Recent advances in the antimicrobial potential of boron cluster compounds
William J.
Price Cunliffe
 a,
Peter J.
Rutledge
a,
Peter J.
Rutledge
 *ab and
Louis M.
Rendina
*ab and
Louis M.
Rendina
 *ab
*ab
aSchool of Chemistry, The University of Sydney, Camperdown, Sydney, NSW 2006, Australia. E-mail: peter.rutledge@sydney.edu.au; louis.rendina@sydney.edu.au
bThe University of Sydney Nano Institute, Sydney, NSW 2006, Australia
First published on 3rd November 2025
Abstract
Since their discovery in the mid-20th century, inorganic boron clusters such as carboranes, metallacarboranes, and dodecaborates have been investigated for a range of medicinal applications, most notably as boron delivery agents for boron neutron capture therapy. Increasingly, boron cluster-containing compounds have also been studied for their antimicrobial activity as scientists seek new ways to address the growing global threat of antimicrobial resistance. Boron cluster compounds have a number of properties that make them promising antimicrobial agents, including high lipophilicity and stability, low toxicity, resistance to enzymatic metabolism, unusual ‘dihydrogen’ bonding, as well as the ability to cross or disrupt cell membranes, or carry other molecules through them. In this review, we summarise and critically evaluate the antibacterial, antifungal, antiviral, and antimalarial boron cluster compounds reported to date, with a focus on the past five years of research. We highlight medicinal chemistry opportunities where boron clusters may deliver anti-infective value beyond that of traditional carbon-based scaffolds.
1. Introduction
Antimicrobial resistance is one of the most pressing global health concerns of the 21st century, with an estimated 10 million lives per year and a cumulative $US100 trillion of economic output at risk by 2050 due to the rise of drug-resistant infections.1,2 As pathogens develop resistance to traditional classes of antimicrobial compounds, there is an urgent need for structural diversification from existing antibacterial, antiviral and antifungal compounds. Boron cluster compounds (BCCs), characterised by their unique three-dimensional cage structures and distinct chemical and biological properties, show promise as novel antimicrobial agents important to the fight against antimicrobial resistance.Boron's use in medicine is not new; borax was used for medicinal and mummification purposes in ancient Egypt, boric acid was commonly used in the 20th century as a mild antiseptic and eye wash, and the first boron-containing natural product, the antibiotic boromycin, was discovered in 1967.3–5 However, long-standing, albeit unsubstantiated, concerns about boron's toxicity due to its biological scarcity meant that boron was often ignored by medicinal chemists until the FDA approval of bortezomib (Velcade®) as a first-line treatment for multiple myeloma in 2003.6 To date, five boron-containing drugs have received FDA approval, all of which contain boronic acid or benzoxaborole moieties with a single boron atom. These boron-containing functional groups are attractive candidates for the diversification of pharmaceutical libraries to combat antimicrobial resistance due the Lewis acidity of boron, its ability to convert readily between sp2 and sp3-hybridised geometries at biological pH, and its relative scarcity in human biological systems.7,8
Boron cluster compounds are a class of synthetic molecules with boron-containing polyhedral cage structures. The boron atoms form closed, multi-atom, cage-like structures, often exhibiting unique, electron-deficient bonding and 3D aromaticity. The most commonly-studied boron clusters include boron hydride clusters (1–3), carboranes (4–9), and metallacarboranes (10) (Fig. 1).
 | ||
| Fig. 1 Boron clusters commonly used for antimicrobial therapy, including boron hydride clusters (1–3), carboranes (4–9), and the metallacarborane COSAN (10). | ||
Boron hydride clusters consist of exclusively boron and hydrogen atoms assembled in cage structures of various shapes and sizes. The closo-dodecaborate ([B12H12]2−) anion (1) is an example of an icosahedral boron hydride cluster with two negative charges delocalised throughout the cage. Its mercapto-substituted derivative, sodium borocaptate (BSH, 2), has been used clinically in Japan for boron neutron capture therapy (BNCT) of patients with brain tumours for many decades,9,10 while its 10-vertex analogue, closo-decaborate ([B10H10]2−, 3), was also employed in early clinical BNCT studies.11
The most common heteroborane clusters, dicarba-closo-dodecaboranes (commonly termed ‘closo-carboranes’ or just ‘carboranes’) are icosahedral C2B10H12 clusters which exist as 1,2-(4), 1,7-(5), and 1,12-(6) isomers according to the relative positions of the two carbon atoms in the cage. These isomers are commonly termed ‘ortho’, ‘meta’, and ‘para’, respectively. ortho-Carboranes are prepared from the reaction of nido-decaborane (B10H14) derivatives with alkynes.12 Thermal cage-rearrangement of the ortho-isomer produces the meta- and para-isomers at 400–500 °C and 600–700 °C, respectively.12,13 Removal of the most electron-deficient boron atom (‘de-boronation’) of a closo-carborane leads to an anionic and hydrophilic nido-carborane analogue (7–9).
The most stable and commonly-studied metallacarborane is the ‘cobalt bis(dicarbollide) ion’ or ‘COSAN’ (10), which consists of a central Co(III) atom ‘sandwiched’ between two deprotonated nido-carborane ([C2B9H11]2−) dianions. Each of these dianions provides six η5-bonded electrons to the Co(III) centre in a bonding arrangement that resembles how cyclopentadienyl ions bond to the metal centre in classical metallocenes like ferrocene. Thus, the COSAN anion is also an 18-electron, low-spin, diamagnetic d6 complex.14
The chemical and biological properties of boron cluster compounds differ greatly from their single boron atom-containing counterparts. In this review, we examine the pharmacological reasons why many BCCs have been found to be promising antimicrobial agents. This discussion is followed by a summary of BCCs possessing antibacterial, antifungal, antiviral, and antimalarial activities, as well as boron clusters with supramolecular properties relevant to antimicrobial therapy. An excellent overview of this area of research was published by Fink and Uchman in early 2021,15 so this review will focus primarily on developments in the field in the past five years while also highlighting the most historically important antimicrobial BCCs reported to date. Table 1 shows an overall summary of the most active compounds reported since 2020.
| Compound | Boron cluster | Pathogen | MIC (μg mL−1) | Ref. |
|---|---|---|---|---|
| 18 | Carborane | S. aureus | 0.5 | 68 |
| 34 | Carborane | M. tuberculosis | 0.092 | 46 |
| 36 | Carborane | M. tuberculosis | 0.40 | 81 |
| 45 | Metallacarborane | MRSA | 1 | 87 |
| 51 | Metallacarborane | Y. enterocolitica | 0.94 (IC50) | 88 |
| 52 | Metallacarborane | S. aureus | 0.050 | 90 |
| 53 | Metallacarborane | S. aureus | 0.054 | 90 |
| 54 | Metallacarborane | C. albicans | 1.2 | 90 |
| 55 | Metallacarborane | C. albicans | 0.64 | 90 |
| 59 | Metallacarborane | S. aureus; MRSA | 1.8 | 92 |
| 63 | Metallacarborane | S. aureus | 0.67 | 94 |
| 65 | Metallacarborane | S. aureus | 0.60 | 94 |
| 67 | Dodecaborate | N. gonorrhoeae | 2 | 97 |
| 83 | Carborane | P. falciparum | 0.06 μM (IC50) | 114 |
| 84 | Carborane | P. falciparum | 0.12 μM (IC50) | 114 |
2. Pharmacological properties of boron clusters
2.1 Structure, stability, pharmacokinetics, and toxicity
The stability of many boron cluster compounds under a wide range of biologically relevant conditions makes them particularly attractive antimicrobial agents. While some boron clusters are highly unstable, those with icosahedral (12-vertex) cages can be exceedingly chemically, thermally and biologically stable. This stability arises from the delocalisation of electron density throughout the σ-bonded framework of the cage, yielding three-dimensional (3D) aromaticity within the σ-bonded cage.16,17 Metallacarboranes possess an even more extensive aromatic system: unlike ferrocene, which displays only local aromaticity on each cyclopentadienyl ligand, COSAN exhibits global aromaticity across the metallacarborane structure.18 Furthermore, unlike simple boron hydrides such as BH4− and B2H6, boron clusters are also kinetically stable to hydrolysis.4Boron clusters display a number of other favourable properties for antimicrobial and other biological uses. Their aromaticity also influences their reaction chemistry – substituent effects are transferred through the cage skeleton much like they are in two-dimensional aromatic π-systems, allowing for the derivatisation of boron clusters with a wide range of halogen or organic substituents at various positions on their cages.16 This enables the fine-tuning of relevant properties such as lipophilicity, molecular weight, and molecular dimensions.
