, S. Shimamura, G. Bergamini, M. Faelth-Savitski, M. Bantscheff, C. Cox, D. A. Gordon, R. R. Willard, J. J. Flanagan, L. N. Casillas, B. J. Votta, W. den Besten, K. Famm, L. Kruidenier, P. S. Carter, J. D. Harling, I. Churcher and C. M. Crews, Catalytic in vivo protein knockdown by small-molecule PROTACs, Nat. Chem. Biol., 2015, 11, 611–617 CrossRef CAS PubMed.
- O. Hsia, M. Hinterndorfer, A. D. Cowan, K. Iso, T. Ishida, R. Sundaramoorthy, M. A. Nakasone, H. Imrichova, C. Schätz, A. Rukavina, K. Husnjak, M. Wegner, A. Correa-Sáez, C. Craigon, R. Casement, C. Maniaci, A. Testa, M. Kaulich, I. Dikic, G. E. Winter and A. Ciulli, Targeted protein degradation via intramolecular bivalent glues, Nature, 2024, 627, 204–211 CrossRef CAS PubMed.
- S. Norris, X. Ba, J. Rhodes, D. Huang, G. Khambatta, J. Buenviaje, S. Nayak, J. Meiring, S. Reiss, S. Xu, L. Shi, B. Whitefield, M. Alexander, E. J. Horn, M. Correa, L. Tehrani, J. D. Hansen, P. Papa and D. S. Mortensen, Design and Synthesis of Novel Cereblon Binders for Use in Targeted Protein Degradation, J. Med. Chem., 2023, 66, 16388–16409 CrossRef CAS PubMed.
- S. A. Kim, A. Go, S.-H. Jo, S. J. Park, Y. U. Jeon, J. E. Kim, H. K. Lee, C. H. Park, C.-O. Lee, S. G. Park, P. Kim, B. C. Park, S. Y. Cho, S. Kim, J. D. Ha, J.-H. Kim and J. Y. Hwang, A novel cereblon modulator for targeted protein degradation, Eur. J. Med. Chem., 2019, 166, 65–74 CrossRef CAS PubMed.
- A. J. Phillips, C. G. Nasveschuk, J. A. Henderson, Y. Liang, M. He, K. Lazarski, G. K. Veits and H. U. Vora, US20190076542A1, 2019.
- E. S. Fischer, K. Böhm, J. R. Lydeard, H. Yang, M. B. Stadler, S. Cavadini, J. Nagel, F. Serluca, V. Acker, G. M. Lingaraju, R. B. Tichkule, M. Schebesta, W. C. Forrester, M. Schirle, U. Hassiepen, J. Ottl, M. Hild, R. E. J. Beckwith, J. W. Harper, J. L. Jenkins and N. H. Thomä, Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide, Nature, 2014, 512, 49–53 CrossRef CAS PubMed.
- P. P. Chamberlain, A. Lopez-Girona, K. Miller, G. Carmel, B. Pagarigan, B. Chie-Leon, E. Rychak, L. G. Corral, Y. J. Ren, M. Wang, M. Riley, S. L. Delker, T. Ito, H. Ando, T. Mori, Y. Hirano, H. Handa, T. Hakoshima, T. O. Daniel and B. E. Cathers, Structure of the human Cereblon–DDB1–lenalidomide complex reveals basis for responsiveness to thalidomide analogs, Nat. Struct. Mol. Biol., 2014, 21, 803–809 CrossRef CAS.
- G. Petzold, E. S. Fischer and N. H. Thomä, Structural basis of lenalidomide-induced CK1α degradation by the CRL4CRBN ubiquitin ligase, Nature, 2016, 532, 127–130 CrossRef CAS PubMed.
- X. Nie, Y. Zhao, H. Tang, Z. Zhang, J. Liao, C. M. Almodovar-Rivera, R. Sundaresan, H. Xie, L. Guo, B. Wang, H. Guan, Y. Xing and W. Tang, Development of Phenyl-substituted Isoindolinone- and Benzimidazole-type Cereblon Ligands for Targeted Protein Degradation, ChemBioChem, 2024, 25, e202300685 CrossRef CAS PubMed.
