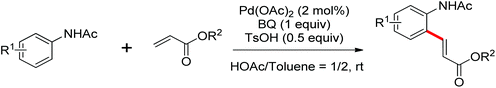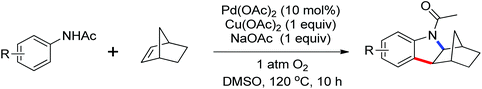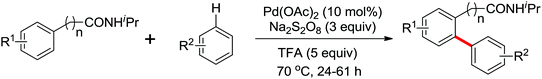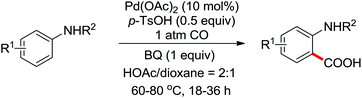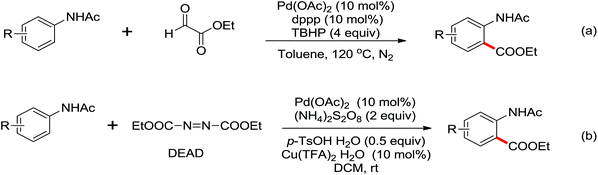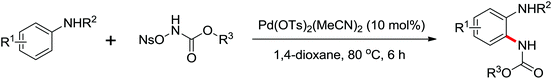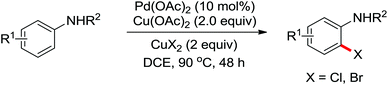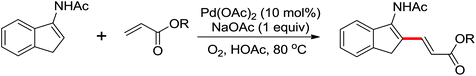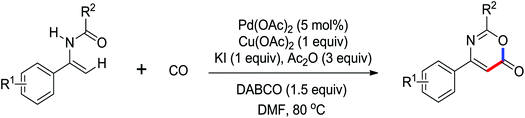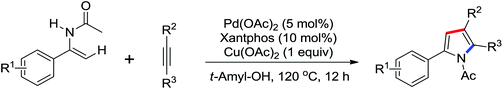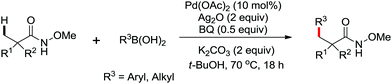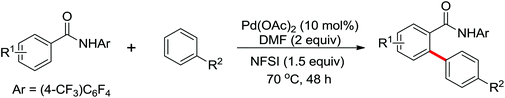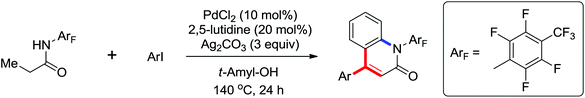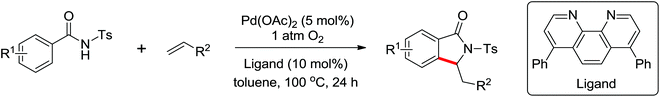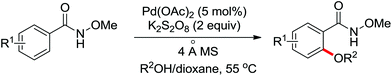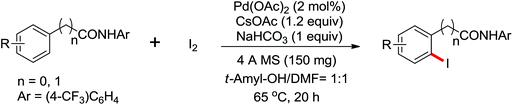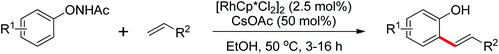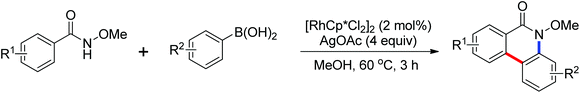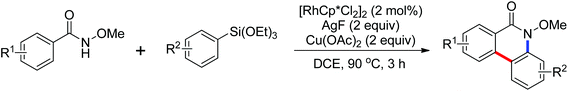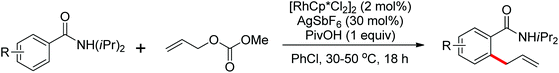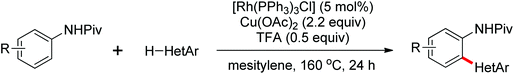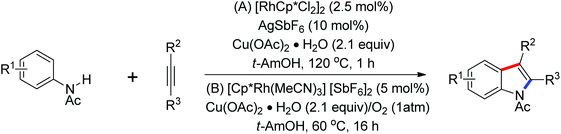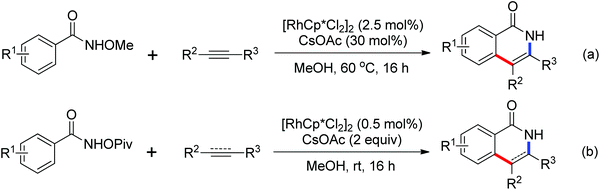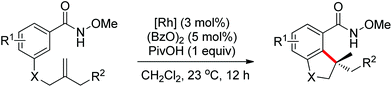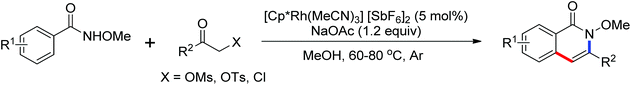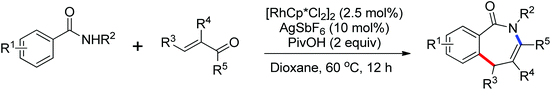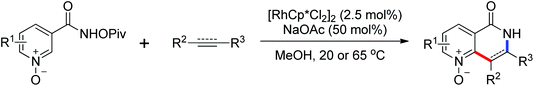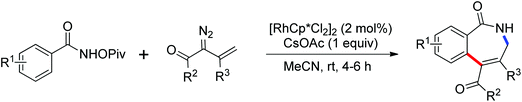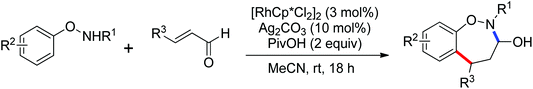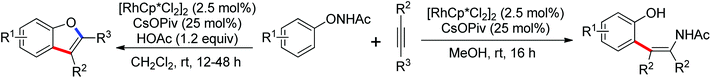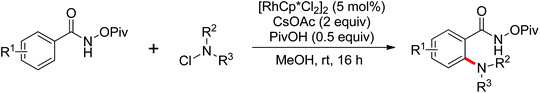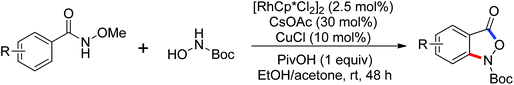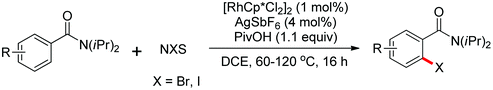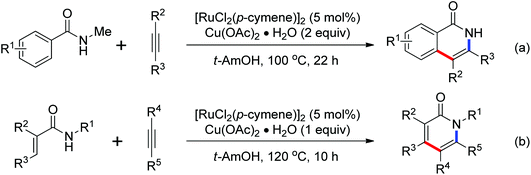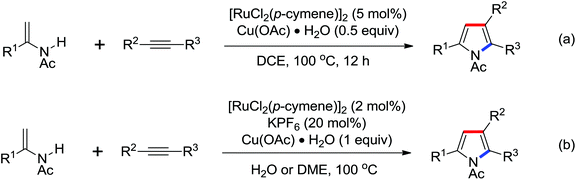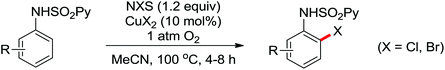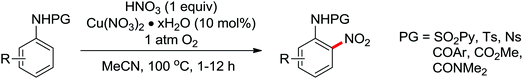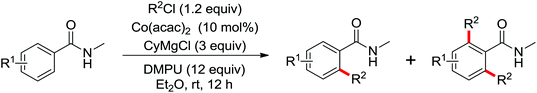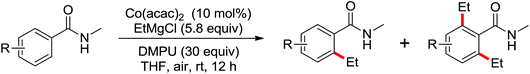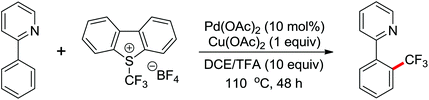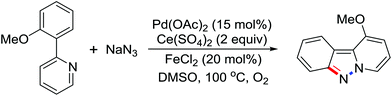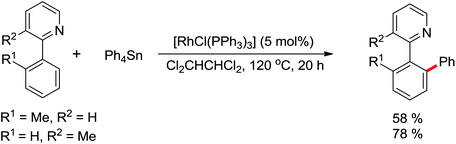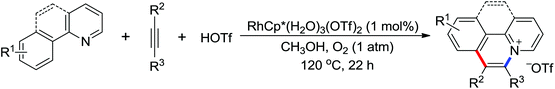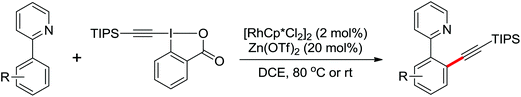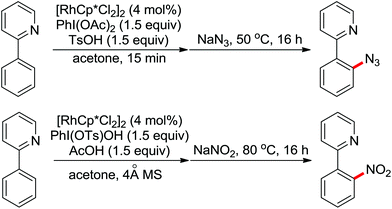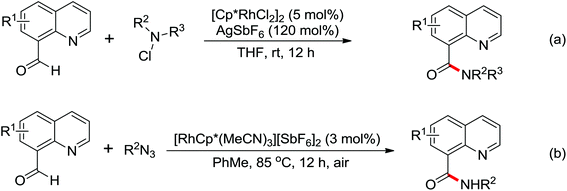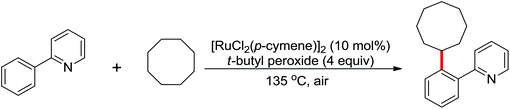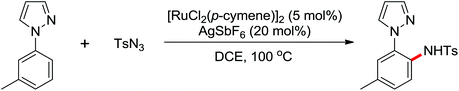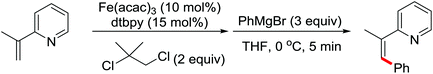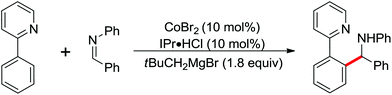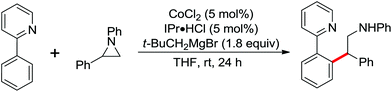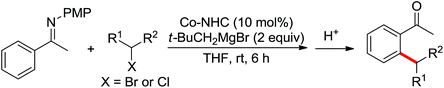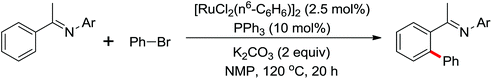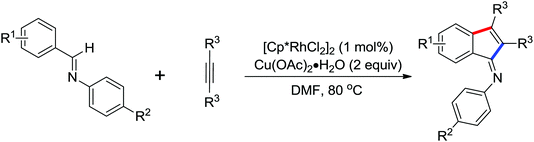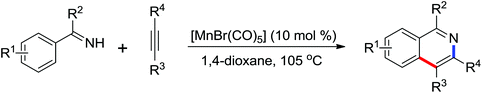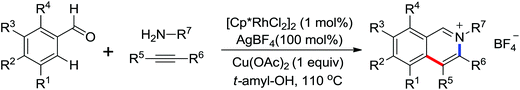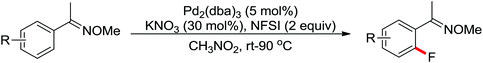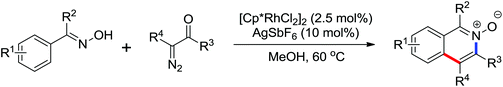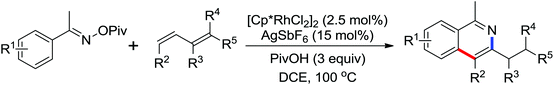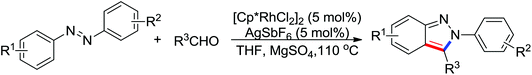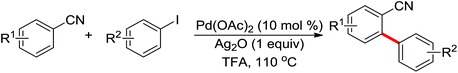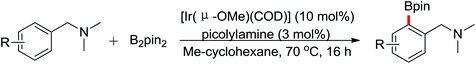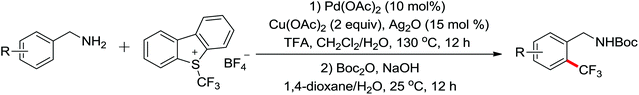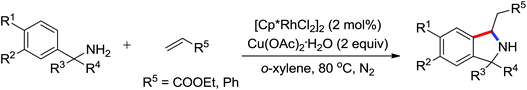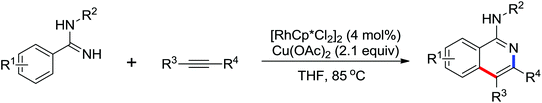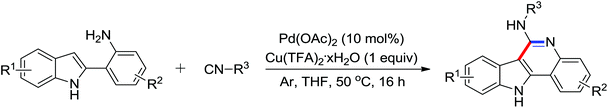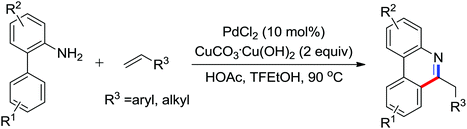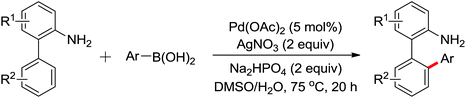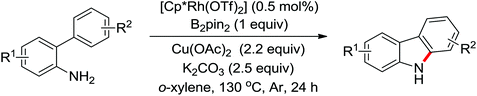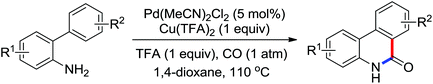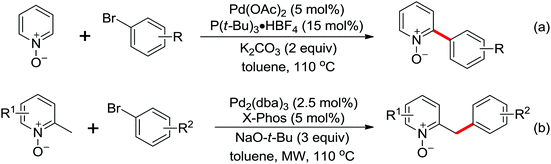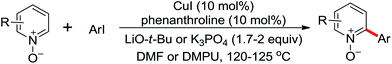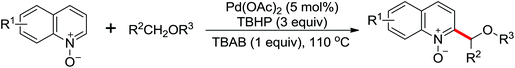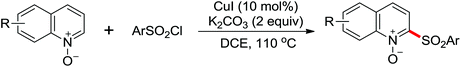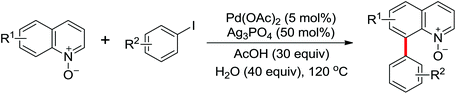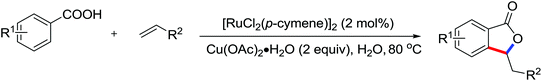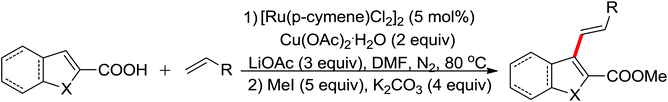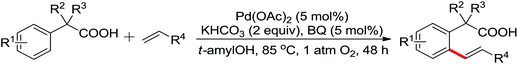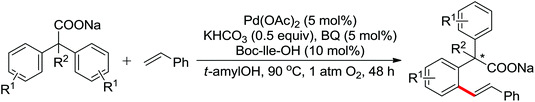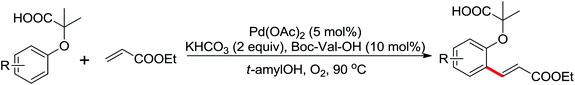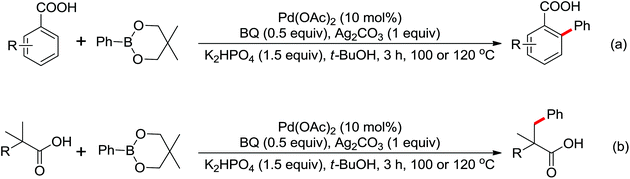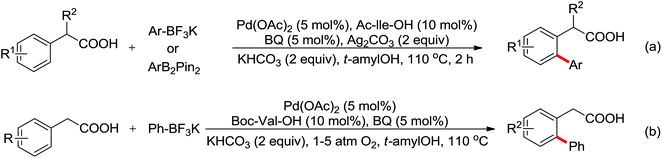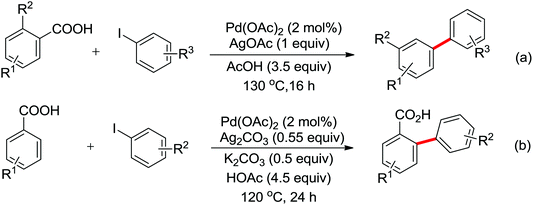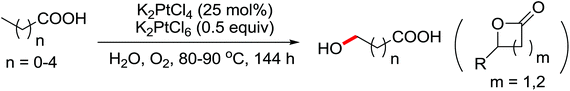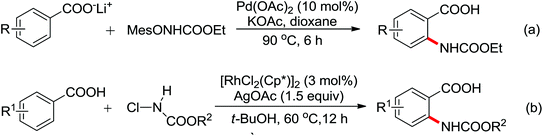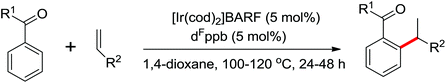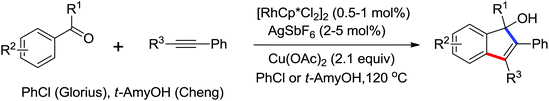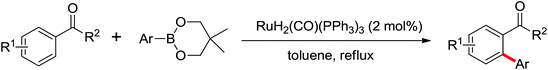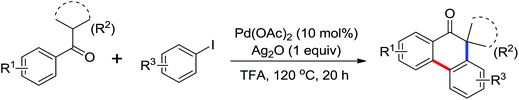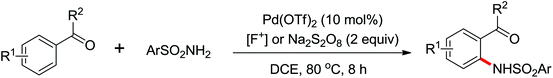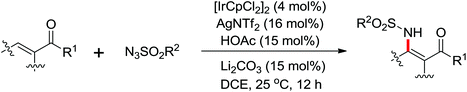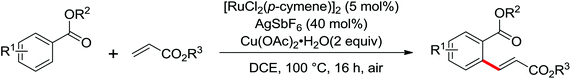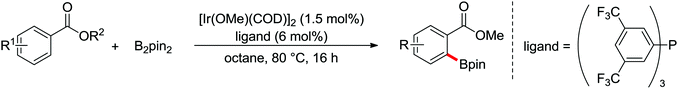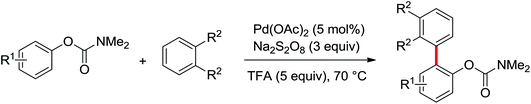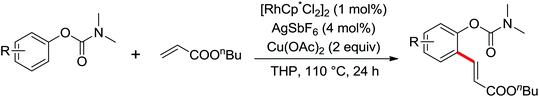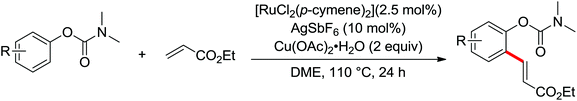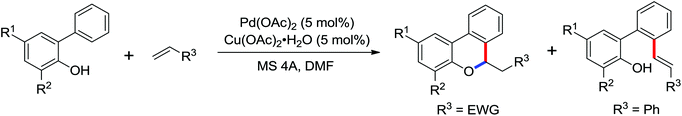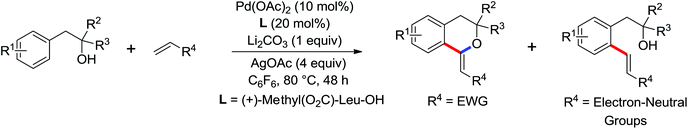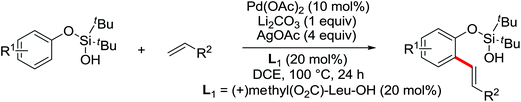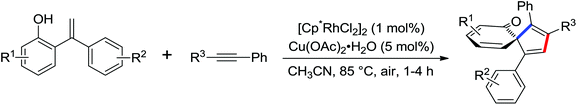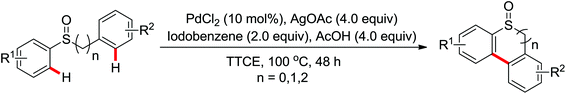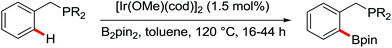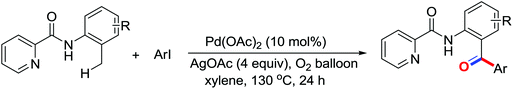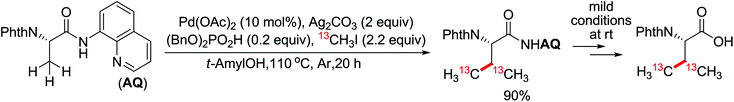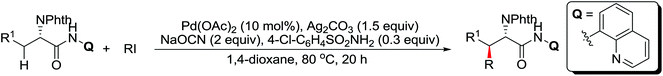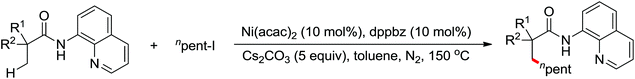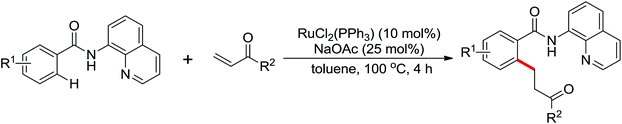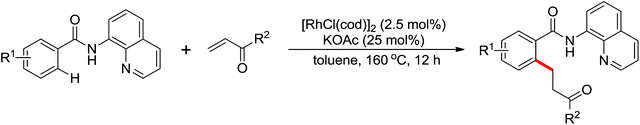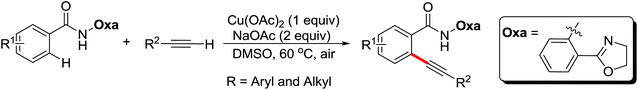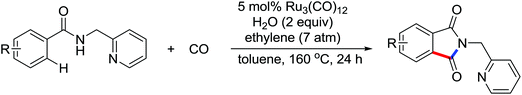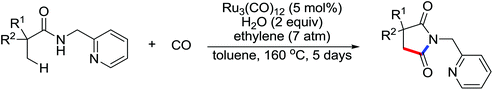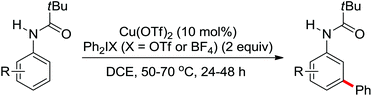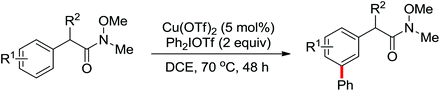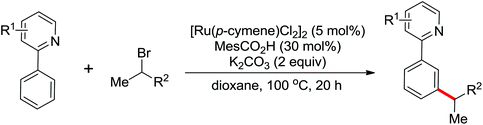Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups
Zhengkai
Chen
a,
Binjie
Wang
a,
Jitan
Zhang
a,
Wenlong
Yu
a,
Zhanxiang
Liu
*a and
Yuhong
Zhang
*ab
aZJU-NHU United R&D Center, Department of Chemistry, Zhejiang University, Hangzhou 310027, China. E-mail: yhzhang@zju.edu.cn
bState Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000, China
First published on 3rd June 2015
Abstract
Transition metal-catalyzed direct functionalization of C–H bonds is one of the key emerging strategies that is currently attracting tremendous attention with the aim to provide alternative environmentally friendly and efficient ways for the construction of C–C and C–hetero bonds. In particular, the strategy involving regioselective C–H activation assisted by various functional groups shows high potential, and significant achievements have been made in both the development of novel reactions and the mechanistic study. In this review, we attempt to give an overview of the development of utilizing the functionalities as directing groups. The discussion is directed towards the use of different functional groups as directing groups for the construction of C–C and C–hetero bonds via C–H activation using various transition metal catalysts. The synthetic applications and mechanistic features of these transformations will be discussed, and the review is organized on the basis of the type of directing groups and the type of bond being formed or the catalyst.
1. Introduction
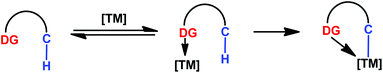
Direct C–H bond functionalization has emerged as a powerful tool in organic synthesis by providing original disconnections of complex molecules from simple starting materials and improving the overall efficiency of the desired transformation, and thus radically transforms the ways for chemists to approach a synthetic challenge. Recent decades have witnessed the development of transition-metal catalysis through the activation of C–H bonds by metal insertion, which has resulted in many pioneering discoveries.1 While the idea of using C–H bonds as starting points for chemical synthesis has become prevalent, with a survey of the development in this area, the sufficient reactivity for the cleavage of the strong C–H bond and the control of site selectivity are two main challenges. In this regard, functional group-assisted C–H bond cleavage shows great promise. Site selectivity can be controlled by the close proximity of C–H bonds to the reactive metal centre and the prerequisite substrate–catalyst interactions are significantly promoted through coordination of heteroatoms with transition metal catalysts (Scheme 1). Consequently, coordination of transition metals with directing groups is usually considered a fundamental step involved in these catalytic C–H bond functionalization processes.Despite the fact that many examples of stoichiometric C–H bond cleavage by various metals were discovered, the development of a catalytic method as a key step towards a synthetically useful process was not realized until the pioneering work of Lewis and Murai. As early as 1986, Lewis and coworkers observed the regioselective mono- and di-ortho-alkylations of phenol with ethylene using an ortho-metalated ruthenium phosphite complex.2a In 1993, an efficient ortho-selective alkylation of aromatic ketones with olefins in the presence of a ruthenium catalyst was reported by Murai and co-workers.2b The high efficiency and site selectivity in this transformation were attributed to the easier insertion of ruthenium into the ortho-C(sp2)–H bond after the coordination of ruthenium with ketone to form the five-membered metallacyclic intermediate. This knowledge about directing groups greatly speeds up the discovery of new approaches in the functionalization of C–H bonds, and the chemistry, by using the chelation-assisted C–H activation strategy in organic synthesis, has rapidly expanded since then.3 Various functional groups, including amide, anilide, imine, heterocyclic, amine, carboxylic acid, ester, ketone, and hydroxyl groups, have been employed as directing groups for catalytic C–H bond functionalizations. The C–H activation transformations directed by functional groups containing sulfur, phosphorus, silicon and π-chelation have been developed. Recently, the use of bidentate-chelation has found broad applications in the direct functionalization of inactivated C(sp3)–H bonds. The more recent approach based on remote-chelation is allowing the meta-position functionalization of C(sp2)–H bonds in the aromatic ring.
In the following sections, we will summarize the recent development of direct C–H bond functionalizations assisted by coordination with commonly occurring functional groups (Table 1). The versatility and limitations of these reactions will be documented by categorizing the functional groups containing nitrogen, oxygen, sulfur, phosphorus, silicon, π-chelation, and the bidentate systems. Given the rapid expansion of this still growing field, it is not possible to cover all of the representative chemistry in the confines of this review. Materials appearing in the previous literature will only be introduced as the background when necessary. Moreover, it should be pointed out that the cited literature sources in this review are mainly from the last 3 years. For detailed information about the early but still widely cited references, the reader is directed to some excellent books and reviews on the subject.
| Directing group | C–H functionalization | Transition metal catalyst | Schemes | Ref. |
|---|---|---|---|---|
|
Olefination | Pd | 2 and 3 | 4–6 |
| Rh | 50 | 67 | ||
| Ru | 96 | 134 | ||
| Arylation (heteroarylation) | Pd | 4–10 | 7–12 | |
| Rh | 64 | 86 | ||
| Ru | 98b | 138 | ||
| Carboxylation | Pd | 11 | 13 | |
| Acylation | Pd | 12 | 14–17 | |
| Ethoxycarboxylation | Pd | 13 | 18,19 | |
| Amination | Pd | 15 and 16 | 21,22 | |
| Amidation | Rh | 68 | 92 | |
| Alkoxylation | Pd | 21 | 27 | |
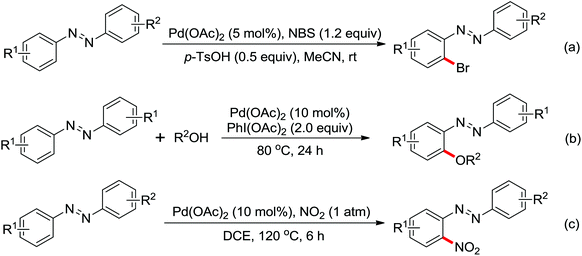
| Halogenation | Pd | 18 and 19 | 23,24 | |
| Trifluoromethylation | Pd | 22 | 29 | |
| Olefination annulation | Ru | 99 | 139 | |
| Oxidative annulation [3 + 2] | Rh | 70 | 96,97 | |
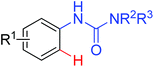
|
Arylation | Pd | 14a | 20d–f |
| Ethoxycarboxylation | Pd | 14c | 20a | |
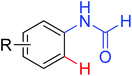
|
Acylation | Pd | 14b | 20g |
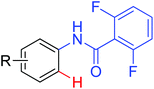
|
Hydroxylation | Ru | 102 | 143 |
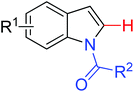
|
Oxidative annulation [3 + 2] | Co | 111 | 153 |
| Alkenylation | ||||
|
Halogenation | Cu | 105 | 147 |
| Nitration | Cu | 106 | 148 | |
|
Iodination | Pd | 20 | 25 |
|
Arylation | Pd | 23 | 30 |
| Olefination | Pd | 24 | 31 | |
| Alkynylation | Pd | 25 | 32 | |
| Rh | 67b | 91 | ||
| Acylation | Pd | 26 | 33 | |
| Acetoxylation | Rh | 92 | 129 | |
| Carbonylation | Pd | 27 | 34 | |
| Trifluoromethylation | Cu | 107 | 149 | |
| Oxidative annulations [3 + 2] | Pd | 28 | 35 | |
| Ru | 100 | 140 | ||
|
Arylation and alkylation | Pd | 29 | 36 |
|
Arylation | Pd | 30 | 37–39 |
| Alkynylation | Pd | 39 | 53 | |
| Alkylation | Pd | 40 | 54 | |
|
Arylation | Pd | 31 | 40 |
| Amination | Pd | 44 | 59 | |
| Ru | 103 | 144 | ||
| Iodination | Pd | 47 | 63 | |
| Borylation | Pd | 48 | 64 | |
| Trifluoromethylation | Pd | 49 | 65 | |
|
Arylation | Pd | 32 | 41 |
|
Arylation/annulation | Pd | 33 | 42–44 |
| Rh | 57–59 | 79–81 | ||
| Acylation/annulation | Pd | 42 | 56 | |
| Alkoxylation | Pd | 45 | 61 | |
| Olefination | Rh | 51 and 55 | 68,76 | |
| Ru | 97 | 136 | ||
| Olefination/annulation | Pd | — | 52 | |
| Rh | 54 and 78 | 77,111 | ||
| Alkylation/annulation | Rh | 63 | 71b | |
| Allenylation | Rh | 56 | 77 | |
| Alkynylation | Rh | 65 and 66a | 87,89 | |
| Ir | 66b | 89 | ||
| Amination | Rh | 89 and 91 | 125,127,128 | |
| Hydroxyamination | Rh | 90 | 126 | |
| Oxidative annulation [4 + 2] | Rh | 72, 73, 76, 79 and 84 | 99,101–103,109,113,119 | |
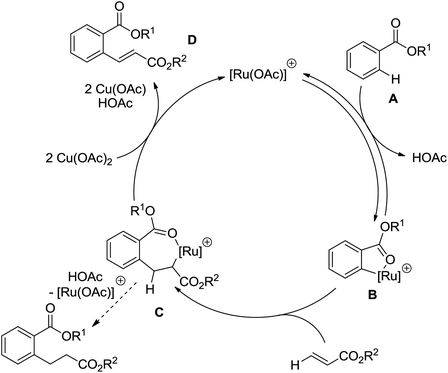
| Ru | 95 | 134 | ||
| Oxidative annulation [4 + 3] | Rh | 77 and 85 | 110,71e | |
| Amidoarylation | Rh | 74 | 104–106 | |
| Hydroarylation | Rh | 75 | 107,108 | |
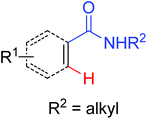
|
Oxidative annulation [4 + 2] | Ru | 94 | 132,133 |
| Arylation | Ru | 98a | 137 | |
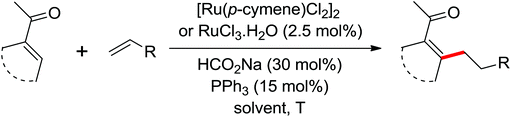
| Olefination | Ru | 96 | 135 | |
| Alkylation | Co | 109 and 110 | 151,152 | |
| Amination | Ir | 104 | 145,146 | |
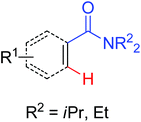
|
Arylation | Rh | 60 and 61 | 82–84 |
| Allylation | Rh | 62 | 85 | |
| Alkynylation | Rh | 67a | 90 | |
| Acylation | Rh | 69a | 55 | |
| Halogenation | Rh | 93 | 130 | |
| Hydroxylation | Ru | 101 | 141 | |
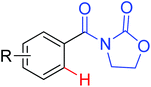
|
Oxidative annulation [3 + 2] | Rh | 81 | 114 |
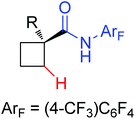
|
Arylation | Pd | 34 | 45 |
|
Arylation/annulation | Pd | 35 and 36 | 47 |
|
Olefination/annulation | Pd | 37 | 49 |
| Alkoxylation and halogenation | Pd | 46 | 62 | |
| Trifluoromethylation | Cu | 108 | 150 | |
| Annulation | Rh | — | 96 | |
|
Olefination/annulation | Pd | 38 | 51 |
|
Acylation | Pd | 41 | 55 |
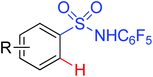
|
Olefination | Pd | 43 | 58 |
| Carboxylation and carbonylation | ||||
| Iodination | ||||
| Arylation | ||||
| Alkylation | ||||
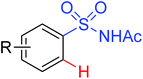
|
Oxidative annulation [4 + 2] | Rh | 83 | 117 |
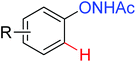
|
Olefination | Rh | 52 and 53 | 70,72 |
| Oxidative annulation [3 + 2] | Rh | 88 | 124 | |
| Oxidative annulation [4 + 3] | Rh | 87 | 123 | |
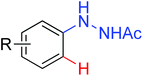
|
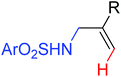
Oxidative annulation [3 + 2] | Rh | 71 | 98 |
|
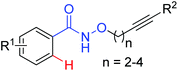
Oxidative annulation [4 + 2] | Rh | 82 | 116 |
|
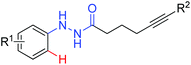
Intramolecular redox-neutral annulation | Rh | 86a | 120 |
|
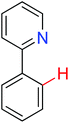
Intramolecular redox-neutral annulation | Rh | 86b | 121,122 |
|
Arylation | Pd | 112 and 113 | 154–159 |
| Rh | 131–133 | 187–192 | ||
| Ru | 153, 155 and 156 | 220–224 | ||
| Cu | 166 | 236–238 | ||
| Fe | 168 and 169 | 244,245 | ||
| Olefination | Pd | 114 and 115 | 160–162 | |
| Rh | 134a, b and 139 | 193,194,201 | ||
| Ru | 157a, b and 158 | 225,226,228 | ||
| Mn | 173 | 248 | ||
| Alkylation | Pd | 116–118 | 163–166 | |
| Rh | 140–142 | 203–206 | ||
| Ru | 159 and 160 | 229,230 | ||
| Co | 171 and 172 | 246,247 | ||
| Carboxylation | Pd | 119 | 167–169 | |
| Rh | 144 and 145 | 207,208 | ||
| Ru | 161 and 162 | 231,232 | ||
| Cyanation | Pd | 120 | 170 | |
| Rh | 147 | 209,210 | ||
| Amination | Pd | 121a and 122 | 171,173 | |
| Rh | 148a, 149a, c, d and 150 | 211,213,215–217 | ||
| Cu | 167 | 163 | ||
| Acetoxylation | Pd | 123 | 174,175 | |
| Hydroxylation | Pd | 124 | 176 | |
| Alkoxylation | Pd | 125 | 177 | |
| Phosphorylation | Pd | 126 | 178,179 | |
| Arylsulfonylation | Pd | 127a | 180 | |
| Thiolation | Pd | 127b | 181 | |
| Rh | 152 | 219 | ||
| Trifluoromethylthiolation | Pd | 127c | 182 | |
| Borylation | Pd | 128 | 183 | |
| Ru | 163 | 233 | ||
| Silylation | Pd | 130 | 186 | |
| Ru | 165 | 235 | ||
| Alkynylation | Rh | 146 | 89 | |
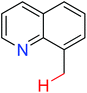
|
Fluorination | Pd | 129 | 184,185 |
| Alkenylation | Rh | 134c | 195 | |
| Amidation | Rh | 121b | 172 | |
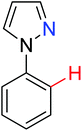
|
Oxidative annulation | Rh | 143 | 71c |
| Alkenylation | Rh | 135 and 137 | 196,197 | |
| Ru | 157c | 227 | ||
| Amidation | Ru | 164 | 234 | |
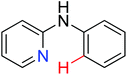
|
Olefination/annulation | Rh | 138a | 199 |
| Oxidative annulations [3 + 2] | ||||
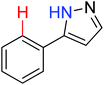
|
Olefination/annulation | Rh | 138b | 200 |
| Oxidative annulations [4 + 2] | ||||
|
Amidation | Rh | 149b and 151 | 214,218 |
|
Alkylation | Rh | 174–176 | 249–251 |
| Co | 177 | 252 | ||
| Olefination | Rh | — | 253 | |
| 194 | 274a,b | |||
| Co | 178 | 254 | ||
|
Self-coupling | Co | — | 246 |
| Annulation | Co | — | 255b | |
| Arylation | Co | — | 255a | |
| Ru | 180 and 181 | 258 | ||
| 182 | 223 | |||
| Pd | 183 | 259 | ||
| Fe | 184 | 260 | ||
| Alkylation/annulation | Rh | 185 | 261 | |
| Olefination/annulation | Ru | — | 274c | |
|
Olefination | Rh | 179 | 256a |
| Oxidative annulation [4 + 2] | Ru | — | 271 | |
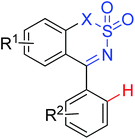
|
Olefination | Rh | — | 256b |
| Olefination/annulation | Rh | — | 257 | |
| Rh | 191b | 270 | ||
| Ir | 191a | 269 | ||
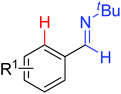
|
Olefination/annulation | Re | 186a | 262 |
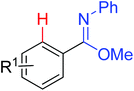
|
Acylation/annulation | Re | 186b and c | 263 |
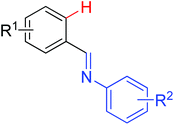
|
Olefination/annulation | Rh | 187 and 188 | 264 |
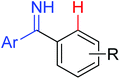
|
Arylation | Rh | 189a | 265 |
| Olefination/annulation | Rh | 189b | 266 | |
| 190 | 268 | |||
| Ru | 189c | 267 | ||
| Mn | 193 | 273 | ||
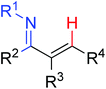
|
Olefination/annulation | Rh | 192a | 272a–c |
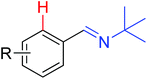
|
Olefination/annulation | Rh | 192b | 272d |
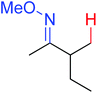
|
Acetoxylation | Pd | 195 | 275 |
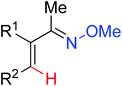
|
Alkylation/annulation | Rh | 208b | 292 |
| Acylation/annulation | Rh | — | 293b | |
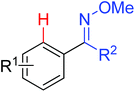
|
Arylation/annulation | Pd | 196 and 197 | 276–278 |
| Fluorination | Pd | 202 | 284 | |
| Alkylation/annulation | Rh | 208a | 291 | |
| Amidation/annulation | Rh | — | 293a | |
| Alkylation | Rh | 198a | 71a | |
| Selenylation | Rh | — | 285 | |
| Olefination | Rh | 198b | 279 | |
| Acylation | Pd | 199 | 280a,d | |
| Acylation | Rh | 199 | 280e | |
| Amidation | Pd | 200a | 171 | |
| — | 282 | |||
| Rh | 200b | 127 | ||
| 200c | 281 | |||
| — | 215 | |||
| Cyanation | Rh | 201 | 283 | |
| Sulfenylation | Rh | — | 219 | |
| Alkylation/annulation | Rh | 208 | 291,292 | |
| Olefination/annulation | Ru | — | 286e | |
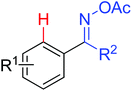
|
Olefination/annulation | Rh | — | 286c |
| 203 and 204 | 287 | |||
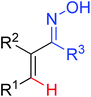
|
Olefination/annulation | Rh | — | 286a,b |
|
Olefination/annulation | Rh | — | 286d |
| Ru | — | 286f | ||
| Alkylation/annulation | Rh | 205 | 71d | |
|
Olefination/annulation | Rh | — | 286g |
| — | 286h | |||
| — | 286i | |||
|
Alkylation/annulation | Rh | 206 | 288 |
|
Olefination/annulation | Rh | 207 | 289,290 |
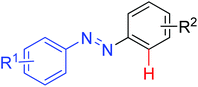
|
Acylation | Pd | 209 | 275,294a,b,d,295,296 |
| Halogenation | Pd | 211a | 298 | |
| Alkylation/annulation | Rh | 210 | 297 | |
| Olefination/annulation | Rh | 212 | 301a,b | |
| Amidation/annulation | Rh | 213a | 302 | |
| Alkoxylation | Pd | 211b | 299 | |
| Nitration | Pd | 211c | 300 | |
| Amidation | Rh | — | 303a,b | |
| 213b | 303c | |||
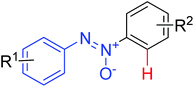
|
Acylation | Pd | — | 294c |
| Olefination | Ru | — | 311 | |
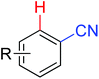
|
Arylation | Pd | 214 | 304 |
| Alkoxylation | Pd | — | 305 | |
| Halogenations | Pd | — | 306 | |
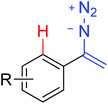
|
Olefination/annulation | Rh | 215 | 307 |
|
Olefination | Rh | 216b | 309 |
| Olefination/annulation | Rh | 216a | 308 | |
|
Olefination | Rh | 217a | 310 |
| Olefination/annulation | Rh | 217b | 312 | |
|
Olefination/annulation | Rh | 218 | 313 |
|
Silylation | Ru | 219 | 314 |
| Olefination | Pd | 220 | 315 | |
| Carboxylation | Pd | 222 | 316 | |
| Borylation | Ir | 223 | 317 | |
| Arylation | Pd | 224 | 318 | |
| Dehydrogenative annulation | Pd | 225 | 319–321 | |
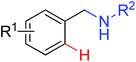
|
Carbonylation/cyclization | Pd | 226 and 227 | 322,323 |
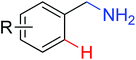
|
Arylation | Pd | 228 | 324 |
| Trifluoromethylation | Pd | 229 | 325 | |
| Olefination | Rh | 230 | 326 | |
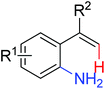
|
Alkylation | Ir | 231 | 327 |
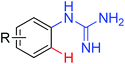
|
Olefination | Pd | 232 | 329 |
| Arylation | ||||
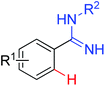
|
Alkenylation/cyclization | Rh | 233 | 330 |
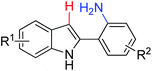
|
Cycloamidination | Pd | 234 | 331 |
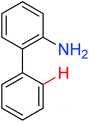
|
Olefination | Ru | 238 | 335 |
| Arylation | Pd | 236 and 237 | 333,334 | |
| Alkenylation/cyclization | Pd | 235 | 332 | |
| Intramolecular amination | Rh | 239 | 336 | |
| Aminocarbonylation | Pd | 240 | 337,338 | |
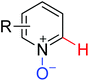
|
Arylation | Pd | 241 | 340 |
| 243 | 342 | |||
| Cu | 242 | 341 | ||
| Alkenylation | Ni | 244a | 343a | |
| Pd | 244b | 342a | ||
| Alkylation | Pd | 246 | 345 | |
| Cu | 247 | 346 | ||
| Acetoxylation | Cu | 248 | 347 | |
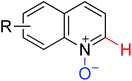
|
Alkenylation | Pd | 244c | 343b |
| Alkylation | Pd | 245 | 344 | |
| Amidation | Cu | 249 | 348 | |
| Sulfonylation | Cu | 250 | 349 | |
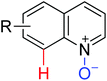
|
Alkenylation | Rh | 251a | 350a |
| Alkylation | Rh | 251b | 350b | |
| 253a | 352 | |||
| Arylation | Pd | 252 | 351 | |
| Alkynylation | Rh | 253b | 352 | |
| Iodination | Rh | 254a | 353 | |
| Amidation | Ir | 254b | 353 | |
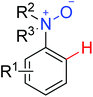
|
Alkenylation | Rh | 255 | 354 |
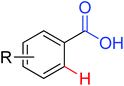
|
Olefination/annulation | Pd | 256 | 355 |
| Ru | 258 | 357 | ||
| Rh | — | 360,361,363 | ||
| Olefination | Rh | 259a | 359 | |
| Arylation | Pd | 266a, 267a, 269 and 270b | 369,370,372,373b | |
| Decarboxylation arylation | Pd | 270a | 373a | |
| Alkylation | Pd | 271a | 369 | |
| Alkylation/annulation | Pd | 272 | 375 | |
| Acylation | Pd | 273b | 377 | |
| Rh | 273a | 376 | ||
| Carboxylation | Pd | 275a | 378 | |
| Alkynylation/annulation | Rh | 282a | 389,390 | |
| Ru | 285 | 394 | ||
| Ir | 284 | 393 | ||
| Olefination/annulation | Rh | 282b | 389 | |
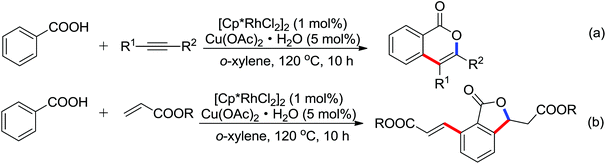
| Intermolecular lactonization | Rh | 287 | 398,399 | |
| Hydroxylation | Pd | 288a | 400 | |
| Amination | Pd | 289a | 402 | |
| Rh | 289b | 403 | ||
| Halogenation | Pd | 290 | 404,405 | |
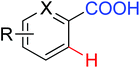
|
Hydroxylation | Cu | 288b | 401 |
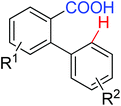
|
Intramolecular lactonization | Cu | 281 | 388 |
| Hydrolysis | Cu | 281 | 388 | |
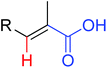
|
Olefination | Rh | 259b | 359 |
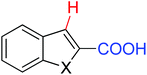
|
Decarboxylation and olefination | Pd | 260b | 362 |
| Olefination | Ru | 261 | 364 | |
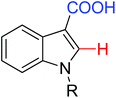
|
Decarboxylation and olefination | Pd | 260a | 362 |
| Oxidative annulation | Pd | 286 | 397 | |
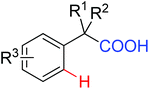
|
Olefination | Pd | 262–264 | 366,367 |
| Arylation | Pd | 267b and 268 | 370,371 | |
| Alkylation | Pd | 271b | 374 | |
| Carboxylation | Pd | 275b | 378 | |
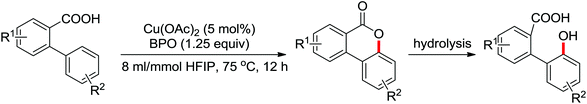
| Intramolecular lactonization | Pd | 280 | 385–387 | |
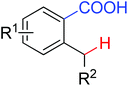
|
Intramolecular lactonization | Pd | 278 | 383 |
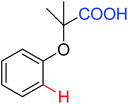
|
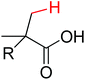
Olefination | Pd | 265 | 368 |
|
Olefination | Pd | 266b | 369 |

|
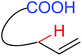
Hydroxylation | Pt | 276 | 379 |
|
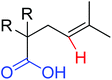
Intramolecular lactonization | Pd | 277 | 382 |
|
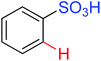
Intramolecular lactonization | Pd | 279 | 384 |
|
Alkynylation | Rh | — | 392 |
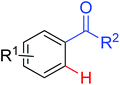
|
Alkylation | Ru | 291 and 292 | 2b,408f |
| Ir | 293 | 410 | ||
| Ru | — | 406,407a–c,408a–f | ||
| Rh | — | 409 | ||
| Olefination | Ru | 294 and 298 | 411,421 | |
| Rh | 297 | 69a | ||
| Ru | — | 412,413,420,422 | ||
| Ir | — | 414 | ||
| Arylation | Ru | 301 | 426a | |
| Ru | — | 426b | ||
| Pd | — | 425 | ||
| Amidation | Pd | 304 | 430 | |
| Ru | 305 | 431–433 | ||
| Ir | 306 | 434 | ||
| Hydroxylation | Pd | 307 | 435 | |
| Pd | — | 436,437 | ||
| Ru | 308 and 309 | 438,439 | ||
| Alkylation/annulation | Rh | 299 | 423 | |
| Ru | 300 | 424 | ||
| Olefination/annulation | Rh | 295 and 296 | 415–418 | |
| Ru | — | 419 | ||
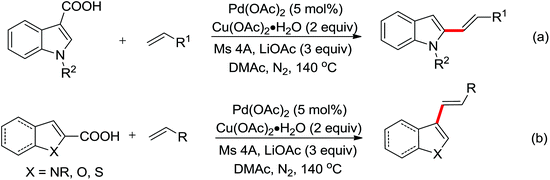
| Arylation/annulation | Pd | 302 | 427 | |
| Intramolecular/annulation | Pd | 303 | 428,429 | |
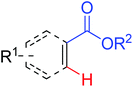
|
Alkylation | Ru | 310 | 406 |
| Olefination | Ru | 311 and 313 | 441,443,444 | |
| Rh | 312 and 315 | 442,445 | ||
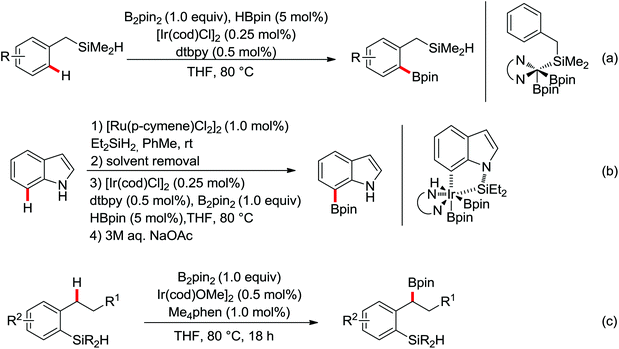
| Borylation | Ir | 316–318 | 419,446–448 | |
| Hydroxylation | Ru | 319 | 450 | |
| Halogenation | Pd | 320 | 451 | |
| Amination | Ir | 321 | 434 | |
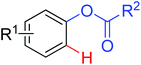
|
Arylation | Pd | 322–324 | 455–457 |
| Olefination | Rh | 326 | 458 | |
| Ru | 327 and 328 | 461,464 | ||
| Halogenation | Pd | 329 | 465–469 | |
| Acylation | Pd | |||
| Hydroxylation | Ru | |||
| Borylation | Ir | |||
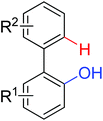
|
Arylation | Pd | 330a | 470 |
| Alkylation/annulation | Pd | 332, 336 and 337 | 473,477,478 | |
| Olefination | Pd | 338 | 479 | |
| Carbonylation | Pd, Ru | 351 and 352 | 493,495 | |
| Intramolecular cyclization | Pd, Cu | 355 and 356 | 498,500 | |
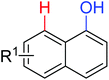
|
Arylation | Pd | 330b | 470 |
| Olefination | Ir | 339 | 480 | |
| Olefination/annulation | Rh, Ru | 340 | 482 | |
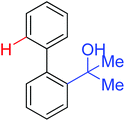
|
Arylation | Pd | 331 | 471,472 |
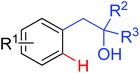
|
Olefination/annulation | Pd | 333 | 474 |
| Carbonylation | Pd | 350 | 492 | |
| Intramolecular cyclization | Pd | 354 | 497 | |
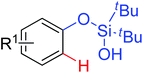
|
Olefination | Pd | 334 | 475 |
| Oxygenation | Pd | 358a | 502 | |
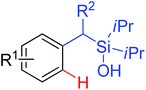
|
Olefination | Pd | 335 | 476 |
| Intramolecular cyclization | Pd | 358b | 503 | |
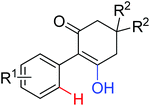
|
Olefination | Pd, Ru | 336 | 477 |
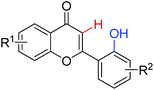
|
Alkylation/annulation | Pd | 337 | 478 |
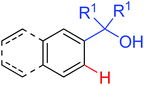
|
Olefination/annulation | Rh | 341 | 483 |
|
Olefination/annulation | Ru | 342 | 484 |
|
Oxidative annulations [3 + 2] or [4 + 2] | Ru, Rh, Pd | 343, 345 and 346 | 485–487 |
|
Oxidative annulations [3 + 2] | Ru | 347 | 488 |
|
Oxidative annulations [5 + 2] or [3 + 2] | Rh | 348 and 349 | 490,491 |
| Carbonylation/annulation | Pd | 353 | 496 | |
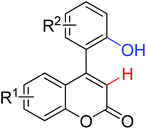
|
Intramolecular cyclization | Cu | 357 | 501 |
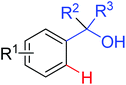
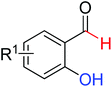
|
Silylation/cyclization | Ir | 359 | 504 |
|
Arylation | Pd | 360 and 362b | 505,510 |
| Alkylation | Rh | 361 | 508 | |
| Olefination/annulation | Rh | 362a and c | 509,511 | |
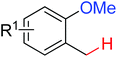
|
Amination | Pd | 363 | 172 |
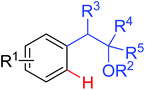
|
Olefination | Pd | 364 | 512 |
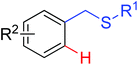
|
Olefination | Pd | 365a | 513 |
| Rh | 366a | 515 | ||
| Arylation | Pd | 365b | 514 | |
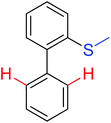
|
Olefination | Pd | 366b | 516 |
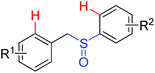
|
Oxidative annulation | Pd | 367 and 368 | 517,518 |
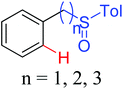
|
Olefination | Pd | 369 | 519 |
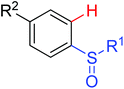
|
Olefination | Rh | 370 | 520 |
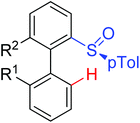
|
Olefination | Pd | 371a | 521 |
| Acetylation | Pd | 371b | 522 | |
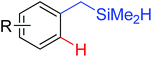
|
Borylation | Ir | 372a | 523 |
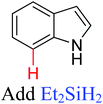
|
Borylation | Ir | 372b | 524 |
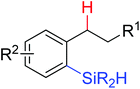
|
Borylation | Ir | 372c | 525 |
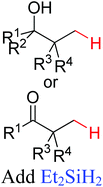
|
Acetylation | Ir | 373 | 526 |
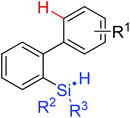
|
Oxidative annulation | Rh | 374 | 527 |
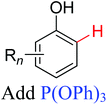
|
Olefination | Ru | 375 | 2a |
| Arylation | Rh | 376 | 528,529 | |
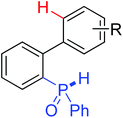
|
Oxidative annulation | Pd | 377 | 530 |
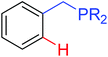
|
Borylation | Ir | 378 | 531 |
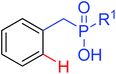
|
Olefination | Pd | 379 | 532 |
| Oxidative annulation | Rh | 380 | 533,534 | |
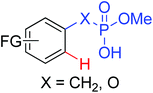
|
Acetylation | Pd | 381 | 535 |
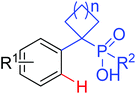
|
Carbonylation | Pd | 382 | 536 |
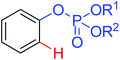
|
Arylation | Pd | 383 | 537,538 |
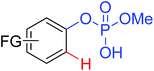
|
Alkenylation | Pd | 384a | 539 |
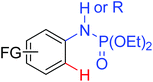
|
Arylation | Pd | 384b | 540 |
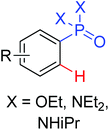
|
Alkenylation | Rh | 385 | 541–544 |
| Arylation | ||||
| Oxidative annulation | ||||
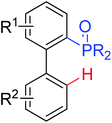
|
Alkenylation | Pd | 386 | 545–548 |
| Hydroxylation | ||||
| Acetoxylation | ||||
| Arylation | ||||
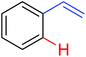
|
Oxidative annulation | Ir | 387 | 550 |
| Carbonylation | Pd | 388 | 551 | |
| Dimerization | Rh | 390 | 552 | |
|
Alkenylation | Pd | 391 | 553 |
| Oxidative annulations [4 + 2] | Pd | 393 | 554 | |
|
Intramolecular C–H activation | Pd | 394 and 398 | 555,559 |
| Arylative cyclization | Pd | 396 | 557 | |
|
Carbocyclization | Pd | 397 and 400 | 558,562 |
|
Cycloaddition [4 + 2] | Pd | 399 | 560,561 |
|
Oxidative annulations | Pd | 401 | 563 |
|
Oxidative annulations [4 + 2] | Rh | 402a | 564 |
| Olefination | Rh | 402b | 564 | |
|
Olefination | Pd | 422 | 592 |
| Fe | 423 | 593 | ||
| Arylation | Pd | 403 | 566 | |
| Cu | 417 | 585 | ||
| Oxidative annulation | Co | 438 | 609,610 | |
| Alkylation | Ni | 426–428 | 597–599 | |
| Ru | 429 | 600a | ||
| Rh | 430 | 600b | ||
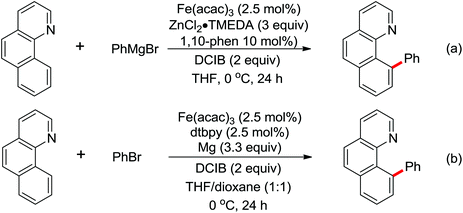
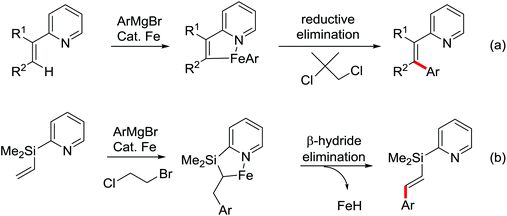
| Fe | 431 | 601 | ||
| Alkynylation | Pd | 433 | 606 | |
| Trifluoromethylation | Cu | 439 | 611 | |
| Amination | Cu | 445 | 618 | |
| Silylation | Pd | 451 | 627 | |
|
Arylation | Pd | 405 | 569 |
| 409–412 | 573–576 | |||
| 415 | 583 | |||
| Fe | 418 and 419 | 586–588 | ||
| Ni | 420 and 421 | 589,590 | ||
| Alkylation | Pd | 424 and 425 | 595,596 | |
| Ni | 428 | 599 | ||
| Alkynylation | Pd | 423 | 605 | |
| Amination | Pd | 441 | 614 | |
| Cu or Ni | 443 | 616 | ||
| Alkoxylation | Pd | 447a | 621a | |
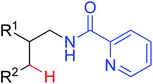
|
Arylation | Pd | 413 and 414 | 580,581 |
| Alkylation | Pd | 594 | ||
| Amination | Pd | 440 | 613 | |
| Alkoxylation | Pd | 446 | 619 | |
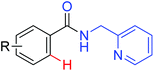
|
Arylation | Pd | 403 | 566 |
| Carbonylation | Ru | 435 | 608a | |
| Oxidative annulation | Ni | 437 | 608c | |
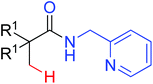
|
Carbonylation | Ru | 436 | 608b |
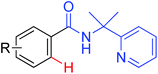
|
Hydroxylation | Cu | 450 | 624 |
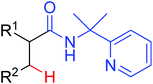
|
Monoarylation/amidation | Pd | 442 | 615 |
| Alkoxylation | Pd | 477b | 621b | |
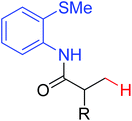
|
Arylation | Pd | 406 | 570 |
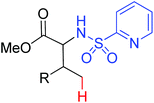
|
Arylation | Pd | 407 | 571 |
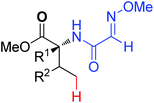
|
Arylation | Pd | 408 | 572 |
|
Halogenation/acetoxylation | Pd | 449 | 623 |
|
Alkynylation | Cu | 434 | 606 |
| Amination | Cu | 408b | 587b | |
| Trifluoromethylation | Cu | 415 | 598 | |
|
Arylation | Fe | 419 | 588 |
|
Amination | Pd | 444 | 617 |
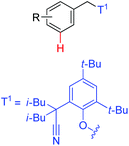
|
Olefination | Pd | 454a | 630 |
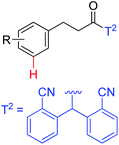
|
Olefination | Pd | 454b | 630 |
| Arylation | Pd | 454c | 631 | |
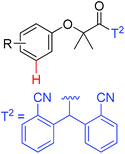
|
Olefination | Pd | 455 and 456 | 368 |
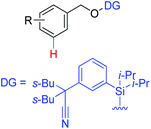
|
Olefination | Pd | 457 | 632 |
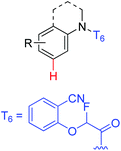
|
Olefination | Pd | 458 | 633 |
| Acetoxylation | ||||
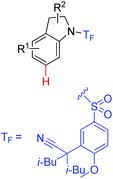
|
Olefination | Pd | 459a | 634 |
| Arylation | Pd | 459b | ||
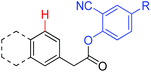
|
Olefination | Pd | 460 | 635 |
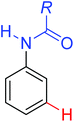
|
meta-Arylation | Cu | 461 and 462 | 637 |
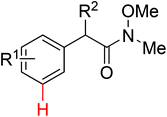
|
meta-Arylation | Cu | 463 | 639 |
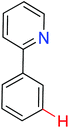
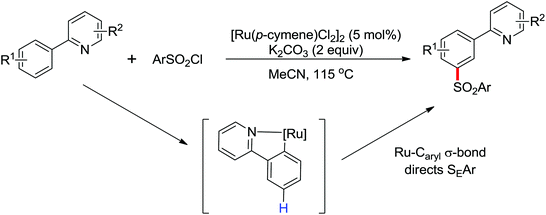
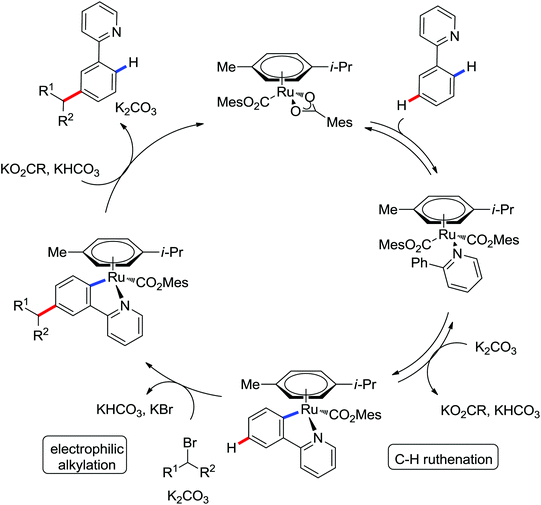
|
meta-Sulfonation | Ru | 464 | 640 |
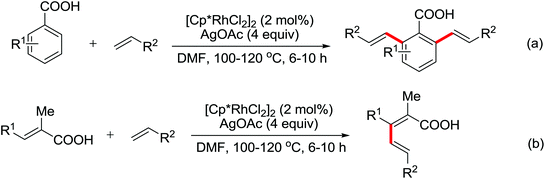
| meta-Alkylation | Ru | 465 and 466 | 641 |
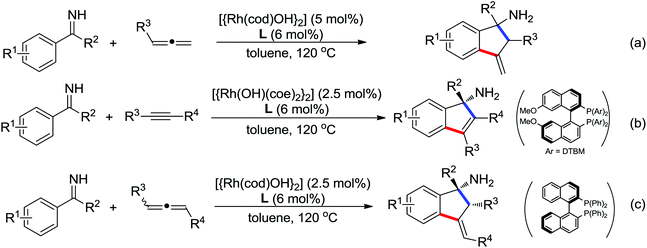
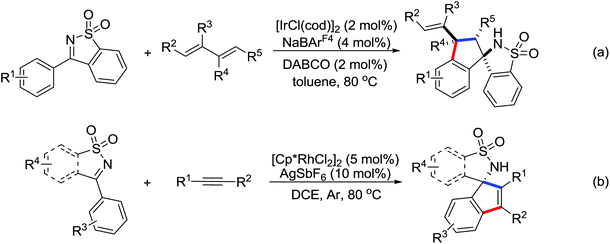
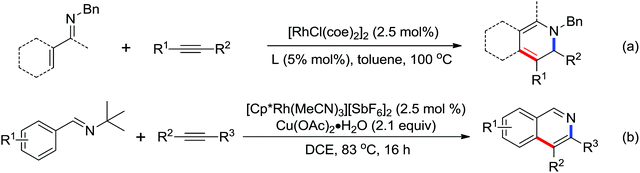
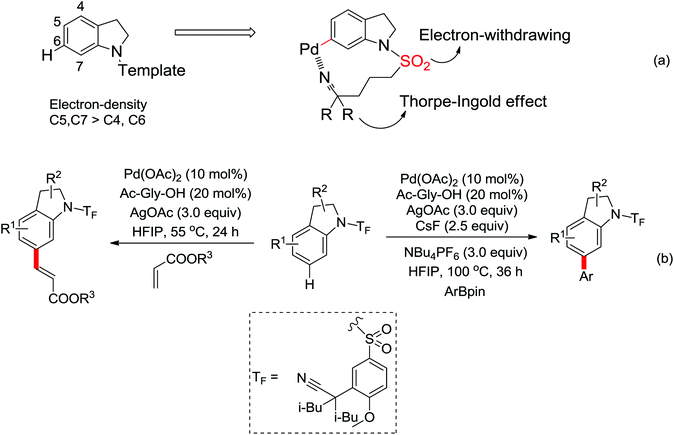
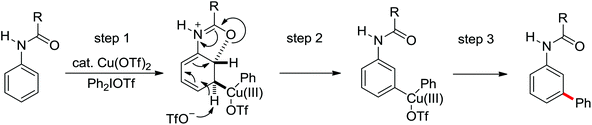
2. The C–H functionalization directed by amide
Among the myriad directing groups tested to date, amide has shown unique reactivity in transition metal-catalyzed C–H functionalizations and has served as a pivotal platform for the discovery and optimization of new transformations via C–H activation. Tremendous attention has been focused on these reactions over the last several years, leading to a variety of transition metal-catalyzed direct C–H functionalizations through the coordination of nitrogen and oxygen atoms with the metal center to fulfill the C–H activation.2.1. Pd catalyzed C–H functionalization
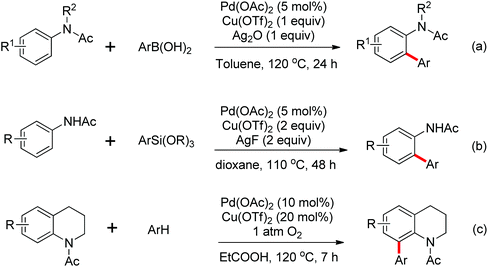
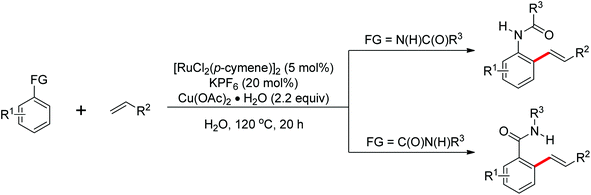
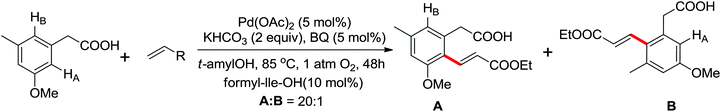
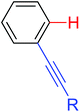
Leeuwen and co-workers demonstrated the ortho-olefination of anilides with acrylates by the palladium-catalyzed oxidative coupling reaction at room temperature in 2002 (Scheme 2).4 Compared with the direct olefination of arenes reported by Fujiwara and others, high ortho-selectivity was observed. It is believed that this was the first example of the use of anilide as the directing group for the C–H functionalization, and many relevant studies about ortho-olefination of anilides were reported in the following years.5While much success was achieved in this selective olefination with acrylates, a significant challenge was represented by aliphatic olefins due to the β-hydrogen elimination. Advances in this respect were recently accomplished by Jiang who showed that palladium-catalyzed transformation of anilides with norbornenes took place to give an array of functionalized indolines (Scheme 3).6 Notably, β-hydride elimination was almost halted due to the strained structure of norbornenes and an oxidative cyclization could be achieved to give the indolines.