Many boron clusters display relatively low levels of toxicity towards mammalian cells.19,20 These include BSH (2),21,22 sodium decaborate,23meta-carborane-substituted amino acids,24meta-carboranylphosphinate-coated magnetic nanoparticles,25 COSAN,26,27 and COSAN derivatives.28,29
In addition, because boron cluster compounds are abiotic and did not exist prior to the 1950s, there are no known enzymatic systems that are able to metabolise them, making them less prone to the development of resistance through enzymatic evolution.30,31 The incorporation of boron clusters into the structures of existing drugs can improve their metabolic stability,32,33 and studies of COSAN derivatives demonstrated a lack of any degradation in cell systems.26,27 Biodistribution studies with radiolabelled iodinated carborane derivatives indicated high levels of these boron clusters intact in the bladder and urine.34,35 A similar molecular imaging study with COSAN derivatives showed high accumulation in the liver with increasing uptake in the lungs and moderate blood clearance.36
2.2 Boron cluster compounds as pharmacophores
A number of boron cluster compounds have been investigated as pharmacophores that can interact with microbial proteins of interest. Often, they are integrated into drug analogues as bioisosteres of phenyl rings due to their similar molecular dimensions. As an example, the van der Waals volume (ca. 148 Å3) of closo-1,2-carborane (4) is comparable to that of adamantane (ca. 136 Å3), and slightly larger than that of a phenyl ring (ca. 79 Å3, rotating: ca. 102 Å3) (Fig. 2A).37,38 Boron clusters are thus often a useful, slightly bulkier substitute for classical hydrophobic groups in drug discovery.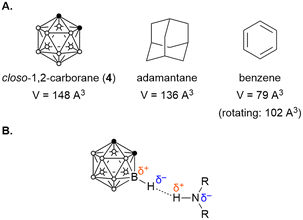 | ||
| Fig. 2 (A) Comparison of the van der Waals volumes of closo-1,2-carborane (4), adamantane, and benzene; (B) dihydrogen bonding interactions between hydridic carborane protons and amino acid residues. | ||
There are three non-covalent bonding interactions between BCCs and proteins that can increase pharmacological activity compared to related organic bioisosteres. First, uncharged boron clusters such as closo-carboranes are slightly more lipophilic than their organic bioisosteres due to the low polarity of B–H bonds, allowing these groups to engage in hydrophobic interactions with protein binding pockets.39–41 Second, boron clusters possess a large number of slightly hydridic Bδ+–Hδ– groups. This polarisation allows the formation of unusual ‘dihydrogen bonds’ in protein receptor sites, which result from dipole–dipole interactions between partial opposite charges in the form B–Hδ–⋯δ+H–X (X = N, O or C) (Fig. 2B).33,42–45 While these interactions are weaker than conventional hydrogen bonds, the three-dimensional cage structure increases bonding accessibility and allows for the simultaneous formation of multiple dihydrogen bonds in three dimensions.33,45,46 Finally, ionically charged BCCs can be stabilised by electrostatic interactions while also binding within hydrophobic protein binding pockets.47,48
Numerous examples of BCC pharmacophores exhibiting a range of biological activities have been reported over previous decades. A carborane-based agonist of oestrogen receptors showed a tenfold increase in potency compared with its bioisostere, 17β-oestradiol, and was able to restore uterine weight and prevent bone loss in ovariectomised mice.49–51 Hydrogen bonding at each end of the molecule and hydrophobic van der Waals contacts across the carborane skeleton contributed to its high binding affinity for the hydrophobic cavity of oestrogen receptors.49 Carborane bioisosteres have also been used as probes for investigating the aromatic recognition sites of enzymes such as chymotrypsin, for which synthetic carborane-containing peptides were found to be more potent inhibitors than their phenyl analogues.52,53
COSAN derivatives have also been investigated as pharmacophores against a range of protein targets. The unmodified cobalt bis(dicarbollide) ion (10) has shown strong nonspecific binding with basic amino acid residues on bovine serum albumin, which it stabilises, preventing chemical and thermal denaturation.54 More recently, COSAN derivatives have been shown to inhibit the carbonic anhydrase IX (CA-IX) zinc metalloenzyme, which promotes hypoxic tumour cell survival by regulating pH and is often associated with cancer growth.55 Two alkylsulfamide COSAN derivatives were shown to exhibit sub-nanomolar in vitro Ki values and high selectivity for CA-IX over the ubiquitous CA-II.28In vivo testing indicated that both compounds significantly inhibited tumour growth in mice transplanted with murine mammary tumour 4T1-12B cells and human colorectal HT-29 cells.56
2.3 Boron cluster interactions with cell membranes
Boron cluster compounds display varied non-covalent interactions with lipid membranes that have increasingly been harnessed for antimicrobial therapies. The intrinsic lipophilicity of many boron clusters promotes interaction with membranes, and this has been shown to allow closo-carboranes to cross not only cell membranes but also the blood brain barrier (BBB).57,58Ionically charged BCCs, including dodecaborate derivatives, nido-carboranes, and COSANs, exhibit charge delocalisation throughout their cage structures, enhancing their hydrophilicity relative to closo-carboranes.16 Because these compounds are large and charge delocalised, they display ‘superchaotropic’ behaviour, meaning they can significantly disrupt the hydrogen bonding structure of water molecules (to a greater extent than traditional chaotropes such as SCN− on the Hofmeister scale). Thus, they tend to dynamically associate to lipid bilayers, neutral surfaces, or other hydrophobic areas in biological environments.59,60
Charged boron clusters can act as supramolecular carriers of hydrophobic bioactive molecules into cells, even where conventional amphiphilic membrane transporters cannot.59,61 Their superchaotropic behaviour differs from that of traditional amphiphilic molecules with ionic head groups and hydrophobic tails; anionic BCCs are typically water-soluble and tend not to encapsulate nor aggregate with their cargo.60 Dodecaborate clusters such as [B12Br12]2− have been shown to act as inorganic membrane carriers for peptides, amino acids, neurotransmitters, vitamins, and antibiotics.60
Similarly, COSANs have shown considerable ability to transport cationic peptides such as heptaarginine into the cytosol without compromising the integrity of either the lipid bilayer or cell function.62 While COSAN derivatives do not display either the broadband carrier activity or the well-defined concentration window of dodecaborates, they exhibit more selectivity towards hydrophilic oligopeptides, and their carrier activity is less affected by cage substitution.
Initial studies suggest that dodecaborate and COSAN-based membrane carriers exhibit low cytotoxicity – B12Br122−, for instance, displayed lower toxicity that its penetrating peptide competitor AcR8.60 However, more thorough investigations into the effects of these superchaotropic membrane carriers on mammalian cells would be welcome additions to this new area of research and increase their promise as antimicrobial agents.
Other boron clusters have been shown to induce membrane disruption, including [B12I12]2−,60 BSH,63 and alkylated dodecaborate derivatives.64 The applications of superchaotropic boron clusters in combination antimicrobial therapy are discussed further in section 6.
3. Antibacterial and antifungal boron clusters
3.1 Antibacterial and antifungal carboranes
The first report of antimicrobial boron cluster compounds was published in 1981 by Totani et al., in which 45 closo- and nido-carborane derivatives were found to display some antibacterial or antifungal activity, with minimum inhibitory concentration (MIC) values in the range 0.4–50 μg mL−1.65 Generally, closo-carboranes (e.g.11) and closo–closo dicage compounds (e.g.12) were less active than nido-carboranes (e.g.13) or closo–nido dicage compounds (e.g.14). Compound 13 displayed the strongest antibacterial activity against Staphylococcus aureus (MIC 0.8 μg mL−1) and Streptococcus pyogenes (MIC 0.4 μg mL−1), while the most active antifungal compound, tetracage carborane 15, exhibited greater antifungal activity than clotrimazole and amphotericin B against Candida albicans (MIC 0.8 μg mL−1) and Aspergillus fumigatus (MIC 1.6 μg mL−1) (Fig. 3).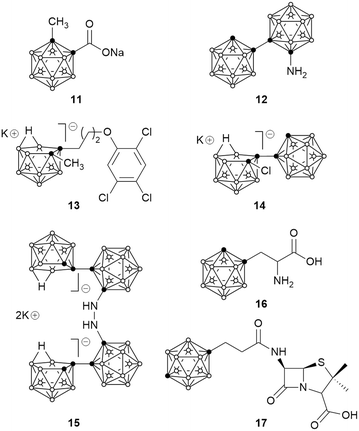 | ||
| Fig. 3 Carborane-containing compounds which exhibit antibacterial or antifungal activity, including bioisosteres of phenylalanine (16) and penicillin G (17). | ||
The next report of antimicrobial activity by this compound class was published in 1999, when racemic ortho-carboranylalanine (16), a carborane-containing amino acid, was shown to possess moderate to weak antibacterial activity against a range of Gram-positive bacteria (MIC 0.86–580 μg mL−1).66 Compound 16 also displayed 103 times stronger antifungal activity against the oomyceteous plant pathogen Plasmopara halstedii (MIC 0.000035–0.074 μg mL−1) than the commercial fungicide tridemorph. It was suggested that 16 interferes with L-His metabolism, acting as a classical antimetabolite, although its specific mechanism of action was not determined.
A number of more recent publications describe carborane-containing compounds with antibacterial activity. In 2018, a para-carborane-containing analogue of penicillin G (17) was shown to display comparable activity (MIC 128 μg mL−1) to penicillin G and ciprofloxacin against two methicillin-resistant S. aureus (MRSA) strains, while ortho- and meta-derivatives were less active.67 This difference was suggested to arise due to the greater lipophilicity of para-carboranes compared with ortho- and meta-carboranes.
