Design of hierarchical zeolite catalysts by desilication†
Danny
Verboekend
and
Javier
Pérez-Ramírez
*
Institute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zurich, Wolfgang-Pauli-Strasse 10, CH 8093, HCI E 125, Zurich, Switzerland. E-mail: jpr@chem.ethz.ch; Fax: +41 44 633 14 05; Tel: +41 44 633 71 20
First published on 4th July 2011
Abstract
Hierarchical (or mesoporous) zeolites have received an ever-increasing attention due to their improved performance in catalysed reactions with respect to conventional (purely microporous) zeolites. Desilication in alkaline media has become a widely applied preparation method to tailor these modified zeolites, due to an optimal combination of efficiency and simplicity. This review presents recent developments that have expanded its general understanding and turned this top-down treatment highly versatile, controllable, and scalable. Design aspects of mesoporous zeolites for catalytic applications are emphasised, encircling the establishment of synthesis–property–function relationships. Alkaline treatment is a key step in strategic combinations with other post-synthesis modifications towards superior zeolite catalysts. The outlook of the field, pinpointing present needs and short-term priorities, is discussed.
 Danny Verboekend | Danny Verboekend (Nieuwegein, the Netherlands, 1983) studied Chemistry at Utrecht University (2008). During this study, he performed an Erasmus project in ICIQ in Tarragona, Spain (2007–2008). At ICIQ he started his PhD studies (2008–2010), and continues at the Institute for Chemical and Bioengineering of the ETH Zurich. His current interests concern post-synthetic modifications on zeolites to improve the molecular transport and performance in catalysed reactions. |
 Javier Pérez-Ramírez | Javier Pérez-Ramírez (Benidorm, Spain, 1974) studied Chemical Engineering at the University of Alicante, Spain (1997) and earned his PhD degree at TUDelft, the Netherlands (2002). He worked for Norsk Hydro and Yara International in Porsgrunn, Norway (2002–2005) and returned to academia as ICREA research professor and group leader at ICIQ in Tarragona, Spain (2005–2009). In 2010, he was appointed full professor and chair of Catalysis Engineering at the Institute for Chemical and Bioengineering of the ETH Zurich. He researches the science and engineering of heterogeneous catalysis to design sustainable processes. |
1. Introduction
In the last decade, substantial efforts have focused on the more effective utilisation of zeolites in heterogeneously catalysed reactions. The sub-optimal use of this class of aluminosilicates is implied by the limited access to, and/or diffusional constraints within, their micropores. Hierarchical (or mesoporous) zeolites alleviate the latter issues by coupling the intrinsic microporosity with an auxiliary mesopore network of inter- or intracrystalline nature.1–7 Each porosity level in the hierarchical structure fulfils a distinct complementary task: the micropores hold catalytically active sites, whose access is facilitated by the newly introduced mesoporosity. A large array of lab-scale approaches to synthesise hierarchical zeolites has been realised.1,4,8–10 Bottom-up routes include the modification of the synthesis protocol resulting in nanosized zeolite crystals,8zeolites including a secondary mesopore template,9 or zeolite composites.4 Top-down routes comprise post-synthetic treatment(s) of previously grown zeolites by demetallation (extraction of framework atoms) or delamination. Examples hereof comprise steam,1 acid,1 or base treatments,10 and more refined approaches that include swelling agents,11,12 irradiation,13,14 and/or strong oxidising reagents.14Although most of the above-mentioned routes are successful in acquiring mesoporosity and improved performance in catalysed reactions, both HSE (health-safety-environment) issues and production costs should be carefully evaluated to progress towards large-scale applications.15,16 For example, the majority of bottom-up methods are not easily amended to industrialisation since they involve substantial amounts of costly templates.7 In contrast, top-down syntheses involving acid, steam, and base treatment are more readily implemented at an industrial scale.1,5,7,17 In fact, both steaming and acid leaching are classically used to prepare ultra-stable Y zeolites with mesopores for fluid catalytic cracking.1 However, these treatments are executed primarily to stabilise the FAU framework and it was shown that the formed mesopores do not significantly affect intracrystalline diffusion, since they are mostly present as cavities inside the crystals.18 The introduction of mesopores by the alkaline-mediated leaching of framework Si has become a very attractive method due to the combination of both experimental simplicity and efficiency of the hierarchical zeolites obtained.5,7 The mesopores induced by alkaline treatment are interconnected and accessible from the external surface of the zeolite crystal,19,20 representing a clear advantage for access-limited and diffusion-constrained reactions. In the past few years, an impressive amount of papers have reported syntheses of mesoporous zeolites by desilication and the corresponding benefits—in terms of activity, selectivity, and/or lifetime—in a wide range of catalysed reactions, including isomerisation, alkylation, acylation, aromatisation, cracking, pyrolysis, methanol-to-hydrocarbons, etc.5,21
The use of base leaching as a post-synthetic modification to increased zeolite performance in adsorption and catalysis was first patented by Dean Arthur Young in the 1960s.22 It was claimed that alkaline-treated mordenite displayed preserved crystallinity and a significantly increased benzene adsorption capacity. Moreover, catalytic evaluation in gas-oil hydrocracking revealed a 3 times higher conversion for an alkaline-treated Pd/mordenite than for the untreated zeolite. Already then, Young speculated that the improved performance of the modified material could be due to better access to the micropores. In the 1970s, other patents claimed superior properties of alkaline-treated zeolites as olefin adsorbents23 and molecular sieves,24 but the obtained benefits remained poorly understood from a scientific ground. Remarkably, the open literature concerning zeolite modification in alkaline media appeared about 25 years after the patent by Young. In 1992, Dessau et al.25 reported the dissolution of large ZSM-5 crystals in an attempt to identify Al gradients. They evidenced the selective removal of framework silicon and an anisotropic dissolution of the ZSM-5 crystals. The latter was implied by the negative charge associated to the lattice Al centers, which inhibit local dissolution. Few years later, Čižmek et al.26 focussed on understanding the dissolution mechanism of zeolites in alkaline media and confirmed the distinctive influence of aluminium on the dissolution kinetics. Mao et al.27 studied the properties of alkaline-treated Y, X, and ZSM-5 zeolites in more detail. They found that treatment in aqueous sodium carbonate led to an increased Al content and enhanced ion-exchange capacity without drastically changing the zeolite structure. In this work, the first N2 isotherm of mesoporous ZSM-5 obtained by base leaching was reported. However the key role of mesopores to increase the intracrystalline diffusion and/or the access to the micropore volume in catalysed reactions was not a topic of discussion.
The first key contribution emphasising the remarkable porous changes implied by alkaline treatment of ZSM-5 was communicated by Matsukata and co-workers in 2000.28 Later, Groen et al. put intense effort into exploring the potential of mesoporous MFI obtained by desilication (Fig. 1). They established optimal conditions29 (especially in terms of time and temperature) and claimed that the long-range order and Brønsted acidity of the zeolite were mostly unchanged upon alkaline treatment.30 Additionally, the superiority of NaOH-treated zeolites in diffusion20 and catalysis31 compared to the corresponding purely microporous parents was demonstrated. On the other hand, by using a single ‘standard’ experimental desilication condition (0.2 M of NaOH, 65 °C, 30 min), they identified a significant limitation in the confined range of molar Si/Al ratios (25–50) for which optimal introduction of intracrystalline mesopores could be achieved. At higher Si/Al ratios, uncontrolled silicon extraction occurred, resulting in the formation of larger pores. For low Si/Al ratios, silicon extraction was hampered resulting in limited extra mesoporosity. Accordingly, framework aluminium was coined as ‘pore-directing agent’ (PDA), due to its regulatory effect on silicon dissolution.32 From then on, the window of Si/Al ratios from 25 to 50 was commonly considered a prerequisite for successful desilication and it has been often noted as the main drawback of this post-synthetic route.5,7 Another intrinsic characteristic of alkaline treatment is the decreased Si/Al ratio in the solid due to the selective silicon dissolution. The high selectively to Si was tentatively attributed to the realumination of extracted framework Al on the external surface of the zeolite. The nature of these aluminium species is not precisely understood, which is remarkable since it could have a significant influence in catalysed reactions. Finally, the generated mesopores are disordered and result into relatively broad size distributions, differently to the more regular distributions achieved by organosilane-directed syntheses.33
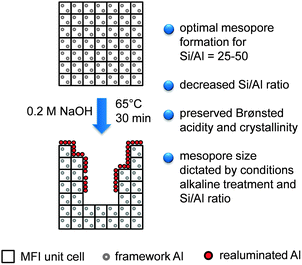 | ||
| Fig. 1 Schematic representation of MFI desilication by NaOH treatment (based on ref. 10). Standard treatment conditions are noted near the arrow. | ||
A first on-topic review was published in 2006,10 focussing on the synthesis of mesoporous ZSM-5 by alkaline treatment. In that work, the less favourable features of desilication were identified but not resolved. Moreover, the scarcity of catalytic data prevented a quantitative assessment of the benefits of hierarchical zeolites in catalysed reactions. Herein we focus on recent key contributions that have enabled to overcome many of the above-mentioned drawbacks and have turned desilication highly versatile, controllable, and scalable. The crucial role that structure–property–function relationships and descriptors play in the design of hierarchical zeolite catalysts is stressed. Future directions in the topic are devised.
