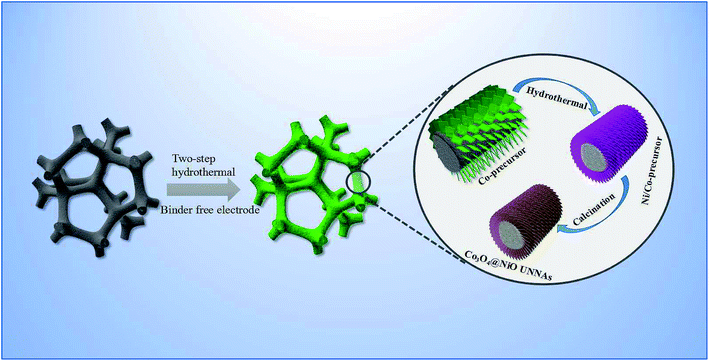
Ultralayered core–shell metal oxide nanosheet arrays for supercapacitors with long-term electrochemical stability†
Dandan
Han
*ab,
Ye
Shen
a,
Yifan
Pan
a,
Zhenyu
Cheng
a,
Yen
Wei
*b,
Guangjian
Zeng
c and
Liucheng
Mao
c
aCollege of Chemistry and Pharmaceutical Engineering, Jilin Institute of Chemical Technology, Jilin 132022, China. E-mail: luckhan2006@163.com; Fax: +86 432 6331 5369; Tel: +86 432 6331 5369
bDepartment of Chemistry, The Tsinghua Center for Frontier Polymer Research, Tsinghua University, Beijing, 100084, China. E-mail: weiyen@tsinghua.edu.cn; Fax: +86 010 6277 1149; Tel: +86 010 6277 2674
cDepartment of Chemistry, Nanchang University, 999 Xuefu Avenue, Nanchang 330031, China
First published on 3rd July 2018
Abstract
High-stability electrodes are highly desirable for practical supercapacitor applications, which is closely linked with the high utilization and degradation of active materials. Herein, core/shell ultralayered Co3O4@NiO nanosheet arrays were developed by a two-step low temperature hydrothermal method. A porous and thin NiO shell was epitaxially grown on the surface of an ultralayered Co3O4 nanosheet. As a supercapacitor electrode material with good electrochemical performance, the prepared ultralayered Co3O4@NiO core/shell nanosheet array electrode demonstrated a notably enhanced specific capacitance (715 F g−1 at 0.5 A g−1) compared with the pure Co3O4 nanosheets. The elegant combination of NiO and Co3O4 nanostructures in the nanosheet arrays showed promising synergistic effects for capacitors with greatly enhanced performance (30.2 W h kg−1 at a power density of 201.5 W h kg−1) and good cycling stability with 102% of the initial capacitance after 6000 cycles. The as-prepared core/shell ultralayered Co3O4@NiO nanosheet arrays with enhanced performance could be considered as a promising electrode material for high-performance supercapacitors.