Two carboranylthiolato bismuth complexes, 18 and 19, were tested for their antibacterial activity against two Gram-positive S. aureus strains and one Gram-negative Escherichia coli strain.68 Heteroleptic complex 18 displayed strong activity (MIC 0.5–1 μg mL−1) against the S. aureus strains, while homoleptic complex 19 was slightly less active (MIC 2–16 μg mL−1) and the homoleptic aryl analogue BiPh3 was inactive (MIC ≥ 128 μg mL−1). This confirmed the results of previous studies into bismuth complexes that suggested the presence of a combination of different ligands was beneficial for antimicrobial activity. Both 18 and 19 were essentially inactive against E. coli (MIC ≥ 128 μg mL−1), likely due to the decreased permeability associated with the double membrane present in Gram-negative bacteria, while both compounds showed very low toxicity to human HEK293 and HepG2 cells at concentrations below 75 μg mL−1 (Fig. 4).
 | ||
| Fig. 4 Carboranylthiolato bismuth complexes (18 and 19) and ferrocene-substituted carborane compounds (20–24) which exhibit antibacterial or antifungal activity. | ||
A series of publications by Li et al. in 2012–2013 reported ferrocene-substituted carborane compounds with antibacterial and antibiofilm activity. First, compounds 20–22 were found to exhibit broad spectrum antibacterial activity against a range of multidrug-resistant (MDR) bacterial isolates and control strains (MIC 12.5–100 μg mL−1).69 Taking advantage of the redox-active properties of ferrocene to follow cellular uptake, electrochemical studies indicated a good level of bacterial cell uptake of these compounds. Combining either 20 or 21 with titanium dioxide nanoparticles considerably increased their antibacterial activity against MDR Acinetobacter baumannii strains.70 A derivative containing two ferrocene units (23) was also found to be active against clinical isolates of Gram-positive S. aureus and Gram-negative P. aeruginosa (MIC 36 μg mL−1) and displayed antibiofilm activity against those same strains (MBIC50 (minimum concentration showing 50% biofilm formation inhibition) 4–6 μg mL−1) with minimal toxicity to normal cells and tissues.71In vivo analysis, including scanning electron microscopy (SEM) studies, showed that 23 could damage bacterial cell walls, destabilise cell membranes, and induce the leakage of nucleic acids, proteins, and other cellular contents. Finally, the carborane ruthenium(II)-arene complex 24 was shown to induce the reversal of biofilm-associated antibiotic resistance of MDR clinical isolates of S. aureus and P. aeruginosa.72 Five known antibiotics (including penicillin G, norfloxacin, and streptomycin) displayed up to eight times greater antibacterial activity against bacterial cells which had been induced beforehand with 24 at a concentration of 8 μg mL−1 compared with those that had not.
Nine closo-carborane-containing analogues of the M. tuberculosis cell growth inhibitor SQ109 (28) were synthesised and tested against M. tuberculosis (H37Rv and Erdman strains), Mycobacterium smegmatis, Bacillus subtilis, E. coli, Saccharomyces cerevisiae, Trypanosoma brucei, and two human cell lines.74 Compared to adamantane-containing analogues, none had potent activity against the M. tuberculosis strains (MIC 12–100 μg mL−1). However, carborane-containing derivatives displayed good activity against the other antimicrobial pathogens (MIC 0.2–29 μg mL−1). Unlike the adamantane-containing analogues, which tended to be less active against E. coli than the other pathogens, the most active carborane-containing compounds, 29 and 30, were similarly active (MIC ∼2 μg mL−1) against E. coli as against the other bacterial, fungal and protozoan species.
Five carborane-bearing thymine derivatives were tested in vitro against M. tuberculosis thymidylate kinase (TMPKmt) and against M. tuberculosis and M. smegmatis strains.75 The best TMPK inhibitor, compound 31, displayed a Ki value of 1.5 μM, while the two most potent mycobacterial growth inhibitors, compounds 32 and 33, fully inhibited M. smegmatis proliferation at a concentration of 100 μg mL−1 (Fig. 6).
Frontline tuberculosis drug isoniazid is a hydrazide prodrug activated by catalase-peroxidase (KatG) to form the isoniazid-nicotinamide adenine dinucleotide (NAD) adduct, which inhibits the enzyme enoyl-acyl carrier protein reductase (InhA) of the fatty acid synthase II (FASII), leading to the growth inhibition of M. tuberculosis cells.76 Mutations in katG and inhA are the primary mechanisms of clinical resistance to isoniazid.77,78 In 2020, a series of carborane-containing isoniazid inhibitors were evaluated in vitro against the M. tuberculosis H37Rv strain and its isoniazid-resistant, KatG-deficient mutant (ΔkatG).46 Hydrazone 34 and was the most active against the wild-type strain (MIC99 0.092 μg mL−1), with comparable activity to isoniazid (MIC99 0.10 μg mL−1). para-Carborane-containing hydrazide derivative 35 was the most potent against ΔkatG, showing a 61-fold increase in activity compared with isoniazid. This suggests that the activity of 35 does not require formation of an adduct with NAD and instead involves other essential molecules within M. tuberculosis. Furthermore, 35 did not display any cytotoxicity towards human cells up to a concentration four times higher than its MIC99 value.
1,8-Difunctionalised cyclam derivatives bearing hydrophobic aromatic pendant groups such as naphthalene and naphthalimide have shown strong activity in vitro against drug-resistant M. tuberculosis (MIC 3.03–6.29 μg mL−1) and in vivo against M. marinum in a zebrafish assay.79,80 Bioisosteric replacement of the pendants with either closo-carboranes (e.g.36) or nido-carboranes (37) resulted in activity improvements against M. tuberculosis (MIC 0.40–2.50 μg mL−1).81 The comparable activity of both closo- and nido-carboranes suggests that their interactions with their (unknown) biomolecular target are primarily governed by their steric bulk and/or dihydrogen bond formation as opposed to purely the hydrophobicity of the closo-carborane cage.
Finally, a series of conjugates of carboranes with the monoterpenes geraniol, nerol, and citronellol were recently tested for activity against M. tuberculosis.82 Only nido-carborane-citronellol conjugate 38 displayed moderate antitubercular activity (MIC ≥ 12.5 μg mL−1).
3.2 Antibacterial and antifungal metallacarboranes
Four reports of COSAN-containing antibacterial and antifungal compounds were published during the 2010s. Ether-linked COSAN derivatives 39 and 40 displayed low micromolar activity (MIC50 1.4–18.0 μg mL−1) against four strains of each the following pathogens: S. aureus, S. pyogenes, E. coli, P. aeruginosa, and Candida species.83 In some cases these compounds were significantly more active than broad-spectrum antibiotic thiamphenicol. Similarly structured alkylammonium COSAN derivative 41 was found to be active against S. aureus (MIC80 3.8 μg mL−1) and the filamentous fungi Trichosporon cutaneum (MIC80 5.0 μg mL−1).84 Subsequent investigations found that compounds 42, 43, and the sodium salt of the parent COSAN 10 all displayed comparable activity against these two pathogens (MIC80 1.0–10.0 μg mL−1), as well as inhibition of biofilm formation.85 Finally, alkoxy COSAN derivative 44 was found to inhibit MRSA (MIC 8.0 μg mL−1) with high selectivity over mammalian cells and high bacterial-killing efficiency, eradicating all MRSA cells within 30 minutes.86 It was shown that 44 can induce irreversible MRSA cell wall and membrane damage by increasing production of reactive oxygen species (ROS), and that it inhibits biofilm formation (Fig. 7). | ||
| Fig. 7 Metallacarborane-containing compounds which exhibit antibacterial or antifungal activity that were reported between 2010 and 2020. | ||
The past five years have seen significantly increased research interest in COSAN-containing antibacterial and antifungal agents. Four COSAN-containing compounds with enhanced water solubility (45–48) were tested against five Gram-negative bacteria (three E. coli and two P. aeruginosa strains), four Gram-positive bacteria (two Enterococcus faecalis and two S. aureus strains), and three fungal C. albicans strains.87 While no activity against Gram-negative strains was observed, all four compounds were active against all of the Gram-positive bacteria and fungi (MIC 1–32 μg mL−1). Polyanionic compounds 47 and 48 were the most active against the C. albicans strains (MIC 4 μg mL−1), whereas compound 45 displayed the strongest bactericidal activity against MRSA (MIC 1 μg mL−1, MBC (minimum bactericidal concentration) 2 μg mL−1). As all these compounds show anti-MRSA activity similar to their activity against wild type S. aureus, it was suggested that the resistance mechanisms deployed by the MRSA strain are ineffective against these COSAN-containing compounds.