- H. Furihata, S. Yamanaka, T. Honda, Y. Miyauchi, A. Asano, N. Shibata, M. Tanokura, T. Sawasaki and T. Miyakawa, Structural bases of IMiD selectivity that emerges by 5-hydroxythalidomide, Nat. Commun., 2020, 11, 4578 CrossRef CAS PubMed.
- X. Ma, B. Leon, E. Ornelas, D. Dovala, L. Tandeske, C. Luu, G. Pardee, S. Widger, J. M. Solomon, R. E. J. Beckwith, H. Moser, M. C. Clifton and C. A. Wartchow, Structural and biophysical comparisons of the pomalidomide- and CC-220-induced interactions of SALL4 with cereblon, Sci. Rep., 2023, 13, 22088 CrossRef CAS.
- I. Sosič, A. Bricelj and C. Steinebach, E3 ligase ligand chemistries: from building blocks to protein degraders, Chem. Soc. Rev., 2022, 51, 3487–3534 RSC.
- E. Villemure, Y. Wang, Y. Zhou, A. R. Wong and C. Nilewski, Tactics and Strategies for the Synthesis of Cereblon Ligands, Synthesis, 2024, 56, 3543–3554 CrossRef CAS.
- H. Schumacher, R. L. Smith and R. T. Williams, The metabolism of thalidomide: the fate of thalidomide and some of its hydrolysis products in various species, Br. J. Pharmacol. Chemother., 1965, 25, 338–351 CrossRef CAS PubMed.
- H. Schumacher, R. L. Smith and R. T. Williams, The metabolism of thalidomide: the spontaneous hydrolysis of thalidomide in solution, Br. J. Pharmacol. Chemother., 1965, 25, 324–337 CrossRef CAS PubMed.
- J. D. Hansen, M. Correa, M. A. Nagy, M. Alexander, V. Plantevin, V. Grant, B. Whitefield, D. Huang, T. Kercher, R. Harris, R. K. Narla, J. Leisten, Y. Tang, M. Moghaddam, K. Ebinger, J. Piccotti, C. G. Havens, B. Cathers, J. Carmichael, T. Daniel, R. Vessey, L. G. Hamann, K. Leftheris, D. Mendy, F. Baculi, L. A. LeBrun, G. Khambatta and A. Lopez-Girona, Discovery of CRBN E3 Ligase Modulator CC-92480 for the Treatment of Relapsed and Refractory Multiple Myeloma, J. Med. Chem., 2020, 63, 6648–6676 CrossRef CAS.
- Y. Ponomaryov, V. Krasikova, A. Lebedev, D. Chernyak, L. Varacheva and A. Chernobroviy, Scalable and green process for the synthesis of anticancer drug lenalidomide, Chem. Heterocycl. Compd., 2015, 51, 133–138 CrossRef CAS.
- S. G. Stewart, D. Spagnolo, M. E. Polomska, M. Sin, M. Karimi and L. J. Abraham, Synthesis and TNF expression inhibitory properties of new thalidomide analogues derived via Heck cross coupling, Bioorg. Med. Chem. Lett., 2007, 17, 5819–5824 CrossRef CAS.
- Y. Zhou, S. L. Garrigues, E. Villemure, N. Ishisoko, H. Q. Nguyen, N. K. Hamidi, R. Vogt, Y. Wang, R. A. Blake, J. Rudolph and C. Nilewski, Heteroaryl Glutarimides and Dihydrouracils as Cereblon Ligand Scaffolds for Molecular Glue Degrader Discovery, ACS Med. Chem. Lett., 2024, 15, 2158–2163 CrossRef CAS PubMed.
- J. A. Seijas, M. P. Vázquez-Tato, C. González-Bande, M. M. Martínez and B. Pacios-López, Microwave Promoted Synthesis of a Rehabilitated Drug: Thalidomide, Synthesis, 2004, 2001, 999–1000 CrossRef.