1. Introduction
Supercapacitors have attracted great attention as long life time energy storage device candidates, which close the gap between traditional capacitors and batteries. Recently, researchers have been devoting a great amount of effort to preparing flexible, free-standing or binder-free electrode materials with diverse morphologies.1,2 As high-performance electrode materials for supercapacitors, metal oxides and their composite materials have received significant technological and scientific interest because of their high theoretical capacities and good long cycling lives compared with their single oxides. Kong’s group designed NiO/NiCo2O4/Co3O4 composite materials with a high capacitance of 1717 F g−1 at 5 mA cm−2, and high rate performance and electrochemical stability.3 Wu et al. reported a simple magnetic field-assisted hydrothermal method to synthesize a wire-like NiO/Co3O4 one dimensional composite for pseudocapacitors, which displayed excellent cycling stability.4 Despite all of those achievements, the preparation of rational heterostructures without conductive additives and polymer binders still remains a challenge.For pseudocapacitors, a new type of energy storage device, such a desired core/shell heterostructured configuration would enlarge the specific capacity and enhance the stability of the electrodes, in contrast to electrodes with a single component. Numerous efforts have given rise to the development of core/shell binder-free electrode materials from transition metal oxides for their supercapacitor properties.5,6 However, it is still a challenge to obtain a well-defined structure with controllable morphology for the excellent electrochemical performance required for energy storage applications. Core/shell metal (Ru, Co, Ni, Mn, Fe, etc.) oxides have synergistic effects, and charge transfer and reversible adsorption properties, which make them excellent candidates for incorporation into high performance supercapacitor electrode designs.7–10 For example, numerous core/shell structures, such as CoO@MnO2,11 Co3O4@MnO2,12 Co3O4@Au@MnO2,13 ZnO@NiO,14 ZnCo2O4@Ni(OH)2,15 and NiCo2O4@MnO2,16 have been synthesized with enhanced electrochemical performance. Hu and coworkers17 designed Co3O4@ NiO hierarchical nanowire arrays that showed enhanced specific capacitance of 1236.67 F g−1 at 1 A g−1, and good cycling retention of 91.35% over 5000 cycles. Sun et al.18 reported Co3O4@Ni–Co–O nanosheet–nanorod arrays with simultaneous high capacitance and an effective synergistic effect. Even with these improvements, it still remains a challenge to increase the stability of electrodes and fully release the electroactivities of individual components for electrode materials with unique structures. One promising strategy is to choose and form ultralayered core/shell nanosheets with porous structures. Designing materials with long range two dimensional ultralayered structures can be an efficient strategy for achieving better rate capabilities and high capacitance because of the possibility of lower internal resistance.19 Particularly, when compared with nanometer or nanoparticle structures, 2-D ultralayered core/shell binder free electrodes can undergo fast redox reactions owing to their long range layered electroconducting frameworks, and their distinct long range, consistent, stable and easy oxide conductivity paths.20,21 For supercapacitors, core/shell heterostructured nanomaterials have been confirmed to be unique, as they facilitate the electroactivities of the electron transfer and ion diffusion components. Therefore, electrochemical performance is dramatically promoted when these materials are combined with highly porous or ultralayered morphologies,10,22,23 and the 2-D core/shell heterostructures represent an attractive configuration for fast reaction kinetics.
In this work, we present a 2D ultralayered core/shell microstructure of dense NiO ultrathin nanosheets grown on ultralayered Co3O4 nanosheet arrays. Especially, the unique two dimensional ultralayered nanostructure was fabricated by using a two-step hydrothermal method as illustrated in Fig. 1. The electrode material demonstrated an excellent specific capacitance of 715 F g−1 at a current density of 0.5 A g−1, which is superior to most reported single Co3O4 or NiO microstructure arrays. More importantly, a new asymmetric supercapacitor (ASC) device, based on 2D NiO@Co3O4 core/shell nanosheet arrays and activated carbon (AC), achieved a higher energy density of 30.2 W h kg−1. In addition, it also exhibited a low charge transfer resistance and a good cycling stability after 6000 cycles (102% of the initial capacitance).
2. Experimental
2.1 Materials
Cobalt nitrate hexahydrate (Co(NO3)2·6H2O), urea ((NH2)2CO), and nickel nitrate hexahydrate (Ni(NO3)2·6H2O) were of analytical grade and were purchased from Tianjin Damao Chemical Reagent Factory and used without further purification. Besides, nickel foam was purchased from Changsha Lyrun Material Co., Ltd. China.2.2 Preparation of the Co3O4@NiO ultralayered nanosheet arrays (UNAs)
The Co3O4@NiO UNAs were fabricated by a two-step hydrothermal method. Firstly, the Co2(OH)2CO3 nanosheet arrays were synthesized according to a previous report.24 Firstly, Co(NO3)2·6H2O (2.5 mmol) and (NH2)2CO (12.5 mmol) were dissolved in 36 mL of ultrapure water under vigorous stirring. Then, a clean Ni foam was immersed into the solution and reacted at 95 °C for 8 h. After being rinsed and dried in a vacuum oven at 60 °C for 3 h, the shell materials with ultralayered nanosheets vertically grown on the substrate were obtained.In a comparable synthesis, 1 piece of Ni foam with Co2(OH)2CO3 nanosheet arrays was immersed in 40 mL of a 0.5 mmol Ni(NO3)2·6H2O and 10 mmol (NH2)2CO solution, then transferred to a 50 mL Teflon-lined stainless steel autoclave at 100 °C for 10 h. Hierarchical Co3O4@NiO UNAs grown on Ni foam were obtained, followed by tempering at 250 °C for 3 h. The average mass loading of nanoarrays on the Ni foam was approximately 7 mg (Co3O4@NiO 1 × 1 cm2).