2. New zeolite frameworks
In the last few years, the number of zeolite frameworks prepared in a hierarchical form by desilication has increased significantly. Table 1 shows that, with respect to the previous review,10 8 new framework topologies were included, speaking in favour of the remarkable versatility of the post-synthetic approach. The entries in this table represent the zeolites most widely used in refining and (petro)chemical industry and/or zeolites of small pore size or low channel dimensionality. The latter zeolites are expected to benefit dramatically from the introduction of auxiliary mesoporosity. In line with Fig. 1, all zeolites comprised a Si/Al ratio within or near the 25–50 range prior to alkaline treatment.| Frameworka | Pore size/Å | Molar Si/Al ratio/— | Crystal sizeb/μm | Type of mesoporosity | Ref. |
|---|---|---|---|---|---|
| a Dimensionality of the micropore system in parentheses. b Crystal morphology in parentheses. c MR = membered ring. d Not available. e Prior to alkaline treatment, the parent Y zeolite (Si/Al ≈ 3) was steam-treated and acid-leached, that is, desilication of the pristine Y zeolite was not attempted. | |||||
| MFI (3D) | 5.1 × 5.5, 5.3 × 5.6 | 25–40 | 0.5 (agglomerates) | Intracrystalline, 6–10 nm | 30 |
| MTW (1D) | 5.6 × 6.0 | 58 | 0.7 (agglomerates) | Intracrystalline, 15–30 nm | 37 |
| MOR (1D) | 6.5 × 7.0, 2.6 × 5.7 | 30 | 4 × 2 × 2 (ellipsoidal particles) | Intracrystalline, 10 nm | 31 |
| BEA (3D) | 7.1 × 7.3, 5.6 × 5.6 | 35 | 3 (truncated bi-pyramids) | Intracrystalline, 2–4 nm | 38 |
| AST (0D) | Apertures formed by 6-MRc | 31 | 0.15 (poorly facetted crystals) | Intercrystalline, 5 nm | 39 |
| FER (2D) | 5.4 × 4.2, 3.5 × 4.8 | 29 | 2 × 2 × 0.1 (platelets) | Inter- and intracrystalline, uncentered | 40 |
| MWW (2D) | 4.0 × 5.5, 4.1 × 5.1 | 40 | 1.25 × 1.25 × 0.1 (platelets) | n.a.d | 41 |
| IFR (1D) | 6.2 × 7.2 | 32 | 1.5 × 0.2 × 0.2 (beams) | Intracrystalline, 3–10 nm | 34 |
| STF (1D) | 5.4 × 5.7 | 29 | 0.5 (agglomerates) | n.a. | 42 |
| CHA (3D) | 3.8 × 3.8 | 14 | 10 (cubes) | Intracrystalline, 2–3 nm | 43 |
| FAU e (3D) | 7.4 × 7.4 | 28e | 0.4 (truncated bi-pyramids) | Intracrystalline, 3 nm | 44 |
| TON (1D) | 4.6 × 5.7 | 42 | 0.15 × 0.04 × 0.04 (nanorods) | Inter- and intracrystalline, uncentered | 45 |
3. Structure–property–function relationships
The establishment of structure–property–function (hereafter spf) relationships is of vital importance to increase the understanding of the nature of mesoporous zeolites and to further optimise their application-oriented design. The most direct way to obtain such relationships should be by associating their key property (mesoporosity) with their application (catalysis). For example, Liet al.34 studied the aromatisation and isomerisation of 1-hexene on parent and alkaline-treated ZSM-5 zeolites (Fig. 2a), and related the mesopore volume (Vmeso) to the aromatisation stability. A maximum was evidenced, which was attributed to the relatively low micropore volume (Vmicro) obtained for the most severely-treated zeolites. van Laak et al.35 prepared mesoporous mordenites by alkaline treatment (after dealumination) and tested them in benzene alkylation with propylene to cumene. Fig. 2b reveals that a linear trend was obtained between the initial reaction rate and the external surface area (Smeso). Verboekend et al.36 studied pyrolysis of low-density polypropylene (LDPE) using hierarchical ITQ-4 and observed an improved catalytic performance (lower T10 in Fig. 2c). However, when the parent zeolite was exposed to a too severe alkaline treatment (sample AT-5), the performance deteriorated, which was attributed to a dramatic drop in Vmicro. These works indicated that the catalytic superiority of the alkaline-treated zeolite depends on a balance between the introduced mesoporosity and the reduction of the micropore volume. This balance should be highly dependent on the nature of the involved catalytic reaction (see Section 4).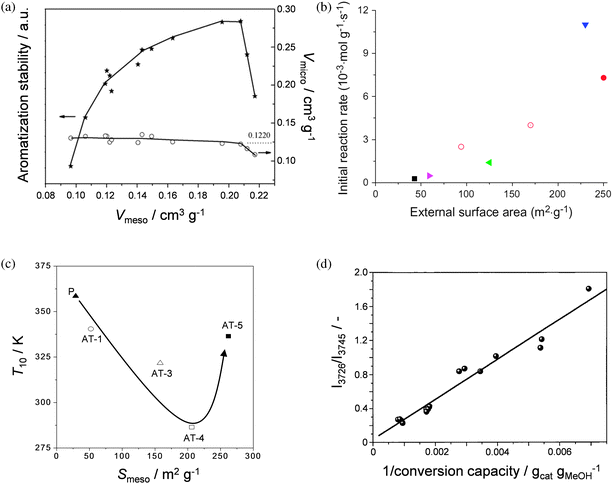 | ||
| Fig. 2 Relation of zeolite porous properties to catalytic parameters. (a) The 1-hexene aromatisation stability versus the micro- and mesopore volume of ZSM-5.34 The stability is maximised for high mesopore volumes coupled to a microporosity larger than ca. 0.12 cm3 g−1. (b) Initial reaction rate in benzene alkylation with propylene as a function of the external surface area of mordenite.35 (c) Correlation between the catalytic activity (T10, temperature at 10% conversion) and the mesopore surface area of parent (P) and alkaline-treated (AT-1, AT-3, AT-4, AT-5) ITQ-4.36 The catalytic activity increases with Smeso up to AT-4. Highly-mesoporous AT-5 proved less active due to the reduced micropore volume. (d) The intensity of the infrared band attributed to crystals defects (3726 cm−1) displays a clear relationship of the conversion of methanol to hydrocarbons over ZSM-5.48 The intensity of the band at 3726 cm−1 is normalised by the intensity of the band attributed to terminal silanols (3745 cm−1). The latter band increases upon introduction of mesoporosity by alkaline treatment. (a) and (b) reproduced with permission from Elsevier, (c) reproduced with permission from Wiley-VCH Verlag. | ||
Although proper relationships can be obtained by linking catalytic performance to the introduced mesopore surface or volume, also other properties can have prominent influence. As shown above, microporosity can be compromised upon alkaline treatment, which proved to detrimentally affect catalysed reactions. Another example of a secondary influence of alkaline treatment is related to the realumination of the zeolites external surface (Fig. 1). It was reported that the realuminated Al, besides decreasing the overall Si/Al ratio, gives rise to Lewis acid sites,36,46,47 which can influence adsorption and catalysis (vide infra). Beato et al.48 claimed that the amount of defects in the zeolite reduced substantially upon alkaline treatment of ZSM-5. It was shown that the infrared band representative of internal/defective silanols (3726 cm−1) correlates to the conversion capacity of methanol to hydrocarbons (Fig. 2d). Unfortunately, the relation of the developed mesoporosity to the catalytic performance was not reported. We stress that experiments decoupling the introduction of mesoporosity from secondary influences on, for example, Vmicro, acidity, and the abundance of defects, are a must in order to precisely understand the catalytic benefits induced by alkaline treatment. This statement is not limited to desilication but applies to any method to prepare hierarchical zeolites.