- P. C. Ray, J. M. C. Tummanepally, J. Rathinapandian and O. D. Tyagi, WO2008035378A2, 2008.
- B. D. Vu, N. M. Ho Ba and D. C. Phan, Facile Synthesis of Thalidomide, Org. Process Res. Dev., 2019, 23, 1374–1377 CrossRef CAS.
- M. E. Matyskiela, W. Zhang, H.-W. Man, G. Muller, G. Khambatta, F. Baculi, M. Hickman, L. LeBrun, B. Pagarigan, G. Carmel, C.-C. Lu, G. Lu, M. Riley, Y. Satoh, P. Schafer, T. O. Daniel, J. Carmichael, B. E. Cathers and P. P. Chamberlain, A Cereblon Modulator (CC-220) with Improved Degradation of Ikaros and Aiolos, J. Med. Chem., 2018, 61, 535–542 CrossRef CAS.
- T. G. Hayhow, R. E. A. Borrows, C. R. Diène, G. Fairley, C. Fallan, S. M. Fillery, J. S. Scott and D. W. Watson, A Buchwald–Hartwig Protocol to Enable Rapid Linker Exploration of Cereblon E3-Ligase PROTACs, Chem. – Eur. J., 2020, 26, 16818–16823 CrossRef CAS PubMed.
- J. Min, A. Mayasundari, F. Keramatnia, B. Jonchere, S. W. Yang, J. Jarusiewicz, M. Actis, S. Das, B. Young, J. Slavish, L. Yang, Y. Li, X. Fu, S. H. Garrett, M.-K. Yun, Z. Li, S. Nithianantham, S. Chai, T. Chen, A. Shelat, R. E. Lee, G. Nishiguchi, S. W. White, M. F. Roussel, P. R. Potts, M. Fischer and Z. Rankovic, Phenyl-Glutarimides: Alternative Cereblon Binders for the Design of PROTACs, Angew. Chem., Int. Ed., 2021, 60, 26663–26670 CrossRef CAS PubMed.
- Y. Zhou, S. L. Garrigues, E. Villemure, N. Ishisoko, H. Q. Nguyen, N. K. Hamidi, R. Vogt, Y. Wang, R. A. Blake, J. Rudolph and C. Nilewski, Heteroaryl Glutarimides and Dihydrouracils as Cereblon Ligand Scaffolds for Molecular Glue Degrader Discovery, ACS Med. Chem. Lett., 2024, 15(12), 2158–2163 CrossRef CAS PubMed.
- X. Zheng, N. Ji, V. Campbell, A. Slavin, X. Zhu, D. Chen, H. Rong, B. Enerson, M. Mayo, K. Sharma, C. M. Browne, C. R. Klaus, H. Li, G. Massa, A. A. McDonald, Y. Shi, M. Sintchak, S. Skouras, D. M. Walther, K. Yuan, Y. Zhang, J. Kelleher, G. Liu, X. Luo, N. Mainolfi and M. M. Weiss, Discovery of KT-474-a Potent, Selective, and Orally Bioavailable IRAK4 Degrader for the Treatment of Autoimmune Diseases, J. Med. Chem., 2024, 67, 18022–18037 CrossRef CAS PubMed.
- F. A. Luzzio, A. V. Mayorov, S. S. W. Ng, E. A. Kruger and W. D. Figg, Thalidomide Metabolites and Analogues. 3. Synthesis and Antiangiogenic Activity of the Teratogenic and TNFα-Modulatory Thalidomide Analogue 2-(2,6-Dioxopiperidine-3-yl)phthalimidine, J. Med. Chem., 2003, 46, 3793–3799 CrossRef CAS.
- S. Liu, Z. Zhuang, J. X. Qiao, K.-S. Yeung, S. Su, E. C. Cherney, Z. Ruan, W. R. Ewing, M. A. Poss and J.-Q. Yu, Ligand Enabled Pd(II)-Catalyzed γ-C(sp3)–H Lactamization of Native Amides, J. Am. Chem. Soc., 2021, 143, 21657–21666 CrossRef CAS PubMed.