Screening a 250-molecule metallacarborane library against P. aeruginosa and the zoonotic plague pathogen Yersinia pestis substitute, Yersinia enterocolitica, identified six COSAN derivatives (including 49–51) with moderate activity against P. aeruginosa (IC50 8.1–48 μg mL−1) and strong activity against Y. enterocolitica (IC50 0.94–5.1 μg mL−1).88 This was significantly stronger anti-Yersinia activity than previously-tested boron-containing compounds (MIC > 1500 μg mL−1).89 Testing using a matrix-type approach of Y. enterocolitica strains exposed to compounds 49–51 for nine cycles of growth revealed that resistance was only generated against N-linked compound 51, regardless of the starting compound used. SEM analysis of these resistant strains revealed that compounds 50 and 51 had a bacteriostatic mode of action that likely targeted bacterial cell division (Fig. 8).
 | ||
| Fig. 8 Metallacarborane-containing compounds which exhibit antibacterial or antifungal activity that have been reported during the 2020s. | ||
Testing of ten COSAN derivatives against C. albicans and a number of bacterial strains showed that compounds 52 and 53 were very active against S. aureus (MIC 0.050–0.054 μg mL−1), while compound 54 displayed equivalent antifungal activity against C. albicans to the positive control amphotericin B (MIC 1.2 μg mL−1).90 Expanded antifungal studies showed that four of the tested compounds exhibited strong anti-Candida activity against five representatives of the Candida genus (MIC 0.61–1.3 μg mL−1). The parent compound, 55, was active against a panel of 100 clinical C. albicans isolates (MIC 0.64–71 μg mL−1), including those resistant to systemic antifungal drugs amphotericin B and fluconazole. Further studies revealed that parent 55 was synergistic with amphotericin B, but that cyclohexane derivative 56 was not, and that the most active compounds of the series exhibited relatively low toxicity against eukaryotic cells and Danio rerio embryos.
A conjugate of COSAN and curcumin (57) displayed moderate activity against Gram-positive bacteria (31–250 μg mL−1) and A. fumigatus (MIC 125 μg mL−1), while ether-linked analogue 58 and three dodecaborate analogues were largely inactive.91 None of the curcumin conjugates displayed any activity against Gram-negative bacteria.
A series of antimicrobial peptide (AMP) mimics combining COSAN or its singly iodinated analogue with cationic di- and tripeptides exhibited broad-spectrum antibacterial activity while displaying low cytotoxicity and low haemolytic activity.92 Iodinated COSAN–AMP conjugates were generally more active than their non-iodinated counterparts. The most active compound, 59, displayed potent antibacterial activity (MIC 1.8–7.4 μg mL−1) against both Gram-positive and Gram-negative strains (S. aureus, P. aeruginosa, MRSA, and E. coli). Further studies indicated that compound 59 works outside the traditional lytic mechanisms of AMPs, instead inducing bacterial membrane depolarization without cell lysis while also causing ATP depletion, ROS overproduction, and morphological changes. In addition, compound 59 resisted proteolytic degradation, overcoming another key shortcoming of many AMPs.
Testing of COSAN compounds containing N-heterocycles, aromatic and aliphatic amines, and α-amino acid amides for antibacterial and cytotoxic properties revealed that compounds 60–62 displayed antibacterial activity against S. aureus (MIC 1.92–5.12 μg mL−1) with reasonable selectivity compared to mammalian cells (selectivity index (SI) 4.58–6.90).94 Iodinated COSAN-amino acid conjugates 63–65 were even more active (MIC 0.60–1.30 μg mL−1) and much more selective (SI 19.9–46.5). These results, in addition to lipophilicity measurements using log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and log
P and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kw, suggest the following structure–activity relationships: (1) while a positively charged side chain (e.g.61–63) may be beneficial for antibacterial activity and selectivity, it appeared to be less important than an amide backbone (e.g.62–65). (2) Introduction of an iodine atom in the 8-position of the COSAN cage improved activity while maintaining low cytotoxicity. (3) Lipophilic–hydrophilic balance was needed for high selectivity – attaching lipophilic substituents can result in increased antibacterial activity but poorer selectivity over mammalian cells. Kill-time kinetic investigations of the most active compound (63) suggested a bactericidal mode of action, while SEM, transmission electron microscopy (TEM), and optical diffraction tomography (ODT) data indicated that the mode of action of compound 63 is also membranolytic (Fig. 9).
kw, suggest the following structure–activity relationships: (1) while a positively charged side chain (e.g.61–63) may be beneficial for antibacterial activity and selectivity, it appeared to be less important than an amide backbone (e.g.62–65). (2) Introduction of an iodine atom in the 8-position of the COSAN cage improved activity while maintaining low cytotoxicity. (3) Lipophilic–hydrophilic balance was needed for high selectivity – attaching lipophilic substituents can result in increased antibacterial activity but poorer selectivity over mammalian cells. Kill-time kinetic investigations of the most active compound (63) suggested a bactericidal mode of action, while SEM, transmission electron microscopy (TEM), and optical diffraction tomography (ODT) data indicated that the mode of action of compound 63 is also membranolytic (Fig. 9).
 | ||
| Fig. 9 COSAN derivatives containing N-heterocycles (60), aliphatic amines (61) and α-amino acid amides (62–65) used to identify trends in metallacarborane antibacterial activity. | ||
A comparison of various mono- or di-halogenated COSAN derivatives' activities against S. aureus, Enterococcus faecium, and mammalian cells presented the following conclusions:95 (1) all halogenated derivatives displayed stronger antibacterial activity than the parent COSAN ion. (2) Antimicrobial activity generally increased with the mass of halogen(s) present. (3) Mono- and homo-disubstituted derivatives of the same halogen had similar activities to each other, except in the case of iodine, where the mono-substituted derivative was found to be more active. (4) As the atomic mass of the halogen(s) increased, so did the SI, but the introduction of a second halogen reduced the SI. (5) Increasing log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P correlated with both increased mass and increased antibacterial activity, but the lipophilicity of COSAN derivatives was not the primary factor determining their biological activity.
P correlated with both increased mass and increased antibacterial activity, but the lipophilicity of COSAN derivatives was not the primary factor determining their biological activity.
These findings indicate that metallacarborane derivatives not only exhibit diverse antimicrobial activity but also significant mechanistic diversity, as demonstrated by the difference between compound 59's non-lytic membrane depolarisation and compound 63's membranolytic activity.
3.3 Antibacterial and antifungal dodecaborates
There are fewer reports of antibacterial or antifungal dodecaborates than carboranes or metallacarboranes. In 2018, a series of dodecaborate derivatives fused with oxazole rings were tested for antibacterial activity.96 While not active against Gram-positive bacteria, many of the compounds displayed strong activity against Neisseria gonorrhoeae, the most potent being compound 66 (MIC 4 μg mL−1). It was determined that the boron cluster was necessary for this antibacterial effect as the corresponding phenyl analogues were inactive (Fig. 10).More recently, a library of 1,2,3-trisubstituted dodecaborate cluster–oxazole conjugates were synthesised using a rhodium and copper-catalysed regioselective direct B–H alkylation–annulation with (−)-menthol and (−)-camphanic acid acting as directing groups.97 Twenty-nine compounds were evaluated against a range of Gram-positive and Gram-negative bacteria, and the menthol derivatives generally had higher potency than the camphor derivatives. Compound 67 was the most active against multidrug-resistant clinical isolates of N. gonorrhoeae (MIC 2 μg mL−1), compound 68 was the most active against Stenotrophomonas maltophilia (MIC 16 μg mL−1), and compound 69 was the most consistently potent against Gram-positive bacteria (MIC 4 μg mL−1 against S. aureus, E. faecalis, and Listeria monocytogenes). Time-kill analyses of compound 67 against N. gonorrhoeae and S. aureus showed rapid bactericidal activity compared with ceftriaxone and vancomycin.
3.4 Antibacterial boron cluster-grafted polymers
Three publications in 2018–2019 reported the antimicrobial activity of boron cluster-grafted polymer foils. Polymer foils such as PET and polystyrene were treated with piranha solution (a mixture of an acid or alkali with hydrogen peroxide), then grafted, either directly or through amino-linkers such as cysteamine, ethylenediamine, or chitosan, to COSAN,98 amine-containing COSAN derivatives,99 or anti-B18 boranes.100 In all three compound classes, strong activity against Gram-positive Staphylococcus epidermidis and the alga Desmodesmus quadricauda was observed, along with limited efficacy against Gram-negative E. coli.4. Antiviral boron clusters
4.1 HIV protease inhibitors
A major breakthrough in the medicinal chemistry of boron cluster compounds occurred in 2005 when it was reported that a series of metallacarboranes, including the parent COSAN (10), displayed potent, specific, and selective competitive inhibition of HIV protease.29 The most active compound (70) displayed a Ki of 2.2 nM, an EC50 of 0.25 μM, and low toxicity in tissue culture. It also weakly inhibited human cathepsin D and pepsin, and was inactive against trypsin, papain, and amylase. A crystal structure of the HIV protease–COSAN complex showed that two COSAN moieties bind to hydrophobic pockets in HIV protease, blocking flap closure and filling the binding pockets. It was then shown that these inhibitors were better at preserving their efficacy in response to uncommon HIV protease mutations than frontline drugs such as saquinavir, indinavir, or nelfinavir.101 A subsequent structure-guided drug design effort found that bulkier analogue 71 had even higher in vitro inhibitory efficiency (Ki 0.27 nM), but lost its potency against four of the seven tested HIV protease variants.102 An investigation into the solubilities and lipophilicities of metallacarborane HIV protease inhibitors found a weak correlation between their octanol–water partition coefficients (Pow) and their solubility in water, and a stronger correlation between their Pow values and the ability of human serum albumin to increase their solubility.103 It also revealed that the potency of these compounds and their inhibition mode did not correlate with lipophilicity and was instead governed by specific binding interactions (Fig. 11).4.2 Nucleoside derivatives
From 2015–2019, a series of conjugates of nucleosides with carboranes,104–106 dodecaborates,107 and metallacarboranes108 were screened for antiviral activity against human cytomegalovirus (HCMV), human parainfluenza virus type 3 (HPIV-3), encephalomyocarditis virus (EMCV), herpes simplex virus type 1 (HSV-1), and vesicular stomatitis virus (VSV). While none of the dodecaborate or metallacarborane conjugates displayed any antiviral activity within non-toxic concentrations, a number of conjugates of carboranes with uridine and 2′-deoxyuridine exhibited promising antiviral activity. Four compounds with particularly notable anti-HCMV activity were para-carborane conjugates 72 (IC50 5.5 μM, SI 182) and 73 (IC50 3.8 μM, SI 17), ortho-carborane conjugate 74 (IC50 25.5 μM, SI 22), and meta-carborane conjugate 75 (IC50 17.9 μM, SI 11), while nido-carborane conjugate 76 displayed moderate inhibition of EMCV (IC50 28.1 μM). Circular dichroism data revealed that carborane modification favoured the syn conformation of uridine derivatives (e.g.73–76) and the anti conformation of 2′-deoxyuridine derivatives (e.g.72) (Fig. 12).106 | ||
| Fig. 12 Conjugates of carboranes with 2′-deoxyuridine (72), uridine (73–76), and purine (77) which exhibit antiviral activity. | ||
In 2022, five nido-carborane–purine conjugates were evaluated against two HSV-1 strains and two influenza strains.109 The most active compound (77) displayed moderate activity against HSV-1 (median infectious dose (ID50) 148.4 μM, SI 13) and low activity against influenzas A and B (SI 6).