2.3 Characterizations
The crystalline information of the as-prepared sample was established by powder X-ray diffraction (XRD, D/max TTR-III, Cu Kα). Structural investigations of the Co3O4@NiO UNAs were performed using a scanning electron microscope (SEM, SUPRA 40, German Zeiss) and a transmission electron microscopy (FEI, Tecnai G2 F20). Elemental mapping of the samples was measured on a FEI Tecnai F20 under the scanning TEM (STEM) mode.2.4 Electrochemical measurements
Electrochemical tests were performed using a three-electrode system with a 2 M KOH electrolyte. Pt foils and a saturated calomel electrode (SCE) were used as the counter electrode and the reference electrode. The specific capacitance was calculated at a variety of current densities from eqn (1):25| Cs = IΔt/mΔV | (1) |
In the asymmetrical supercapacitors, the corresponding energy and power densities were calculated using the following equations:26,27
| E = 0.5CΔV2 | (2) |
| P = E/Δt | (3) |
3. Results and discussion
The SEM image in Fig. 2a shows the ultralayered morphology with well-arranged 2D nanosheet arrays. In fact, a hierarchy is self-assembled by many highly layered multilateral Co3O4 nanosheets, forming a multi-hierarchy with many individual irregular interspaces, as shown in high-magnification SEM imaging (Fig. 2b). Amazingly, the nanosheet arrays with good orientation distribution enhance the transition of ions and reduce the charge transfer resistance, distinguishing them from the nanosheets that are aligned and cross-linked with each other. | ||
| Fig. 2 (a and b) SEM images of the Co3O4 nanosheet arrays grown on Ni foam. (c–f) Ultralayered 2D Co3O4@NiO nanosheet arrays grown on Ni foam. | ||
To achieve synergy, the core shell materials are required to possess following essential features: the materials in the core are required to have superior conductivity to assist the diffusion of ions and the transport of electrons. Meanwhile, the core materials also should possess stable and uniform structures to curtail ion transmission. Finally, the shell grown on the boundary of the core materials should have excellent electrochemical properties, enlarge the specific surface area and assist ions to penetrate into the core region to release the potential properties of the core materials.28,29 For this purpose, the dense and porous NiO shell was grown homogeneously on the robust scaffold to form a 2D ultralayered core/shell structure. Fig. 2c–f show the SEM images of the as obtained core/shell Co3O4@NiO UNAs. As shown, the composed structures have a spongy morphology. The major nanoflake has a length of 2 μm, while the aligned nanosheets are as thin as 5–15 nm, and the morphology of the Co3O4@NiO UNA electrode could be tuned by the hydrothermal reaction time, as illustrated in Fig. S1.† Thus, the ultralayered Co3O4 nanoarray was entirely covered by these unique NiO nanosheets, leading to large interspaces and a hierarchical surface. These results conform to the XRD measurements (Fig. S2 see ESI†). It is believed that such an absorbent sponge morphology should facilitate the entry of electrolyte ions into the internal regions, through the porous nanosheets, to realize the release of the potential electrochemical properties of the core materials.