- S. Yamanaka, H. Furihata, Y. Yanagihara, A. Taya, T. Nagasaka, M. Usui, K. Nagaoka, Y. Shoya, K. Nishino, S. Yoshida, H. Kosako, M. Tanokura, T. Miyakawa, Y. Imai, N. Shibata and T. Sawasaki, Lenalidomide derivatives and proteolysis-targeting chimeras for controlling neosubstrate degradation, Nat. Commun., 2023, 14, 4683 CrossRef CAS PubMed.
- P. Filippakopoulos, J. Qi, S. Picaud, Y. Shen, W. B. Smith, O. Fedorov, E. M. Morse, T. Keates, T. T. Hickman, I. Felletar, M. Philpott, S. Munro, M. R. McKeown, Y. Wang, A. L. Christie, N. West, M. J. Cameron, B. Schwartz, T. D. Heightman, N. La Thangue, C. A. French, O. Wiest, A. L. Kung, S. Knapp and J. E. Bradner, Selective inhibition of BET bromodomains, Nature, 2010, 468, 1067–1073 CrossRef CAS.
- A. Lejava, G. A. Miseo, T. Phan, J. Zhu, H. L. Powers, J. Li, D. S. Mortensen, C. W. Zapf, G. Khambatta, J. Buenviaje and N. Holmberg-Douglas, Development of a Buchwald–Hartwig Amination for an Accelerated Library Synthesis of Cereblon Binders, ACS Med. Chem. Lett., 2025, 16, 89–95 CrossRef CAS.
- Z. Zhong, C. Besnard and J. Lacour, General Ir-Catalyzed N–H Insertions of Diazomalonates into Aliphatic and Aromatic Amines, Org. Lett., 2024, 26, 983–987 CrossRef CAS PubMed.
- E. Levashova, L. Bischof, A. Bunev, A. Sapegin, O. Grygor'eva, A. Kudinov, S. Ebeling, I. Tatarinov, D. Dar'in, G. Kantin, M. D. Hartmann and S. Kalinin, Extending the chemical space of glutarimide-based cereblon ligands through an efficient Rh(II)-catalyzed X–H insertion reaction, Eur. J. Med. Chem., 2026, 301, 118235 CrossRef CAS PubMed.
- M. Krasavin, A. Bubyrev, A. Kazantsev, C. Heim, S. Maiwald, D. Zhukovsky, D. Dar'in, M. D. Hartmann and A. Bunev, Replacing the phthalimide core in thalidomide with benzotriazole, J. Enzyme Inhib. Med. Chem., 2022, 37, 527–530 CrossRef CAS PubMed.
- A. J. Phillips, C. G. Nasveschuk, J. A. Henderson, Y. Liang, M. He, M. Duplessis and C. Chen, WO2018237026A1, 2018.
- J. W. Gu, M. S. Oderinde, H. Li, F. Roberts, J. M. Ganley and M. D. Palkowitz, Expedited Aminoglutarimide C–N Cross-Coupling Enabled by High-Throughput Experimentation, J. Org. Chem., 2024, 89, 17738–17743 CrossRef CAS.
- J. M. Ronnebaum and F. A. Luzzio, Synthesis of 1,2,3-triazole ‘click’ analogues of thalidomide, Tetrahedron, 2016, 72, 6136–6141 CrossRef CAS.
- Y. Gemechu, D. Millrine, S. Hashimoto, J. Prakash, K. Sanchenkova, H. Metwally, P. Gyanu, S. Kang and T. Kishimoto, Humanized cereblon mice revealed two distinct therapeutic pathways of immunomodulatory drugs, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 11802–11807 CrossRef CAS.
- Y. Ueda, T. Ando, Y. Eikyu, T. Okamoto, H. Yokoyama, N. Kunimura, D. Kanaoka, S. Izuno and M. Watanabe, Design, synthesis, and evaluation of a novel protein degrader FPFT-2216, Bioorg. Med. Chem. Lett., 2025, 123, 130193 CrossRef CAS PubMed.
- K. Baek, R. J. Metivier, S. S. Roy Burman, J. W. Bushman, H. Yoon, R. J. Lumpkin, J. K. Ryan, D. M. Abeja, M. Lakshminarayan, H. Yue, S. Ojeda, Y. Xiong, J. Che, A. L. Verano, A. M. Schmoker, N. S. Gray, K. A. Donovan and E. S. Fischer, Unveiling the hidden interactome of CRBN molecular glues, Nat. Commun., 2025, 16, 6831 CrossRef CAS PubMed.
- P. Neigenfind, L. Massaro, Á. Péter, A. P. Degnan, M. A. Emmanuel, M. S. Oderinde, C. He, D. Peters, T. El-Hayek Ewing, Y. Kawamata and P. S. Baran, Simplifying Access to Targeted Protein Degraders via Nickel Electrocatalytic Cross-Coupling, Angew. Chem., Int. Ed., 2024, 63, e202319856 CrossRef CAS PubMed.
- L.-M. Chen, C. Shin, T. J. DeLano, A. Carretero-Cerdán, G. Gheibi and S. E. Reisman, Ni-Catalyzed Asymmetric Reductive Arylation of α-Substituted Imides, J. Am. Chem. Soc., 2024, 146, 29523–29530 CrossRef CAS PubMed.
- E. Tokunaga, T. Yamamoto, E. Ito and N. Shibata, Understanding the Thalidomide Chirality in Biological Processes by the Self-disproportionation of Enantiomers, Sci. Rep., 2018, 8, 17131 CrossRef PubMed.
- T. Yamamoto, E. Tokunaga, S. Nakamura, N. Shibata and T. Toru, Synthesis and Configurational Stability of (S)- and (R)-Deuteriothalidomides, Chem. Pharm. Bull., 2010, 58, 110–112 CrossRef CAS PubMed.
- C. D. Wong, L. C. Bradford, N. Hirbawi and E. R. Jarvo, Nickel-Catalyzed Suzuki–Miyaura Cross-Coupling Reaction of Aliphatic Alcohol Derivatives, Angew. Chem., Int. Ed., 2025, e202509657 CAS.
- W. F. Tracy, G. H. M. Davies, L. N. Grant, J. M. Ganley, J. Moreno, E. C. Cherney and H. M. L. Davies, Anhydrous and Stereoretentive Fluoride-Enhanced Suzuki–Miyaura Coupling of Immunomodulatory Imide Drug Derivatives, J. Org. Chem., 2024, 89, 4595–4606 CrossRef CAS PubMed.
- F. Lovering, J. Bikker and C. Humblet, Escape from flatland: increasing saturation as an approach to improving clinical success, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed.
- S. M. Szewczyk, I. Verma, J. T. Edwards, D. R. Weiss and E. L. P. Chekler, Trends in Neosubstrate Degradation by Cereblon-Based Molecular Glues and the Development of Novel Multiparameter Optimization Scores, J. Med. Chem., 2024, 67, 1327–1335 CrossRef CAS PubMed.
- A. Steiner, J. Krieger, R. Jones, D. Böse, Y. Wang, H. Eggenweiler, J. D. Williams and C. O. Kappe, Photoredox Csp3–Csp2 Reductive Cross-Couplings of Cereblon Ligands for PROTAC Linker Exploration in Batch and Flow, ChemCatChem, 2022, 14, e202201184 CrossRef CAS.
- P. Zhang, C. “Chip” Le and D. W. C. MacMillan, Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling, J. Am. Chem. Soc., 2016, 138, 8084–8087 CrossRef CAS PubMed.
- K. Lovato, D. Loskot, C. Gelin, L. Zhu, C. Chaudhry, N. A. Vellore, A. Del Rosario, T. Courtney, S. Miller, J.-H. Cho, H. Bell-Temin, J. D. Venable and J.-F. Brazeau, Reductive C(sp2)-C(sp3) Coupling Protocol to Enable Linker Exploration of Cereblon E3-Ligase BRD4 Proteolysis-Targeting Chimeras, J. Med. Chem., 2025, 68, 10061–10074 CrossRef CAS PubMed.
- J. Tsien, Á. Péter, X. Zheng, S. Wang, B. Jiang, M. Emmanuel, M. Oderinde, P. Bolduc, M. Nicastri, S. Dey, M. Collins, J. Lee, M. Bravo, M. Palkowitz, J. Qiao, Y. Kawamata and P. Baran, Accelerating Medicinal Chemistry: A C(sp3)-rich Fragment Toolbox for Redox-Neutral Cross-Coupling, ChemRxiv, 2025, preprint, DOI:10.26434/chemrxiv-2025-2k4tc.
- J. Wang, L. E. Ehehalt, Z. Huang, O. M. Beleh, I. A. Guzei and D. J. Weix, Formation of C(sp2)–C(sp3) Bonds Instead of Amide C–N Bonds from Carboxylic Acid and Amine Substrate Pools by Decarbonylative Cross-Electrophile Coupling, J. Am. Chem. Soc., 2023, 145, 9951–9958 CrossRef CAS PubMed.
- J. L. Sloane, A. B. Santos, E. M. Simmons and T. C. Sherwood, Metallaphotoredox-Mediated Decarboxylative Cross-Coupling of α-Oxy Morpholine Carboxylic Acids and (Hetero)Aryl Halides, Org. Lett., 2023, 25, 4219–4224 CrossRef CAS PubMed.
- O. P. Datsenko, A. Baziievskyi, I. Sadkova, B. Campos, J. T. Brewster, J. Kowalski, R. J. Hinklin and P. K. Mykhailiuk, Alkyl Azetidines Via Batch and Flow Photochemistry, Org. Lett., 2025, 27, 5318–5323 CrossRef CAS PubMed.
- W. F. Tracy, G. H. M. Davies, L. Jia, E. D. Evans, Z. Sun, J. Buenviaje, G. Khambatta, S. Yu, L. Shi, V. Shanmugasundaram, J. Moreno, E. C. Cherney and H. M. L. Davies, Asymmetric Dirhodium-Catalyzed Modification of Immunomodulatory Imide Drugs and Their Biological Assessment, ACS Med. Chem. Lett., 2024, 15, 1575–1583 CrossRef CAS PubMed.
- R. P. Nowak, J. Che, S. Ferrao, N. R. Kong, H. Liu, B. L. Zerfas and L. H. Jones, Structural rationalization of GSPT1 and IKZF1 degradation by thalidomide molecular glue derivatives, RSC Med. Chem., 2023, 14(3), 501–506 RSC.
- W. F. Tracy, J. C. Sharland, D. Ly, G. H. M. Davies, D. G. Musaev, H. Fang, J. Moreno, E. C. Cherney and H. M. L. Davies, Diversity Synthesis Using Glutarimides as Rhodium Carbene Precursors in Enantioselective C–H Functionalization and Cyclopropanation, J. Am. Chem. Soc., 2025, 147, 11336–11345 CrossRef CAS PubMed.
- J. Zhang, J. Che, X. Luo, M. Wu, W. Kan, Y. Jin, H. Wang, A. Pang, C. Li, W. Huang, S. Zeng, W. Zhuang, Y. Wu, Y. Xu, Y. Zhou, J. Li and X. Dong, Structural Feature Analyzation Strategies toward Discovery of Orally Bioavailable PROTACs of Bruton's Tyrosine Kinase for the Treatment of Lymphoma, J. Med. Chem., 2022, 65, 9096–9125 CrossRef CAS PubMed.
- Y. Li, J. Yang, A. Aguilar, D. McEachern, S. Przybranowski, L. Liu, C.-Y. Yang, M. Wang, X. Han and S. Wang, Discovery of MD-224 as a First-in-Class, Highly Potent, and Efficacious Proteolysis Targeting Chimera Murine Double Minute 2 Degrader Capable of Achieving Complete and Durable Tumor Regression, J. Med. Chem., 2019, 62, 448–466 CrossRef CAS PubMed.