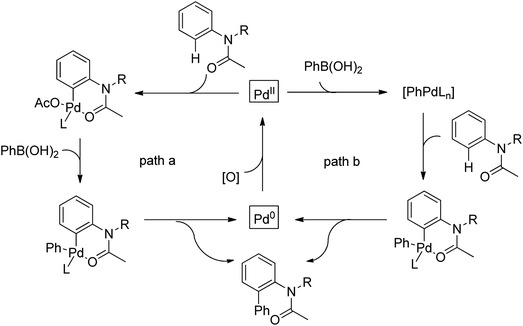
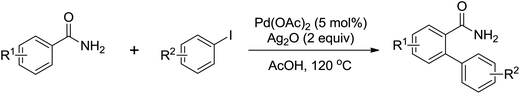
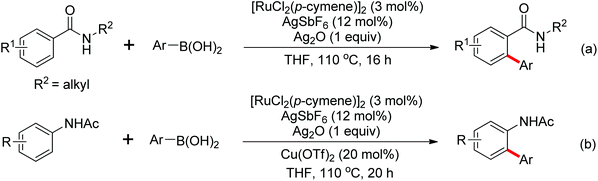
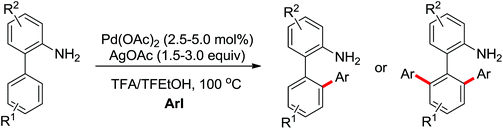
The palladium-catalyzed ortho-arylation of anilides was reported by Daugulis and co-workers in 2005 (Scheme 4).7 They found that the combination of aryliodides and AgOAc was equally effective with the diphenyliodonium salts, which significantly simplified the reaction substrates to give the diverse diarylated anilides in good yields. In comparison, direct arylation through the oxidative coupling of C–H bonds with organometallic reagents is much more challenging.8a The first beautiful example to apply for aryl boronic acids in the ortho-arylation directed by an acetamino group was demonstrated by Shi and co-workers (Scheme 5a).8b The electrophilic attack of a Pd(II) species at the aromatic ring with the assistance of the acetamino group may initiate the coupling reaction. The subsequent transmetalation and reductive elimination produced the arylated product (Scheme 6, Path a). Another possibility was the first formation of arylated Pd(II) species by transmetalation of the boronic acid with Pd(II) salts, followed by an electrophilic attack of Pd(II) at the aromatic ring to form a diaryl palladium species, which underwent reductive elimination to give the arylation product (Scheme 6, Path b). Shi and co-workers also, for the first time, applied arylsilanes as coupling partners to achieve the direct arylation to construct biaryls through this oxidative coupling strategy (Scheme 5b).8c
The direct hydrogenative arylation of acetanilides with another molecule of arenes through the double C–H activation strategy was described by Shi and co-workers (Scheme 5c).8d A remarkable feature of this reaction was the use of arenes as the coupling substrates rather than aryl halides or metallic aromatics, which allowed efficient biaryl synthesis via dual C–H coupling with high regioselectivity. However, it was necessary to use the arenes in excess (often as a solvent) in order to obtain higher yields in this reaction. Further study by Buchwald and co-workers indicated that the arylation of anilides with arenes could occur by the use of 4–11 equiv. of the arene substrates in TFA and a metal-containing cocatalyst was not required under an oxygen atmosphere (Scheme 7).9
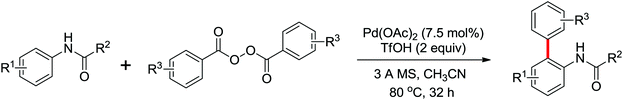
A notable advance of the above transformations was achieved by Dong and co-workers by the use of sodium persulfate (Na2S2O8) as the oxidant (Scheme 8).10 In their reaction, the scope of the coupling partners could be extended to phenylacetamides and benzamides, and an intramolecular cross-coupling for preparing lactams was also described. The relevant trifluoroacetate-bridged bimetallic Pd complexes derived from anilides were prepared and their stoichiometric reactivity was investigated.
More recently, this arylation was achieved at ambient temperature using highly electrophilic cationic palladium (Pd[TFA]+) as a catalyst by You and co-workers (Scheme 9).11 In addition, the ortho-acetoxylation of anilides with acetic acid was developed by the same group under similar conditions.
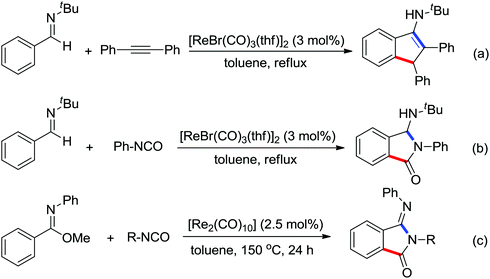
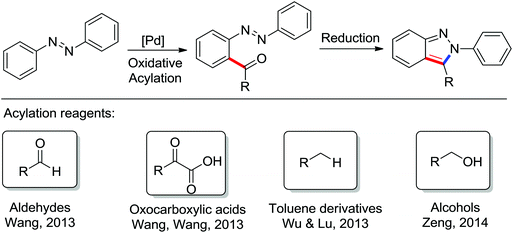
The Pd-catalyzed decarboxylative ortho-arylation of amides using aryl acylperoxides as arylation agents was reported by Wang and co-workers (Scheme 10).12 With the release of CO2, a variety of ortho-arylated anilide compounds could be prepared. When N-methoxyarylamides were used as the substrates under the reaction conditions, the cyclization reaction occurred after the decarboxylative arylation to give various phenanthridinones in moderate to good yields.
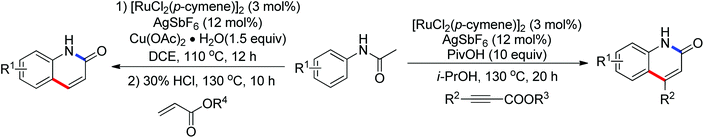
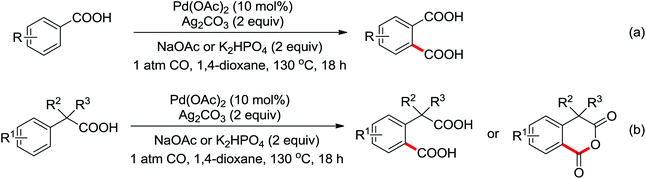
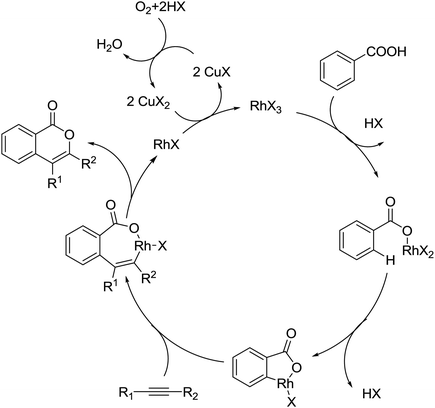
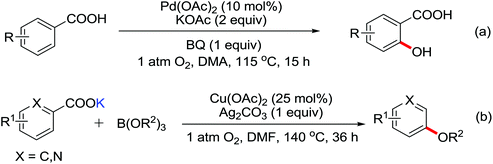
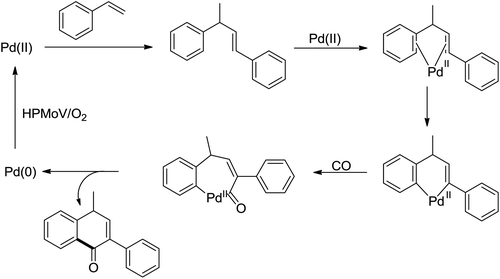
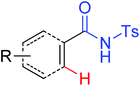
The palladium-catalyzed ortho-carboxylation of anilides with carbon monoxide was demonstrated in 2010 by Yu and co-workers (Scheme 11).13 Since carbon monoxide may impede the C–H activation process and reduce Pd(II) to Pd(0), highly electrophilic cationic Pd(II) species and relatively acidic conditions were required. The author adopted p-TsOH as an additive and HOAc as a cosolvent to carry out the reaction smoothly. Besides the preparation of useful anthranilic acids, the mechanistic insights of the reaction were also explored in detail by the authors. Strong evidence for the key intermediates from a mixture of anhydride and benzoxazinone was displayed and the reaction mechanism was well elucidated. This methodology afforded a rapid and convenient route to the assembly of diverse biologically active benzoxazinone and quinazolinone derivatives from simple anilides.
The Pd-catalyzed decarboxylative ortho-acylation of anilides with α-oxocarboxylic acids was described by Ge and co-workers in 2010 (Scheme 12).14 The reaction was conducted at room temperature and ortho-acylated anilides were afforded in good to excellent yields after the release of a molecule of CO2. This protocol also exhibited good feasibility and compatibility with both aryl and aliphatic α-oxocarboxylic acids. The method could be extended to prepare the corresponding C7-acylated indolines.15 Later, commercially available aromatic and aliphatic aldehydes were employed as the acylation reagents by the groups of Li, Kwong, Wang and Yu, respectively, which allowed the easier formation of ortho-acylated anilides.16 More than 2 equiv. TBHP was required for these transformations. Benzylic alcohols and toluene were found to be good substrates for the ortho-acylation of anilides under similar reaction conditions by the use of more TBHP.17
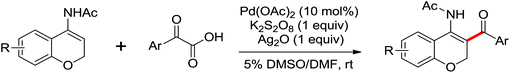
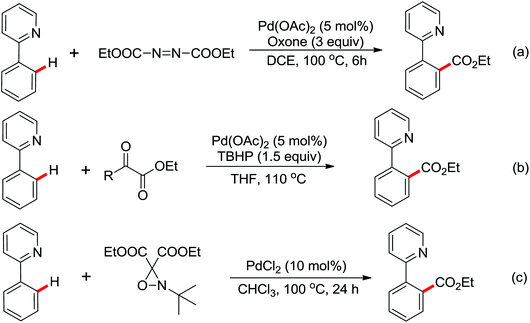
In 2012, Tan and co-workers successfully developed the palladium-catalyzed ortho-esterification of anilides by the use of glyoxylates (Scheme 13a).18 A bidentate phosphine ligand dppp was found to be beneficial for the reaction. The exploration of using a readily available agent, azodicarboxylate (DEAD), as the esterification reagent was reported by You's group (Scheme 13b).19 The reaction could be carried out at ambient temperature with (NH4)2S2O8 as an oxidant, allowing the facile synthesis of anthranilic acid derivatives. According to the controlled experiments, a Pd(II)/Pd(IV) catalytic cycle may be involved in the ethoxycarboxylation process via a radical pathway.
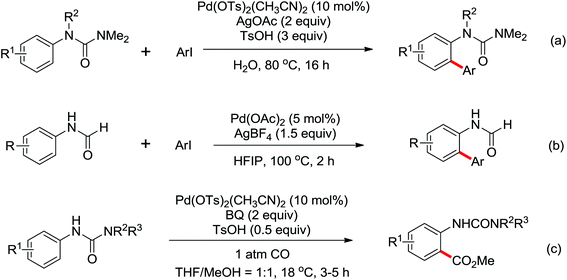
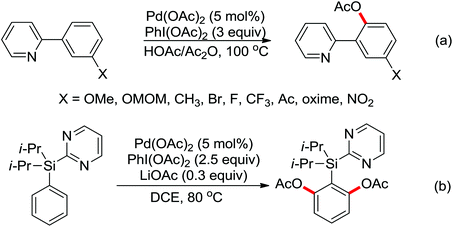
Based on amide-assisted C–H activation of anilides, a range of urea, formamide, guanidine and carbamate controlled ortho-olefination and -arylation of arenes were established.20 For example, the palladium-catalyzed ortho-olefination of aryl ureas with acrylates20b,c and ortho-arylations of aryl ureas with aryl iodides (Scheme 14a) or other arylation agents20d–f has been developed. In a related study, Xu and Huang's group developed a palladium-catalyzed ortho-arylation of formanilides (Scheme 14b), in which formamide was used as a directing group.20g The obtained biarylformanilide products could be easily converted to the relevant biarylisocyanides or N-heterocycles. Recently, Lloyd-Jones’ and Booker-Milburn's group reported a urea directed palladium-catalyzed carbonylation at room temperature in CO (1 atmosphere) to afford biologically and synthetically important anthranilic acid derivatives and heterocycles (Scheme 14c).20a [Pd(OTs)2(MeCN)2] was chosen as an efficient precatalyst and presented the exclusive activating effect of the urea substrates.
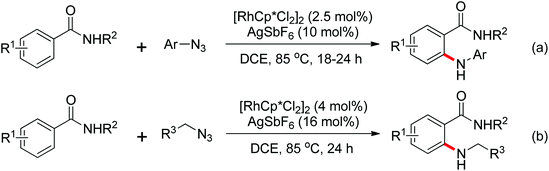
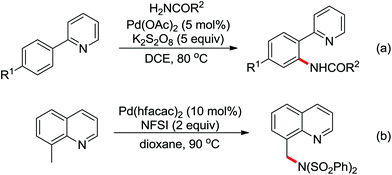
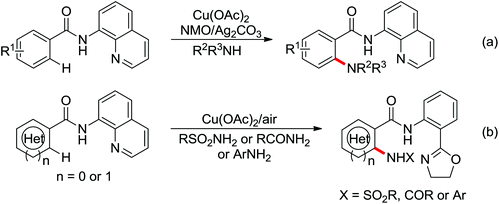
A palladium catalyzed intermolecular ortho-C–H amidation of anilides using N-nosyloxycarbamates as a nitrogen source for the synthesis of diverse 2-aminoanilines was developed by Yu and co-workers in 2010 (Scheme 15).21 This reaction exhibited good regioselectivity and functional group tolerance, and could proceed under relatively mild and simple reaction conditions. The N-nosyloxycarbamates were supposed to produce nitrene to insert into the cyclopalladated complex via a metal–nitrene pathway. A Pd(IV) or a dimeric Pd(III) complex were also possibly involved in the reaction process.
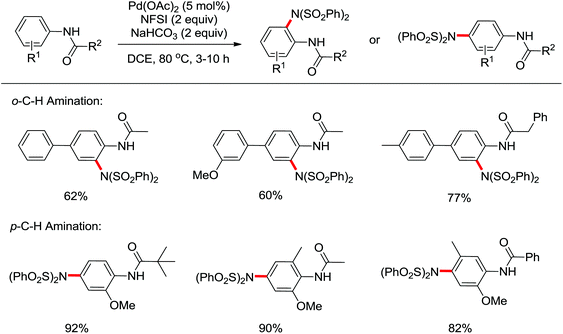
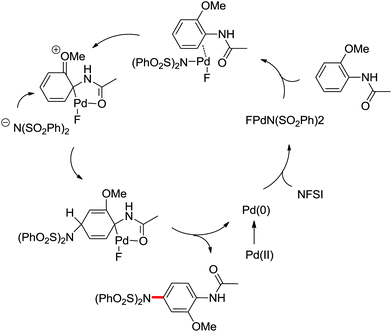
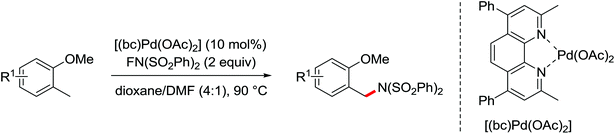
The direct construction of ortho-C–N bonds of anilides was explored by Zhang and co-workers in 2011 using N-fluorobenzenesulfonimide (NFSI) as a nitrogen source (Scheme 16).22 NFSI is a popular oxidant and can also be used as the fluorine reagent. Surprisingly, NFSI showed excellent reactivity as a nitrogen source in their experiments and the ortho C–H amination products were generated with high regioselectivity by the palladium catalysis. Interestingly, an active FPdN(SO2Ph)2 species was supposed to undergo the electrophilic palladation with the assistance of the amide group, leading to the formation of a dearomatized spiro-cyclopalladium intermediate. The key intermediate could be captured by N(SO2Ph)2 anions via nucleophilic amination followed by hydride elimination to afford ortho-amination products. It was reasonable that when 2-OMe-substituted anilides were used as the substrates under the optimal conditions, the para-amination products were obtained in high yields. An important observation by Zhang was that the addition of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) had little effect on the reaction, suggesting that a radical process was not involved in the reaction pathway.
The proposed mechanism of this remarkable transformation involved initial oxidative addition of the NFSI to a palladium(0) species, followed by the electrophilic palladation resulting in the dearomatized spiro-palladacycle intermediate. Thereafter, an amination reaction took place, and subsequent hydrogen elimination yielded the amination product (Scheme 17).
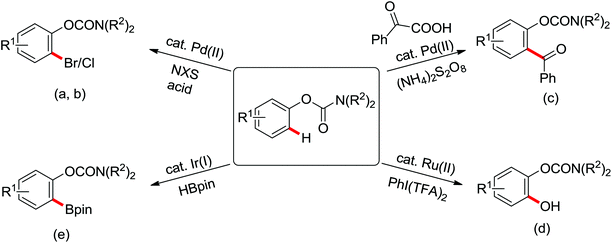
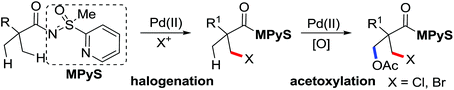
Pioneering research on the formation of C–X bonds using amide as a directing group was reported by Shi and co-workers in 2006 (Scheme 18).23 The halogenation reaction proceeded regioselectively as a result of the chelation effect of the amide directing group with CuCl2 or CuBr2 as halide sources. A cyclopalladation complex of acetanilide was prepared and was presumably involved in the reaction as the key intermediate through a Pd(II)/Pd(IV) or Pd(II)/Pd(0) mechanism.
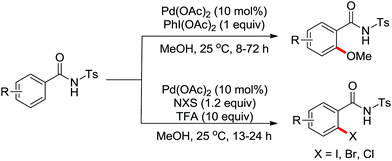
Advances were achieved by Bedford and co-workers using p-toluenesulfonic acid (PTSA) as the crucial additive (Scheme 19).24 Thus, the Pd-catalyzed ortho-halogenation smoothly took place at room temperature in air within 1–4 hours with N-halosuccinimide (NXS) as the halide source. The formation of para-bromination by-products through the electrophilic pathway was suppressed under their reaction conditions although electrophilic bromination was the major product in the presence of PTSA without a palladium catalyst.
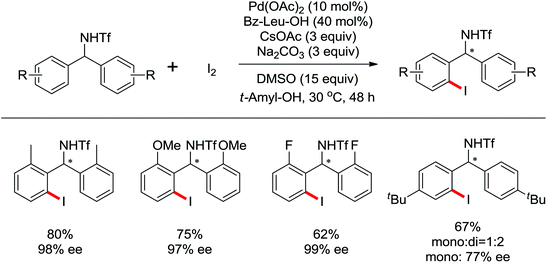
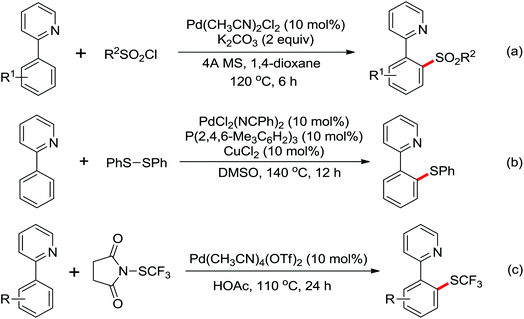
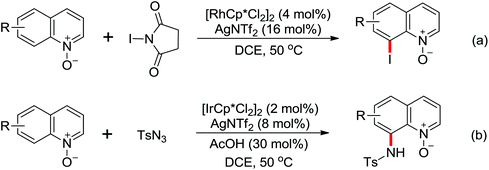
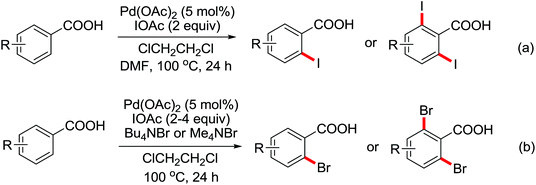
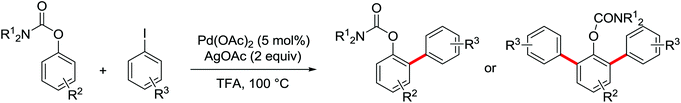
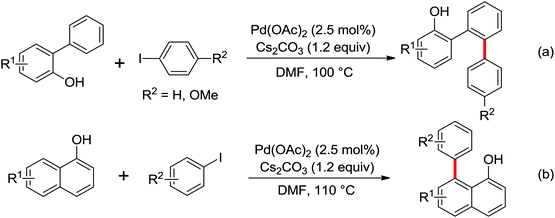
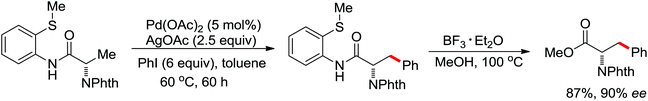
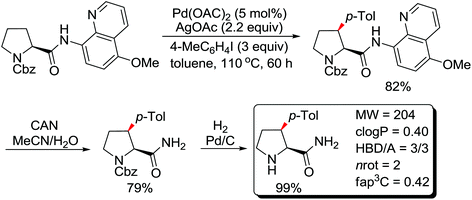
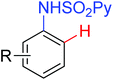
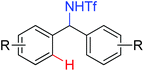
The more challenging ortho-iodination was reported by Yu and co-workers recently using cheaper and milder molecular I2 as the sole oxidant (Scheme 20).25 Notably, a wide range of heterocycles that were previously incompatible with directed C–H activation showed excellent reactivity to give the corresponding iodinated heterocycles in good yields. Since the product separation of this catalytic system was much simpler, they successfully developed the first enantioselective C–H iodination reaction to synthesize a variety of chiral diarylmethylamines using a mono-N-benzoyl-protected amino acid as the chiral ligand by the employment of trifluoromethanesulfonyl-protected diarylmethylamines as the substrates and readily available molecular iodine as the sole oxidant.26 In this reaction, the choice of a suitable base and additive had a significant influence on both the yield and enantioselectivity. The useful iodination reaction could also be extended to a gram-scale reaction under mild reaction conditions with a slight decrease of enantioselectivity.
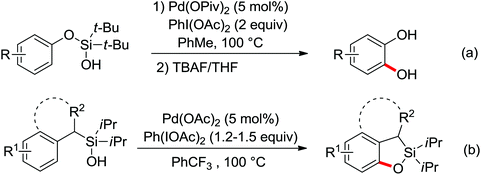
Palladium-catalyzed directed ortho-alkoxylation of anilides with primary and secondary alcohols was developed by Wang and co-workers (Scheme 21).27 In their study of the palladium-catalyzed ortho-alkoxylation of N-methoxybenzamides, a poor yield was obtained for the methoxylation of acetanilide.28 After extensive optimization of the reaction conditions, the addition of catalytic methanesulfonic acid (MSA) was found to have a significant effect on the reaction efficiency and a wide variety of aryl alkyl ethers could be synthesized in moderate to excellent yields at room temperature in the presence of 20 mol% MSA.
The palladium-catalyzed ortho-trifluoromethylation of anilides using Umemoto's reagent as a trifluoromethyl source was described recently by Shi and co-workers (Scheme 22).29 The reaction exhibited broad substrate scope and high reactivity and efficiency. A series of control experiments for mechanism research indicated that the C–H cleavage was involved in the rate-determining step as the catalytic process. Furthermore, a palladacycle complex was synthesized and successfully used as a catalyst in the reaction, and the stoichiometric reaction from the palladacycle complex could also afford the desired product. All of the relevant studies provided solid evidence of the reaction mechanism. In addition, various ortho-trifluoromethyl anilide products could be easily transformed into different patterns of active molecules through existing methods.
Enamides are important synthetic intermediates and stable enamine surrogates that have been widely used in both academia and industry. Due to the special electron-donating properties of nitrogen atoms, enamides are quite rarely used as coupling partners. The simple extension of catalytic systems to C(sp2)–H bond activation of enamides has also been proved to be difficult.
In 2009, Loh and co-workers developed the first palladium-catalyzed direct coupling of cyclic enamides with aryl boronic acids to generate various cyclic enamide derivatives in moderate to good yields (Scheme 23a).30a A six-membered palladacycle with the metal coordinated to the acetamino group was supposed to be the key intermediate for the C–H activation. By using the same strategy, they developed a palladium-catalyzed arylation of cyclic enamides with Hiyama organosilanes in the presence of 3 equiv. AgF (Scheme 23b).30b Both of these procedures required the aryl–metal species as the coupling partners. Recently, this group made a significant improvement by using the simple inactivated arenes as the coupling partner (Scheme 23c).30c Absolute stereocontrol of the double bond to form highly substituted enamides with perfect Z selectivity was achieved in their transformation.
The Fujiwara oxidative olefination of enamides with acrylates and acrylonitrile was reported by Loh and co-workers using ambient oxygen as the sole oxidant with excellent regioselectivity (Scheme 24).31 A key six-membered cyclic vinylpalladium complex was isolated and characterized by 1H NMR spectroscopy and single crystal X-ray analysis, which was propounded to be the intermediate during the olefination reaction of enamides. Good functional group tolerance and excellent regioselectivities were the notable features of the reaction. Very recently, a palladium catalyzed direct ethynylation reaction between N-vinylacetamides and (bromoethynyl)triisopropylsilanes was reported by the same group (Scheme 25).32 A wide range of conjugated enyne products were obtained in moderate to good yields. In order to obtain the high reactivity, a solvent mixture of DCM and DMSO was necessary, and DMSO was supposed to act as a weak ligand to stabilize the palladium catalyst via coordination. A Pd(II)/Pd(IV) pathway was proposed for the reaction.
The palladium-catalyzed acylation of enamides using α-oxocarboxylic acids as a decarboxylative acylation agent was demonstrated by Duan and Guo in 2012 (Scheme 26).33 This elegant method provided an efficient route for the preparation of various acylated enamides from readily available α-oxocarboxylic acids under mild reaction conditions.
The palladium-catalyzed synthesis of heterocycles from enamides was investigated by Guan and co-workers (Scheme 27).34 They discovered that 1,3-oxazin-6-ones could be generated by the palladium-catalyzed carbonylation of enamides with CO (1 atm). The use of KI and DABCO in the reaction remarkably increased the efficiency of the reaction. It was a convenient and environmentally friendly method for the preparation of this important six-membered heterocycles. More recently, they described an oxidative annulation reaction between enamides and alkynes by palladium catalysis to give highly substituted pyrroles via the C(sp2)–H bond activation (Scheme 28).35 In this case, the phosphine ligand (Xantphos) could significantly improve the conversion of the reaction. Various functional groups could be tolerated, and the poly-substituted pyrroles, which were not easy to prepare by other methods, could be produced in high yields.
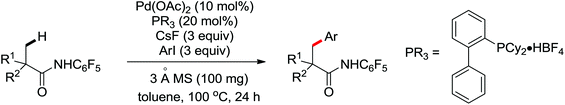
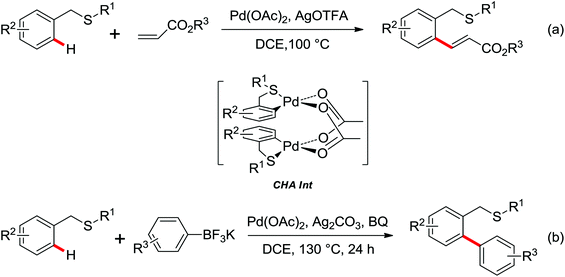
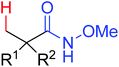
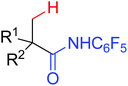
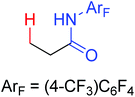
The first palladium catalyzed functionalization of a C(sp3)–H bond with aryl and alkyl boronic acids by the use of CONHOMe as a directing group was reported by Yu and co-workers in 2008 (Scheme 29).36 The reaction employed air as a stoichiometric oxidant to replace the expensive silver salts and the CONHOMe group can be easily transformed into some other useful functional groups. The potential utility of this elegant protocol was broad and could be extended to afford some biologically active molecules. Shortly afterwards, the same group developed a novel Pd(0)/PR3-catalyzed intermolecular arylation of C(sp3)–H bonds directed by the CONH-C6F5 group (Scheme 30).37 Compared with the useful C(sp3)–H functionalizations using Pd(II)/Pd(IV) and Pd(II)/Pd(0) catalysis reported by Yu,38 this reaction employed Pd(0)/PR3 as a catalytic system. Buchwald's Cyclohexyl JohnPhos and CsF were utilized as the ligand and the base respectively to improve the reactivity of the reaction. This protocol provided an efficient approach to the arylation of carboxylic acids. To gain a deeper understanding of the reactivity of Pd(0)/PR3-catalyzed intermolecular arylation of sp3 C–H bonds, a further detailed mechanistic investigation of this reaction was demonstrated by the same group in 2013.39
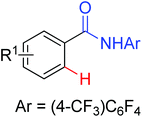
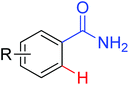
The Pd-catalyzed C(sp2)–H arylation directed by amides using simple arenes as the arylating agents was reported by Yu's group in 2011 (Scheme 31).40 The reaction featured the exceedingly high para-selectivity for the C–H/C–H coupling reactions. The F+ reagents were used as bystanding oxidants for the high yield and regioselectivity, in which NFSI was the optimal one. The reaction pathway was supposed to be divided into two C–H activation processes. The first C–H activation step was a well-known procedure directed by the acidic amide group, and the second C–H activation step presumably underwent an electrophilic palladation mechanism based on the result of the KIE experiment. The elegant method provided an alternative route for the synthesis of a series of synthetically useful biaryl products. In 2012, Wang and co-workers developed a palladium-catalyzed ortho-arylation of benzamides by aryl iodides with simple amide CONH2 as a directing group (Scheme 32).41 It was the first time to use N-unsubstituted amide CONH2 as a directing group. The acquired biphenyl-2-carboxamide products could be readily transformed to various biaryl derivatives.
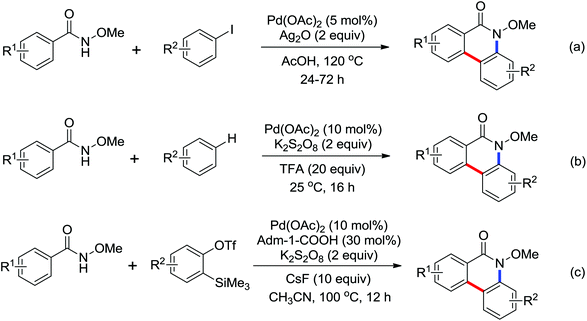
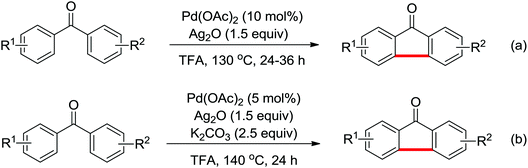
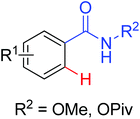
Phenanthridinones are regarded as the key units of important biologically active molecules and natural products. A one-pot cascade reaction to synthesize phenanthridinones through the palladium-catalyzed reaction of N-methoxybenzamides and aryl iodides was developed by Wang and co-workers in 2011 (Scheme 33a).42 The dual C–H activation included intermolecular C–C bond formation by directed arylation and intramolecular C–N bond formation in one pot. The Pd(II)–Pd(IV)–Pd(II) and Pd(II)–Pd(0)–Pd(II) catalytic cycles were proposed to be involved in the reaction system. Later, Cheng and co-workers reported a more convenient method to produce the phenanthridinones by palladium-catalyzed multiple C–H activation reactions of N-methoxybenzamides with simple arenes at ambient temperatures (Scheme 33b).43 It was a great improvement for replacing aryl iodines with more readily available arenes. K2S2O8 was used as an oxidant and TFA was chosen to be an important additive to promote the reactivity of the reaction. This methodology was efficient and practical for the synthesis of diverse phenanthridinone derivatives. More recently, a palladium-catalyzed cyclization of benzamides with highly reactive benzynes to afford phenanthridinones in one pot was described by Jeganmohan and co-workers (Scheme 33c).44a The sterically hindered 1-adamantanecarboxylic acid (Adm-1-COOH) acted as an effective additive and enhanced the reaction yields. The highly reactive benzyne was used as a π-component to insert into an ortho-metalated five-membered palladacycle for the cyclization reaction. Almost at the same time, Xu and co-workers reported a similar study of palladium-catalyzed oxidative annulation of arynes and cyclooctynes with benzamides for the synthesis of phenanthridinones and isoquinolones at high efficiency.44b
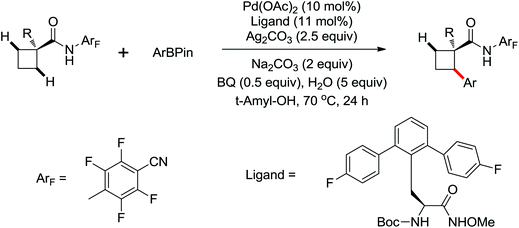
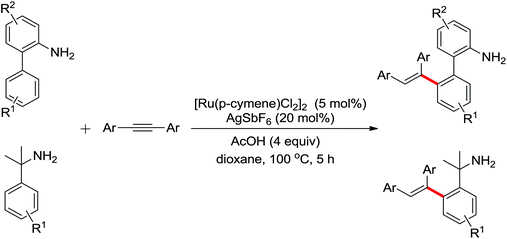
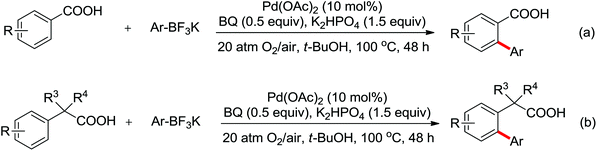
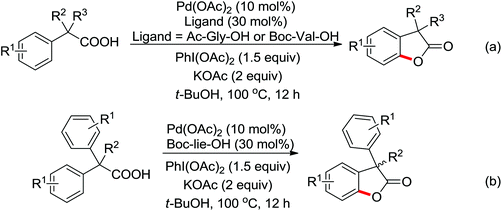
Enantioselective functionalization of prochiral inactivated C–H bonds is of great significance and remains a great challenge. An enantioselective method for Pd(II)-catalyzed arylation of methylene β-C(sp3)–H bonds in cyclobutanecarboxylic acid derivatives using arylboron reagents as arylating agents was developed by Yu and co-workers recently (Scheme 34).45 The chiral mono-N-protected α-amino-O-methylhydroxamic acid (MPAHA) ligands played a key role in achieving high yields and enantioselectivities of the reaction. As early as 2011, the same group had reported the first Pd(II)-catalyzed enantioselective C–H activation of cyclopropanes using a novel mono-N-protected amino acid as a ligand.46 These protocols provided new methods to enantioselectively construct a variety of biologically active cyclopropane and cyclobutanecarboxylic acid derivatives.
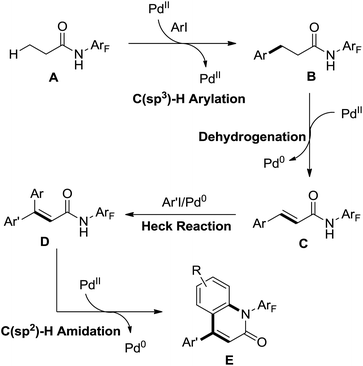
A ligand promoted triple sequential arylation of the β-C(sp3)–H bonds of propionamide followed by amidation to prepare diverse 4-aryl-2-quinolinones in one pot was developed by Yu and co-workers in 2014 (Scheme 35).47 These cascade reactions contained C(sp3)–H arylation, dehydrogenation, Heck reaction and C(sp2)–H amidation. Similar to the previous studies of ligand-controlled β-arylation reactions of primary and secondary C(sp3)–H bonds by the same group,48 the crucial role of a suitable ligand could not be ignored. 2,5-Lutidines were chosen as the effective ligand to promote the triple sequential C–H activation reactions and a stereospecific Heck reaction. The plausible mechanism was proposed: at first, hydrocinnamide B was afforded by the β-arylation of the primary C(sp3)–H bond, followed by the β-hydride elimination assisted by Pd insertion to give cinnamide intermediate C. Then the intermediate C underwent Heck coupling with a second aryl iodide to yield the arylated cinnamide intermediate D, which underwent intramolecular amidation to provide the final product 4-aryl-2-quinolinone E (Scheme 36).
A Pd-catalyzed tandem C–H olefination/annulation sequence that employed tosyl amide as a novel directing group to afford a wide range of isoindolinones was developed by Zhu and co-workers in 2011 (Scheme 37).49 Various aliphatic alkenes as well as conjugated alkenes were all compatible with this protocol. Bathophenanthroline was utilized as a superior ligand to promote the reaction. Eco-friendly molecular oxygen functioned as the sole oxidant. The reaction was green and practical with water as the only waste. In the same year, Booker-Milburn's and Wang's groups also reported a similar methodology for the synthesis of isoindolinones via Pd-catalyzed C–H activation of N-alkoxybenzamides respectively.50 A series of isoindolinone products were obtained by the use of these general cascade reactions. Furthermore, the substituted phthalimides could also be afforded by carbonylation with CO.
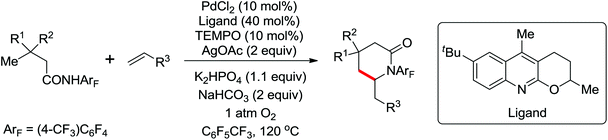
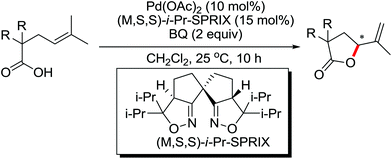
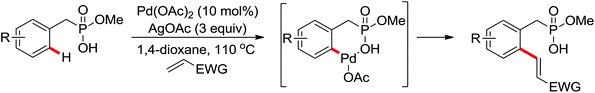
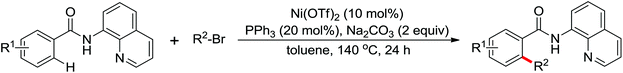
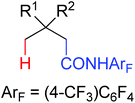
The development of C(sp3)–H olefination is more difficult and remains a challenge. Recently, Yu and co-workers established a quinoline-based ligand enabled γ-C–H olefination and carbonylation of aliphatic acids directed by a weakly coordinating amide directing group (Scheme 38).51 After detailed screening of different pyridine- and quinoline-based ligands, the developed quinoline-based ligand containing a pyran structural motif was vital for the smooth transformation of γ-C–H to furnish a variety of highly functionalized all-carbon quaternary centers. Compared with the ligand-promoted β-C–H functionalizations of aliphatic acids developed by the same group,52 the γ-C–H activation of aliphatic acids had more challenges and greater synthetic significance.
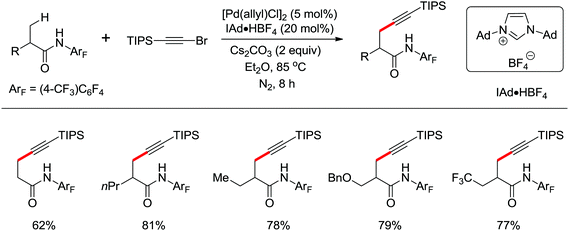
Concerning the C(sp2)–H alkynylation reactions, the alkynylation of inert C(sp3)–H bonds is not easy to achieve. The first alkynylation of β-C(sp3)–H bonds with alkynyl bromides using Pd(0)/N-heterocyclic carbene (NHC) and Pd(0)/phosphine (PR3) catalysts was reported by Yu and co-workers in 2013 (Scheme 39).53 Suitable ligands played a remarkable influence on the reactivity of the reaction and a wide range of PR3 and NHC ligands were screened for this reaction. PCy3·HBF4 and IAd·HBF4 were optimal, respectively. Polar, strongly coordinating solvents gave no desired product. A variety of amides from commercially available carboxylic acids could be readily installed on the alkynyl group at the β-position using this protocol.
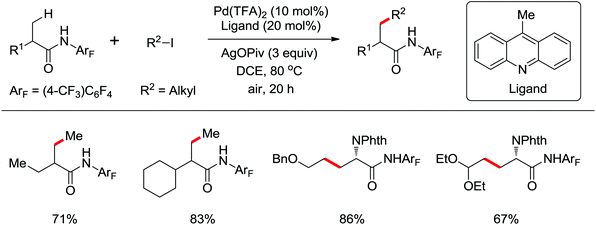
The C–H alkylation catalyzed by transition metals constitutes a useful alternative tool for C–C bond formation. Besides alkylborons, diverse alkylhalides were utilized as coupling partners in transition metal-catalyzed C–H alkylation for the past few years. A ligand-promoted Pd(II)-catalyzed C(sp3)–H and C(sp2)–H alkylation of simple amides utilizing alkyl iodides as an alkylating agent was presented by Yu's group in 2014 (Scheme 40).54 Pyridine- or quinoline-based ligands were identified to have some positive effect on Pd-catalyzed C(sp3)–H functionalization.47,48,51 As for the oxidant, AgOPiv was more effective than Ag2CO3 in improving the yield of the reaction. A broad series of biologically active unnatural amino acids and geometrically controlled tri- and tetrasubstituted acrylic acids could be produced smoothly by this efficient alkylated methodology.
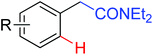
An efficient method for Pd-catalyzed oxidative decarboxylative acylation of phenylacetamides with α-oxocarboxylic acids under mild conditions was developed by Kim and co-workers in 2013 (Scheme 41).55 (NH4)2S2O8 was identified as a superior oxidant and DCE was used as the optimal solvent. Under the reaction system, a wide range of bisacylated products of symmetrical phenylacetamides and monoacylated products of unsymmetrical phenylacetamides were successfully obtained, which could also be further transformed into various synthetically important 3-isochromanone derivatives via the reduction and intramolecular cyclization process.
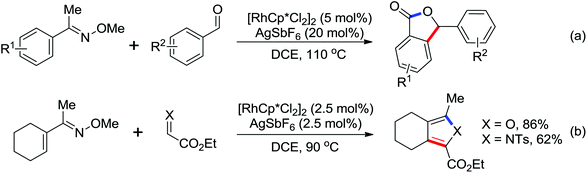
The first Pd-catalyzed C–H activation/annulation reaction for the convenient synthesis of hydroxyl isoindolones using N-OMe benzamide and benzaldehyde as the starting substrates was presented by Zhao and co-workers in 2013 (Scheme 42).56 The readily available TBHP was the optimal oxidant for the reaction. Compared with Rh-catalyzed double-activation reactions for the synthesis of hydroxyl isoindolones reported by Kim and co-workers,57 this reaction exhibited many notable features, such as short reaction times, wide substrate scope and high atom efficiency. The reaction mechanism was supposed to undergo a radical process after a series of control experiments were carried out.
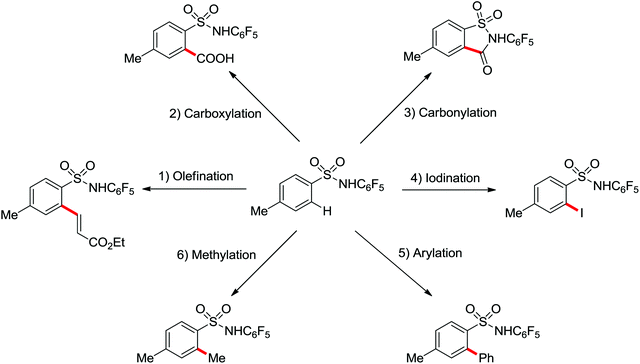
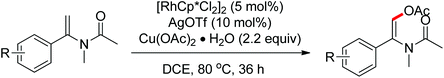
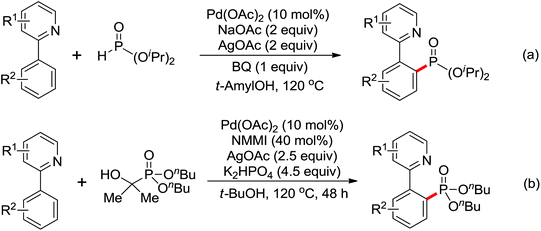
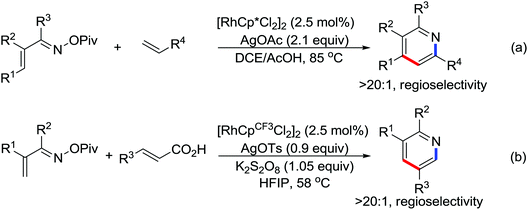
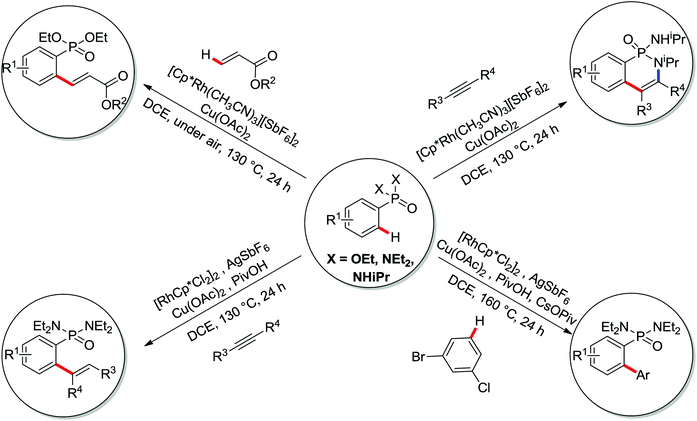
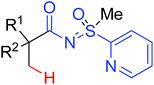
A diverse range of reactions directed by the sulfonamide functionality under different catalytic conditions were reported by Yu and co-workers in 2011 (Scheme 43).58 After extensive screening and optimizations, a series of divergent ortho-C–H functionalization processes, including olefination, carboxylation, carbonylation, iodination, arylation, and alkylation, were successfully achieved, providing a straightforward route for the synthesis of various sulfonamide analogues.
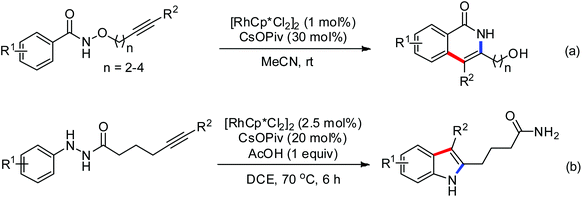
Besides the direct C–C bond construction from a C–H bond, the direct C–H amination is a significant issue in C–H functionalization. Yu and co-workers explored the palladium-catalyzed intermolecular C–H amination of N-aryl benzamides utilizing electrophilic O-benzoyl hydroxylamines as nitrogen sources (Scheme 44).59 The O-benzoyl hydroxylamines could also be in situ generated from secondary amines and benzoyl peroxide in one pot. This useful finding had vital significance in the study of intermolecular C–H amination with alkylamines, providing a complementary route to synthesize diverse biologically active arylamines. As early as 2008, the same group reported a catalytic C–H lactamization reaction using a Pd(II) catalyst.60 Various N-methoxyhydroxamic acids were used as substrates for the preparation of β-, γ-, and δ-lactams. A Pd(II)/Pd(IV) pathway was possibly involved in the catalytic C–H amination process.
Wang and co-workers developed a Pd-catalyzed ortho-alkoxylation using the N-methoxy amide group (CONHOMe) as an ortho-directing group (Scheme 45).61 K2S2O8 was superior for other oxidants and the molecular sieve was necessary for the high yield of the reaction. A Pd(II)/Pd(IV) pathway catalytic cycle was presumably involved in the reaction. A wide range of alkoxylations of N-methoxybenzamides could be furnished and further converted to various derivatives for potential synthetic application.
Under simple and mild conditions, Fabis and coworkers demonstrated a palladium-catalyzed ortho-C–H alkoxylation and halogenation of substituted arenes directed by the N-tosylcarboxamide group (Scheme 46).62 MeOH and NXS acted as alkoxylated and halogenated sources, respectively. Under the efficient methodology, various ortho-alkoxylations and ortho-halogenations of N-tosylbenzamides were afforded in high yields, which could be further transformed into different valuable synthetic intermediates by some ready manipulations. A palladacycle was isolated and characterized by X-ray analysis to investigate the process of C–X and C–O bond formation.
Recently, Yu and co-workers reported a Pd(II)-catalyzed ortho-C–H iodination of various arenes as well as a wide range of heterocycles directed by a weakly coordinating amide auxiliary using cheaper and milder I2 as the sole oxidant (Scheme 47).63 CsOAc was identified as an iodide scavenger and NaHCO3 was used as a coadditive to form the imidate structure by N–H deprotonation of the amide, which could dramatically improve the reactivity of the reaction. This convenient protocol showed wide substrate scope including various heterocycles that showed poor reactivity in many directed C–H activation reactions.
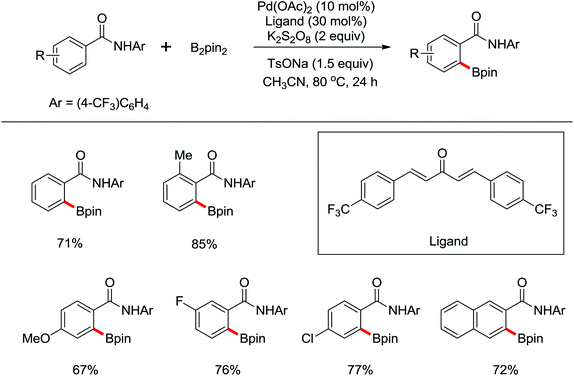
The first Pd-catalyzed ortho-C–H borylation with N-arylbenzamides and diboron reagent (B2Pin2) under oxidative conditions was developed by Yu and co-workers in 2012 (Scheme 48).64 The usage of electron-deficient dba ligand was necessary for significantly improving the yield of the borylated product with excellent monoselectivity. The choice of a suitable base was also essential for the good efficiency of the reaction and TsONa was the optimal one. This borylation reaction showed broad substrate scope and could be easily extended to the gram scale in moderate yield. Notably, the borylated products could also be transformed into a wide range of synthetically useful synthons in excellent yields.
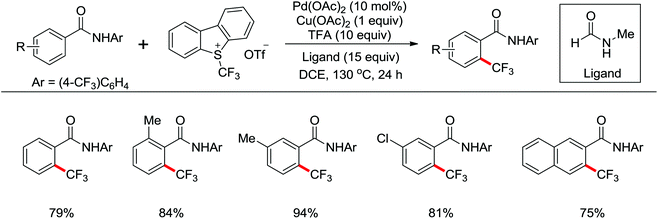
Trifluoromethylated arenes are important structural motifs and widely exist in diverse pharmaceuticals, agrochemicals and organic materials. Trifluoromethylation of aryl C–H bonds catalyzed by transition metal has emerged as a powerful tool to construct C–CF3 bonds. A Pd(II)-catalyzed trifluoromethylation of a range of N-arylbenzamides using Umemoto's reagent as a trifluoromethylating agent was developed by Yu and co-workers (Scheme 49).65 The ligand had a significant impact on this reaction and N-methylformamide was the best choice, which was identified as both a ligand and a base. Different potential coordination modes of acidic amides with the palladium(II) catalyst were proposed, which might promote the reactivity of directed C–H activation. A wide array of biologically active trifluoromethylated products were delivered by this elegant strategy from readily available synthetically versatile arenes.
2.2. Rh catalyzed C–H functionalization
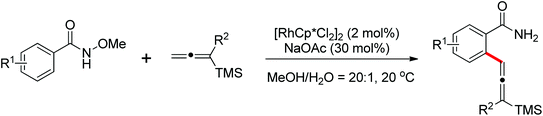
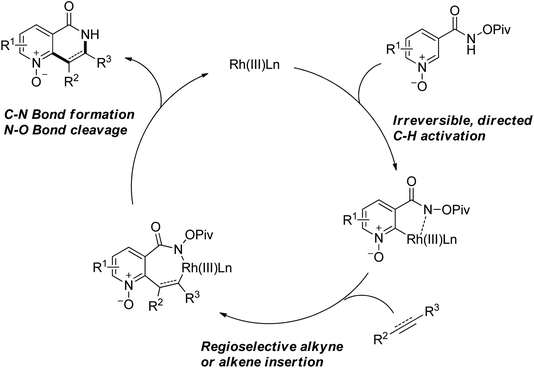
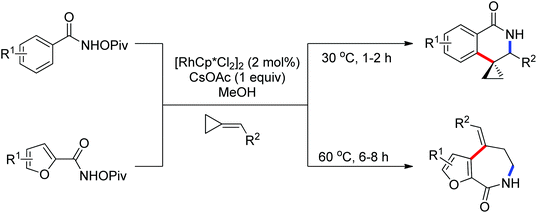
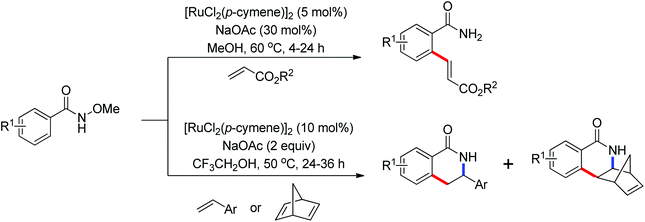
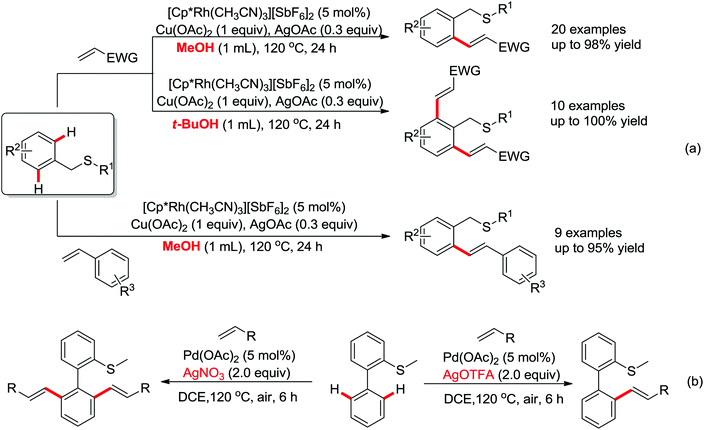
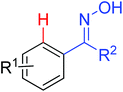
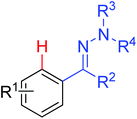
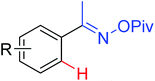
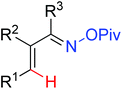
As a powerful catalyst, the widespread use of the rhodium catalyst in C–H functionalizations has made drastic progress in recent years.1u,3d,66 In the catalyzed oxidative olefination of acetanilides or benzamides, Rh catalysts exhibited many notable features in contrast to common Pd catalysts, such as lower catalyst loadings, broad functional group tolerance and high reactivity of electron-neutral olefins. A Rh(III) catalyzed oxidative ortho-olefination and vinylation of various acetanilides derived from the corresponding anilines was demonstrated by Glorius and co-workers in 2010 (Scheme 50).67 Both electron-withdrawing and electron-donating groups were compatible with the reaction and the yield was up to 98%. It is worthwhile to note that ethylene could also be involved in this reaction and a satisfactory yield of styrenes could be smoothly prepared, which were difficult to access by other direct catalytic oxidative–Heck reactions. The produced olefins had synthetical versatility and could be applied to natural product total synthesis.Later, an efficient Rh(III)-catalyzed C–H olefination reaction of N-methoxybenzamides under mild conditions was reported by the same group (Scheme 51).68 It is important to note that they exploited CONH(OMe) as a directing group and N–O bonds as an internal oxidant, avoiding the addition of an external oxidant. This reaction had more advantages than similar previous reports:69 mild reaction conditions, excellent regio- and mono-selectivity and broad substrate scope and high yields. In addition, when the –OMe group on the benzamide N was replaced with a more accessibly Rh-chelated O-pivaloyl group, diverse tetrahydroisoquinolinone derivatives were afforded. This strategy provided a practical route for the olefination of benzamide derivatives and selective construction of valuable tetrahydroisoquinolinone products.
By a similar strategy, Lu and Liu developed an efficient Rh(III) catalyzed C–H olefination reaction with N-phenoxyacetamides and alkenes to afford ortho-alkenyl phenols by the use of an oxidizing directing group in 2013 (Scheme 52).70 The N–O bond was identified as an internal oxidant to promote the olefination process and could be cleaved after the completion of the reaction. This reaction offered a convenient route for the synthesis of ortho-alkenyl phenols under mild conditions.
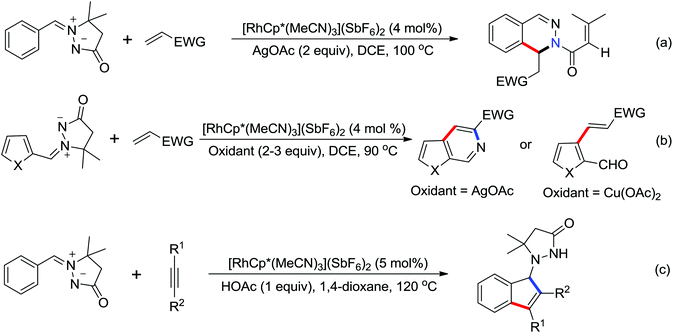
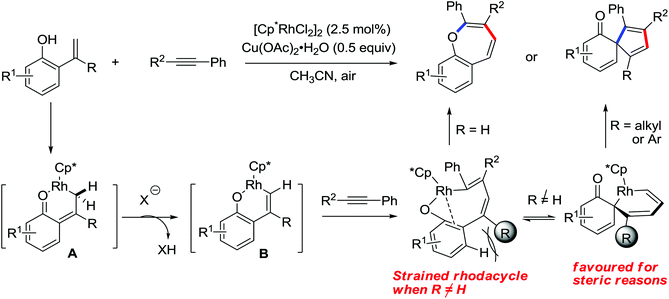
N-Tosylhydrazones were regarded as the precursors for the in situ generation of diazo substrates, which were extensively involved in the Rh(III)-catalyzed directed ortho C–H bond functionalization reactions.71 A Rh(III)-catalyzed ortho-alkenylation of N-phenoxyacetamides with N-tosylhydrazones or diazoesters via C–H activation was demonstrated by Wang and co-workers in 2014 (Scheme 53).72 As a new type of coupling partners, N-tosylhydrazones were successfully applied in the chelation-assisted C–H functionalizations for the first time. The oxidizing directing group, which contained an N–O bond, was chosen to orient the ortho-alkenylated reaction. Under mild reaction conditions, a wide range of ortho-alkenyl phenols were obtained in moderate to good yields. The Rh(III)–carbene migratory insertion process was proposed to exist in the catalytic cycles.
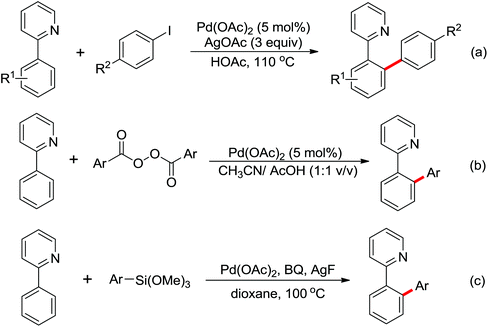
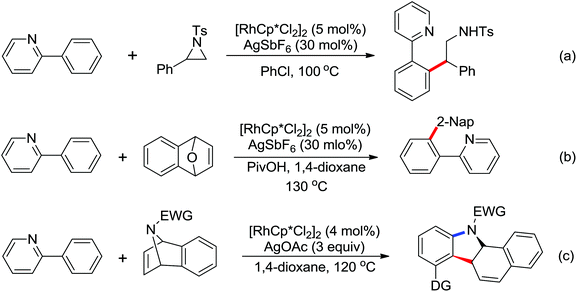
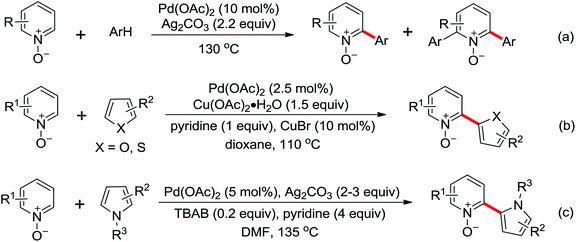
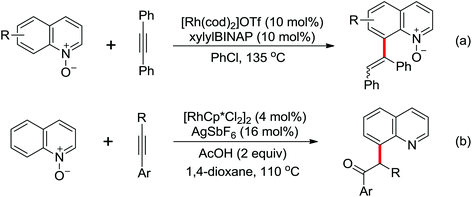
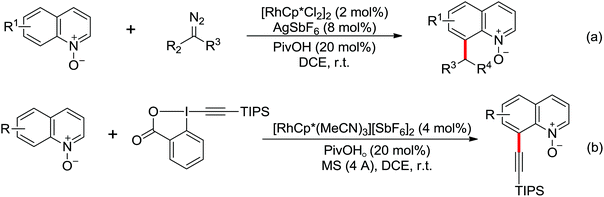
Due to the poor electron density of the pyridyl ring and the strong coordination of the nitrogen atom, few examples of oxidative olefination of pyridines have been reported. A Rh(III)-catalyzed oxidative olefination of pyridines and quinolines using pivalamides at the ortho-position as the directing group was developed by Shi and co-workers in 2013.73 Cu(OAc)2 was supposed to be coordinated with the pyridine nitrogen to afford high reactivity. This efficient protocol was successfully applied to synthesize pharmaceutically important molecules. After one year, the same group reported a rhodium(III)-catalyzed selective olefination of picolinamide derivatives with carboxamides as directing groups, providing a convenient method for the synthesis of diverse 3-alkenylpicolinamides.74
In contrast to alkenes and alkynes, the application of allenes as the coupling partners in Rh-catalyzed C–H functionalization transformation remains sparse. Recently, an efficient Rh(III)-catalyzed intermolecular annulation of N-pivaloyloxy benzamide derivatives with allenes for the rapid construction of biologically important 3,4-dihydroisoquinolin-1(2H)-ones was disclosed by Glorius and co-workers in 2012 (Scheme 54).75 The deuteration experiments were conducted and the C–H bond cleavage was presumably involved in the rate-determining step. The reaction might proceed in a concerted metalation/deprotonation (CMD) pathway and the steric interference had a distinct influence on the regioselectivity of the allenes. Later, the same group reported a Rh(III)-catalyzed alkenyl coupling reaction directed by amide groups with allenyl carbinol carbonates to afford a variety of [3]dendralenes (Scheme 55).76 This protocol provided a practical and straightforward way to access [3]dendralenes with diverse substitution patterns.
The first example of Rh(III)-catalyzed ortho allenylation of N-methoxybenzamides with allenylsilanes to produce diverse poly-substituted allenylsilanes under very mild reaction conditions was developed by Ma and co-workers in 2013 (Scheme 56).77 The reaction was supposed to undergo C–H bond cleavage, allene insertion and β-H elimination to give the final 2-(3-silylallenyl)benzamide products. The transformation showed many noteworthy advantages, such as ambient reaction temperature, insensitive to air and moisture, high efficiency and broad functional group tolerance. The products could be further transformed into a wide range of useful corresponding derivatives and the presented protocol had a broad application potential from the viewpoint of synthesis. A rhodium-catalyzed coupling–cyclization reaction of 2,3-allenols with N-methoxybenzamides to synthesize various optically active 2,5-dihydrofurans via C–H functionalization was reported by the same group.78 The tetramethylcyclopentadienyl anion was used as an effective ligand and highly efficient axial-to-central chirality transfer was observed in the process of the reaction.
A Rh(III)-catalyzed selective coupling of N-methoxy-1H-indole-1-carboxamide and aryl boronic acids was described by Cui and co-workers recently (Scheme 57).79 Through the switch of different reaction conditions or substitutions, three distinct reaction patterns of arylation, [4 + 2] cyclization, and [4 + 1] cyclization were achieved smoothly, producing divergent products. The C–H activation and electrophilic addition were proposed to be involved in the reaction pathway.
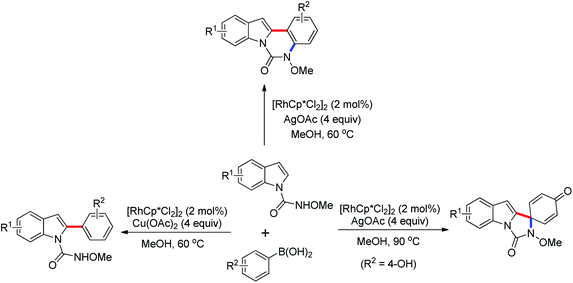
The Rh(III)-catalyzed dual C–H activation of N-methoxybenzamides with aryl boronic acids through one-pot C–C/C–N bond formation to provide various substituted phenanthridinones in good yields was developed by Cheng and co-workers in 2012 (Scheme 58).80 Previous methods to synthesize phenanthridinones by the use of palladium catalysts have been reported by Wang42 and Cheng43 in 2011. The presence of a silver salt was crucial to the reaction and 4 equivalents of Ag2O were necessary for the highest yield of the product. High regioselectivity and efficiency of this catalytic reaction were achieved. Two years later, the same group reported a similar Rh(III)-catalyzed dual C–H bond activation and annulation reactions for the one-pot synthesis of substituted phenanthridinone derivatives using aryltriethoxysilanes in the place of aryl boronic acids (Scheme 59).81 AgF was used as the scavenger of organosilicon reagents.