More details about the core/shell and porous structure were revealed by TEM and HRTEM. From the TEM image (Fig. 3a), it can be observed that the 2D Co3O4 nanosheets exhibit a two dimensional structure with an almost pentagonal shape with a flat surface. The high-resolution TEM (HRTEM) image in Fig. 3b shows the Co3O4 nanosheet is single crystal in nature. The lattice spacings of 0.24 nm and 0.29 nm can be indexed as the (311) and (220) planes of the cubic Co3O4. Fig. 3c and d show the Co3O4@NiO UNAs core/shell layered structures, in which the thin and freestanding NiO nanosheets are grown on the outside of the core with powerful bonding. The inset of the HRTEM image shows the interplanar spacing from the lattice fringes, and is measured to be 0.24 nm, corresponding to (111) lattice planes of the NiO crystal. STEM EDS mapping (Fig. 3e) was applied to further confirm the core/shell structure of the Co3O4@NiO UNAs. As shown, the Ni and O are homogeneously dispersed in the whole structure. Obviously, Co is primarily located in the core region, since the 2D Co3O4 scaffold was uniformly coated by the ultrathin NiO nanosheets.
 | ||
| Fig. 3 TEM and HRTEM images of (a and b) Co3O4 nanosheet arrays and (c and d) ultralayered 2D Co3O4@NiO nanosheet arrays. (e) Mapping analysis of the ultralayered 2D Co3O4@NiO nanosheet arrays. | ||
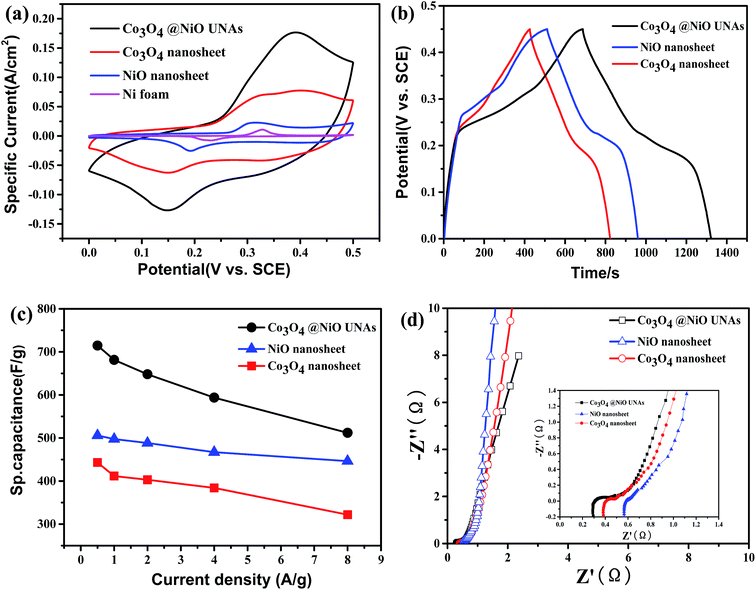
In order to identify whether such architectures are favorable for high-rate capacitive energy storage, the electrochemical performance of the obtained electrode material was determined by CV, galvanostatic charge–discharge tests, and electrochemical impedance spectroscopy. The capacitance from the Ni foam was insignificant as shown in Fig. 4a and S3.† As shown in Fig. 4a, the CV curves are recorded at a voltage ranging from 0 to 0.5 V for Co3O4@NiO UNAs, single NiO and Co3O4 electrodes at 10 mV s−1. Both the CV curves show vital faradaic responses. However, the CV curves demonstrate slight differences because the core surface becomes rough as well as containing another transition metal oxide after the addition of NiO. The ultrathin NiO nanosheets are beneficial to promotion of several faradaic reactions between NiO and NiOOH,30 and Fig. 4b shows the charge/discharge curves of both Co3O4@NiO UNAs, NiO and Co3O4 nanosheet electrodes at 0.5 A g−1. As expected, the Co3O4@NiO UNA electrode delivers a much longer discharge time than the single NiO, or Co3O4, which is consistent with the CV curves. CV curves of the as-fabricated Co3O4@NiO UNAs core/shell nanosheet hierarchies and NiO nanosheet hierarchies at different scan rates and the corresponding charge/discharge curves at different current density are presented in Fig. S4.†
Based on eqn (1), the specific capacitance of the Co3O4@NiO UNA electrode at 0.5 A g−1 was 715 F g−1 (about 5.07 F cm−1), which is 1.63 times the capacitance of the single Co3O4 nanosheets (443 F g−1 about 4.30 F cm−1). As shown in Fig. 4c the Co3O4@NiO UNAs have specific capacitances of 715, 681, 648, 594 and 512 F g−1 at the current densities of 0.5, 1, 2, 4 and 8 A g−1, respectively, which are much higher than those of the Co3O4 nanosheets (443, 412, 403, 384 and 322 F g−1 at the current densities of 0.5, 1, 2, 4 and 8 A g−1, respectively) and the NiO nanosheets (505.75, 497.5, 488.19, 467.01 and 446.39 F g−1 at the current densities of 0.5, 1, 2, 4 and 8 A g−1, respectively). The considerable enhancement of the supercapacitive performance is derived from the following: firstly, the microscopic layered structure accelerates the permeation process of the electrolyte by reducing the diffusion time of OH− ions and also accommodates the strain arising due to the high rate of insertion and extraction of OH− ions.31 Furthermore, the porous and ultrathin shells do not prevent contact between the hierarchical Co3O4 nanosheet arrays and the ions in the electrolyte whilst maintaining their structural integrity.32
Fig. 4d shows the impedance Nyquist plots of the Co3O4@NiO UNA and Co3O4 nanosheet electrodes. At low frequency, the linear sections in the impedance plots at lower frequencies indicate Warburg impedance (W), which is described as a diffusive resistance of OH− ions within the electrode. As observed, the Co3O4@NiO UNA electrodes show a higher slope of the straight line with regard to that of the single oxide electrodes, indicating the facile penetration of the electrolyte and easy diffusion of ions into the internal regions of the material. At high frequency, the real part of the intersection of the curves corresponds to the resistance of the solution (Rs). The core/shell Co3O4@NiO UNAs exhibit a much lower Rs value (0.3 Ω), which is lower than that of the single Co3O4 electrodes. Moreover, the ultralayered Co3O4@NiO UNAs display a smaller semi-circle diameter (Rct) as well, which suggests that the core/shell materials have a lower interfacial resistance and higher charge transfer conductivity than those of the pure single oxide electrodes.
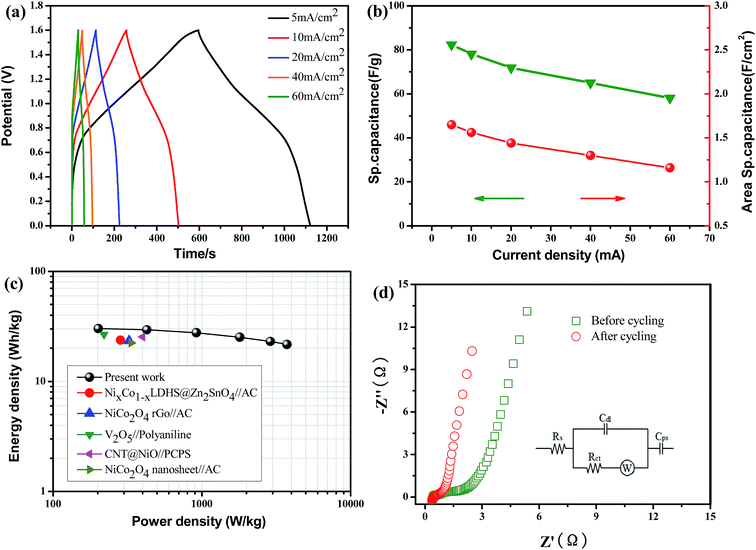
To further explore the practical applications of the active materials, an ASC was fabricated in a KOH aqueous electrolyte by using Co3O4@NiO UNAs as the anode and activated carbon as the cathode, as is schematically illustrated in Fig. 5a.