- J. A. Jarusiewicz, S. Yoshimura, A. Mayasundari, M. Actis, A. Aggarwal, K. McGowan, L. Yang, Y. Li, X. Fu, V. Mishra, R. Heath, S. Narina, S. M. Pruett-Miller, G. Nishiguchi, J. J. Yang and Z. Rankovic, Phenyl Dihydrouracil: An Alternative Cereblon Binder for PROTAC Design, ACS Med. Chem. Lett., 2023, 14, 141–145 CrossRef CAS PubMed.
- H. Xie, C. Li, H. Tang, I. Tandon, J. Liao, B. L. Roberts, Y. Zhao and W. Tang, Development of Substituted Phenyl Dihydrouracil as the Novel Achiral Cereblon Ligands for Targeted Protein Degradation, J. Med. Chem., 2023, 66, 2904–2917 CrossRef CAS PubMed.
- I. D. G. Nixon, J. M. Bateman, I. N. Michaelides, G. Fairley, M. J. Pemberton, E. L. Braybrooke, K. Sutton and P. J. Lindsay-Scott, One-Step Regioselective Synthesis of N-1-Substituted Dihydrouracils: A Motif of Growing Popularity in the Targeted Protein Degradation Field, J. Org. Chem., 2024, 89, 18301–18312 CrossRef CAS PubMed.
- Z. Wang, S. Shaabani, X. Gao, Y. L. D. Ng, V. Sapozhnikova, P. Mertins, J. Krönke and A. Dömling, Direct-to-biology, automated, nano-scale synthesis, and phenotypic screening-enabled E3 ligase modulator discovery, Nat. Commun., 2023, 14, 8437 CrossRef CAS PubMed.
- J. Li, C. Li, Z. Zhang, Z. Zhang, Z. Wu, J. Liao, Z. Wang, M. McReynolds, H. Xie, L. Guo, Q. Fan, J. Peng and W. Tang, A platform for the rapid synthesis of molecular glues (Rapid-Glue) under miniaturized conditions for direct biological screening, Eur. J. Med. Chem., 2023, 258, 115567 CrossRef CAS PubMed.
- R. Stevens, H. J. Shrives, J. Cryan, D. Klimaszewska, P. Stacey, G. A. Burley, J. D. Harling, D. J. Battersby and A. H. Miah, Expanding the reaction toolbox for nanoscale direct-to-biology PROTAC synthesis and biological evaluation, RSC Med. Chem., 2025, 16, 1141–1150 RSC.
- G. Petzold, P. Gainza, S. Annunziato, I. Lamberto, P. Trenh, L. A. McAllister, B. DeMarco, L. Schwander, R. D. Bunker, M. Zlotosch, R. SriRamaratnam, S. Gilberto, G. Langousis, E. J. Donckele, C. Quan, V. Strande, G. M. De Donatis, S. B. Alabi, J. Alers, M. Matysik, C. Staehly, A. Dubois, A. Osmont, M. Garskovas, D. Lyon, L. Wiedmer, V. Oleinikovas, R. Lieberherr, N. T. Rubin, D. T. Lam, X. Lucas, E. Liardo, N. I. Widlund, A. Ritzén, R. M. Caceres, D. Vigil, J. Tsai, O. Wallace, M. Peluso, A. Sadok, R. Tiedt, A. M. Paterson, V. Zarayskiy, B. Fasching, D. Bonenfant, M. Warmuth, J. C. Castle and S. A. Townson, Mining the CRBN target space redefines rules for molecular glue–induced neosubstrate recognition, Science, 2025, 389, eadt6736 CrossRef CAS PubMed.
- J. T. Cruite, G. P. Dann, J. Che, K. A. Donovan, S. Ferrao, S. B. Ficarro, E. S. Fischer, N. S. Gray, F. Huerta, N. R. Kong, H. Liu, J. A. Marto, R. J. Metivier, R. P. Nowak, B. L. Zerfas and L. H. Jones, Cereblon covalent modulation through structure-based design of histidine targeting chemical probes, RSC Chem. Biol., 2022, 3, 1105–1110 RSC.
| This journal is © The Royal Society of Chemistry 2026 |