4.3 Antiviral decaborate derivatives
A series of 10-vertex decaborate derivatives were synthesised and evaluated for antiviral activity against influenza A and SARS-CoV-2. Compound 78, containing a decaborate cage linked to a L-histidine methyl ether, displayed superior antiviral activity against an influenza A/H1N1 strain compared with an adamantane analogue and rimantadine hydrochloride.110 Molecular docking studies indicated that the mechanism of action of 78 is similar to that of rimantadine on the M2 channel of the influenza A virus, and that the boron hydride cluster acts as a membrane carrier. Compound 78 was also shown to display some inhibitory activity against SARS-CoV-2, and it has been proposed that it may disrupt SARS-CoV viroporin E function by capturing positively charged ions into its coordination sphere.111 Subsequent studies identified that compounds 79 and 80 also displayed minor antiviral activity against SARS-CoV-2 (IC50 312 and 625 μg mL−1, respectively),112 while amino acid conjugates 81 and 82 were active against another influenza A/H1N1 strain (IC50 5.0 and 20.0 μg mL−1, respectively) (Fig. 13).1135. Antimalarial boron clusters
The Open Source Malaria consortium explored boron cluster compounds as part of an effort to improve the solubility and metabolic stability of triazolopyrazine derivatives with potent (IC50 <100 nM) activity against Plasmodium falciparum. Thus, representative compounds from this series were modified by replacement of a phenyl ring with ortho-carborane (83), meta-carborane (84), para-carborane (85), and nido-carborane (86) moieties, as well as a range of organic bioisosteres.114 Of 32 compounds screened against P. falciparum, including the parent phenyl compound, compounds 83 and 84 were the most potent (IC50 0.06 and 0.12 μM, respectively). The trend in closo-carborane activities followed the order ortho > meta > para, indicating that the more polar and less hydrophobic the closo-carborane isomer, the more potent the final triazolopyrazine derivative. The charged nido-carborane 85 was significantly less active than its closo-analogue 83, likely indicative of weaker binding to a hydrophobic receptor pocket. Both closo- and nido-carborane compounds were inactive against HepG2 cells (IC50 > 10 μM), but compound 83 did display moderate inhibitory activity (IC50 3.62 μM) against the human ether-à-go-go-related gene (hERG), which encodes an essential potassium channel in the heart and smooth muscle tissue. It also exhibited relatively poor metabolic and physicochemical properties compared to the parent phenyl analogue, including lower solubility as well as higher clearance and shorter half-lives in human liver and mouse liver microsomes (Fig. 14).6. Combination antimicrobial therapy
In recent years, the superchaotropic properties of anionic boron clusters have increasingly been harnessed for new combination antimicrobial therapies. The landmark 2022 Nature publication describing the ability of dodecaborate derivatives to act as broadband membrane carriers also found that the prototype superchaotropic cluster carrier [B12Br12]2− effectively transported antibiotics ampicillin and kanamycin A into vesicles where the conventional amphiphile pyrenebutyrate could not.60 Further investigations found that [B12Br12]2− significantly enhanced the antibacterial effect of kanamycin A against Gram-negative E. coli cells, reducing E. coli viability from 60% (kanamycin A alone) to <1% (kanamycin A and [B12Br12]2−). Aminoglycoside antibiotics like kanamycin A require effective passage through the cell wall and plasma membrane to reach their intracellular targets, so this result indicates that anionic boron clusters can transport membrane-impermeable antimicrobial agents into Gram-negative bacterial cells.It was also reported in 2022 that, in addition to displaying strong inhibition against three S. epidermidis strains (MIC80 2.0–3.1 μg mL−1), the COSAN ion (10) enhanced the inhibitory action of tetracycline, erythromycin, and vancomycin against this microbe.115 Compound 10 had a synergistic or additive effect with each of these antibiotics, reducing the concentrations required to deliver inhibitory effects by up to a factor of ten. Moreover, no toxicity against human HEK 293T cells was found at relevant MIC80 concentrations of any of the combination therapies. Propidium iodide uptake assays confirmed that the combination of 10 with each of these three antibiotics significantly increased the cytoplasmic membrane permeabilization of S. epidermidis cells, while TEM data indicated that the combination of tetracycline and 10 induced complete cell lysis and leakage of intracellular contents.
A series of recent publications have demonstrated that closo-dodecaborate 1 ions can also form antimicrobial supramolecular complexes with a range of compounds. The formation of these complexes is guided by the superchaotropic effect of the boron clusters, which means they have promise as efficient photosensitisers for antimicrobial photodynamic therapy (PDT) and photothermal therapy (PTT). When combined with 1, the antibacterial activity of berberine against S. aureus and E. coli was reduced to almost zero.116 However, light irradiation of the supramolecular complex induced enhanced ROS generation, reactivating the antibacterial behaviour of berberine in a controlled manner suitable for antibacterial PDT. Self-assembly of 1 with malachite green was shown to form a cubic-type supramolecular complex which both suppressed the toxicity of malachite green and displayed significant photothermal conversion efficiency upon laser irradiation at 660 nm, making it suitable for antimicrobial PTT.117 Similar principles were used to design a photoinduced water-stable supramolecular radical platform for efficient antibacterial PTT,118 as well as a supramolecular photosensitiser-loaded spray hydrogel for PDT which demonstrated strong antibacterial activity against S. aureus and E. coli.119 These studies represent an exciting emerging application of the superchaotropic properties of boron clusters, and build on previous findings that modification of the photosensitiser chlorin e6 with carboranes can significantly improve its antimicrobial photodynamic inactivation efficacy against Gram-positive bacteria.120
7. Conclusions
Traditionally, interest in boron cluster compounds has been focused on their potential as BNCT agents and pharmacophores with anticancer activity. Increasingly, however, boron clusters have been investigated for their antimicrobial activity. These compounds are unconventional yet attractive antimicrobial agents for a number of reasons, including their high chemical and biological stability, generally low toxicity, resistance to enzymatic metabolism, lipophilicity, capacity to form dihydrogen bonds with amino acid residues in protein receptor sites, and ability to act as broadband membrane carriers by virtue of their superchaotropic properties.As highlighted in this review, a wide and diverse range of boron clusters have been found to be potent antibacterial, antifungal, antiviral or antimalarial agents. Current areas of antimicrobial boron cluster research receiving particularly strong interest include (1) metallacarborane derivatives displaying antibacterial and antifungal activity, (2) carborane derivatives exhibiting antitubercular and other antimycobacterial activity, and (3) combination therapy harnessing the superchaotropic properties of anionic boron clusters, including the molecular self-assembly of supramolecular complexes for PDT and PTT.
Even after extended research interest in boron clusters since the mid-20th century, their physicochemical and biological properties are not yet fully understood. Notably, a significant proportion of the publications reviewed did not report cytotoxicity or selectivity data for antimicrobial boron cluster compounds. While many boron cluster compounds are generally considered to be relatively non-toxic, the collection and reporting of more comprehensive toxicity data for novel antimicrobial boron cluster compounds would significantly improve their value as alternatives to traditional carbon-based scaffolds in medicinal chemistry.
It is encouraging to see a number of studies investigating structural patterns in antimicrobial boron clusters, especially for metallacarboranes. The modes of action of many of these compound classes have been revealed, but more mechanistic investigations into the observed antimicrobial activities would be a significant asset to this growing area of research. Ultimately, antimicrobial boron cluster compounds have been shown to be useful and interesting alternatives to more traditional carbon-based analogues, and are likely to remain a promising area of antimicrobial research into the future.