The individual CV curves of the positive and negative electrodes were measured in a three-electrode system at a scan rate of 20 mV s−1 (Fig. 5b). It was revealed that the ASC could have a operation voltage of 1.6 V. Fig. 5c presents the CV curves of the Co3O4@NiO//AC ASC device at different voltage windows, and it demonstrates that the whole voltage window of the ASC can be increased up to 1.6 V as expected. Fig. 5d shows the CV curves of the ASC device at different scan rates of 5 to 50 mV s−1, revealing that the CV curves present a large current area without obvious redox peaks, which is a combined property of pseudocapacitive and EDLCs types, and this implies ideal capacitive behavior. The galvanostatic charge–discharge curves at different current densities are shown in Fig. 6a. The specific capacitances and areal capacitances calculated from the charge/discharge curves as a function of current density were presented as shown in Fig. 6b. Impressively, the Co3O4@NiO//AC ASC device exhibits high specific capacitances of 82, 78, 71, 65 and 58 F g−1 at current densities of 5, 10, 20, 40 and 60 mA cm−2. The asymmetric supercapacitor demonstrated a maximum energy density of 30.2 W h kg−1 at a power density of 201.5 W h kg−1 (Fig. 6c), which reveals a much better performance than most pseudocapacitors.33–37 To further verify the effect of the 2D long-range layered structure on the decreasing Rct of the ASC, electrochemical impedance spectroscopy (EIS) was used to better understand the remarkable electrochemical performance (Fig. 6d). Such a pattern can be fitted by an equivalent circuit for impedance analysis and is shown in the inset of Fig. 6d, where Rs is the solution resistance of the electrochemical system, Cdl is a double layer capacitor, Cps is a faradaic pseudocapacitor, W is the Warburg impedance, and Rct is the faradaic interfacial charge transfer resistance. Noticeably, the devices have negligible semicircles at the high frequency region before and after cycling, demonstrating relatively low charge transfer resistance. At the high frequency region, after 6000 cycles, the Co3O4@NiO//AC exhibits a much lower real axis intercept and a smaller semi-circle diameter, exhibiting excellent kinetics and outstanding stability during cycling.
The cycling stability of the ASC was also performed at a current density of 30 mA cm−1 and shown in Fig. 7. It is evident that the capacitance of ASC stabilizes at 3000 cycles, suggesting the improvement in pore stability, electrolyte ions infiltration and surface activation of the electrodes during the repeated cycling.38–40 It is noted that the ASC displays good cycling stability with about 2% capacitance increase after 6000 cycles. Compared with previous reports, it shows desirable cycle lifetime. Moreover, the data from cyclic stability reveal the influence of the shape and type of the material on the cyclic stability (see ESI, SI-5†).41–44
Tracing the source of the high cycling stability and the capacitance was of great importance for constructing efficient electrodes. The cycling stability of the Co3O4@NiO//AC UNAs is superior to other structured materials. The reasons are as follows: (1) the layered morphology, due to the enhanced ordered structure, possesses well-defined long-range, uniform, stable and smooth oxide conductivity paths as compared to the nanometer length flakes or nanoparticles of highly disordered microstructures. (2) Ultralayered layered structures can be resistant to flaking and can be more tolerant to high-rate redox reactions for long term cycling processes. (3) This hierarchical core–shell nanosheet array architecture allows for synergistic effects of both the Co3O4 nanosheet core and NiO nanoflake shell.
4. Conclusions
In summary, we report a simple procedure for fabricating asymmetric supercapacitors, and 3D ultralayered core/shell Co3O4@NiO UNAs were fabricated utilizing a facile and efficient two-step hydrothermal process. The long-range ultralayered structure is beneficial for improving electroconductivity and enhancing the stability of the active materials. Compared with other 2D core/shell nanosheet materials, the designed device based on the Co3O4@NiO UNAs demonstrates improved electrochemical performance at a large potential of 1.6 V and possess a high energy density of 30.2 W h kg−1 at the power density of 201.5 W h kg−1. The ∼102% capacitance retention after 6000 cycles manifests excellent cycling stability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21401073), the Natural Science Foundation of Jilin Province of China (20170101211JC), and the Science and Technology Innovative Development Program of Jilin City (20166022).Notes and references
- W. J. Ma, S. H. Chen, S. Y. Yang, W. P. Chen, W. Weng and M. F. Zhu, ACS Appl. Mater. Interfaces, 2016, 8, 14622 CrossRef PubMed.
- W. Wang, W. Y. Liu, Y. X. Zeng, Y. Han, M. H. Yu, X. H. Lu and Y. X. Tong, Adv. Mater., 2015, 27, 3572 CrossRef PubMed.
- M. C. Liu, L. B. Kong, C. Lu, X. M. Li, Y. C. Luo and L. Kang, ACS Appl. Mater. Interfaces, 2012, 4, 4631 CrossRef PubMed.
- T. Liu, Y. Li, G. Y. Quan, P. Dai, X. X. Yu, M. Z. Wu, Z. Q. Sun and G. Li, Mater. Lett., 2015, 139, 208 CrossRef.
- S. J. He and W. Chen, Nanoscale, 2015, 7, 6957 RSC.
- Y. L. Huang, Y. X. Zeng, M. H. Yu, P. Liu, Y. X. Tong, F. L. Cheng and X. H. Lu, Small Methods, 2017, 1700230 Search PubMed.
- K. Ghosh, C. Y. Yue, M. M. Sk, R. K. Jena and S. Bi, Sustainable Energy Fuels, 2018, 2, 280 RSC.
- D. Cai, H. Huang, D. Wang, B. Liu, L. Wang and Y. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 15905 CrossRef PubMed.
- H. Chuo, H. Gao, Q. Yang, N. Zhang, W. Bu and X. Zhang, J. Colloid Interface Sci., 2017, 497, 50 CrossRef PubMed.
- R. Li, S. L. Wang, Z. C. Huang, F. X. Lu and T. B. He, J. Power Sources, 2016, 312, 156 CrossRef.
- X. Z. Wang, Y. H. Xiao, D. C. Su, S. G. Xu, L. M. Zhou, S. D. Wu, L. F. Han, S. M. Fang and S. K. Cao, Int. J. Hydrogen Energy, 2016, 41, 13540 CrossRef.
- M. Huang, Y. Zhang, F. Li, L. Zhang, Z. Wen and Q. Liu, J. Power Sources, 2014, 252, 98 CrossRef.
- W. Li, G. Li, J. Sun, R. Zou, K. Xu, Y. Sun, Z. Chen, J. Yang and J. Hu, Nanoscale, 2013, 5, 2901 RSC.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, X. L. Wang, C. D. Gu, X. B. Zhao and J. F. Hong, ACS Nano, 2012, 6, 5531 CrossRef PubMed.
- H. Chuo, H. Gao, Q. Yang, N. Zhang, W. Bu and X. Zhang, J. Mater. Chem. A, 2014, 2, 20462 RSC.
- L. Yu, G. Zhang, C. Yuan and X. W. Lou, Chem. Commun., 2013, 49, 137 RSC.
- Q. Q. Hu, Z. X. Gu, X. T. Zheng and X. J. Zhang, Chem. Eng. J., 2016, 304, 223 CrossRef.
- Z. Y. Lu, Q. Yang, W. Zhu, Z. Chang, J. F. Liu, X. M. Sun, D. G. Evans and X. Duan, Nano Res., 2012, 5, 369 CrossRef.
- M. S. Kumar and G. R. Rao, J. Phys. Chem. C, 2011, 115, 15646 CrossRef.
- A. K. Das, R. Bera, A. Maitra, S. K. Karan, S. Paria, L. Halder, S. K. Si, A. Bera and B. B. Khatua, J. Mater. Chem. A, 2017, 5, 22242 RSC.
- D. Cai, D. Wang, B. Liu, L. Wang, Y. Liu and H. Li, ACS Appl. Mater. Interfaces, 2014, 6, 5050 CrossRef PubMed.
- X. D. Zhang, J. L. Xiao, X. Y. Zhang, Y. Meng and D. Xiao, Electrochim. Acta, 2016, 191, 758 CrossRef.
- X. S. Chen, G. B. Liu, W. Zheng, W. Feng, W. W. Cao, W. P. Hu and P. A. Hu, Adv. Funct. Mater., 2016, 26, 8537 CrossRef.
- K. W. Qiu, Y. Lu, J. B. Cheng, H. L. Yan, X. Y. Hou, D. Y. Zhang, M. Lu, X. M. Liu and Y. S. Luo, Electrochim. Acta, 2015, 157, 62 CrossRef.
- Y. Xiao, S. Liu, F. Li, A. Zhang, J. Zhao, S. Fang and D. Z. Jia, Adv. Funct. Mater., 2012, 22, 4052 CrossRef.
- Y. F. Yang, D. Cheng, S. J. Chen, Y. L. Guan and J. Xiong, Electrochim. Acta, 2016, 193, 116 CrossRef.
- J. Wen, S. Z. Li, B. R. Li, Z. C. Song, H. N. Wang, R. Xiong and G. J. Fang, J. Power Sources, 2015, 284, 279 CrossRef.
- B. Liu, D. Z. Kong, Z. X. Huang, R. W. Mo, Y. Wang, Z. J. Han, C. W. Cheng and H. Y. Yang, Nanoscale, 2016, 8, 10686 RSC.
- Y. F. Li, H. Wang, J. M. Jian, Y. Fan, L. Yu, G. Cheng, J. L. Zhou and M. Sun, RSC Adv., 2016, 6, 13957 RSC.
- S. C. Hou, G. H. Zhang, W. Zeng, J. Zhu, F. L. Gong, F. Li and H. G. Duan, ACS Appl. Mater. Interfaces, 2014, 6, 13564 CrossRef PubMed.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987 CrossRef.
- J. P. Liu, J. Jiang, M. Bosman and H. J. Fan, J. Mater. Chem., 2012, 22, 2419 RSC.
- X. Wang, W. S. Liu, X. Lu and P. S. Lee, J. Mater. Chem., 2012, 43, 23114 RSC.
- X. Wang, A. Sumboja, M. Lin, J. Yan and P. S. Lee, Nanoscale, 2012, 22, 7266 RSC.
- W. F. Mak, G. Wee, V. Aravindan, N. Gupta, S. G. Mhaisalkar and S. Madhavi, J. Electrochem. Soc., 2012, 159, A1481 CrossRef.
- H. Yi, H. Wang, Y. T. Jing, T. Q. Peng and X. F. Wang, J. Power Sources, 2015, 285, 281 CrossRef.
- Z. B. Wu, X. L. Pu, X. B. Ji, Y. R. Zhu, M. J. Jing, Q. Y. Chen and F. P. Jiao, Electrochim. Acta, 2015, 174, 238 CrossRef.
- K. Xu, W. Li, Q. Liu, B. Li, X. Liu, L. An, Z. Chen, R. Zou and J. Hu, J. Mater. Chem. A, 2014, 2, 4795 RSC.
- J. Wang, L. Shen, P. Nie, X. Yun, Y. Xu, H. Dou and X. Zhang, J. Mater. Chem. A, 2015, 3, 2853 RSC.
- Z. Li, Z. Xu, H. Wang, J. Ding, B. Zahiri, C. M. B. Holt, X. Tan and D. Mitlin, Energy Environ. Sci., 2014, 7, 1708 RSC.
- W. Hong, J. Q. Wang, Z. P. Li and S. R. Yang, Energy, 2015, 93, 435 CrossRef.
- P. Sennu, V. Aravindan and Y. S. Lee, J. Power Sources, 2016, 306, 248 CrossRef.
- J. Q. Sun, W. Y. Li, B. J. Zhang, G. Li, L. Jiang, Z. G. Chen, R. J. Zou and J. Q. Hu, Nano Energy, 2014, 4, 56 CrossRef.
- G. D. Nie, X. F. Lu, M. Q. Chi, Y. Zhu, Z. Z. Yang, N. Song and C. Wang, Electrochim. Acta, 2017, 231, 36 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00290h |
| This journal is © The Royal Society of Chemistry 2018 |