Apart from relating properties directly to catalytic performance, it is important to consider the changed zeolite's constitution to other critical functions as adsorption and diffusion. Efforts concerning the establishment of spf-relationships on mesoporous ITQ-4 zeolites showed that the rate of n-butane uptake increased for alkaline-treated samples, which was assigned to the enhanced diffusion due to the presence of intracrystalline mesopores (Fig. 3a). The same work demonstrated a dual tendency of the propene uptake as a function of pressure. At relatively low pressures it was favoured on the parent sample, which was attributed to its intrinsic microporous character. On the other hand, above 1 kPa, the adsorption is favoured on the alkaline-treated samples, which was related to their increased Lewis acidity (Fig. 3b).36 This result suggests that especially reactants that can interact with Lewis sites could display altered diffusion inside the mesoporous zeolite crystals.
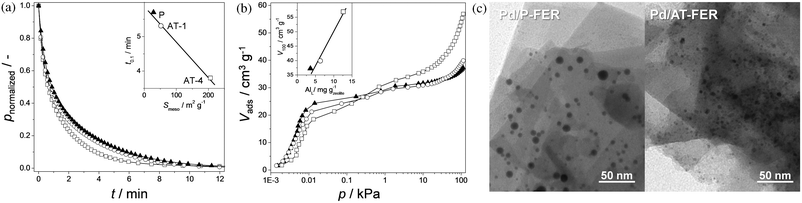 | ||
| Fig. 3 Influence of mesoporosity on the dynamic and static adsorption of hydrocarbons and on the dispersion of deposited metal particles. (a) Normalised pressure profiles during the transient uptake of n-butane at 25 °C on ITQ-4 samples.36 The time at which a fraction of 90% is adsorbed (t0.1) relates linearly to the mesopore surface area (inset in a). (b) Adsorption isotherms of propene at 25 °C on ITQ-4 samples.36 In the low-pressure range (p < 0.1 kPa), the uptake is highest for the untreated zeolite (solid triangle). In the high-pressure range (p > 1 kPa), the uptake is highest for the most mesoporous sample (open square). The inset shows that the total uptake (V100) increases linearly with the Lewis acidity. (c) TEM images of Pd/ferrierite samples.49 The introduction of mesopores (Pd/AT-FER) led to the formation of smaller palladium particles. (a) and (b) reproduced with permission from Wiley-VCH Verlag, (c) reproduced with permission from the American Chemical Society. | ||
The functions of mesopores prove not restricted to overcoming diffusional constraints and increasing accessibility. Work on alkaline-treated ferrierite49 showed that the presence of mesoporosity leads to a reduced size of deposited metallic particles (Fig. 3c). Other recent contributions showed that the external surface of zeolites of alkaline-treated ZSM-5 zeolites could successfully be silanised, which facilitated the immobilisation of lipase enzymes for further use as biocatalyst.50,51
4. Descriptors
Significant progress concerning the in-depth characterisation and categorisation of hierarchical zeolites has been made in the form of descriptors. These tools help to quantify and characterise mesoporous zeolites by the critical evaluation and combination of certain properties. For example, the accessibility index (ACI) enables to standardise acid site accessibility in zeolites.52,53 The ACI is determined by relating the amount of acid sites probed by alkylpyridines of different sizes to the total amount of acid sites in the zeolite. For example, pyridine (0.57 nm), able to enter the ZSM-5 micropore (0.56 nm), can probe all sites resulting in an ACI near unity. On the other hand, the more bulky lutidine (0.67 nm) and collidine (0.74 nm) can access only part of the acid sites. The introduction of mesoporosity in ZSM-5 by desilication increased the adsorption for the latter two probes, hereby evidencing an increased accessibility of the micropore volume (Fig. 4).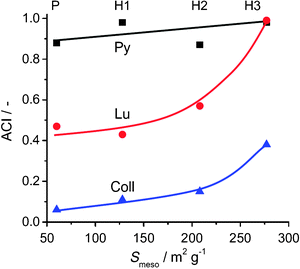 | ||
| Fig. 4 Accessibility index (ACI) of pyridine (Py), lutidine (Lu), and collidine (Coll) versus the mesopore surface area of ZSM-5.52 For pyridine, a full accessibility of the parent (P) and alkaline-treated zeolites (H1, H2, H3) is evidenced (ACI ≈ 1). For the larger molecules lutidine and collidine accessibility is limited to 0.5 and 0.1, respectively, in the parent MFI zeolite. Upon the introduction of intracrystalline mesopores by alkaline treatment the accessibility increases to unity for lutidine, whereas for collidine the ACI increased about 4-fold. Reprinted with permission from Elsevier. | ||
As mentioned in Section 3, the introduction of mesoporosity in hierarchical zeolites is frequently coupled to a lowered micropore volume. This trend sparked the development of the generic tool termed the hierarchy factor (HF).54 The HF is expressed as the relative mesopore surface area (Smeso/Stotal) multiplied by a relative microporosity (Vmicro/Vtotal), and can be used to classify the porous characteristics of any material. Accordingly, the HF enabled to compare hierarchical zeolites independent of the synthetic methodology (Fig. 5a). The presence of alkaline-treated samples scattered over the full length and width of the graph exemplifies the large tuning ability of desilication. Pérez-Ramírez et al.54 tailored, by alkaline treatment in the presence of tetrapropyl ammonium cations (TPA+), the porous properties of mesoporous ZSM-5 and obtained accordingly a gradual variation of HFs (Fig. 5b). The original hierarchy factor has been recently complemented with a variant specific to framework and preparative approach: the indexed hierarchy factor (IHF).55 In the latter case, the normalisation proceeds by dividing Vmicro and Smeso, not by the total pore volume and surface area, but by their maximum values, i.e. IHF = ((Vmicro/Vmaxmicro) × (Smeso/Smaxmeso)).