Author contributions
W. J. P. C. conceived of the manuscript, wrote the manuscript and prepared the figures. P. J. R. and L. M. R. supervised the project and edited and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
We acknowledge and pay respect to the Gadi people of the Eora Nation, the traditional owners of the land on which we research, teach, and collaborate at the University of Sydney.References
- C. S. Ho, C. T. H. Wong, T. T. Aung, R. Lakshminarayanan, J. S. Mehta, S. Rauz, A. McNally, B. Kintses, S. J. Peacock, C. de la Fuente-Nunez, R. E. W. Hancock and D. S. J. Ting, Lancet Microbe, 2025, 6, 100947 CrossRef.
- J. O'Neill, Tackling drug-resistant infections globally: final report and recommendations, 2016 Search PubMed.
- R. J. Grams, W. L. Santos, I. R. Scorei, A. Abad-García, C. A. Rosenblum, A. Bita, H. Cerecetto, C. Viñas and M. A. Soriano-Ursúa, Chem. Rev., 2024, 124, 2441–2511 CrossRef CAS PubMed.
- F. Issa, M. Kassiou and L. M. Rendina, Chem. Rev., 2011, 111, 5701–5722 CrossRef CAS.
- R. Hütter, W. Keller-Schien, F. Knüsel, V. Prelog, G. C. Rodgers jr, P. Suter, G. Vogel, W. Voser and H. Zähner, Helv. Chim. Acta, 1967, 50, 1533–1539 CrossRef.
- R. C. Kane, P. F. Bross, A. T. Farrell and R. Pazdur, Oncologist, 2003, 8, 508–513 CrossRef.
- D. B. Diaz and A. K. Yudin, Nat. Chem., 2017, 9, 731–742 CrossRef CAS.
- S. J. Baker, J. W. Tomsho and S. J. Benkovic, Chem. Soc. Rev., 2011, 40, 4279–4285 RSC.
- Y. Mishima, C. Honda, M. Ichihashi, H. Obara, J. Hiratsuka, H. Fukuda, H. Karashima, T. Kobayashi, K. Kanda and K. Yoshino, Lancet, 1989, 334, 388–389 CrossRef.
- R. F. Barth, N. Gupta and S. Kawabata, Cancer Commun., 2024, 44, 893–909 CrossRef.
- R. M. Murilla, G. G. Edilo, M. L. M. Budlayan and E. S. Auxtero, Nano TransMed, 2025, 4, 100081 CrossRef.
- R. N. Grimes, in Carboranes, ed. R. N. Grimes, Academic Press, 3rd edn, 2016, ch. 1, pp. 1–5 Search PubMed.
- D. Grafstein and J. Dvorak, Inorg. Chem., 1963, 2, 1128–1133 CrossRef CAS.
- L. Pazderová, E. Z. Tüzün, D. Bavol, M. Litecká, L. Fojt and B. Grűner, Molecules, 2023, 28(19), 6971 CrossRef PubMed.
- K. Fink and M. Uchman, Coord. Chem. Rev., 2021, 431, 213684 CrossRef CAS.
- R. N. Grimes, in Carboranes, ed. R. N. Grimes, Academic Press, 2016, ch. 2, pp. 7–18 Search PubMed.
- P. v. R. Schleyer and K. Najafian, Inorg. Chem., 1998, 37, 3454–3470 CrossRef CAS PubMed.
- J. Poater, C. Viñas, I. Bennour, S. Escayola, M. Solà and F. Teixidor, J. Am. Chem. Soc., 2020, 142, 9396–9407 CrossRef CAS.
- B. Schwarze, M. Gozzi and E. Hey-Hawkins, in Boron-Based Compounds, 2018, pp. 60–108 Search PubMed.
- G. Calabrese, A. Daou, E. Barbu and J. Tsibouklis, Drug Discovery Today, 2018, 23, 63–75 CrossRef CAS PubMed.
- T. Kageji, Y. Nakagawa, K. Kitamura, K. Matsumoto and H. Hatanaka, J. Neuro-Oncol., 1997, 33, 117–130 CrossRef CAS.
- J. Li, O. Janoušková, R. Fernandez-Alvarez, S. Mesíková, Z. Tošner, S. Kereïche, M. Uchman and P. Matějíček, Chem. – Eur. J., 2020, 26, 14283–14289 CrossRef CAS PubMed.
- A. Diaz, K. Stelzer, G. Laramore and R. Wiersema, Res. Dev. Neutron Capture Ther., Proc. Int. Congr., 10th, 2002, 993–999 CAS.
- T. He, S. V. Chittur and R. A. Musah, ACS Chem. Neurosci., 2019, 10, 1524–1534 CrossRef CAS PubMed.
- E. Oleshkevich, A. Morancho, A. Saha, K. M. O. Galenkamp, A. Grayston, S. G. Crich, D. Alberti, N. Protti, J. X. Comella, F. Teixidor, A. Rosell and C. Viñas, Nanomed.: Nanotechnol., Biol. Med., 2019, 20, 101986 CrossRef CAS.
- M. Tarrés, E. Canetta, E. Paul, J. Forbes, K. Azzouni, C. Viñas, F. Teixidor and A. J. Harwood, Sci. Rep., 2015, 5, 7804 CrossRef PubMed.
- I. Fuentes, T. García-Mendiola, S. Sato, M. Pita, H. Nakamura, E. Lorenzo, F. Teixidor, F. Marques and C. Viñas, Chem. – Eur. J., 2018, 24, 17239–17254 CrossRef CAS.
- B. Grüner, J. Brynda, V. Das, V. Šícha, J. Štěpánková, J. Nekvinda, J. Holub, K. Pospíšilová, M. Fábry, P. Pachl, V. Král, M. Kugler, V. Mašek, M. Medvedíková, S. Matějková, A. Nová, B. Lišková, S. Gurská, P. Džubák, M. Hajdúch and P. Řezáčová, J. Med. Chem., 2019, 62, 9560–9575 CrossRef.
- P. Cígler, M. Kožíšek, P. Řezáčová, J. Brynda, Z. Otwinowski, J. Pokorná, J. Plešek, B. Grüner, L. Dolečková-Marešová, M. Máša, J. Sedláček, J. Bodem, H.-G. Kräusslich, V. Král and J. Konvalinka, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 15394–15399 CrossRef.
- Z. J. Leśnikowski, J. Med. Chem., 2016, 59, 7738–7758 CrossRef.
- J. F. Valliant, K. J. Guenther, A. S. King, P. Morel, P. Schaffer, O. O. Sogbein and K. A. Stephenson, Coord. Chem. Rev., 2002, 232, 173–230 CrossRef CAS.
- A. Buzharevski, S. Paskaš, M.-B. Sárosi, M. Laube, P. Lönnecke, W. Neumann, B. Murganić, S. Mijatović, D. Maksimović-Ivanić, J. Pietzsch and E. Hey-Hawkins, Sci. Rep., 2020, 10, 4827 CrossRef CAS PubMed.
- A. Marfavi, P. Kavianpour and L. M. Rendina, Nat. Rev. Chem., 2022, 6, 486–504 CrossRef.
- M. E. El-Zaria, N. Janzen, M. Blacker and J. F. Valliant, Chem. – Eur. J., 2012, 18, 11071–11078 CrossRef CAS.
- D. S. Wilbur, D. K. Hamlin, R. R. Srivastava and M.-K. Chyan, Nucl. Med. Biol., 2004, 31, 523–530 CrossRef CAS PubMed.
- K. B. Gona, A. Zaulet, V. Gómez-Vallejo, F. Teixidor, J. Llop and C. Viñas, Chem. Commun., 2014, 50, 11415–11417 RSC.
- M. Scholz and E. Hey-Hawkins, Chem. Rev., 2011, 111, 7035–7062 CrossRef CAS PubMed.
- B. J. Eleazer and D. V. Peryshkov, Comments Inorg. Chem., 2018, 38, 79–109 CrossRef CAS.
- A. Dąbrowska, M. Matuszewski, K. Zwoliński, A. Ignaczak and A. B. Olejniczak, Eur. J. Pharm. Sci., 2018, 111, 226–237 CrossRef PubMed.
- K. Yamamoto and Y. Endo, Bioorg. Med. Chem. Lett., 2001, 11, 2389–2392 CrossRef CAS.
- S. Fujii, H. Masuno, Y. Taoda, A. Kano, A. Wongmayura, M. Nakabayashi, N. Ito, M. Shimizu, E. Kawachi, T. Hirano, Y. Endo, A. Tanatani and H. Kagechika, J. Am. Chem. Soc., 2011, 133, 20933–20941 CrossRef CAS.
- J. Fanfrlík, M. Lepšík, D. Horinek, Z. Havlas and P. Hobza, ChemPhysChem, 2006, 7, 1100–1105 CrossRef.
- R. N. Grimes, Dalton Trans., 2015, 44, 5939–5956 RSC.
- P. Mader, A. Pecina, P. Cígler, M. Lepšík, V. Šícha, P. Hobza, B. Grüner, J. Fanfrlík, J. Brynda and P. Řezáčová, BioMed Res. Int., 2014, 2014, 389869 Search PubMed.
- R. Otero, S. Seoane, R. Sigüeiro, A. Y. Belorusova, M. A. Maestro, R. Pérez-Fernández, N. Rochel and A. Mouriño, Chem. Sci., 2016, 7, 1033–1037 RSC.
- D. Różycka, M. Korycka-Machała, A. Żaczek, J. Dziadek, D. Gurda, M. Orlicka-Płocka, E. Wyszko, K. Biniek-Antosiak, W. Rypniewski and A. B. Olejniczak, Pharmaceuticals, 2020, 13(12), 465 CrossRef PubMed.
- E. Hey-Hawkins and C. Viñas Texidor, Boron-Based Compounds: Potential and Emerging Applications in Medicine, John Wiley & Sons, Incorporated, 2018 Search PubMed.
- A. Pecina, M. Lepšík, J. Řezáč, J. Brynda, P. Mader, P. Řezáčová, P. Hobza and J. Fanfrlík, J. Phys. Chem. B, 2013, 117, 16096–16104 CrossRef CAS PubMed.
- Y. Endo, T. Iijima, Y. Yamakoshi, M. Yamaguchi, H. Fukasawa and K. Shudo, J. Med. Chem., 1999, 42, 1501–1504 CrossRef CAS.
- Y. Endo, T. Yoshimi and C. Miyaura, Pure Appl. Chem., 2003, 75, 1197–1205 CrossRef CAS.
- M. Hirata, M. Inada, C. Matsumoto, M. Takita, T. Ogawa, Y. Endo and C. Miyaura, Biochem. Biophys. Res. Commun., 2009, 380, 218–222 CrossRef CAS PubMed.
- W. Fischli, O. Leukart and R. Schwyzer, Helv. Chim. Acta, 1977, 60, 959–963 CrossRef CAS PubMed.
- P. E. Johnson and J. A. Stewart, Arch. Biochem. Biophys., 1972, 149, 295–306 CrossRef CAS.
- I. Fuentes, J. Pujols, C. Viñas, S. Ventura and F. Teixidor, Chem. – Eur. J., 2019, 25, 12820–12829 CrossRef CAS PubMed.
- S. Pastorekova and R. J. Gillies, Cancer Metastasis Rev., 2019, 38, 65–77 CrossRef CAS.
- M. Kugler, J. Nekvinda, J. Holub, S. El Anwar, V. Das, V. Šícha, K. Pospíšilová, M. Fábry, V. Král, J. Brynda, V. Kašička, M. Hajdúch, P. Řezáčová and B. Grüner, ChemBioChem, 2021, 22, 2741–2761 CrossRef CAS.
- W. Chen, S. C. Mehta and D. R. Lu, Adv. Drug Delivery Rev., 1997, 26, 231–247 CrossRef CAS.
- T. O. B. Olusanya, G. Calabrese, D. G. Fatouros, J. Tsibouklis and J. R. Smith, Biophys. Chem., 2019, 247, 25–33 CrossRef CAS PubMed.
- K. I. Assaf and W. M. Nau, Angew. Chem., Int. Ed., 2018, 57, 13968–13981 CrossRef CAS PubMed.
- A. Barba-Bon, G. Salluce, I. Lostalé-Seijo, K. I. Assaf, A. Hennig, J. Montenegro and W. M. Nau, Nature, 2022, 603, 637–642 CrossRef CAS PubMed.
- M. Gos, J. Cebula and T. M. Goszczyński, J. Med. Chem., 2024, 67, 8481–8501 CrossRef CAS PubMed.
- Y. Chen, A. Barba-Bon, B. Grüner, M. Winterhalter, M. A. Aksoyoglu, S. Pangeni, M. Ashjari, K. Brix, G. Salluce, Y. Folgar-Cameán, J. Montenegro and W. M. Nau, J. Am. Chem. Soc., 2023, 145, 13089–13098 CrossRef CAS PubMed.
- D. Gabel, D. Awad, T. Schaffran, D. Radovan, D. Dărăban, L. Damian, M. Winterhalter, G. Karlsson and K. Edwards, ChemMedChem, 2007, 2, 51–53 CrossRef CAS.
- T. Schaffran, J. Li, G. Karlsson, K. Edwards, M. Winterhalter and D. Gabel, Chem. Phys. Lipids, 2010, 163, 64–73 CrossRef CAS PubMed.
- T. Totani, K. Aono, K. Yamamoto and K. Tawara, J. Med. Chem., 1981, 24, 1492–1499 CrossRef CAS PubMed.
- G. Oros, I. Ujváry and R. J. Nachman, Amino Acids, 1999, 17, 357–368 CrossRef CAS PubMed.
- D. Różycka, Z. J. Leśnikowski and A. B. Olejniczak, J. Organomet. Chem., 2019, 881, 19–24 CrossRef.
- C. Selg, T. Grell, A. Brakel, P. C. Andrews, R. Hoffmann and E. Hey-Hawkins, ChemPlusChem, 2024, 89, e202300759 CrossRef CAS.
- S. Li, C. Wu, X. Lv, X. Tang, X. Zhao, H. Yan, H. Jiang and X. Wang, Sci. China: Chem., 2012, 55, 2388–2395 CrossRef CAS.
- S. Li, C. Wu, X. Zhao, H. Jiang, H. Yan and X. Wang, J. Biomed. Nanotechnol., 2013, 9, 393-402(310) Search PubMed.
- S. Li, Z. Wang, Y. Wei, C. Wu, S. Gao, H. Jiang, X. Zhao, H. Yan and X. Wang, Biomaterials, 2013, 34, 902–911 CrossRef CAS.
- S. Li, C. Wu, X. Tang, S. Gao, X. Zhao, H. Yan and X. Wang, Sci. China: Chem., 2013, 56, 595–603 CrossRef CAS.
- R. C. Reynolds, S. R. Campbell, R. G. Fairchild, R. L. Kisliuk, P. L. Micca, S. F. Queener, J. M. Riordan, W. D. Sedwick, W. R. Waud, A. K. W. Leung, R. W. Dixon, W. J. Suling and D. W. Borhani, J. Med. Chem., 2007, 50, 3283–3289 CrossRef CAS.
- K. Li, Y. Wang, G. Yang, S. Byun, G. Rao, C. Shoen, H. Yang, A. Gulati, D. C. Crick, M. Cynamon, G. Huang, R. Docampo, J. H. No and E. Oldfield, ACS Infect. Dis., 2015, 1, 215–221 CrossRef CAS PubMed.
- A. Adamska, A. Rumijowska-Galewicz, A. Ruszczynska, M. Studzińska, A. Jabłońska, E. Paradowska, E. Bulska, H. Munier-Lehmann, J. Dziadek, Z. J. Leśnikowski and A. B. Olejniczak, Eur. J. Med. Chem., 2016, 121, 71–81 CrossRef CAS PubMed.
- C. Vilchèze and W. R. Jacobs, J. Mol. Biol., 2019, 431, 3450–3461 CrossRef.
- Y. Zhang, B. Heym, B. Allen, D. Young and S. Cole, Nature, 1992, 358, 591–593 CrossRef CAS.
- D. Machado, J. Perdigão, J. Ramos, I. Couto, I. Portugal, C. Ritter, E. C. Boettger and M. Viveiros, J. Antimicrob. Chemother., 2013, 68, 1728–1732 CrossRef CAS.
- M. Yu, G. Nagalingam, S. Ellis, E. Martinez, V. Sintchenko, M. Spain, P. J. Rutledge, M. H. Todd and J. A. Triccas, J. Med. Chem., 2016, 59, 5917–5921 CrossRef CAS.
- M. Spain, J. K. H. Wong, G. Nagalingam, J. M. Batten, E. Hortle, S. H. Oehlers, X. F. Jiang, H. E. Murage, J. T. Orford, P. Crisologo, J. A. Triccas, P. J. Rutledge and M. H. Todd, J. Med. Chem., 2018, 61, 3595–3608 CrossRef CAS.
- N. Smith, D. Quan, G. Nagalingam, J. A. Triccas, L. M. Rendina and P. J. Rutledge, RSC Med. Chem., 2022, 13, 1234–1238 RSC.
- D. A. Gruzdev, G. L. Levit, I. N. Ganebnykh, D. V. Belyaev, D. V. Vakhrusheva and V. P. Krasnov, Monatsh. Chem., 2025, 156, 885–896 CrossRef CAS.
- T. Popova, A. Zaulet, F. Teixidor, R. Alexandrova and C. Viñas, J. Organomet. Chem., 2013, 747, 229–234 CrossRef CAS.
- E. Kvasničková, J. Masák, J. Čejka, O. Maťátková and V. Šícha, J. Organomet. Chem., 2017, 827, 23–31 CrossRef.
- E. Vaňková, K. Lokočová, O. Maťátková, I. Křížová, J. Masák, B. Grüner, P. Kaule, J. Čermák and V. Šícha, J. Organomet. Chem., 2019, 899, 120891 CrossRef.
- Y. Zheng, W. Liu, Y. Chen, H. Jiang, H. Yan, I. Kosenko, L. Chekulaeva, I. Sivaev, V. Bregadze and X. Wang, Organometallics, 2017, 36, 3484–3490 CrossRef CAS.
- I. Romero, M. Martinez-Medina, C. Camprubí-Font, I. Bennour, D. Moreno, L. Martínez-Martínez, F. Teixidor, M. A. Fox and C. Viñas, Organometallics, 2020, 39, 4253–4264 CrossRef CAS.