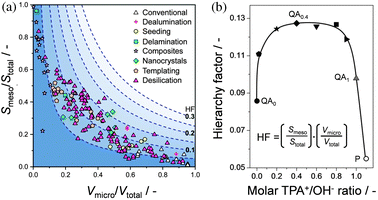 | ||
| Fig. 5 (a) The hierarchy factor (HF), determined as the product (Vmicro/Vtotal) and (Smeso/Stotal), plotted in a contour plot as a function of the relative mesoporous surface area and the relative microporous volume of different zeolite types prepared by different methods.54 The nature of each hierarchical zeolite dictates its location in the plot. (b) HFs of the parent (P) and ZSM-5 zeolites obtained by desilication in the absence (QA0) and in the presence of tetrapropyl ammonium (QA0.4, QA1).54 HFs are particularly high in zeolites treated with tetrapropyl ammonium (TPA+)-containing solutions in the TPA+/OH− range of 0.2–0.6, attending to the high mesopore surface areas coupled to preserved micropore volumes. Reprinted with permission from Wiley-VCH Verlag. | ||
Although the hierarchy factor enables to categorise efficiently zeolites by their porosity, its true value becomes clear when related to catalytic parameters. For example, catalytic tests using the zeolites described in Fig. 5b in benzene alkylation with ethylene resulted into a straight line of productivity versusHF (Fig. 6a). It was concluded that the reaction is equally sensitive to both the introduced Smeso and the sacrificed Vmicro. Zheng et al.56 found similar linear relationships with the HF of conversion in the catalytic cracking of isopropylbenzene. Work by Verboekend et al.36 showed a different relationship of the hierarchy factor versus the catalytic activity of desilicated ITQ-4 zeolites in the pyrolysis of LDPE (Fig. 6b). Alkaline treatment of ITQ-4 samples led to increased HFs (AT-1, AT-3), attending to relatively large development of mesoporosity. On the other hand, when the samples were more severely treated (higher alkalinity), the HF lowered due to the relatively strong decrease of the micropore volume (AT-4, AT-5). However, instead of a linear, a clockwise trend of the T10versus the HF was obtained, suggesting a strong dependency of this reaction on the mesopore surface area. It should be stressed that the HF describes purely the porous properties and does not take other changes, e.g. composition and acidity, into account. Therefore, correlation of the HF to catalytic properties is suitable mostly when the porosity is the dominant factor.
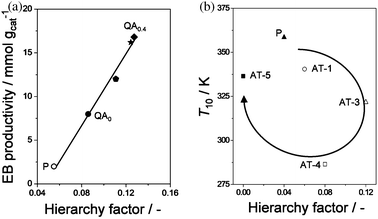 | ||
| Fig. 6 The hierarchy factor coupled to catalysis. (a) The productivity of ethylbenzene (EB) during benzene alkylation over ZSM-5 shows a linear dependence with the hierarchy factor.54 This trend indicates that the latter catalytic parameter is equally benefited from a high micropore volume as mesopore surface area. (b) Catalytic activity (T10) of ITQ-4 in LDPE pyrolysis.36 A clockwise evolution of the T10versus the HF is obtained, which proves that the relative mesoporosity contributes more strongly in this particular reaction than the relative microporosity. Reproduced with permission from Wiley-VCH Verlag. | ||
Apart from a possible reduction in micropore volume, the associated weight loss upon desilication should not be overlooked. Since mesopores are created by the partial dissolution of the crystal, a substantial weight loss (typically 30% upon standard alkaline treatment on ZSM-5)10 appears a prerequisite to obtain a high mesopore surface. The ‘desilication efficiency’ has been introduced to quantify the degree of zeolite dissolution upon introducing the external surface area.45 This efficiency, expressed as the external surface area developed per % of weight loss (Fig. 7), proved to depend on at least three factors: the Si/Al ratio in the parent zeolite, the crystal morphology, and the conditions or sequence of the applied treatment(s).
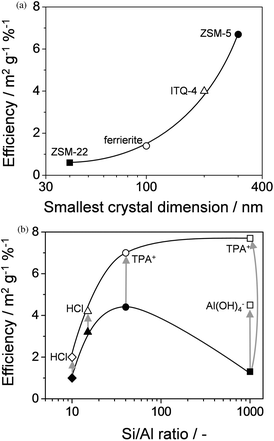 | ||
| Fig. 7 The desilication efficiency, expressed as external surface introduced per percent of weight loss (m2 g−1 %−1) as a function of (a) the smallest crystal dimension of different zeolites,45 and (b) the Si/Al ratio for various MFI zeolites. With a decrease of the smallest crystal dimension the efficiency decreases substantially (see Section 5). The efficiency of conventional alkaline-treatment is highest for zeolites with Si/Al ratio in the range of 25–50 (solid symbols in b). Increased efficiencies (open symbols in b) are obtained by modification of the experimental protocol by either subsequent acid washing (H+) (see Section 8), or the use of external pore directing agents as tetrapropyl ammonium (TPA+) or Al(OH)4− (see Section 6). | ||
5. Zeolite morphology
It is established that the introduction of mesopores by desilication in large crystals (prepared with TPA+ as template) can be inefficient because of Al-zoning.19,57 Recent focus was placed on desilication of zeolites with different morphological features, i.e. small crystals and zeolites with defects and/or intergrowths.Verboekend et al.45 suggested that the limited development of mesopore surface area (maximum ca. 100 m2 g−1) and low desilication efficiency obtained upon alkaline treatment of ZSM-22 and ferrierite zeolites should be related to their crystal morphology (see Table 1). Fig. 7a shows that the desilication efficiency decreases dramatically upon reducing the smallest crystal dimension. It proved that especially desilication of zeolites with platelet (ferrierite) or needle crystals (ZSM-22) is less favourable since the larger part of the created mesopore surface is derived from intercrystalline mesoporosity. Likely, the relatively limited degree of mesoporosity obtained by van Laak et al.58 (maximum 106 m2 g−1) in the alkaline treatment of mordenite was related to the small crystal dimensions. We emphasise that unfavourable crystal morphologies are particularly common for zeolites with micropore networks of limited dimensionality, hence relatively limited accessible pore mouths. In the latter cases the micropore channels are typically grown along the longest dimensions of the crystal, implying an even lower number of pore mouths. Therefore, the resulting ‘less-efficient’ introduction of Smeso upon alkaline treatment should nevertheless increase the access to the micropores significantly.
Svelle et al.59 showed that the amount of intergrowths in ZSM-5 crystals determines the characteristics of desilication (Fig. 8). A large amount of intergrowths reduces the importance of the framework Si/Al of the parent zeolite and leads to mesopore formation derived from defect removal. Work done by Haldor Topsøe confirms that both the morphology and crystals defects have a major influence on the desilication behaviour.48 Clearly, the optimal alkaline treatment strongly depends on the unique nature of each particular batch of zeolites. For example, properties to be taken into account include framework type, Si/Al ratio, Al distribution, crystal morphology, and relative abundance of defects.
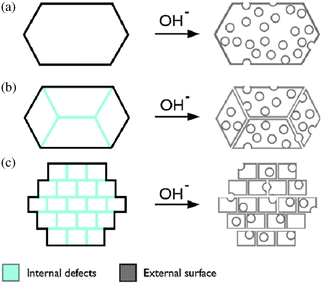 | ||
| Fig. 8 Schematic representation of different mesopore formation mechanisms.59 (a) Little intergrowths or defects require the optimal Si/Al ratio = 20–50 to introduce mesopores. (c) In the case many intergrowths are present, the Si/Al ratio is less important and the mesopores are mostly formed due to intergrowths/defect removal. (b) Intermediate cases lead to a combination of the two. Reproduced with permission from Elsevier. | ||
6. External pore-directing agents
The use of external pore-directing agents proved a great development in the control over the alkaline treatment. First efforts pointed out that alkylammonium hydroxides, i.e. common structure directing agents in zeolite synthesis, could also be used as alternative bases.60 The absence of inorganic cations in the alkaline solution enabled hereby the one-pot introduction of intracrystalline mesopores and ion-exchange.61 Later, Pérez-Ramírez et al.54 proved that the specific interaction of tetraalkylammonium cations (TAAs) with the zeolite surface under alkaline conditions can provide a tuneable protection against zeolite dissolution. This protection enabled to prepare zeolites with similar mesopore surface areas compared to standard alkaline treatment, but with largely preserved micropore volumes and, concomitantly, higher HFs (see Section 4). Moreover, higher yields and control over the size of the mesopores could be achieved. In the latter sense, the TAA+ cations function as ‘pore-growth moderators’. Additionally, in case TPA+ was used, a higher desilication efficiency was evidenced, attending to the similar Smeso but higher yield (Fig. 7b). Nevertheless, at this stage, framework Al (or other framework trivalent heteroatoms)62 was still considered pore-directing agent.Verboekend and Pérez-Ramírez63 explored the deliberate addition of ‘external’ agents to the alkaline solution in more detail in the synthesis of mesoporous all-silica MFI, that is hierarchical silicalite-1. By addition of Al(OH)4− to the alkaline solution, they were able to steer the otherwise-uncontrolled dissolution to the formation of intracrystalline mesopores (Fig. 9). It hereby was proven that framework aluminium is not a prerequisite to obtain highly-mesoporous zeolites by desilication. By making use of different ‘external’ PDAs (Al(OH)4−, Ga(OH)4−, and TAAs) the authors deduced that the specific interaction between the pore-directing agent and the zeolite surface leads to a partial protection of the zeolite's surface, which directs the mesopore formation. They concluded that, in conventional desilication, framework Al is first dissolved from the zeolite after which it re-aluminates back onto the zeolite's external surface. There it fulfils the pore-directing role leading to the controlled leaching of the zeolite crystal. By using external PDAs, alkaline treatments could now be applied to introduce mesoporosity in zeolites with compositional Si/Al ratios of 25 till infinite, hereby substantially increasing the versatility of desilication. Furthermore, the addition of different amounts and types of PDAs enabled to obtain various mesopore sizes. However, we must emphasise that the size and the uniformity of mesopores has not yet been found to be of particular advantage for access- or diffusion-limited reactions. Rather, the synthetic route should secure the interconnectivity of the meso- and micropore networks. Of course, the enhanced alkaline treatment on silicate-1 inferred an increased desilication efficiency (Fig. 7b). The outcome of this investigation is graphically summarised in Fig. 10; the successful introduction of intracrystalline mesopore namely depends on three aspects: the zeolite, the PDA, and the treatment conditions.
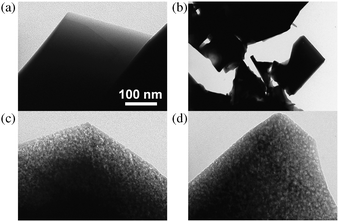 | ||
| Fig. 9 TEM micrographs of parent and alkaline-treated silicalite-1: (a) represents the parent zeolite, (b) standard alkaline treatment (AT), (c) AT including Al(OH)4−, and (d) AT including TPA+. The scale bar in (a) applies also to (b–d). The addition of PDA enabled the control of the dissolution process which led to the formation of intracrystalline mesopores of various diameters. Reproduced after ref. 63 with permission from Wiley-VCH Verlag. | ||
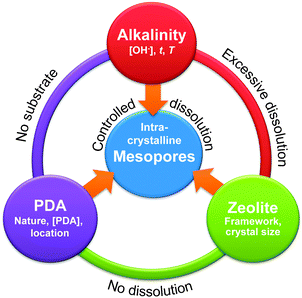 | ||
| Fig. 10 Schematic representation of the three main parameters required to form mesopores by desilication. Only the correct combination of alkalinity, pore-directing agent (PDA), and zeolite results in controlled dissolution of the zeolite leading to intracrystalline mesoporosity. Reproduced after ref. 63 with permission from Wiley-VCH Verlag. | ||
7. Template-containing zeolites
The desilication of template-containing zeolites was first explored by Čižmek et al.64 They showed that template-containing zeolites require higher alkalinities to dissolve compared to template-free zeolites. Pérez-Ramírez et al.65 used this knowledge to prepare beta zeolites containing different degrees of template by controlled calcination. Subsequent alkaline treatments (at 0.2 M NaOH) followed by complete template removal resulted in hierarchical beta crystals containing tailored degrees of mesoporosity (Fig. 11).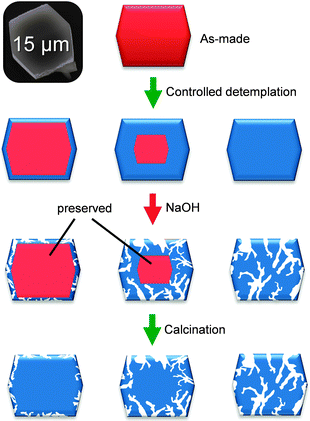 | ||
| Fig. 11 Schematic representation of the partial detemplation and desilication to tailor mesoporosity development in zeolite crystals. By preserving part of the template in the crystals part of the crystal is ‘protected’ during (standard) alkaline treatment. | ||
van Laak et al.66 explored the influence on porosity of concentrated alkaline solutions (1 M NaOH) on template-containing beta, ZSM-5, and ZSM-12 zeolites. The treatments led to the formation of large intercrystalline mesopores (ca. 40 nm), but substantially lower degrees of mesoporosity compared to standard alkaline treatment. Nonetheless, the advantage of the latter approach is that the realumination of Al was prevented, which in principle enabled to decouple the introduction of mesopores by alkaline treatment from changes in acidity. Possibly, in the latter scenario, the pore formation was directed by released template molecules. Interestingly, besides playing a crucial role in the conventional synthesis of zeolites, tetraalkylammonium cations have contributed dramatically making desilication more versatile and tuneable.
8. Sequential post-synthetic treatments
The use of sequential post-synthetic treatments has long been applied to improve zeolite properties, both in research laboratories and in the industrial scale.67–69 Probably the best known example is the modification of zeolite Y by sequential steam and acid treatment to increase stability.1,69 Alkaline treatments (aimed at the introduction of secondary porosity) have also been performed on numerous occasions in combination with other treatments. Groen et al.70 were the first to report sequences of steam, acid, and alkaline treatments aimed at creating superior mesoporous zeolites. They showed that the highly-mesoporous alkaline-treated zeolites could successfully be steamed, but that the introduction of mesoporosity in the steamed zeolite was inhibited by the presence of extra-framework Al species.Later, the use of sequential acid and alkaline treatment led to the synthesis of hierarchical zeolites starting from parent samples with framework Si/Al ratios far below the established range of 25–50 (Fig. 12g and i). In these cases the zeolites were exposed to a severe acid treatment to increase the Si/Al ratio to within the optimal range, facilitating the introduction of mesoporosity by the successive alkaline treatment. For example, Liet al.71 and van Laak et al.35 used the latter approach to synthesise mesoporous mordenite. They started from a parent with Si/Al ≈ 13, and increasing it to Si/Al ≈ 28, after which a subsequent alkaline treatment led to the introduction of mesoporosity, as well as the typical reduction in Si/Al ratio (Fig. 12d–f). A similar approach was followed by de Jong et al.44 in the preparation of mesoporous Y by desilication. These authors used a commercial steamed and acid-leached Y, which comprised a bulk Si/Al ratio of 28 (Table 1), that is, within the optimal range for desilication. Although successful in the introduction of intracrystalline mesopores, sequential acid and alkaline treatments do not yield mesoporous zeolites of high (framework) Al content.
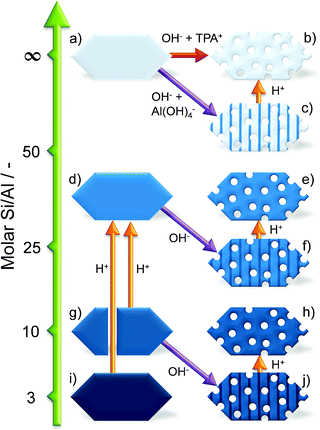 | ||
| Fig. 12 Overview of strategies aimed at introducing intracrystalline mesopores in zeolites by desilication. The y-axis indicates the influence of either acid (H+) or alkaline (OH−) treatments on the Si/Al ratio. Alkaline treatment typically results in the increase of the Si/Al ratio by realumination of the external surface (indicated by zeolites with the striped pattern, see c, f, and j). Acid treatments are applied to remove this extra-framework Al hereby restoring the intrinsic Si/Al ratio, or to remove framework Al to facilitate subsequent alkaline treatment of a more siliceous framework (going from g and i to d). In the case of all-silica zeolites, external PDAs are required to introduce intracrystalline mesoporosity. | ||
Fernandez et al.72 showed that a subsequent acid wash can be used to uncouple the introduction of mesoporosity from the alkaline-induced surface enrichment of aluminium (Fig. 12e). The acid wash led to the removal of the surface-deposited Al, which in turn restored the Si/Al ratio and reduced the amount of Lewis acid sites. Consequently, the acid-washed sample displayed an increased selectivity in the isomerisation of o-xylene to p-xylene. Possibly, the lower p-xylene selectivity for the unwashed hierarchical sample was due to the interaction of the benzene rings with the generated Lewis acidity.
The synthesis of mesoporous all-silica zeolites by desilication is illustrated at the top of Fig. 12. Obviously, using tetraalkyl ammonium cations as PDA, the Si/Al ratio remains unaltered upon alkaline treatment (Fig. 12a and b). However, when Al(OH)4− is used as PDA the Si/Al drops due to the realuminated Al (Fig. 12c). Again, a subsequent acid wash can be used to increase the Si/Al ratio towards that of the parent.
Other work by Pérez-Ramírez et al. revealed that besides generating Lewis acidity, the Al-rich debris can have a dramatic influence on both porosity and crystallinity.45,49,55 For example, alkaline treatments on ZSM-22 (Si/Al = 42) led to a severe drop in micropore volume upon alkaline treatment.45 The latter was attributed to the uni-directionality of the elliptical 10MR micropores, which implied a high tendency to be blocked. A sequential acid wash restored the micropore volume nearly completely. Later work55 included desilication of MFI zeolites as a function of both the Si/Al ratio of the parent zeolite and the concentration of the applied aqueous NaOH solution. This approach enabled to introduce mesopores in a range much wider than the preferred range established by Groen et al.32 (Fig. 13a). Moreover, in a case study on high-alumina zeolites (Si/Al = 10–20),55 it was shown that the high Al content of the zeolites led to a large amount of amorphous Al-rich debris that, besides reducing the Si/Al ratio (Fig. 12j), blocked large part of the meso- and microporosity (hence Brønsted acidity) and reduced crystallinity. A proper acid wash a dilute aqueous HCl solution enabled to remove these debris, hereby restoring the Si/Al ratio, both meso- and microporosity, the acidity, and part of the crystallinity.
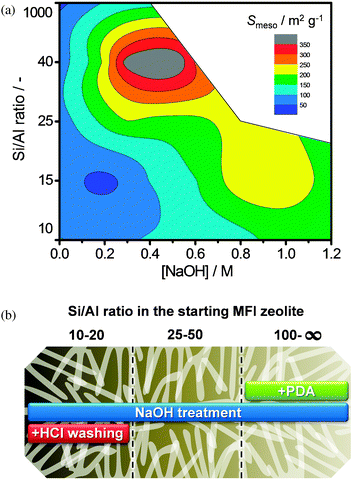 | ||
| Fig. 13 (a) Contour plot illustrating the development of mesoporosity in MFI zeolites, as a function of Si/Al ratio (y-axis) and concentration NaOH (x-axis). The Si/Al range leading to substantial (>200 m2 g−1) mesoporosity is significantly larger as originally established by Groen et al.32 (b) Schematic representation of the protocol for the desilication of MFI zeolites of different framework Si/Al ratios. In the 25–50 range alkaline treatment using aqueous NaOH suffices. High-Al zeolites require sequential alkaline and acid treatment, while high-Si zeolites require the addition of external pore directing agents to the alkaline solution. Reproduced after ref. 55 with permission from the American Chemical Society. | ||
The use of sequential alkaline and acid treatment enabled the successful alkaline treatment of zeolites with Si/Al ratios as low as 10. Herewith, the application range of desilication on MFI was extended to 10–1000, i.e. practically the full compositional spectrum (Fig. 13b). This implies that besides high-silica zeolites, also Al-rich zeolites should now be considered as candidates for desilication. Naturally, the desilication protocol should be carefully adjusted to the Si/Al ratio of the parent zeolite. When the latter is assured, it is clear that significantly higher desilication efficiencies can be attained (Fig. 7b).
9. Comparison to other methods
Recent works have compared the properties and efficiency of hierarchical zeolites obtained by various approaches. Particular focus was on the comparison of mesoporous ZSM-5 zeolites derived from desilication and carbon templating and catalytic testing in the conversion of methanol to hydrocarbons (MTH). Both Svelle et al.59 and Sazama et al.73 concluded that zeolites prepared by secondary templating display a lot of defect sites. In contrast, zeolites prepared by desilication proved relatively free of defects, in line with the findings of Beato et al.48Catalytic testing evidenced that the alkaline-treated zeolites displayed the slowest rate of deactivation. Moreover, the data provided by Svelle et al. showed that both the conversion capacity and the deactivation rate relate well to the corresponding IHFs (Fig. 14a and b).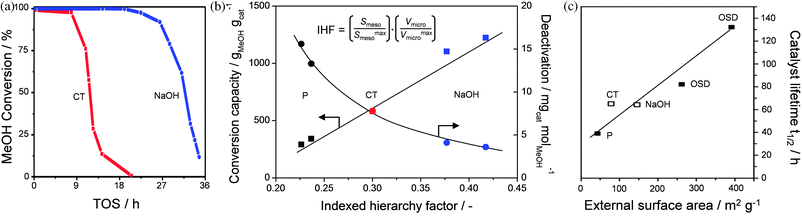 | ||
| Fig. 14 Comparison of hierarchical ZSM-5 zeolites obtained by different methods in the conversion of methanol to hydrocarbons. (a) Conversion as a function of time on stream (TOS) over mesopores zeolites prepared by carbon templating (CT) and desilication (NaOH).73 (b) Conversion capacity and deactivation as a function of the indexed hierarchy factor (equation included) of parent (P) and hierarchical ZSM-5 zeolites prepared by carbon templating and desilication.59 (c) Correlation between catalyst lifetime (t1/2) and external surface areas of zeolites prepared by alkaline treatment, carbon templating and organosilane-directed synthesis (OSD).74 (a) and (c) reproduced with permission from Elsevier, (b) produced using data from ref. 59. | ||
Kim et al.74 compared mesoporous zeolites derived organosilane-directed synthesis, carbon templating, and desilication, and found a good correlation between the external surface area and the lifetime of ZSM-5 in MTH (Fig. 14c). They observed that the base-treated zeolite showed moderate performance compared to the organosilane-directed ZSM-5 and similar performance to the carbon-templated zeolite. The relatively poor performance of the base-treated sample could be related to sub-optimal treatment conditions applied, i.e. a mild alkalinity employed (0.2 M NaOH) coupled to a relatively low Si/Al ratio (13) (see Fig. 13a). Liet al.71 also compared different approaches including dealumination, hard- and soft-templating and sequential dealumination and desilication. They observed that the desilicated samples displayed higher reactivity and large number of Brønsted sites. Although they are not straightforward to directly compare, these works suggest that the efficiency of the mesopores obtained upon desilication is quite high, particularly compared to those generated by carbon templating.
10. Scale up
Besides leading to efficient pore structures, the preparation of hierarchical zeolites by desilication is of high relevance due to its scalable nature. In fact, the only scale up of mesoporous zeolites reported in the open literature was performed using alkaline treatments. Groen et al.17 executed alkaline treatments using a 6-L lab reactor producing about 500 g of mesoporous ZSM-5. They also executed alkaline treatments on binder-containing ZSM-5 extrudates, and observed that mesoporosity could successfully be introduced. However, the influence of the alkaline treatment on the inorganic binder could not be ascertained, because the amount and its nature were unknown. Additionally, the performed experiments made use of highly controllable lab facilities, i.e. not industrial equipment.Unpublished work by our group has evidenced that the desilication protocol can be successfully executed on zeolite powders using pilot-scale reactors (50 L and 1.5 m3). The obtained products had the same properties irrespective of the scale of the alkaline treatment. These results will be shortly disclosed, as well as the influence of binder addition in the preparation of shaped hierarchical zeolites.
11. Conclusions and outlook
In the last several years major progress has been achieved in synthesis and application of mesoporous zeolites prepared by desilication, both in terms of reported benefits in catalysed reactions and general understanding of the desilication mechanism. We therefore expect the industrial application of these exciting materials within few years, that is, if they are not applied in a large scale already. Nevertheless, a number of subjects deserve more dedicated study.For example, some fundamental issues concerning the mesopore formation by desilication have not been fully addressed. Although some general rules and guidelines were devised, the process of mesopore formation on a molecular level is still unclear. Both techniques with sufficient spatial and time resolution as well as theoretical studies could shed more light on this. The exact nature and moreover the catalytic potential of the aluminium covering the external surface has not been identified. Besides, it is worth mentioning that most fundamental studies of desilication have focussed on the MFI framework, particularly ZSM-5 with Si/Al ratios of about 25–50. It is probable that some of the established theories deviate, or even do not apply at all, for different zeolite families.
The need to expand comprehension of structure–property–function relationships remains. An extended understanding of each part of catalysis, adsorption, transport, reaction, desorption will enable to tune the design towards a targeted catalytic application. Studies of hierarchical zeolites as a function of Smeso and/or mesopore sizes versuscatalytic parameters should help establish the optimal degree and type of mesoporosity for particular reactions. Moreover, whereas the positive influence of stability and conversion are frequently reported and understood, the influence of the introduction of mesopores on the catalytic selectively remains unclear. We expect the decoupling of the introduction of mesoporosity from secondary influences on, for example, micropore volume, acidity, and/or defects, to play a key role.
We anticipate the synthesis of hierarchical zeolites starting from parent samples which were previously considered unsuited for the desilication. For example Si- and Al-rich zeolites may attract increasing attention. This expansion of candidates should go hand in hand with a refinement of post-synthetic tools. For example, the acid wash, used to remove the surface aluminates, may need refinement attending the different framework types and Si/Al ratios. Following an increased amount of types of mesoporous zeolites, we expect a concomitant increase of their application in general. For example, the potential of hierarchical zeolites as improved ion exchangers and adsorbents may constitute another avenue of research. Finally, the functionalisation of mesopore surface area provides a chance to combine the unique characteristics of zeolites to novel applications.
The large-scale synthesis of hierarchical zeolites initiates a shift towards a key step in catalysts design: shaping. The forming of mesoporous zeolites shapes enables the detailed study of a truly hierarchical zeolite catalyst. In the latter sense, each size range comprises a unique function: the zeolite micropores provide the activity; the mesopores facilitate intracrystalline transport; and the shaped geometry enables practical implementation. A crucial aspect lies in the realisation whether the structure–property–function relationships obtained for lab-scale powders apply also for shaped mesoporous zeolites. The latter is in question since shaped catalysts commonly include additives as, for example, binders (Fig. 15).
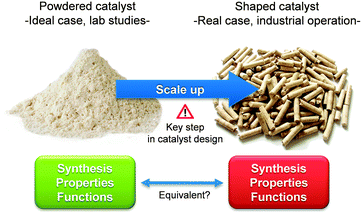 | ||
| Fig. 15 The scale up of hierarchical zeolites is a key step in catalysts design. It remains unsure whether the structure–property–function relationships established from lab-scale powders are equivalent to those of ton-scale shaped catalysts. | ||
Finally, with an open eye to industrialisation, critical economic analyses of any approach aimed at synthesising hierarchical zeolites are encouraged. For example, in the case of desilication, reporting yields is of high relevance. Moreover, reported benefits in catalysis should be evaluated financially and weighed against the increased cost the zeolite production. In the latter case, a close collaboration with industrial partners is of crucial importance.
Acknowledgements
Financial support by ETH Zurich and the Swiss National Science Foundation (Project number 200021-134572) is acknowledged.References
- S. van Donk, A. H. Janssen, J. H. Bitter and K. P. de Jong, Catal. Rev. Sci. Eng., 2003, 45, 297 CrossRef CAS
.
- M. Hartmann, Angew. Chem., Int. Ed., 2004, 116, 6004 CrossRef
.
- Y. Tao, H. Kanoh, L. Abrams and K. Kaneko, Chem. Rev., 2006, 106, 896 CrossRef CAS
.
- J. Čejka and S. Mintova, Catal. Rev. Sci. Eng., 2007, 49, 457 CAS
.
- J. Pérez-Ramírez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530 RSC
.
- W. Schmidt, ChemCatChem, 2009, 1, 53 Search PubMed
.
- R. Chal, C. Gérardin, M. Bulut and S. van Donk, ChemCatChem, 2011, 3, 67 Search PubMed
.
- L. Tosheva and V. P. Valtchev, Chem. Mater., 2005, 17, 2494 CrossRef CAS
.
- K. Egeblad, C. H. Christensen, M. Yu. Kustova and C. H. Christensen, Chem. Mater., 2008, 20, 946 CrossRef CAS
.
- J. C. Groen, L. A. A. Peffer, J. A. Moulijn and J. Pérez-Ramírez, J. Mater. Chem., 2006, 16, 2121 RSC
.
- A. Corma, V. Fornes, S. B. Pergher, Th. L. M. Maesen and J. G. Buglass, Nature, 1998, 396, 353 CrossRef CAS
.
- W. J. Roth and J. Čejka, Catal. Sci. Technol., 2011, 1, 43 RSC
.
- S. Abelló and J. Pérez-Ramírez, Phys. Chem. Chem. Phys., 2009, 11, 2959 RSC
.
- C. C. Pavel, R. Palkovits, F. Schüth and W. Schmidt, J. Catal., 2008, 254, 84 CrossRef CAS
.
- J. L. Casci, Microporous Mesoporous Mater., 2005, 82, 217 CrossRef CAS
.
- S. Zones, Microporous Mesoporous Mater., 2011 DOI:10.1016/j.micromeso.2011.03.039
.
- J. C. Groen, J. A. Moulijn and J. Pérez-Ramírez, Ind. Eng. Chem. Res., 2007, 46, 4193 CrossRef CAS
.
- P. Kortunov, S. Vasenkov, J. Kärger, R. Valiullin, P. Gottschalk, M. F. Elía, M. Perez, M. Stöcker, B. Drescher, G. McElhiney, C. Berger, R. Gläser and J. Weitkamp, J. Am. Chem. Soc., 2005, 127, 13055 CrossRef CAS
.
- J. C. Groen, T. Bach, U. Ziese, A. M. Paulaime-van Donk, K. P. de Jong, J. A. Moulijn and J. Pérez-Ramírez, J. Am. Chem. Soc., 2005, 127, 10792 CrossRef CAS
.
- J. C. Groen, W. Zhu, S. Brouwer, S. J. Huynink, F. Kapteijn, J. A. Moulijn and J. Pérez-Ramírez, J. Am. Chem. Soc., 2007, 129, 355 CrossRef CAS
.
- M. S. Holm, E. Taarning, K. Egeblad and C. H. Christensen, Catal. Today, 2011, 168, 3 CAS
.
-
D. A. Young, US3326797, 1967 Search PubMed
.
-
D. H. Rosback and R. W. Neuzil, US4048111, 1977 Search PubMed
.
-
A. J. Rein, D. D. Saperstein and S. H. Pines, US4134965, 1979 Search PubMed
.
- R. M. Dessau, E. W. Valyocsik and N. H. Goeke, Zeolites, 1992, 12, 776 CrossRef CAS
.
- A. Čižmek, B. Subotić, I. Šmit, A. Tonejc, A. Rosario, F. Crea and A. Nastro, Microporous Mater., 1997, 8, 159 CrossRef
.
- R. Le Van Mao, A. Ramsaran, S. Xiao, J. Yao and V. Semmer, J. Mater. Chem., 1995, 5, 533 RSC
.
- M. Ogura, S. Y. Shinomiya, J. Tateno, Y. Nara, E. Kikuchi and H. Matsukata, Chem. Lett., 2000, 82
.
- J. C. Groen, L. A. A. Peffer, J. A. Moulijn and J. Pérez-Ramírez, Colloids Surf., A, 2004, 241, 53 CrossRef CAS
.
- J. C. Groen, L. A. A. Peffer, J. A. Moulijn and J. Pérez-Ramírez, Microporous Mesoporous Mater., 2004, 69, 29 CrossRef CAS
.
- J. C. Groen, T. Sano, J. A. Moulijn and J. Pérez-Ramírez, J. Catal., 2007, 251, 21 CrossRef CAS
.
- J. C. Groen, J. C. Jansen, J. A. Moulijn and J. Pérez-Ramírez, J. Phys. Chem. B, 2004, 108, 13062 CrossRef CAS
.
- M. Choi, H. S. Cho, R. Srivastava, C. Venkatesan, D.-H. Choi and R. Ryoo, Nat. Mater., 2006, 5, 718 CrossRef CAS
.
- Y. Li, S. Liu, Z. Zhang, S. Xie, C. Zhu and L. Xu, Appl. Catal., A, 2008, 338, 100 CrossRef CAS
.
- A. N. C. van Laak, S. L. Sagala, J. Zečević, H. Friedrich, P. E. de Jongh and K. P. de Jong, J. Catal., 2010, 276, 170 CrossRef CAS
.
- D. Verboekend, J. C. Groen and J. Pérez-Ramírez, Adv. Funct. Mater., 2010, 20, 1441 CrossRef CAS
.
- X. Wei and P. G. Smirniotis, Microporous Mesoporous Mater., 2006, 97, 97 CrossRef CAS
.
- J. C. Groen, S. Abelló, L. A. Villaescusa and J. Pérez-Ramírez, Microporous Mesoporous Mater., 2008, 114, 93 CrossRef CAS
.
- J. Pérez-Ramírez, S. Abelló, L. A. Villaescusa and A. Bonilla, Angew. Chem., Int. Ed., 2008, 47, 7913
.
- A. Bonilla, D. Baudouin and J. Pérez-Ramírez, J. Catal., 2009, 265, 170 CrossRef CAS
.
- Ł. Mokrzycki, B. Sulikowski and Z. Olejniczak, Catal. Lett., 2009, 127, 296 CrossRef CAS
.
- Z. Musilová-Pavlačková, S. I. Zones and J. Čejka, Top. Catal., 2010, 53, 273 CrossRef CAS
.
- L. Sommer, D. Mores, S. Svelle, M. Stöcker, B. M. Weckhuysen and U. Olsbye, Microporous Mesoporous Mater., 2010, 132, 384 CrossRef CAS
.
- K. P. de Jong, J. Zečević, H. Friedrich, P. E. de Jongh, M. Bulut, S. van Donk, R. Kenmogne, A. Finiels, V. Hulea and F. Fajula, Angew. Chem., Int. Ed., 2010, 49, 10074 CrossRef CAS
.
- D. Verboekend, A. M. Chabaneix, K. Thomas, J.-P. Gilson and J. Pérez-Ramírez, CrystEngComm, 2011, 13, 3408 RSC
.
- M. S. Holm, S. Svelle, F. Joensen, P. Beato, C. H. Christensen, S. Bordiga and M. Bjørgen, Appl. Catal., A, 2009, 356, 23 CrossRef CAS
.
- A. van Miltenburg, J. Pawlesa, A. M. Bouzga, N. Žilkova, J. Čejka and M. Stöcker, Top. Catal., 2009, 52, 1190 CrossRef
.
-
P. Beato, WO2010099885, 2010 Search PubMed
.
- D. Verboekend, R. Caicedo-Realpe, A. Bonilla, M. Santiago and J. Pérez-Ramírez, Chem. Mater., 2010, 22, 4679 CrossRef CAS
.
- S. Mitchell, A. Bonilla and J. Pérez-Ramírez, Mater. Chem. Phys., 2011, 127, 278 CrossRef CAS
.
- S. Mitchell and J. Pérez-Ramírez, Catal. Today, 2011, 168, 28 CAS
.
- F. Thibault-Starzyk, I. Stan, S. Abelló, A. Bonilla, K. Thomas, C. Fernandez, J.-P. Gilson and J. Pérez-Ramírez, J. Catal., 2009, 264, 11 CrossRef CAS
.
- D. Verboekend, I. Stan, K. Thomas, L. A. Villaescusa and J. Pérez-Ramírez, Catal. Today., 2010, 152, 11 CrossRef CAS
.
- J. Pérez-Ramírez, D. Verboekend, A. Bonilla and S. Abelló, Adv. Funct. Mater., 2009, 19, 3972 CrossRef CAS
.
- D. Verboekend, S. Mitchell, M. Milina, J. C. Groen and J. Pérez-Ramírez, J. Phys. Chem. C, 2011 DOI:10.1021/jp201671s
.
- J. Zheng, Q. Zeng, Y. Yi, Y. Wang, J. Ma, B. Qin, X. Zhang, W. Sun and R. Li, Catal. Today., 2011, 168, 124 CAS
.
- N. Danilina, F. Krumeich, S. A. Castelanelli and J. A. van Bokhoven, J. Phys. Chem. C, 2010, 114, 6640 CrossRef CAS
.
- A. N. C. van Laak, R. W. Gosselink, S. L. Sagala, J. D. Meeldijk, H. Friedrich, P. E. de Jongh and K. P. de Jong, Appl. Catal., A, 2010, 382, 65 CrossRef CAS
.
- S. Svelle, L. Sommer, K. Barbera, P. N. R. Vennestrøm, U. Olsbye, K. P. Lillerud, S. Bordiga, Y.-H. Pan and P. Beato, Catal. Today, 2011, 168, 38 CAS
.
- S. Abelló, A. Bonilla and J. Pérez-Ramírez, Appl. Catal., A, 2009, 364, 191 CrossRef CAS
.
- M. S. Holm, M. K. Hansen and C. H. Christensen, Eur. J. Inorg. Chem., 2009, 1194 CrossRef CAS
.
- J. C. Groen, R. Caicedo-Realpe, S. Abelló and J. Pérez-Ramírez, Mater. Lett., 2009, 63, 1037 CrossRef CAS
.
- D. Verboekend and J. Pérez-Ramírez, Chem.–Eur. J., 2011, 17, 1137 CrossRef CAS
.
- A. Čižmek, B. Subotić, R. Aiello, F. Crea, A. Nastro and C. Tuoto, Microporous Mater., 1995, 4, 159 CrossRef CAS
.
- J. Pérez-Ramírez, S. Abelló, A. Bonilla and J. C. Groen, Adv. Funct. Mater., 2009, 19, 164 CrossRef CAS
.
- A. N. C. van Laak, L. Zhang, A. N. Parvulescu, P. C. A. Bruijnincx, B. M. Weckhuysen, K. P. de Jong and P. E. de Jongh, Catal. Today, 2011, 168, 48 CAS
.
-
P. E. Eberly, S. M. Laurent and H. E. Robson, US3506400, 1970 Search PubMed
.
-
S. A. Melikyan, A. A. Melikyan, S. V. Gevorkyan and E. S. Khalatyan, SU1407904, 1988 Search PubMed
.
-
R. Eckehart, P. Kleinschmit, A. Kiss and F. Heindl, EP0413138, 1991 Search PubMed
.
- J. C. Groen, J. A. Moulijn and J. Pérez-Ramírez, Microporous Mesoporous Mater., 2005, 87, 153 CrossRef CAS
.
- X. Li, R. Prins and J. A. van Bokhoven, J. Catal., 2009, 262, 257 CrossRef CAS
.
- C. Fernandez, I. Stan, J.-P. Gilson, K. Thomas, A. Vicente, A. Bonilla and J. Pérez-Ramírez, Chem.–Eur. J., 2010, 16, 6224 CrossRef CAS
.
- P. Sazama, B. Wichterlova, J. Dedecek, Z. Tvaruzkova, Z. Musilova, L. Palumbo, S. Sklenak and O. Gonsiorova, Microporous Mesoporous Mater., 2011, 143, 87 CrossRef CAS
.
- J. Kim, M. Choi and R. Ryoo, J. Catal., 2010, 269, 219 CrossRef CAS
.
Footnote |
| † This minireview is dedicated to the memory of Dr Dean A. Young, a pioneer in the improvement of zeolite catalysts by post-synthetic modifications. |
| This journal is © The Royal Society of Chemistry 2011 |