- W. Swietnicki, W. Goldeman, M. Psurski, A. Nasulewicz-Goldeman, A. Boguszewska-Czubara, M. Drab, J. Sycz and T. M. Goszczyński, Int. J. Mol. Sci., 2021, 22(13), 6762 CrossRef CAS PubMed.
- Z. Sayin, U. S. Ucan and A. Sakmanoglu, Biol. Trace Elem. Res., 2016, 173, 241–246 CrossRef CAS PubMed.
- K. Kubiński, M. Masłyk, M. Janeczko, W. Goldeman, A. Nasulewicz-Goldeman, M. Psurski, A. Martyna, A. Boguszewska-Czubara, J. Cebula and T. M. Goszczyński, J. Med. Chem., 2022, 65, 13935–13945 CrossRef PubMed.
- A. A. Druzina, N. E. Grammatikova, O. B. Zhidkova, N. A. Nekrasova, N. V. Dudarova, I. D. Kosenko, M. A. Grin and V. I. Bregadze, Molecules, 2022, 27(9), 2920 CrossRef CAS PubMed.
- K. Fink, B. Szermer-Olearnik, A. Kędziora, B. Dudek, G. Bugla-Płoskońska, W. Goldeman, M. Gos, M. Cuprych-Belter, M. Psurski, P. Migdał, M. Uchman and T. M. Goszczyński, J. Med. Chem., 2025, 68, 16076–16092 CrossRef CAS PubMed.
- I. Bennour, M. N. Ramos, M. Nuez-Martínez, J. A. M. Xavier, A. B. Buades, R. Sillanpää, F. Teixidor, D. Choquesillo-Lazarte, I. Romero, M. Martinez-Medina and C. Viñas, Dalton Trans., 2022, 51, 7188–7209 RSC.
- J. Cebula, K. Fink, W. Goldeman, B. Szermer-Olearnik, A. Nasulewicz-Goldeman, M. Psurski, M. Cuprych, A. Kędziora, B. Dudek, G. Bugla-Płoskońska, M. Chaszczewska-Markowska, M. Gos, P. Migdał and T. M. Goszczyński, J. Med. Chem., 2023, 66, 14948–14962 CrossRef CAS PubMed.
- K. Zakret-Drozdowska, B. Szermer-Olearnik, W. Goldeman, M. Gos, D. Drozdowski, A. Gągor and T. M. Goszczyński, Inorg. Chem. Front., 2025, 12, 191–204 RSC.
- Y. Sun, J. Zhang, Y. Zhang, J. Liu, S. van der Veen and S. Duttwyler, Chem. – Eur. J., 2018, 24, 10364–10371 CrossRef CAS PubMed.
- R. Varkhedkar, F. Yang, R. Dontha, J. Zhang, J. Liu, B. Spingler, S. van der Veen and S. Duttwyler, ACS Cent. Sci., 2022, 8, 322–331 CrossRef CAS PubMed.
- T. Knapova, J. Matousek, K. Kolarova, P. Slepicka, V. Sicha, J. Trogl and Z. Kolska, ChemistrySelect, 2019, 4, 4382–4391 CrossRef CAS.
- M. Benkocká, K. Kolářová, J. Matoušek, A. Semerádtová, V. Šícha and Z. Kolská, Appl. Surf. Sci., 2018, 441, 120–129 CrossRef.
- M. Benkocká, S. Lupínková, J. Matoušek, K. Kolářová and Z. Kolská, RSC Adv., 2018, 8, 15001–15008 RSC.
- M. Kožíšek, P. Cígler, M. Lepšík, J. Fanfrlík, P. Řezáčová, J. Brynda, J. Pokorná, J. Plešek, B. Grüner, K. Grantz Šašková, J. Václavíková, V. Král and J. Konvalinka, J. Med. Chem., 2008, 51, 4839–4843 CrossRef PubMed.
- P. Řezáčová, J. Pokorná, J. Brynda, M. Kožíšek, P. Cígler, M. Lepšík, J. Fanfrlík, J. Řezáč, K. Grantz Šašková, I. Sieglová, J. Plešek, V. Šícha, B. Grüner, H. Oberwinkler, J. Sedláček', H.-G. Kräusslich, P. Hobza, V. Král and J. Konvalinka, J. Med. Chem., 2009, 52, 7132–7141 CrossRef PubMed.
- J. Rak, B. Dejlová, H. Lampová, R. Kaplánek, P. Matějíček, P. Cígler and V. Král, Mol. Pharmaceutics, 2013, 10, 1751–1759 CrossRef CAS PubMed.
- M. Białek-Pietras, A. B. Olejniczak, E. Paradowska, M. Studzińska, P. Suski, A. Jabłońska and Z. J. Leśnikowski, J. Organomet. Chem., 2015, 798, 99–105 CrossRef.
- M. Białek-Pietras, A. B. Olejniczak, E. Paradowska, M. Studzińska, A. Jabłońska and Z. J. Leśnikowski, J. Organomet. Chem., 2018, 865, 166–172 CrossRef.
- D. Saftić, M. Studzińska, E. Paradowska, I. Piantanida, G. Baranović, M. Białek-Pietras and Z. J. Leśnikowski, Bioorg. Chem., 2020, 94, 103466 CrossRef PubMed.
- J. Laskova, A. Kozlova, M. Białek-Pietras, M. Studzińska, E. Paradowska, V. Bregadze, Z. J. Leśnikowski and A. Semioshkin, J. Organomet. Chem., 2016, 807, 29–35 CrossRef CAS.
- I. Kosenko, I. Ananyev, A. Druzina, I. Godovikov, J. Laskova, V. Bregadze, M. Studzinska, E. Paradowska, Z. J. Leśnikowski and A. Semioshkin, J. Organomet. Chem., 2017, 849–850, 142–149 CrossRef CAS.
- D. A. Gruzdev, A. A. Telegina, V. A. Ol'shevskaya, V. L. Andronova, G. A. Galegov, V. V. Zarubaev, G. L. Levit and V. P. Krasnov, Russ. Chem. Bull., 2022, 71, 2375–2382 CrossRef CAS.
- V. V. Avdeeva, T. M. Garaev, N. V. Breslav, E. I. Burtseva, T. V. Grebennikova, A. P. Zhdanov, K. Y. Zhizhin, E. A. Malinina and N. T. Kuznetsov, J. Biol. Inorg. Chem., 2022, 27, 421–429 CrossRef CAS PubMed.
- T. M. Garaev, T. V. Grebennikova, V. V. Avdeeva, V. V. Lebedeva and V. F. Larichev, Probl. Virol., 2023, 68, 18–25 CrossRef CAS.
- E. Y. Matveev, T. M. Garaev, S. S. Novikov, A. I. Nichugovskii, I. E. Sokolov, V. F. Larichev, V. V. Lebedeva, T. V. Grebennikova, V. V. Avdeeva, E. A. Malinina, K. Y. Zhizhin and N. T. Kuznetsov, Russ. J. Inorg. Chem., 2023, 68, 670–677 CrossRef CAS.
- T. M. Garaev, I. I. Yudin, N. V. Breslav, T. V. Grebennikova, E. Y. Matveev, E. A. Eshtukova-Shcheglova, V. V. Avdeeva, K. Y. Zhizhin and N. T. Kuznetsov, Molecules, 2024, 29(24), 5886 CrossRef CAS PubMed.
- E. G. Tse, S. D. Houston, C. M. Williams, G. P. Savage, L. M. Rendina, I. Hallyburton, M. Anderson, R. Sharma, G. S. Walker, R. S. Obach and M. H. Todd, J. Med. Chem., 2020, 63, 11585–11601 CrossRef CAS PubMed.
- E. Vaňková, K. Lokočová, P. Kašparová, R. Hadravová, I. Křížová, O. Maťátková, J. Masák and V. Šícha, Pharmaceuticals, 2022, 15(5), 534 CrossRef PubMed.
- Z.-Z. Peng, S.-Y. Xu, W.-Z. Li, L. Li, X.-Q. Wang and W. Wang, New J. Chem., 2022, 46, 5470–5473 RSC.
- Z.-X. Wang, X. Chen, X. Liu, W.-Z. Li, Y.-Y. Ye, S.-Y. Xu, H. Zhang and X.-Q. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 55486–55494 CrossRef CAS PubMed.
- J. Xiao, W.-Z. Li, R.-Y. Xiong, S.-Y. Xu, C.-S. Liu, Y. Ruan, H. Li, H. Zhang, W. Wang and X.-Q. Wang, ACS Appl. Mater. Interfaces, 2024, 16, 26537–26546 CrossRef CAS PubMed.
- X.-J. Fang, L. Wang, N. Zhou, Y.-R. Ruan, R.-Y. Xiong, Z. Wang and W. Wang, ACS Appl. Polym. Mater., 2024, 6, 13341–13349 CrossRef CAS.
- E. O. Omarova, P. A. Nazarov, A. M. Firsov, M. G. Strakhovskaya, A. Y. Arkhipova, M. M. Moisenovich, I. I. Agapov, V. A. Ol'shevskaya, A. V. Zaitsev, V. N. Kalinin, E. A. Kotova and Y. N. Antonenko, PLoS One, 2015, 10, e0141990 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |