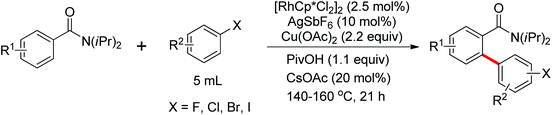
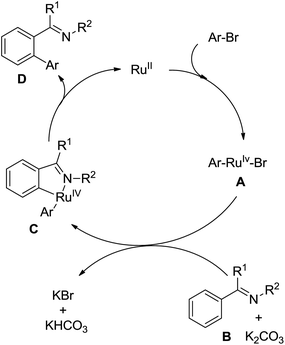
A unique Rh(III)-catalyzed selective dehydrogenative cross-coupling between benzamides and aryl halides (I, Br, Cl) to form biaryl products by means of double C–H activation was disclosed by Glorius and co-workers in 2012 (Scheme 60).82 Importantly, the halogen did not participate in the reaction and survived under the reaction conditions. The combination of a catalytic amount of CsOPiv (20 mol%) together with 1.1 equiv. of PivOH inhibited the undesired homocoupling of aryl halides. The benzamide bearing a NiPr2 group as the directing group could further improve the reactivity of the cross-coupling reaction. Mechanistic investigations indicated that this catalytic system proceeded by two separate C–H activation steps on each coupling partner. At the same year, Glorius and co-workers extended the Rh(III)-catalyzed selective dehydrogenative cross-coupling into vinylic substrates bearing a directing group.83 A wide range of synthetically valuable tri- and tetrasubstituted olefins were furnished in moderate yields.
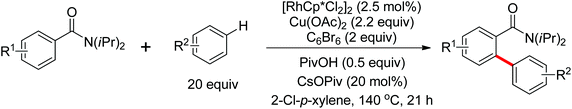
A novel Rh(III)-catalyzed dehydrogenative cross-coupling reaction of benzamide derivatives with simple arenes as arylating agents was demonstrated by Glorius and co-workers in the same year (Scheme 61).84 The directed oxidative cross-dehydrogenative coupling (CDC) of two non-prefunctionalized arenes was achieved with the assistance of the directing group. Polybrominated benzene derivative (C6Br6) was chosen to be the optimal and inexpensive additive/cooxidant to promote the reaction. Pivalic acid (PivOH) and cesium pivalate (CsOPiv) had also some positive effects to improve the efficiency of the reaction. Under the reaction conditions, a wide range of synthetically useful biaryl patterns were readily afforded in moderate to good yields.
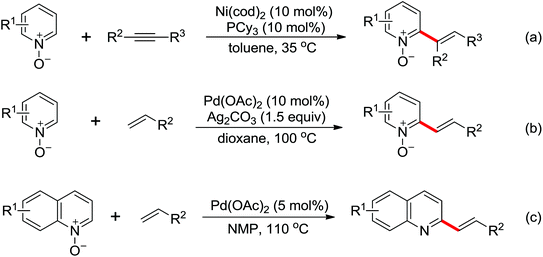
Allylarene is a kind of important scaffold in natural products and biologically active compounds. The methods for the direct C–H allylation of arenes have been rarely studied. In 2013, Glorius and co-workers reported a Rh(III)-catalyzed intermolecular direct C–H allylation reaction with readily available allyl carbonates as the allyl source under mild reaction conditions (Scheme 62).85a A slight excess of AgSbF6 had some notable effect to facilitate the process of the allylation reaction. Some mechanistic investigations implied that the C–H bond cleavage was involved in the rate-limiting step and a concerted metalation–deprotonation (CMD) process was the key step. This reaction was an excellent alternative to existing methods to access allylated aromatics. In the same year, a similar study of Rh(III)-catalyzed direct olefination of arenes using allyl acetate as an olefinating agent via C–H activation was developed by Loh and co-workers.85b
A Rh(III)-catalyzed coupling reaction/annulation of O-pivaloyl benzhydroxamic acids with donor/acceptor diazo compounds to give diverse isoindolones with a stereogenic carbon was demonstrated by Rovis and co-workers (Scheme 63).71b In contrast with CO and isocyanides, diazo compounds were chosen as one-carbon components in the cyclization reaction. A broad range of benzhydroxamic acids and diazo compounds were compatible with the reaction conditions and various isoindolones were smoothly afforded. In particular, isoindolones containing quaternary carbons with a trifluoromethyl group were successfully obtained using this elegant method.
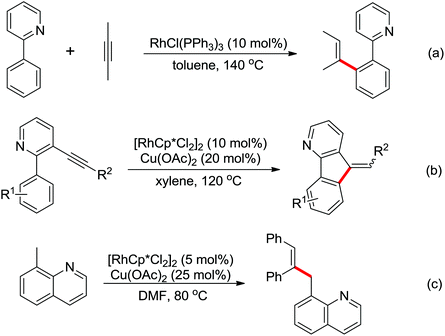
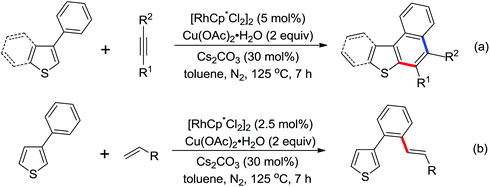
An unprecedented Wilkinson catalyst [Rh(PPh3)3Cl] catalyzed highly regioselective cross-coupling of aromatic amine derivatives with various heteroarenes via dual C–H activations was reported by You and co-workers recently (Scheme 64).86 The reaction overcame the intrinsic obstacles of the common C–H/C–H oxidative cross-coupling reactions: the poor regioselectivity and the requirement of a large excess of the arenes. A wide variety of important aryl–heteroaryl frameworks were rapidly constructed by the use of this efficient protocol, and some highly extended π-conjugated heteroacenes that had great air stability as organic semiconductors in electronic devices were synthesized. Compared with the classical catalytic system of [RhCp*Cl2]2/AgSbF6, the current innovative catalyst system was much less expensive and had more potential to be applied to other C–H activation reactions.
Alkynes are important functional groups in organic synthesis because of their versatility to be readily converted into a lot of useful structural motifs. Besides the Sonogashira coupling reaction, the comparable C–H alkynylation is another powerful and practical tool to construct alkyne compounds. The rhodium(III)-catalyzed ortho-C–H alkynylation of electron-poor arenes bearing the amide directing group by taking advantage of the hypervalent iodine reagent as the alkyne source was discovered by Loh and co-workers (Scheme 65).87 The reaction showed many notable features, such as mild reaction conditions, broad substrate scope and high efficiency and selectivity for monoalkynylation. This useful protocol could also have applications for further functionalization of natural products or related complex molecules. Shortly afterwards, a Rh(III)-catalyzed C–H alkynylation of an acrylamide derivative employing tosyl-imide as a weakly coordinating directing group was demonstrated by the same group.88
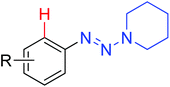
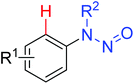
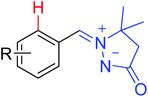
Using the hypervalent iodine–alkyne reagents, Li and co-workers also developed the chelation-assisted C–H alkynylation of various (hetero)arenes (Scheme 66).89 Both Rh(III) and Ir(III) catalysts could catalyze the transformation of alkynylation, providing complementary insights into C–H alkynylation of different substrates. The reaction system was compatible with many directing groups, such as heterocycles, N-methoxy imines, azomethine imines, secondary carboxamides, azo compounds, N-nitrosamines, and nitrones. The selectivity of mono- and dialkynylation was controllable for the N-heterocyclic directing groups. Systematic mechanistic investigations were conducted and the Rh(III) alkynyl complex and Rh(III) vinyl complex were presumably identified as key intermediates in the reaction.
The Rh(III)-catalyzed direct alkynylation of alkenes using hypervalent iodine–alkyne reagents was studied by Glorius’ group90 and Loh's group91 respectively (Scheme 67). In these transformations, valuable building blocks of conjugated 1,3-enynes were generated.
Ellman and co-workers presented the first example of Rh-(III)-catalyzed coupling of anilides and enamides with isocyanates to form N-acyl anthranilamides and enamine amides (Scheme 68).92 [Cp*Rh(MeCN)3](SbF6)2 was identified as an efficient catalyst. Some kinetic examinations of the reaction were conducted to gain more insights into the reaction mechanism. An efficient method for Rh(III)-catalyzed oxidative carbonylation of aromatic amides via C–H/N–H activation to form a wide variety of phthalimides was described by Rovis’ group93 and a rhodium-catalyzed annulation of N-benzoylsulfonamide with isocyanides through C–H activation to provide a straightforward approach to a wide range of 3-(imino)isoindolinones was developed by Zhu and co-workers.94
The rhodium-catalyzed regioselective acylation of benzamides via C–H bond activation using aldehydes as the acyl sources was reported by Kim and co-workers in 2011 (Scheme 69a).57 Silver carbonate was the optimal oxidant of the reaction. A wide variety of benzamides and aldehydes were successful involved in the reaction and the relevant aryl ketones, which were crucial structural motifs in biologically active compounds and functional materials, could be afforded in moderate to good yields. Based on the above studies, another different reaction pattern using similar substrates was developed by Kim (Scheme 69b).95 The rhodium-catalyzed oxidative acylation followed by an intramolecular cyclization took place, affording a range of 3-hydroxyisoindolin-1-one building blocks, which were ubiquitous structural motifs in synthetic and naturally occurring bioactive compounds. High temperature (150 °C) was necessary for the reaction. The relevant competition reaction was conducted to gain insight into the catalytic process and the insertion of aldehyde into a rhodacycle intermediate was presumably involved in the rate-limiting step of the transformation.
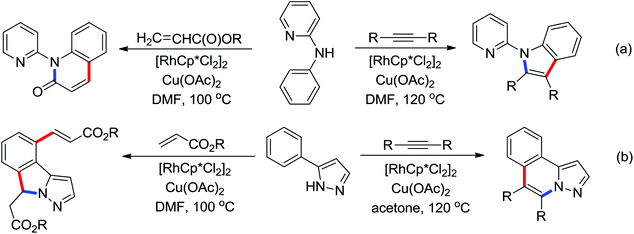
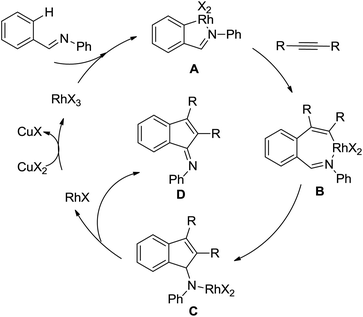
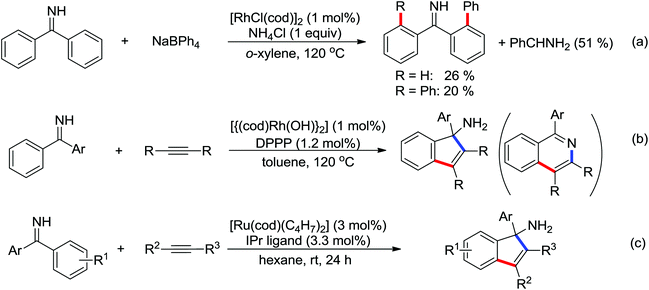
Indoles and pyrroles are the most common motifs and are widely found in natural products, drugs, and other bioactive molecules. The rhodium(III)-catalyzed acetanilides and enamines C–H bond functionalization/annulation reaction with internal alkynes to afford a wide variety of indoles and pyrroles was presented by Stuart and co-workers in 2010 (Scheme 70).96 Compared with the rhodium(III)-complex [Cp*RhCl2]2, which has been applied in the previous study of indole synthesis by the same group,97 the dicationic analogue [Cp*Rh(MeCN)3][SbF6]2 was involved in the reaction and had some positive effect on the reactivity of the reaction. In addition, molecular oxygen was regarded as terminal oxidant and milder conditions were allowed for the reaction. Detailed mechanistic investigations were conducted to obtain a better understanding of the reaction mechanism. As for the indole synthesis, Glorius developed an efficient rhodium(III)-catalyzed redox-neutral C–H activation/cyclization of 2-acetyl-1-arylhydrazines with various alkynes to prepare unprotected indoles (Scheme 71).98 The N–N bond was utilized as an innovative directing group and an internal oxidant in the reaction. A variety of different symmetric alkynes and unsymmetrical alkynes all smoothly participated in high regioselectivity, providing a powerful alternative to the Fischer indole synthesis.
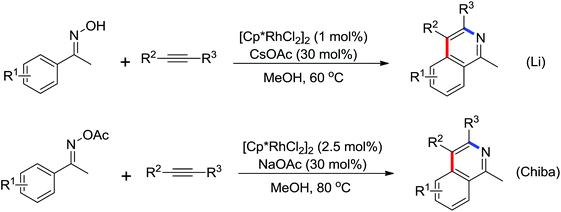
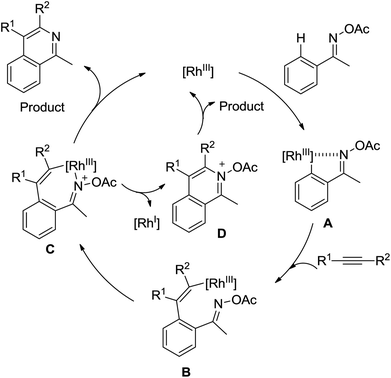
A conceptually new method of rhodium(III)-catalyzed isoquinolone synthesis from benzhydroxamic acid precursors in the absence of an external oxidant was demonstrated by Guimond and co-workers in 2010 (Scheme 72a).99 The inner N–O bond was used as an internal oxidant, which has been successfully applied to C–N bond formation by Hartwig.100 The redox neutral isoquinolone synthesis proceeded under mild reaction conditions, and was also insensitive to air and moisture with broad functional group tolerance. Shortly after this, the same group developed a more reactive internal oxidant/directing group that could readily afford a wide variety of isoquinolones at room temperature with lower catalyst loadings (0.5 mol%) (Scheme 72b).101 It was a significant improvement over the previous study. The terminal alkynes and alkenes were compatible with the reaction conditions, expanding the substrate scope of the reaction and furnishing various monosubstituted isoquinolones and 3,4-dihydroisoquinolones in good yields. Extensive mechanistic investigations and DFT calculations were conducted, indicating that a concerted metalation deprotonation (CMD) process was proposed to be the turnover limiting step and a stepwise C–N bond reductive elimination/N–O bond oxidative addition mechanism was suitable for the current reaction.
A rhodium(III)-catalyzed oxidative cycloaddition of benzamides and alkynes via C–H/N–H activation to form a series of isoquinolones in good yields was disclosed by Rovis and co-workers.102 Cu(OAc)2 was regarded as a stoichiometric oxidant and AgSbF6 was used to sequester the halides. Many competition experiments between different alkynes were carried out and the results revealed that more electron-rich alkynes were favored in the reaction but the regioselectivity of insertion was supposed to be largely dependent on steric factors.
Based on the above pioneering studies, a rhodium(III)-catalyzed versatile and flexible C–H activation/1,3-diyne general strategy for the rapid assembly of diverse polysubstituted bisheterocycles in high efficiency and selectivity was achieved by Glorius recently (Scheme 73).103
The rhodium(III)-catalyzed distinct reaction patterns of tethered olefin-containing benzamides directed by the amide group were developed by Rovis and co-workers (Scheme 74).104 Intramolecular hydroarylation, amidoarylation, and dehydrogenative Heck-type reaction were achieved with different substrates. A wide variety of tethered alkenes could be involved in the reaction and the corresponding five- or six-membered biologically useful fused oligocyclic lactam products were produced in good to excellent yields. The diastereoselectivity of the amidoarylation product could be controlled using a substrate containing a pre-existing stereocenter. More recently, Glorius’ group reported an almost identical study through intramolecular redox-neutral cyclization process of a wide variety of tethered olefin-containing benzamides via C–H activation.105 A rhodium(III)-catalyzed intramolecular amidoarylation of alkyne via a C–H activation pathway for the synthesis of 3,4-fused tricyclic indoles was independently reported by Xu106a and Li,106b further expanding the synthetic utility of the intramolecular redox-neutral annulation reaction.
An enantioselective intramolecular rhodium(III)-catalyzed hydroarylation to afford functionalized dihydrobenzofurans that possessed a quaternary stereocenter directed by the CONHOMe group under mild conditions was demonstrated by Cramer and co-workers in 2014 (Scheme 75).107 The existence of a special chiral Cp ligand could significantly enhance the enantioselectivity of the hydroarylation of aryl hydroxamates. The scope of the enantioselective hydroarylation was broad and a wide range of substituents were tolerant. The meta-alkoxy group of the olefin side chain played the role of a secondary directing group for Rh-catalyzed reactions. Diverse dihydrofurans bearing methyl-substituted quaternary stereocenters were produced in moderate to good yields. As early as 2012, Rovis and Cramer independently reported a chiral rhodium complex catalyzed directed C–H bond functionalization for the synthesis of asymmetric tetrahydroisoquinolinone derivatives with high enantioselectivity.108
Since α-(pseudo)halo ketones are widely utilized as electrophiles in cross-coupling reactions, they could be applied in transition-metal-catalyzed C–H activation. More recently, the first example of using α-halo and pseudohalo ketones as C(sp3)-based electrophiles in Rh(III)-catalyzed C–H activation to synthesize diverse N-heterocycles under mild and redox-neutral reaction conditions was developed by Glorius and co-workers (Scheme 76).109 In this reaction, α-(pseudo)halo ketones were identified as oxidized alkyne equivalents to undergo Rh(III) catalyzed redox-neutral annulations. Various important N-heterocycles, such as 3-aryl- and 3-alkyl-substituted isoquinolones, 2-pyridones, 1,2-benzothiazines, and 2-methylated indole, could be obtained in moderate to excellent yields under mild conditions.
Azepine and its derivatives are important skeletal motifs in numerous natural products and synthetic compounds. Extensive attention has been paid to the development of effective methods towards these useful molecules. In 2013, Glorius and co-workers developed an efficient methodology of rhodium(III)-catalyzed intermolecular annulations for the synthesis of azepine derivatives utilizing simple and readily available benzamides and α,β-unsaturated aldehydes or ketones as starting materials (Scheme 77).110 PivOH was the best additive to improve the reaction efficiency by releasing H2O as the only waste. The scope of the substrates was broad and a wide range of azepine derivatives could be readily prepared in moderate to excellent yields. Some control experiments indicated that the catalytically active species [RhIIICp*] was identified as a transition-metal catalyst and a Lewis acid catalyst was used for the dehydration process simultaneously. The annulation procedure was presumably supposed to undergo C–H activation, cyclization and condensation steps.
A rhodium(III)-catalyzed alkene migratory insertion in a range of benzhydroxamic acids for the synthesis of dihydroisoquinolones was reported by Rovis and co-workers in 2015 (Scheme 78).111 The sterically bulky di-tert-butylcyclopentadienyl ligand (Cpt) was used as an effective ligand to increase the regioselectivity of the reaction, because the classical Cp* ligand exhibited relatively low selectivity. Crystallographic results of the 5-membered RhCpt metallacycle offered some evidence for the enhanced regioselectivity. Other transformations employing rhodium(III)-catalyzed C–H activation/annulation reactions of benzhydroxamic acids with different coupling partners were reported and the relevant N-heterocycles were afforded in various manners.112
Rh(III)-catalyzed [4 + 2] annulation reaction of aromatic carboxamides with alkynes is a powerful and efficient route for the preparation of some fused nitrogen-containing heterocycles. However, the application of this method to electron-deficient pyridine derivatives has proved to be quite difficult. Poor selectivity and low reaction rates of pyridines constituted the main challenges to relevant C–H functionalization reactions. A Rh(III)-catalyzed C–H activation/annulation of nicotinamide N-oxides with alkynes or alkenes to afford naphthyridinone N-oxide or dihydronaphthyridinone N-oxide products under mild conditions was demonstrated by Huckins and co-workers in 2013 (Scheme 79).113 The existence of N-oxide could increase the reaction rates and enhance the desired regioselectivity. Many marked features, such as low catalyst loadings, mild reaction conditions, good yields and regioselectivities, were achieved.
The postulated mechanism was given based on deuterated experiments (Scheme 80). Initially, a C–H cyclometallation at the 2-position of the pyridine N-oxide ring was performed to produce a rhodium cycle complex, which underwent regioselective alkyne/alkene insertion into the Rh–C bond to afford a seven-membered ring metallocycle. The subsequent reductive elimination gave the final naphthyridinone N-oxide product by using the N–O bond as an internal oxidant to re-oxidize the Rh(I) to Rh(III).
Shi and co-workers reported a rhodium(III)-catalyzed C–H activation/annulation of benzimides from abundant and essentially unfunctionalized benzoic acids with alkynes to form diverse synthetically valuable indenones (Scheme 81).114N-Acyloxazolidinone with proper directing ability and electrophilicity was identified as the optimal directing group and the highest yield was obtained. Decahydronaphthalene was the best solvent for the reaction. A wide range of functionalized indenone products were prepared from readily available benzimide derivatives under redox neutral conditions. Later, Li and coworkers reported a Rh(III)-catalyzed C–H functionalization of 1-benzoylpyrrolidine with propargyl alcohols for the synthesis of (4-benzylidene)isochroman-1-ones.115 Highly enantio-enriched lactones could be obtained by the use of optically pure propargyl alcohols as the starting materials.
A rhodium(III)-catalyzed C–H activation for the efficient construction of pyridines and cyclopentenone rings from readily available N-allyl sulfonamides and alkynes using sulfonamides as effective directing groups was demonstrated by Li and co-workers in 2012 (Scheme 82).116 When N-allyl p-tolylsulfonamide was used as the substrate, AgOAc (4.5 equiv.) proved to be an ideal oxidant to support a twofold oxidation process, affording 3-sulfonylpyridine product in good yield. Several cross-over experiments evidenced the possible involvement of a formal oxidative intermolecular 1,3-shift process of the sulfonyl group. When introducing a methyl (blocking) group into the olefin unit to inhibit the 1,3-shift process of the sulfonyl group, unexpected cyclopentenone products were generated. The corresponding ketone oxygen atom had likely come from the water. The reaction exhibited broad substrate scope and the high selectivity was determined by the allyl moiety of the sulfonamide.
A rhodium(III)-catalyzed oxidative C–H activation of simple and widely available sulfonamides and subsequent addition of internal alkynes for the convenient synthesis of the benzosultams was established by Cramer and co-workers (Scheme 83).117 The acylated sulfonamide was chosen as a suitable directing group to construct a wide range of pharmaceutically important sulfonamides. Catalytic amounts of copper(I) acetate and molecular oxygen (50 mbar) as a terminal oxidant were beneficial for the high conversions and yields of the reaction. This reaction was a general and attractive method to access the synthetically and medicinally important aryl sultams. Furthermore, there have been many other good examples of sulfonamide-assisted C–H activation in recent years.118
Cui and co-workers demonstrated a Rh(III)-catalyzed C–H activation/cycloaddition of benzamides and methylenecyclopropanes for the divergent synthesis of biologically valuable spiro dihydroisoquinolinones and furan-fused azepinones under mild conditions (Scheme 84).119 A carboxylate assisted C–H activation via a concerted metalation/deprotonation (CMD) pathway and MCP mild insertion process was presumably involved in the reaction. For the formation of azepinone, the fused electron-rich furan moiety could stabilize the metal intermediate and promote a cyclopropyl carbinyl–butenyl rearrangement via a ring opening process to give azepinone product. They also investigated a Rh(III)-catalyzed C–H activation/[4 + 3] cycloaddition of benzamides with vinylcarbenoids, which could be utilized as suitable three-carbon coupling partners to give azepinones (Scheme 85).71e CsOAc was regarded as the optimal additive and rendered the desired product in 95% yield. A wide variety of azepinones were formed in moderate to good yields by this general and versatile approach. It was the first example of utilization of vinylcarbenoids as three-carbon components in Rh(III)-catalyzed cycloaddition reactions.
An efficient rhodium(III)-catalyzed intramolecular annulation of alkyne tethered hydroxamic esters for the facile synthesis of hydroxyalkyl-substituted isoquinolone/2-pyridone derivatives was demonstrated by Park and co-workers (Scheme 86a).120 This reaction showed reverse regioselectivity compared to the previous intermolecular version of rhodium-catalyzed C–H/N–O bond functionalization reactions. The reaction proceeded under mild reaction conditions and obviated the use of external oxidants. It was noteworthy that the total synthesis of some phenanthroindolizidine alkaloids was successfully accomplished by this method. After two years, Li and Zhou reported a similar Rh(III)-catalyzed intramolecular redox-neutral C–H activation/annulation of a tethered alkyne to afford 2-amidealkyl indoles with completely reversed regioselectivity (Scheme 86b).121 The strategy was also applied to the highly efficient formal total synthesis of goniomitine using Rh(III)-catalyzed C–H activation/annulation as a key step. A rhodium(III)-catalyzed intramolecular annulation to synthesize tricyclic isoquinoline derivatives in good yields was also developed by Gulías and co-workers in 2013.122
A Rh(III)-catalyzed intermolecular [4 + 3] annulation method for the rapid synthesis of 1,2-oxazepines from N-phenoxyacetamides and α,β-unsaturated aldehydes was reported by Zhao and co-workers (Scheme 87).123 This atom-economical protocol might involve C–H activation, alkene insertion, and intramolecular nucleophilic attack from a Rh amide to yield 1,2-oxazepine products, which were readily transformed to biologically important chroman derivatives. Previously, Lu and Liu described a mild rhodium(III) catalyzed redox-neutral coupling of N-phenoxyacetamides and alkynes for the synthesis of ortho-hydroxyphenyl substituted enamides or benzofurans with high regioselectivity and yields (Scheme 88).124
A rhodium(III)-catalyzed direct C–H amination of N-pivaloyloxy benzamides with N-chloroamines under mild conditions was achieved by Glorius and co-workers (Scheme 89).125 The versatile N-pivaloyloxy amide and N-chloroalkylamines were used as the directing group and the readily accessible nitrogen source. This novel method provided a complementary route to construct some important arylamine structures.
Recently, Zhang and co-workers developed a novel aryl C–H bond hydroamination catalyzed by the synergistic combination of rhodium and copper catalysis using commercially available N-Boc-hydroxyamine as a nitrogen source (Scheme 90).126 A wide variety of bioactive benzo[c]isoxazole derivatives were readily prepared by this efficient protocol. The electrophile nitrosocarbonyl compound was in situ generated by copper-catalyzed aerobic oxidation and used as a coupling partner under mild reaction conditions.
Chang and co-workers presented the first example of a rhodium-catalyzed direct arene C–H amination of benzamides utilizing sulfonyl azides as the amine source to afford a range of diarylamines, which were widely existing in biologically active natural products (Scheme 91a).127 The procedure did not require an external oxidant and released N2 as a single by-product, providing a practical and environmentally benign amination process. The elegant method was also readily extended to the amination of ketoximes with high reactivity and functional group tolerance, emphasizing the generality of this method. Later, they extended the scope of the rhodium-catalyzed direct amination reaction to various alkyl azides (Scheme 91b).128 Both benzyl and aliphatic azides bearing a wide range of functional groups were identified as the nitrogen source and were involved in the reaction to give synthetically more important and diverse N-alkylaniline products successfully. Besides amides, the reaction could also be extended to some substrates bearing weak coordinating groups such as aromatic ketones and aryl ketoximes. A plausible and rational amination pathway was also proposed based on the mechanistic studies.
The first rhodium-catalyzed acetoxylation of an olefinic C(sp2)–H bond in enamides with high regio- and stereoselectivity to afford a variety of substituted vinyl acetates with absolute Z-configuration was accomplished by Zhang and co-workers recently (Scheme 92).129 Cu(OAc)2 was utilized as the oxidant and the source of acetate in the reaction. The isotope effect studies indicated that the step of carbometalation by abstracting the olefinic hydrogen was reversible and the C–H activation might not be involved in the rate-determining step.
The first example of Rh(III)-catalyzed direct ortho bromination and iodination reaction of different aromatic compounds via C–H activation for the convenient synthesis of ortho-brominated and -iodinated aromatic products was reported by Glorius and co-workers in 2012 (Scheme 93).130 This general and practical strategy was compatible with many different directing groups, offering new possibilities to prepare a wide range of aromatic halides. The Rh(III)-catalyzed halogenation reaction of vinylic C–H bonds was reported by the same group later, which provided a general and efficient approach to a variety of substituted haloacrylic acid derivatives.131
2.3. Ru catalyzed C–H functionalization
Compared with a lot of well-developed rhodium-catalyzed oxidative annulations to synthesize diverse heterocycles, the use of less-expensive ruthenium catalysts for oxidative annulations was far from studied at the same stage.1x Due to its extraordinary reactivity and selectivity, the [RuCl2(p-cymene)]2 complex has been used as a catalyst for diverse C–H bond functionalization reactions with high efficiency. The first ruthenium-catalyzed oxidative annulation reaction of alkynes with benzamides for the facile synthesis of a wide range of isoquinolones with broad scope was reported by Ackermann and co-workers (Scheme 94a).132 Detailed mechanistic investigations showed that a carboxylate assisted C–H bond metalation was the rate-limiting step, which was different from the rhodium-catalyzed isoquinolone synthesis.99,101,102 They also investigated the ruthenium-catalyzed oxidative annulations of acrylamides and alkynes with stoichiometric amounts of external oxidants to synthesize 2-pyridones (Scheme 94b).133 The ruthenium-catalyzed C–H functionalization under mild conditions to deliver a series of isoquinolone motifs was reported by Ackermann's and Wang's groups respectively (Scheme 95).134A ruthenium catalyzed cross-dehydrogenative alkenylation of various anilides and benzamides was reported by Ackermann and co-workers (Scheme 96).135 A cationic ruthenium(II) complex was chosen as the optimal catalyst and water was used as a green solvent. Detailed mechanistic studies provided strong evidence for the different mechanisms of the two transformations. The cycloruthenation step in oxidative alkenylation of acetanilide was reversible, whereas an irreversible C–H bond metalation was involved in cross-dehydrogenative alkenylations of benzamides.
The first ruthenium catalyzed oxidative C–H bond olefination of N-methoxybenzamides using the CONH(OMe) group as an oxidizing directing group was developed by Wang and co-workers (Scheme 97).136 With the variance of olefinic partners and solvents, two types of products were afforded in moderate to good yields. On the basis of the mechanistic studies, the reaction presumably proceeded by an intermolecular carboruthenation of alkene via rate-determining C–H bond activation followed by reductive elimination to form the desired products.
In 2012, Jeganmohan and co-workers reported a ruthenium-catalyzed ortho-arylation of N-alkyl benzamides with substituted aromatic boronic acids with high regioselectivity (Scheme 98a).137 When substituted alkenylboronic acids were used as starting reactants, the corresponding ortho-alkenylation of N-alkyl benzamides was achieved successfully. The ortho-arylated N-alkyl benzamides were readily converted into fluorenones under the current methodology. Later, the same group developed a ruthenium-catalyzed oxygen atom directed ortho-arylation of acetanilides with aromatic boronic acids (Scheme 98b).138 It was noteworthy that diarylated products or N-arylated acetanilides were not observed under the reaction conditions. The obtained ortho-arylated N-substituted anilines were key synthetic intermediates for various organic transformations and could be further converted into biologically useful phenanthridine and carbazole derivatives. Shortly after this, they demonstrated a Ru-catalyzed cyclization of anilides with propiolates or acrylates to deliver 2-quinolinones bearing diverse functional groups in good to excellent yields, extending the application of Ru-catalyzed C–H functionalization reactions (Scheme 99).139
Wang and Liu respectively reported an efficient and regioselective ruthenium-catalyzed oxidative annulation of enamides with alkynes via the cleavage of C(sp2)–H/N–H bonds to afford a wide range of biological and pharmaceutical important pyrrole derivatives (Scheme 100a).140a With the addition of AgSbF6 and MeOH, some N-unsubstituted pyrroles could be smoothly obtained by Wang's work. The reaction developed by Liu could be carried out in the aqueous medium (Scheme 100b).140b
A ruthenium-catalyzed ortho-selective C–H bond hydroxylation on benzamides with PhI(OAc)2 as the terminal oxidant was disclosed by Ackermann and co-workers (Scheme 101).141 The reaction had some notable features, such as weakly coordinating benzamides as readily modifiable directing groups, a low catalyst loading, high efficiency, and regioselectivity. One year later, they developed a similar strategy of ruthenium-catalyzed site selective C(sp2)–H bond oxygenations on aryl Weinreb amides, which could be readily transformed into the corresponding ketones and aldehydes.142 Rao and co-workers reported the first Ru(II)-catalyzed C–H mono- and dihydroxylation of anilides for the synthesis of 2-aminophenols and heterocycles with a new directing group strategy (Scheme 102).143 The 2,6-difluorobenzoyl group was chosen as a double-functional directing group, which could serve not only as a readily cleavable coordinating group but also as a potential structural component for further complex molecule synthesis.
The Ru(II)-catalyzed ortho-C–H amination directed by a weakly coordinating amide group using electrophilic O-benzoyl hydroxylamines as the N-source at room temperature was developed by Yu and Dai (Scheme 103).144 The scope of this reaction for heteroarene substrates was substantially broader than the Pd-catalyzed C–H amination reaction reported by the same group,59 showing the excellent reactivity of the weakly coordinating directing group with Ru(II) catalysts.
2.4. Other metal (Ir, Cu, Co) catalyzed C–H functionalization
Chang and co-workers developed the iridium-catalyzed direct C–H amidation of arenes and alkenes using acyl azides as the nitrogen source under mild conditions (Scheme 104a).145 The acyl azides were utilized as the nitrogen donor of an acyl amino group in the reaction to afford a broad range of amidated arenes and olefins with high functional group tolerance. An in situ generated cationic half-sandwich iridium complex was proposed to be involved in the reaction and was used as an active catalyst. The amidation reaction featured the absence of external oxidants, molecular nitrogen as a single byproduct and wide substrate scope, enabling this environmentally benign method to be practical in organic synthesis and medicinal chemistry. An iridium-catalyzed direct C–H amination of benzamides by employing anilines as the nitrogen source at room temperature was reported by the same group (Scheme 104b).146 For the first time, this transformation achieved the amination by the use of simple and readily available anilines in the place of azides. A unique iridacyclic species was synthesized and unambiguously characterized, revealing that the Ir metal was coordinated to the carbonyl O atom rather than the amide nitrogen atom, which was different from the previously reported palladacycle complexes. It was the first example of Ir-catalyzed direct C–H amination of benzamides using simple aniline as coupling partners to construct a wide variety of synthetically and medically useful arylamine products.A practical Cu-catalyzed direct ortho-halogenation of anilines employing a readily removable N-sulfonyl directing group under aerobic conditions was developed by Carretero and co-workers (Scheme 105).147N-Halosuccinimides (NXS, X = Cl, Br) were identified as convenient X+ sources to participate in the reaction. Various halogenated anilines, which were versatile precursors for a lot of heterocyclic frameworks, could be produced by this efficient method. They disclosed a practical copper-catalyzed direct nitration of protected anilines employing nitric acid as the nitrating agent (Scheme 106).148 This reaction had broad substrate scope in terms of N-protecting groups (sulfonamides, carbamates, amides, and ureas) and remarkable functional group tolerance. The dinitrated aniline derivatives were also prepared by using two equivalents of HNO3. The final products could be regarded as valuable building blocks to some relevant nitrogenated architectures.
The first example of copper-catalyzed C–H trifluoromethylation of enamides for the direct formation of olefinic C–CF3 bonds at room temperature was reported by Loh and co-workers (Scheme 107).149 Togni's reagent was used as a trifluoromethylation agent. The amido group introduced onto the olefin moiety had a dual role: stabilizing the putatively formed α-carbonium and inducing the subsequent proton elimination or a migration process during the reaction. The Lewis acidity of the copper catalyst was also beneficial for the reactivity of the transformation. The oxytrifluoromethylation of enamides was realized by a slight modification of the reaction conditions.
The copper-catalyzed C–H trifluoromethylation of electron-deficient alkenes was demonstrated by Loh and co-workers (Scheme 108).150 Compared with the electron-rich alkenes, the trifluoromethylation of electron-deficient alkenes had more challenges. The directing group was deemed to have a vital effect on the success of this C–CF3 bond formation. Based on the sufficient results of a set of control experiments, the involvement of radical species was proposed in the catalytic process and olefinic C–H bond activation was not involved in the rate-determining step. This reaction tolerated various substituted acrylate derivatives and a broad range of synthetically useful functionalities.
A cobalt-catalyzed coupling of a secondary benzamide with an alkyl chloride in the presence of cyclohexyl magnesium chloride (CyMgCl) at room temperature was developed by Nakamura and co-workers (Scheme 109).151 It was a rare example of using the cobalt catalyst to introduce a saturated hydrocarbon group directly into benzamide substrates through C–H bond activation. An inexpensive ligand, 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU), could remarkably promote the transformation through increasing the reactivity of alkyl cobalt species. Alkyl chlorides bearing a wide range of functional groups could readily take part in the reaction to give the desired products. Later, a cobalt-catalyzed coupling of alkyl Grignard reagent with benzamide and 2-phenylpyridine derivatives using atmospheric air as the sole oxidant under mild conditions was demonstrated by the same group (Scheme 110).152 The reaction mechanism might be different from the previously reported coupling with alkyl chlorides.
Recently, a Co(III)-catalyzed C2-selective C–H alkenylation/annulation cascade of N-carbamoyl indoles to afford diverse pyrroloindolones in one-pot was demonstrated by Kanai and co-workers (Scheme 111).153 A cationic high-valent Cp*Co(III) complex, [Cp*Co(III)(C6H6)](PF6)2, was utilized as an effective catalyst due to the unique nucleophilic activity of the Co–C bond. The catalytic activity of the Cp*Co(III) complex in the reaction was significantly different from those of Cp*Rh(III) complexes. A wide range of C2-alkenylated indoles and pyrroloindolones were delivered by this method, showing the high functional tolerance. Detailed mechanistic studies on the catalytic process shed light on the reaction mechanism. The indolyl–Co species was produced by regioselective C–H metalation at the C2-position of indole via a concerted metalation–deprotonation mechanism with the aid of an acetate unit.
3. The C–H functionalization directed by nitrogen-containing unsaturated bonds
3.1. The C–H functionalization directed by heterocycle
The first palladium-catalyzed heterocycle-directed arylation was demonstrated by Sanford and co-workers in 2005 (Scheme 112a).154 The selective ortho-arylation of diverse arylpyridines, quinolines, pyrrolidinones, oxazolidinones, and benzodiazepines could be achieved via the Pd(II)/Pd(IV) catalytic cycle using diphenyliodonium salts as arylation reagents. However, a mixture of the analogous phenylated compound was obtained using [Ph–I–Ar]BF4 as the arylation reagent. Notably, the unsymmetrical mesityl/aryl-substituted iodonium reagents [Mes–I–Ar]BF4 provided a single arylated product in good to excellent isolated yields. Importantly, detailed mechanistic studies supported a Pd(II)/Pd(IV) catalytic cycle that was different from the traditional Pd(0)/Pd(II) cycle. A related example of a Pd-catalyzed oxidative cross-coupling of diverse L = CAr–H and Ar–H substrates was reported by the same group, in which the BQ could bind to the initially formed cyclometalated Pd(II) complex to promote the subsequent C–H activation of Ar–H (Scheme 112b).155 They also studied the oxidative homocoupling coupling of two 2-arylpyridine derivatives (Scheme 112c).156Iodobenzenes were used as coupling reagents in the palladium-catalyzed arylation using pyridines or pyrazoles as directing groups by Daugulis and co-workers (Scheme 113a).157 Notably, the transformation allowed the functionalization of not only aromatic but also benzylic and even inactivated sp3 C–H bonds. Longer reaction time was required when electron-poor iodides were used. Further attempts to use acyl peroxides and aryl trimethoxysilanes as arylation reagents in the palladium-catalyzed arylation of C–H bonds of heteroarenes were studied by Yu158 and Sun,159 respectively (Scheme 113b and c).
Pd-catalyzed pyridine-directed alkenylation of inactivated C(sp3)–H bonds was reported by Sanford and co-workers (Scheme 114).160 The transformation underwent the initial C–H olefination and the subsequent intramolecular Michael addition to afford the 6,5-N-fused bicyclic cores. Notably, this reaction utilized air as the terminal oxidant and proceeded efficiently with various 2-alkylpyridines and α,β-unsaturated alkenes.
Meanwhile, palladium-catalyzed C–H olefination of arenes using pyridines as directing groups via remote coordination was developed. For example, Carretero and co-workers found that N-(2-pyridyl)sulfonyl anilines could serve as a removable directing group in the Pd-catalyzed oxidative olefination (Scheme 115a).161 Subsequently, You and Lan applied 2-pyridylmethyl ether as an efficient directing group to achieve the C–H alkenylation, in which a presumed seven-membered cyclopalladated intermediate was formed via the chelation-assisted ortho C–H cleavage (Scheme 115b).162 It was notable that the non-activated olefins such as styrene derivatives, n-decene, could efficiently couple with the substrates to provide the products in good to excellent yields.
The first palladium-catalyzed alkylation of sp2 and sp3 C–H bonds with methylboroxine and alkylboronic acids was demonstrated by Yu and co-workers in 2006 (Scheme 116a).163 The author supposed that benzoquinone played a key role in the C–H activation and reductive-elimination process. Cu(OAc)2 was the oxidant to regenerate Pd(II) in the catalytic cycle. Using the same strategy, they developed the asymmetric sp2 C–H alkylation reaction of arenes with boronic acids as alkylating reagents (Scheme 116b).164 The use of monoprotected amino acids as chiral ligands allowed the success of the enantioselective C–H alkylation reaction.
A novel palladium-catalyzed methylation of aryl C–H bond with peroxides was achieved by Li in 2008 (Scheme 117).165 The key methylpalladium intermediate was generated from dicumyl peroxide, which was used as both a methylating reagent and a hydrogen acceptor. The amount of peroxide exerted an important influence on the products, in which 4 equiv. of the peroxide was used to generate the dimethylation product as a single product.
Yu and co-workers demonstrated the first palladium-catalyzed trifluoromethylation of arenes with 5-(trifluoromethyl)dibenzothiophenium tetrafluoroborate (Scheme 118).166 The presence of copper(II) acetate significantly improved the yield, while other oxidants proved to be ineffective. The use of TFA was found to be indispensible for the success of this Ar–CF3 bond-forming protocol. A wide range of substituents such as keto, ester, and nitro groups were compatible in the reaction.
The palladium-catalyzed oxidative ethoxycarbonylation of aromatic C–H bonds with diethyl azodicarboxylate (DEAD) was reported by Yu and co-workers (Scheme 119a).167 The transformation was operated without the use of carbon monoxide and protection against air/moisture. The authors proposed that the ethoxyacyl radical, which was generated from thermal decomposition of DEAD, might undergo the insertion into Pd–C bonds via the formation of Pd(IV) species to afford the product. A related example of the palladium-catalyzed direct alkoxycarbonylation of aromatic C–H bonds with α-keto esters was achieved by Wang and co-workers (Scheme 119b).168 The reactions of 2-arylpyridines, 2-arylquinolines, benzo[h]quinolines, 2-phenylpyrimidines, N-pyrimidinylpyrroles and N-pyrimidinylindoles with diverse benzoylformates proceeded smoothly to generate the desired alkoxycarbonylation products in good yields. Recently, oxaziridine was also found to be an excellent CO2Et group donor by Shi and co-workers (Scheme 119c).169 The key step of the ring opening mechanism of oxaziridines involved the initial insertion of Pd(II) into the N–O bond of oxaziridine to generate the Pd(IV) species and the subsequent rearrangement by a shift of CO2Et from the carbon to the Pd via a C–C bond cleavage process.
Palladium-catalyzed C–H cyanation of 2-phenylpyridine using CuCN as the cyanation source was demonstrated by Cheng and co-workers in 2009 (Scheme 120).170 A ligand exchange of CN− after the initial cycle metalation of Pd(OAc)2 was involved in the key step of the reaction. The procedure was successfully applied in the synthesis of Menispermum dauricum DC.
A pioneering work on the palladium-catalyzed C–H amidation of inactivated sp2 and sp3 C–H bonds was reported by Che and co-workers in 2006 (Scheme 121a).171 Various amides, including carbamates, acetamides, and sulfonamides, were proven to be effective amidation reagents to give the products in middle to high yields. Importantly, the catalytic amidation of inactivated C(sp3)–H bonds of 8-methylquinoline was achieved. The authors believed that the nitrene species, which were generated from oxidation of amides by K2S2O8, were crucial to the mechanism. Other nitrogen sources, such as iodobenzene diacetate/bistosylimide, PhI(OAc)NTs2, and N-fluorobis(phenylsulfonyl)imide (NFSI), has been used to construct the C–N bond via the palladium-catalyzed C–H activation (Scheme 121b).172 The substrates bearing various substituents, such as methyl, bromine, fluorine and nitro, were tolerated to give the corresponding products in good to excellent yields.
A palladium-catalyzed tandem C–H azidation and N–N bond formation of arylpyridines was developed by Jiao and co-workers (Scheme 122).173 Ce(SO4)2 was used as an oxidant for the generation of Pd(IV) species and the yields of the products dramatically decreased when Ce(SO4)2 was replaced by other oxidants. It was interesting that FeCl2 or O2 could increase the efficiency of this reaction. The method provided an alternative concise approach for the construction of bioactively important pyrido[1,2-b]indazoles.
Sanford and co-workers reported a palladium-catalyzed acetoxylation of meta-substituted arene substrates using PhI(OAc)2 (Scheme 123a).174 The reactions showed good functional group tolerance with respect to the meta-substituent on the arenes. A silicon-tethered pyridine was subsequently developed as the directing group in the palladium-catalyzed C–H oxygenation of arenes by Gevorgyan and co-workers (Scheme 123b).175 Compared with traditional heterocycle directing groups, the 2-pyrimidyldiisopropylsilyl (PyrDipSi) group had the advantage of being easy to remove and transform. A wide range of functional groups, such as methoxy, carbomethoxy, amide, and acetyl, could be tolerated. LiOAc was found to be crucial for the successful second C–H oxygenation, suggesting that the reaction went through a concerted metalation–deprotonation (CMD) pathway.
The PdCl2 and N-hydroxyphthalimide co-catalyzed C–H hydroxylation of 2-arylpyridines was achieved in the presence of oxygen by Jiao and co-workers (Scheme 124).176 The results of O18-labeling experiments indicated that the oxygen atom in the hydroxy group originated from the molecular oxygen. Hydroxyl radical was involved in the NHPI catalytic process in this transformation, which was demonstrated by EPR (electron paramagnetic resonance spectroscopy) with DMPO (5,5-dimethyl-1-pyrroline N-oxide) as the radical trap reagent. This chemistry provided a green and practical method to synthesize a variety of substituted 2-(pyridin-2-yl)phenols.
The palladium-catalyzed ortho-alkoxylation of 2-aryl-1,2,3-triazoles was developed by Kuang and co-workers (Scheme 125).177 A Pd(IV) intermediate was supposed to be involved in the catalytic reaction, which was generated from the oxidation of Pd(II) by K2S2O8. The subsequent ligand exchange and reductive elimination gave the final alkoxylation products and regenerated the palladium(II) catalyst. Functional groups such as halide, ester, ether, and ketone were tolerated under the oxidizing reaction conditions. Various primary alcohols, including EtOH, n-BuOH, and HO(CH2)2OH, were applied in this transformation to give the corresponding alkoxylated products.
Yu and co-workers demonstrated the first palladium-catalyzed phosphorylation of arenes using H-phosphonates (Scheme 126a).178 It should be noted that slow addition of H-phosphonate to the reaction with a syringe pump was essential to avoid the expeditious deactivation of the catalyst. The use of BQ was found to be necessary for the formation of the products, which promoted the reductive elimination in a similar manner to that observed in the coupling of C–H bonds with organometallic reagents. H-phosphonates bearing different substituents, such as Cl, F, CF3, CN and OMe, could couple with 2-arylpyridines smoothly to give the products in moderate yields. Murakani and co-workers also described an analogous phosphonation reaction of 2-arylpyridines, in which α-hydroxyalkylphosphonate was used to generate the H-phosphonate in situ by treatment with a base under the reaction conditions (Scheme 126b).179
The construction of C–S bonds via palladium-catalyzed C–H functionalization with arylsulfonyl chlorides was reported by Dong and co-workers (Scheme 127a).180 When DMF was used as the solvent and CuCl2 was applied as a cocatalyst, the C–Cl bond could be constructed. The direct thiolation of aryl C–H bonds in arenes was also achieved using disulfides or thiols by Nishihara and co-workers (Scheme 127b).181 It was proposed that the phosphine ligands played a crucial role in the dissociation of the palladium dimer to form the active monomeric species. The reaction proceeded efficiently with just 0.6 equivalents of disulfides, indicating that the arenethiols were oxidized to regenerate the corresponding disulfides under the oxidative reaction conditions. Very recently, the monotrifluoromethylthiolation of arene C–H bonds was achieved using a structural analogue of NCS under the Pd-catalyzed conditions by Shen and co-workers (Scheme 127c).182
The first palladium-catalyzed regioselective borylation of 2-phenylpyridine derivatives was reported by Takai and co-workers (Scheme 128).183 The Lewis acidity of the borane reagents influenced the reactivity of the reaction, and the pinacolborane as well as bis(pinacolate)diboron failed to afford the corresponding products. When 9-borabicyclo[3.3.1]nonane (9-BBN) was used, the reaction proceeded successfully even at room temperature. The interaction between Lewis basic nitrogen and Lewis acidic boron atoms was crucial to the high activity. It was interesting that the reaction proceeded even in the absence of the palladium catalyst at higher temperature.
Pd-catalyzed formation of aromatic and benzylic C–F bonds using electrophilic fluorinating reagents was described by Sanford (Scheme 129a).184 With the microwave irradiation, the N-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate was used as a highly effective F+ source to give the fluorination products in the moderate yields. A diversity of functional groups, including aryl halides, nonenolizable ketones and esters, trifluoromethyl substituents, and methyl ethers, were tolerated in the reaction. The reaction might involve a Pd(II)/Pd(IV) process. They also investigated a palladium-catalyzed C–H fluorination of 8-methylquinoline derivatives using nucleophilic fluoride (Scheme 129b).185 The major side product was the oxygenation product, and among the different hypervalent iodine reagents, PhI(OPiv)2 led to the best ratio of the fluorination products/oxygenation products. However, the replacement of expensive AgF with CsF, RbF, NBu4F, or KF under the optimized reaction conditions almost halted the reaction.
Kanai and co-workers reported a new palladium-catalyzed C–H fluorosilylation of 2-phenylpyridines (Scheme 130).186 Treatment of 2-phenylpyridines with amino(1,3,2-dioxaborolan-2-yl)diphenylsilane produced fluorosilylated 2-phenylpyridines in good to excellent yields by palladium catalysis. The pyridyl group acted as both a directing group to promote C–H fluorosilylation before the reaction and a component of the silafluorene equivalents in the products. This fluorosilylation reaction presented high functional-group tolerance and proceeded in good to excellent yields, even on a gram scale.
The rhodium-catalyzed direct ortho-arylation of 2-arylpyridines with arylstannanes represented the first effective catalytic cross-coupling of an aryl C–H bond with aryl metal compounds to give biaryls (Scheme 131).187 The pyridyl group was proposed to direct the transition metal-catalyzed C–H bond cleavage of an aromatic ring. In general, the reaction gave both mono- and di-arylated products. The selective generation of mono-arylated products could be achieved with ortho- or meta-substituted substrates.
A rhodium-catalyzed pyridine directed arylation of arenes with less toxic arylboronic acids was reported by Studer (Scheme 132a).188 The commercially available 2,2,6,6-tetramethylpiperidine-N-oxyl radical (TEMPO) was used as the oxidant and the phosphine ligand played the crucial role in the reactivity. Later on, a rhodium-catalyzed arylation of arenes with aroyl chlorides as the non-organometallic coupling partners was developed by Yu and co-workers (Scheme 132b).189 A decarbonylation process took place under the reaction conditions, and an increase of rhodium catalyst loading to 10 mol% could provide the double arylated compound as the major products.
A rhodium-catalyzed pyridyl-directed intermolecular formation of C(sp2)–C(sp2) bonds via double C–H bond cleavage was reported by Kambe and co-workers (Scheme 133a).190 In this transformation, the C(sp2)–H bond of the electron-rich heterocycles such as thiophene and selenophene directly coupled with the arenes to give the biaryl heterocyclic derivatives in moderate to high yields. More recently, an efficient rhodium(III)-catalyzed C–H bond arylation of (hetero)arenes with organosilanes was developed by Loh and co-workers (Scheme 133b).191 This reaction could took place at relatively low temperature in aqueous media and a variety of functional groups were well tolerated. A rhodium(III)-catalyzed arylation of arenes bearing a directing group using 4-hydroxycyclohexa-2,5-dienones as the arylating reagents for the synthesis of 3-arylated phenols was disclosed by Li and co-workers (Scheme 133c).192 The rearomatization process was identified to be the driving force of the reaction.
The rhodium-catalyzed C–H alkenylation of 2-phenylpyridine was reported by Lim and Kang, in which PPh3 was indispensible to keep the activity of the Rh(I) catalyst (Scheme 134a).193 Unsymmetric alkynes with a single bulky substituent, such as 1-(trimethylsilyl)-1-propyne, showed high regioselectivity and favored the mono-alkenylated product. The primarily polymeric materials were generated when terminal alkynes were used, while regioisomeric mixtures were obtained by the use of unsymmetric alkyne, such as 2-hexyne. Recently, the Rh(III)-catalyzed intramolecular olefination of 3-alkynyl-2-arylpyridines to afford 4-azafluorene compounds was reported by Shibata, in which a nitrogen-containing multi-cyclic skeleton could be realized through the pyridine-directed C–H bond activation (Scheme 134b).194 The more challenging rhodium-catalyzed C(sp3)–H olefination of 8-methylquinoline with alkynes was demonstrated by Wang and co-workers (Scheme 134c).195 A five-membered rhodacycle has been structurally characterized, thus revealing the key intermediate in the C–H activation step.
A novel Rh(III)-catalyzed heterocycle-assisted arylation was developed by Miura in 2008, in which polyarylated naphthyl- and anthrylazole derivatives could be provided by the direct coupling of phenylazoles with internal alkynes (Scheme 135).196 The reaction involved the cleavage of multiple C–H bonds and the fluorescence properties of the products were studied. The coordination of the nitrogen atom in the 2-position of 1a to a Rh(III) species formed the rhodacycle intermediate A, which was inserted into the triple bond of alkyne to give the intermediate B. The second C–H activation of intermediate B generated the intermediate C, which underwent the second insertion to the alkyne. The subsequent reductive elimination gave the product and liberated the Rh(I). Cu(OAc)2 was required as the external oxidant to regenerate the Rh(III) catalyst (Scheme 136). Later on, they investigated the selective Rh(III)-catalyzed alkenylation of 1-phenylpyrazoles, in which the installation of two different vinyl groups was possible by a simple one-pot strategy (Scheme 137).197 A Rh(III)-catalyzed cascade C–H activation of pyridines via oxidative annulation of functionalized pyridines with two alkyne molecules for the synthesis of diverse quinolines was demonstrated by Li and co-workers in 2011.198
The Rh(III)-catalyzed indole synthesis from N-aryl-2-aminopyridines with alkenes was reported by Li and co-workers (Scheme 138a).199 The authors proposed that the N-aryl-2-aminopyridines went through the initial olefination and the subsequent intramolecular annulation afforded the indole derivatives. The corresponding quinolone products could also be afforded using acrylates as coupling reagents. After this work, they applied the strategy in the synthesis of pyrazole derivatives (Scheme 138b).200
To overcome the drawbacks of the use of stoichiometric metal oxidants in the Rh(III)-catalyzed C–H activation reaction, an efficient Rh/O2 catalytic system for the synthesis of isoquinolinium salts from arenes and alkynes was developed by Huang (Scheme 139).201 The symmetrical diarylalkynes containing various electron rich or deficient functional groups reacted smoothly to give the corresponding products in moderate to high yields, while the unsymmetrical alkynes gave a mixture of isomers in a moderate ratio. Stoichiometric reaction of the cyclometalated rhodium complexes provided strong evidence of facile oxidation of Rh(I) to Rh(III) by molecular oxygen facilitated by the additive acid. By using the same catalytic system, they achieved the synthesis of 2-vinylindoles.202
Another dream reaction based on the C–H activation is the nucleophilic addition reaction toward C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X motifs.1y The rhodium(III)-catalyzed pyridine-directed C–H addition to N-Ts-imines or Boc-imines was reported for the first time by Shi's group203a and Ellman's group203b in 2011, simultaneously (Scheme 140). These reactions showed excellent functional group compatibility. Shi and co-workers also systematically studied the mechanism by isolating the key intermediates and kinetic studies to clearly support the C–H activation pathway.204 Bergman and co-workers proposed that the arylation reaction was initiated by the electrophilic deprotonation of phenyl C–H bond of 2-phenylpyridine to form an Ar–Rh(III) intermediate. The subsequent coordination of the N-Boc-imine would promote the imine addition, and the protonolysis regenerated the catalyst. By the use of [RhCp*(CH3CN)3][SbF6]2 as the pre-activated catalyst, the pyridine-directed C–H addition to other polar multiple bonds (Scheme 141a),205a ethyl glyoxylate (Scheme 141b),205b and aryl aldehydes (Scheme 141c)205c was achieved recently. With the same strategy, alkenyl C–H could also nucleophilic addition to the C
X motifs.1y The rhodium(III)-catalyzed pyridine-directed C–H addition to N-Ts-imines or Boc-imines was reported for the first time by Shi's group203a and Ellman's group203b in 2011, simultaneously (Scheme 140). These reactions showed excellent functional group compatibility. Shi and co-workers also systematically studied the mechanism by isolating the key intermediates and kinetic studies to clearly support the C–H activation pathway.204 Bergman and co-workers proposed that the arylation reaction was initiated by the electrophilic deprotonation of phenyl C–H bond of 2-phenylpyridine to form an Ar–Rh(III) intermediate. The subsequent coordination of the N-Boc-imine would promote the imine addition, and the protonolysis regenerated the catalyst. By the use of [RhCp*(CH3CN)3][SbF6]2 as the pre-activated catalyst, the pyridine-directed C–H addition to other polar multiple bonds (Scheme 141a),205a ethyl glyoxylate (Scheme 141b),205b and aryl aldehydes (Scheme 141c)205c was achieved recently. With the same strategy, alkenyl C–H could also nucleophilic addition to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X bonds under mild conditions.205d
X bonds under mild conditions.205d
The rhodium-catalyzed ring-opening of aziridines was applied in the pyridine-directed alkylation by Li and co-workers (Scheme 142a).206a Both electron-donating and electron-withdrawing substituents at the meta and para positions of the phenyl group were tolerated to afford the corresponding β-branched amines. The chelation of the pyridine and the sulfonamide N atoms with the Rh atom furnished a rather rare eight-membered ring, which was further stabilized by fusing with a four-membered ring. They demonstrated that the 7-azabenzonorbornadienes or 7-azabenzonorbornadienes could be used as coupling reagents in the Rh(III)-catalyzed C–H functionalization of (hetero)arenes, in which dihydrocarbazoles and dehydrative naphthylation products could be afforded, respectively (Scheme 142b and c).206b Li also reported other Rh(III)-catalyzed C–H functionalization reactions using pyridine as the directing group to construct C–C bonds through varying the type of the reaction substrates.206c,d
The rhodium(III)-catalyzed C–C coupling of diazomalonates with arenes was reported by Li and co-workers (Scheme 143).71c Several directing groups, such as pyrazole, pyrimidine and oxazole, were proved effective in the alkylation of (hetero)aromatics with good functional group compatibility. The carbenoid C–H coupling mechanism was proposed by the authors, and the isolation of the key intermediate supported the proposed pathway.
The first rhodium-catalyzed direct oxidative carbonylation of aromatic C–H bond with CO and alcohols was reported by Zhang and co-workers (Scheme 144).207 The reaction showed high regioselectivity and good functional group tolerance, but the yields of carbonylation products were highly influenced by the steric hindrance and boiling point of the alcohol used.
The use of non-toxic CO2 as the carbon source to construct the C–C bond in the Rh-catalyzed acylation of arenes was investigated by Iwasawa (Scheme 145).208 Various substituted and functionalized 2-arylpyridines and 1-arylpyrazoles underwent the carboxylation in the presence of the rhodium catalyst and a stoichiometric methylating reagent, AlMe2(OMe), to give carboxylated products in good yields. Methylrhodium(I) species was proposed to be the key intermediate, which underwent C–H activation to afford rhodium(III). The subsequent reductive elimination of methane gave the nucleophilic arylrhodium(I). This approach had a promising application in the field of carbon dioxide fixation.
Alkynes as fundamental building blocks are widely used in synthetic chemistry and materials science. Recently, alkynylated hypervalent iodines was developed as a versatile and powerful alkynylating reagent, which was applied in the rhodium-catalyzed C–H alkynylation of arenes by Li and co-workers (Scheme 146).89 The high yield of the product was attributed to the use of Zn(OTf)2, which might play a dual role in both activating the catalyst by reversible chloride abstraction and activating the alkyne substrate. A rhodium(III) alkynyl complex and a Rh(III) vinyl complex was established as key intermediates.
Anbarasan and co-workers reported a rhodium catalyzed cyanation of arenes using N-cyano-N-phenyl-p-methylbenzenesulfonamide (NCTS) as an environmentally benign electrophilic cyanating reagent (Scheme 147a).209 The authors proposed that the key step was the transformation of the aryl motif to the nitrile carbon atom from the cyanating reagent after the initial rhodium-catalyzed C–H activation. More recently, Xu and co-workers reported the rhodium-catalyzed C–H bond cyanation of arenes with tert-butyl isocyanide, in which the additive Cu(TFA)2 was used as an oxidant to regenerate the Rh(III) catalyst (Scheme 147b).210
The pioneering work on rhodium-catalyzed intermolecular amidation of arenes with sulfonylazides via chelation-assisted C–H bond activation has been reported by Chang and co-workers (Scheme 148a).211 The amidation required no external oxidants and released N2 as the single byproduct, thus offering an environmentally benign C–N arylation procedure that can be readily scaled-up. The more challenging rhodium-catalyzed C(sp3)–H amidation with azides was achieved by Wang and co-workers (Scheme 148b).212 The key step was a C(sp3)–H activation process to afford a five-membered rhodacyclic intermediate, which was partially supported by the isolation and the subsequent characterization of the intermediates.
Other electrophilic nitrogen sources, such as hydroxylamines (Scheme 149a),213 chloroamines (Scheme 149b)214 were also used in the C–H bond activation to construct the C–N bond. The 2,4,6-trichlorobenzoyl groups as substituents on the N-atom gave the amidation product in excellent yields under the redox-neutral reaction conditions. When N-hydroxycarbamates were used as coupling reagents, Ag2CO3 was found to be the ideal oxidant for the reaction (Scheme 149c).215 Su and co-workers reported a rhodium-catalyzed intermolecular N-chelating-directed aromatic C–H amidation with amides (Scheme 149d).216a A wide range of substituents of 2-arylpyridines, such as aldehydes and bromide groups were tolerated. The success was attributed to the additive PhI(OAc)2, which may serve as an oxidant to regenerate Rh(III) intermediate, or react with sulfonamide to afford the nitrene precursor. A Rh(III)-catalyzed C–H amidation of arenes bearing different chelating groups using readily available N-arenesulfonated imides as efficient amidating reagents was reported by Wan, Li and co-workers.216b
Inorganic salts, such as sodium azide, sodium nitrite, have been used to construct the C–N bond via rhodium-catalyzed C–H activation, presenting alternative routes to fulfill the current C–H azidation and nitration reactions (Scheme 150).217 Pyridine, pyrimidine, and pyrazole heterocycles were found to be efficient directing groups to control the regioselectivity in the reaction. The authors proposed that the hypervalent iodine reagent was important to generate the active azidation agent PhI(N3)(OTs), which would go through the subsequent electrophilic azidation via a five-membered-ring transition state.
The rhodium-catalyzed intermolecular direct amidation of aldehyde C–H bonds with N-chloroamines was developed by Li and co-workers (Scheme 151a).218a A variety of primary and secondary amines were applied to afford the corresponding amides in moderate to excellent yields in the absence of external oxidants. The first step of the catalytic cycle was supposed to be the heterocycle-directed C–H activation of aldehyde C–H bonds to form the five-membered cyclometalated acyl–Rh(III) complex, which was demonstrated by the X-ray crystallography. Further improvement using azides as the nitrogen source in the Rh(III)-catalyzed amidation of aldehyde was studied by the same group (Scheme 151b).218b
The construction of C–S bonds from 2-phenylpyridines with aryl or alkyl disulfides via Rh(III)-catalyzed C–H activation has been reported by Li (Scheme 152).219 The selective mono- or dithiolation of arenes could be controlled by the reaction temperature. Significantly, this C–H thioetherification reaction was compatible with many directing groups (pyridine, pyrimidine, pyrazole, and oxime) and various synthetically useful functional groups.
Oi and Inoue reported the first example of Ru-catalyzed C–H arylation of 2-arylpyridines using arylhalides (Scheme 153).220 Bromobenzene, iodobenzene and phenyltriflate were effective arylation reagents in the reaction. The oxidative addition of Ru(II) to ArBr generated the tetravalent arylruthenium complex A, which reacted electrophilically with 2-phenylpyridine to give the zwitterionic intermediate B. Elimination of HBr formed the arylated ruthenacycle C, which underwent the reductive elimination to afford the product (Scheme 154).
A related example of ruthenium-catalyzed arylation of 2-alkenylpyridines with aryl bromides was developed by the same group later (Scheme 155a).221 By the use of an air-stable secondary phosphine oxide, Ackermann and co-workers further realized the arylation with inexpensive, but less reactive aryl chlorides or aryl tosylates (Scheme 155b and c).222,223 Meanwhile, the oxidative homocoupling reaction of 2-arylpyridines was reported by Li, in which FeCl3 was used as an oxidant to regenerate the Ru(II) catalyst (Scheme 156).224
Zhang and co-workers first reported a new catalytic method for the alkenylation of arylpyridines at the ortho-C–H bond with terminal alkynes in the presence of 5 mol% of RuCl3 and 1 equiv. of benzoyl peroxide or benzoic acid in NMP at 150 °C, with high stereoselectivity toward (E)-stereoisomers. Various terminal alkynes such as aryl terminal alkynes, alkyl terminal alkynes and vinyl terminal alkynes were tolerated and gave the products in moderate to high yields. However, the internal alkynes were ineffective under these reaction conditions. A plausible mechanism involved the initial formation of a cyclometalated intermediate, regioselective insertion of alkyne, and protonation of the resulting Ru–C bond (Scheme 157a).225 Very recently, the ruthenium-catalyzed alkenylation of N-(2-pyridyl)-indoles was achieved by Zeng (Scheme 157b).226 Various arenes and alkynes, including electron-deficient and electron-rich internal alkynes and terminal alkynes, were compatible in this transformation. Remarkably, the directing group-pyridyl moiety can be easily removed to afford free (N–H) indoles under mild conditions.
Ruthenium-catalyzed C–H alkenylation with styrene and alkyl acrylates was reported by Dixneuf (Scheme 157c).227 The acetic acid solvent played a key role in the reaction and the yields dramatically decreased without the use of the acetic acid. Importantly, alkenylation with electrophilic acrylates required a stoichiometric amount of the oxidant while alkenylation with styrene proceeded with a catalytic amount of Cu(OAc)2·H2O in acetic acid.
An oxidative annulation reaction of 2-arylindoles with alkynes by ruthenium-catalyzed C–H bond cleavage was achieved by Ackermann in 2012 (Scheme 158).228 Remarkably, the stoichiometric amounts of the oxidant could be avoided and catalytic amounts of Cu(OAc)2·H2O were found to be sufficient. Substrates bearing different functional groups, such as fluoro, nitro, ester, ketone or aldehyde substituents, were well tolerated with the catalytic system.
Li and co-workers reported a ruthenium-catalyzed oxidative cross-coupling of chelating arenes with cycloalkanes (Scheme 159).229 The use of t-butyl peroxide as an oxidant and cycloalkanes as a solvent was crucial to achieve the best yield. Other ruthenium catalysts, such as Ru3(CO)12, Ru(acac)3, [Ru(cod)Cl2]2, Ru(CO)H2(PPh3)3 and [Ru(benzene)Cl2]2, led to a mixture of mono- and dialkylation products.
A stable and easy-operated ruthenium catalytic system was developed by Ackermann (Scheme 160).230 The additives such as metal carboxylates were necessary to generate the active ruthenium catalyst, and steric KO2CMes led to the best yield. Notably, inactivated alkenes, such as alkyl alkenes and vinyl silanes, could be used as excellent coupling reagents to provide the alkylated heteroarenes.
Ruthenium-catalyzed amino- and alkoxycarbonylations of arenes was described by Kakiuchi and co-workers (Scheme 161).231 The use of carbamoylchlorides and alkyl chloroformates offered an alternative route to synthesize the carbonylation products that required the consume of CO in the traditional method. A broad generality of amide and ester groups were achieved, taking advantage of the wide availability of carbonylating agents. Moreover, alkyl chloroformates, which were inactive under usual Friedel–Crafts conditions, could be used in this direct catalytic alkoxycarbonylation.
In 2013, Beller reported a ruthenium-catalyzed carbonylation of arenes in water under a CO atmosphere (Scheme 162).232 The monoaroylation products were obtained exclusively under the optimal conditions. Interestingly, silver acetate or silver hexafluoroantimonate which had a positive effect on the generation of the active species in the Ru(II)-catalyzed C–H activation, inhibited the reactivity of this catalyst system completely.
A new 3-phenylindenyl dihydridosilyl ruthenium complex was developed to promote the selective ortho-borylation of aromatic C–H bonds by Nolan and co-workers (Scheme 163).233 The [RuH2(3-phenylindenyl)(SiEt3)] complex exhibited high efficiency in the C–H activation step to generate the ruthenium(IV) intermediate, which reacted with B2Pin2 to form a new ruthenium intermediate, with concomitant releasing of HBpin. The final reductive elimination afforded the desired borylated products. Importantly, the Ir and Rh catalytic systems could also be used and the tandem reactions such as a one-pot borylation/Suzuki–Miyaura sequence could be performed.
The ruthenium-catalyzed C–H amidation of arenes with sulfonyl azides was reported by Ackermann and co-workers (Scheme 164).234 Various alkyl and aryl sulfonyl azides were tolerated to give the products in moderate to high yields. Furthermore, pyrazolyl-, pyrimidyl- or pyridyl-substituted arenes and heteroarenes could successfully be transferred into the corresponding amidation derivatives.
In 2004, Kakiuchi reported a novel Ru3(CO)12-catalyzed silylation of benzylic C–H bonds in arylpyridines and arylpyrazoles with hydrosilanes (Scheme 165).235 Norbornene was used as an important hydrogen acceptor. The silylation took place selectively at the ortho-position of the CH3 group rather than the para-position, indicating that the coordination of the nitrogen of heterocycle was involved in this silylation reaction. The reaction proceeded exclusively at the methyl group instead of methylene group in an ethyl substituted substrate, which indicated that steric congestion had an important influence on the reactivity of benzylic C–H bonds. This method provided an alternative route for preparing benzylsilane derivatives.
In the last few decades, late transition metal-catalyzed C–H activation, such as palladium, ruthenium, rhodium, iridium, gold, and platinum, had been extensively studied. However, the relatively high price and considerable toxicity limited their widespread applications. Therefore, more and more chemists shifted their attention to the first-row transition metals, especially copper, iron, and cobalt, which have obvious advantages and unique features. Miura and co-workers reported a copper-mediated intermolecular biaryl coupling reaction (Scheme 166a).236 The reaction proceeded without palladium catalysts and enabled an unprecedented coupling between 2-arylazines with azoles. Carboxylic acid additives generally improved the reaction efficiency, among which PivOH afforded the best yields. Later, the copper-mediated cross-coupling of indoles and 1,3-azoles was also reported by the same group (Scheme 166b).237 Compared with the previous work, the use of atmospheric oxygen was found to render the reaction in a catalytic manner. More recently, they found that pyridine could be used as a removable directing group in the copper-mediated dehydrogenative arylation of 2-pyridones with 1,3-azoles (Scheme 166c).238 The authors proposed that the initial cupration of the 1,3-azole and the subsequent chelation of the pyridine moiety to the Cu center afforded the Cu(III) species. The final reductive elimination liberated the product and Cu(I) species, which could be oxidated by molecular oxygen to regenerate the starting Cu(II) species.
The Cu(II)-catalyzed C–H cyanation, amination, etherification and thioetherification reactions of 2-arylpyridine were investigated by Yu group.239 A radical–cation pathway was proposed by the authors: (1) the initial single electron transfer (SET) from the aryl ring to the coordinated Cu(II) led to the cation–radical intermediate. (2) The subsequent intramolecular anion transfer from the Cu(I) complex to the ortho position of arene. (3) The final SET led to the generation of ortho-functionalized products.
The Cu(II)-mediated C–H functionalization of 2-arylpyridine has been rapidly developed using different anion sources by several groups. For example, Cu(II)-catalyzed ortho-cyanation of 2-arylpyridine using different CN sources, such as benzyl nitrile,240 acetonitrile,241 azobisisobutyronitrile,242 was achieved. Recently, Liu and co-workers demonstrated that AgNO3 could provide the nitrate anion in the copper-mediated ortho-nitration of (hetero)arenes (Scheme 167).243
In the last few decades, late transition metal-catalyzed C–H activation, such as palladium, ruthenium, rhodium, iridium, gold, and platinum, had been extensively studied. However, the relatively high price and considerable toxicity limited their applications and possible improvements. Therefore, more and more chemists shifted their attention to the first-row transition metals, especially copper and iron, which have obvious advantages and unique features. A iron-catalyzed direct arylation of α-benzoquinoline with phenylmagnesium bromide was first developed by Nakamura and co-workers in 2008 (Scheme 168).244 The arylation products were obtained using 10 mol% of Fe(acac)3/phen, 3 equiv. of ZnCl2·TMEDA and 6 equiv. of arylmagnesium bromide at 0 °C. 1,2-Dichloroisobutane (DCIB) had an important role in the reaction as an oxidant. The oxidation state and the counteranion of the iron salt had only a little influence on the catalytic activity and various iron catalysts could be applied in this reaction.
In 2011, Nakamura and co-workers reported an iron-catalyzed olefinic C–H arylation with the Grignard reagent (Scheme 169).245 A wide range of vinyl groups, such as cyclic and acyclic in the pyridine could be tolerated under the reaction conditions. The solvent had an important influence on the E/Z ratio of products, which could be raised from 3/97 to 95/5 when the solvent was changed from to PhCl/Et2O to THF. It was noted that the byproducts could be retarded by the addition of the 4,4-di-tert-butyl bipyridine (dtbpy) ligand and the slow addition of the Grignard reagent.
Based on the results of the control experiments, the author proposed two different reaction pathways: (a) the C–H bond activation resulted in a five-membered metallacycle which underwent reductive elimination to give the substituted olefin; (b) the five-membered metallacycle underwent the oxidative Heck reaction to give the products (Scheme 170). The aliphatic halides of 1,2-dichloroisobutane and 1-bromo-2-chloroethane were required to oxidize the iron hydride species which led to the formation of the byproduct 2-(ethyldimethylsilyl)pyridine.
Yoshikai and co-workers reported a cobalt-catalyzed arylation of aldimines (Scheme 171),246 in which the polar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond could serve as a reaction partner. The choice of the Grignard reagent of t-BuCH2MgBr was critical, and the use of other alkyl Grignard reagents such as MeMgCl, n-BuMgBr, iPrMgBr, and Me3SiCH2MgCl, resulted in lower yields. The ligand 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride (IPr) was indispensable for the reaction and a wide functional group was observed.
N bond could serve as a reaction partner. The choice of the Grignard reagent of t-BuCH2MgBr was critical, and the use of other alkyl Grignard reagents such as MeMgCl, n-BuMgBr, iPrMgBr, and Me3SiCH2MgCl, resulted in lower yields. The ligand 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride (IPr) was indispensable for the reaction and a wide functional group was observed.
Yoshikai and co-workers reported a cobalt-catalyzed C–H alkylation of 2-arylpyridines with 1,2-diarylaziridines (Scheme 172).247 A cobalt-N-heterocyclic carbene catalyst was found to promote ortho-C–H functionalization of 2-arylpyridines with 1,2-diarylaziridines through ring-opening alkylation. The reaction afforded the 1,1-diarylethanes bearing 2-amino functional groups in moderate to good yields under mild room-temperature conditions. The combination of a cobalt salt with an NHC ligand as well as a Grignard reagent was indispensible to offer versatile catalytic systems for ortho-C–H functionalization of arenes with electrophiles.
As early as in 1971, Bruce and co-workers reported seminal work on stoichiometric ortho-manganation of benzylideneaniline. However, there were only a few examples of Mn-catalyzed C–C bond formation reactions via inert C–H activation. In 2013, Wang and co-workers demonstrated the first manganese-catalyzed aromatic C–H alkenylations with terminal alkynes (Scheme 173).248 The alkenylate products were obtained by the reaction of 2-phenylpyridine with terminal alkynes in the presence of MnBr(CO)5 (20 mol%), Cy2NH (10 mol%) in Et2O at 80 °C. Importantly, the secondary amines bearing steric bulkiness were found to be beneficial for the C–H activation step. A wide range of functional groups were compatible with the catalytic system. Steric substituents on the benzene moiety showed little influence on the reaction outcome. The reaction occurred readily in a highly chemo-, regio-, and stereoselective manner, delivering anti-Markovnikov E-configured olefins in high yields.
3.2. The C–H functionalization directed by imine
Imines are common reactive functionalities that allow access to a diversity of synthetically useful architectures through demonstrated reactivities. In recent years, the strong coordination ability of imine with transition metals has been found wide applications in challenging C–H bond functionalizations.The first example of imine directed ortho-alkylation of arenes with various olefins was demonstrated by Jun and co-workers in 2000 (Scheme 174).249 Compared with the previous alkylation of aryl ketones by Murai and co-workers, in which ketone was used as the directing group,2b this process exhibited a broad substrate scope of alkenes. The alkenes bearing allylic hydrogens, electron-deficient alkenes, and even internal alkenes, took part in the reaction smoothly to give the corresponding alkylated aryl ketones after acidic hydrolysis. The over alkylation that plagued aryl ketone alkylation could also be avoided in this transformation.
As shown in Scheme 175, the mechanism involved the initial precoordination of the imine nitrogen atom to the Rh(I) center, followed by the activation of an aromatic C–H bond. The resulting metallacycle C went through olefin coordination and hydride insertion to give D, which underwent reductive elimination to furnish the ortho-alkylated product E.
Bergman, Ellman and co-workers demonstrated the imine-assisted intramolecular alkylation of arenes (Scheme 176a).250 Allyl ethers and allylamines tethered to the ortho-position could function as efficient olefin coupling partners to undergo the intramolecular annulation to afford the functionalized indane, tetralane, dihydrobenzofuran and dihydroindole derivatives. However, the reaction suffered from the low functional group tolerance and high reaction temperature. After the optimization of the reaction conditions, this intramolecular alkylation reaction could be performed at lower temperature by the use of [RhCl(coe)2]2 as a catalyst, which allowed the enantioselective cyclization of aromatic imines in the presence of the chiral phosphoramidite ligands (Scheme 176b).251 The observed high enantioselectivities were attributed to the unique binding properties of phosphoramidite ligands with the rhodium catalyst.
The ortho-alkylation of imines using alkyl halides in the presence of a cobalt-N-heterocyclic carbene (NHC) catalyst was reported by Yoshikai and co-workers (Scheme 177).252 The choice of different N-heterocyclic carbene ligands was crucial for the yields and selectivities of the reaction under mild reaction conditions. These mild conditions afforded the alkylated products in high efficiencies with primary and secondary alkyl chlorides and bromides. The stereochemical outcomes by the use of secondary alkyl halides suggested that the reaction mechanism involved the formation of the alkyl radical through a single-electron transfer from cobalt species.
Jun and co-workers reported the first example of the Rh(I)-catalyzed ortho-olefination of common aromatic ketimines with alkynes.253 The product could further undergo the cyclization to result in the generation of isoquinoline derivatives at the high reaction temperature. Recently, Yoshikai group accomplished this addition of aromatic imines to alkynes via cobalt-catalyzed C–H bond activation (Scheme 178).254 A quaternary catalytic system, including cobalt salt, triarylphosphine ligand, Grignard reagent and pyridine, was developed for the transformation. The role of each components of the system has been proposed: (1) the active cobalt intermediate could be stabilized by the phosphine ligand; (2) the Grignard reagent may reduce cobalt(II) to give rise to an organocobalt(0) species as the catalytically active species; (3) pyridine appeared to serve as a coligand for the cobalt catalyst. Interestingly, the reaction displayed a unique regioselectivity to form the C–C bond at the more sterically hindered ortho positions, which was ascribed to the electron-withdrawing substituents to strengthen a metal carbon bond at the ortho position or the coordination ability of substituents with the metal center in the oxidative C–H activation process. By the use of cobalt catalysts, Yoshikai group also achieved the ortho-arylation reaction of aromatic imines with aryl chlorides under their well-established quaternary catalytic system at room temperature.246,255
The application of acrylates and styrenes in the ortho-olefination directed by an N-Ts imine group was demonstrated by Li and Wu group respectively, in which an excess of copper(II) salt was used as an oxidant (Scheme 179).256 A blocking group was introduced to the ortho and meta positions of the aromatic ring to obtain good isolated yield of mono-olefinated products when styrene was used.256a The N-sulfonyl ketimines were further applied to the synthesis of the functionalized pyridines via imine-directed ortho-olefination and subsequent annulation by Dong and co-workers.257 It was proposed that the N–S bond of N-sulfonyl ketimines served as an internal oxidant.
The ruthenium-catalyzed ortho-arylation of aryl imines with aryl and alkenyl bromide was reported by Oi in 2002 (Scheme 180).258 The tetravalent aryl- or alkenyl-ruthenium species should be the key intermediate, which was generated from oxidative addition of aryl bromide to the ruthenium(II) complex. The proposed mechanism is shown in Scheme 181. The coordination of A to the imine moiety afforded the arylated ruthenacycle intermediate C, followed by the reductive elimination to generate the ortho-arylated product D.
The use of aryl chlorides in the ruthenium-catalyzed arylation of imines was developed by Ackermann (Scheme 182).223 Both electron-rich and electron-poor aryl chlorides were tolerated in this reaction and a wide variety of functional groups were compatible, including enolizable ketones, nitriles, and esters. The successful employment of inactive aryl chlorides in the C(sp2)–H arylation was attributed to the use of air-stable, electron-rich phosphine oxides as preligands. The corresponding mono-arylated ketones were isolated upon hydrolysis in high yields.
A Pd(II)-catalyzed direct C–H bond arylation on electron-deficient arenes that proceeded at room temperature was described by Gaunt and co-workers (Scheme 183).259 Compared with previous efforts, the increased steric bulk around the aniline moiety was important to increase stability of the imine towards hydrolytic cleavage. The key cyclopalladation intermediate was obtained by the treatment of imine with 1 equivalent of Pd(OAc)2 at room temperature in methanol, which gave the ortho-arylated benzaldimine in excellent yields when treated with PhB(OH)2. Further achievement was made by the use of benzene as a source of the aryl group for this ortho-arylation reaction.
Iron-catalyzed coupling of an aromatic imine with a diarylzinc reagent was developed by Nakamura and co-workers (Scheme 184).260 By the use of cheap, ubiquitous and nontoxic iron catalysts, the reaction took place under very mild conditions (0 °C in THF) and tolerated the presence of reactive functional groups, including halides, nitriles, triflate and tosylate groups.
The oxidative coupling of aldehydes to form phthalides by Rh(III) catalysis was reported by Seayad and co-workers (Scheme 185).261 The presence of catalytic amounts of amines played the key role by reacting with the aldehydes to in situ form imine, iminium, or aminal intermediates, which could act as better directing groups, and thus would be able to enhance the reactivity of the Rh-catalyzed ortho C–H activation in comparison with the aldehyde functionality.249 Interestingly, primary aryl amines such as 4-trifluoromethylaniline showed the high efficiency, while nucleophilic amines such as p-anisidine, pyrrolidine, and cyclohexyl amine gave only trace amount of the products.
The rhenium-catalyzed insertion of aromatic aldimine into isocyanate or acetylene to give phthalimidine or indene derivatives was described by Kuninobu and Takai (Scheme 186a and b).262 These authors proposed a reaction pathway: (1) an ortho C–H bond activation by rhenium catalyst; (2) insertion of the acetylene into the rhenium–carbon bond; (3) intramolecular nucleophilic attack of the formed alkenylrhenium at the carbon atom of the imine; (4) reductive elimination and 1,3-rearrangement of hydrogen atoms. The directing groups of oxime or hydrazone from an aldehyde did not work. Recently, a new transformation was reported by the same group for the rhenium-catalyzed synthesis of 3-imino-1-isoindolinones (Scheme 186c).263
The Rh-catalyzed reaction of aromatic imines with alkynes to achieve indenone imine and isoquinoline derivatives was reported by Satoh and Miura (Scheme 187).264 A plausible mechanism for the reaction of benzylideneaniline with alkynes is illustrated in Scheme 188. The initial coordination of the nitrogen atom of imine to Rh(III)X3 species led to the regioselective C–H bond cleavage to afford rhodacycle intermediate A. The subsequent alkyne insertion formed intermediate B, which underwent the intramolecular nucleophilic addition of the amido-rhodium moiety to the imine group to give the intermediate C. The β-hydrogen elimination generated the final product and Rh(I)X species, which could be oxidized by a copper(II) salt to regenerate the Rh(III)X3 catalyst.
During the study of Suzuki–Miyaura-type cross-coupling of arylboron compounds with aryl halides, Satoh and Miura unexpectedly found the Rh-catalyzed ortho-phenylation of the N-unsubstituted benzophenone imine (Scheme 189a).265 The subsequent studies by Zhao and co-workers demonstrated that N-unsubstituted aromatic ketimines and internal alkynes could form indenamines through a Rh-catalyzed [3 + 2] annulation (Scheme 189b).266 Notably, the formation of byproduct implicated that a Rh-catalyzed [4 + 2] annulation occurred during the reaction to give isoquinolines. The high selectivity of [3 + 2] versus [4 + 2] was attributed to the presence of DPPP ligands. Recently, they developed the catalytic system of a Ru(II)/π-allyl catalyst and N-heterocyclic carbine (NHC) ligands to allow this [3 + 2] annulation to occur at 20–60 °C without the need for other additives (Scheme 189c).267
In 2010, Cramer and coworkers developed a Rh-catalyzed [3 + 2] annulation of unsubstituted ketimines with terminal allenes to give the useful substituted indanylamines (Scheme 190a).268a In most cases, a racemic annulation product was obtained and only one example gave the moderate enantioselectivity. The subsequent research by the same group demonstrated that 2,2′-bis(diphenylphosphino)-1,1′-binaphthalene (BINAP) could be used as the effective chiral ligand to provide the excellent enantioselectivity of stereogenic center of the amino group. Both alkynes and racemic allenes were proved to be the excellent coupling partners (Scheme 190b and c).268b,c
Nishimura and co-workers obtained aminoindane derivatives by iridium-catalyzed [3 + 2] annulation involving cyclic N-sulfonyl ketimines and 1,3-dienes (Scheme 191a).269 The reaction enjoyed high regio- and diastereoselectivity and the deuterium intermolecular competition experiment of kH/kD (2.1) revealed that the C–H bond cleavage occurred during the turnover-limiting step. More recently, Dong and co-workers reported an alternative approach, in which spirocyclic benzosultams could be generated through Rh(III)-catalyzed C–H activation of cyclic N-sulfonyl ketimines with internal alkynes (Scheme 191b).270 As early as 2012, Li and co-workers had demonstrated that the redox-neutral annulative coupling of N-sulfonyl imines with alkynes could be achieved using the Ru catalyst.271
Direct ortho-alkenylation of common aromatic ketimines and its sequential rhodium-catalyzed [4 + 2] annulation offered a one-pot synthetic protocol of isoquinoline or pyridine derivatives.272 The first application of this strategy was achieved by Jun in 2003 at high temperatures (170 °C).253 A Rh(I)-catalyzed synthesis of highly substituted dihydropyridines derivatives from alkynes and α,β-unsaturated N-benzyl aldimines or ketimines under mild reaction conditions was developed by Bergman and Ellman later (Scheme 192a).272a–c The reaction was proposed to go through the C–H alkenylation/electrocyclization/aromatization sequence, and the isolated rhodium intermediate supported the supposed reaction pathway. In 2009, Fagnou and co-workers made significant contributions to carry out the isoquinoline synthesis via rhodium(III)-catalyzed oxidative cross-coupling/cyclization of aryl aldimines and alkyne, in which Cu(OAc)2·H2O was used as an oxidant in the Rh(III)/Rh(I) mechanism (Scheme 192b).272d A variety of alkynes could be employed with high regioselectivity, including unsymmetrical alkynes.
Wang's group presented the first example of manganese-catalyzed C–H dehydrogenative [4 + 2] annulation of N-unsubstituted imines and alkynes to give the isoquinolines. This reaction showed the high efficiency and broad substrate scope (Scheme 193).273 The key five-membered manganacycle intermediate was prepared and investigated in the subsequent mechanistic study. The C–H bond cleavage induced by catalytic manganese(I) in the rate-determining step was verified through kinetic isotope effect (KIE) experiments.
In 2012, Cheng and co-workers demonstrated the regioselective Rh-catalyzed synthesis of substituted isoquinolinium salts from the reaction of benzaldehydes, amines and alkynes. The success was attributed to the coordination of the nitrogen atom of the imine, which was generated in situ from benzaldehydes and amines, to the rhodium species, followed by the subsequent C–H bond activation (Scheme 194).274a The role of AgBF4 was crucial for the success of the present catalytic reaction, which provided an inert anion necessary for the isolation of isoquinolinium salt, and removed the chloride on the rhodium complex to facilitate the catalytic reaction. The synthesis of substituted isoquinolinium salts from ketimines274b and Ru-catalyzed synthesis of substituted isoquinolinium salts from benzaldehydes, amines and alkynes274c were reported by the same group.
3.3. The C–H functionalization directed by oxime
Oximes are important organic scaffolds and widely exist in pharmaceuticals and functional materials. Pioneer work made by Sanford and co-workers demonstrated that the Pd-catalyzed acetoxylation of inactivated sp3 C–H bonds could be achieved by employment of oxime as the directing group in the presence of stoichiometric oxidant PhI(OAc)2 (Scheme 195).275 The high selectivity and efficiency of the reaction was attributed to the coordination of O-methyl oxime with the palladium catalyst. In general, oxime presented improved stability than imine as the directing group under the reaction conditions.The palladium-catalyzed oxime-directed arylation of substituted benzaldehyde oxime ethers with aryl halides was reported by Cheng and co-workers for the synthesis of fluorenones (Scheme 196).276 The plausible catalytic pathway was proposed by the authors as shown in Scheme 197. First, the intermediate B was formed from the intermediate A through the oxime directed C–H activation. The arylation product D was achieved via the reductive elimination of Pd(IV) intermediate C that was generated from the oxidative addition of the iodobenzene to the Pd(II) intermediate B. The second coordination of arylated products D to palladium(II) generated the seven-membered palladacycle E, which went subsequent intramolecular insertion of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group into the Pd–aryl bond to produce intermediate F. The final β-elimination afforded the cyclization product. The stoichiometric reaction of prepared anionic palladacycle B with aryl iodide and the reaction of isolated intermediate D with aryl iodide resulted in the fluorenone product, which consisted with the proposed mechanism. Subsequent investigation demonstrated that the scope of the arylation reagent could be extended to arylboronic acids277 and simple arenes.278
N group into the Pd–aryl bond to produce intermediate F. The final β-elimination afforded the cyclization product. The stoichiometric reaction of prepared anionic palladacycle B with aryl iodide and the reaction of isolated intermediate D with aryl iodide resulted in the fluorenone product, which consisted with the proposed mechanism. Subsequent investigation demonstrated that the scope of the arylation reagent could be extended to arylboronic acids277 and simple arenes.278
Azamalonates were applied as the coupling reagent to afford α-aryl carbonyl compounds with excellent regioselectivities and functional groups tolerance by Yu and coworkers (Scheme 198a).71a The carbenoid cross-coupling with arene C–H bonds did not require a strongly basic medium and gave the benign N2 as the only by-product. Two pathways were proposed by the authors, in which the Rh–carbene formation, or 1,2-aryl shift without the Rh–carbene intermediates were both possible. The arylated products could readily be transformed into other useful compounds, such as naphthalenes and isocoumarins.
The Rh(III)-catalyzed oxidative coupling reaction of aryl O-methyl oximes with alkenes was reported by Bergman and Ellman group (Scheme 198b).279 The coupling of inactivated terminal alkenes could be accomplished in moderate to good yields. The para-cyano aryl oxime gave a poor yield of the coupled product (10% by NMR), probably due to coordination of the nitrile to the catalyst.
In 2010, Yu and co-workers developed a Pd-catalyzed ortho-acylation of aryl ketone oximes with aldehydes using TBHP as an oxidant for the convenient synthesis of 1,2-diacylbenzene oximes (Scheme 199).280a It was believed that a radical process was involved in the reaction and the acyl radicals could be in situ generated from the aldehydes with the aid of TBHP. The transformation was proposed to begin with the ortho-palladation of O-methyl oxime with Pd(OAc)2 to provide the 5-membered palladacycle, followed by reacting with acyl radicals, which were generated in situ by hydrogen atom abstraction of the aldehydes, affording the desired products via Pd(III) or Pd(IV) intermediate. Subsequently, several studies about Pd-catalyzed acylation reaction directed by oximes using different acylating reagents, such as toluenes,280b α-keto acids,280c benzylic ethers280d were successfully reported in the following years. Furthermore, Zhou and Li developed a Rh-catalyzed intermolecular oxidative cross-coupling of simple aldehydes with acetophenone O-methyl oxime via C–H bond activation.280e The reaction featured broad substrate scopes, high regioselectivities and mild reaction conditions, providing a complementary route to a wide range of diaryl ketones.
Palladium-catalyzed C–N bond formation via oxime-directed C–H amidation reactions was reported by Che, Yu and co-workers in 2006 (Scheme 200a).171 Some primary amides, such as carbamates, acetamides, and sulfonamides, could be acted as effective nucleophiles for the reaction. In addition, catalytic amidation of inactivated sp3 C–H bonds with high regio- and chemoselectivities was also achieved under the current protocol. The authors proposed that a key nitrene intermediate generated from the oxidation of benzamide, might undergo the insertion to the chelation-directed palladacycle to give the final amidated product.
Recently, a Rh-catalyzed direct arene C–H amidation of ketoxime using sulfonyl azides as the amine source was developed by Chang and co-workers (Scheme 200b).127 Many aryl ketoximes could be smoothly aminated to give the desired products in moderate to good yields. In addition, phenyl azides bearing not only electron-withdrawing substituents, but also electron-neutral and even electron-donating groups were all tolerant in this amination reaction. The rhodium(III)-catalyzed intermolecular direct amidation of ketoxime employing N-chloroamines as amine reagents was demonstrated by Yu and co-workers (Scheme 200c).281 It was worth mentioning that monochlorinated primary amines could be well compatible with the Rh-catalyzed amination.281b This protocol presented a notable advancement compared to other related reactions with secondary amines and amides as coupling partners. More recently, Zhou found that readily available N-hydroxycarbamates could also be used as a nitrogen source to proceed rhodium(III)-catalyzed C–H amidation.215 Liu reported the palladium-catalyzed oxime-assisted aromatic C–H nitration using AgNO2 as the nitro source.282
Recently, a Rh-catalyzed C–H cyanation reaction reagent for the synthesis of aromatic nitriles was developed by Fu and co-workers (Scheme 201).283 The N-cyano-N-phenyl-p-toluenesulfonamide (NCTS) was a less toxic, readily available and easily handled crystalline salt, and could be used as a practical cyanation reagent. Ag2CO3 was found to be an effective additive and the relatively high temperature was necessary for the high efficiency of the reaction. The kinetic isotope effect (KIE) experiment was conducted to have an understanding of the reaction mechanism and the results indicated a C–H activation process was involved in the rate-determining step. The reaction tolerated a wide range of synthetically important functional groups and a number of aromatic and heteroaromatic nitriles were readily afforded using this method. Furthermore, the obtained nitrile products could be easily transformed into many other synthetically useful groups, providing a convenient method for the preparation of functional molecules.
A palladium-catalyzed C–H bond fluorination under mild conditions in the presence of a catalytic amount of simple, cheap, and nontoxic nitrate as the promoter was developed by Xu and co-workers (Scheme 202).284O-Methyl oxime ethers was chosen as an efficient directing group, and both aromatic and olefinic C(sp2)–H bond could be successfully selective fluorination in this transformation. The role of nitrate anion was deemed as a pivotal promoter in the reaction. The author speculated that a highly reactive cationic [Pd(NO3)]+ species generated in situ from the Pd precatalyst and nitrate initiated the C–H bond activation process. This elegant selective fluorination reaction was also readily performed on a gram scale and no decrease of the yield was observed.
Disulfides could also be used as effective coupling reagents in the Rh-catalyzed C–H thiolation of ketoximes reported by Zhou and Li.219 The mono or dithiolation products were smoothly achieved with good selectivity using different oxidants in the reaction, affording a straightforward way for selective construction of aryl thioethers and dithioethers. A Rh(III)-catalyzed, chelation-assisted C–H activation and selenylation of arenes was reported by Wan and Li.285 The oxime, azo, pyridyl and N-oxide all could be used as useful directing groups, and electrophilic selenyl chlorides and diselenides were employed as selenylating reagents. The catalytic transformation could tolerate a broad range of functional groups and exhibited high efficiency under mild conditions.
Although the previous Rh-catalyzed oxidative coupling reactions of aryl imines and internal alkynes were extensively investigated, the stoichiometric addition of external oxidants, such as Cu(OAc)2, was necessary for the reaction to regenerate the active Rh(III) catalyst. The N–O bond of oxime derivatives could act as an internal oxidant to maintain the catalytic cycle, thus providing redox-neutral reaction conditions.286 In 2010, Li286d and Chiba287 independently reported a synthetic method for the synthesis of isoquinolines from aryl ketone oxime derivatives and internal alkynes under the catalytic redox-neutral [Cp*RhCl2]2–NaOAc system (Scheme 203). Several control experiments were performed to shed light on the reaction mechanism and the results revealed that the catalytic cycle was initiated by the C–H rhodation process and the C–H activation was likely the rate-determining step.
Based on the mechanistic investigations and the relevant results, a plausible mechanism is outlined in Scheme 204. The initial coordination of O-acetyloximes with Rh(III) led to the generation of arylrhodium intermediate A, followed by insertion into alkynes to afford vinyl rhodium species B. The subsequent intramolecular annulation and reductive elimination would provide the N-acetoxyisoquinolinium cation D, which could serve as an oxidant for the regeneration of the Rh(III) catalyst and afforded the isoquinoline as the final product.
Glorius and co-workers reported the Rh(III)-catalyzed coupling reaction of oximes and diazo compounds to afford multisubstituted isoquinoline and pyridine N-oxides (Scheme 205).71d The employment of diazo compounds could act as the equivalents of electron-deficient alkynes in Rh(III)-catalyzed isoquinoline synthesis. Other noteworthy advantages of the reaction were the high regioselectivities and reactivities. This elegant intermolecular annulation reaction for the synthesis of isoquinoline and pyridine N-oxides presumably underwent tandem C–H activation, cyclization, and condensation steps in the absence of any external oxidants.
Subsequently, the same group developed a Rh(III)-catalyzed C–H functionalization/aromatization cascade of the aromatic oxime esters with diverse 1,3-dienes to prepare isoquinolines with complete regioselectivity (Scheme 206).288 A variety of important functional groups were compatible, such as halogens (F, Cl, and Br), cyano, nitro, trifluoromethyl, and methoxy groups.
Very recently, Rovis demonstrated the rhodium-catalyzed coupling of α,β-unsaturated oxime esters and alkenes to provide pyridines (Scheme 207a).289 The authors found that high regioselectivity of 6-substituted pyridines could be achieved when electron-deficient alkenes were used as coupling reagents, while inactivated alkenes resulted in the mixture of regioisomeric products. They also developed the synthesis of pyridine via the Rh(III)-catalyzed decarboxylative coupling of acrylic acids with unsaturated oxime esters. The carboxylic acid, which would be removed by decarboxylation, served as a traceless activating group, giving 5-substituted pyridines with high regioselectivity. Potassium persulfate (K2S2O8) was selected as the best oxidant to avoid the use of superstoichiometric silver reagents, which was usually necessary for the decarboxylation step (Scheme 207b).290
The [3 + 2] annulation pathway of oxime-directed C–H functionalization to afford phthalide by the use of aldehydes as coupling reagents was demonstrated by Bergman and Ellman (Scheme 208a).291 The imine directing group served to capture the alcohol intermediate upon reversible aldehyde addition. Afterwards, the strategy was applied in the synthesis of furans and pyrroles by the same group (Scheme 208b).292 The annulation was initiated by the rhodium(III)-catalyzed addition of an alkenyl C–H bond across an aldehyde C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or imine C
O or imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond with subsequent cyclization and aromatization. Annulations with ethyl glyoxylate and the corresponding N-tosyl imine provided access to 2-carboxyethyl-substituted furans and pyrroles, respectively. More recently, Li and co-workers demonstrated that isocyanates could be applied in the synthesis of 3-methyleneisoindolin-1-ones using aryl ketone O-methyl oximes as directing groups via a cascade annulation.293
N bond with subsequent cyclization and aromatization. Annulations with ethyl glyoxylate and the corresponding N-tosyl imine provided access to 2-carboxyethyl-substituted furans and pyrroles, respectively. More recently, Li and co-workers demonstrated that isocyanates could be applied in the synthesis of 3-methyleneisoindolin-1-ones using aryl ketone O-methyl oximes as directing groups via a cascade annulation.293
3.4. The C–H functionalization directed by diazo group
Due to the unique structures of the azo moiety in the field of dyes, indicators, photochemical switches and the rapeutic agents, the methods to synthesize ortho-substituted azo compounds via C–H activation have been developed in recent years. In the early work by Sanford, the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the azo group was used as a directing group in the palladium-catalyzed acetoxylation of (E)-1,2-diphenyldiazene with PhI(OAc)2 in CH3CN at 100 °C.275 Recently, various coupling reagents were developed in the palladium-catalyzed acylation of azobenzenes (Scheme 209).294 For example, Wang demonstrated that azobenzenes could be readily acylated at the ortho position through the azo-directed C–H activation.294a The key step of the mechanism was the oxidation of cyclopalladated intermediate by the reactive benzoyl radical, which was generated from the reaction of benzaldehyde with TBHP. Both electron- and electron-withdrawing groups were tolerated under the acylation conditions. The resulting acylated azobenzenes could be further transformed into synthetically important indazoles through a Zn/NH4Cl/MeOH reduction in high yields. They also demonstrated that the α-oxocarboxylic acids could be used as coupling reagents in the palladium-catalyzed decarboxylative ortho-acylation of azobenzenes at room temperature.294b–d
N bond of the azo group was used as a directing group in the palladium-catalyzed acetoxylation of (E)-1,2-diphenyldiazene with PhI(OAc)2 in CH3CN at 100 °C.275 Recently, various coupling reagents were developed in the palladium-catalyzed acylation of azobenzenes (Scheme 209).294 For example, Wang demonstrated that azobenzenes could be readily acylated at the ortho position through the azo-directed C–H activation.294a The key step of the mechanism was the oxidation of cyclopalladated intermediate by the reactive benzoyl radical, which was generated from the reaction of benzaldehyde with TBHP. Both electron- and electron-withdrawing groups were tolerated under the acylation conditions. The resulting acylated azobenzenes could be further transformed into synthetically important indazoles through a Zn/NH4Cl/MeOH reduction in high yields. They also demonstrated that the α-oxocarboxylic acids could be used as coupling reagents in the palladium-catalyzed decarboxylative ortho-acylation of azobenzenes at room temperature.294b–d
Due to the improved stability and availability, much attention has been paid to the use of toluene and its derivatives as acylation reagents. Lu and Wu independently realized the regioselective acylation of azobenzene with toluene, in which the key benzoyl radical was formed in situ from the oxidation of toluene.295 Readily available alcohols were further used as the acyl sources by Zeng and co-workers.296 Notably, the substrate scope could be extended to heteroaryl methanols and aliphatic alcohols, albeit giving low yields.
The direct synthesis of indazole was developed from the rhodium(III)-catalyzed addition of azobenzenes to aldehydes by Ellman and co-workers (Scheme 210).297 The azo moiety served as not only a directing group, but also a nucleophile to trap the initial aldehyde addition product. The regioselective functionalization of unsymmetrical azobenzenes could be controlled by either electronic or steric effects. The major product was obtained by C–H functionalization on the less electron-rich phenyl ring of the azobenzene except for the 4-nitro derivatives. But when meta-substitution was introduced to the azobenzene, the C–H activation occurred at the less hindered arene, in which the selectivity was determined by steric effects. A broad range of aldehydes and azobenzenes could participate in the reaction to provide access to a variety of substituted indazoles.
Tian and co-workers demonstrated the palladium-catalyzed regioselective halogenation of aromatic azo compounds by the use of N-bromo-succinimide at room temperature (Scheme 211a).298 The substrate scope was extended to unsymmetrical aromatic azo compounds, in which electron-rich aryl groups prefer to be mono-brominated.
By the use of PhI(OAc)2 as oxidants, Sun and co-workers realized the palladium-catalyzed ortho-alkoxylation of aromatic azo compounds with both primary and secondary alcohols via Pd(II)/Pd(IV) catalytic cycle (Scheme 211b).299 The same group also reported the palladium-catalyzed direct ortho-nitration reaction of azoarenes in which NO2 was used as both a nitro source and an oxidant for the first time (Scheme 211c).300 When unsymmetrical azoarenes were employed, the reaction took place exclusively on the benzene ring with an electron-donating group in moderate to good yields. The nitration products could be converted into O-aminoazoarenes or benzotriazole derivatives by zinc.
Cheng and co-workers developed a rhodium-catalyzed synthesis of cinnolinium salts from azobenzenes and alkynes in good yields (Scheme 212).301a The successful isolation of a five-membered rhodacycle and the result of the subsequent stoichiometric reaction sufficiently supported the proposed C–H activation process. Interestingly, the isomeric ratio of the product using unsymmetrical alkynes appeared to be sensitive to the used metal oxidant, in which a combination of AgBF4 and Cu(OAc)2·H2O could greatly improve the regioselectivity. Almost at the same time, a rhodium(III)-catalyzed oxidative C–H activation/cyclization of azo compounds with alkynes for the synthesis of cinnoline and cinnolinium frameworks was demonstrated by You and co-workers.301b
The [3 + 3] annulations of aromatic azides with azobenzenes to give phenazines was reported by Ellman (Scheme 213a).302 The transformation proceeded through chelation-assisted ortho-amidation, the intramolecular electrophilic aromatic substitution, and aromatization processes. An additional acid was essential for the protonation of rhodacycle intermediate and the electrophilic substitution step. When unsymmetrical azobenzenes were used, regioselective C–N bond formation occurred in the electron-deficient arene. In addition to the azobenzenes, the annulation of aromatic azides with aromatic imines to afford acridines was also demonstrated. In 2014, Lee and other groups further studied the synthesis of benzotriazoles from azobenzenes with N-sulfonyl azides. The amidated azobenzenes could go through the subsequent oxidative cyclization in the presence of PhI(OAc)2 to provide the benzotriazoles in one pot (Scheme 213b).303c
3.5. The C–H functionalization directed by other nitrogen-containing unsaturated bonds
The coordination ability of the nitrogen atom in unsaturated bonds with transition metals was widely investigated in chelation-assisted C–H functionalizations. Much effort was made by Sun and co-workers, who studied the synthesis of biphenyl-2-carbonitrile derivatives via a palladium-catalyzed C–H arylation of arylnitriles with aryl halides using cyano as the directing group (Scheme 214).304 Due to the linear configuration of arylnitriles, it was proposed that the coordination of the π-electron between carbon and nitrogen to palladium was possible. An acidic medium was necessary to promote the transformation and TFA was used as the best solvent. When common functional groups were introduced to the substrates, the directing effect of the cyano group was not influenced. Subsequently, the palladium-catalyzed ortho-alkoxylation305 and halogenations306 of arylnitrile were developed by the same group.Chiba and co-workers reported the synthesis of highly substituted isoquinolines from α-aryl vinyl azides and internal alkynes under a rhodium/copper bimetallic catalytic system (Scheme 215).307 The addition of acetic acid (1 equiv.) proved to be optimal for the isoquinoline formation, which could reduce the reaction temperature. This method showed wide substrate tolerance for unsymmetrical alkyne, resulting in isoquinoline formation in a regioselective manner. The authors proposed that the reductive formation of imine derivatives from vinyl azides could serve as directing groups to generate a rhodacycle intermediate, which might be followed by a C–C and C–N bond formation sequence to form aza-heterocyclic frameworks.
Recently, Wang and co-workers developed a strategy to synthesis indoles through rhodium catalyzed triazene-directed C–H annulation using alkynes (Scheme 216a).308 They proposed that an active Rh(III) acetate species was generated from [Cp*RhCl2]2, AgSbF6, and copper acetate, which could coordinate to the middle nitrogen atom of the triazene moiety. The value of KIE (kH/kD = 2.7) indicated that the C–H activation was the rate-determining step. The N–N bond cleavage might be promoted by HOAc that was in situ generated in the reaction. Excellent regioselectivity was achieved by asymmetrically substituted alkyne. They studied the rhodium-catalyzed olefination of aromatic C–H bond using triazene as a directing group later (Scheme 216b).309 The triazene directing group could be conveniently removed or further transformed into other useful functional groups.
The coordination characteristics of the N-nitroso moiety as a directing group was investigated in the Rh(III)-catalyzed functionalization of arenes.310,311 Zhu and co-workers found that the unique coordination characteristics of the N-nitroso group could enable the use of the inactivated olefins as coupling reagents (Scheme 217a).310 During the mechanistic studies, a five-membered rhodacycle was prepared and characterized, in which the nitrogen atom of nitroso coordinated to the Rh centre. The intermolecular kinetic isotope effect experiment gave a KIE value of 4.5, indicating that the C–H activation might be involved in the turnover-limiting step. The synthetic versatility of this method was further demonstrated by the transformation of the N-nitroso moiety to amine with high efficiency under mild conditions.
Zhu group and Huang group independently investigated the Rh-catalyzed synthesis of indole from N-nitrosoanilines and alkynes (Scheme 217b).312 The N–N bond of N-nitrosoanilines served as an intramolecular oxidant, which was also observed when O-methyl oxime was used as the directing group.286 The electron-rich and electron-deficient substrates showed the equal reactivity in the competition experiment, which supported the concerted metalation–deprotonation (CMD) reaction pathway.
In 2012, Li and co-workers reported the Rh(III)-catalyzed oxidative coupling of azomethine ylides with olefins (Scheme 218a and b).313a Depending on the following cascade reactions, the cleavage of C–N or N–N bonds in the azomethine ylides of (hetero)arylaldehydes after the C–H activation was occurred. For example, azomethines of benzaldehydes went through selective C–H and C–N cleavage to produce 1,2-dihydrophthalazines. When different oxidants were used in the reaction, the pyridine or aldehyde derivatives could be smoothly afforded by C–H and N–N cleavage of azomethines of heteroaldehydes. Later, Li and co-workers developed a rhodium(III)-catalyzed oxidative annulation of azomethine ylides with alkynes via C–H activation for the synthesis of indenamines (Scheme 218c).313b The obtained indenamine product could readily be oxidized to an indenone and derivatives under aerobic conditions.
4. The C–H functionalization directed by amine
4.1. The C–H functionalization directed by N,N-dimethylamino group
Amines are common structural units in both naturally occurring molecules and pharmaceutical agents. The large applications of amines in synthetic, medicinal, materials, and coordination chemistry have fueled interest in the development of methods for their construction. However, the involvement of amines as a directing group in directed C–H functionalization is relatively less studied due to its property of strong Brønsted base.The first example of utilizing amine as the directing group was reported by Murai and co-workers in 2002 (Scheme 219).314 By the use of the N,N-dimethylaminomethyl group as the directing group, they achieved a Ru-catalyzed C–H silylation reaction of N,N-dimethyl-1-phenylmethanamine with triethylsilane via non-π-conjugated chelation-assistance. The norbornene functioned as a hydrogen scavenger and facilitated the reaction process. The result of reaction demonstrated that a catalytic reaction involving aromatic C–H bond cleavage could proceed smoothly, albeit in the non-π-conjugated chelation-assistance manner. It was believed that this protocol was the first example of transition-metal-catalyzed directed C–H functionalization using N,N-dimethylbenzylamine as the directing group.
Inspired by Murai's work, the Pd-catalyzed regioselective olefination of substituted N,N-dimethylbenzylamines via directed C–H functionalization was developed by Shi and co-workers (Scheme 220).315 The acidity of the reaction conditions had a huge influence on the efficiency of the reaction due to the strong Brønsted base property of the N,N-dimethylbenzylamines. After detailed screening, the combination of 2,2,2-trifluoroethanol (TFEtol) and AcOH was chosen as the best solvent system for the transformation. Mechanistic studies indicated that electrophilic attack on the phenyl ring by the Pd(II) ion assisted by the N,N-dimethylaminomethyl group to form intermediate A was a key step. The acetic acid played dual important role: tuning the concentration of N,N-dimethyl amines through acid–base balance and promoting the dissociation of the anionic ligand from the catalyst center to generate coordinately unsaturated and reactive catalyst species for C–H activation (Scheme 221). In addition, the N,N-dimethylaminomethyl group could be further transformed into different useful functional groups.
Subsequently, Shi and co-workers developed a LiCl-promoted Pd(II)-catalyzed highly regioselective ortho-carbonylation of N,N-dimethylbenzylamines via selective aryl C–H activation (Scheme 222).316 Compared to other Pd(II)-catalyzed transformations, the carbonylation still faced many challenges because the depalladation process was complicated by reduction of Pd(II) to Pd(0) under a CO atmosphere. Further hydrogenation of desired product could achieve ortho-methyl benzoate derivatives in one pot. In the reaction, LiCl was found to be the most effective additive and was regarded as Lewis acid to promote the insertion of CO into the C–Pd bond. The successful control of the reactivity and binding ability of N,N-dimethylbenzylamine through tuning the acidity of the reaction system was crucial.
An iridium-catalyzed C–H borylation reaction of benzylic amines using picolylamine as the effective ligand to provide ortho-substituted boronate esters was reported by Clark and co-workers (Scheme 223).317 The reaction generally featured good yields and high selectivities of the mono ortho-borylation products when a basic amine ligand was employed. Preliminary experiments demonstrated that the partial dissociation of one amine of the hemilabile diamine ligand was necessary for providing the coordination site for ortho C–H borylation.
Based on Shi's olefination of N,N-dimethylbenzylamines, Zhang and co-workers developed a Pd-catalyzed C–H ortho-arylation of various arenes directed by the N,N-dimethylaminomethyl group (Scheme 224).318 The protocol allowed the ortho-diarylation of p-substituted N,N-dimethylbenzylamine using various aryl iodide as coupling partner in the presence of AgOAc and Cu(OAc)2·H2O. The exclusive monoarylation of the meta-substituted arenes occurred at the less hindered ortho position of the dimethylaminomethyl groups owing to the steric effect. The addition of Cu(OAc)2·H2O was quite important to the high efficiency of this transformation.
A new Pd-catalyzed direct dehydrogenative annulations of N,N-dimethylaminomethyl ferrocene with internal alkynes to afford a wide range of ferrocene functionalized naphthalenes was developed by Wu, Cui and co-workers (Scheme 225).319 The relatively high reaction temperature was beneficial for the rapid conversion of the substrates and the air was necessary for this transformation. Mechanistic studies demonstrated that N,N-dimethylaminomethyl ferrocenium was generated in situ and served as a terminal oxidant. This procedure utilized the redox activity of ferrocene to make this approach “greener” and easier to handle. Ferrocene functionalized naphthalenes could also be widely applied in π-conjugated functional materials. Recently, the Pd(II)-catalyzed enantioselective C–H direct acylation of ferrocene derivatives utilizing diphenyl diketone as a carbonyl source was developed by Wu and co-workers.320 A variety of 2-acyl-1-dimethylaminomethyl ferrocenes with planar chirality were produced with high enantioselectivity. Additionally, an enantioselective palladium-catalyzed direct functionalization of aminomethylferrocene derivatives with boronic acids was realized by You and Gu.321 Commercially available amino acid Boc-L-Val-OH was found to be the most effective ligand in terms of enantioselectivity and reactivity. This transformation provided a general and practical route for the synthesis of enantiopure ferrocene compounds.
4.2. The C–H functionalization directed by secondary amine
The secondary amine also can be used as a directing group for ortho C–H activation. In 2004, Orito and co-workers described a Pd-catalyzed direct aromatic carbonylation under an atmosphere of CO gas (Scheme 226).322 A broad series of five- or six-membered benzolactams from secondary ω-phenylalkylamines was afforded in high yields. It was proposed that this carbonylation process started from well-known ortho-palladation and the subsequent insertion of a molecular CO into the resultant cyclopalladation product resulted in the formation of an acylpalladium complex. After a nucleophilic attack of an internal amino group at the carbonyl group, the desired benzolactam products were obtained.A Pd(II)-catalyzed C–H bond carbonylation and intramolecular cyclization of β-arylethylamines for the synthesis of a diversity of dihydro-2-quinolones was presented by Gaunt and co-workers (Scheme 227).323 The carbonylation reaction proceeded smoothly at room temperature and tolerated complex functionality and stereocentres. The use of AcOH as a solvent was supposed to suppress the CO mediated reduction of the Pd(II) to palladium black. This useful protocol provided more opportunities for synthetic application of β-arylethylamine motifs.
4.3. The C–H functionalization directed by free amine
Free amine could be also used as a directing group in catalytic C–H functionalizations. In 2006, Daugulis and co-workers reported a direct Pd-catalyzed ortho-arylation of benzylamines in the presence of AgOAc and CF3COOH (Scheme 228).324 The reaction proceeded well in trifluoroacetic acid and the amount of acid strongly influenced the reaction. In order to study the properties of directing groups, N-trifluoroacetylbenzylamine was treated with iodobenzene under the standard reaction conditions. The desired arylation product did not observed, indicating the free amine should be the directing group.Recently, a Pd(II)-catalyzed ortho-C–H trifluoromethylation of N-unsubstituted benzylamines utilizing an electrophilic CF3 reagent was reported by Yu and co-workers (Scheme 229).325 The presence of H2O and Ag2O proved to be crucial for the high yields of the reaction. The oxidative addition of the electrophilic trifluoromethylation reagent to the [Ar–Pd(II)] species led to the formation of an octahedral Pd(IV) intermediate and the reductive elimination afforded the final ortho-trifluoromethylation product. The reaction was expected to be applicable in medicinal chemistry to prepare diverse functionalized benzylamines.
Recently, Miura and co-workers demonstrated that the dehydrogenative ortho-alkenylation and cyclization of α,α-disubstituted benzylamines with acrylates could be performed efficiently at room temperature by rhodium or ruthenium catalysis (Scheme 230).326 Notably, the styrenes could participate in this transformation to give the corresponding stilbenes smoothly by rhodium catalysis.
An Ir-catalyzed cross-coupling of styrene derivatives with allylic carbonates via the free amine directed vinyl C(sp2)–H bond activation was originally developed by You and co-workers in 2009 (Scheme 231).327 The cross-coupling reaction proceeded well under the efficient catalytic system of [Ir(COD)Cl]2/Feringa's ligand. The unprecedented skipped Z, E diene products were obtained instead of the possible amination product. Moreover, the cis-Heck-type product was formed exclusively and the example offered a complementary method for the preparation of the useful diene compounds. Subsequently, the same group also reported an iridium-catalyzed allylic vinylation and asymmetric allylic amination reactions with o-amino styrene derivatives.328
Guanidines are important structural motifs and have found wide applications in a range of biologically important molecules. In 2012, Yu and co-workers reported a Pd-catalyzed C–H functionalization method for the arylation and olefination of arenes using guanidine as the directing group (Scheme 232).329 The combination of K2S2O8 and TFA was vital for the high efficiency of the arylation, and in contrast, when BQ was identified as an oxidant, the best results of olefination were given. It was proposed that the arylation reaction presumably underwent a PdII/PdIV pathway and the olefination seemed more likely to follow a Pd0/PdII catalytic cycle.
A Rh(III)-catalyzed oxidative coupling of N-aryl and N-alkyl benzamidines with alkynes for the synthesis of N-substituted 1-aminoisoquinolines with high efficiency was developed by Li and co-workers (Scheme 233).330 A broad range of functionalized isoquinolines was afforded in moderate to good yields. It was noteworthy that the steric bulk of the C–aryl ring had a vital influence on the reaction selectivity and efficiency.
An efficient Pd(II)-catalyzed cycloamidination with isocyanide insertion for the synthesis of 6-aminoindolo[3,2-c]quinoline and 4-aminopyrrolo[1,2-a]quinoxaline derivatives was described by Zhu and co-workers (Scheme 234).331 The reaction featured broad substrate scope and neutral as well as mild reaction conditions without the racemization of the chiral substrates. Diverse nitrogen heterocycles containing a cyclic amidine subunit could be afforded by this practical method.
A new method for the palladium-catalyzed free-amine-directed alkenylation of C(sp2)–H bonds and cyclization to give the corresponding functionalized phenanthridines was described by Zhang (Scheme 235).332 The reaction was highly regioselective and showed good functional-group tolerance. Various alkenes, including electron-deficient alkenes, styrenes and alkyl alkenes, could participate in this transformation. Moreover, the involvement of α-branched styrenes led to the formation of tricyclic compounds with a seven-membered amine ring. The key six-membered palladacycle was successfully synthesized and shed light on the reaction mechanism. The kinetic isotope experiment indicated that the C–H cleavage might be involved in the catalytic alkenylation and proceed through an electrophilic palladation pathway.
Zhang's group also developed an efficient protocol for Pd-catalyzed free-amine directed arylation of the C(sp2)–H bond by the use of aryl iodide as an arylating agent in the presence of trifluoroacetic acid (Scheme 236).333 In this reaction, silver salt might act as a halide scavenger to improve the reaction. The reaction exhibited excellent reactivity and regioselectivity, and a wide range of mono- or diarylated products were prepared after the modification of reaction conditions.
They developed a palladium-catalyzed free-amine-directed Suzuki–Miyaura-type coupling reaction of biaryl-2-amines with aryl boronic acids in aqueous medium later (Scheme 237).334 The employment of soluble silver nitrate was a spotlight in the transformation. Silver nitrate was supposed to influence the reaction in two manners: acting as an oxidant to oxidize Pd0 to PdII and serving as a promoter to facilitate the cyclopalladation process. In the presence of silver salts and additional water, high reactivity and chemoselectivity for the formation of carbon–carbon bonds were achieved.
At the same time, the ruthenium-catalyzed alkenylation reaction of 2-aminobiphenyls and cumylamine with alkynes directed by the free amine group was demonstrated by Murai and co-workers, producing the corresponding regioselectively alkenylated products with high efficiency (Scheme 238).335 The addition of AcOH was crucial for the reaction, which was supposed to facilitate the C–H bond activation step. Because of their biological activities, the obtained ortho-alkenylated α,α-dialkylbenzylamine derivatives including cumylamines had potential applications in diverse functional molecules.
A Rh-catalyzed amino-directed C–H bond borylation and Cu(II)-mediated ring closure of the resulting borylated anilines was developed by Yan and Chen to achieve N–H carbazole products (Scheme 239).336 This reaction adopted a free-amino group as the directing group to form a C–B bond, which undergoes the copper-mediated formation of an intramolecular C–N bond. It was the first dehydrogenative cyclization of non-protected 2-aminobiaryl derivatives for the synthesis of medically important non-protected carbazoles in one-pot with good to excellent yields.
A Pd-catalyzed C(sp2)–H aminocarbonylation reaction of unprotected o-arylanilines under an atmospheric pressure of CO using the amine group as a directing group to prepare free (NH)-phenanthridinones was reported by Zhu and co-workers (Scheme 240).337 The incompatibility of CO with the oxidative reaction conditions and the formation of urea byproduct were main obstacles in the C–H aminocarbonylation reaction in the presence of a free amine group. The addition of 1 equivalent of TFA to increase the electrophilicity of the palladium(II) species might inhibit the side-reaction effectively. A wide variety of free (NH)-phenanthridinone derivatives were successfully synthesized by this strategy and the scope of the reaction could also extend to some ortho-heteroarene substituted anilines to give polyheterocycles containing a free (NH)-lactam moiety. Meanwhile, Zhang and co-workers reported a similar Pd-catalyzed aminocarbonylation of o-arylanilines under a carbon monoxide atmosphere to synthesize various phenanthridinones.338
5. The C–H functionalization directed by polar N+–O− bonds
Pyridines are important heterocycles and widely present in natural products, pharmaceuticals, and functional materials. However, the ortho-functionalization of pyridines through electrophilic aromatic substitution is quite difficult due to its electron-poor nature. In recent years, the pyridine N-oxides have served as an attractive platform for the regioselective functionalization of pyridines via transition metal-catalyzed C–H bond cleavage.339In 2005, Fagnou and co-workers disclosed the palladium-catalyzed regioselective arylation of pyridine N-oxides, in which the arylation occurred in excellent yields with complete selectivity at the 2-position with a wide range of aryl bromides (Scheme 241a).340a The presence of both electron-donating and withdrawing groups in the pyridine N-oxide made little difference on the efficiency of the transformation. The detected value of intermolecular primary KIE was 4.7, which indicated that the C–H bond cleavage might be involved in the rate-limiting step. Further investigation of the mechanism by Fagnou and others revealed that the reaction may undergo a concerted metallation–deprotonation pathway.340b,c This palladium-catalyzed direct arylation could also be performed with a broad range of azine and azole N-oxides. The arylation of benzylic C(sp3)–H bonds of pyridine N-oxides was demonstrated by the same group (Scheme 241b).340d In this case, the arylation took place at the 2-methyl position of pyridine N-oxide rather than the ortho C(sp2)–H bond, which might be attributed to the easier deprotonation of more acidic benzylic C(sp3)–H bonds under the strong NaOt-Bu. The sequential sp2/sp3 arylation of pyridine N-oxides under controlled reaction conditions has been achieved by the authors, which was important for the derivation of heterocyclic compounds.
The copper-catalyzed arylation of pyridine N-oxide using aryl iodide as a coupling reagent was reported by Daugulis and co-workers in 2008 (Scheme 242).341a The acidic pyridine N-oxides could be arylated at the 2-position with high selectivity using K3PO4 base. If the substrates were less acidic (pKa 27–35), a stronger lithium alkoxide base was required. A phenanthroline ligand was necessary for this efficient transformation, which could stabilize the copper catalyst and facilitate the halide displacement step. The extension of coupling reagents from aryl iodides to aryl bromides in this arylation was realized by You and co-workers,341b in which pyridine, quinoline, and 2-p-tolylpyridine N-oxides could smoothly undergo the reaction to afford the desired products in moderate yields.
The application of inactivated arenes in the Pd-catalyzed C–H/C–H cross-coupling of pyridine N-oxides was reported by Chang and co-workers in 2008 (Scheme 243a).342a In this work, a range of simple arenes were suitable coupling reagents to give the corresponding monoarylated products with N-oxides of pyridine, pyrazine, quinoxaline, quinoline and benzo[h]quinolone at the ortho-position in good yields.
The thiophenes or furans were used as coupling reagents in the Pd(II)-catalyzed arylation of pyridine N-oxides by You's group (Scheme 243b).342b Stoichiometric Cu(II) salts were essential for the transformation, and a catalytic amount of CuBr was used as an activator to enhance the reactivity. Other N-oxides, such as pyrazine, quinoline and isoquinoline, were applicable to this transformation. Later on, the Pd(II)-catalyzed regioselective C3 heteroarylation of indoles and pyrroles with pyridine N-oxides was demonstrated by Li and co-workers (Scheme 243c).342c
The first Ni(0)-catalyzed alkenylation of pyridine N-oxides by the regio- and stereoselective insertion of alkynes was reported by Nakao's and Hiyama's group in 2007 (Scheme 244a).343a The resulting alkenylated pyridine-N-oxides could be readily deoxygenated with PCl3 to provide 2-alkenylpyridines in excellent yields. Unsymmetric alkynes were excellent substrates to afford (E)-2-alkenylpyridine-N-oxides, in which the steric repulsion between the bulkier R3 and the pyridyl group might be responsible for the observed good regioselectivities. Primary mechanism investigation revealed that the N-oxide moiety played an important role in directing the metal catalyst to the proximal C2–H bond, and also improved the corresponding C–H bond acidity to undergo the oxidative addition to nickel(0).
In 2008, Chang described the Pd(II)-catalyzed selective alkenylation of pyridine N-oxides using olefins (Scheme 244b).342a The alkenylation reaction showed broad substrate scope: both electron-deficient olefins (conjugated with ester, amide, ketone or diethyl vinylphosphonate groups) and aliphatic olefins smoothly went through the alkenylation at the 2-position of pyridine N-oxides. The dialkenylation of the pyridine N-oxides was not observed, showing excellent chemoselectivity. A palladium complex was isolated by the treatment of pyridine N-oxide with PdCl2(PPh3)2, which demonstrated the interaction between the oxygen atom of the pyridine N-oxide and the palladium catalyst. However, the reaction of this palladium complex with olefins failed to provide the alkenylated products under the reaction conditions, suggesting that the N-oxide-bound Pd complex was probably a resting species outside the catalytic cycle.
An external-oxidant free condition was developed by Wu and co-workers for the Pd(II)-catalyzed alkenylation of heteroarene N-oxides, in which the 2-alkenylated quinolones or 1-alkenylated isoquinolines were produced in chemo- and regioselective manners (Scheme 244c).343b The authors proposed that the initial alkenylated quinoline-N-oxides could act as the oxidant to convert Pd(0) into Pd(II) and complete the catalytic cycle.
The Pd(II)-catalyzed C-2 alkylation of quinolone N-oxides with ethers was demonstrated by Cui, Wu and co-workers in 2013 (Scheme 245).344 A series of quinoline-containing heterocyclic compounds was obtained in moderate to excellent yields using Pd(OAc)2 as a catalyst and TBHP (tert-butyl hydroperoxide) as an oxidant. TBAB (tetrabutylammonium bromide) and H2O were used as additives in order to obtain the best yield. In the mechanism investigation, the authors proposed that a key Pd(III) intermediate might be involved by the reaction of Pd(II) species with an ether radical, which was generated in situ by the hydrogen N-atom abstraction by TBHP.
The use of nonactivated secondary and tertiary alkyl bromides as coupling reagents in the Pd(II)-catalyzed ortho-alkylation of pyridine N-oxides was reported by Fu and co-workers (Scheme 246).345 The author proposed that the radical-type C–Br cleavage was engaged in the Pd-catalyzed C–H functionalization reaction, which was consistent with the fact that the primary bromides were less reactive during the transformation. The reaction showed good compatibility with many synthetically relevant functional groups, presenting a good method for the preparation of alkylpyridine derivatives.
More recently, Larionov reported the Cu(II)-catalyzed coupling reaction of heterocyclic N-oxides with Grignard reagents, in which the N–O bond was reduced spontaneously to provide the 2-aryl-, alkyl-, and alkenyl-substituted N-heterocycles in good to excellent yields (Scheme 247).346 Both primary and secondary alkylmagnesium halides proved to be suitable nucleophiles in the reaction with substituted quinoline, pyridine, and isoquinoline N-oxides. The chemoselective preference for the C2-addition in 4-chloroquinoline N-oxides has been exploited for a regioselective synthesis of new structural analogues of amodiaquine and chloroquine.
The 2-acetoxylation of quinoline N-oxides catalyzed by a cheap copper(I) salt via C–H activation was described by Cui, Wu and co-workers (Scheme 248).347 The reaction proceeded smoothly under a nitrogen atmosphere, in which TBHP served as a terminal oxygen source. The authors proposed that quinoline N-oxide radical formed by TBHP was an important intermediate in the catalytic cycle. However, electron-deficient aldehydes and heterocyclic aldehydes failed to give the desired products.
The challenging C–N bond construction of quinoline N-oxides with lactams/cyclamines in the presence of the Cu(OAc)2 catalyst was reported by Li and co-workers in 2013 (Scheme 249a).348a In their reaction, lactams and cyclamines were excellent coupling substrates to provide 2-aminoquinoline derivatives in good yields. The detected intermolecular deuterium kinetic isotope effect value of the amidation was 1.3, suggesting that the C–H bond cleavage in quinoline N-oxide might not be involved in the rate-limiting step. Later on, Wu, Cui and co-workers developed a simple and efficient method for the direct amination of quinoline N-oxides with secondary aliphatic amines via a copper-catalyzed dehydrogenative coupling reaction (Scheme 249b).348b O2 served as the only oxidant in the catalytic system to give the 2-aminoquinoline derivatives in good yields.
The copper-catalyzed sulfonylation of quinoline N-oxides via C–H bond activation was reported by Cui, Wu and co-workers (Scheme 250).349 Commercially available aryl sulfonyl chlorides were used as the sulfonylation reagents to provide various 2-aryl sulfonylquinolines in chemo- and regioselective manners. Generally, the reaction efficiency was slightly sensitive to the electronic properties of the sulfonyl chlorides, in which the electron-rich substrates gave higher yields. The improved acidity of quinolone N-oxides was supposed to promote the deprotonation–metallation of the C–H bond at the 2-carbon under basic conditions. Other heteroarene N-oxides, such as pyrazine, pyrimidine, 1-methyl-imidazole, pyridine, and their derivatives, failed to provide the corresponding heterocyclic aromatic sulfone under the reaction conditions.
In the above studies, the transition metal-catalyzed ortho functionalization of pyridine N-oxides was generally supposed to undergo the electrophilic aromatic substitution pathway. Shibata and co-workers demonstrated for the first time that an N-oxide could be used as a directing group to promote the rhodium-catalyzed alkenylation at the C-8 position of quinoline N-oxides (Scheme 251a).350a The reaction took place at high temperature, leading to an E/Z mixture of isomeric products. Based on the preliminary mechanistic study, the authors proposed that C–H bond cleavage occurred at both the C-2 and C-8 positions of the substrate to give two intermediates. But the stable 5-membered metallacycle intermediate preferred to undergo the alkyne insertion, followed by the reductive elimination to afford the alkenylated products.
Li and co-workers developed a rhodium(III)-catalyzed redox-neutral coupling of quinoline N-oxide with internal alkynes, leading to the synthesis of substituted acetophenones (Scheme 251b).350b The reaction featured a good functional group tolerance, in which both electron-donating and electron-withdrawing groups in quinoline N-oxides were well tolerated to provide the products in moderate to good yields. When the reaction was conducted in the presence of H2O18, no 18O incorporation was observed, implicating an intramolecular O-atom transfer pathway. The authors proposed a RhIII/RhI/RhIII mechanism, in which the N–O bond in quinoline N-oxides could readily undergo O-atom transfer to alkynes to afford an α-oxocarbenoid intermediate.
Very recently, Larionov and co-workers reported the Pd(II)-catalyzed C8–H arylation of quinoline N-oxides with iodoarenes (Scheme 252).351 The method tolerated a number of functional groups in quinolines and iodoarenes. Detailed experiments were carried out to study the reactivity and selectivity of Pd-catalyzed arylation under ligand-free conditions. The observed KIE (kH/kD = 2.0) indicated that C8–H bond cleavage may be involved in the turnover-determining step. The Hammett analysis suggested that the reaction was accelerated by the electron-withdrawing substituents in the iodoarene and electron-donating substituents in the 5- and 6-positions of the quinoline system. The computational studies using DFT demonstrated that the C8 cyclopalladation was the lower energy pathway under phosphine-free conditions and explained the reversal of site selectivity to C2 in the presence of a phosphine ligand.
By the use of diazocarbonyl compounds, Chang's group demonstrated the synthetic utility of carbenoid chemistry in the Rh(III)-catalyzed direct C–H alkylation reactions at the C-8 position of quinoline N-oxides (Scheme 253a).352 The reaction proceeded efficiently at room temperature with excellent regioselectivity. The common functional groups, such as carbamate, silyloxy, acetoxy, acetal, aldehyde, ketone, and ester, were compatible with the reaction conditions. The authors also realized the introduction of the alkynyl moiety at the C-8 position of quinoline N-oxides (Scheme 253b). The pregenerated cationic species [RhCp*(MeCN)3][SbF6]2 presented high efficiency in the reaction of quinoline N-oxides and 1-[(triisopropylsilyl)ethynyl]-1,2-benziodoxol-3(1H)-one (TIPS-EBX) to provide the alkylated products in moderate to good yields. The resulting alkylated and alkynylated quinoline N-oxides could easily be deoxygenated with the use of zinc reagent under mild conditions.
The Rh(III)-catalyzed regioselective C–H bond iodination of quinoline N-oxides at the 8-position using N-iodosuccinimide (NIS) was reported by Chang and co-workers (Scheme 254a).353 The reaction took place at moderate temperatures in the presence of an in situ generated cationic Cp*Rh(III) catalyst. The authors also achieved the direct C-8 amidation of quinolone N-oxide using iridium catalysts (Scheme 254b). An iridacycle complex was isolated from the reaction of quinolone N-oxide and [Cp*IrCl2]2, revealing that the unique regioselectivity was attributed to the formation of N-oxide-chelated iridacycle. Catalytic acetic acid was essential, which was believed to influence the bond strength between the metal and inserted amido moieties and facilitate the proto-demetalation process.
The Rh-catalyzed ortho-olefination of tertiary aniline N-oxides was reported by You and co-workers. Electron-deficient olefins, such as acrylates, acrylamide, acrylonitrile and vinyl phosphonate, could undergo the reaction conditions to prepare 2-alkenylated tertiary anilines in good yields (Scheme 255).354 The five-membered cyclometalated Rh(III) complex from the starting material was isolated and its structure was established by X-ray crystallographic analysis, suggesting the directed C–H metalation in the catalytic cycle. The N–O bond was identified as an internal oxidant to achieve the ortho C–H functionalization of tertiary anilines.
6. The C–H functionalization directed by carboxylic acid
Carboxylic acids widely exist in great structural diversity both from natural and synthetic sources. The weak coordination ability of carboxylic acid to transition metal catalysts renders it an attractive and valuable directing group in C–H bond functionalization reactions. Moreover, carboxylic acid is highly convenient to remove or transform to other functional groups through demonstrated reactions.6.1. Alkenylation of C–H bond and subsequent annulation reaction
In 1998, the Miura group reported the pioneering work of palladium-catalyzed carboxylate-directed C–H olefination. Benzoic acids and naphthoic acids could react with alkenes to give either phthalide or isocoumarin derivatives in moderate to good yields (Scheme 256).355 The regioselectivity of the reaction was presumably determined in the alkene addition step, in which the nucleophilic cyclization or Wacker-type oxidative cyclization was involved (Scheme 257). When acrylates were used as the coupling reagents, phthalides could be obtained as single products. Further studies demonstrated that the selectivities of the products between phthalides and isocoumarins could be controlled by the introduction of ortho-substituent of benzoic acids, probably due to the steric effect between the neighboring ortho-substituent and carboxylate group, which prevented the O atom from attacking the distant C2 of the vinyl group.356The Ru-catalyzed carboxylate-directed C–H alkenylation was developed by Ackermann and co-workers (Scheme 258).357 Interestingly, the phthalide was generated as a single product from the corresponding benzoic acid and acrylate or acrylonitrile with 2 mol% [RuCl2(p-cymene)]2 as the catalyst. It is important that water is used as an environmentally benign reaction medium. The kinetic isotope effect (kH/kD = 3.6) indicated that the oxidative alkenylation proceeded through an irreversible C–H bond metalation. Recently, the oxidative C–H alkenylation of aromatic sulfonic acids was achieved with an in situ generated Ru(II) catalyst by the same group.358
Miura and co-workers demonstrated the Rh-catalyzed carboxylate-directed ortho-olefination of benzoic acids with alkenes using silver or copper salts as oxidants (Scheme 259).359 The reaction exhibited broad substrate scope, such as naphthoic acids, α,β-unsaturated carboxylic acids, and heteroarene carboxylic acids. Notably, the directing group could be readily removed during the olefination reaction or post-treatment with AgOAc/K2CO3. Recently, the Rh-catalyzed ortho-C–H olefination of arenes directed by the acid group was further developed by Liu's group and Miura's group.360,361 A wide range of acid substrates including substituted thiophene or furan-2-carboxylic acids, 2-substituted benzoic acids, 1-naphthoic acids and aromatic sulfonic acids could successfully undergo alkenylation with the carboxyl groups intact.
The Pd-catalyzed olefination of the heteroarene carboxylic acid, such as indole-3-carboxylic acids, with alkenes to give the corresponding 3-vinylated indoles was also reported by Miura and co-workers (Scheme 260).362 It was proposed that the ortho-olefination and the subsequent decarboxylation process were involved in the mechanism. Despite the highly selectivity with respect to C3-vinylation of N-methyl-pyrrole-2- and benzofuran-2-carboxylic acids, the selectivity for the vinylation of thiophene and benzothiophene carboxylic acids in the reaction was still problematic, in which the mixtures of 2- and 3-vinylated products were obtained. Furthermore, they demonstrated the Rh(III)-catalyzed synthesis of meta-substituted stilbene derivatives via the ortho-olefination/decarboxylation of benzoic acids.363
Miura and Satoh demonstrated the Ru-catalyzed vinylation of heteroaromatic carboxylic acids to give the 3-vinylated products. Since the decarboxylation process was sluggish under Ru catalysis, the carboxyl function remained in the obtained vinylated products, which could undergo further catalytic transformations (Scheme 261).364 A wide variety of heteroaromatic carboxylic acids were well compatible with the reaction conditions.
Using the readily synthesized catalyst [Cp*IrCl2]2, Ison and co-workers developed the carboxylate-directed reaction of C–H bonds with benzoquinone, affording the benzochromenones as the final products.365 The reaction presented a rare example of C–H functionalization with an electrophilic Ir(III) catalyst, in which the benzoquinone served not only as the coupling reagent but also as an intramolecular oxidant to regenerate the Ir(III) catalyst.
Besides benzoic acid or heteroarene carboxylic acids, the more synthetically useful phenyl acetic acids and 3-phenylpropionic acids could also be used as efficient substrates in the Pd-catalyzed C–H functionalization reactions. In 2010, Yu's group developed an operationally simple, atom-economical, carboxylate-directed Pd-catalyzed C–H olefination of phenylacetic acids using O2 as a terminal oxidant (Scheme 262).366 BQ was chosen as a useful ligand to prevent minor formation of the meta- and di-ortho-olefinated products. A variety of phenylacetic acids were involved in this catalyst system to give the desired olefinated products in good to excellent yields. As for the substrates with chiral centers at the α position, the addition of Li2CO3 as the base could prevent racemization and give a product with high ee value.
In the same work, Yu's group also reported the pioneering ligand-enabled regioselective C–H olefination of phenylacetic acids (Scheme 263).366 The positional selectivities of multiple substituted aromatic rings with two approximately electronically equivalent ortho-C–H bonds could be achieved by the addition of specific mono-N-protected amino acids. In addition, the olefination of 3-phenylpropionic acids could also be achieved by using this transformation via remote coordination.
The discovery that amino acid ligand could promote C–H activation provided new opportunities for developing enantioselective C–H activation. In 2010, Yu's group developed a carboxylate-directed enantioselective C–H olefination reaction of α,α-diphenylacetic acids using mono-protected amino acids as effective ligands (Scheme 264).367 Substrates with alkyl, electron-donating, or moderately electron-deficient substituents on the benzene rings coupled with olefin partners such as styrenes or acrylates efficiently, affording the ortho-vinylated products in good to excellent yields and enantioselectivities. This new development represented an encouraging step toward the realization of synthetically useful Pd-catalyzed enantioselective C–H activation reactions. Unfortunately, the selectivities were poor for the substrates containing α-hydrogen, which tended to racemize under the reaction conditions.
Yu's group has also demonstrated that ligands that can cooperate with the weak coordination of the distal carbonyl group to promote C–H activation (Scheme 265).368 Therefore, phenol derivatives were olefinated with acrylates in the presence of Pd(OAc)2 and KHCO3 under atmospheric oxygen, using Boc-Val-OH as the ligand. These C–H activation reactions apply the distal chelating atoms (six bonds away), which still remain challenging due to the difficulty of forming larger membered palladacycles.
6.2. Arylation of C–H bonds
The first Pd(II)-catalyzed carboxylate-directed arylation of benzoic acids using phenyl boronates as aryl donors was demonstrated by Yu and co-workers (Scheme 266).369 The generality of this transformation was further demonstrated by β-arylation of aliphatic acids, in which Pd(II)/Pd(IV) catalysis was likely to be involved. Stoichiometric Ag2CO3 was used as the oxidant and the use of BQ was crucial for the success of this transformation. The reaction presumably proceeds via the in situ generation of the corresponding carboxylate, which would bind the Palladium center to undergo the C–H activation/transmetallation/reductive elimination sequence, giving rise to the β-arylated products.Subsequently, Yu's group reported Pd-catalyzed C–H arylation of benzoic and phenyl acetic acids using aryltrifluoroborates as coupling reagents (Scheme 267).370 This new protocol substantially expanded the scope of the reaction, in which the ortho C–H arylation of phenylacetic acids and electron-deficient arenes containing α-H was achieved for the first time. However, the drawbacks of the use of high-pressure O2 or air, long reaction times, and limited substrate scope limited the application of this protocol. To overcome these limitations, a ligand-acceleration strategy has been developed by the authors. With the use of Ac-Ile-OH as the ligand and Ag2CO3 as the oxidant, the coupling of phenylacetic acids with aryltrifluoroborates or pinacol esters of arylboronic acids gave arylation products in nearly quantitative yields (Scheme 268).371 Mechanistic studies demonstrated that the initial rate of electron-poor phenylacetic acids was improved by the amino acid ligands and the catalytic cycle was proposed to proceed via a concerted metallation/deprotonation pathway.
In 2007, Daugulis’ group developed the direct ortho-arylation of benzoic acids with benzene halides (Scheme 269).372 When iodobenzenes were applied as coupling partners, acetic acid was used as the solvent to impede the competitive decarboxylation process, and stoichiometric AgOAc was applied for the removal of iodide. The chloride and bromide substitutions of benzoic acids were tolerated and the reaction was most likely to go through a Pd(II)/Pd(IV) pathway. A modified reaction condition allowed the ortho-arylation of benzoic acids by the use of chlorobenzene as the coupling partner.
In 2011, Larrosa's group reported the first example of meta-C–H arylation and decarboxylation using iodo-arenes as coupling partners in the presence of Pd(OAc)2 and Ag2CO3 (Scheme 270a).373a In contrast to the previously reported arylation, the arylation products underwent further decarboxylation at higher reaction temperature to provide meta-substituted biaryl compounds, which would be difficult to obtain by traditional Friedel–Crafts methods. They found that the use of K2CO3 could remarkably suppress the Pd-mediated decarboxylation to give the biaryl carboxylic acids (Scheme 270b).373b
6.3. Alkylation of C–H bonds
The Pd-catalysed ortho-methylation of benzoic acids using methylboronate as the coupling partner in the presence of benzoquinone and Ag2CO3 was reported by Yu's group (Scheme 271a).369 However, the ortho-alkylation of arylcarboxylic acid failed when other alkylboron reagents were used as the coupling partner due to the potential β-hydride elimination. This challenge was successfully overcome by Yu and co-workers by using the ligand strategy in 2013. Thus, the Pd-catalyzed ligand-accelerated C–H alkylation of phenylacetic and benzoic acids using alkyl boron reagents was achieved smoothly in the presence of mono-N-protected amino acid (MPAA) as the ligand. The accelerated C–H cleavage reactivity was attributed to the additional role of mono-N-protected amino acid (MPAA) in the catalysis. Potassium alkyl trifluoroborates and alkyl boronic acids were the compatible coupling partners (Scheme 271b).374 A variety of primary alkyl boron coupling partners, including fragments possessing trifluoromethyl, phenyl, Boc-amine, ester, or ketone functional groups, were compatible. Despite these advances, alkyltrifluoroborates containing α-heteroatoms, olefins, or alkynes did not give the desired coupling products.The Pd-catalyzed sequential alkylation/lactonization reaction of benzoic acids with alkyl halides to afford lactones was described in 2009 by Yu and co-workers (Scheme 272).375 This transformation involved the Pd-catalyzed carboxylate-directed ortho-C–H alkylation and subsequent SN2 substitution. The use of alkyl halides, such as 1,2-dichloroethane, dichloromethane, and dibromomethanes, led the catalytic cycle to be closed in the presence of an inexpensive base instead of Ag+ salts.
6.4. Acetylation and carboxylation of C–H bonds
The first example of Rh-catalyzed ortho-acylation of aromatic carboxylic acids was reported by Gooßen and co-workers (Scheme 273a).376 The transformations would be of considerable interest due to the ortho selectivity when compared with traditional Friedel–Crafts acylation. [Rh(cod)Cl]2 was found to be the best catalyst to promote the acylation of benzoic acids with anhydrides in the ortho position. At the same time, Ge's group showed the palladium-catalyzed chemoselective decarboxylative cross-coupling of benzoic acids with α-oxocarboxylic acids (Scheme 273b).377 The method provided efficient access to 2-acylbenzoic acid derivatives. Ag2CO3 was used to generate acyl silver species through the silver-mediated decarboxylation of α-oxocarboxylic acids and reoxidize Pd(0) into Pd(II). The synthesis of pitofenone has been achieved applying this transformation as the key step.The transformation was proposed to start with the palladation of silver benzoate A to produce the Pd(II) intermediate B. The subsequent transmetalation step with the acyl silver species C which was formed by the silver-mediated decarboxylation of F, generated the Pd(II) intermediate D. The reductive elimination of D provided the silver salt E and Pd(0), which could be reoxidized into Pd(II) by Ag2CO3. Protonation of intermediate E provided the desired product (Scheme 274).
Direct C–H carbonylation using CO has been extensively studied as an expedient route to access carboxylic acid derivatives. In 2008, Yu's group disclosed a new Pd(II)-catalyzed direct carboxylation reaction of aryl and vinyl carboxylic acids using a C–H activation/CO insertion sequence to form dicarboxylic acids (Scheme 275).378 Due to steric effects of substrates, excellent regioselectivities were observed in the carboxylation of meta-substituted carboxylic acids. The key cyclopalladated intermediate was characterized and studied.
6.5. Intramolecular oxidative cyclization
An early example involving Pt-catalyzed C–H hydroxylation of aliphatic acids was demonstrated by Sen and Kao in 1991 (Scheme 276).379 The β-C–H or γ-C–H of aliphatic acids could be hydroxylated via C–H bond cleavage by carboxylate-chelated Pt(II) species, followed by the cyclization to form lactones. However, the reactions gave only moderate yields and the substrate scope was limited. In 2001, Sames and co-workers developed the catalytic process for the Pt-catalyzed functionalization of α-amino acids in water.380 The work presented a rare example of catalytic heteroatom-directed functionalization of remote alkyl groups in complex substrates. The success was attributed to the chelation of Pt with both the carboxylate and amino groups. In addition, the Pt-catalyzed benzylic C(sp3)–H acetoxylation of ortho-alkyl-substituted aromatic carboxylic acids to obtain aryl lactone products was reported by Chang's group, in which the C–H activation could be assisted by the chelation of the platinum catalyst to the carboxylate group.381The Pd-catalyzed macrolactonization of ω-alkenoic acids to furnish 14- to 19-membered alkyl and arylmacrolides via allylic C–H oxidation was reported by White and co-workers (Scheme 277).382 The reaction exhibited remarkable selectivity and a wide range of functional groups were tolerated. Mechanistic studies indicated that the macrolactonization proceeded via a Pd-templated π-allyl carboxylate intermediate.
Later, a Pd-catalyzed lactonization via benzylic C(sp3)–H oxidation was developed by Martin group (Scheme 278).383 This Pd-catalyzed process could be drastically accelerated by the employment of an N-protected amino acid as ligand in the synthesis of highly functionalized benzolactones. Mechanistic studies revealed that the transformation proceeded via a Pd(0)/Pd(II) catalytic cycle and the reductive elimination of C(sp3)–O was the rate-limiting step.
Importantly, the Pd-catalyzed enantioselective allylic C–H functionalization was developed by Sasai's group by the use of chiral ligand spiro-bis(isoxazoline) (SPRIX), in which the 4-alkenoic acids underwent intramolecular oxidative cyclization to give γ-lactone derivatives with moderate to good enantioselectivities (Scheme 279).384 It was proposed that a π-allyl Pd intermediate was involved in the catalytic process and the carboxylate group played a crucial role in the enantioselective C–H acetoxylation reaction.
The carboxylic group could be used as both a directing and a reacting group in the Pd-catalyzed intramolecular C(sp2)–H activation/C–O bond formation. In 2013, Wang and co-workers disclosed a novel synthetic method for benzofuranones via carboxylate-directed C–H activation/C–O cyclization with the use of Pd(OAc)2 as the catalyst and PhI(OAc)2 as the oxidant (Scheme 280a).385 It should be noted that the previous acetoxylation of the C(sp2)–H bond was not observed under the optimized reaction conditions. Moreover, the use of MPAA ligands such as Boc-Ile-OH allowed for the synthesis of benzofuran-2-ones with excellent enantioselectivities, thus providing a complementary strategy to current methods (Scheme 280b).385 This reaction was the first example of Pd-catalyzed enantioselective C–H functionalization through a Pd(II)/Pd(IV) redox catalytic cycle. Simultaneously, Shi's group reported the Pd-catalyzed direct lactonization of 2-arylacetic acids through the same reaction sequence, in which amino acids were not needed.386 Subsequently, Wang and co-workers further applied the protocol in the synthesis of biaryl lactone from 2-aryl carboxylic acids.387 The synthetic utility of the new transformation was demonstrated in a concise total synthesis of the natural product cannabinol from commercially available starting materials.
Recently, copper catalysts were studied in the lactonization of 2-arylbenzoic acids, as exemplified by Martin's group, who developed a novel C(sp2)–H hydroxylation assisted by benzoic acids in the presence of Cu(OAc)2 catalyst and BPO oxidant under mild reaction conditions (Scheme 281).388 Mechanistically, a single electron transfer process was likely involved in this transformation, which could be inhibited by radical scavengers such as TEMPO, BHT, and 1,1-diphenylethylene.
6.6. Intermolecular oxidative cyclization
Carboxylate groups can also act as directing groups in the oxidative coupling of benzoic acids and alkynes/alkenes using a Rh–Cu co-catalytic system. In 2007, Miura's group developed a Rh–Cu system to synthesize isocoumarins from benzoic acids and internal alkynes (Scheme 282a).389,390 The reaction produced isocoumarins and proceeded regioselectively with respect to substituted benzoic acids and unsymmetrical internal alkynes. The resulting Rh(I)X species from the reductive elimination step could be oxidized in the presence of the copper cocatalyst under air to regenerate Rh(III)X3 to render the transformation catalytic (Scheme 283). When acrylates were employed in this transformation, the cyclization was supposed to occur after the first vinylation (Scheme 282b).389 The same group also demonstrated the rhodium-catalyzed coupling of acrylic acids with alkynes to provide the corresponding α-pyrone, which proceeded efficiently via the vinylic C–H bond cleavage.391 Recently, Li's group described the Rh-catalyzed synthesis of sulfones via the oxidative coupling of sulfonic acids with internal alkyne.392Ir-catalyzed oxidative coupling of benzoic acids with alkynes to produce naphthalene derivatives was reported by Miura and co-workers, in which 2 equiv. of the alkyne should be incorporated (Scheme 284).393 In contrast to the rhodium-catalyzed reactions (Scheme 263), the iridium-catalyzed reactions went through decarboxylation of the seven-membered iridacycle and subsequent insertion to the second alkyne to afford naphthalene.
Jeganmoha's group reported Ru-catalyzed oxidative cyclization of aryl carboxylic acids with alkynes (Scheme 285).394 By the use of the Ru/Ag catalyst system, the isocoumarin could be produced as the final product and no naphthalene derivatives were observed. Simultaneously, Ackermann's group reported a similar catalyst system to synthesize isocoumarins and α-pyrones through the coupling of carboxylic acids with alkynes in the presence of cationic Ru(II) catalyst.395,396 In this reaction, AgSbF6 was much less effective than KPF6 in promoting the transformation.
 | ||
| Scheme 285 Rh-catalyzed oxidative coupling of substituted arene or heteroarene carboxylic acids with alkynes. | ||
A Pd-catalyzed oxidative annulation of allenes with N-alkyl-indole-2-carboxylic acid derivatives via direct C–H functionalization was described by Swamy's group in 2012 (Scheme 286).397 The reaction underwent the initial palladation at the C3-position of indole to form the palladacycle intermediate, followed by the subsequent insertion of C–Pd bonds into allenes and reductive elimination to generate the lactones in moderate to good yields. This method was fairly general for a wide range of allenes, even terminal substituted allenes.
Besides carbon–carbon multiple bonds, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond can also be inserted into the immediate C–M species generated from transition metal-catalyzed C–H cleavage. In 2012, Li group described a novel Rh(III)-catalyzed synthesis of 3-substituted phthalides from benzoic acids and aldehydes (Scheme 287a).398 The reaction should proceed through carboxylate-directed ortho-C–H functionalization and subsequent intramolecular cyclization to generate 3-substituted phthalides in moderate to good yields. The initial coordination of Rh(III) species to the carboxylic oxygen atom was attributed to the use of Ag2CO3 as the base. In 2013, Gooßen and co-workers reported the [Rh(cod)Cl]2/CsF catalyst system to promote the straightforward synthesis of 3-alkylidenephthalides from benzoic acids and aliphatic acids or anhydrides in one-pot (Scheme 287b).399
O bond can also be inserted into the immediate C–M species generated from transition metal-catalyzed C–H cleavage. In 2012, Li group described a novel Rh(III)-catalyzed synthesis of 3-substituted phthalides from benzoic acids and aldehydes (Scheme 287a).398 The reaction should proceed through carboxylate-directed ortho-C–H functionalization and subsequent intramolecular cyclization to generate 3-substituted phthalides in moderate to good yields. The initial coordination of Rh(III) species to the carboxylic oxygen atom was attributed to the use of Ag2CO3 as the base. In 2013, Gooßen and co-workers reported the [Rh(cod)Cl]2/CsF catalyst system to promote the straightforward synthesis of 3-alkylidenephthalides from benzoic acids and aliphatic acids or anhydrides in one-pot (Scheme 287b).399
6.7. C–X bond formation
The C–O bonds are privileged in organic structures, including pharmaceuticals and agricultural chemicals, and C–O bond formation has been a research topic of great importance for C–H activation. In 2009, Yu's group discovered a versatile Pd-catalyzed ortho-hydroxylation of benzoic acids with O2 as the oxygen source (Scheme 288a).400 The addition of 1 equiv. of benzoquinone significantly increased the rate of hydroxylation and improved the yields of the products. The reaction exhibited many notable advantages, such as a wide substrate scope and a high atom efficiency. Direct oxygenation of the aryl–Pd species by molecular O2 was proposed, which was consistent with the labeling results using 18O2 and H218O. The method was important in the field of hydroxylation of inert C–H bonds using molecular oxygen.Gooßen's group further developed a Cu/Ag-catalyzed C–H alkoxylation of aromatic carboxylates, in which the carboxylate substituent was used as a cleavable directing group (Scheme 288b).401 Best yields could be achieved performing the reaction under an O2 atmosphere. Benzoates with electron-donating or electron-withdrawing groups could be smoothly converted, and a broad range of functional groups, including keto, cyano, nitro, sulfonyl, N-heterocyclic, and even bromo substituents, were tolerated. High selectivity was achieved, in which neither double alkoxylation nor non-decarboxylative alkoxylation was observed. The reaction represented a rare example of an aromatic functionalization, in which the original directing group was removed during the transformation.
Besides the C–C and C–O bonds, other C–heteroatom bonds could be formed via carboxylate-directed C–H activation. In 2012, Yu's group reported a novel method to synthesize anthranilic acid by intermolecular C–H amination of N-arylbenzamides with O-benzoyl hydroxylamines in the presence of Pd(OAc)2 and KOAc (Scheme 289a).402 The reaction presented excellent regioselectivity and functional group compatibilities. Mechanistic studies demonstrated that the amidation was probably initiated by rate-limiting cyclo-palladation (kH/kD = 2.6) of the benzoic acids. Recently, a Rh(III)-catalyzed direct ortho-C–H amidation/amination of benzoic acids with N-chlorocarbamates/N-chloromorpholines was also achieved to give anthranilic acids in up to 85% yields with excellent ortho-selectivity and functional group tolerance (Scheme 289b).403
In 2008, Yu and co-workers developed the Pd-catalyzed ortho halogenation of carboxylic acids (Scheme 290).404 The IOAc was generated in situ from PhI(OAc)2 and I2, which served as the key iodination reagent in the transformation. When tetrabutylammonium bromide was used, the corresponding brominated products could be afforded with improved monoselectivity. The authors proposed that the large tetraalkyl ammonium cation appeared to assist in the displacement of the monoiodinated (or monobrominated) product from the Pd(II) center to prevent the undesired dihalogenation. Subsequently, the Pd(II)-catalyzed ortho-C–H iodination reactions of phenylacetic acids were achieved by the same group.405 The synthetic utility of this iodination reaction was further demonstrated by carrying out the subsequent amidation, cyanation, acetylenation and homologation reactions.
7. The C–H functionalization directed by carbonyl group of ketone and aldehyde
Ketones are one of the most common and popular functionalities in organic chemistry. In 1993, Murai and co-workers studied the transition metal catalyzed C–H activation by using ketone as the directing group (Scheme 291).2b It was demonstrated that the ortho-alkylation of acetophenones with olefins could be achieved in the presence of low-valent ruthenium catalyst. The reaction exhibited good efficiency, selectivity and generality, which were of significance for the practical organic synthesis. A wide range of aromatic ketones could be involved in this reaction. One of the most important features of this reaction was the achievement of complete regioselectivity by employing an acyl group as the directing group. The coordination of the ketone carbonyl oxygen brought the low-valent ruthenium close to the ortho-C–H bond to drive the formation of a stable five-membered ruthenacycle. The subsequent insertion of an olefin into the Ru–H bond (or into the Ru–C bond) produced another cyclometallated intermediate, which underwent the reductive elimination to give the final alkylation products. However, the low-valent ruthenium catalysis was not easy to handle due to its air- and moisture-sensitive properties. Furthermore, the olefin scope was only limited to very electron-rich generally silylated olefins. Further research was carried out by Murai and Trost illustrating that this reaction could also be extended to olefinic C–H bonds.406Based on Murai's pioneering work, Weber and co-workers reported the ruthenium-catalyzed regioselective copolymerization of acetophenones with α,ω-dienes407a under similar conditions to Murai's work. The further mechanistic study by Weber indicated that RuH2CO(PPh3)2 could activate C–H bonds which were ortho to an aromatic carbonyl group, and also transfer its hydrogen atoms to one of the C–C double bonds of α,ω-dienes. The active ruthenium species was determined through extensive investigations.407b,c
Taking some disadvantages of low-valent ruthenium catalysts into consideration, Genet and Darses realized a series of ortho-alkylation reactions with olefins using a more stable and readily available [Ru(p-cymene)Cl2]2 catalyst in recent years.408a–e In the presence of sodium formate and triphenylphosphine, a highly active catalyst could be in situ generated from the [Ru(p-cymene)Cl2]2 catalyst, providing the desired coupling products in high yields. These catalytic systems showed high versatility by modifying the electronic and steric properties of the catalyst by adopting different ligands. These novel protocols were compatible with a wide range of substrates such as heteroaromatic ketones, conjugate enones, and aromatic imines. Subsequently, a simple catalytic system to achieve ortho-alkylation of ketones using the cheaper RhCl3·H2O as the catalyst was developed by the same group (Scheme 292).408f
The first rhodium-catalyzed ortho-alkylation of ketones with diverse olefins was demonstrated by Brookhart and co-workers in 1999.409 The rhodium bis-olefin complex [C5Me5Rh(C2H3SiMe3)2] was identified as an efficient catalyst for the selective addition of olefins to the ortho position of aromatic ketones. In contrast to the ruthenium-catalyzed ortho-alkylation reaction reported by Murai, the rhodium complex was not involved in the carbonyl coordination process for the ortho C–H activation, but activation of all sites (ortho, meta, and para) of the substrate was observed. The chelation of the carbonyl group to the metal center lowered the barrier for the reductive elimination to product.
Among previous studies reported, the anti-Markovnikov addition product was preferentially formed. Recently, Bower and co-workers developed an efficient system for branch-selective carbonyl-directed ortho-alkylation monosubstituted olefins using cationic Ir(I) catalyst (Scheme 293).410 Ligands had a significant impact on the reaction efficiency and branch selectivity. After detailed screening of the ligands, dFppb, a previously unreported ligand that was the pentafluorophenyl analogue of dppb, was the optimal ligand for providing the desired product in quantitative yield with complete branch selectivity. The catalytic system was compatible with a wide range of directing groups and afforded a series of branched hydroarylation products with high efficiency.
The ruthenium-catalyzed ortho-alkenylation of ketones with various internal acetylenes was described by Murai and co-workers in 1995 (Scheme 294).411 An E/Z mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 coupling products was afforded in good yields for symmetrically substituted dialkyl- and diarylacetylenes. The present protocol also provided a useful method for the preparation of diverse substituted vinylsilanes. Subsequently, this reaction was extended to vinylic C–H bonds of α,β-conjugate enones by Murai, giving a variety of conjugated dienones with the assistance of RuH2CO(PPh3)2 as the catalyst.412 Woodgate and co-workers demonstrated a similar alkenylation reaction of fused aromatic ketones. Cyclopenta[a]naphthalene derivatives could be yielded through a one-pot insertion–cyclisation sequence.413 Cationic iridium-BINAP complex-catalyzed addition ortho-C–H bonds in aryl ketones to alkynes was reported by Shibata and co-workers in 2008.414
1 coupling products was afforded in good yields for symmetrically substituted dialkyl- and diarylacetylenes. The present protocol also provided a useful method for the preparation of diverse substituted vinylsilanes. Subsequently, this reaction was extended to vinylic C–H bonds of α,β-conjugate enones by Murai, giving a variety of conjugated dienones with the assistance of RuH2CO(PPh3)2 as the catalyst.412 Woodgate and co-workers demonstrated a similar alkenylation reaction of fused aromatic ketones. Cyclopenta[a]naphthalene derivatives could be yielded through a one-pot insertion–cyclisation sequence.413 Cationic iridium-BINAP complex-catalyzed addition ortho-C–H bonds in aryl ketones to alkynes was reported by Shibata and co-workers in 2008.414
Tanaka415 and Shibata416 independently developed a cationic rhodium(I)-catalyzed cascade reaction of aromatic ketones with 1,6-diynes to give 1,3-dienes in high yields in 2007 (Scheme 295). Carbonyl-directed activation of aromatic and vinylic C–H bonds was regarded as the key step in the transformation. Shibata also pointed out that when ketone substrates were treated with 1,6-enynes, chiral products could be obtained with high ee value using a chiral Rh catalyst.
Substituted indenol derivatives are an important structural motif present in various biologically active compounds. Glorius's and Cheng's groups independently developed a rhodium(III)-catalyzed directing group-assisted C–H activation/carbocyclization of aryl ketones with alkynes to afford substituted indenols in good to excellent yields (Scheme 296).417,418 Glorius found that, with regard to electron-neutral and electron-rich phenones bearing protons at dehydrative positions (α or γ), the corresponding fulvene derivatives were successfully afforded after changing PhCl to 1,4-dioxane at higher temperature. Subsequently, Jeganmohan and co-workers also reported a similar reaction of Ru-catalyzed C–H activation of substituted ketones with alkynes, providing substituted indenol derivatives in good to excellent yields.419
In contrast to the Ru-catalyzed ortho-alkylation reaction of ketones with olefins reported by Murai, rhodium(III)-catalyzed oxidative ortho-olefination reaction of aromatic ketones with a wide range of olefins was realized by Glorius and co-workers (Scheme 297).69a This reaction exhibited broad substrate scope and both electron-poor and electron-rich styrenes were well tolerated. Subsequently, a similar olefination reaction of aromatic ketones using [Ru(p-cymene)Cl2]2 as the catalyst was reported by Jeganmohan and co-workers.420
A ruthenium(II)-catalyzed ortho-alkenylation of aromatic and heteroaromatic aldehydes with activated alkenes to give substituted alkene derivatives in good to moderate yields was developed by Jeganmohan (Scheme 298).421 It was the first example of ortho-alkenylation reaction directed by a weakly coordinating aldehyde group. The incident side reactions of decarbonylation of aldehydes, hydroacylation of aldehydes with alkenes, and oxidation of aldehydes to acids were not observed in the reaction. Through a photochemical rearrangement, the obtained alkene products could be further converted into some unusual four-membered cyclic ketones or polycyclic isochromanone derivatives. Based on Jeganmohan's work, Prabhu and co-workers demonstrated a ruthenium(II)-catalyzed highly regioselective alkenylation of indole at the C-4 position using an aldehyde functional group as a directing group under mild conditions, giving various biologically active 4-substituted indole derivatives in high yields.422
An unprecedented rhodium(III)-catalyzed direct functionalization of ortho-C–H bonds of aromatic ketone derivatives followed by an intramolecular cyclization sequence to prepare indene derivatives was presented by Li and co-workers (Scheme 299).423 A conjugate addition of α,β-unsaturated ketone and subsequent aldol condensation was regarded to be involved in the cascade reaction. Notably, the reaction was insensitive to air and water, providing a practical and convenient route to synthesize indene derivatives.
More recently, a ruthenium-catalyzed C–H alkenylation using arylacetophenones and olefins as substrates was developed by Greaney and co-workers (Scheme 300).424 According to the different choices of substrate and reaction conditions, three orthogonal cascade C–H functionalization processes were undergone to access 1-indanones, indeno indenes, and indeno furanones through cascade pathways. A wide series of polycyclic architectures could be readily afforded in a single operation with ruthenium-catalyzed multiple C–H functionalization steps.
A Pd-catalyzed multiple arylation of benzyl phenyl ketones with aryl bromides was developed by Miura and co-workers in 1999.425 In the presence of excess aryl bromides, the α- and two ortho-positions of carbonyl group were completely arylated and diphenylmethyl 2,6-diphenylphenyl ketone derivatives were predominant products.
The Ru-catalyzed ortho-arylation of aromatic ketones using arylboronates as the arylating reagents was reported by Kakiuchi and co-workers in 2003, giving the arylated phenyl ketones with high efficiency and regioselectivity (Scheme 301).426a Further intensive investigations of the reaction by the same group426b indicated that the use of aliphatic ketones, such as pinacolone and acetone, as an additive or solvent dramatically suppressed the undesired reduction of the aromatic ketones, producing the final ortho-arylation products in high yields. Detailed mechanistic studies revealed that the pinacolone functioned as a scavenger of ortho-hydrogens of the aromatic ketones or as the acceptor of the HB species. The results of kinetic isotope effects showed that the ketone carbonyl group coordinated to the ruthenium catalyst prior to C–H bond cleavage. This useful transformation provided an alternative method for the preparation of biaryl compounds.
A palladium(II)-catalyzed novel and mechanistically interesting method for the synthesis of phenanthrone derivatives from sec-alkyl aryl ketones and aryl iodides via dual C–H activation and enolate cyclization was developed by Cheng and co-workers (Scheme 302).427 The five-membered palladacycle dimer from the reactions of Pd(OAc)2 with aryl ketones in TFA was isolated and determined by single crystal X-ray diffraction, affording some evidence of the reaction mechanism. After completing the first C–H activation to deliver the arylation product, further C–H activation of another Pd complex gave a seven-membered palladacycle, which underwent enolization, rearrangement and subsequent reductive elimination to give the final product.
A palladium-catalyzed intramolecular oxidative dehydrogenative cyclization of aromatic ketones to afford the fluorenone derivatives was reported by Cheng428 and Shi,429 independently (Scheme 303). The choice of solvent was crucial for the transformation, and trifluoroacetic acid (TFA) was used as the optimal solvent to give the desired fluorenone product in the highest yield. The reaction showed broad substrate scope and provided a convenient and effective route toward the synthesis of fluorenone derivatives.
In contrast to a lot of previous studies on ketone-directed C–C bond formation, ketone-directed construction of C–N bonds has rarely been reported. Until 2011, Liu and co-workers made a significant breakthrough on Pd-catalyzed intermolecular oxidative directed C–H amidation of aromatic ketones with amides (Scheme 304).430 The electrical properties of Pd species were crucial for the success of this transformation and electron-deficient Pd(OTf)2 was chosen as the best one. As for ketone substrates, electron-rich ketones could more strongly coordinate to Pd, showing a better performance than that of electron-deficient ketones. Furthermore, the superiority was embodied by the fact that ketones with more electron-rich tertiary alkyl groups exhibited a higher efficiency than ketones with a primary or secondary alkyl group. Some relevant mechanical investigations indicated that the reaction might not involve a nitrene intermediate and the C–H activation was presumably the rate-limiting step of the transformation.
More recently, the Ru-catalyzed ortho-C–H amination of aromatic ketones by using sulfonyl azides as the nitrogen source was reported by Chang, Sahoo and Jiao, respectively.431–433 The reaction showing high functional group tolerance and a wide range of sulfonylazides could be successfully installed on arylketones, providing a new complementary approach to the practical ketone-directed intermolecular C–N bond formation (Scheme 305). In addition to Chang's amination of ketones, some strong coordinating nitrogen groups such as benzamide, 2-pyridyl, pyrazole, and ketoxime moieties all enabled the ortho-C–H amination smoothly in the presence of AgSbF6 salt and acetate additive.
The iridium-catalyzed direct intermolecular C–H amidation of weakly coordinating substrates, particularly of ketone derivatives, was developed by Chang and co-workers (Scheme 306).434 During the optimization of the reaction, the lithium counteraction was found to have a significantly stronger effect on the transformation than other cations and the combination of lithium carbonate with acetic acid gave the desired products in almost quantitative yield. With the successful isolation of relevant iridacyclic intermediates, some convincing evidence of the mechanistic aspects was proposed and the role of additives was also speculated therein. The facile conversion of ester and ketone functional group further extended the application of this useful transformation.
ortho-Acylphenol is an important structural motif found in diverse bioactive molecules from natural products to drugs and can be readily used for the preparation of various oxygen-containing heterocycles. The Pd-catalyzed regioselective ortho-hydroxylation of ketones, benzoates, benzamides, acetanilides, and sulfonamides at room temperature was reported by Rao and co-workers in 2012 (Scheme 307).435 A broad range of functionalized phenols were accessed in high yields by this method. Preliminary mechanistic investigations revealed that the TFA/TFAA solvent system served as both the essential factor for C–H activation and as the oxygen source. The reaction showed excellent reactivity, good functional group tolerance, and high regioselectivity. The obtained phenol derivatives could be easily transformed into some oxygen-containing heterocycles and key intermediates of drugs.
Simultaneously, Dong and co-workers also developed a Pd-catalyzed ortho-selective formal hydroxylation of arenes to produce various 2-acylphenols.436 Moreover, a Pd-catalyzed ortho-carboxylation of simple aryl ketones to afford an unusual ketal–lactone motif was also disclosed concomitantly. Soon after this, similar research on palladium-catalyzed ortho-oxygenation of aromatic ketones by using PhI(OTFA)2 as an oxidant was described by Kwong and co-workers.437
The Ru-catalyzed ortho-oxygenation of aromatic ketones was developed by Ackermann in 2012 (Scheme 308).438 [Ru(O2CMes)2(p-cymene)]2 was an effective catalyst and PhI(OAc)2 was used as a terminal oxidant to give the desired product in highest yields. The reaction proceeded with excellent functional group tolerance and broad substrate scope.
Recently, the Ru-catalyzed ortho-C–H oxygenation using very weakly coordinating aldehydes as directing groups was also developed by Ackermann and co-workers (Scheme 309).439 Among a variety of ruthenium complexes, [RuCl2(p-cymene)]2 was the optimal catalyst. Detailed mechanistic studies revealed that the oxygenation reaction initially occurred by a kinetically relevant C–H metalation and the obtained 2-hydroxy benzaldehydes could be easily converted into various valuable heterocycles.
8. The C–H functionalization directed by ester
8.1. The C–H functionalization directed by ester from acid
In contrast, the application of ester functionality as the feasible directing group in the transition-metal-catalyzed C–H bond activation has not been widely reported. However, as a weakly coordinating group, the ester moiety serves as a promising and valuable directing group as it possesses numerous advantages. First, it presents weak coordination to catalysts to induce selective C–H bond cleavage and facilitate the release of the catalyst species. Second, this functional group is omnipresent in numerous natural products and synthetic compounds. Third, the ester unit may be used as a readily manageable protecting group in organic synthesis and it can be converted into various functional groups under ambient conditions.The first transition metal catalyzed ester directed ortho-alkylation of functional alkenes was reported in 1995 by Trost and co-workers (Scheme 310a).406b Using Ru(H2)(CO)(PPh3)3 as an efficient catalyst, the methyl-1-cyclopentenecarboxylate or cyclohexenecarboxylate reacted effectively with alkene to give the desired C–H/olefin coupling products. However, the substrates such as methyl benzoates were incompatible with this methodology. Simultaneously, Murai and co-workers also tried this reaction using the same catalyst and substituted alkyl benzoates could be successfully involved in this reaction (Scheme 310b).440a They found that the introduction of an electron-withdrawing group into the aromatic ring dramatically changed the reactivity. The reaction of aromatic ester substituted with CF3 or F with triethoxyvinylsilane occurred smoothly to give the coupling products. Further studies showed that the substrates were limited to a benzo-δ-lactone, heteroaromatic esters and aromatic ester substituted with CF3 or F. Other benzoates with electron-withdrawing groups, such as o-CO2Me, o-CN, o-NO2 and p-NO2, failed to afford coupling products.440b
Plietker and co-workers revealed a ruthenium-catalyzed broadly applicable hydrovinylation of terminal and internal alkynes with highly substituted electron-deficient olefins (Scheme 311).441 The efficient ruthenium hydride complex was air and moisture stable, and could be readily prepared from RuCl3. The addition of catalytic amounts of NaOMe had a significant effect on the derivatization of the catalyst based on important preliminary studies by Murai, allowing the reaction to proceed smoothly in the case of some substituted acrylates. Some deuterated experiments were conducted to shed light on the reaction mechanism. A variety of highly substituted 1,3-dienes were obtained using this method in good to excellent yields.
Chang and co-workers reported a Rh(III)-catalyzed ortho-olefination of benzoates under oxidative conditions (Scheme 312).442 They found that an ester moiety could work as an efficient directing group in the Rh(III)-catalyzed olefination reaction. A catalytic copper oxidant was enough for the high yield of the olefination product. The alteration of an alkoxy part of benzoates and the position of an additional substituent on benzoates had little influence on the reaction efficiency. A significant kinetic isotope effect (KIE) based on the deuterated experiment was observed (kH/kD = 2.3), indicating that the C–H bond cleavage at the 2-position of ethyl benzoate was likely involved in the rate-determining step.
Oxidative Fujiwara–Moritani-type alkenylations with weakly coordinating aromatic esters as the directing groups were accomplished by employing a cationic ruthenium(II) complex (Scheme 313). The two-fold C–H bond functionalizations offered a convenient method for the synthesis of various highly substituted alkene derivatives in a highly regio- and stereoselective manner. Jeganmohan443 and Ackermann444 independently reported the site-selective ruthenium-catalyzed oxidative alkenylations of arenes bearing weakly coordinating esters. The obtained substituted alkene derivatives could be easily transformed into substituted acrylic acid derivatives in excellent yields. Mechanistic studies were indicative of a reversible acetate-assisted cycloruthenation step to form complex B. Subsequent migratory insertion of alkene furnished intermediate C followed by β-hydride elimination gave the desired product D, and the catalytically active cationic species were regenerated by the oxidation of Cu(OAc)2 (Scheme 314).
Very recently, Zhang's group successfully developed a general and selective method to prepare conjugated dienes by Rh(III)-catalyzed direct olefination of acrylates with electron-rich alkenes via double C(sp2)–H bond activation (Scheme 315).445 It was noteworthy that the ester group of acrylates was found to be important to improve the activation of vinylic C–H bonds through chelation. Furthermore, this direct cross-coupling reaction exhibited high stereoselectivity to give the (Z, E) diene isomer as the major product (the ratio of (Z, E) and (E, E) isomers is 96/4). This protocol provided an efficient route for the preparation of diverse substituted 1,3-butadienes.
Direct borylation of C–H bonds is a highly active recent research subject in the catalytic use of C–H bonds. Sawamura and co-workers reported the ester directed ortho-borylation of aromatic C–H bonds with bis(pinacolato)diboron using a solid-supported monophosphine–Ir system. Silica-SMAP-Ir, which was readily formed in situ from [Ir(OMe)-(cod)]2 and silica-SMAP, showed high activities and selectivities for the borylation of aromatic C–H bonds (Scheme 316a).446 The monocoordinating feature of the immobilized phosphine ligand had an obviously positive role in highly efficient ortho-directing effects, whereas electronic effects of the strongly σ-donating SMAP ligand might have an additional influence on the catalytic properties. Later, they also developed the ester-directed regioselective borylation of heteroarenes catalyzed by a silica-supported iridium complex, a wide range of heteroarenes, including thiophene, pyrrole, furan, benzothiophene, benzofuran, indole, and carbazole derivatives, could be well involved in the reaction (Scheme 316b).447
Miyaura and co-workers disclosed an iridium catalyzed ortho-C–H borylation of benzoate ester derivatives with bis(pinacolato)diboron (Scheme 317).448 The iridium complexes were readily in situ generated from [Ir(OMe)(COD)]2 and commercial tris[3,5-bis(trifluoromethyl)-phenyl]phosphine. The choice of the iridium precursor was crucial for the borylation, and [Ir(OMe)(COD)]2 in a non-polar solvent showed the best reactivity. The corresponding aromatic boron compounds were produced in high yields with excellent regioselectivities.
Compared with Ir-catalyzed borylations using surface supported phosphine ligands, which were the focus of pioneering studies by Sawamura, Smith and co-workers developed a novel homogeneous Ir catalyst that could mimic these heterogeneous ones using bidentate silyl ligands that contained P- or N-donors, which were shown to effect ortho-borylations for a range of substituted aromatics (Scheme 318).449 The novel bidentate silyl ligand exhibited high reactivity and a number of substituted methyl benzoates could successfully participate in the reaction, providing the mono- and di-borylated products.
Rao and co-workers reported the first example of ruthenium(II)-catalyzed ortho-hydroxylation of benzoates using ester as an efficient directing group (Scheme 319).450 Both the TFA/TFAA cosolvent system and oxidants (K2S2O8, Selectfluor, or iodic acid) served as the critical factors in this transformation. The ratio of TFA/TFAA also had an indispensable role in the high yield of the reaction. Higher temperature was necessary for improving the yields. It was possible that the catalytic pathway might undergo a Ru(IV) intermediate followed by reductive elimination to afford the final product. A wide range of ortho-hydroxylated ethyl benzoates could be generated at high efficiency by this method.
Rao and co-workers reported the first palladium(II)-catalyzed regio- and chemoselective ortho-chlorination/bromination reaction of benzoates and 2-phenylacetates (Scheme 320).451 This method was developed for the synthesis of a broad range of arene chlorides/bromides by the use of NCS/NBS as the halide source. The use of a suitable oxidant was an essential factor for the regioselectivity of this reaction and Na2S2O8 was the best one. In addition, the amount of TfOH also significantly affected both the rate and outcome of the reaction. Mechanistic investigations indicated that the C–H bond cleavage might be involved in the rate-limiting step of this transformation. It should be noted that this protocol was conducted without the need for air- or moisture-free reaction conditions, making the method more convenient and practical.
Chang and co-workers described the Ir(III)-catalyzed direct amidation of aryl and alkenyl C(sp2)–H bonds using esters as viable chelating groups under very mild conditions (Scheme 321).434 The para-toluenesulfonyl azide was used as the nitrogen source. A combination of AcOH and Li2CO3 would generate lithium acetate and lithium bicarbonate in situ, and an acetate ion was expected to coordinate more favorably to the cationic iridium center to form the iridium acetate resting species. The reaction exhibited a broad substrate scope and proceeded under very mild conditions with excellent functional group tolerance. In addition, the protocol could be readily carried out on a gram scale. It was noteworthy that the ester unit could be converted into various functional groups under ambient conditions.
8.2. The C–H functionalization directed by ester from aryl phenol
Because substituted phenols are important organic intermediates with broad synthetic utility, protected phenols (either in the carbamate or ester form) have attracted intensive interest from the community studying transition metal-catalyzed C–H functionalizations.452Snieckus and co-workers developed a widespread and popular strategy for accessing elaborate phenol derivatives via directed ortho-metalation (DoM) of O-phenylcarbamates.453 Notably, the O-carbamate not only served as a useful protecting group but also acted as a functional handle for further transformations, including anionic Snieckus–Fries rearrangements454 and cross couplings.
In 2009, Bedford and co-workers reported a palladium-catalyzed ortho-arylation of phenols utilizing carbamates as protecting/directing groups (Scheme 322).455 Aryl carbamates could be readily prepared and are easy to convert back to free phenols. Aryl iodides and diaryliodonium salts were chosen as arylating reagents, in which the former gave both mono- and di-arylated products, whilst the latter favoured mono-arylation. When diaryliodonium salts were used as the arylating reagent, deprotection of the phenol occurred, which could in turn be exploited in the one-pot synthesis of dibenzopyranone derivatives via a novel sequential arylation–deprotection–lactonization process.
Cross-coupling of simple arenes was developed as a green strategy for making biaryl linkages. This arylation involved two aromatic C–H bonds (Ar–H and Ar′–H) undergoing concomitant functionalization to form a new biaryl C–C bond (Ar–Ar′). Dong and co-workers developed an ortho-arylation of O-phenyl carbamates by employing simple arenes as the cross-coupling partner (Scheme 323).456 Na2S2O8 was used as the best effective oxidant and TFA was the optimal additive to promote the efficiency of the reaction. Based on the convincing mechanistic investigations, a possible mechanism was proposed where two C–H bond activations occurred through a Pd(0)/Pd(II) catalytic cycle involved in cyclopalladation and electrophilic metalation, respectively.
Liu and Fu developed an example of an acyloxy-directed Pd(II)-catalyzed ortho-C–H arylation reaction of phenol esters (Scheme 324).457 This reaction provided a practical strategy for preparing 2-arylphenol derivatives. HOTf was identified as an important additive because the presence of HOTf could tune the electrophilicity of Pd(II) and stabilize the active Pd intermediates. It was notable that the selectivity of mono- versus diarylation could be controlled by modifying the reaction temperature and reactant ratio. In addition, the first palladacycle of phenol ester was successfully isolated and characterized by X-ray crystallography. The plausible mechanism of the arylation reaction possibly involved an acyloxy-directed Pd insertion into the C–H bond and subsequent oxidation of the Pd(II) complex to a Pd(IV) intermediate by Ar2I+OTf−, followed by the reductive elimination from the Pd(IV) complex to afford the desired product (Scheme 325).
Liu and co-workers further reported a systematic theoretical study of Rh(III)-catalyzed ortho-olefination of phenol carbamates (Scheme 326).458 This reaction constituted a complementary method for the Pd-catalyzed processes for the ortho-arylation of phenol esters and carbamates. Both substituted styrenes and acrylates well underwent the process to afford the corresponding olefination products in good yields. High regioselectivity was observed for most of the substrates, which favored the C–H functionalization at a sterically less hindered position. Extensive mechanistic studies were conducted and the results indicated that the overall catalytic cycle consisted of three main steps: the C–H activation of arene through the acetate-assisted CMD mechanism, alkene insertion, and β-H elimination. Simultaneously, direct ortho-C–H olefination of phenol derivatives catalyzed by rhodium was developed by Loh and co-workers.459 Later on, a mechanistic rationale for Rh(III)-catalyzed oxidative heck coupling of phenol carbamates with alkenes was proposed by Huang and Fu.460
The directing ability of the carbamate group was also exploited by Ackermann and co-workers for highly efficient oxidative Fujiwara–Moritani-type alkenylations of aromatic C–H bonds with a less expensive ruthenium catalyst (Scheme 327).461 The air and moisture stable ruthenium complexes were identified as viable catalysts for environmentally benign twofold C–H bond alkenylations recently. The presence of stoichiometric amounts of Cu(OAc)2·H2O could proceed the reaction successfully under an atmosphere of ambient air. Intermolecular competition experiments with differently substituted arenes indicated that electron-rich substrates were preferentially converted. Almost at the same time, similar oxidative ortho-alkenylation of phenyl carbamates was independently achieved by Wang and co-workers.462 Jeganmohan and co-workers also achieved alkenylations of aryl acetates with acrylates in the presence of catalytic amounts of [RuCl2(p-cymene)]2 and Cu(OAc)2·H2O with ambient air as the terminal oxidant. Base-mediated cleavage of the directing group in product using K2CO3 in MeOH provided the desired ortho-alkenylated phenols.463
A new process for alkenylation of ortho-C–H bond of aryl carbamates was developed by Jeganmohan and co-workers with alkynes in the presence of a ruthenium catalyst, AgSbF6 and pivalic acid, in which highly substituted alkene derivatives could be afforded in good to excellent yields in a highly regio- and stereoselective manner (Scheme 328).464 Notably, the regiochemistry of the products was determined by the alkyne substituents. The substrate scope of the present hydroarylation reaction could be easily extended to various substituted aryl carbamates and alkynes.
By the use of phenyl carbamates as substrates, a series of C–H functionalization reactions, such as C–H bromination, chlorination, decarboxylative acylation, oxygenation, and borylation, were successively reported (Scheme 329). Nicholas and co-workers reported a palladium-catalyzed ortho-bromination of aryl-O-carbamates using NBS as a bromine source via C–H bond activation (Scheme 329a).465 A series of cyclometalated palladium complexes derived from O-phenylcarbamates with different acids were synthesized and characterized to gain insight into the reaction pathway. A plausible mechanism for the present ortho-bromination reaction presumably involved the Pd(II)/Pd(IV) catalytic cycle. Initial C–H activation of the carbamate substrate would generate the palladacycle, which could be oxidized by the NBS to give the corresponding Pd(IV) intermediate. Subsequent reductive elimination from the Pd(IV) manifold leads to the release of the desired products.
Most recently, a practical, room-temperature Pd(II)-catalyzed regioselective ortho-chlorination/bromination reaction was developed by Rao and co-workers for a facile one-pot synthesis of a broad range of chloro/bromophenol derivatives from easily accessible phenols (Scheme 329b).466 NCS or NBS was utilized as a halogenation agent in the presence of TfOH. This protocol represented one of the rare examples of mild C–H functionalization at ambient temperature and demonstrated an excellent regioselectivity and reactivity for C–H halogenations.
Kim and co-workers described an efficient method for oxidative acylation of O-phenyl carbamates with α-oxocarboxylic acids catalyzed by palladium using ammonium persulfate as a convenient oxidant (Scheme 329c).467 The presence of a catalytic amount of triflic acid was crucial for the transformation. A wide range of O-phenyl carbamates with electron-donating groups and halogen groups were favored in the process, whereas substrates with electron-withdrawing groups (e.g., NO2 and CO2Et) at the para-position failed to deliver the acylation products under the optimal reaction conditions.
Regioselective hydroxylations of ortho-C–H bonds in aryl carbamates were achieved by Ackermann and co-workers (Scheme 329d).468 By employing [RuCl2(p-cymene)]2 as the catalyst and PhI(OAc)2 as the effective oxidant, a number of oxygenation products of phenol derivatives could be obtained with excellent chemo- and ortho-selectivities. The intramolecular competition experiments indicated that the carbamates were found to be more potent directing groups as compared to esters, but appeared to be less efficient than the amide substituents.
The directed ortho-borylation of phenol carbamates was reported by Sawamura and co-workers with an immobilized monophosphine–Ir catalysis system (silica-SMAP-Ir) (Scheme 329e).469 Compared with the higher reactivity of pinB–Bpin, H-Bpin was used as a boron source in this study in the case of atom economy. Various aryl carbamate derivatives with one or two additional substituents on the aromatic ring were applicable to the reaction. Notably, the directing carbamoyloxy group could also act as a leaving group in the cross-coupling reactions as demonstrated in the synthesis of a terphenyl derivative.
9. The C–H functionalization directed by hydroxyl derivatives
Hydroxyl is a very important functional group in organic chemistry, which exhibits weak coordination to transition metals.3c This characteristic enables it to play an important role in several transition-metal-catalyzed transformations.A key application of hydroxyl as a directing group in a selective intermolecular arylation of 2-phenylphenols by palladium catalysis was reported by Miura and co-workers (Scheme 330).470 This arylation regioselectively took place at the spatially neighboring positions of the phenolic function to give the corresponding 2-(2′-arylphenyl)phenol products. The reaction was supposed to be initiated by the oxidative addition of aryl iodide to give an arylpalladium(II) species, which reacted with the phenolate generated aryl(aryloxy)palladium intermediate. The subsequent C–H activation and reductive elimination afforded monoarylated product. The coordination of phenolic oxygen to intermediary arylpalladium(II) species was considered to be crucial for the activation of C–H bonds. The reaction could be extended to 1-naphthols to give the 8-aryl-1-naphthol products. The use of cesium carbonate as the base was important to the success of the formation of phenolate which was soluble in DMF to enhance the rate of deprotonation.
The palladium-catalyzed arylation of 2-(biphenyl-2-yl)-2-propanol with aryl halides was later developed by the same research group (Scheme 331).471,472 In this transformation, both the processes of ortho-phenylation and β-carbon elimination occurred in the initial step, and the resulting ortho-phenylated product underwent further coupling with aryl halides to give a series of multiple arylation products. One particular interesting example was the reaction of alcohol with 1,2-dibromo-4,5-dimethylbenzene to afford triphenylene in 60% yield.
 | ||
| Scheme 331 Palladium-catalyzed arylative carbon–carbon bond cleavage of α,α-disubstituted arylmethanols. | ||
A related precedent for this approach came in the form of a study by Miura and co-workers, who disclosed that 2-phenylphenol could effectively undergo oxidative coupling with alkenes using a palladium–copper catalyst in air (Scheme 332).473 The subsequent oxa-Michael addition furnished benzopyrans in moderate yields when the electron-deficient alkenes were used as the substrates.
Because many directing groups only effect the C–H activation of proximate bonds via five-membered palladacycles, the range of carbon skeletons that can be accessed are therefore restricted. The use of spatially remote, unprotected tertiary, secondary, and primary alcohols as the directing groups in a Pd(II)-catalyzed ortho-C–H olefination was developed by Yu and co-workers (Scheme 333).474 They discovered that the mono-N-protected amino acid ligands could significantly accelerate the olefination, which represented a rare example of the ligand-promoted C–H activation reaction. Olefination with styrenes and simple olefins gave the corresponding olefinated products without further cyclization. However, the resulting olefination products with electron-deficient alkenes underwent subsequent Pd(II)-catalyzed oxidative intramolecular cyclization to give the corresponding pyran products, which could be converted into ortho-alkylated alcohols after hydrogenolysis.
Although the hydroxyl group has been used as an efficient directing group in C–H bond activation, direct ortho-functionalization of simple phenols is quite difficult because the five- or six-membered metallacycles are unable to be established. Gevorgyan and co-workers designed a traceless/modifiable silicon-tethered directing group (PyDipSi) for ortho-acyloxylation and halogenation of arenes.244 By the use of this strategy, they successfully achieved the Pd-catalyzed ortho-alkenylation of phenols by the use of phenols with a silanol group (Scheme 334).475 The silanol group could be easily removed after the reaction with tetrabutylammonium fluoride (TBAF) in a “semi-one-pot” approach, and the diverse alkenylated phenols could be efficiently prepared in good to excellent yields.
Ge and co-workers described a palladium(II)-catalyzed alkenylation of benzyldiisopropylsilanols with olefins (Scheme 335).476 In contrast with the procedures of Yu and Gevorgyan, the ligand was not required in this transformation, and both the electron-rich and electron-poor arenes showed good reactivity to give the ortho-olefinated products in good yields. However, the electron-rich olefins failed to afford the desired olefination product. The ortho-alkenyl-substituted alkylarenes could be obtained after the smooth “deprotection” of the olefinated benzyldiisopropylsilanols.
Given the electronic similarity between the enol group of cyclic 1,3-dicarbonyls and the hydroxyl group of phenols, Lam and co-workers extended the above methodology to the enol-directed oxidative annulation of 2-aryl-3-hydroxy-2-cyclohexenones with terminal alkene (Scheme 336).477 The reaction proceeded not only by the well-established palladium catalysis, but also with ruthenium catalysis. In general, electron-deficient alkenes such as acrylates delivered benzopyrans, which were formed by the subsequent intramolecular oxa-Michael addition of the alkenylation products. The cyclization was not observed for styrenes and the alkenylated products were isolated in moderate yields.
Very recently, a practical and efficient method for the tandem C–H alkenylation/C–O cyclization reactions via the C–H functionalization of flavone derivatives was developed by Hong and co-workers (Scheme 337).478 This synthetic process provided concise access to a variety of flavone-fused benzopyrans.
A palladium-catalyzed phenolic hydroxyl-directed alkenylation of 2-arylphenols was reported by Sun and co-workers (Scheme 338).479 The reaction proceeded under an atmosphere of air using the acrylates as coupling partners to give the corresponding coupling products in moderate to good yields. All of the products obtained were proved to be E isomers. The electron rich olefin such as styrene or 1-hexene failed to give the desired alkenylation products under the standard reaction conditions.
The metal-catalyzed oxidative annulation of aryl or alkenyl substrates with alkynes via C–H cleavage has been proved to be an efficient and atom-economical strategy to access a range of valuable heterocyclic products.66b The reaction of 1-naphthols with internal alkynes in the presence of iridium catalyst to afford the corresponding 8-substituted 1-naphthol derivatives was reported by Miura and co-workers (Scheme 339).480 They found that the naphtho[1,8-bc]pyran heterocycles were generated with the reaction of 1-naphthols and diarylacetylenes when a rhodium/copper catalytic system in air was employed (Scheme 340).481 The reaction was supposed to be initiated by the coordination of the phenolic oxygen to rhodium to form a five-membered rhodacycle intermediate via the C–H activation. The insertion of this key intermediate to the alkyne and the subsequent reductive elimination afforded the naphthopyran heterocycles. The Rh(I) species was oxidized by the copper(II) salt to regenerate the Rh(III) catalyst and the oxygen in air achieved the reoxidation of Cu(I) species to CuII. However, poor site selectivity was observed with unsymmetrical alkynes.
The control of site selectivity in the reaction of 1-naphthols and unsymmetrical alkynes was demonstrated by Ackermann and co-workers using a ruthenium catalyst (Scheme 340).482 The control experiments revealed that the electron-deficient alkynes converted to the product faster than electron-rich alkynes and the C–H bond ruthenation step was reversible. The effective annulation of alkynes was also proved viable with 4-hydroxycoumarin.
Important isochromene derivatives were synthesized by Satoh, Miura and co-workers through the rhodium-catalyzed oxidative coupling of α,α-disubstituted benzyl alcohols with alkynes (Scheme 341).483 This method could be extended to α,α-dimethylallyl alcohols to give the corresponding 2H-pyran heterocycles.
The use of less expensive ruthenium catalysts for the formation of isochromene derivatives was demonstrated by Ackermann and co-workers (Scheme 342).484 The highly effective oxidative annulation of alkynes with benzyl alcohols proceeded efficiently under an atmosphere of air. Mechanistic studies indicated that an irreversible, kinetically relevant C–H ruthenation step might be involved in the reaction.
A new mode of Ru-catalyzed oxidative annulation of alkyne involving the functionalization of C(sp3)–H and C(sp2)–H bonds was reported by Lam and co-workers (Scheme 343).485 This enolate-directed ruthenium-catalyzed oxidative annulation of alkynes with 2-aryl-1,3-dicarbonyl compounds resulted in a diverse range of spiroindenes with high levels of regioselectivity, which were important structures in biologically active compounds. A notable feature of this process was the formation of an all-carbon quaternary center, which has not been described previously in the alkyne oxidative annulations. A wide range of unsymmetrical alkynes were tolerated and a high regioselectivity with initial C–C bond formation occurring at the alkyne carbon atom of the alkyl substituent was observed. The terminal alkynes gave a complex mixture.
A plausible catalytic cycle was proposed by the authors (Scheme 344). The deprotonation of the cyclic 1,3-dicarbonyl substrate generated an enolate, which was able to direct the cycloruthenation of the [Ru(OAc)2(p-cymene)]2 complex to form the six-membered ruthenacycle A. Coordination and insertion of the alkyne with ruthenacycle A gave the unusual eight-membered ruthenacycle B with the preference of C–C bond formation at the alkyne carbon atom bearing the alkyl substituent. The reductive elimination released the spiroindene products and the Ru(0) species was oxidized by Cu(OAc)2 to regenerate the Ru(II) catalyst.
The development of corresponding processes for the synthesis of chiral spiroindenes and benzopyran products was demonstrated by the same research group (Scheme 345).486 Site selectivity in the initial C–H functionalization to spiroindene or benzopyran products could be achieved by the use of palladium- or ruthenium-based catalysts, respectively. A palladium-N-heterocyclic carbene complex as the precatalyst mainly resulted in the oxidative annulation with alkynes to provide spiroindenes exclusively. In contrast, a [RuCl2(p-cymene)]2/Cu(OAc)2 catalyst system gave benzopyrans as the major products. This reaction was an elegant example of successful site selectivity control by the suitable choice of a catalytic system.
When the 1,3-enynes were used as the substrates of unsymmetrical alkynes in a Rh(III)-catalyzed annulation, besides the expected spiroindene product, a benzopyran compound was also isolated (Scheme 346).487 A reasonable reaction pathway for the one-carbon annulation to form the benzopyran compound was presented by the authors and a rare 1,4-migration of Rh(III) species was proposed to be involved in the transformation. The related selectivity was almost determined by the properties of substituent R1. The spiroindenes were given as the major products with the electron-donating substituent and the benzopyran compounds were isolated in high yields when the substituent was replaced by the electron-withdrawing groups. Further investigation demonstrated that enynes containing allylic hydrogens cis to the alkyne, which facilitated the 1,4-Rh(III) migration, were required for the effective oxidative annulation.
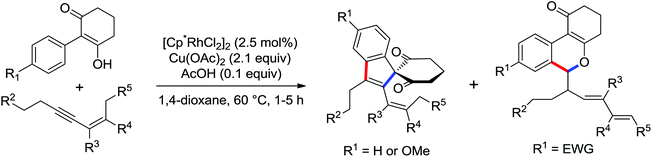 | ||
| Scheme 346 Oxidative annulation reactions of various 2-aryl-3-hydroxy-2-cyclohexenones with 1,3-enyne. | ||
Recently, Luan and co-workers demonstrated the first example of Ru-catalyzed vinylative dearomatization of α-aryl-β-naphthols through an unprecedented C–H activation/dearomatization tandem process (Scheme 347).488 In this transformation, an all-carbon quaternary centre was efficiently constructed by employing the inexpensive [RuCl2(p-cymene)]2 as the catalyst and Cu(OAc)2 as the oxidant. Various symmetrical and unsymmetrical alkynes participated in the reaction selectively and a variety of synthetically important functional groups were found to be tolerable in this process. However, the 2-phenylphenols showed low reactivity and gave the products in extremely low yields. The evolution of dearomatization of naphthyl rings in the substrates compelled by the C–H activation was established. This catalytic method provided a straightforward way to access the useful spirocyclic compounds bearing the spiro[indene-1,1′-naphthalene] skeleton.
The synthesis of benzoxepines by the rhodium-catalyzed oxidative [5 + 2] annulation of 2-vinylphenols with alkynes was developed by Mascareñas, Gulías and co-workers (Scheme 348, R = H).489 In contrast with the α-aryl-β-naphthols, the dearomatization of phenols was not observed with 2-vinylphenols and a readily [5 + 2] cycloaddition to alkynes occurred in the presence of [Cp*RhCl2]2 catalyst and Cu(OAc)2 oxidant. The reaction took place with high regioselectivity with unsymmetrical alkynes, leading to the production of benzoxepines in which the phenyl group was in the carbon tethered to the oxygen group. The mechanistic study gave evidence of the attack of the conjugated alkene to form the electrophilic Rh complex A. Rearomatization resulted in the intermediate B, which underwent insertion to alkynes and liberated the products after reductive elimination. However, the reaction failed with the substrates with alkyl substituents at the terminal position of the alkene such as (E)-2-(prop-1-en-1-yl)phenol. To further study the scope of the process and gain more mechanistic insights, Mascareñas, Gulías and co-workers explored the performance of alkenylphenol derivatives equipped with a substituent at the internal position of the alkene. They found that a formal [3C + 2C] cycloaddition between 2-alkenylphenols and alkynes took place by the rhodium-catalysis under oxidative conditions to give the spirocyclic products (Scheme 348, R = alkyl or Ar).490 These results pointed out the potential of using substituents in key strategic positions of substrates to change reaction outcomes (oxepine vs. spirocycle) because of the generation of steric interferences that affected key steps of the mechanism.
At the same time, Lam and co-workers reported the synthesis of spirocyclic enones by rhodium-catalyzed dearomatizing oxidative annulation of 2-alkenylphenols with alkynes and enynes (Scheme 349).491
A carbonylation of phenethyl alcohols by palladium catalysis was developed by Yu and co-workers (Scheme 350).492 The reaction was significantly improved by the use of mono-N-protected amino acids as the ligands. It was supposed that the amino acids were able to reduce the conversion of Pd(II) to palladium black in the presence of reductive carbon monoxide. Importantly, a histamine release inhibitor was successfully synthesized, which showed the potential of the methodology for rapid synthesis of an entire library of histamine release inhibitors.
The synthesis of dibenzopyranones through Pd-catalyzed phenol-directed C–H activation/carbonylation of 2-phenylphenol derivatives was described by Shi and co-workers (Scheme 351).493 The important combination of carbonylation chemistry and selective C–H activation strategy led to the facile syntheses of dibenzopyranones. The reaction was found to be sensitive to the electronic nature of substituents on the aromatic ring with the hydroxyl group, and electron-neutral groups, such as methyl and phenyl, gave higher yields. The mechanistic study revealed that the C–H activation step might go through an SEAr mechanism. Based on this work, Chuang, Cheng and coworkers reported palladium-catalyzed C–H activation and CO insertion into 2-arylphenols under acid–base free and mild conditions.494
Kondo's group demonstrated a new Ru-catalyzed carbonylative C–H cyclization of 2-arylphenols under relatively mild conditions (Scheme 352).495 This method employed a combination of a catalytic Ru/N-heterocyclic carbene (NHC) system with a balloon pressure of CO and O2. Functional groups, such as the alkoxycarbonyl, acetyl groups and the halogen atoms (F, Cl, and Br), were well tolerated.
The more challenging Pd-catalyzed carboxylation of inactivated alkenyl C–H bonds of 2-hydroxystyrenes using CO2 was investigated by Iwasawa and co-workers in 2013 (Scheme 353).496 Treatment of 2-hydroxystyrenes and a catalytic amount of Pd(OAc)2 with Cs2CO3 under the atmospheric pressure of CO2 afforded the corresponding coumarins in good yields. The bromophenyl moiety could be tolerated under the reaction conditions, implying that the Pd(0) species might not formed in the reaction process. The reaction was proposed to undergo reversible nucleophilic addition of the alkenylpalladium intermediate to CO2. The substrates bearing various functional groups could provide the desired carboxylation products in good yields. However, β-substituted 2-hydroxystyrenes did not give the desired products under the reaction conditions.
Yu and co-workers developed a new method for the construction of dihydrobenzofurans with Pd(II)-catalyzed C–H activation/C–O cyclization reaction directed by a proximate hydroxyl group (Scheme 354).497 The presence of a base was essential to reduce the decomposition of the starting material and PhI(OAc)2 was used as the most effective oxidant for the Pd(II)–Pd(IV) catalytic cycle. It is notable that the ortho-brominated substrates could participate in the reaction to afford dihydrobenzofuran products with the remaining bromide, which provided a useful handle for the subsequent transformations. The process could be applied in the synthesis of natural products and drug molecules containing spirocyclic dihydrobenzofurans.
Later on, Liu and co-workers reported a practical phenol-directed C–H activation/C–O cyclization reaction as an efficient route to substituted dibenzofurans (Scheme 355).498 The addition of sodium pivalate (PivONa) could significantly improve the reaction and air was used as the oxidant for the Pd(0)/Pd(II) catalytic cycle. The X-ray diffraction analysis of a four-coordinate Pd(II) complex carrying an anionic pivalate ligand provided clear evidence that the phenol group coordinated with Pd(II) as a neutral σ donor. The mechanism investigation revealed that the turnover-limiting step of the reaction was found to be the reductive elimination instead of C–H activation. Yoshikai and co-workers reported a similar Pd-catalyzed process involving 3-nitropyridine as a ligand and tert-butyl peroxybenzoate as an oxidant.499 The mechanistic experiment suggested that the reaction involved Pd(II)-mediated C–H bond cleavage as the rate-limiting step.
The Cu(I)-catalyzed process for the synthesis of dibenzofuran derivatives was developed by Zhu and co-workers (Scheme 356).500 A catalytic amount of CuBr could promote the cycloetherification reaction using Cs2CO3 and PivOH as additives under the air atmosphere. The presence of a strong para-electron-withdrawing group (e.g., NO2) on the phenol was essential for the success of the reaction. Hong and co-workers further extended the methodology to the C–H functionalization of chromones and coumarins, providing a straightforward process for the preparation of heterocyclic-fused benzofurans with good functional group tolerance (Scheme 357).501
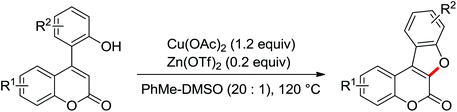 | ||
| Scheme 357 Synthesis of heterocyclic-fused benzofurans via C–H functionalization of flavones and coumarins. | ||
The Pd-catalyzed silanol-directed ortho-C–H oxygenation of phenol derivatives was presented by Gevorgyan and co-workers (Scheme 358a).502 The reaction presented high site selectivity and a broad functional group tolerance. Both electron-neutral and electron-poor phenols could undergo the Pd-catalyzed C–H acetoxylation/C–O cyclization reaction to form silacycle, followed by the desilylation to furnish catechol products. The corresponding 18O-labeling studies demonstrated that the acetoxylated product was involved in the stepwise route instead of the direct C–O reductive cyclization from the starting silanol. The same group further discovered that the benzylsilanol could be used as a substrate in the efficient synthesis of oxasilacycles, which were valuable intermediates containing an easily cleavable Si–O bond and a modifiable C–Si bond (Scheme 358b).503
Hartwig demonstrated the Ir-catalyzed ortho-silylation of phenol derivatives, in which the in situ generated diethyl(hydrido)silyl ethers went through dehydrogenative cyclization to form benzoxasilole products (Scheme 359).504 The transformation proceeded with low catalyst loadings (1 mol%), and a range of functional groups were found to be compatible with the reaction conditions. The synthetic utility was further demonstrated by the conversion of the benzoxasilole products to phenol and biaryl derivatives by Tamao–Fleming oxidation and Hiyama cross coupling.
The Pd-catalyzed hydroxyl-directed aldehyde C–H bond cleavage could be involved as the key step in the cross-coupling reactions of salicylaldehydes with aryl iodides, which provided new routes for the synthesis of 2-aroylphenols derivatives. In 1996, Miura and co-workers reported the Pd-catalyzed coupling of salicylaldehydes with phenyl iodides to produce biaryl ketones, in which the phenolic function could act as a good anchor for the catalytic intermolecular C–C coupling (Scheme 360).505 The addition of LiCl (20 mol%) could promote the reaction and enhance the yield of the product. Furthermore, Miura and co-workers developed the coupling reaction of salicylaldehydes with alkynes/alkenes/allenes using a rhodium-based catalyst system.506 Various alkynes, alkenes or allenes were applicable to the process, affording the corresponding ketones. Tanaka, Suemune and coworkers reported the Rh-catalyzed hydroacylation between salicylaldehyde and alkenylnitriles at room temperature, in which an additional effect of the CN ligand on transition metal-catalyzed reactions was observed.507
Rhodium-catalyzed asymmetric intermolecular hydroacylation reaction of norbornenes with salicylaldehydes was developed by Bolm and co-workers (Scheme 361).508 It was found that monodentate phosphoramidite ligands gave rise to endo products, while bidentate phosphine ligands catalyzed the reaction to form exo products predominantly.
The rhodium-catalyzed oxidative coupling of salicylaldehydes with internal alkynes was achieved with the cleavage of the aldehyde C–H bond to provide 2,3-disubstituted chromone derivatives by Miura, Satoh and coworkers (Scheme 362a).509 A cyclopentadiene ligand C5H2Ph4 was crucial for the success of the transformation, which was supposed to stabilize the unstable Rh(I) species during the reoxidation step to prolong the lifetime of the catalyst. Later, the synthesis of xanthones was reported by Peng's group via Pd-catalyzed annulation of 1,2-dibromoarenes and salicylaldehydes (Scheme 362b).510 Recently, Glorius’ group reported a Rh(III)-catalyzed regioselective dehydrogenative Heck reaction of aldehyde C–H bonds with different classes of olefins to afford aurone derivatives (Scheme 362c).511 Both aldehyde C–H olefination and oxidative cyclization were involved in the mechanism in the presence of a hydroxy directing group. When less electrophilic olefin substrates such as styrene and ethylene were used, the reaction could afford only olefinated products without further cyclization even when the amount of oxidant was increased. A variety of salicylaldehydes could be converted to the desired aurone derivatives, and this method can be applied to the synthesis of lonchocarpin and 4-methoxylonchocarpin.
Álvarez and Muñiz developed an arylether-directed palladium-catalyzed oxidative amidation of C(sp3)–H bonds with NFSI as the decisive reagent (Scheme 363).172 By the use of the preformed bathocuproine palladium complex [(bc)Pd(OAc)2], a series of 2-methylanisole derivatives could undergo selective C(sp3)–H amidation under the optimized conditions.
Yu's group demonstrated a new method for the functionalization of a major class of ethers, namely arylethyl ethers (Scheme 364).512 They successfully achieved an aliphatic ether-directed C–H olefination via a six-membered cyclopalladation which was promoted by monoprotected amino acid ligands (MPAA). This reaction provided a new method for chemically modifying readily available and synthetically useful ethers.
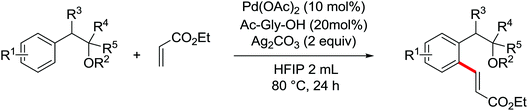 | ||
| Scheme 364 Ether-directed ortho-C–H olefination with a palladium(II)/monoprotected amino acid catalyst. | ||
10. The C–H functionalization directed by S, P, Si-containing functional groups
10.1. The C–H functionalization directed by thioether and sulfoxide
Sulfur compounds have attracted much attention from chemists in organic synthesis, coordination chemistry, biomaterials, and functional materials. Many of the sulfur containing molecules present unique properties and thus have found wide applications in pharmaceuticals and functional materials. Straightforward synthesis of sulfur containing molecules is highly desirable. However, the investigation of using sulfur containing functional groups as directing groups in C–H activation poses a challenge due to their ability to poison metal catalysts in some cases. In 2012, Zhang and co-workers developed a Pd-catalyzed thioether directed C–H alkenylation of arenes, which should be the first example of employing thioether as the directing group in direct C–H functionalization reactions (Scheme 365a).513 Using AgOTFA as an oxidant, the reaction of arylthioethers and acrylic esters was carried out in DCE to afford the dialkenylation products. It was found that the length of the tether group between arene and sulfur exerted a drastic effect on the reaction, and the benzyl thioethers showed the best efficiency. Besides, the authors succeeded in isolating a key five-membered palladacycle intermediate, which demonstrated that sulfur was indeed the anchoring atom. It should be noted that the thioether could be easily removed, which is an important issue for the application of the chelation-assisted C–H activation strategy. Furthermore, the thioether can be readily transformed to other useful functionalities. The palladium-catalyzed thioether-assisted C–H arylation of arenes was reported by the same group later using Ag2CO3 as an oxidant and BQ as an additive at 130 °C in DCE (Scheme 365b).514The rhodium-catalyzed thioether-directed ortho-alkenylation of arenes was reported by Shi and co-workers (Scheme 366a).515 With benzyl-(methyl)sulfane as the model substrate, both methyl acrylate and butyl acrylate gave the corresponding products in high yields. Impressively, positional selectivities of multiple substituted aromatic rings with two approximately electronically equivalent ortho-C–H bonds could be controlled by using different solvents: MeOH favored mono-alkenylation whereas t-BuOH favored dialkenylation using the Cu(OAc)2 as an oxidant and AgOAc as an additive. When styrenes were used as the resource of olefin, the mono-alkenylation products were obtained in DCE. However, the homobenzyl methyl sulfide showed very low reactivity in their rhodium catalytic system. After intensive screening of the reaction conditions, they found the combination of palladium catalyst with silver salt led to the formation of the alkenylated product of biphenyl-2-yl(methyl)sulfane in good yields (Scheme 366b).516 Interestingly, the selectivity of the mono-alkenylation and di-alkenylation could be controlled by the choice of oxidant. The mono-alkenylated arenes were obtained as the major products when AgOTFA was used as an oxidant, and the di-alkenylated arenes were given as the products in the presence of 2 equiv. AgNO3.
The palladium-catalyzed double C–H activation of simple benzyl phenyl sulfoxides to synthesize dibenzothiophenes was described by Antonchick and co-workers (Scheme 367).517 They proposed that the sulfoxide functionalized as a directing group to undergo the first ortho-C–H activation in the benzyl phenyl ring. The second C–H activation gave the intermediate A, which underwent the reductive elimination to give the sulfur-bridged six-membered polycycle B. However, polycycle B was unstable under the acidic reaction conditions and underwent Pummerer rearrangement to produce the thiophenyl derivative C, which underwent palladium-catalyzed cyclization to afford the dibenzothiophenes. It was unusual that aryliodide played an important role in this transformation and the reaction was sluggish without the use of aryl iodide.
The sulfur-bridged six-membered polycycle was prepared by Zhang and co-workers using the less acidic reaction conditions. They found that when the amount of acetic acid was decreased to 4 equivalents, the Pummerer rearrangement could be thoroughly suppressed and the five-, six-, and seven-membered sulfur-bridged polycycles could be isolated using different sulfoxide substrates (Scheme 368).518 The solvent had a significant effect on the reaction efficiency, and TTCE gave the best results. A sulfide-bridged six-membered pyrene-thienoacene molecule was synthesized readily using this method, and a preliminary study revealed its excellent fluorescence performance to give a photoluminescence quantum yield as high as 0.48.
Sulfoxide directed ortho-alkenylation of arenes was disclosed by Zhang and co-workers (Scheme 369).519 Because the Pd(OAc)2/AgOTFA system for thioether-assisted ortho-olefination was inactive to its sulfoxide analogues, much effort was made to find an effective catalytic system for sulfoxide substrates. Fortunately, after extensive optimization of the reaction conditions, the use of Selectfluor as an oxidant was found to be superior, and various ortho-alkenylated products were obtained with broad substrate scope in high yields. It was established that five-membered and six-membered palladacycles of sulfoxide could be formed, which led to the successful ortho-olefination of benzyl and 2-phenylethyl sulfoxide substrates. Importantly, 3-phenylpropenyl sulfoxide with a three carbon tether length could participate in the ortho-alkenylation smoothly, showing the excellent directing property of sulfoxide group. The sulfoxide group could be easily transformed to other functionalities such as hydroxyl and aldehyde.
The rhodium-catalyzed ortho-alkenylation of phenyl sulfoxides using alkenes or alkynes as substituent sources was developed by Satoh, Miura, and co-workers (Scheme 370).520 Both acrylates and internal alkynes showed good reactivity to give the ortho-alkenylated phenylsulfoxides. Although solid mechanism evidence was not presented, the authors believed that the oxygen rather than sulfur was the anchoring atom to form a more stable five-membered rhodacycle key intermediate. A further transformation of the sulfoxide group to sulfide and useful diphenylbenzothiophenes after the alkenylation was achieved by convenient reduction or treatment of the sulfoxide with acid respectively.
The use of chiral sulfoxide as both directing group and chiral auxiliary was explored by Colobert and co-workers. They found that 2-p-tolylsulfinylbiphenyl derivatives could be olefinated with methyl acrylate in the presence of 10 mol% Pd(OAc)2 and 6 equiv. AgOAc at 80 °C, using DCE as the solvent (Scheme 371a).521 An atropodiastereoselective olefination was desired by the introduction of the chiral sulfoxide group in the biaryl. However, a relatively low atropodiastereoselectivity was observed under the reaction conditions, in which an 80 °C temperature was required for the olefination. The high temperature might cause the rotation of the olefinated product around the biaryl axis and led to the low stereoselectivity. Nevertheless, this work showed the possibility of asymmetric C–H bond functionalization by the use of a chiral sulfoxide. In the light of this concept, the stereoselective acetoxylation and iodination of biaryls was successfully achieved through a mild C–H activation/DKR (dynamic kinetic resolution) sequence by Colobert and co-workers (Scheme 371b).522 The replacement of PhI(OAc)2 by persulfate oxidant/acetic acid drastically improved the efficiency of the reaction and the acetoxylation could be performed at room temperature. Mechanistic studies suggested that the isomerization of the diastereomeric mixture of biaryl substrates might occur when bridging palladacyclic intermediates with a decreased configurational stability were generated. This was a successful example to access the atropisomeric scaffold by the direct C–H functionalization strategy.
10.2. The C–H functionalization directed by Si-containing functional group
The hydrosilanes were explored by Hartwig and co-workers to function as the directing groups in an Ir-catalyzed ortho-borylation of benzyldimethylsilane. The formation of a bisboryl monosilyl complex through the reaction of hydrosilane and [Ir(cod)Cl]2 in the presence of B2pin2 was supposed to be the key intermediate, which allowed the selective ortho-C–H bond activation and functionalization (Scheme 372a).523 Substrates with more readily cleavable silicon linkers such as silyl ether and silylamine worked well under the reaction conditions to give the corresponding ortho-borylation products. It should be emphasized that the preparation of these siloxide or silylamine substrates and the borylation reaction could be performed in one pot. This method provided a valuable novel route for the synthesis of borylated products from benzylic silanes, phenols, and anilines. By the use of a similar strategy, a change in borylation selectivity of indoles from 2-position to 7-position was accomplished (Scheme 372b).524 The directed borylation reaction at 7-position of the indole framework tolerated a variety of substituents such as halogen, cyano group, and alkoxy and benzyloxy groups at the 3-, 4-, and 5-positions. Extension of this strategy to alkylarylsilane brought out the successful iridium-catalyzed borylation of secondary benzylic C–H bonds in the presence of 3,4,7,8-tetramethyl-1,10-phenanthroline as the ligand (Scheme 372c).525In 2012, Hardwig and co-workers achieved the site-selective functionalization of aliphatic C–H bonds by the combination of an iridium–phenanthroline catalyst and a dihydridosilane reagent (Scheme 373).526 The site-selective γ-functionalization of primary C–H bonds was controlled by a hydroxyl group, and the scope of the reaction encompassed alcohols and ketones bearing different substitution patterns and auxiliary functional groups. Importantly, this superior method was suitable for the site-selective functionalization of complex natural products under mild conditions.
The Rh-catalyzed synthesis of silafluorenes from biphenylhydrosilanes was described by Kuninobu, Takai and co-workers (Scheme 374).527 The pathway by a Friedel–Crafts-type reaction was excluded. The oxidative addition of rhodium into the hydrosilane was supposed to be an important step, which allowed the subsequent selective C–H activation through binding the rhodium to deliver selectively the catalyst to the proximal C–H bond. This dehydrogenation reaction could be applied for the synthesis of the ladder-type bis-silicon-bridged p-terphenyl compounds.
10.3. The C–H functionalization directed by P-containing functional group
Phosphorus is one of the most important atoms in organic compounds and can be found in a wide range of organic molecules such as natural products, polymers, liquid crystals, and ligands. The utility of the phosphate group as a directing group in C–H activated reactions was reported as early as 1986 by Lewis and Smith (Scheme 375).2a They found that the regioselective mono and double ortho-alkylations of phenol with ethylene could be achieved by using an ortho-metalated ruthenium phosphite complex. The initial exchange of the aryloxy moieties of the phosphite ligands with phenols led to the formation of an ortho-metalated ruthenium complex via the phosphorus-assisted C–H bond cleavage. The subsequent substitution of phosphite by ethylene, insertion of ethylene into the Ru–C bond, and finally reductive elimination gave the ethylated product. This was the first time that the ortho-metalated complex was used for catalytic C–C bond formation via C–H cleavage.The use of Wilkinson's catalyst with an appropriate phosphinite cocatalyst as the catalytic system for ortho-arylation of phenols was reported by Bedford and co-workers in 2003 (Scheme 376).528 In this transformation, the facile ortho-metalation occurred through the aryloxide group incorporation into the phosphinite cocatalyst phosphorus donors to give low-strain five-membered metallacycles. The cocatalyst influenced the reaction significantly; the reaction worked with the aryl dialkylphosphinite very well, but the bulky triarylphosphite ligand required a large excess of aryl bromide and the homocoupled biphenol was observed. A variety of aryl halides could be selectively coupled with phenols, leading to the synthesis of 2-arylated phenols. A similar chemistry was reported by Oi, Inoue and co-workers.529 The direct ortho arylation of phenols with aryl bromides was accomplished using a rhodium complex and hexamethylphosphorous triamide (HMPT).
In 2011, Kuninobu, Takai and co-workers explored a new strategy for the synthesis of dibenzophosphole oxides as shown in Scheme 377.530 In the presence of a catalytic amount of palladium(II) acetate, dehydrogenation of phosphine–hydrogen and carbon–hydrogen bonds of the secondary hydrophosphine oxides occurred through the palladium-catalyzed successive P–H and C–H bond cleavage steps. The palladium species formed from the oxidative addition of Pd(0) to P–H bonds or from the elimination of P–H bonds with Pd(OAc)2 played a key role in the activation of C–H bonds. By the use of this strategy, a ladder-type dibenzophosphole oxide was synthesized by the double intramolecular dehydrogenative cyclization.
An elegant ortho-C–H borylation of aryldiphenylphosphines catalyzed by iridium was developed by Clark and co-workers (Scheme 378).531 In this reaction, the phosphine was used as a directing group to assist the activation of C–H bonds and the diamine ligand was crucial for the displacement of the borylated substrate to allow catalyst turnover. The use of toluene as a solvent could improve the mono-substituted product. The resulting boronate ester was air sensitive and an air-stable crystalline product was obtained by the protection of the phosphine with borane. It was quite easy to remove the protecting group and pure ambiphilic phosphine boronate esters were obtained. This method provided an efficient new route to a variety of ambiphilic ligands.
The phosphonic acid and its derivatives have extensively served as essential compounds in organic, bioorganic, and medicinal chemistry. The Pd-catalyzed direct ortho-olefination of the benzylic phosphonic acid monoesters with ethyl acrylate or alkene was developed by Kim and co-workers in 2013 (Scheme 379).532 The reaction was carried out in the presence of Pd(OAc)2 catalyst using AgOAc as an oxidant to give a mixture of mono- and bisolefinated products. The benzylic phosphonic acid monoesters with electron-withdrawing substituents could give moderate yields, but the reaction was incomplete for the substrates with an electron-donating substituent. The most important step for the reaction was supposed to be the formation of six-membered palladacycle with the assistance of the phosphoryl–hydroxyl group through the coordination of the oxygen atom. The following insertion of C–Pd into double bonds and β-hydrogen elimination generated the ortho-alkenylated product.
The Rh-catalyzed ortho-alkenylation of phenylphosphinic acids with alkynes was reported by Satoh, Miura and co-workers (Scheme 380).533 They found that phenylphosphinic acids could react with internal alkynes under rhodium catalysis efficiently to give the corresponding phosphaisocoumarin derivatives. The key step of this transformation involved the coordination of hydroxyl group in phenylphosphinic acids to the Rh(III) center and subsequent cyclorhodation to form a stable five-membered rhodacycle intermediate. This phosphinoxy group directed oxidative coupling could be successfully extended to alkenes, and a variety of ortho-alkenylated arylphosphine products were obtained. A similar chemistry using different phenylphosphinic or alkenylphosphonic acids was described by Lee and co-workers.534
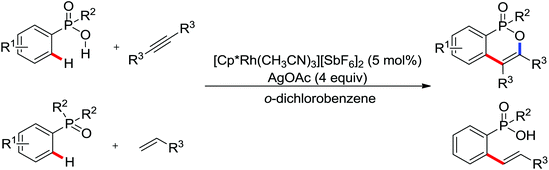 | ||
| Scheme 380 Rh(III)-catalyzed oxidative coupling through phosphinoxy group directed C–H bond cleavage. | ||
The ortho-acetoxylation using the phosphoryl–hydroxyl directing group under Pd(OAc)2 catalysis was reported by Kim and co-workers (Scheme 381).535 In this transformation, the solvent played the key role. The use of 1,2-dichloroethane (DCE) significantly improved the reaction efficiency to give the product in high yields. Other solvents, including dioxane, MeOH, DMSO, DMF, AcOH and toluene, gave poor yields. However, the reaction exhibited a relatively low selectivity. Mono-acetoxylated products were obtained when using the benzylic phosphonic and aryl phosphoric monoacids with substituents at the ortho- or meta-position. However, a mixture of mono- and di-acetoxylated products resulted when using the para-substituted substrates.
The Pd-catalyzed ortho-carbonylation and phosphaannulation of phosphonic and phosphinic acids was developed by Lee and co-workers (Scheme 382).536 The substituents at the α-positions of ethyl hydrogen benzylphosphonates influenced remarkably the efficiency of the reaction. The phosphinic acid substrates with one substituent or without substituent in the α-position were inactive. Only the substrates with two substituents in the α-position conducted the reaction smoothly to give the phosphaannulation product in high yields. Similar to the above phosphonic substrate, the hydroxyl group was supposed to be the directing group.
The Pd(II)-catalyzed arylation of aryl phosphates and aryl hydrogen phosphates using the phosphoryl as the ortho-directing group was developed by Kim and co-workers (Scheme 383).537 Various functionalized aryl groups were introduced into the ortho-position of organophosphates to successfully synthesize the biaryl compounds. They proposed that the mechanism of this reaction underwent the Pd(II)/Pd(IV) catalytic pathway and the key step of this transformation involved the weak coordination of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond with Pd(II) to generate the six-membered palladacycle intermediate. The Ar2IOTf was used as both an arylation reagent and an oxidant in this reaction. Later on, a similar transformation of ortho-arylation via Pd(OTf)2·2H2O catalyzed C–H bond activation with phosphate as a directing group was reported by Kang and co-workers.538
O bond with Pd(II) to generate the six-membered palladacycle intermediate. The Ar2IOTf was used as both an arylation reagent and an oxidant in this reaction. Later on, a similar transformation of ortho-arylation via Pd(OTf)2·2H2O catalyzed C–H bond activation with phosphate as a directing group was reported by Kang and co-workers.538
An efficient Pd-catalyzed ortho-alkenylation was subsequently described by Lee and co-workers using a mono-phosphoric acid-directing group (Scheme 384a).539 This reaction offered a new approach to synthesize various ortho-alkenylated phosphoric products. Importantly, various alkenes, including the electron-deficient alkenes, styrene, vinyl sulfone, vinyl phosphate, and phenyl vinyl ketone derivatives, could be successfully employed to give the ortho-alkenylation products. The Pd(II)-catalyzed arylation of phosphoramidate in the presence of TfOH and CuO was reported by the same research group (Scheme 384b).540
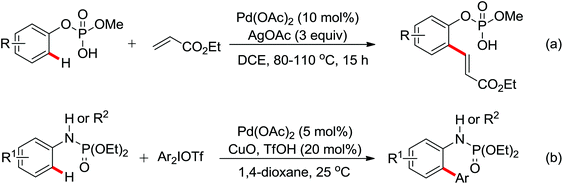 | ||
| Scheme 384 Palladium-catalyzed ortho-alkenylation of mono-phosphoric acid and ortho-arylation of phosphoramidate. | ||
The Rh(III)-catalyzed oxidative C–H activation using the phosphoryl-related directing groups was demonstrated by Glorius and co-workers.541 After modification of the reaction conditions, the ortho-alkenylation, the ortho-arylation, and the oxidative cyclization products could be obtained by rhodium catalysis. The catalytic process was supposed to undergo the five-membered rhodacycle intermediate generated by coordination of rhodium with the phosphoryl group. Almost at the same time, Kim542 and Lee543 reported similar transformations respectively. The Ru-catalyzed ortho-alkenylation of the phenylphosphine oxides with alkynes has been described by Satoh, Miura and co-workers.544 The corresponding (o-alkenylphenyl)phosphines were the useful ligands. The added acid was supposed to play a role in the step of reductive elimination to promote the reaction (Scheme 385).
The application of palladium-catalyzed phosphine oxide as a directing group was described by Yang and co-workers. They initially reported an alkenylation of diarylphosphine oxides with diverse alkenes. They found that the amino acid ligand Ac-Gly-OH could significantly improve the alkenylation to afford the useful alkene–phosphine ligands in high yields. In addition, a seven-membered cyclopalladium intermediate might be involved in this transformation via the Pd(II)/Pd(IV) catalytic pathway (Scheme 386a).545 Later on, they reported the palladium-catalyzed hydroxylation546 and acetoxylation547 of diarylphosphine oxides (Scheme 386b and c). Very recently, they developed a palladium-catalyzed arylation of diarylphosphine oxides to give the terphenyl electron-rich phosphorus compounds (Scheme 386d).548
11. The π-bond coordination assisted C–H functionalization
The π-bonds are the most common functional groups in organic molecules and play an indispensable role in organic synthesis. Because several metal complexes with unsaturated π-bonding ligands are known in the literature, such as Zeise's salt, Ni(COD)2, Pd2(dba)3 and [RuCl2(p-cymene)2]2, it is unsurprising that such ligands can also be used as directing groups in these reactions. The π-bond coordination is generally weaker than σ-bond coordination with regard to metals because π-bonding electron pairs tend to be lower in energy than lone pairs. However, a recent study has demonstrated that π-coordinating functional groups can assist in guiding metal complexes for site-selective C–H bond activation.54911.1. The C–H functionalization assisted by alkene
Kiyooka and co-workers demonstrated π-bond coordination-assisted C–H bond activation using iridium complexes (Scheme 387).550 The styrene reacted with N-benzylmaleimide in the presence of [{IrCl(cod)}2] and 2,2′-bipyridine (bpy) in toluene at 150 °C for 3 days to give product in 63% yield, in addition to various byproducts. The reaction was likely to be initiated by the coordination of styrene to iridium complex to form the coordinatively unsaturated intermediate, which underwent oxidative addition to the ortho-C–H bond to afford the C–H activation intermediate.In 2007, a palladium-catalyzed carbonylation of styrenes and carbon monoxide to produce arylnaphthalene-1(4H)-ones in the presence of Pd(OAc)2 combined with H5PMo10V2O40·nH2O was described by Ishii and co-workers (Scheme 388).551 Styrenes substituted with electron-withdrawing groups failed to give the product. The reaction was found to proceed in two stages involving the head-to-tail dimerization of styrenes, followed by carbonylation of the resulting dimers.
A possible mechanism for this transformation is depicted in Scheme 389. First, but-1-ene-1,3-diyldibenzene coordinated to Pd(OAc)2 at two of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds and facilitated ortho-C–H activation of the aryl group to give palladacycle intermediate. Insertion of CO into the Pd–C bond followed by reductive elimination afforded product and palladium(0). The palladium(0) species was then oxidized back to the active palladium(II) species by HPMoV/O2.
C double bonds and facilitated ortho-C–H activation of the aryl group to give palladacycle intermediate. Insertion of CO into the Pd–C bond followed by reductive elimination afforded product and palladium(0). The palladium(0) species was then oxidized back to the active palladium(II) species by HPMoV/O2.
The rhodium-catalyzed dimerization of styrenes involving ortho-C–H bond cleavage was reported by Tobisu and Chatani (Scheme 390).552 In contrast to the conventional head-to-tail, tail-to-tail, or head-to-head dimers, they found the unusual dimerization product in moderate yields after reacting various styrenes in the presence of 10 mol% [{RhCl(cod)}2], 20 mol% t-BuOH, and 40 mol% Na2CO3 in 1,4-dioxane. In this reaction the new carbon–carbon bond was formed between the benzene ring at the 2-position and the vinylic carbon. The reaction represented the first rhodium catalyzed ortho C–H functionalization of styrenes.
In 2012, Cheng and Gandeepan demonstrated a Pd-catalyzed allylic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond directed oxidative ortho-olefination of arenes (Scheme 391).553 The reaction proceeded under mild conditions and used oxygen as an oxidant to give ortho-olefinated allylarenes in good to excellent yields. The choice of solvent and oxidant was crucial for the success of the present catalytic reaction. TFA appeared to be the best additive for this catalytic reaction. The reaction was highly selective and effectively cleaved the ortho C–H bond of the arene attached to the allylic C
C bond directed oxidative ortho-olefination of arenes (Scheme 391).553 The reaction proceeded under mild conditions and used oxygen as an oxidant to give ortho-olefinated allylarenes in good to excellent yields. The choice of solvent and oxidant was crucial for the success of the present catalytic reaction. TFA appeared to be the best additive for this catalytic reaction. The reaction was highly selective and effectively cleaved the ortho C–H bond of the arene attached to the allylic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. No C–H activation was observed when using vinylic or butenylic C
C bond. No C–H activation was observed when using vinylic or butenylic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds as directing groups. The proposed mechanism is shown in Scheme 392.
C bonds as directing groups. The proposed mechanism is shown in Scheme 392.
Recently, Cheng's group reported the synthesis of highly substituted naphthalenes from substituted allylarenes and internal alkynes using a catalytic amount of Pd(OAc)2 (Scheme 393).554 The reaction of allylarene with an alkyne gave substituted naphthalenes in good yields in the presence of 10 equivalents of TFA under an oxygen atmosphere. This reaction also worked in the absence of Cu(OAc)2 with decreasing efficiency and no reaction was observed without TFA. Two regioisomeric products were produced when unsymmetrical alkynes were used as starting materials. Substrates with meta substituents selectively underwent C–H activation at less hindered sites owing to steric hindrance. This reaction proceeded through the π-coordination of the allylic carbon–carbon double bond to Pd(II) and ortho-selective C–H bond activation.
11.2. The C–H functionalization assisted by alkyne
In 2008, Gevorgyan and Chernyak discovered the palladium catalyzed exclusive 5-exo-dig hydroarylation of o-alkynylbiaryls (Scheme 394).555 The method allowed for efficient cyclization of a variety of o-alkynylbiaryls possessing electron-neutral and electron-deficient aryl rings into the corresponding 9-benzylidene-9H-fluorenes. The reaction proceeded through alkyne-assisted C–H activation instead of the more common Friedel–Crafts-type electrophilic aromatic substitution, which predominantly followed 6-endo-dig carbocyclization.556 Based on the high efficiency of the cyclization of substrates bearing electron deficient aryl rings and the observed high values of kinetic isotope effects, as well as on the exclusive cis-selectivity of cyclization, a plausible mechanism involving the C–H activation motif has been proposed for this transformation.The mechanistic studies suggested the possible catalytic cycle outlined in Scheme 395. Coordination of the alkyne to the palladium complex, followed by activation of the aromatic C–H bond, afforded ortho-palladation intermediate. Intramolecular migratory insertion of the alkyne into the Pd–C bond provided the vinylpalladium species, which gave the final product and regenerated the catalyst. This reaction could also be applied to the synthesis of fluorenes that were fully substituted at C-10 by adding an aryl bromide and slightly modifying the reaction conditions (Scheme 396).557
Later on, the same group further extended this strategy to synthesize alkylideneindanones as single E stereoisomers in good to excellent yields from ortho-alkynyl ketones (Scheme 397).558 It was demonstrated that the Pd-catalyzed regio- and stereoselective carbocyclization of o-alkynyl ketones, featuring the carbopalladation of the triple bond with Pd enolate species.
Recently, Jin and co-workers developed a novel palladium-catalyzed alkyne directed dual C–H activation of bis-biaryl alkynes to form the important products 9,9′-bifluorenylidene derivatives in high yields with a broad range of functional group compatibility (Scheme 398).559 The use of the PdCl2 catalyst combined with the MnO2 and PivOH was vital for the accomplishment of the catalytic cycle sufficiently. Unexpected mechanistic evidence indicated that this transformation was in sharp contrast with the previously reported hydroarylation. The reaction seemed to proceed through an unusual mechanism involving alkyne-directed dual C–H activation followed by carbopalladation with a C–C triple bond.
A palladium(0)-catalyzed C–H activation assisted by alkynyl coordination from alkynyl aryl ethers and internal alkynes for the synthesis of 2-methylidene-2H-chromenes was reported by Hiyama and co-workers (Scheme 399a).560 In this reaction, the alkynoxy group acted as a directing group to promote ortho C–H functionalization. The presence of oxygen and alkynyl moieties in substrates was essential for the success of the annulation. PCy3 was the most efficient ligand for the transformation catalyzed by the Pd(OAc)2/Zn system. Other phosphine ligands, such as PPh3 and PBu3, were less effective, whereas the presence of bulky silyl substituents on the alkynyl groups was also imperative. A wide variety of alkynes were compatible with the reaction conditions, and it was noteworthy that unsymmetrical alkynes afforded single regioisomer products. Deuterium-labeling experiments indicated that the arylpalladium hydride complex formed by oxidative addition was a key intermediate in the reaction.
By using the same strategy, Hiyama and co-workers demonstrated the palladium(0)-catalyzed cycloaddition reaction of alkynyl aryl ethers and allenes to form 2,3-bismethylidene-2,3-dihydro-4H-1-benzopyrans in good yields (Scheme 399b).561a It seemed that an arylpalladium hydride complex intermediate was involved in the reaction. Insertion of the allene into the Pd–C bond followed by intramolecular migratory insertion to the alkynyl moiety offered the final product. Recently, this group made a further improvement of this kind of reaction by the use of isocyanates as the coupling partner (Scheme 399c).561b The palladium-catalyzed reaction of alkynyl aryl ethers with isocyanates via C–H activation gave methylidene-2H-1,4-benzoxazin-3(4H)-one derivatives in moderate to good yields.
Hiyama and co-workers also extended their alkynyl-assisted C–H activation strategy for the synthesis of functionalized benzofurans through intramolecular sp3-C–H activation (Scheme 400).562 The reaction of silyl-substituted ortho-tolylalkynyl ethers in the presence of zinc metal gave the Z-exocyclic alkylidene product in high yield and the product could be readily transformed into benzofuran upon treatment with acetic acid. The alkynoxy moiety containing bulky substituents could act as a directing group and play a key role in the present transformation. It was noteworthy that Zn(OAc)2, generated in situ from the reaction of Pd(OAc)2 with Zn, could effectively promote the cyclization process. The control experiment indicated that in situ generated Zn(OAc)2 acted as a Lewis acid and an acetate ion donor to facilitate the cleavage of benzylic C–H bonds.
A palladium-catalyzed C–H activation and annulation reaction from diarylacetylenes for the synthesis of dibenzo[a,e]pentalenes was demonstrated by Segawa and co-workers (Scheme 401).563 A mechanistic study indicated that the new annulation occurred through alkyne-directed, ortho-selectively electrophilic aromatic C–H palladation. Both symmetric diarylacetylenes and unsymmetric arylacetylenes were applicable to this reaction and afforded the corresponding products.
11.3. The C–H functionalization directed by arene π-bond coordination
Besides alkene and alkyne groups, the heterocycles π bond can also be used as a directing group. Miura and co-workers demonstrated a rhodium(III)-catalyzed C–H activation reaction by using the thiophene π bond as a directing group.564 This strategy could be used to synthesize substituted naphthothiophenes through annulation of 3-phenylthiophenes with alkynes (Scheme 402a). Alkenylation of 3-phenylthiophene could also occur through the same procedure (Scheme 402b).12. The C–H functionalization assisted by a bidentate directing group
Despite the great advances in the field of catalytic directed C–H bond functionalization, a large number of transformations remain to be discovered and some inherent limitations still exist. To extend the concept of directed C–H bond functionalization beyond its limits, the development of new types of directing groups is the crucial point for achieving the catalytic transformations that cannot be completed with existing directing groups. Besides the monodentate directing group, the bidentate directing groups are found to promote the activation of C–H bonds via the formation of stable metallacycle and show a rigid coordination to transition metals. The stoichiometric amount of metallacycle of the bidentate directing groups has been well studied in a previous paper.56512.1. Arylation of C–H bonds
In 2005, Daugulis and co-workers first demonstrated the palladium-catalyzed C–H functionalization of C(sp3)–H and C(sp2)–H bonds by utilizing the bidentate directing group (Scheme 403).566 They showed that an 8-aminoquinolyl directing group could promote the palladium-catalyzed arylation of C(sp3)–H and C(sp2)–H bonds, thus offering an elegant method for the functionalization of various aliphatic and aromatic acids or amides. Significantly, the selective activation of C(sp3)–H bonds, which was very difficult to achieve, could be performed smoothly by the use of the bidentate system. It was believed that the double coordination of the metal by those auxiliaries would facilitate the reaction by stabilizing Pd(IV) species, which was suspected to be involved as the key step in the mechanism.567 As shown in Scheme 404, the coordination of amide to a Pd(II) species followed by a ligand exchange process generated palladium complex A, which gave rise to the intermediate B by a cyclometalation process via a concerted metalation–deprotonation mechanism.1n,568 The oxidative addition of intermediate B with an aryl iodide followed by reductive elimination generated the intermediate D, which produced the desired product E upon protonation and regenerated the Pd(II) intermediate A by ligand exchange. This methodology opened up a new avenue of research in the C–H functionalization.After this pioneering example, Corey and co-workers successfully employed this strategy for the synthesis of a broad range of unnatural (S)-α-amino acids (Scheme 405).569 The diastereoselective arylation of β-C(sp3)–H in α-amino acid derivatives was smoothly achieved. When the β-hydrogen was attached to a tertiary carbon, the rate of C–H activation drastically decreased and the arylation of the γ-methyl C–H bond occurred due to the bulky nature of the substrate. For instance, N-phthaloylated phenylalanine amide performed in this reaction with p-iodoanisole by producing β-C–H arylation product in a yield of 91%, while a fascinating γ-CH3 arylation product was isolated in 87% yield by employing the isoleucine derivative in the reaction with p-iodoanisole under the same conditions.
The scope of this reaction to a range of N-protected phthaloyl nonnatural α-amino acids and aryl partners was extended by Tran and Daugulis (Scheme 406).570 They found that a high selectivity of monoarylation could be achieved when 2-thiomethylaniline was employed as a directing group. Meanwhile, the use of an 8-aminoquinolyl directing group allowed for the diarylation of methyl groups and diastereoselective monoarylation of methylene groups of the amino acids. The acetoxylation and alkylation of C–H bonds in amino acid derivatives could also be furnished with the assistance of an 8-aminoquinolyl directing group. N-(2-Pyridyl)sulfamide as a new directing group in the palladium-catalyzed arylation of amino acid derivatives was studied by Carretero and co-workers (Scheme 407).161,571 A variety of N-(2-pyridyl)sulfonamide amino acid derivatives, including α-quaternary amino acids and β-amino acids, reacted with iodoarenes in the presence of Pd(OAc)2 to provide γ-arylated products in good yields. An unprecedented remote C(sp3)–H arylation of dipeptides occurred, illustrating the compatibility of the method with the peptidic bonds. Notably, the auxiliary could be easily removed by treatment with zinc/NH4Cl to give amino acid derivatives. The key role of a bimetallic γ-metalated five-membered ring palladacycle complex of the (2-pyridyl)sulfonyl unit was demonstrated.
Ma and co-workers developed an effective protocol for palladium-catalyzed direct γ-arylation of substituted 2-aminobutanates by employing 2-methoxyiminoacetyl (MIA) as the directing group (Scheme 408).572 In comparison with Carretero's work, this reaction resulted in a better selectivity and higher yield.
Palladium-catalyzed mono-arylation of inactivated β-C(sp3)–H bonds was developed by Chen and co-workers via the palladium-catalyzed N-quinolylcarboxamide-directed coupling of phthaloyl alanine with aryl iodides under very mild reaction conditions (Scheme 409).573 A broad range of aryl iodides, including unprotected phenol and indole derivatives, could be selectively installed into the β-methyl group to produce various natural and unnatural aromatic α-amino acid compounds. From an operational perspective, these mono-arylation reactions were compatible with broad functional groups, insensitive to water and air, and could be operated on a gram scale. The further C–H functionalization in a diastereoselective of these monoarylated compounds could be performed smoothly, thereby providing various β-disubstituted aromatic α-amino acids which were difficult to synthesize by other ways.
The stereospecific installation of (hetero)aryl and vinyl substituents at the inactivated 3-position of N-protected prolines via Pd-catalyzed C–H functionalization was independently developed by Bull574 and Zhang575 (Scheme 410). These methods enabled the efficient preparation of small yet complex enantiopure cis-2,3-disubstituted pyrrolidines. It was noteworthy that in Bull's report the products could be isolated as a single stereoisomer (>98% ee). The reaction was operative under an air atmosphere and was also successful on a larger scale. This 3-arylation reaction occurred in high yields with aminoquinolyl and methoxyaminoquinolyl directing groups which could be readily removed to give primary amide derivatives. The subsequent removal of the N-protected groups via hydrogenolysis afforded the 2-amido-3-aryl pyrrolidines as the cis-isomers.
Based on the previous study involving Pd-catalyzed C–H bond arylation of amino acid derivatives, Chen and coworkers synthesized the celogentin C by an efficient and stereoselective palladium-catalyzed indolylation of the β-C–H bond in N-Phth Leu (Scheme 411).576 They proposed that the quinoline moiety served as a chelating auxiliary for palladium coordination, and promoted the formation of trans-palladacycle intermediate. The palladium(II) intermediate presumably underwent cross-coupling with an aryl iodide partner to provide the final arylated product, which had an erythro stereochemical preference. Based on this, they successfully achieved the high-yielding and stereoselective 6-indolylation of N-phthaloyl leucine. In light of the model reaction, the total synthesis of the celogentin C in a total of 23 steps from simple amino acid building blocks was completed. This was the first example of utilizing the bidentate chelation strategy for the total synthesis of a natural product. Later, Baran and co-workers reported the example of sequential Pd-catalyzed C–H arylation in an inactivated cyclobutane ring with two different aryl iodides to complete the total synthesis of pipercyclobutanamide B (Scheme 412a).577 They recently described a concise total synthesis of pipercyclobutanamide A based on the bidentate directing system of sequential palladium-catalyzed arylation/vinylation of the C(sp3)–H bonds with aryl iodide and styrenyl iodide (Scheme 412b).578 These examples demonstrated the power of guided C–H functionalization to enable the fundamentally new approach to natural product synthesis.
The strategy of bidentate auxiliary-directed C–H functionalization has been applied in arylation of aromatic and aliphatic amines.579 In 2011, Chen's group developed a practical synthetic strategy based on the picolinamide-directed palladium-catalyzed arylation and alkenylation of the remote C(sp3)–H bonds in a variety of aliphatic amines (Scheme 413).580 A broad range of aryl iodides were used successfully in this transformation. O-TIPS, NO2, tosylamide, and CO2Me groups were tolerated well under the reaction conditions, and the use of iodophenol gave the desired product in moderate yield. More importantly, various disubstituted cyclic vinyl iodides could participate in the reaction to afford the alkenylated products in moderate to good yields, which could undergo further transformations. Interestingly, the installation of a methylene hydroxy group in the ortho-position of the picolinamide (PA) auxiliary made this directing group much easier to remove under mild conditions. This modified PA directing group was exploited in the formal synthesis of (+)-obafluorin.
A palladium-catalyzed benzylic C–H arylation/oxidation of aniline substrates by the utilization of picolinamide as the directing group was reported by Zhang and co-workers (Scheme 414).581 This reaction could take place under argon or air, but a dioxygen atmosphere was superior. Screening of the additives indicated that AgOAc gave the best yield. Other palladium catalysts such as PdCl2, Pd(CH3CN)2Cl2, and Pd(PPh3)2Cl2 also worked in this transformation but delivered lower yields. Control experiments confirmed that the palladium catalyst and silver additive were necessary in this process. The transformation tolerated a broad range of substrates, and aryl iodides as well as arenes bearing both electron-withdrawing and electron-donating substituents could well participate in the transformation to give the corresponding diarylketones in moderate to good yields. The control experiments indicated that a Pd(II)/Pd(0) process might be involved in the reaction.
For the arylation of C–H bonds, different aryl resources other than aryl iodides have been explored.582 Shi and co-workers reported a successful example of Pd-catalyzed primary and secondary C(sp3)–H arylation by using diarylhyperiodonium salts as the arylation reagents (Scheme 415).583 Various acid derivatives and different diarylhyperiodonium reagents were compatible with this reaction. They hypothesized that the trifluoromethanesulfonate anion might participate in the rate-limiting proton-abstraction step. To evaluate the reactivity of the corresponding ArI from Ar2IOTf, the PhI was used as the substrates and the reaction was sluggish under standard reaction conditions. This method could be applied to the direct arylation of naturally important structural units such as the derivatives of amino acids and oleic acid.
The Ru(II)-catalyzed ortho-arylation of aromatic amides with a bidentate directing group was reported by Chatani and co-workers (Scheme 416).584 The presence of a bidentate directing group, such as 2-pyridynylmethylamino or 8-aminoquinoline, was essential for the arylation to proceed smoothly. A variety of aryl bromides as well as heteroaromatic bromides were applicable to the arylation reaction. The use of phenyl chloride as a coupling reagent gave the corresponding product in low yield. This reaction proceeded in a highly selective manner at the less hindered C–H bonds of meta-substituted aromatic amides, indicating that the regioselectivity was controlled by the steric nature of substrates. Although the reaction mechanism for Ru(II)-catalyzed arylation was not elucidated, the electronic effects of substituents on the efficiency of the reactions were examined in detail.
A copper-mediated C–H/C–H coupling of benzoic acid derivatives and 1,3-azoles with the assistance of bidentate directing groups was developed by Miura and co-workers (Scheme 417).585 After extensive screening, the 8-aminoquinolinyl group showed excellent directing properties among the bidentate directing groups to allow the C–H/C–H coupling efficiently. A better result was achieved when anhydrous Cu(OAc)2 was used. In addition, the relatively acidic NH was also believed to play a pivotal role during the transformation. Through the mechanism investigation, the C–H bond cleavage of benzoxazole was found to occur reversibly, while the benzamide was an irreversible process under the reaction conditions. The authors proposed that stoichiometric Cu(OAc)2 was necessary to provide the azolylcopper intermediate, followed by the generation of the Cu(III) complexes before reductive elimination. A variety of 1,3-azoles were applicable to this direct C–H/C–H coupling, and both electron-rich and electron-deficient 5-aryloxazoles benzoxazoles underwent the arylation smoothly.
The iron-catalyzed β-arylation of 2,2-disubstituted propionamide with an organozinc reagent in the presence of an organic oxidant and a bisphosphine ligand was disclosed by Nakamura's group (Scheme 418).586 The 8-aminoquinolinyl (NH-Q) directing group and 1,2-bis(diphenylphosphino)benzene (dppbz) ligand were found to be essential for the transformation. The success of the reaction was mostly attributed to the careful design of the ligand. For example, the bidentate ligand 1,2-bis(diphenylphosphino)-ethane (dppe), which was similar to dppbz except for its slightly larger bite angle and a more flexible backbone, gave the product in 9% yield. The reaction was also sensitive to the structure of the substrate. For instance, cyclohexanecarboxamide and cyclopentanecarboxamide participated in the reaction smoothly to give the corresponding products, whereas the corresponding cyclobutane- and cyclopropanecarboxamides failed to give the desired products. They proposed that the CH3–C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bond angle might be crucial for efficient C–H activation, and a wider or smaller angle resulted in the formation of a chelation intermediate less stable. The overwhelmingly higher reactivity of a methyl group over a benzylic group excluded a radical mechanism that was previously observed in iron catalysis,587 and the high sensitivity of the yield of the structure of the substrates and the ligands suggested the involvement of organoiron intermediate in the crucial step. Kinetic isotope effect (KIE) experiments with a primary KIE of 2.4 indicated that the cleavage of the C–H bond might be the rate-determining step of the reaction.
O) bond angle might be crucial for efficient C–H activation, and a wider or smaller angle resulted in the formation of a chelation intermediate less stable. The overwhelmingly higher reactivity of a methyl group over a benzylic group excluded a radical mechanism that was previously observed in iron catalysis,587 and the high sensitivity of the yield of the structure of the substrates and the ligands suggested the involvement of organoiron intermediate in the crucial step. Kinetic isotope effect (KIE) experiments with a primary KIE of 2.4 indicated that the cleavage of the C–H bond might be the rate-determining step of the reaction.
A novel triazolyldimethylmethyl (TAM) amide-based bidentate auxiliary was developed in the iron-catalyzed arylation of inactivated C(sp3)–H bonds by Ackermann and co-workers (Scheme 419).588 Detailed optimization studies revealed that this C–H functionalization was viable with a catalyst system consisting of FeCl3 as the metal source and dppe as the ligand. The triazole auxiliary proved to be superior to the hitherto employed bidentate directing groups, such as the widely used 8-aminoquinolyl auxiliary, under the reaction conditions. The iron catalytic system could also be applied in the direct β-arylation of α,β-unsaturated amide derivatives.
The Ni-catalyzed β-arylation of aliphatic amides with aryl iodides was achieved by Chatani and coworkers (Scheme 420).589 The base and the additive of benzoic acid were crucial for the efficiency of the reaction, and the choice of Na2CO3 and MesCOOH gave the best yield. Various nickel complexes, including Ni(II) and Ni(0) catalysts, showed a high catalytic activity for this transformation. To gain insights into the mechanism for the reaction, deuterium-labeling experiments were carried out, which revealed that the C–H bond cleavage step was fast and reversible. The detailed mechanistic investigation and the control experiments illustrated that the reaction proceeded through a Ni(II)/Ni(IV) catalytic cycle. Shortly after this, an analogous chemistry with a broad scope of aryl halides as substrates was demonstrated by You and co-workers (Scheme 421).590
12.2. Alkenylation of C–H bonds
Due to the easy β-hydrogen elimination, the olefination of inactivated aliphatic alkenes remains a big challenge in palladium-catalyzed coupling reactions.315,591 Recently, the palladium-catalyzed ortho C–H bond olefination of phenylacetic acid derivatives using inactivated aliphatic alkenes with high regio- and stereoselectivities was reported by Maiti and co-workers (Scheme 422).592 Compared with the previous Pd-catalyzed C–H olefination, this significant breakthrough of the restricted substrate scope, in particular non-compliance of inactivated aliphatic olefins, was owing to the bidentate directing strategy. A racemic 1,1′-binaphthyl-2,2′-diamine (rac-BINAM) ligand was supposed to enhance the catalytic activity of active Pd(II) species. The versatility of this operationally simple method was illustrated through the sequential C–H olefination in synthesizing divinylbenzene derivatives.By the use of organoborate reagent as the olefinic coupling partner, Nakamura and co-workers demonstrated an efficient protocol for the C–H alkenylation of a quinolinyl amide in the presence of iron and zinc catalysts (Scheme 423).593 They found that a stoichiometric organoiron(III) in the absence of DCIB was quite effective to give the product in nearly quantitative yield, indicating that the phenyliron(III) intermediate played the key role in the alkenylation. The zinc salt was crucial to promote the transfer of the organic group from the boron atom to iron(III) catalyst. This reaction allowed the coupling of a variety of aromatic, heteroaromatic and olefinic substrates with alkenyl and aryl boron compounds under mild oxidative conditions.
12.3. Alkylation of C–H bonds
Compared with the C–H bond arylation and alkenylation reactions, the direct alkylation of C–H bonds with inactivated alkyl halides remains in an early stage of development. During the past few years, Chen's group reported a series of Pd-catalyzed alkylation reactions of C(sp2)–H and C(sp3)–H bonds directed by the PA group [PA = picolinamide].594 In 2013, Chen and co-workers reported the Pd-catalyzed alkylation of inactivated methylene C(sp3)–H bonds of aminoquinolyl aliphatic carboxamides with α-haloacetate and methyl iodide (Scheme 424).595 This reaction was highly efficient with the tolerance of a wide range of functional groups. The reaction enabled a streamlined strategy for the synthesis of various natural and unnatural amino acids, particularly β-alkylated α-amino acids, in a diastereoselective manner. With simple isotope-enriched reagents, they also provided a convenient and powerful solution to site-selectively incorporate isotopes into the carbon scaffolds of amino acid substrates. They postulated that the C–H alkylation reaction proceeded through a C–H palladation/coupling sequence and that a Pd(II)/(IV) manifold was operative. Notably, (BnO)2PO2H was the most effective additive among the carboxylic acid and organic phosphate tested. It was proposed that (BnO)2PO2H could form a soluble complex with Ag2CO3 to improve the concentration of Ag+ in the reaction medium, and might also serve as a ligand for palladium during the oxidative addition and reductive elimination steps. They also suspected that (BnO)2PO2H could facilitate the protonolysis of the Pd-complexed alkylated intermediate to promote the release of the target product, thus accelerating the turnover of Pd catalyst.A sulfonamide-promoted palladium(II)-catalyzed alkylation of inactivated β-methylene C(sp3)–H bonds of α-amino acid substrates with alkyl iodides was reported by Shi and co-workers (Scheme 425).596 The reaction tolerated a diverse array of functional groups and proceeded with high diastereoselectivity. Alkyl iodides bearing β-hydrogen atoms could be used as substrates under the optimized reaction conditions. This transformation provided a versatile strategy for the stereoselective synthesis of unnatural β-disubstituted α-amino acids. Interestingly, a series of stoichiometric and catalytic experiments indicated that the presence of 4-Cl-C6H4SO2NH2 and NaOCN was crucial for the reaction to proceed efficiently.
A new Ni-catalyzed 8-aminoquinoline directed ortho C–H bond alkylation of benzamides and acrylamide derivatives with inactivated primary alkyl halides was developed by Chatani and co-workers (Scheme 426).597 This reaction represented the first example of Ni-catalyzed ortho alkylation of amide derivatives with high functional group compatibility and proceeded in a highly selective manner at the less hindered C–H bond when the meta-substituted aromatic amides were employed. The deuterated experiment indicated that cleavage of the C–H bond appeared to be a reversible and rapid step, which was not the rate-determining step.
Compared with the primary alkyl halides, C–H functionalizations with secondary alkyl halides are considered to be more difficult because of their reluctance to undergo oxidative additions to transition metals and the subsequent β-hydride eliminations. In 2014, Ackermann developed a nickel-catalyzed C–H bond alkylation with challenging secondary alkyl bromides and chlorides. Catalytic nickel precursors [(DME)NiCl2] in combination with BDMAE [bis(2-dimethylaminoethyl)ether] and LiOtBu in toluene have been proved to be the optimal conditions (Scheme 427).598 In this synthesis, a robust nickel(II) catalyst enabled a C–H transformation with cyclic and acyclic alkyl bromides and chlorides with ample substrate scope. Notably, this versatile nickel(II) catalyst also formed the basis for unprecedented trifluoroethylations of inactivated C–H bonds.
A highly regioselective alkylation of 2,2-disubstituted propionamide with 8-aminoquinolinyl group as the amide moiety via a nickel-catalyzed C(sp3)–H bond functionalization process was demonstrated by Ge and co-workers (Scheme 428).599 It was also found that not only alkyl iodides but also alkyl bromides or chlorides with the addition of CsI could participate in the reaction to generate the corresponding products. However, both secondary alkyl halides and benzyl bromide failed to give the corresponding products, presumably due to the steric effect or the instability of the intermediate. Moreover, this reaction showed a predominant preference for C(sp3)–H bonds of methyl groups via a five-membered ring intermediate over the C(sp2)–H bonds of arenes in the cyclometalation step. The deuterium-labeling experiment indicated that the cyclometalation of amide with a nickel species was the rate-determining step. The addition of TEMPO resulted in the decreased yield, and 2,2,6,6-tetramethyl-1-(pentyloxy)piperidine was isolated as the major product, which was consistent with the radical involving the Ni(II)/Ni(III) catalytic cycle.
Besides the alkyl halides, various α,β-unsaturated carbonyl compounds could be employed in the alkylation reaction. For instance, Chatani and co-workers developed Ru(II) (Scheme 429) and Rh(I)-catalyzed (Scheme 430) alkylation of ortho C(sp2)–H bonds in aromatic amides with the assistance of bidentate directing groups.600 These reactions had a broad substrate scope, in which acrylic esters, acrylamide, fumarate, maleate, and phenyl vinyl sulfone could be tolerated. The transformation provided fast and economical access to the functionalizable molecular backbones, which could be achieved by the potential transformation of the amide moiety to various functional groups.
Following the pioneering work by Nakamura and co-workers, Cook's group disclosed a robust iron-catalyzed ortho alkylation of aryl amides under mild reaction conditions (Scheme 431).601 In particular, this reaction did not require a co-oxidant and could be accomplished in less than 10 min, proceeding in high yields with exceptional regioselectivity. Although several Grignard reagents were viable in the reaction, phenylmagnesium bromide offered the highest conversion rate to the product. In addition, the rate of Grignard reagent addition was critical to the success of the reaction (i.e., dropwise additions over less than nine minutes compromised the yield of the reaction). In contrast to Pd-, Co-, and Ni-catalyzed C–H alkylations, the reaction provided exclusively the monosubstituted products without any detectable dialkylation product. Moreover, the presence of a meta substituent provided very high levels of regioselectivity, favoring the less-hindered position. The small intermolecular KIE (kH/kD = 1.5) and the large intramolecular KIE (kH/kD = 2.9) observed in the control experiment implied that substrate coordination was irreversible and occurred prior to C–H cleavage.
12.4. Alkynylation of C–H bonds
Alkynes are powerful building blocks in chemical synthesis due to the diverse reactivity of carbon–carbon triple bonds toward addition, cycloaddition, and metathesis reactions.602 Encouraged by the success of C–H bond functionalizations in recent years, chemists have paid much attention to the direct alkynylation of C–H bonds.603 Chatani and co-workers accomplished the palladium catalyzed alkynylation of inactivated C(sp3)–H bonds (Scheme 432).604 These reactions allowed for the straightforward introduction of an ethynyl group into aliphatic acid derivatives. The reaction tolerated a variety of functional groups and could be applied to the natural-product-based substrates, thus offering a new method for preparing complex alkyne components in a more straightforward manner. Furthermore, this Pd-catalyzed strategy for alkynylation of C–H bonds could be extended to the transformation of benzoic acid derivatives (Scheme 433).605 Alternatively, Yu and co-workers recently developed Cu(II)-promoted ortho alkynylation of arenes and heteroarenes with terminal alkynes to prepare aryl alkynes (Scheme 434).606 This reaction tolerated a variety of arenes and terminal alkynes bearing different substituents. More importantly, aliphatic alkynes, including n-pentyl-, tert-butyl-, cyclopropyl-, and cyclohexyl-substituted acetylenes, were all compatible with the reaction conditions, thus providing an alternative synthetic disconnection to Sonogashira coupling. Moreover, the resulting alkynylated benzoic acids could be converted to various useful heterocycles and valuable synthons.12.5. Annulation of C–H bonds
Bidentate auxiliary assisted transition-metal-catalyzed C–H bond functionalization has also emerged as a powerful tool for the synthesis of heterocycles.607 In 2009, Chatani and co-workers608a,b demonstrated the Ru-catalyzed carbonylation of aromatic, aliphatic amides to synthesize phthalimides and pyrrolidine-2,5-dione derivatives with tolerance of a variety of functional groups (Schemes 435 and 436). In these processes, the choice of ethylene and H2O as additives was crucial to result in an effective catalytic system. Notably, for the carbonylation of inactivated C(sp3)–H bonds, the reaction proceeded selectively at a methyl C–H bond over a methylene C–H bond.The Ni-catalyzed oxidative annulation of aromatic amides with alkynes to produce isoquinolones has also been disclosed (Scheme 437).608c This transformation represented the first example of Ni-catalyzed transformations of ortho C–H bonds by utilizing chelation assistance. Various functional groups in the aromatic ring of amides were tolerated under the reaction conditions, and substrates with an electron-donating group were less reactive than those with an electron-withdrawing group. Moreover, a highly regioselective result was achieved when the unsymmetrical alkynes were used as substrates.
A cobalt catalyzed coupling of alkynes with C(sp2)–H bonds was demonstrated by Daugulis and co-workers (Scheme 438).609 Both terminal and internal alkynes were compatible in the reaction. The reaction proceeded more efficiently in trifluoroethanol, presumably due to higher solubility of the cobalt catalyst. Furthermore, this reaction demonstrated the first use of cobalt catalysis by employing bidentate, monoanionic auxiliaries. Subsequently, with the modified catalytic system, they accomplished the cobalt-catalyzed aminoquinoline-directed ortho-functionalization of C(sp2)–H bonds with alkenes and carbon monoxide to synthesize six-membered and five-membered nitrogen-containing heterocycles.610
12.6. C–X bond formation
In addition to the Pd-catalyzed arylation, alkenylation and alkylation using removable bidentate directing groups, the challenging carbon–heteroatom bond formation through C–H bond activation was also developed. Daugulis and co-workers reported a copper mediated, auxiliary-assisted direct sulfenylation of β-sp2 C–H bonds of benzoic acid derivatives and γ-C(sp2)–H bonds of benzylamine derivatives (Scheme 439).611 The reaction showed high generality, excellent selectivity toward ortho C–H bonds, as well as good functional group tolerance. This method provided a novel and straightforward way to form C–S bonds, thereby accomplishing the preparation of aryl trifluoromethyl thioethers.The C–N bond construction612 by palladium-catalyzed C–H bond intramolecular amination reactions of inactivated C(sp3)–H and C(sp2)–H bonds of amine substrates to form azetidines, pyrrolidines, and indulines has been studied (Scheme 440).613 For instance, Chen and co-workers developed a novel protocol for the synthesis of pyrrolidones and indolinones by the palladium-catalyzed intramolecular amination of inactivated C(sp3)–H and C(sp2)–H bonds at the γ position of secondary-amide precursors (Scheme 441).614 These lactamization reactions were efficient and versatile, and required only inexpensive reagents. Notably, combining these reactions with other palladium-catalyzed C(sp3)–H functionalization reactions in a sequential manner could provide the alternative to diverse pyrrolidinone products from readily accessible carboxylic acid.
A palladium(II)-catalyzed sequential C(sp3)–H monoarylation/amidation for the stereoselective synthesis of α-amino-β-lactams from simple aniline derivatives was developed by Shi and co-workers (Scheme 442).615 The N,N-bidentate PIP amide directing group was found to be effective in both controlling the selectivity in the arylation step for monoarylation and enhancing the reactivity in the amination step. This procedure tolerated a wide range of functional groups, and the desired products were obtained in moderate to high yields. This procedure showed the power of sequential C–H functionalization to streamline the synthesis of complex, enantiomerically pure heterocycles from readily available starting materials.
Copper- and nickel-catalyzed intramolecular dehydrogenative amidation of aliphatic amides was reported by Kanai and Ge, respectively (Scheme 443).616 The amination reactions occurred at a terminal methyl group, as well as at the internal benzylic position of an alkyl chain. More importantly, β-lactams could be obtained in high yields even on a gram scale.
A palladium-catalyzed intramolecular amination of C(sp2)–H and C(sp3)–H bonds assisted by an accessible N,O-bidentate auxiliary was developed by Yao and Zhao (Scheme 444).617 The oxalyl amide derivative was used as a newly bidentate directing group for the first time. Through this strategy, both C(sp2)–H and C(sp3)–H bonds at δ- and ε-positions were effectively activated. Some pharmaceutically-important nitrogen-containing heterocycles, such as tetrahydroquinolines (THQs), benzomorpholines (BMPs), pyrrolidines, and indulines, could be smoothly synthesized. In addition, the developed directing group could be easily introduced and removed under mild reaction conditions.
The intermolecular C(sp2)–H bond amination catalyzed or promoted by copper was reported by Daugulis and Yu, respectively (Scheme 445).618 The exceptional compatibility of this amination with multiple heteroatoms present in both reactants rendered this reaction highly valuable for the synthesis of medicinally important compounds, such as 2-benzamidobenzoic acids and N-phenylaminobenzoates, by using readily available benzoic acids (Scheme 445b). Remarkably, a variety of sulfonamides, amides, and anilines could be tolerated in this reaction to give the desired products.
Chen's group reported the efficient synthesis of alkyl ethers by the functionalization of inactivated sp3- and sp2-hybridized C–H bonds. Picolinamide-protected amine substrates underwent facile alkoxylation at the γ or δ positions with a range of alcohols, including t-BuOH, to give alkoxylated products under the Pd(OAc)2-catalyzed, PhI(OAc)2-mediated reaction conditions. This reaction featured a broad substrate scope, inexpensive reagents, and convenient operating conditions and highlighted the emerging value of inactivated C–H bonds, particularly the C(sp3)–H bond of methyl groups (Scheme 446).619 In addition to the inactivated methyl C(sp3)–H bonds,620 the palladium-catalyzed alkoxylation of inactivated methylene positions was achieved using hypervalent iodine as the oxidants by Rao and Shi, respectively (Scheme 447).621 These reactions were complementary to previous methods for the synthesis of dialkyl ethers.
Subsequently, Rao and co-workers developed a Pd(II) catalyzed double inactivated C(sp3)–H alkoxylation to prepare symmetric or unsymmetric acetals with the addition of Ag2CO3 as the co-oxidant (Scheme 448).622 Sahoo and co-workers demonstrated the unprecedented halogenation and acetoxylation sequence on two β-CH3 moieties by using S-methyl-S-2-pyridyl-sulfoximine as a directing group, providing access to highly functionalized quaternary carbon centers bearing carboxylic acids (Scheme 449).623
Very recently, Shi and co-workers demonstrated an effective copper-mediated hydroxylation of arenes and heteroarenes directed by their newly developed PIP directing group (Scheme 450).624 This method was scalable and compatible with a wide range of functional groups and heteroarenes, providing an operationally simple protocol for the synthesis of o-hydroxybenzamides. This new directing group was applied in the synthesis of benzoisothiazolones via Cu(II)-mediated C–S/N–S bond formation. The direct diversifications of the benzoisothiazolone products into a variety of sulfur-containing compounds were also investigated.625
The direct C–H bond functionalization for the synthesis of organosilanes has become more and more attractive during the development of new methods for efficient strategic installation of silyl groups into organic compounds. Several transition metals such as Sc, Ru, Rh, Ir, and Pt catalyzed silylation of aromatic, vinylic, allylic, and aliphatic C–H bonds have made innovative contributions to this field.235,626 Kanai's group demonstrated a palladium-catalyzed regioselective silylation of C(sp2)–H and C(sp3)–H bonds of benzamides and carboxamides with the aid of 8-aminoquinoline as a directing group (Scheme 451).627 Silver salts were superior to other oxidants, and calcium sulfate was the best choice of additive. Moreover, this study gave the first example of transition-metal-catalyzed germanylation of C(sp2)–H bonds and intermolecular C(sp3)–H silylation at the internal positions of alkyl chains.
Intrigued by the previous reports,29,65,166,325,628 Yu and co-workers established a method for the copper(II)-mediated ortho-trifluoromethylation of arenes, including heteroarenes, with TMSCF3. This copper(II)-promoted C–H activation reaction has taken advantage of the oxazoline-based bidentate auxiliary to complete the transformation within 30 minutes. Significant isotope effects observed in both intra- and intermolecular kinetic isotope experiments indicated that a copper-mediated C–H cleavage step was involved in the rate-determining step (Scheme 452).585a,629 Initially, copper(II) complex coordinated to the substrate, followed by the C–H activation and disproportionation of intermediate A to yield a chelated organocopper(III) complex B. Subsequent transmetallation of the organocopper(III) species with TMSCF3 afforded Cu(III)–CF3 intermediate C, and then reductive elimination would deliver the desired ortho-trifluoromethylated arene.
13. The remote meta-C–H bond activation
As shown above, functionalization of ortho arene C–H bonds could be achieved by chelation-directed metalation with different directing groups via five or six membered cyclometalation (Scheme 453). However, the remote control of site selectivity, especially meta-selective functionalization of electron-rich aromatic rings, is still one of the greatest challenges in this active research field. A breakthrough in this area was achieved by Yu and co-workers in 2012.630 They designed a class of easily removable nitrile-containing templates that direct the activation of distal meta-C–H bonds (more than ten bonds away) of a tethered arene through the formation of a macropalladacycle. The template was designed based on the weak interaction between Pd(II) and a nitrile group, and the nitrile group coordinated in an ‘end-on’ coordination geometry which relieved the strain of a macrocyclic cyclophane-like pre-transition state of the meta-C–H activation event.In this pioneering report, Yu and co-workers demonstrated the new mode of C–H activation that nitrile-containing templates deliver palladium to the vicinity of tethered arene substrates to promote C–H olefination of distal meta-C–H bonds.630 This reaction proceeded with excellent selectivity and high functional group compatibility. In addition, this template overrode the intrinsic electronic and steric biases as well as ortho-directing effects with two broadly useful classes of arene substrates (toluene derivatives and hydrocinnamic acids) (Scheme 454a and b). Unlike in conventional directed ortho-C–H olefination reactions where the coordinated directing group could prevent sterically hindered olefins from binding, in this case the nitrile group on the template was weakly coordinated to the [Pd(II)–Ar] intermediate and could be effectively displaced by disubstituted olefins, allowing carbopalladation to proceed. Finally, the template could be readily removed to afford meta-alkylated toluenes or hydrocinnamic acids in excellent yields.
Later on, they developed meta-C–H arylation and methylation of 3-phenylpropanoic acid and phenolic derivatives using organoborons as coupling partners (Scheme 454c).631 The combination of a weakly coordinating U-shaped template and a mono-protected amino acid ligand was crucial for the cross-coupling of C–H bonds with organoborons. Tetrabutylammonium (TBA) salts showed a dramatic influence on the catalytic performance of palladium in cross coupling reactions for the ability of surfactants to prevent undesired agglomeration of Pd(0) species to form unreactive palladium black. Furthermore, the anionic counterion played an important role in stabilizing cationic palladium intermediates.
Recently, they investigated the meta-C–H olefination of electron-rich phenol derivatives (Scheme 455).368 The end-on coordinating nitrile template was easily introduced to α-phenoxyacetic acids which was derived from the phenol. This palladium catalyzed olefination proceeded with high meta-selectivity in the presence of the Boc-Val-OH ligand. The reaction was unprecedented and orthogonal to previous electrophilic substitution of phenols in terms of regioselectivity. This protocol was successfully applied to the parent α-phenoxycarboxylic acids of drug molecules fenofibrate, clofibrate, and etofibrate. To gain insights into the mechanism for the reaction, an intermolecular isotope kinetic experiment was carried out and a significant kinetic isotope effect (kH/kD = 3.8) was observed, indicating that the C–H cleavage might be involved in the rate-determining step. A tentative catalytic cycle could also be proposed (Scheme 456).
Based on Yu's nitrile-based directing groups, Tan and co-workers reported meta-selective C–H olefination using a silicon-tethered nitrile-based directing group (Scheme 457).632 The directing group with a silicon atom attachment could be synthesized in three steps from inexpensive starting materials and easily removed by tetrabutylammonium fluoride at room temperature, allowing for a facile installation/deprotection strategy increasing the synthetic practicality of this template. To investigate the substrate scope, they found that the bis-substituted products were produced simultaneously by the use of 2-substituted substrates. The alkenylation of 3-substituted substrates clearly showed that the method was applicable to a wide variety of functional groups. meta Selectivity decreased slightly with 4-substituted compounds due to steric hindrance. Various olefin partners revealed that electron-deficient olefins bearing amide, ketone, and sulfone groups produced functionalized compounds with moderate yields and high selectivity. Moreover, 1,2-disubstituted trans-methyl crotonate also proceeded well, affording a single stereoisomer as the major product. An additional advantage of this chemistry was the potential to reuse the silicon-directing group.
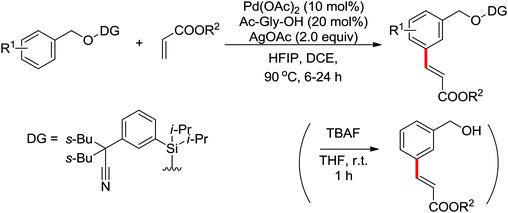 | ||
| Scheme 457 Remote meta-C–H olefination of using a nitrile-based directing group and cleavable Si-tether. | ||
Very recently, Yu and co-workers reported the first olefination and acetoxylation of remote meta-C–H bonds as far as 11 bonds away of tetrahydroquinolines, anilines and benzylamines by using a new recyclable template (Scheme 458a).633 The template could be readily installed through acylation of the amine substrates with the commercially available 2-(2-cyanophenoxy)-2-fluoroacetic acid. This template was able to direct the meta selective C–H functionalization of bicyclic heterocycles via a highly strained, tricyclic-cyclophane-like palladated intermediate. X-ray and nuclear magnetic resonance studies revealed that the conformational biases induced by a single fluorine substitution in the template could be enhanced by using a ligand to switch from ortho- to meta-selectivity (Scheme 458b). Directed by this versatile template, the remote meta-C–H bond activation of tetrahydroquinoline, benzoxazines, anilines, benzylamines, 2-phenylpyrrolidines and 2-phenylpiperidines proceeded smoothly under the Pd(OAc)2/Ac-Gly-OH/Ag salt system.
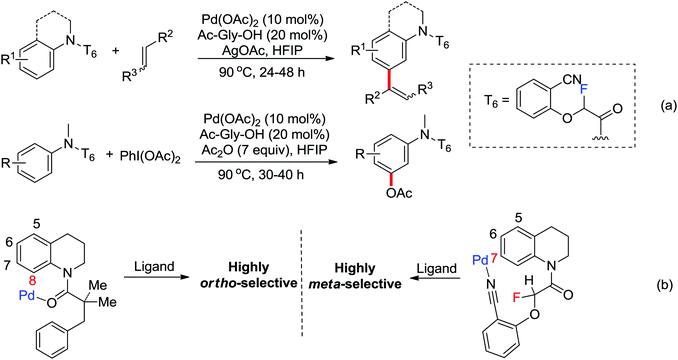 | ||
| Scheme 458 Remote meta-C–H olefination and acetoxylation of tetrahydroquinolines, anilines and benzylamines. | ||
However, the previously developed templates for amines did not give meta-selectivity for indoline substrates, presumably due to both conformational and electronic properties of indolines. To override this intrinsic site-selectivity, Yu and co-workers designed a novel nitrile-containing template based on two key principles: first, a more electron-withdrawing template would reduce the electron density at the ortho- and para-positions of the indoline substrate, and second, a maximization of the Thorpe–Ingold effect in the template backbone would enhance the directing power of the nitrile (Scheme 459a). This new template was attached to the indolinyl nitrogen via a removable sulfonamide linkage. The meta-C–H olefination, arylation, and acetoxylation of indolines were achieved by using this template (Scheme 459b).634 The combination of the nitrile template and a monoprotected amino acid ligand was crucial for the meta-selective C–H functionalization of electron-rich indulines that were shown to be highly reactive toward electrophilic palladation at the para-positions. This method provides a useful tool for the preparation of a wide range of meta-substituted indoline derivatives, including biologically important natural products and drug molecules.
Maiti and co-workers reported a meta-selective C–H bond olefination using a 2-hydroxybenzonitrile template attached with a phenylacetic acid derived scaffold (Scheme 460).635 Introduction of an electron-donating methoxy (OMe) substituent at the para position to the hydroxyl group in the template (in Scheme 460, R1 = OMe) enhanced the reactivity, and the selective desired monoalkenylated product was obtained in moderate to good yield, which was easily transferred to trans-esterification compounds in (CF3)2CHOH (1,1,1,3,3,3-hexafluoro-2-propanol, HFIP). This palladium-catalyzed protocol was applicable to a wide range of substituted phenylacetic acids and tolerated a variety of functional groups. The directing group (2-hydroxy-5-methoxybenzonitrile) could be easily installed and removed to form pharmaceutically relevant phenylacetic acid derivatives.
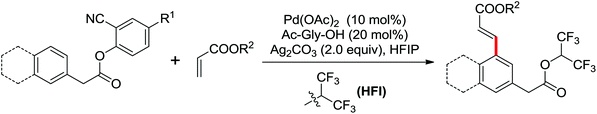 | ||
| Scheme 460 meta-Selective arene C–H bond olefination of arylacetic acid using a nitrile-based directing group. | ||
There is another type of meta C–H activation achieved via regular ortho C–H activation followed by functionalization at the para position to the M–C bond, and herein they result in a formal meta C–H activation. Recently, extensive and significant progress toward meta- or para-selective C–H functionalizations has aroused much interest of chemists.636
A copper-catalyzed meta-arylation reaction of anilide using Ph2IOTf as the arylating agent was developed by Gaunt and co-workers, which was highly different from the usual ortho-C–H bond functionalization (Scheme 461).637 The elegant C–H bond functionalization strategy provided a direct method to afford the meta isomer on highly versatile electron-rich aromatic structures. The nature of the acyl group of anilide had an obvious influence on the yield of the reaction but without changing the meta-selectivity. The protocol showed many notable features, such as simple operation, mild conditions, broad substrate scope and exclusive regioselectivity. Although the precise mechanism of the reaction was unclear, a highly electrophilic Cu(III)–aryl species was proposed to be involved in the reaction (Scheme 462). The Cu(III)–aryl species was regarded as a Lewis acid to activate the aromatic ring to undergo an anti-oxy-cupration process across the 2,3 position on the arene ring. The Cu(III)–aryl species was placed at the meta position via the dearomatizing process, followed by rearomatizing deprotonation and reductive elimination to deliver the desired product.
In 2011, Wu and co-workers638 described their theoretical study of the novel mechanism of the copper-catalyzed anilide meta C–H bond arylation reaction reported by Gaunt. The density functional theory (DFT) calculations and experimental investigations were carried out to elucidate the reaction mechanism. A Heck-like four-membered-ring transition state involving a Cu(III)–Ph intermediate was considered to be involved in the meta arylation reaction process.
A copper-catalyzed meta-selective arylation of the α-aryl carbonyl scaffold with diaryliodonium salts using a remote and versatile Weinreb amide group as the directing group was developed by Gaunt and co-workers (Scheme 463).639 The location of the carbonyl group was supposed to have a significant role in determining the site selectivity of the reaction. Moreover, the arene nucleophilicity of the electronically neutral α-aryl carbonyl motif was different from the electron-rich anilide. A range of simple alkyl substituted α-arylketones could work well in the meta-arylation reaction. Interestingly, the meta-arylation reaction proceeded well with exquisite selectivity even in the absence of the copper catalyst, just at the elevated temperature. Hence the Cu(III)–aryl intermediate that was previously proposed in their original working model was not that convincing. The precise mechanism still needed to be further investigated.
A ruthenium-catalyzed selective meta sulfonation of 2-phenylpyridines was demonstrated by Frost and co-workers (Scheme 464).640 The mechanistic pathway of the reaction is quite different from Gaunt's work and the catalytic chelation-assisted cyclometalation was proposed to be involved in the Ru catalyzed meta sulfonation reaction. It was surmised that the chelating group might facilitate the formation of a stable Ru–Caryl σ bond that could induce a strong para-directing effect. In addition, the observed kinetic isotope effect indicated that the C–H bond cleavage was the rate-determining step in the catalytic cycle. The reaction was the first example of a catalytic σ-activation process.
This first ruthenium-catalyzed direct C–H bond alkylation with excellent levels of unusual meta-selectivity under nonacidic reaction conditions was disclosed by Ackermann and co-workers (Scheme 465).641 The ruthenium(II) biscarboxylate complexes proved to be the key factor of the highly meta selective alkylation and the carboxylic acid MesCO2H was indispensable. A wide variety of arenes with ample scope could be smoothly compatible with the reaction system. Isotope labeling experiments suggested that an initial reversible ortho-C–H bond cyclometalation was involved in the reaction process and the meta-C–H bond cleavage was not supposed to be kinetically relevant. It was rationalized that an SEAr-type alkylative substitution occurred in the meta-position, facilitated by the strong activation and the ortho-/para-directing effect induced by the Ru–C(sp2)-σ bond. The relevant mechanistic studies were also conducted and the possible catalytic cycle was proposed (Scheme 466). Initially, the cyclometalated complex was reversibly formed via C–H ruthenation. An SEAr-type alkylation of the aromatic substrates with the secondary alkyl halides activated by cyclometalation occurred to generate an ortho or para functionalization of the Ru–C(sp2) bond. Finally, the proton-demetalation process delivered the desired meta-substituted product and regenerated the catalytically active Ru(II) species.
14. Conclusion and outlook
The field of transition-metal-catalyzed group directed C–H bond functionalization reactions has expanded rapidly over the last ten years. Various functionalities have been designed and employed as directing groups successfully for C–C bonds or C–heteroatom bond formation. A broad range of useful transformations, including arylation, olefination, alkylation, alkynylation, carbonylation, amination halogenations, and so on, have appeared in the literature. Owing in part to their good functional group compatibility and high efficiency, these reactions have been utilized rapidly in the syntheses of complex molecules.Despite the impressive number of contributions and the results obtained, there are still significant demand and great room for further development in this area. For instance, compared with the huge number of functionalities existing in the organic molecules, the directing properties of many functionalities, especially the less basic ones, are unknown and are waiting for investigation. In many cases the directing groups are not an intrinsic part of the substrate and/or product, which need to be installed and removed or converted to the final desired functional group. It will be important to develop more general methods that are applicable to a broad spectrum of functionalities or removable directing groups. On the other hand, most of the current chelation-directed metalation of C–H bonds is limited to ortho-selectivity or β-selectivity. The control of the positional selectivity of C–H cleavage, especially to achieve the remote C–H functionalization, remains an outstanding challenge. Moreover, most of the efficient catalytic systems for C–H activation rely on expensive precious metals. The development of more powerful novel catalytic processes adopting inexpensive, environmentally benign, and relatively nontoxic metals is of significance for the widespread application of the direct C–H functionalization methodology. It should also be noted that the asymmetric C–H activation is still in its infancy and its true potential remains open ended. To address all of these issues, the evolution of a new system that allows access to a greater diversity of products is highly desirable. Generally, the mechanistic investigations will allow a thorough understanding of the C–H activation process and conclude the disciplines of the complex interplay between catalyst, ligands, solvent, additives, and substrates, and thus guide the design and discovery of new highly active and selective catalytic systems. Nevertheless, due to the highly sustainable nature of direct C–H bond functionalizations, advances in this area will change the way of functional molecule construction and speed up the industrial applications.
Acknowledgements
We gratefully acknowledge NSFC (no. 21272205 and 21472165) and the Program for Zhejiang Leading Team of S&T Innovation (2011R50007) for their financial support.References
- For selected reviews on transition-metal catalyzed C–H fictionalization, see: (a) C. Jia, T. Kitamura and Y. Fujiwara, Acc. Chem. Res., 2001, 34, 633 CrossRef CAS PubMed; (b) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173 RSC; (c) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2001, 42, 1074 CrossRef PubMed; (d) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (e) R. Giri, B.-F. Shi, K. M. Engle, N. Maugel and J.-Q. Yu, Chem. Soc. Rev., 2009, 38, 3242 RSC; (f) P. Thansandote and M. Lautens, Chem. – Eur. J., 2009, 15, 5874 CrossRef CAS PubMed; (g) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed; (h) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (i) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Commun., 2010, 46, 677 RSC; (j) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer and O. Baudoin, Chem. – Eur. J., 2010, 16, 2654 CrossRef CAS PubMed; (k) H. Li, B.-J. Li and Z.-J. Shi, Catal. Sci. Technol., 2011, 1, 191 RSC; (l) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; (m) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; (n) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed; (o) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed; (p) W. R. Gutekunst and P. S. Baran, Chem. Soc. Rev., 2011, 40, 1976 RSC; (q) J. Wencel-Delord, T. Dröge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC; (r) O. Baudoin, Chem. Soc. Rev., 2011, 40, 4902 RSC; (s) S. R. Neufeldt and M. S. Sanford, Acc. Chem. Res., 2012, 45, 936 CrossRef CAS PubMed; (t) L. Yang and H. Huang, Catal. Sci. Technol., 2012, 2, 1099 RSC; (u) G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651 RSC; (v) D.-G. Yu, B.-J. Li and Z.-J. Shi, Tetraedron, 2012, 68, 5130 CrossRef CAS PubMed; (w) B.-J. Li and Z.-J. Shi, Chem. Soc. Rev., 2012, 41, 5588 RSC; (x) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed; (y) X.-S. Zhang, K. Chen and Z.-J. Shi, Chem. Sci., 2014, 5, 2146 RSC; (z) C. Zheng and S.-L. You, RSC Adv., 2014, 4, 6173 RSC; (a a) L. Ackermann, Acc. Chem. Res., 2014, 47, 281 CrossRef CAS PubMed; (a b) M. Zhang, Y. Zhang, X. Jie, H. Zhao, G. Li and W. Su, Org. Chem. Front., 2014, 1, 843 RSC; (a c) P. L. Arnold, M. W. McMullon, J. Rieb and F. Kühn, Angew. Chem., Int. Ed., 2015, 54, 82 CrossRef CAS PubMed.
- (a) L. N. Lewis and J. F. Smish, J. Am. Chem. Soc., 1986, 108, 2728 CrossRef CAS; (b) S. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda and N. Chatani, Nature, 1993, 366, 529 CrossRef CAS PubMed.
- (a) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed; (b) G. Rousseau and B. Breit, Angew. Chem., Int. Ed., 2011, 50, 2450 CrossRef CAS PubMed; (c) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed; (d) D. A. Colby, A. S. Tsai, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2012, 45, 814 CrossRef CAS PubMed; (e) C. Zhu, R. Wang and J. R. Falck, Chem. – Asian J., 2012, 7, 1502 CrossRef CAS PubMed; (f) Y. Wang, G. Cheng and X. Cui, Chin. J. Org. Chem., 2012, 32, 2018 CrossRef CAS; (g) D. Li, C. He, H. Cai and G. Wang, Chin. J. Org. Chem., 2013, 33, 203 CrossRef CAS; (h) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed; (i) B. Zhang, H. Guan, B. Liu and B. Shi, Chin. J. Org. Chem., 2014, 34, 1487 CrossRef CAS; (j) G. Shi and Y. Zhang, Adv. Synth. Catal., 2014, 365, 1419 CrossRef PubMed; (k) F. Zhang and D. R. Spring, Chem. Soc. Rev., 2014, 43, 6906 RSC.
- M. D. K. Boele, G. P. F. van Strijdonck, A. H. M. de Vries, P. C. J. Kamer, J. G. de Vries and P. W. N. M. vanLeeuwen, J. Am. Chem. Soc., 2002, 124, 1586 CrossRef CAS PubMed.
- (a) C. Amatore, C. Cammoun and A. Jutand, Adv. Synth. Catal., 2007, 349, 292 CrossRef CAS PubMed; (b) J.-R. Wang, C.-T. Yang, L. Liu and Q.-X. Guo, Tetrahedron Lett., 2007, 48, 5449 CrossRef CAS PubMed; (c) B. S. Kim, C. Jang, D. J. Lee and S. W. Youn, Chem. – Asian J., 2010, 5, 2336 CrossRef CAS PubMed; (d) W. Rauf, A. L. Thompson and J. M. Brown, Dalton Trans., 2010, 39, 10414 RSC; (e) X. Liu and K. K. (Mimi) Hii, J. Org. Chem., 2011, 76, 8022 CrossRef CAS PubMed; (f) L. L. Chng, J. Zhang, J. Yang, M. Amoura and J. Y. Ying, Adv. Synth. Catal., 2011, 353, 2988 CrossRef CAS PubMed; (g) B. Schmidt and N. Elizarov, Chem. Commun., 2012, 48, 4350 RSC.
- Y. Gao, Y. Huang, W. Wu, K. Huang and H. Jiang, Chem. Commun., 2014, 50, 8370 RSC.
- O. Daugulis and V. G. Zaitsev, Angew. Chem., Int. Ed., 2005, 44, 4046 CrossRef CAS PubMed.
- (a) B.-J. Li, S.-D. Yang and Z.-J. Shi, Synlett, 2008, 949 CAS; (b) Z. Shi, B. Li, X. Wan, J. Cheng, Z. Fang, B. Cao, C. Qin and Y. Wang, Angew. Chem., Int. Ed., 2007, 46, 5554 CrossRef CAS PubMed; (c) S. Yang, B. Li, X. Wan and Z. Shi, J. Am. Chem. Soc., 2007, 129, 6066 CrossRef CAS PubMed; (d) B.-J. Li, S.-L. Tian, Z. Fang and Z.-J. Shi, Angew. Chem., Int. Ed., 2008, 47, 1115 CrossRef CAS PubMed.
- G. Brasche, J. García-Fortanet and S. L. Buchwald, Org. Lett., 2008, 10, 2207 CrossRef CAS PubMed.
- C. S. Yeung, X. Zhao, N. Borduas and V. M. Dong, Chem. Sci., 2010, 1, 331 RSC.
- F. Yang, F. Song, W. Li, J. Lan and J. You, RSC Adv., 2013, 3, 9649 RSC.
- D. Li, N. Xu, Y. Zhang and L. Wang, Chem. Commun., 2014, 50, 14862 RSC.
- R. Giri, J. K. Lam and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 686 CrossRef CAS PubMed.
- P. Fang, M. Li and H. Ge, J. Am. Chem. Soc., 2010, 132, 11898 CrossRef CAS PubMed.
- M. Kim, N. K. Mishra, J. Park, S. Han, Y. Shin, S. Sharma, Y. Lee, E.-K. Lee, J. H. Kwak and I. S. Kim, Chem. Commun., 2014, 50, 14249 RSC.
- (a) O. Baslé, J. Bidange, Q. Shuai and C.-J. Li, Adv. Synth. Catal., 2010, 352, 1145 CrossRef PubMed; (b) Y. Wu, B. Li, F. Mao, X. Li and F. Y. Kwong, Org. Lett., 2011, 13, 3258 CrossRef CAS PubMed; (c) C. Li, L. Wang, P. Li and W. Zhou, Chem. – Eur. J., 2011, 17, 10208 CrossRef CAS PubMed; (d) C.-W. Chan, Z. Zhou and W.-Y. Yu, Adv. Synth. Catal., 2011, 353, 2999 CrossRef CAS PubMed.
- (a) F. Xiao, Q. Shuai, F. Zhao, O. Basle, G. Deng and C.-J. Li, Org. Lett., 2011, 13, 1614 CrossRef CAS PubMed; (b) Y. Yuan, D. Chen and X. Wang, Adv. Synth. Catal., 2011, 353, 3373 CrossRef CAS PubMed; (c) Y. Wu, P. Y. Choy, F. Mao and F. Y. Kwong, Chem. Commun., 2013, 49, 689 RSC.
- S. Wang, Z. Yang, J. Liu, K. Xie, A. Wang, X. Chen and Z. Tan, Chem. Commun., 2012, 48, 9924 RSC.
- Y. Huang, G. Li, J. Huang and J. You, Org. Chem. Front., 2014, 1, 347 RSC.
- Urea as DG: (a) C. E. Houlden, M. Hutchby, C. D. Bailey, J. G. Ford, S. N. G. Tyler, M. R. Gagné, G. C. Lloyd-Jones and K. I. Booker-Milburn, Angew. Chem., Int. Ed., 2009, 48, 1830 CrossRef CAS PubMed; (b) W. Rauf, A. L. Thompson and J. M. Brown, Chem. Commun., 2009, 3874 RSC; (c) L. Wang, S. Liu, Z. Li and Y. Yu, Org. Lett., 2011, 13, 6137 CrossRef CAS PubMed; (d) T. Nishikata, A. R. Abela and B. H. Lipshutz, Angew. Chem., Int. Ed., 2010, 49, 781 CrossRef CAS PubMed; (e) T. Nishikata, A. R. Abela, S. Huang and B. H. Lipshutz, J. Am. Chem. Soc., 2010, 132, 4978 CrossRef CAS PubMed; (f) Z. Jiang, L. Zhang, C. Dong, X. Su, H. Li, W. Tang, L. Xu and Q. Fan, RSC Adv., 2013, 3, 1025 RSC; (g) C. Wan, J. Zhao, M. Xu and J. Huang, J. Org. Chem., 2014, 79, 4751 CrossRef CAS PubMed; (h) N. Uhlig and C.-J. Li, Chem. – Eur. J., 2014, 20, 12066 CrossRef CAS PubMed.
- K.-H. Ng, A. S. C. Chan and W.-Y. Yu, J. Am. Chem. Soc., 2010, 132, 12862 CrossRef CAS PubMed.
- K. Sun, Y. Li, T. Xiong, J. Zhang and Q. Zhang, J. Am. Chem. Soc., 2011, 133, 1694 CrossRef CAS PubMed.
- X. Wan, Z. Ma, B. Li, K. Zhang, S. Cao, S. Zhang and Z. Shi, J. Am. Chem. Soc., 2006, 128, 7416 CrossRef CAS PubMed.
- R. B. Bedford, M. F. Haddow, C. J. Mitchell and R. L. Webster, Angew. Chem., Int. Ed., 2011, 50, 5524 CrossRef CAS PubMed.
- X.-C. Wang, Y. Hu, S. Bonacorsi, Y. Hong, R. Burrell and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 10326 CrossRef CAS PubMed.
- L. Chu, X.-C. Wang, C. E. Moore, A. L. Rheingold and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 16344 CrossRef CAS PubMed.
- T.-S. Jiang and G.-W. Wang, J. Org. Chem., 2012, 77, 9504 CrossRef CAS PubMed.
- G.-W. Wang and T.-T. Yuan, J. Org. Chem., 2010, 75, 476 CrossRef CAS PubMed.
- L.-S. Zhang, K. Chen, G. Chen, B.-J. Li, S. Luo, Q.-Y. Guo, J.-B. Wei and Z.-J. Shi, Org. Lett., 2013, 15, 10 CrossRef CAS PubMed.
- (a) H. Zhou, W.-J. Chung, Y.-H. Xu and T.-P. Loh, Chem. Commun., 2009, 3472 RSC; (b) H. Zhou, Y.-H. Xu, W.-J. Chung and T.-P. Loh, Angew. Chem., Int. Ed., 2009, 48, 5355 CrossRef CAS PubMed; (c) S. Pankajakshan, Y.-H. Xu, J. K. Cheng, M. T. Low and T.-P. Loh, Angew. Chem., Int. Ed., 2012, 51, 5701 CrossRef CAS PubMed.
- Y.-H. Xu, Y. K. Chok and T.-P. Loh, Chem. Sci., 2011, 2, 1822 RSC.
- Y.-H. Xu, Q.-C. Zhang, T. He, F.-F. Meng and T.-P. Loh, Adv. Synth. Catal., 2014, 356, 1539 CrossRef CAS PubMed.
- H. Wang, L.-N. Guo and X.-H. Duan, Org. Lett., 2012, 14, 4358 CrossRef CAS PubMed.
- M. Chen, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Angew. Chem., Int. Ed., 2013, 52, 14196 CrossRef CAS PubMed.
- M.-N. Zhao, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2014, 16, 608 CrossRef CAS PubMed.
- D.-H. Wang, M. Wasa, R. Giri and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 7190 CrossRef CAS PubMed.
- M. Wasa, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 9886 CrossRef CAS PubMed.
- R. Giri, J. Liang, J.-G. Lei, J.-J. Li, D.-H. Wang, X. Chen, I. C. Naggar, C. Guo, B. M. Foxman and J.-Q. Yu, Angew. Chem., Int. Ed., 2005, 44, 7420 CrossRef CAS PubMed.
- T. M. Figg, M. Wasa, J.-Q. Yu and D. G. Musaev, J. Am. Chem. Soc., 2013, 135, 14206 CrossRef CAS PubMed.
- X. Wang, D. Leow and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 13864 CrossRef CAS PubMed.
- D.-D. Li, T.-T. Yuan and G.-W. Wang, J. Org. Chem., 2012, 77, 3341 CrossRef CAS PubMed.
- G.-W. Wang, T.-T. Yuan and D.-D. Li, Angew. Chem., Int. Ed., 2011, 50, 1380 CrossRef CAS PubMed.
- J. Karthikeyan and C.-H. Cheng, Angew. Chem., Int. Ed., 2011, 50, 9880 CrossRef CAS PubMed.
- (a) S. Pimparkar and M. Jeganmohan, Chem. Commun., 2014, 50, 12116 RSC; (b) X. Peng, W. Wang, C. Jiang, D. Sun, Z. Xu and C.-H. Tung, Org. Lett., 2014, 16, 5354 CrossRef CAS PubMed.
- K.-J. Xiao, D. W. Lin, M. Miura, R.-Y. Zhu, W. Gong, M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 8138 CrossRef CAS PubMed.
- M. Wasa, K. M. Engle, D. W. Lin, E. J. Yoo and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 19598 CrossRef CAS PubMed.
- Y. Deng, W. Gong, J. He and J.-Q. Yu, Angew. Chem., Int. Ed., 2014, 53, 6692 CrossRef CAS PubMed.
- (a) J. He, S. Li, Y. Deng, H. Fu, B. N. Laforteza, J. E. Spangler, A. Homs and J.-Q. Yu, Science, 2014, 343, 1216 CrossRef CAS PubMed; (b) M. Wasa, K. S. L. Chan, X.-G. Zhang, J. He, M. Miura and J.-Q. Yu, J. Am. Chem. Soc., 2012, 134, 18570 CrossRef CAS PubMed.
- C. Zhu and J. R. Falck, Org. Lett., 2011, 13, 1214 CrossRef CAS PubMed.
- (a) J. W. Wrigglesworth, B. Cox, G. C. Lloyd-Jones and K. I. Booker-Milburn, Org. Lett., 2011, 13, 5326 CrossRef CAS PubMed; (b) D.-D. Li, T.-T. Yuan and G.-W. Wang, Chem. Commun., 2011, 47, 12789 RSC.
- S. Li, G. Chen, C.-G. Feng, W. Gong and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 5267 CrossRef CAS PubMed.
- M. Wasa, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 3680 CrossRef CAS PubMed.
- J. He, M. Wasa, K. S. L. Chan and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 3387 CrossRef CAS PubMed.
- R.-Y. Zhu, J. He, X.-C. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 13194 CrossRef CAS PubMed.
- J. Park, M. Kim, S. Sharma, E. Park, A. Kim, S. H. Lee, J. H. Kwak, Y. H. Jung and I. S. Kim, Chem. Commun., 2013, 49, 1654 RSC.
- Q. Yu, N. Zhang, J. Huang, S. Lu, Y. Zhu, X. Yu and K. Zhao, Chem. – Eur. J., 2013, 19, 11184 CrossRef CAS PubMed.
- J. Park, E. Park, A. Kim, Y. Lee, K. Chi, J. H. Kwak, Y. H. Jung and I. S. Kim, Org. Lett., 2011, 13, 4390 CrossRef CAS PubMed.
- H.-X. Dai, A. F. Stepan, M. S. Plummer, Y.-H. Zhang and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 7222 CrossRef CAS PubMed.
- E. J. Yoo, S. Ma, T.-S. Mei, K. S. L. Chan and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 7652 CrossRef CAS PubMed.
- M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 14058 CrossRef CAS PubMed.
- G.-W. Wang and T.-T. Yuan, J. Org. Chem., 2010, 75, 476 CrossRef CAS PubMed.
- F. Péron, C. Fossey, J. S. O. Santos, T. Cailly and F. Fabis, Chem. – Eur. J., 2014, 20, 7507 CrossRef PubMed.
- X.-C. Wang, Y. Hu, S. Bonacorsi, Y. Hong, R. Burrell and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 10326 CrossRef CAS PubMed.
- H.-X. Dai and J.-Q. Yu, J. Am. Chem. Soc., 2012, 134, 134 CrossRef CAS PubMed.
- X.-G. Zhang, H.-X. Dai, M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2012, 134, 11948 CrossRef CAS PubMed.
- For recent reviews on Cp*Rh(III)-catalyzed C–H functionalizations, see: (a) F. W. Patureau, J. Wencel-Delord and F. Glorius, Aldrichimica Acta, 2012, 45, 31 CAS; (b) T. Satoh and M. Miura, Chem. – Eur. J., 2010, 16, 11212 CrossRef CAS PubMed.
- F. W. Patureau and F. Glorius, J. Am. Chem. Soc., 2010, 132, 9982 CrossRef CAS PubMed.
- S. Rakshit, C. Grohmann, T. Besset and F. Glorius, J. Am. Chem. Soc., 2011, 133, 2350 CrossRef CAS PubMed.
- (a) F. W. Patureau, T. Besset and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 1064 CrossRef CAS PubMed; (b) F. Wang, G. Song and X. Li, Org. Lett., 2010, 12, 5430 CrossRef CAS PubMed.
- Y. Shen, G. Liu, Z. Zhou and X. Lu, Org. Lett., 2013, 15, 3366 CrossRef CAS PubMed.
- (a) W.-W. Chan, S.-F. Lo, Z. Zhou and W.-Y. Yu, J. Am. Chem. Soc., 2012, 134, 13565 CrossRef CAS PubMed; (b) T. K. Hyster, K. E. Ruhl and T. Rovis, J. Am. Chem. Soc., 2013, 135, 5364 CrossRef CAS PubMed; (c) X. Yu, S. Yu, J. Xiao, B. Wan and X. Li, J. Org. Chem., 2013, 78, 5444 CrossRef CAS PubMed; (d) Z. Shi, D. C. Koester, M. Boultadakis-Arapinis and F. Glorius, J. Am. Chem. Soc., 2013, 135, 12204 CrossRef CAS PubMed; (e) S. Cui, Y. Zhang, D. Wang and Q. Wu, Chem. Sci., 2013, 4, 3912 RSC.
- F. Hu, Y. Xia, F. Ye, Z. Liu, C. Ma, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2014, 53, 1364 CrossRef CAS PubMed.
- J. Zhou, B. Li, F. Hu and B.-F. Shi, Org. Lett., 2013, 15, 3460 CrossRef CAS PubMed.
- J. Zhou, B. Li, Z.-C. Qian and B.-F. Shi, Adv. Synth. Catal., 2014, 356, 1038 CrossRef CAS PubMed.
- H. Wang and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 7318 CrossRef CAS PubMed.
- H. Wang, B. Beiring, D.-G. Yu, K. D. Collins and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 12430 CrossRef CAS PubMed.
- R. Zeng, S. Wu, C. Fu and S. Ma, J. Am. Chem. Soc., 2013, 135, 18284 CrossRef CAS PubMed.
- R. Zeng, J. Ye, C. Fu and S. Ma, Adv. Synth. Catal., 2013, 355, 1963 CrossRef CAS PubMed.
- J. Zheng, Y. Zhang and S. Cui, Org. Lett., 2014, 16, 3560 CrossRef CAS PubMed.
- J. Karthikeyan, R. Haridharan and C.-H. Cheng, Angew. Chem., Int. Ed., 2012, 51, 12343 CrossRef CAS PubMed.
- N. Senthilkumar, K. Parthasarathy, P. Gandeepan and C.-H. Cheng, Chem. – Asian J., 2013, 8, 2175 CrossRef CAS PubMed.
- J. Wencel-Delord, C. Nimphius, F. W. Patureau and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 2247 CrossRef CAS PubMed.
- J. Wencel-Delord, C. Nimphius, F. W. Patureau and F. Glorius, Chem. – Asian J., 2012, 7, 1208 CrossRef CAS PubMed.
- J. Wencel-Delord, C. Nimphius, H. Wang and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 13001 CrossRef CAS PubMed.
- (a) H. Wang, N. Schröder and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 5386 CrossRef CAS PubMed; (b) C. Feng, D. Feng and T.-P. Loh, Org. Lett., 2014, 16, 4540 CrossRef PubMed.
- Y. Huang, D. Wu, J. Huang, Q. Guo, J. Li and J. You, Angew. Chem., Int. Ed., 2014, 53, 12158 CrossRef CAS PubMed.
- C. Feng and T.-P. Loh, Angew. Chem., Int. Ed., 2014, 53, 2722 CrossRef CAS PubMed.
- C. Feng, D. Feng, Y. Luo and T.-P. Loh, Org. Lett., 2014, 16, 5956 CrossRef CAS PubMed.
- F. Xie, Z. Qi, S. Yu and X. Li, J. Am. Chem. Soc., 2014, 136, 4780 CrossRef CAS PubMed.
- K. D. Collins, F. Lied and F. Glorius, Chem. Commun., 2014, 50, 4459 RSC.
- C. Feng, D. Feng and T.-P. Loh, Chem. Commun., 2014, 50, 9865 RSC.
- K. D. Hesp, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2011, 133, 11430 CrossRef CAS PubMed.
- Y. Du, T. K. Hyster and T. Rovis, Chem. Commun., 2011, 47, 12074 RSC.
- C. Zhu, W. Xie and J. R. Falck, Chem. – Eur. J., 2011, 17, 12591 CrossRef CAS PubMed.
- S. Sharma, E. Park, J. Park and I. S. Kim, Org. Lett., 2012, 14, 906 CrossRef CAS PubMed.
- D. R. Stuart, P. Alsabeh, M. Kuhn and K. Fagnou, J. Am. Chem. Soc., 2010, 132, 18326 CrossRef CAS PubMed.
- D. R. Stuart, M. Bertrand-Laperle, K. M. N. Burgess and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 16474 CrossRef CAS PubMed.
- D. Zhao, Z. Shi and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 12426 CrossRef CAS PubMed.
- N. Guimond, C. Gouliaras and K. Fagnou, J. Am. Chem. Soc., 2010, 132, 6908 CrossRef CAS PubMed.
- Y. Tan and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 3676 CrossRef CAS PubMed.
- N. Guimond, S. I. Gorelsky and K. Fagnou, J. Am. Chem. Soc., 2011, 133, 6449 CrossRef CAS PubMed.
- T. K. Hyster and T. Rovis, J. Am. Chem. Soc., 2010, 132, 10565 CrossRef CAS PubMed.
- D.-G. Yu, F. de Azambuja, T. Gensch, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2014, 53, 9650 CrossRef CAS PubMed.
- T. A. Davis, T. K. Hyster and T. Rovis, Angew. Chem., Int. Ed., 2013, 52, 14181 CrossRef CAS PubMed.
- Z. Shi, M. Boultadakis-Arapinis, D. C. Koester and F. Glorius, Chem. Commun., 2014, 50, 2650 RSC.
- (a) X. Zhang, Y. Li, H. Shi, L. Zhang, S. Zhang, X. Xu and Q. Liu, Chem. Commun., 2014, 50, 7306 RSC; (b) B. Zhou, Y. Yang, H. Tang, J. Du, H. Feng and Y. Li, Org. Lett., 2014, 16, 3900 CrossRef CAS PubMed.
- B. Ye, P. A. Donets and N. Cramer, Angew. Chem., Int. Ed., 2014, 53, 507 CrossRef CAS PubMed.
- (a) T. K. Hyster, L. Knorr, T. R. Ward and T. Rovis, Science, 2012, 338, 500 CrossRef CAS PubMed; (b) B. Ye and N. Cramer, Science, 2012, 338, 504 CrossRef CAS PubMed.
- D.-G. Yu, F. de Azambuja and F. Glorius, Angew. Chem., Int. Ed., 2014, 53, 2754 CrossRef CAS PubMed.
- Z. Shi, C. Grohmann and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 5393 CrossRef CAS PubMed.
- T. K. Hyster, D. M. Dalton and T. Rovis, Chem. Sci., 2015, 6, 254 RSC.
- (a) T. K. Hyster and T. Rovis, Synlett, 2013, 1842 CAS; (b) M. Presset, D. Oehlrich, F. Rombouts and G. A. Molander, Org. Lett., 2014, 16, 1528 Search PubMed; (c) H. Wang, C. Grohmann, C. Nimphius and F. Glorius, J. Am. Chem. Soc., 2012, 134, 19592 CrossRef CAS PubMed; (d) N. J. Webb, S. P. Marsden and S. A. Raw, Org. Lett., 2014, 16, 4718 CrossRef CAS PubMed.
- J. R. Huckins, E. A. Bercot, O. R. Thiel, T.-L. Hwang and M. M. Bio, J. Am. Chem. Soc., 2013, 135, 14492 CrossRef CAS PubMed.
- B.-J. Li, H.-Y. Wang, Q.-L. Zhu and Z.-J. Shi, Angew. Chem., Int. Ed., 2012, 51, 3948 CrossRef CAS PubMed.
- F. Wang, Z. Qi, J. Sun, X. Zhang and X. Li, Org. Lett., 2013, 15, 6290 CrossRef CAS PubMed.
- D. Wang, F. Wang, G. Song and X. Li, Angew. Chem., Int. Ed., 2012, 51, 12348 CrossRef CAS PubMed.
- M. V. Pham, B. Ye and N. Cramer, Angew. Chem., Int. Ed., 2012, 51, 10610 CrossRef CAS PubMed.
- (a) M. Miura, T. Tsuda, T. Satoh, S. Pivsa-Art and M. Momura, J. Org. Chem., 1998, 63, 5211 CrossRef CAS; (b) X. Li, X. Gong, M. Zhao, G. Song, J. Deng and X. Li, Org. Lett., 2011, 13, 5808 CrossRef CAS PubMed; (c) S. Hu, D. Wang, J. Liu and X. Li, Org. Biomol. Chem., 2013, 11, 2761 RSC; (d) T. Zhang, Z. Qi, X. Zhang, L. Wu and X. Li, Chem. – Eur. J., 2014, 20, 3283 CrossRef CAS PubMed; (e) W. Dong, K. Parthasarathy, Y. Cheng, F. Pan and C. Bolm, Chem. – Eur. J., 2014, 20, 15732 CrossRef CAS PubMed.
- S. Cui, Y. Zhang and Q. Wu, Chem. Sci., 2013, 4, 3421 RSC.
- X. Xu, Y. Liu and C.-M. Park, Angew. Chem., Int. Ed., 2012, 51, 9372 CrossRef CAS PubMed.
- B. Zhou, J. Du, Y. Yang and Y. Li, Chem. – Eur. J., 2014, 20, 12768 CrossRef CAS PubMed.
- N. Quiñones, A. Seoane, R. García-Fandiño, J. L. Mascareñas and M. Gulías, Chem. Sci., 2013, 4, 2874 RSC.
- P. Duan, X. Lan, Y. Chen, S.-S. Qian, J. J. Li, L. Lu, Y. Lu, B. Chen, M. Hong and J. Zhao, Chem. Commun., 2014, 50, 12135 RSC.
- G. Liu, Y. Shen, Z. Zhou and X. Lu, Angew. Chem., Int. Ed., 2013, 52, 6033 CrossRef CAS PubMed.
- C. Grohmann, H. Wang and F. Glorius, Org. Lett., 2012, 14, 656 CrossRef CAS PubMed.
- W. Yang, J. Sun, X. Xu, Q. Zhang and Q. Liu, Chem. Commun., 2014, 50, 4420 RSC.
- J. Ryu, K. Shin, S. H. Park, J. Y. Kim and S. Chang, Angew. Chem., Int. Ed., 2012, 51, 9904 CrossRef CAS PubMed.
- K. Shin, Y. Baek and S. Chang, Angew. Chem., Int. Ed., 2013, 52, 8031 CrossRef CAS PubMed.
- W. Yu, J. Chen, K. Gao, Z. Liu and Y. Zhang, Org. Lett., 2014, 16, 4870 CrossRef CAS PubMed.
- N. Schröder, J. Wencel-Delord and F. Glorius, J. Am. Chem. Soc., 2012, 134, 8298 CrossRef PubMed.
- N. Kuhl, N. Schröder and F. Glorius, Org. Lett., 2012, 14, 3860 Search PubMed.
- L. Ackermann, A. V. Lygin and N. Hofmann, Angew. Chem., Int. Ed., 2011, 50, 6379 CrossRef CAS PubMed.
- L. Ackermann, A. V. Lygin and N. Hofmann, Org. Lett., 2011, 13, 3278 CrossRef CAS PubMed.
- (a) L. Ackermann and S. Fenner, Org. Lett., 2011, 13, 6548 CrossRef CAS PubMed; (b) B. Li, H. Feng, S. Xu and B. Wang, Chem. – Eur. J., 2011, 17, 12573 CrossRef CAS PubMed.
- L. Ackermann, L. Wang, R. Wolfram and A. V. Lygin, Org. Lett., 2012, 14, 728 CrossRef CAS PubMed.
- B. Li, J. Ma, N. Wang, H. Feng, S. Xu and B. Wang, Org. Lett., 2012, 14, 736 CrossRef CAS PubMed.
- R. K. Chinnagolla and M. Jeganmohan, Org. Lett., 2012, 14, 5246 CrossRef CAS PubMed.
- R. K. Chinnagolla and M. Jeganmohan, Chem. Commun., 2014, 50, 2442 RSC.
- R. Manikandan and M. Jeganmohan, Org. Lett., 2014, 16, 3568 CrossRef CAS PubMed.
- (a) B. Li, N. Wang, Y. Liang, S. Xu and B. Wang, Org. Lett., 2013, 15, 136 CrossRef CAS PubMed; (b) K. Murugan and S.-T. Liu, Tetrahedron Lett., 2013, 54, 2608 CrossRef CAS PubMed.
- V. S. Thirunavukkarasu, J. Hubrich and L. Ackermann, Org. Lett., 2012, 14, 4210 CrossRef CAS PubMed.
- F. Yang and L. Ackermann, Org. Lett., 2013, 15, 718 CrossRef CAS PubMed.
- X. Yang, G. Shan and Y. Rao, Org. Lett., 2013, 15, 2334 CrossRef CAS PubMed.
- M. Shang, S.-H. Zeng, S.-Z. Sun, H.-X. Dai and J.-Q. Yu, Org. Lett., 2013, 15, 5286 CrossRef CAS PubMed.
- J. Ryu, J. Kwak, K. Shin, D. Lee and S. Chang, J. Am. Chem. Soc., 2013, 135, 12861 CrossRef CAS PubMed.
- H. Kim, K. Shin and S. Chang, J. Am. Chem. Soc., 2014, 136, 5904 CrossRef CAS PubMed.
- B. Urones, Á. M. Martínez, N. Rodríguez, R. G. Arrayás and J. C. Carretero, Chem. Commun., 2013, 49, 11044 RSC.
- E. Hernando, R. R. Castillo, N. Rodríguez, R. G. Arrayás and J. C. Carretero, Chem. – Eur. J., 2014, 20, 13854 CrossRef CAS PubMed.
- C. Feng and T.-P. Loh, Chem. Sci., 2012, 3, 3458 RSC.
- C. Feng and T.-P. Loh, Angew. Chem., Int. Ed., 2013, 52, 12414 CrossRef CAS PubMed.
- Q. Chen, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2011, 133, 428 CrossRef CAS PubMed.
- Q. Chen, L. Ilies, N. Yoshikai and E. Nakamura, Org. Lett., 2011, 13, 3232 CrossRef CAS PubMed.
- (a) H. Ikemoto, T. Yoshino, K. Sakata, S. Matsunaga and M. Kanai, J. Am. Chem. Soc., 2014, 136, 5424 CrossRef CAS PubMed; (b) C.-H. Jun, J.-B. Hong, Y.-H. Kim and K. Y. Chung, Angew. Chem., Int. Ed., 2000, 39, 3440 CrossRef CAS.
- D. Kalyani, N. R. Deprez, L. V. Desai and M. S. Sanford, J. Am. Chem. Soc., 2005, 127, 7330 CrossRef CAS PubMed.
- K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2007, 129, 11904 CrossRef CAS PubMed.
- K. L. Hull, E. L. Lanni and M. S. Sanford, J. Am. Chem. Soc., 2006, 128, 14047 CrossRef CAS PubMed.
- D. Shabashov and O. Daugulis, Org. Lett., 2005, 7, 3657 CrossRef CAS PubMed.
- W.-Y. Yu, W. N. Sit, Z. Zhou and A. S.-C. Chan, Org. Lett., 2009, 11, 3174 CrossRef CAS PubMed.
- W. Li, Z. Yin, X. Jiang and P. Sun, J. Org. Chem., 2011, 76, 8543 CrossRef CAS PubMed.
- K. J. Stowers, K. C. Fortner and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 6541 CrossRef CAS PubMed.
- A. Garcia-Rubia, B. Urones, R. G. Arrayas and J. C. Carretero, Angew. Chem., Int. Ed., 2011, 50, 10927 CrossRef CAS PubMed.
- X. Cong, J. You, G. Gao and J. Lan, Chem. Commun., 2013, 49, 662 RSC.
- X. Chen, C. E. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 12634 CrossRef CAS PubMed.
- B.-F. Shi, N. Maugel, Y.-H. Zhang and J.-Q. Yu, Angew. Chem., Int. Ed., 2008, 47, 4882 CrossRef CAS PubMed.
- Y. Zhang, J. Feng and C.-J. Li, J. Am. Chem. Soc., 2008, 130, 2900 CrossRef CAS PubMed.
- X. Wang, L. Truesdale and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 3648 CrossRef CAS PubMed.
- W.-Y. Yu, W. N. Sit, K.-M. Lai, Z. Zhou and A. S. C. Chan, J. Am. Chem. Soc., 2008, 130, 3304 CrossRef CAS PubMed.
- W. Zhou, H. Li and L. Wang, Org. Lett., 2012, 14, 4594 CrossRef CAS PubMed.
- X. Peng, Y. Zhu, T. A. Ramirez, B. Zhao and Y. Shi, Org. Lett., 2011, 13, 5244 CrossRef CAS PubMed.
- X. Jia, D. Yang, S. Zhang and J. Cheng, Org. Lett., 2009, 11, 4716 CrossRef CAS PubMed.
- H.-Y. Thu, W.-Y. Yu and C.-M. Che, J. Am. Chem. Soc., 2006, 128, 9048 CrossRef CAS PubMed.
- Á. Iglesias, R. Álvarez, Á. R. de Lera and K. Muñiz, Angew. Chem., Int. Ed., 2012, 51, 2225 CrossRef PubMed.
- Q.-Z. Zheng, P. Feng, Y.-F. Liang and N. Jiao, Org. Lett., 2013, 15, 4262 CrossRef CAS PubMed.
- D. Kalyani and M. S. Sanford, Org. Lett., 2005, 7, 4149 CrossRef CAS PubMed.
- A. V. Gulevich, F. S. Melkonyan, D. Sarkar and V. Gevorgyan, J. Am. Chem. Soc., 2012, 134, 5528 CrossRef CAS PubMed.
- Y. Yan, P. Feng, Q.-Z. Zheng, Y.-F. Liang, J.-F. Lu, Y. Cui and N. Jiao, Angew. Chem., Int. Ed., 2013, 52, 5827 CrossRef CAS PubMed.
- S. Shi and C. Kuang, J. Org. Chem., 2014, 79, 6105 CrossRef CAS PubMed.
- C.-G. Feng, M. Ye, K.-J. Xiao, S. Li and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 9322 CrossRef CAS PubMed.
- C. Li, T. Yano, N. Ishida and M. Murakani, Angew. Chem., Int. Ed., 2013, 52, 9081 Search PubMed.
- X. Zhao, E. Dimitrijevic and V. M. Dong, J. Am. Chem. Soc., 2009, 131, 3466 CrossRef CAS PubMed.
- M. Iwasaki, M. Iyanaga, Y. Tsuchiya, Y. Nishimura, W. Li, Z. Li and Y. Nishihara, Chem. – Eur. J., 2014, 20, 2459 CrossRef CAS PubMed.
- C. Xu and Q. Shen, Org. Lett., 2014, 16, 2046 CrossRef CAS PubMed.
- Y. Kuninobu, T. Iwanaga, T. Omura and K. Takai, Angew. Chem., Int. Ed., 2013, 52, 4431 CrossRef CAS PubMed.
- K. L. Hull, W. Q. Anani and M. S. Sanford, J. Am. Chem. Soc., 2006, 128, 7134 CrossRef CAS PubMed.
- K. B. McMurtrey, J. M. Racowski and M. S. Sanford, Org. Lett., 2012, 14, 4094 CrossRef CAS PubMed.
- Q. Xiao, X. Meng, M. Kanai and Y. Kuninobu, Angew. Chem., Int. Ed., 2014, 53, 3168 CrossRef CAS PubMed.
- S. Oi, S. Fukita and Y. Inoue, Chem. Commun., 1998, 2439 RSC.
- T. Vogler and A. Studer, Org. Lett., 2008, 10, 129 CrossRef CAS PubMed.
- X. Zhao and Z. Yu, J. Am. Chem. Soc., 2008, 130, 8136 CrossRef CAS PubMed.
- V. P. Reddy, R. Qiu, T. Iwasaki and N. Kambe, Org. Lett., 2013, 15, 1290 CrossRef CAS PubMed.
- M.-Z. Lu, P. Lu, Y.-H. Xu and T.-P. Loh, Org. Lett., 2014, 16, 2614 CrossRef CAS PubMed.
- X. Zhang, F. Wang, Z. Qi, S. Yu and X. Li, Org. Lett., 2014, 16, 1586 CrossRef CAS PubMed.
- Y.-G. Lim, K.-H. Lee, B. Koo and J.-B. Kang, Tetrahedron Lett., 2001, 42, 7609 CrossRef CAS.
- T. Shibata, S. Takayasu, S. Yuzawa and T. Otani, Org. Lett., 2012, 14, 5106 CrossRef CAS PubMed.
- B. Liu, T. Zhou, B. Li, S. Xu, H. Song and B. Wang, Angew. Chem., Int. Ed., 2014, 53, 4191 CrossRef CAS PubMed.
- N. Umeda, H. Tsurugi, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2008, 47, 4019 CrossRef CAS PubMed.
- N. Umeda, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 7094 CrossRef CAS PubMed.
- G. Song, X. Gong and X. Li, J. Org. Chem., 2011, 76, 7583 CrossRef CAS PubMed.
- J. Chen, G. Song, C.-L. Pan and X. Li, Org. Lett., 2010, 12, 5426 CrossRef CAS PubMed.
- X. Li and M. Zhao, J. Org. Chem., 2011, 76, 8530 CrossRef CAS PubMed.
- G. Zhang, L. Yang, Y. Wang, Y. Xie and H. Huang, J. Am. Chem. Soc., 2013, 135, 8850 CrossRef CAS PubMed.
- L. Yang, G. Zhang and H. Huang, Adv. Synth. Catal., 2014, 356, 1509 CrossRef CAS PubMed.
- (a) Y. Li, B.-J. Li, W.-H. Wang, W.-P. Huang, X.-S. Zhang, K. Chen and Z.-J. Shi, Angew. Chem., Int. Ed., 2011, 50, 2115 CrossRef CAS PubMed; (b) A. S. Tsai, M. E. Tauchert, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2011, 133, 1248 CrossRef CAS PubMed.
- Y. Li, X.-S. Zhang, H. Li, W.-H. Wang, K. Chen, B.-J. Li and Z.-J. Shi, Chem. Sci., 2012, 3, 1634 RSC.
- (a) K. Parthasarathy, A. R. Azcargorta, Y. Cheng and C. Bolm, Org. Lett., 2014, 16, 2538 CrossRef CAS PubMed; (b) L. Yang, C. A. Correia and C.-J. Li, Adv. Synth. Catal., 2011, 353, 1269 CrossRef CAS PubMed; (c) Y. Li, X.-S. Zhang, K. Chen, K.-H. He, F. Pan, B.-J. Li and Z.-J. Shi, Org. Lett., 2012, 14, 636 CrossRef CAS PubMed; (d) Y. Li, X.-S. Zhang, Q.-L. Zhu and Z.-J. Shi, Org. Lett., 2012, 14, 4498 CrossRef CAS PubMed.
- (a) X. Li, S. Yu, F. Wang, B. Wan and X. Yu, Angew. Chem., Int. Ed., 2013, 52, 2577 CrossRef CAS PubMed; (b) Z. Qi and X. Li, Angew. Chem., Int. Ed., 2013, 52, 8995 CrossRef CAS PubMed; (c) S. Yu and X. Li, Org. Lett., 2014, 16, 1200 CrossRef CAS PubMed; (d) S. Yu and X. Li, Org. Lett., 2014, 16, 1220 CrossRef CAS PubMed.
- Z.-H. Guan, Z.-H. Ren, S. M. Spinella, S. Yu, Y.-M. Liang and X. Zhang, J. Am. Chem. Soc., 2008, 131, 729 CrossRef PubMed.
- H. Mizuno, J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2011, 133, 1251 CrossRef CAS PubMed.
- M. Chaitanya, D. Yadagiri and P. Anbarasan, Org. Lett., 2013, 15, 4960 CrossRef CAS PubMed.
- X. Hong, H. Wang, G. Qian, Q. Tan and B. Xu, J. Org. Chem., 2014, 79, 3228 CrossRef CAS PubMed.
- J. Y. Kim, S. H. Park, J. Ryu, S. H. Cho, S. H. Kim and S. Chang, J. Am. Chem. Soc., 2012, 134, 9110 CrossRef CAS PubMed.
- N. Wang, R. Li, L. Li, S. Xu, H. Song and B. Wang, J. Org. Chem., 2014, 79, 5397 Search PubMed.
- C. Grohmann, H. Wang and F. Glorius, Org. Lett., 2013, 15, 3014 CrossRef CAS PubMed.
- B. Zhou, J. Du, Y. Yang and Y. Li, Org. Lett., 2013, 15, 2934 CrossRef CAS PubMed.
- B. Zhou, J. Du, Y. Yang, H. Feng and Y. Li, Org. Lett., 2014, 16, 592 CrossRef CAS PubMed.
- (a) H. Zhao, Y. Shang and W. Su, Org. Lett., 2013, 15, 5106 CrossRef CAS PubMed; (b) S. Yu, B. Wan and X. Li, Org. Lett., 2013, 15, 3706 CrossRef CAS PubMed.
- F. Xie, Z. Qi and X. Li, Angew. Chem., Int. Ed., 2013, 52, 11862 CrossRef CAS PubMed.
- (a) B. Zhou, J. Du, Y. Yang and Y. Li, Org. Lett., 2013, 15, 2934 CrossRef CAS PubMed; (b) B. Zhou, Y. Yang, J. Shi, H. Feng and Y. Li, Chem. – Eur. J., 2013, 19, 10511 CrossRef CAS PubMed.
- Y. Yang, W. Hou, L. Qin, J. Du, H. Feng, B. Zhou and Y. Li, Chem. – Eur. J., 2014, 20, 416 CrossRef CAS PubMed.
- S. Oi, S. Fukita, N. Hirata, N. Watanuki, S. Miyano and Y. Inoue, Org. Lett., 2001, 3, 2579 CrossRef CAS PubMed.
- S. Oi, K. Sakai and Y. Inoue, Org. Lett., 2005, 7, 4009 CrossRef CAS PubMed.
- L. Ackermann, A. Althammer and R. Born, Angew. Chem., Int. Ed., 2006, 45, 2619 CrossRef CAS PubMed.
- L. Ackermann, Org. Lett., 2005, 7, 3123 CrossRef CAS PubMed.
- X. Guo, G. Deng and C.-L. Li, Adv. Synth. Catal., 2009, 351, 2071 CrossRef CAS PubMed.
- K. Cheng, B. Yao, J. Zhao and Y. Zhang, Org. Lett., 2008, 10, 5309 CrossRef CAS PubMed.
- L. Liang, S. Fu, D. Lin, X.-Q. Zhang, Y. Deng, H. Jiang and W. Zeng, J. Org. Chem., 2014, 79, 9472 CrossRef CAS PubMed.
- P. B. Arockiam, C. Fischmeister, C. Bruneau and P. H. Dixneuf, Green Chem., 2011, 13, 3075 RSC.
- L. Ackermann, L. Wang and A. V. Lygin, Chem. Sci., 2012, 3, 177 RSC.
- G. Deng, L. Zhao and C.-J. Li, Angew. Chem., Int. Ed., 2008, 47, 6278 CrossRef CAS PubMed.
- M. Schinkel, I. Marek and L. Ackermann, Angew. Chem., Int. Ed., 2013, 52, 3977 CrossRef CAS PubMed.
- T. Kochi, S. Urano, H. Seki, E. Mizushima, M. Sato and F. Kakiuchi, J. Am. Chem. Soc., 2009, 131, 2792 CrossRef CAS PubMed.
- A. Tlili, J. Schranck, J. Pospech, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 6293 CrossRef CAS PubMed.
- J. A. Fernandez-Salas, S. Manzini, L. Piola, A. M. Z. Slawin and S. P. Nolan, Chem. Commun., 2014, 50, 6782 RSC.
- V. S. Thirunavukkarasu, K. Raghuvanshi and L. Ackermann, Org. Lett., 2013, 15, 3286 CrossRef CAS PubMed.
- F. Kakiuchi, K. Tsuchiya, M. Matsumoto, E. Mizushima and N. Chatani, J. Am. Chem. Soc., 2004, 126, 12792 CrossRef CAS PubMed.
- M. Kitahara, N. Umeda, K. Hirano, T. Satoh and M. Miura, J. Am. Chem. Soc., 2011, 133, 2160 CrossRef CAS PubMed.
- M. Nishino, K. Hirano, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2012, 51, 6993 CrossRef CAS PubMed.
- R. Odani, K. Hirano, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2014, 53, 10784 CrossRef CAS PubMed.
- X. Chen, X.-S. Hao, C. E. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 6790 CrossRef CAS PubMed.
- J. Jin, Q. Wen, P. Lu and Y. Wang, Chem. Commun., 2012, 48, 9933 RSC.
- X. Kou, M. Zhao, X. Qiao, Y. Zhu, X. Tong and Z. Shen, Chem. – Eur. J., 2013, 19, 16880 CrossRef CAS PubMed.
- H. Xu, P.-T. Liu, Y.-H. Li and F.-S. Han, Org. Lett., 2013, 15, 3354 CrossRef CAS PubMed.
- L. Zhang, Z. Liu, H. Li, G. Fang, B.-D. Barry, X. Bi and Q. Liu, Org. Lett., 2011, 13, 6536 CrossRef CAS PubMed.
- (a) J. Norinder, A. Matsumoto, N. Yoshikai and E. Nakamura, J. Am. Chem. Soc., 2008, 130, 5858 CrossRef CAS PubMed; (b) L. Ilies, M. Kobayashi, A. Matsumoto, N. Yoshikai and E. Nakamura, Adv. Synth. Catal., 2012, 354, 593 CrossRef CAS PubMed.
- L. Ilies, S. Asako and E. Nakamura, J. Am. Chem. Soc., 2011, 133, 7672 CrossRef CAS PubMed.
- K. Gao and N. Yoshikai, Chem. Commun., 2012, 48, 4305 RSC.
- K. Gao, R. Paira and N. Yoshikai, Adv. Synth. Catal., 2014, 356, 1486 CrossRef CAS PubMed.
- B. Zhou, H. Chen and C. Wang, J. Am. Chem. Soc., 2013, 135, 1264 CrossRef CAS PubMed.
- C.-H. Jun, J.-B. Hong, Y.-H. Kim and K. Y. Chung, Angew. Chem., Int. Ed., 2000, 39, 3440 CrossRef CAS.
- R. K. Thalji, K. A. Ahrendt, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2001, 123, 9692 CrossRef CAS.
- (a) R. K. Thalji, J. A. Ellman and R. G. Bergman, J. Am. Chem. Soc., 2004, 126, 7192 CrossRef CAS PubMed; (b) S. J. O'Malley, K. L. Tan, A. Watzke, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2005, 127, 13496 CrossRef PubMed; (c) K. A. Ahrendt, R. G. Bergman and J. A. Ellman, Org. Lett., 2003, 5, 1301 CrossRef CAS PubMed; (d) R. M. Wilson, R. K. Thalji, R. G. Bergman and J. A. Ellman, Org. Lett., 2006, 8, 1745 CrossRef CAS PubMed.
- K. Gao and N. Yoshikai, J. Am. Chem. Soc., 2013, 135, 9279 CrossRef CAS PubMed.
- S.-G. Lim, J. H. Lee, C. W. Moon, J.-B. Hong and C.-H. Jun, Org. Lett., 2003, 5, 2759 CrossRef CAS PubMed.
- P.-S. Lee, T. Fujita and N. Yoshikai, J. Am. Chem. Soc., 2011, 133, 17283 CrossRef CAS PubMed.
- (a) K. Gao, P.-S. Lee, C. Long and N. Yoshikai, Org. Lett., 2012, 14, 4234 CrossRef CAS PubMed; (b) T. Yamakawa and N. Yoshikai, Org. Lett., 2013, 15, 196 CrossRef CAS PubMed.
- (a) T. Zhang, L. Wu and X. Li, Org. Lett., 2013, 15, 6294 CrossRef CAS PubMed; (b) N.-J. Wang, S.-T. Mei, L. Shuai, Y. Yuan and Y. Wei, Org. Lett., 2014, 16, 3040 CrossRef CAS PubMed.
- Q.-R. Zhang, J.-R. Huang, W. Zhang and L. Dong, Org. Lett., 2014, 16, 1684 CrossRef CAS PubMed.
- S. Oi, Y. Ogino, S. Fukita and Y. Inoue, Org. Lett., 2002, 4, 1783 CrossRef CAS PubMed.
- M. J. Tredwell, M. Gulias, N. G. Bremeyer, C. C. C. Johansson, B. S. L. Collins and M. J. Gaunt, Angew. Chem., Int. Ed., 2011, 50, 1076 CrossRef CAS PubMed.
- N. Yoshikai, A. Matsumoto, J. Norinder and E. Nakamura, Angew. Chem., Int. Ed., 2009, 48, 2925 CrossRef CAS PubMed.
- P. W. Tan, N. A. B. Juwaini and J. Seayad, Org. Lett., 2013, 15, 5166 CrossRef CAS PubMed.
- Y. Kuninobu, Y. Tokunaga, A. Kawata and K. Takai, J. Am. Chem. Soc., 2006, 128, 202 CrossRef CAS PubMed.
- S. Sueki, Y. Guo, M. Kanai and Y. Kuninobu, Angew. Chem., Int. Ed., 2013, 52, 11879 CrossRef CAS PubMed.
- T. Fukutani, N. Umeda, K. Hirano, T. Satoh and M. Miura, Chem. Commun., 2009, 5141 RSC.
- K. Ueura, T. Satoh and M. Miura, Org. Lett., 2005, 7, 2229 CrossRef CAS PubMed.
- Z.-M. Sun, S.-P. Chen and P. Zhao, Chem. – Eur. J., 2010, 16, 2619 CrossRef CAS PubMed.
- J. Zhang, A. Ugrinov and P. Zhao, Angew. Chem., Int. Ed., 2013, 52, 6681 CrossRef CAS PubMed.
- (a) D. N. Tran and N. Cramer, Angew. Chem., Int. Ed., 2010, 49, 8181 CrossRef CAS PubMed; (b) D. N. Tran and N. Cramer, Angew. Chem., Int. Ed., 2011, 50, 11098 CrossRef CAS PubMed; (c) D. N. Tran and N. Cramer, Angew. Chem., Int. Ed., 2013, 52, 10630 CrossRef CAS PubMed.
- T. Nishimura, Y. Ebe and T. Hayashi, J. Am. Chem. Soc., 2013, 135, 2092 CrossRef CAS PubMed.
- L. Dong, C.-H. Qu, J.-R. Huang, W. Zhang, Q.-R. Zhang and J.-G. Deng, Chem. – Eur. J., 2013, 19, 16537 CrossRef CAS PubMed.
- P. Zhao, F. Wang, K. Han and X. Li, Org. Lett., 2012, 14, 5506 CrossRef CAS PubMed.
- (a) D. A. Colby, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2008, 130, 3645 CrossRef CAS PubMed; (b) S. Duttwyler, C. Lu, A. L. Rheingold, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2012, 134, 4064 CrossRef CAS PubMed; (c) S. Duttwyler, S. Chen, M. K. Takase, K. B. Wiberg, R. G. Bergman and J. A. Ellman, Science, 2013, 339, 678 CrossRef CAS PubMed; (d) N. Guimond and K. Fagnou, J. Am. Chem. Soc., 2009, 131, 12050 CrossRef CAS PubMed.
- R. He, Z.-T. Huang, Q.-Y. Zheng and C. Wang, Angew. Chem., Int. Ed., 2014, 53, 4950 CrossRef CAS PubMed.
- (a) J. Jayakumar, K. Parthasarathy and C.-H. Cheng, Angew. Chem., Int. Ed., 2012, 51, 197 CrossRef CAS PubMed; (b) N. Senthilkumar, P. Gandeepan, J. Jayakumar and C.-H. Cheng, Chem. Commun., 2014, 50, 3106 RSC; (c) K. Parthasarathy, N. Senthilkumar, J. Jayakumar and C.-H. Cheng, Org. Lett., 2012, 14, 3478 CrossRef CAS PubMed.
- L. V. Desai, K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2004, 126, 9542 CrossRef CAS PubMed.
- V. S. Thirunavukkarasu, K. Parthasarathy and C.-H. Cheng, Angew. Chem., Int. Ed., 2008, 47, 9462 CrossRef CAS PubMed.
- C.-L. Sun, N. Liu, B.-J. Li, D.-G. Yu, Y. Wang and Z.-J. Shi, Org. Lett., 2010, 12, 184 CrossRef CAS PubMed.
- V. S. Thirunavukkarasu and C.-H. Cheng, Chem. – Eur. J., 2011, 17, 14723 CrossRef CAS PubMed.
- A. S. Tsai, M. Brasse, R. G. Bergman and J. A. Ellman, Org. Lett., 2011, 13, 540 CrossRef CAS PubMed.
- (a) C.-W. Chan, Z. Zhou, A. S. C. Chan and W.-Y. Yu, Org. Lett., 2010, 12, 3926 CrossRef CAS PubMed; (b) S. Guin, S. K. Rout, A. Banerjee, S. Nandi and B. K. Patel, Org. Lett., 2012, 14, 5294 CrossRef CAS PubMed; (c) M. Kim, J. Park, S. Sharma, A. Kim, E. Park, J. H. Kwak, Y. H. Jung and I. S. Kim, Chem. Commun., 2013, 49, 925 RSC; (d) S. Han, S. Sharma, J. Park, M. Kim, Y. Shin, N. K. Mishra, J. J. Bae, J. H. Kwak, Y. H. Jung and I. S. Kim, J. Org. Chem., 2014, 79, 275 CrossRef CAS PubMed; (e) Y. Yang, B. Zhou and Y. Li, Adv. Synth. Catal., 2012, 354, 2916 CrossRef CAS PubMed.
- (a) K.-H. Ng, Z. Zhou and W.-Y. Yu, Org. Lett., 2012, 14, 272 CrossRef CAS PubMed; (b) K.-H. Ng, Z. Zhou and W.-Y. Yu, Chem. Commun., 2013, 49, 7031 RSC.
- W. Zhang, S. Lou, Y. Liu and Z. Xu, J. Org. Chem., 2013, 78, 5932 CrossRef CAS PubMed.
- T.-J. Gong, B. Xiao, W.-M. Cheng, W. Su, J. Xu, Z.-J. Liu, L. Liu and Y. Fu, J. Am. Chem. Soc., 2013, 135, 10630 CrossRef CAS PubMed.
- S.-J. Lou, D.-D. Xu and Z.-Y. Xu, Angew. Chem., Int. Ed., 2014, 53, 10330 CrossRef CAS PubMed.
- S. Yu, B. Wan and X. Li, Org. Lett., 2015, 17, 58 CrossRef CAS PubMed.
- (a) K. Parthasarathy, M. Jeganmohan and C.-H. Cheng, Org. Lett., 2008, 10, 325 CrossRef CAS PubMed; (b) T. K. Hyster and T. Rovis, Chem. Commun., 2011, 47, 11846 RSC; (c) P. C. Too, S. H. Chua, S. H. Wong and S. Chiba, J. Org. Chem., 2011, 76, 6159 CrossRef CAS PubMed; (d) X. Zhang, D. Chen, M. Zhao, J. Zhao, A. Jia and X. Li, Adv. Synth. Catal., 2011, 353, 719 CrossRef CAS PubMed; (e) R. K. Chinnagolla, S. Pimparkar and M. Jeganmohan, Org. Lett., 2012, 12, 3032 CrossRef PubMed; (f) C. Kornhhaß, J. Li and L. Ackemann, J. Org. Chem., 2012, 77, 9190 CrossRef PubMed; (g) S.-C. Chuang, P. Gandeepan and C.-H. Cheng, Org. Lett., 2013, 15, 5750 CrossRef CAS PubMed; (h) X.-C. Huang, X.-H. Yang, R.-J. Song and J.-H. Li, J. Org. Chem., 2014, 79, 1025 CrossRef CAS PubMed; (i) W. Han, G. Zhang, G. Li and H. Huang, Org. Lett., 2014, 16, 3532 CrossRef CAS PubMed; (j) Z.-W. Zhang, A. Lin and J. Yang, J. Org. Chem., 2014, 79, 7041 CrossRef CAS PubMed.
- P. C. Too, Y.-F. Wang and S. Chiba, Org. Lett., 2010, 12, 5688 CrossRef CAS PubMed.
- D. Zhao, F. Lied and F. Glorius, Chem. Sci., 2014, 5, 2869 RSC.
- J. M. Neely and T. Rovis, J. Am. Chem. Soc., 2013, 135, 66 CrossRef CAS PubMed.
- J. M. Neely and T. Rovis, J. Am. Chem. Soc., 2014, 136, 2735 CrossRef CAS PubMed.
- Y. Lian, R. G. Bergman and J. A. Ellman, Chem. Sci., 2012, 3, 3088 RSC.
- Y. Lian, T. Huber, K. D. Hesp, R. G. Bergman and J. A. Ellman, Angew. Chem., Int. Ed., 2013, 52, 629 CrossRef CAS PubMed.
- (a) B. Zhou, W. Hou, Y. Yang and Y. Li, Chem. – Eur. J., 2013, 19, 4701 CrossRef CAS PubMed; (b) W. Hou, B. Zhou, Y. Yang, H. Feng and Y. Li, Org. Lett., 2013, 15, 1814 CrossRef CAS PubMed.
- (a) H. Li, P. Li and L. Wang, Org. Lett., 2013, 15, 620 CrossRef CAS PubMed; (b) H. Li, P. Li, H. Tan and L. Wang, Chem. – Eur. J., 2013, 19, 14432 CrossRef CAS PubMed; (c) H. Li, P. Li, Q. Zhao and L. Wang, Chem. Commun., 2013, 49, 9170 RSC; (d) Z.-Y. Li, D.-D. Li and G.-W. Wang, J. Org. Chem., 2013, 78, 10414 CrossRef CAS PubMed.
- (a) F. Xiong, C. Qian, D. Lin, W. Zeng and X. Lu, Org. Lett., 2013, 15, 5444 CrossRef CAS PubMed; (b) H. Song, D. Chen, C. Pi, X. Cui and Y. Wu, J. Org. Chem., 2014, 79, 2955 CrossRef CAS PubMed.
- H. Tang, C. Qian, D. Lin, H. Jiang and W. Zeng, Adv. Synth. Catal., 2014, 356, 519 CrossRef CAS PubMed.
- Y. Lian, R. G. Bergman, L. D. Lavis and J. A. Ellman, J. Am. Chem. Soc., 2013, 135, 7122 CrossRef CAS PubMed.
- X.-T. Ma and S.-K. Tian, Adv. Synth. Catal., 2013, 355, 337 CAS.
- Z. Yin, X. Jiang and P. Sun, J. Org. Chem., 2013, 78, 10002 CrossRef CAS PubMed.
- J. Dong, B. Jin and P. Sun, Org. Lett., 2014, 16, 4540 CrossRef CAS PubMed.
- (a) K. Muralirajan and C.-H. Cheng, Chem. – Eur. J., 2013, 19, 6198 CrossRef CAS PubMed; (b) D. Zhao, Q. Wu, X. Huang, F. Song, T. Lv and J. You, Chem. – Eur. J., 2013, 19, 6239 CrossRef CAS PubMed.
- Y. Lian, J. R. Hummel, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2013, 135, 12548 CrossRef CAS PubMed.
- (a) X. Jia and J. He, J. Org. Chem., 2014, 79, 4180 CrossRef CAS PubMed; (b) H. Wang, Y. Yu, X. Hong, Q. Tan and B. Xu, J. Org. Chem., 2014, 79, 3279 CrossRef CAS PubMed; (c) T. Ryu, J. Min, W. Choi, W. H. Jeon and P. H. Lee, Org. Lett., 2014, 16, 2810 CrossRef CAS PubMed.
- W. Li, Z. Xu, P. Sun, X. Jiang and M. Fan, Org. Lett., 2011, 13, 1286 CrossRef CAS PubMed.
- W. Li and P. Sun, J. Org. Chem., 2012, 77, 8362 CrossRef CAS PubMed.
- B. Du, X. Jiang and P. Sun, J. Org. Chem., 2013, 78, 2786 CrossRef CAS PubMed.
- Y.-F. Wang, K. K. Toh, J.-Y. Lee and S. Chiba, Angew. Chem., Int. Ed., 2011, 50, 5927 CrossRef CAS PubMed.
- Y.-F. Wang, K. K. Toh, J.-Y. Lee and S. Chiba, Angew. Chem., Int. Ed., 2011, 50, 5927 CrossRef CAS PubMed.
- C. Wang, H. Chen, Z. Wang, J. Chen and Y. Huang, Angew. Chem., Int. Ed., 2012, 51, 7242 CrossRef CAS PubMed.
- B. Liu, Y. Fan, Y. Gao, C. Sun, C. Xu and J. Zhu, J. Am. Chem. Soc., 2013, 135, 468 CrossRef CAS PubMed.
- H. Li, X. Xie and L. Wang, Chem. Commun., 2014, 50, 4218 RSC.
- (a) B. Liu, C. Song, C. Sun, S. Zhou and J. Zhu, J. Am. Chem. Soc., 2013, 135, 16625 CrossRef CAS PubMed; (b) C. Wang and Y. Huang, Org. Lett., 2013, 15, 5294 CrossRef CAS PubMed.
- (a) W. Zhen, F. Wang, M. Zhao, Z. Du and X. Li, Angew. Chem., Int. Ed., 2012, 51, 11819 CrossRef CAS PubMed; (b) Y. Chen, F. Wang, W. Zhen and X. Li, Adv. Synth. Catal., 2013, 355, 353 CAS.
- F. Kakiuchi, K. Igi, M. Matsumoto, T. Hayamizu, N. Chatani and S. Murai, Chem. Lett., 2002, 30, 396 CrossRef.
- G. Cai, Y. Fu, Y. Li, X. Wan and Z. Shi, J. Am. Chem. Soc., 2007, 129, 7666 CrossRef CAS PubMed.
- H. Li, G.-X. Cai and Z.-J. Shi, Dalton Trans., 2010, 39, 10442 RSC.
- A. J. Roering, L. V. A. Hale, P. A. Squier, M. A. Ringgold, E. R. Wiederspan and T. B. Clark, Org. Lett., 2012, 14, 3558 CrossRef CAS PubMed.
- R. Feng, J. Yao, Z. Liang, Z. Liu and Y. Zhang, J. Org. Chem., 2013, 78, 3688 CrossRef CAS PubMed.
- H. Zhang, X. Cui, X. Yao, H. Wang, J. Zhang and Y. Wu, Org. Lett., 2012, 14, 3012 CrossRef CAS PubMed.
- C. Pi, X. Cui, X. Liu, M. Guo, H. Zhang and Y. Wu, Org. Lett., 2014, 16, 5164 CrossRef CAS PubMed.
- D.-W. Gao, Y.-C. Shi, Q. Gu, Z.-L. Zhao and S.-L. You, J. Am. Chem. Soc., 2012, 135, 86 CrossRef PubMed.
- K. Orito, A. Horibata, T. Nakamura, H. Ushito, H. Nagasaki, M. Yuguchi, S. Yamashita and M. Tokuda, J. Am. Chem. Soc., 2004, 126, 14342 CrossRef CAS PubMed.
- B. Haffemayer, M. Gulias and M. J. Gaunt, Chem. Sci., 2011, 2, 312 RSC.
- A. Lazareva and O. Daugulis, Org. Lett., 2006, 6, 5211 CrossRef PubMed.
- M. Miura, C.-G. Feng, S. Ma and J.-Q. Yu, Org. Lett., 2013, 15, 5258 CrossRef CAS PubMed.
- C. Suzuki, K. Morimoto, K. Hirano, T. Satoh and M. Miura, Adv. Synth. Catal., 2014, 356, 1521 CrossRef CAS PubMed.
- H. He, W.-B. Liu, L.-X. Dai and S.-L. You, J. Am. Chem. Soc., 2009, 131, 8346 CrossRef CAS PubMed.
- K.-Y. Ye, H. He, W.-B. Liu, L.-X. Dai, G. Helmchen and S.-L. You, J. Am. Chem. Soc., 2011, 133, 19006 CrossRef CAS PubMed.
- J. Shao, W. Chen, M. A. Giulianotti, R. A. Houghten and Y. Yu, Org. Lett., 2012, 14, 5452 CrossRef CAS PubMed.
- X. Wei, M. Zhao, Z. Du and X. Li, Org. Lett., 2011, 13, 4636 CrossRef CAS PubMed.
- Y. Wang and Q. Zhu, Adv. Synth. Catal., 2012, 354, 1902 CrossRef CAS PubMed.
- Z. Liang, L. Ju, Y. Xie, L. Huang and Y. Zhang, Chem. – Eur. J., 2012, 18, 15816 CrossRef CAS PubMed.
- Z. Liang, R. Feng, H. Yin and Y. Zhang, Org. Lett., 2013, 15, 4544 CrossRef CAS PubMed.
- Z. Liang, J. Yao, K. Wang, H. Li and Y. Zhang, Chem. – Eur. J., 2013, 19, 16825 CrossRef CAS PubMed.
- C. Suzuki, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2013, 15, 3990 CrossRef CAS PubMed.
- Q. Jiang, D. Duan-Mu, W. Zhong, H. Chen and H. Yan, Chem. – Eur. J., 2013, 19, 1903 CrossRef CAS PubMed.
- D. Liang, Z. Hu, J. Peng, J. Huang and Q. Zhu, Chem. Commun., 2013, 49, 173 RSC.
- Z. Liang, J. Zhang, Z. Liu, K. Wang and Y. Zhang, Tetrahedron, 2013, 69, 6519 CrossRef CAS PubMed.
- (a) G. Yan, A. J. Borah and M. Yang, Adv. Synth. Catal., 2014, 356, 2375 CrossRef CAS PubMed; (b) Y. Wang and L. Zhang, Synthesis, 2015, 289 Search PubMed.
- (a) L.-C. Campeau, S. Rousseaux and K. Fagnou, J. Am. Chem. Soc., 2005, 127, 18020 CrossRef CAS PubMed; (b) H.-Y. Sun, S. I. Gorelsky, D. R. Stuart, L.-C. Campeau and K. Fagnou, J. Org. Chem., 2010, 75, 8180 CrossRef CAS PubMed; (c) Y. Tan, F. Barrios-Landeros and J. F. Hartwig, J. Am. Chem. Soc., 2012, 134, 3683 CrossRef CAS PubMed; (d) L.-C. Campeau, D. J. Schipper and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 3266 CrossRef CAS PubMed.
- (a) H.-Q. Do, R. M. K. Khan and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 15185 CrossRef CAS PubMed; (b) D. Zhao, W. Wang, F. Yang, J. Lan, L. Yang, G. Gao and J. You, Angew. Chem., Int. Ed., 2009, 48, 3296 CrossRef CAS PubMed.
- (a) S. H. Cho, S. J. Hwang and S. Chang, J. Am. Chem. Soc., 2008, 130, 9254 CrossRef CAS PubMed; (b) P. Xi, F. Yang, S. Qin, D. Zhao, J. Lan, G. Gao, C. Hu and J. You, J. Am. Chem. Soc., 2010, 132, 1822 CrossRef CAS PubMed; (c) X. Gong, G. Song, H. Zhang and X. Li, Org. Lett., 2011, 13, 1766 CrossRef CAS PubMed.
- (a) K. S. Kanyiva, Y. Nakao and T. Hiyama, Angew. Chem., Int. Ed., 2007, 46, 8872 CrossRef CAS PubMed; (b) J. Wu, X. Cui, L. Chen, G. Jiang and Y. Wu, J. Am. Chem. Soc., 2009, 131, 13888 CrossRef CAS PubMed.
- Z. Wu, C. Pi, X. Cui, J. Bai and Y. Wu, Adv. Synth. Catal., 2013, 355, 1971 CrossRef CAS PubMed.
- B. Xiao, Z.-J. Liu, L. Liu and Y. Fu, J. Am. Chem. Soc., 2013, 135, 616 CrossRef CAS PubMed.
- O. V. Larionov, D. Stephens, A. Mfuh and G. Chavez, Org. Lett., 2014, 16, 864 CrossRef CAS PubMed.
- X. Chen, C. Zhu, X. Cui and Y. Wu, Chem. Commun., 2013, 49, 6900 RSC.
- (a) G. Li, C. Jia and K. Sun, Org. Lett., 2013, 15, 5198 CrossRef CAS PubMed; (b) C. Zhu, M. Yi, D. Wei, X. Chen, Y. Wu and X. Cui, Org. Lett., 2014, 16, 1840 CrossRef CAS PubMed.
- Z. Wu, H. Song, X. Cui, C. Pi, W. Du and Y. Wu, Org. Lett., 2013, 15, 1270 CrossRef CAS PubMed.
- (a) T. Shibata and Y. Matsuo, Adv. Synth. Catal., 2014, 356, 1516 CrossRef CAS PubMed; (b) X. Zhang, Z. Qi and X. Li, Angew. Chem., Int. Ed., 2014, 53, 10794 CrossRef CAS PubMed.
- D. E. Stephens, J. Lakey-Beitia, A. C. Atesin, T. A. Atesin, G. Chavez, H. D. Arman and O. V. Larionov, ACS Catal., 2015, 5, 167 CrossRef CAS PubMed.
- J. Jeong, P. Patel, H. Hwang and S. Chang, Org. Lett., 2014, 16, 4598 CrossRef CAS PubMed.
- H. Hwang, J. Kim, J. Jeong and S. Chang, J. Am. Chem. Soc., 2014, 136, 10770 CrossRef CAS PubMed.
- X. Huang, J. Huang, C. Du, X. Zhang, F. Song and J. You, Angew. Chem., Int. Ed., 2013, 52, 12970 CrossRef CAS PubMed.
- M. Miura, T. Tsuda, T. Satoh, S. Pivsa-Art and M. Nomura, J. Org. Chem., 1998, 63, 5211 CrossRef CAS.
- D. Nandi, D. Ghosh, S.-J. Chen, B.-C. Kuo, N. M. Wang and H. M. Lee, J. Org. Chem., 2013, 78, 3445 CrossRef CAS PubMed.
- L. Achermann and J. Pospech, Org. Lett., 2011, 13, 4153 CrossRef PubMed.
- W. Ma, R. Mei, G. Tenti and L. Ackermann, Chem. – Eur. J., 2014, 20, 15248 CrossRef CAS PubMed.
- S. Mochida, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2011, 76, 3024 CrossRef CAS PubMed.
- Y. Dong and G. Liu, Chem. Commun., 2013, 49, 8066 RSC.
- T. Iitsuka, P. Schaal, K. Hirano, T. Satoh, C. Bolm and M. Miura, J. Org. Chem., 2013, 78, 7216 CrossRef CAS PubMed.
- A. Maehara, H. Tsurugi, T. Satoh and M. Miura, Org. Lett., 2008, 10, 1159 CrossRef CAS PubMed.
- S. Mochida, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2010, 12, 5776 CrossRef CAS PubMed.
- T. Ueyama, S. Mochida, T. Fukutani, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2011, 13, 706 CrossRef CAS PubMed.
- K. L. Engelman, Y. Feng and E. A. Ison, Organometallics, 2011, 30, 4572 CrossRef CAS.
- D.-H. Wang, K. M. Engle, B.-F. Shi and J.-Q. Yu, Science, 2010, 327, 315 CrossRef CAS PubMed.
- B.-F. Shi, Y.-H. Zhang, J. K. Lam, D.-H. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 460 CrossRef CAS PubMed.
- H.-X. Dai, G. Li, X.-G. Zhang, A. F. Stepan and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 7567 CrossRef CAS PubMed.
- R. Giri, N. Maugel, J.-J. Li, D.-H. Wang, S. P. Breazzano, L. B. Saunders and J.-Q. Yu, J. Am. Chem. Soc., 2007, 129, 3510 CrossRef CAS PubMed.
- D.-H. Wang, T.-S. Mei and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 17676 CrossRef CAS PubMed.
- K. M. Engle, P. S. Thuy-Boun, M. Dang and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 18183 CrossRef CAS PubMed.
- H. A. Chiong, Q.-N. Pham and O. Daugulis, J. Am. Chem. Soc., 2007, 129, 9879 CrossRef CAS PubMed.
- (a) J. Cornella, M. Righi and I. Larrosa, Angew. Chem., Int. Ed., 2011, 50, 9429 CrossRef CAS PubMed; (b) C. Arroniz, A. Ironmonger, G. Rassias and I. Larrosa, Org. Lett., 2013, 15, 910 CrossRef CAS PubMed.
- P. S. Thuy-Boun, G. Villa, D. Dang, P. Richardson, S. Su and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 17508 CrossRef CAS PubMed.
- Y.-H. Zhang, B.-F. Shi and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 6097 CrossRef CAS PubMed.
- P. Mamone, G. Danoun and L. J. Gooßen, Angew. Chem., Int. Ed., 2013, 52, 6704 CrossRef CAS PubMed.
- J. Miao and H. Ge, Org. Lett., 2013, 15, 2930 CrossRef CAS PubMed.
- R. Giri and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 14082 CrossRef CAS PubMed.
- L. C. Kao and A. Sen, J. Chem. Soc., Chem. Commun., 1991, 1242 RSC.
- B. D. Dangel, J. A. Johnson and D. Sames, J. Am. Chem. Soc., 2001, 123, 8149 CrossRef CAS.
- J. M. Lee and S. Chang, Tetrahedron Lett., 2006, 47, 1375 CrossRef CAS PubMed.
- K. J. Fraunhoffer, N. Prabagaran, L. E. Sirois and M. C. White, J. Am. Chem. Soc., 2006, 128, 9032 CrossRef CAS PubMed.
- P. Novak, A. Correa, J. Gallardo-Donaire and R. Martin, Angew. Chem., Int. Ed., 2011, 50, 12236 CrossRef CAS PubMed.
- K. Takenaka, M. Akita, Y. Tanigaki, S. Takizawa and H. Sasai, Org. Lett., 2011, 13, 3506 CrossRef CAS PubMed.
- X.-F. Cheng, Y. Li, Y.-M. Su, F. Yin, J.-Y. Wang, J. Sheng, H. U. Vora, X.-S. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 1236 CrossRef CAS PubMed.
- M. Yang, X. Jiang, W.-J. Shi, Q.-L. Zhu and Z.-J. Shi, Org. Lett., 2013, 15, 690 CrossRef CAS PubMed.
- Y. Li, Y.-J. Ding, J.-Y. Wang, Y.-M. Su and X.-S. Wang, Org. Lett., 2013, 15, 2574 CrossRef CAS PubMed.
- J. Gallardo-Donaire and R. Martin, J. Am. Chem. Soc., 2013, 135, 9350 CrossRef CAS PubMed.
- K. Ueura, T. Satoh and M. Miura, Org. Lett., 2007, 9, 1407 CrossRef CAS PubMed.
- M. Shimizu, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 3478 CrossRef CAS PubMed.
- S. Mochida, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 6295 CrossRef CAS PubMed.
- Z. Qi, M. Wang and X. Li, Chem. Commun., 2014, 50, 9776 RSC.
- K. Ueura, T. Satoh and M. Miura, J. Org. Chem., 2007, 72, 5362 CrossRef CAS PubMed.
- R. K. Chinnagolla and M. Jeganmohan, Chem. Commun., 2012, 48, 2030 RSC.
- L. Ackermann, J. Pospech, K. Graczyk and K. Rauch, Org. Lett., 2012, 14, 930 CrossRef CAS PubMed.
- M. Deponti, S. I. Kozhushkov, D. S. Yufit and L. Ackermann, Org. Biomol. Chem., 2013, 11, 142 CAS.
- R. R. Suresh and K. C. K. Swamy, J. Org. Chem., 2012, 77, 6959 CrossRef CAS PubMed.
- X. Shi and C.-J. Li, Adv. Synth. Catal., 2012, 354, 2933 CrossRef CAS PubMed.
- G. Danoun, P. Mamone and L. J. Gooßen, Chem. – Eur. J., 2013, 19, 17287 CrossRef CAS PubMed.
- Y.-H. Zhang and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 14654 CrossRef CAS PubMed.
- S. Bhadra, W. I. Dzik and L. J. Gooßen, Angew. Chem., Int. Ed., 2013, 52, 2959 CrossRef CAS PubMed.
- K.-H. Ng, F.-N. Ng and W.-Y. Yu, Chem. Commun., 2012, 48, 11680 RSC.
- F.-N. Ng, Z. Zhou and W.-Y. Yu, Chem. – Eur. J., 2014, 20, 4474 CrossRef CAS PubMed.
- T.-S. Mei, R. Giri, N. Maugel and J.-Q. Yu, Angew. Chem., Int. Ed., 2008, 47, 5215 CrossRef CAS PubMed.
- T.-S. Mei, D.-H. Wang and J.-Q. Yu, Org. Lett., 2010, 12, 3140 CrossRef CAS PubMed.
- (a) F. Kakiuchi, Y. Tanaka, T. Sato, N. Chatani and S. Murai, Chem. Lett., 1995, 679 CrossRef CAS; (b) B. M. Trost, K. Imi and W. Davies, J. Am. Chem. Soc., 1995, 117, 5371 CrossRef CAS.
- (a) H. Guo and W. P. Weber, Polym. Bull., 1994, 32, 525 CrossRef CAS; (b) H. Guo, G. Wang, M. A. Tapsak and W. P. Weber, Macromolecules, 1995, 28, 5686 CrossRef CAS; (c) P. Lu, J. Paulasaari, K. Jin, R. Bau and W. P. Weber, Organometallics, 1998, 17, 584 CrossRef CAS.
- (a) R. Martinez, R. Chevalier, S. Darses and J.-P. Genet, Angew. Chem., Int. Ed., 2006, 45, 8232 CrossRef CAS PubMed; (b) R. Martinez, J.-P. Genet and S. Darses, Chem. Commun., 2008, 3855 RSC; (c) R. Martinez, M.-O. Simon, R. Chevalier, C. Pautigny, J.-P. Genet and S. Darses, J. Am. Chem. Soc., 2009, 131, 7887 CrossRef CAS PubMed; (d) M.-O. Simon, R. Martinez, J.-P. Genet and S. Darses, Adv. Synth. Catal., 2009, 351, 153 CrossRef CAS PubMed; (e) M.-O. Simon, R. Martinez, J.-P. Genet and S. Darses, J. Org. Chem., 2010, 75, 208 CrossRef CAS; (f) M.-O. Simon, J.-P. Genet and S. Darses, Org. Lett., 2010, 12, 3038 CrossRef CAS PubMed.
- C. P. Lenges and M. Brookhart, J. Am. Chem. Soc., 1999, 121, 6616 CrossRef CAS.
- G. E. M. Crisenza, N. G. McCreanor and J. F. Bower, J. Am. Chem. Soc., 2014, 136, 10258 CrossRef CAS PubMed.
- F. Kakiuchi, Y. Yamamoto, N. Chanati and S. Murai, Chem. Lett., 1995, 681 CrossRef CAS.
- F. Kakiuchi, T. Uetsuhara, Y. Tanaka, N. Chanati and S. Murai, J. Mol. Catal. A: Chem., 2002, 182, 511 CrossRef.
- P. W. R. Harris, C. E. F. Rickard and P. D. Woodgate, J. Organomet. Chem., 1999, 589, 168 CrossRef CAS.
- K. Tsuchikama, M. Kasagawa, Y.-K. Hashimoto, K. Endo and T. Shibata, J. Organomet. Chem., 2008, 693, 3939 CrossRef CAS PubMed.
- K. Tanaka, Y. Otake, A. Wada, K. Noguchi and M. Hirano, Org. Lett., 2007, 9, 2203 CrossRef CAS PubMed.
- K. Tsuchikama, Y. Kuwata, Y.-K. Tahara, Y. Yoshinami and T. Shibata, Org. Lett., 2007, 9, 3097 CrossRef CAS PubMed.
- F. W. Patureau, T. Besset, N. Kuhl and F. Glorius, J. Am. Chem. Soc., 2011, 133, 2154 CrossRef CAS PubMed.
- K. Muralirajan, K. Parthasarathy and C.-H. Cheng, Angew. Chem., Int. Ed., 2011, 50, 4169 CrossRef CAS PubMed.
- R. K. Chinnagolla and M. Jeganmohan, Eur. J. Org. Chem., 2012, 417 CrossRef CAS PubMed.
- K. Padala and M. Jeganmohan, Org. Lett., 2011, 13, 6144 CrossRef CAS PubMed.
- K. Padala and M. Jeganmohan, Org. Lett., 2012, 14, 1134 CrossRef CAS PubMed.
- V. Lanke and K. R. Prabhu, Org. Lett., 2013, 15, 6262 CrossRef CAS PubMed.
- X.-Y. Shi and C.-J. Li, Org. Lett., 2013, 15, 1476 CrossRef CAS PubMed.
- V. P. Mehta, J.-A. García-López and M. F. Greaney, Angew. Chem., Int. Ed., 2014, 53, 1529 CrossRef CAS PubMed.
- T. Satoh, Y. Kametani, Y. Terao, M. Miura and M. Nomura, Tetrahedron Lett., 1999, 40, 5345 CrossRef CAS.
- (a) F. Kakiuchi, S. Kan, K. Igi, N. Chanati and S. Murai, J. Am. Chem. Soc., 2003, 125, 1689 CrossRef PubMed; (b) F. Kakiuchi, Y. Matsuura, S. Kan and N. Chanati, J. Am. Chem. Soc., 2005, 127, 5936 CrossRef CAS PubMed.
- P. Gandeepan, K. Parthasarathy and C.-H. Cheng, J. Am. Chem. Soc., 2010, 132, 8569 CrossRef CAS PubMed.
- P. Gandeepan, C.-H. Hung and C.-H. Cheng, Chem. Commun., 2012, 48, 9379 RSC.
- H. Li, R.-Y. Zhu, W.-J. Shi, K.-H. He and Z.-J. Shi, Org. Lett., 2012, 14, 4850 CrossRef CAS PubMed.
- B. Xiao, T.-J. Gong, J. Xu, Z.-J. Liu and L. Liu, J. Am. Chem. Soc., 2011, 133, 1466 CrossRef CAS PubMed.
- M. Bhanuchandra, M. R. Yadav, R. K. Rit, M. R. Kuram and A. K. Sahoo, Chem. Commun., 2013, 49, 5225 RSC.
- Q.-Z. Zheng, Y.-F. Liang, C. Qin and N. Jiao, Chem. Commun., 2013, 49, 5654 RSC.
- J. Kim, J. Kim and S. Chang, Chem. – Eur. J., 2013, 19, 7328 CrossRef CAS PubMed.
- J. Kim and S. Chang, Angew. Chem., Int. Ed., 2014, 53, 2203 CrossRef CAS PubMed.
- G. Shan, X. Yang, L. Ma and Y. Rao, Angew. Chem., Int. Ed., 2012, 51, 13070 CrossRef CAS PubMed.
- F. Mo, L. J. Trzepkowski and G. Dong, Angew. Chem., Int. Ed., 2012, 51, 13075 CrossRef CAS PubMed.
- P. Y. Choy and F. Y. Kwong, Org. Lett., 2013, 15, 270 CrossRef CAS PubMed.
- V. S. Thirunavukkarasu and L. Ackermann, Org. Lett., 2012, 14, 6206 CrossRef CAS PubMed.
- F. Yang, K. Rauch, K. Kettelhoit and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 11285 CrossRef CAS PubMed.
- (a) M. Sonoda, F. Kakiuchi, A. Kamatani, N. Chatani and S. Murai, Chem. Lett., 1996, 25, 109 CrossRef; (b) F. Kakiuchi, H. Ohtaki, M. Sonoda, N. Chatani and S. Murai, Chem. Lett., 2001, 30, 918 CrossRef.
- N. M. Neisius and B. Plietker, Angew. Chem., Int. Ed., 2009, 48, 5752 CrossRef CAS PubMed.
- S. H. Park, J. Y. Kim and S. Chang, Org. Lett., 2011, 13, 2372 CrossRef CAS PubMed.
- K. Graczyk, W. Ma and L. Ackermann, Org. Lett., 2012, 14, 4110 CrossRef CAS PubMed.
- K. Padala, S. Pimparkar, P. Madasamy and M. Jeganmohan, Chem. Commun., 2012, 48, 7140 RSC.
- R. Feng, W. Yu, K. Wang, Z. Liu and Y. Zhang, Adv. Synth. Catal., 2014, 356, 1501 CrossRef CAS PubMed.
- S. Kawamorita, H. Ohmiya, K. Hara, A. Fukuoka and M. Sawamura, J. Am. Chem. Soc., 2009, 131, 5058 CrossRef CAS PubMed.
- S. Kawamorita, H. Ohmiya and M. Sawamura, J. Org. Chem., 2010, 75, 3855 CrossRef CAS PubMed.
- T. Ishiyama, H. Isou, T. Kikuchi and N. Miyaura, Chem. Commun., 2010, 46, 159 RSC.
- B. Ghaffari, S. M. Preshlock, D. L. Plattner, R. J. Staples, P. E. Maligres, S. W. Krska, R. E. Maleczka Jr. and M. R. Smith, J. Am. Chem. Soc., 2014, 136, 14345 CrossRef CAS PubMed.
- Y. Yang, Y. Lin and Y. Rao, Org. Lett., 2012, 14, 2874 CrossRef CAS PubMed.
- X. Sun, G. Shan, Y. Sun and Y. Rao, Angew. Chem., Int. Ed., 2013, 52, 4440 CrossRef CAS PubMed.
- S. D. Sarkar, W. Liu, S. I. Kozhushkov and L. Ackermanna, Adv. Synth. Catal., 2014, 356, 1461 CrossRef PubMed.
- For a review, see: V. Snieckus, Chem. Rev., 1990, 90, 879 CrossRef CAS.
- M. P. Sibi and V. Snieckus, J. Org. Chem., 1983, 48, 1935 CrossRef CAS.
- R. B. Bedford, R. L. Webstera and C. J. Mitchell, Org. Biomol. Chem., 2009, 7, 4853 CAS.
- X. Zhao, C. S. Yeung and V. M. Dong, J. Am. Chem. Soc., 2010, 132, 5837 CrossRef CAS PubMed.
- B. Xiao, Y. Fu, J. Xu, T.-J. Gong, J.-J. Dai, J. Yi and L. Liu, J. Am. Chem. Soc., 2010, 132, 468 CrossRef CAS PubMed.
- T.-J. Gong, B. Xiao, Z.-J. Liu, J. Wan, J. Xu, D.-F. Luo, Y. Fu and L. Liu, Org. Lett., 2011, 13, 3235 CrossRef CAS PubMed.
- C. Feng and T.-P. Loh, Chem. Commun., 2011, 47, 10458 RSC.
- Q. Zhang, H.-Z. Yu, Y.-T. Li, L. Liu, Y. Huang and Y. Fu, Dalton Trans., 2013, 42, 4175 RSC.
- J. Li, C. Kornhaaß and L. Ackermann, Chem. Commun., 2012, 48, 11343 RSC.
- B. Li, J. Ma, Y. Liang, N. Wang, S. Xu, H. Song and B. Wang, Eur. J. Org. Chem., 2013, 1950 CrossRef CAS PubMed.
- M. C. Reddy and M. Jeganmohan, Eur. J. Org. Chem., 2013, 1150 CrossRef CAS PubMed.
- M. C. Reddy and M. Jeganmohan, Chem. Commun., 2013, 49, 481 RSC.
- A. John and K. M. Nicholas, J. Org. Chem., 2012, 77, 5600 CrossRef CAS PubMed.
- X. Y. Sun, Y. H. Sun, C. Zhang and Y. Rao, Chem. Commun., 2014, 50, 1262 RSC.
- S. Sharma, A. Kim, E. Park, J. Park, M. Kim, J. H. Kwak, S. H. Lee, Y. H. Jung and I. S. Kima, Adv. Synth. Catal., 2013, 355, 667 CrossRef CAS PubMed.
- W. P. Liu and L. Ackermann, Org. Lett., 2013, 15, 3484 CrossRef CAS PubMed.
- K. Yamazaki, S. Kawamorita, H. Ohmiya and M. Sawamura, Org. Lett., 2010, 12, 3978 CrossRef CAS PubMed.
- (a) T. Satoh, Y. Kawamura, M. Miura and M. Nomura, Angew. Chem., Int. Ed. Engl., 1997, 36, 1740 CAS; (b) T. Satoh, J. Inoh, Y. Kawamura, M. Miura and M. Nomura, Bull. Chem. Soc. Jpn., 1988, 71, 2239 CrossRef.
- Y. Terao, H. Wakui, T. Satoh, M. Miura and M. Nomura, J. Am. Chem. Soc., 2001, 123, 10407 CrossRef CAS.
- Y. Terao, H. Wakui, M. Nomoto, T. Satoh, M. Miura and M. Nomura, J. Org. Chem., 2003, 68, 5236 CrossRef CAS PubMed.
- M. Miura, T. Tsuda, T. Satoh and M. Nomura, Chem. Lett., 1997, 1103 CrossRef CAS.
- Y. Lu, D.-H. Wang, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 5916 CrossRef CAS PubMed.
- C. Huang, B. Chattopadhyay and V. Gevorgyan, J. Am. Chem. Soc., 2011, 133, 12406 CrossRef CAS PubMed.
- C. Wang and H. Ge, Chem. – Eur. J., 2011, 17, 14371 CrossRef CAS PubMed.
- S. R. Chidipudi, M. D. Wieczysty, I. Khan and H. W. Lam, Org. Lett., 2013, 15, 570 CrossRef PubMed.
- Y. Kim, Y. Moon, D. Kang and S. Hong, Org. Biomol. Chem., 2014, 12, 3413 CAS.
- C. Zhang, J. Ji and P. Sun, J. Org. Chem., 2014, 79, 3200 CrossRef CAS PubMed.
- T. Satoh, Y. Nishinaka, M. Miura and M. Nomura, Chem. Lett., 1999, 615 CrossRef CAS.
- S. Mochida, M. Shimizu, K. Hirano, T. Satoh and M. Miura, Chem. – Asian J., 2010, 5, 847 CrossRef CAS PubMed.
- V. S. Thirunavukkarasu, M. Donati and L. Ackermann, Org. Lett., 2012, 14, 3416 CrossRef CAS PubMed.
- K. Morimoto, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2011, 76, 9548 CrossRef CAS PubMed.
- S. Nakanowatari and L. Ackermann, Chem. – Eur. J., 2014, 20, 5409 CrossRef CAS PubMed.
- S. R. Chidipudi, I. Khan and H. W. Lam, Angew. Chem., Int. Ed., 2012, 51, 12115 CrossRef PubMed.
- J. D. Dooley, S. R. Chidipudi and H. W. Lam, J. Am. Chem. Soc., 2013, 135, 10829 CrossRef CAS PubMed.
- D. J. Burns and H. W. Lam, Angew. Chem., Int. Ed., 2014, 53, 9931 CrossRef CAS PubMed.
- J. Nan, Z. Zuo, L. Luo, L. Bai, H. Zheng, Y. Yuan, J. Liu, X. Luan and Y. Wang, J. Am. Chem. Soc., 2013, 135, 17306 CrossRef CAS PubMed.
- A. Seoane, N. Casanova, N. Quiñones, J. L. Mascareñas and M. Gulías, J. Am. Chem. Soc., 2014, 136, 834 CrossRef CAS PubMed.
- A. Seoane, N. Casanova, N. Quiñones, J. L. Mascareñas and M. Gulías, J. Am. Chem. Soc., 2014, 136, 7607 CrossRef CAS PubMed.
- S. Kujawa, D. Best, D. J. Burns and H. W. Lam, Chem. – Eur. J., 2014, 20, 8599 CrossRef CAS PubMed.
- Y. Lu, D. Leow, X. Wang, K. M. Engle and J.-Q. Yu, Chem. Sci., 2011, 2, 967 RSC.
- S. Luo, F.-X. Luo, X.-S. Zhang and Z.-J. Shi, Angew. Chem., Int. Ed., 2013, 52, 10598 CrossRef CAS PubMed.
- T.-H. Lee, J. Jayakumar, C.-H. Cheng and S. Chuang, Chem. Commun., 2013, 49, 11797 RSC.
- K. Inamoto, J. Kadokawa and Y. Kondo, Org. Lett., 2013, 15, 3962 CrossRef CAS PubMed.
- K. Sasano, J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2013, 135, 10954 CrossRef CAS PubMed.
- X. Wang, Y. Lu, H.-X. Dai and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 12203 CrossRef CAS PubMed.
- B. Xiao, T.-J. Gong, Z.-J. Liu, J.-H. Liu, D.-F. Luo, J. Xu and L. Liu, J. Am. Chem. Soc., 2011, 133, 9250 CrossRef CAS PubMed.
- Y. Wei and N. Yoshikai, Org. Lett., 2011, 13, 5504 CrossRef CAS PubMed.
- J. Zhao, Y. Wang, Y. He, L. Liu and Q. Zhu, Org. Lett., 2012, 14, 1078 CrossRef CAS PubMed.
- Y. Moon, Y. Kim, H. Hong and S. Hong, Chem. Commun., 2013, 49, 8323 RSC.
- C. Huang, N. Ghavtadze, B. Chattopadhyay and V. Gevorgyan, J. Am. Chem. Soc., 2011, 133, 17630 CrossRef CAS PubMed.
- C. Huang, N. Ghavtadze, B. Godoi and V. Gevorgyan, Chem. – Eur. J., 2012, 18, 9789 CrossRef CAS PubMed.
- E. M. Simmons and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 17092 CrossRef CAS PubMed.
- T. Satoh, T. Itaya, M. Miura and M. Nomura, Chem. Lett., 1996, 823 CrossRef CAS.
- (a) K. Kokubo, K. Matsumasa, M. Miura and M. Nomura, J. Org. Chem., 1997, 62, 4564 CrossRef CAS; (b) K. Kokubo, K. Matsumasa, Y. Nishinaka, M. Miura and M. Nomura, Bull. Chem. Soc. Jpn., 1999, 72, 303 CrossRef CAS.
- M. Imai, M. Tanaka, S. Nagumo, N. Kawahara and H. Suemune, J. Org. Chem., 2007, 72, 2543 CrossRef CAS PubMed.
- R. T. Stemmler and C. Bolm, Adv. Synth. Catal., 2007, 349, 1185 CrossRef CAS PubMed.
- M. Shimizu, H. Tsurugi, T. Satoh and M. Miura, Chem. – Asian J., 2008, 3, 881 CrossRef CAS PubMed.
- S. Wang, K. Xie, Z. Tan, X. An, X. Zhou, C.-C. Guo and Z. Peng, Chem. Commun., 2009, 6469 RSC.
- Z. Shi, N. Schrçder and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 8092 CrossRef CAS PubMed.
- G. Li, D. Leow, L. Wan and J.-Q. Yu, Angew. Chem., Int. Ed., 2013, 52, 1245 CrossRef CAS PubMed.
- M. Yu, Y. Xie, C. Xie and Y. Zhang, Org. Lett., 2012, 14, 2164 CrossRef CAS PubMed.
- J. Yao, M. Yu and Y. Zhang, Adv. Synth. Catal., 2012, 354, 3205 CrossRef CAS PubMed.
- X.-S. Zhang, Q.-L. Zhu, Y.-F. Zhang, Y.-B. Li and Z.-J. Shi, Chem. – Eur. J., 2013, 19, 11898 CrossRef CAS PubMed.
- X.-S. Zhang, Y.-F. Zhang, K. Chen and Z.-J. Shi, Org. Chem. Front., 2014, 1, 1096 RSC.
- R. Samanta and A. P. Antonchick, Angew. Chem., Int. Ed., 2011, 50, 5217 CrossRef CAS PubMed.
- B. Wang, Y. Liu, C. Lin, Y. Xu, Z. Liu and Y. Zhang, Org. Lett., 2014, 16, 4574 CrossRef CAS PubMed.
- B. Wang, C. Shen, J. Yao, H. Yin and Y. Zhang, Org. Lett., 2014, 16, 46 CrossRef CAS PubMed.
- K. Nobushige, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2014, 16, 1188 CrossRef CAS PubMed.
- T. Wesch, F. R. Leroux and F. Colobert, Adv. Synth. Catal., 2013, 355, 2139 CrossRef CAS PubMed.
- C. K. Hazra, Q. Dherbassy, J. W.-Delord and F. Colobert, Angew. Chem., Int. Ed., 2014, 53, 13871 CrossRef CAS PubMed.
- T. A. Boebel and J. F. Hartwig, J. Am. Chem. Soc., 2008, 130, 7534 CrossRef CAS PubMed.
- D. W. Robbins, T. A. Boebel and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 4068 CrossRef CAS PubMed.
- S. H. Cho and J. F. Hartwig, J. Am. Chem. Soc., 2013, 135, 8157 CrossRef CAS PubMed.
- E. M. Simmons and J. F. Hartwig, Nature, 2012, 483, 70 CrossRef CAS PubMed.
- T. Ureshino, T. Yoshida, Y. Kuninobu and K. Taka, J. Am. Chem. Soc., 2010, 132, 14324 CrossRef CAS PubMed.
- R. B. Bedford, S. J. Coles, M. B. Hursthouse and M. E. Limmert, Angew. Chem., Int. Ed., 2003, 42, 112 CrossRef CAS PubMed.
- S. Oi, S.-i. Watanabe, S. Fukita and Y. Inoue, Tetrahedron Lett., 2003, 44, 8665 CrossRef CAS PubMed.
- Y. Kuninobu, T. Yoshida and K. Takai, J. Org. Chem., 2011, 76, 7370 CrossRef CAS PubMed.
- K. M. Crawford, T. R. Ramseyer, C. J. A. Daley and T. B. Clark, Angew. Chem., Int. Ed., 2014, 53, 7589 CrossRef CAS PubMed.
- X. Meng and S. Kim, Org. Lett., 2013, 15, 1910 CrossRef CAS PubMed.
- Y. Unoh, Y. Hashimoto, D. Takeda, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2013, 15, 3258 CrossRef CAS PubMed.
- (a) J. Seo, Y. Park, I. Jeon, T. Ryu, S. Park and P. H. Lee, Org. Lett., 2013, 15, 3358 CrossRef CAS PubMed; (b) T. Ryu, J. Kim, Y. Park, S. Kim and P. H. Lee, Org. Lett., 2013, 15, 3986 CrossRef CAS PubMed; (c) Y. Park, J. Seo, S. Park, E. J. Yoo and P. H. Lee, Chem. – Eur. J., 2013, 19, 16461 CrossRef CAS PubMed.
- L. Y. Chan, X. Meng and S. Kim, J. Org. Chem., 2013, 78, 8826 CrossRef CAS PubMed.
- S. Shin, Y. Jeong, W. H. Jeon and P. H. Lee, Org. Lett., 2014, 16, 2930 CrossRef CAS PubMed.
- L. Y. Chan, L. Cheong and S. Kim, Org. Lett., 2013, 15, 2186 CrossRef CAS PubMed.
- W. H. Jeon, T. S. Lee, E. J. Kim, B. Moon and J. Kang, Tetrahedron, 2013, 69, 5152 CrossRef CAS PubMed.
- L. Y. Chan, S. Kim, T. Ryu and P. H. Lee, Chem. Commun., 2013, 49, 4682 RSC.
- B. C. Chary, S. Kim, Y. Park, J. Kim and P. H. Lee, Org. Lett., 2013, 15, 2692 CrossRef CAS PubMed.
- D. Zhao, C. Nimphius, M. Lindale and F. Glorius, Org. Lett., 2013, 15, 4504 CrossRef CAS PubMed.
- B. C. Chary and S. Kim, Org. Biomol. Chem., 2013, 11, 6879 CAS.
- J. Mo, S. Lim, S. Park, T. Ryu, S. Kim and P. H. Lee, RSC Adv., 2013, 3, 18296 RSC.
- M. Itoh, Y. Hashimoto, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2013, 78, 8098 CrossRef CAS PubMed.
- H.-L. Wang, R.-B. Hu, H. Zhang, A.-X. Zhou and S.-D. Yang, Org. Lett., 2013, 15, 5302 CrossRef CAS PubMed.
- H.-Y. Zhang, H.-M. Yi, G.-W. Wang, B. Yang and S.-D. Yang, Org. Lett., 2013, 15, 6186 CrossRef CAS PubMed.
- H. Zhang, R.-B. Hu, X.-Y. Zhang, S.-X. Li and S.-D. Yang, Chem. Commun., 2014, 50, 4686 RSC.
- R.-B. Hu, H. Zhang, X.-Y. Zhang and S.-D. Yang, Chem. Commun., 2014, 50, 2193 RSC.
- P. Gandeepan and C. H. Cheng, Chem. – Asian J., 2015, 10, 824 CrossRef CAS PubMed.
- S.-i. Kiyooka and Y. Takeshita, Tetrahedron Lett., 2005, 46, 4279 CrossRef CAS PubMed.
- S. Yamada, Y. Obora, S. Sakaguchi and Y. Ishii, Bull. Chem. Soc. Jpn., 2007, 80, 1194 CrossRef CAS.
- M. Tobisu, I. Hyodo, M. Onoe and N. Chatani, Chem. Commun., 2008, 6013 RSC.
- P. Gandeepan and C.-H. Cheng, J. Am. Chem. Soc., 2012, 134, 5738 CrossRef CAS PubMed.
- P. Gandeepan and C.-H. Cheng, Org. Lett., 2013, 15, 2084 CrossRef CAS PubMed.
- N. Chernyak and V. Gevorgyan, J. Am. Chem. Soc., 2008, 130, 5636 CrossRef CAS PubMed.
- V. Mamane, P. Hannen and A. Furstner, Chem. – Eur. J., 2004, 10, 4556 CrossRef CAS PubMed.
- N. Chernyak and V. Gevorgyan, Adv. Synth. Catal., 2009, 351, 1101 CrossRef CAS PubMed.
- N. Chernyak, S. I. Gorelsky and V. Gevorgyan, Angew. Chem., Int. Ed., 2011, 50, 2342 CrossRef CAS PubMed.
- J. Zhao, N. Asao, Y. Yamamoto and T. Jin, J. Am. Chem. Soc., 2014, 136, 9540 CrossRef CAS PubMed.
- (a) Y. Minami, Y. Shiraishi, K. Yamada and T. Hiyama, J. Am. Chem. Soc., 2012, 134, 6124 CrossRef CAS PubMed; (b) Y. Minami, T. Anami and T. Hiyama, Chem. Lett., 2014, 43, 1791 CrossRef.
- (a) Y. Minami, M. Kanda and T. Hiyama, Chem. Lett., 2014, 43, 181 CrossRef CAS; (b) Y. Minami, M. Kanda and T. Hiyama, Chem. Lett., 2014, 43, 1408 CrossRef CAS.
- Y. Minami, K. Yamada and T. Hiyama, Angew. Chem., Int. Ed., 2013, 52, 10611 CrossRef CAS PubMed.
- T. Maekawa, Y. Segawa and K. Itami, Chem. Sci., 2013, 4, 2369 RSC.
- T. Iitsuka, K. Hirano, T. Satoh and M. Miura, Chem. – Eur. J., 2014, 20, 385 CrossRef CAS PubMed.
- P. L. Alsters, P. F. Engel, M. P. Hogerheide, M. Copijn, A. L. Spek and G. van Koten, Organometallics, 1993, 12, 1831 CrossRef CAS.
- V. G. Zaitsev, D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2005, 127, 13154 CrossRef CAS PubMed.
- D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2010, 132, 3965 CrossRef CAS PubMed.
- (a) D. L. Davies, S. M. A. Donald and S. A. Macgregor, J. Am. Chem. Soc., 2005, 127, 13754 CrossRef CAS PubMed; (b) D. Lapointe and K. Fagnou, Chem. Lett., 2010, 39, 1118 CrossRef.
- B. V. S. Reddy, L. R. Reddy and E. J. Corey, Org. Lett., 2006, 8, 3391 CrossRef CAS PubMed.
- L. D. Tran and O. Daugulis, Angew. Chem., Int. Ed., 2012, 51, 5188 CrossRef CAS PubMed.
- (a) A. Garcia-Rubía, R. G. Arrayás and J. C. Carretero, Angew. Chem., Int. Ed., 2009, 48, 6511 CrossRef PubMed; (b) A. Garcia-Rubía, B. Urones, R. G. Arrayás and J. C. Carretero, Chem. – Eur. J., 2010, 16, 9676 CrossRef PubMed; (c) N. Rodríguez, J. A. Romero-Revilla, M. A. Fernández-Ibánez and J. C. Carretero, Chem. Sci., 2013, 4, 175 RSC.
- M. Fan and D. Ma, Angew. Chem., Int. Ed., 2013, 52, 12152 CrossRef CAS PubMed.
- B. Wang, W. A. Nack, G. He, S.-Y. Zhang and G. Chen, Chem. Sci., 2014, 5, 3952 RSC.
- D. P. Affron, O. A. Davis and J. A. Bull, Org. Lett., 2014, 16, 4956 CrossRef CAS PubMed.
- R. Feng, B. Wang, Y. Liu, Z. Liu and Y. Zhang, Eur. J. Org. Chem., 2015, 142 CrossRef CAS PubMed.
- Y. Feng and G. Chen, Angew. Chem., Int. Ed., 2010, 49, 958 CrossRef CAS PubMed.
- W. R. Gutekunst and P. S. Baran, J. Am. Chem. Soc., 2011, 133, 19076 CrossRef CAS PubMed.
- W. R. Gutekunst, R. Gianatassio and P. S. Baran, Angew. Chem., Int. Ed., 2012, 51, 7507 CrossRef CAS PubMed.
- (a) Y. Zhao, G. He, W. A. Nack and G. Chen, Org. Lett., 2012, 14, 2948 CrossRef CAS PubMed; (b) W. Cui, S. Chen, J.-Q. Wu, X. Zhao, W. Hu and H. Wang, Org. Lett., 2014, 16, 4288 CrossRef CAS PubMed.
- G. He and G. Chen, Angew. Chem., Int. Ed., 2011, 50, 5192 CrossRef CAS PubMed.
- Y. Xie, Y. Yang, L. Huang, X. Zhang and Y. Zhang, Org. Lett., 2012, 14, 1238 CrossRef CAS PubMed.
- (a) E. T. Nadres, G. I. Franco Santos, D. Shabashov and O. Daugulis, J. Org. Chem., 2013, 78, 9689 CrossRef CAS PubMed; (b) N. Hoshiya, T. Kobayashi, M. Arisawa and S. Shuto, Org. Lett., 2013, 15, 6202 CrossRef CAS PubMed; (c) Q. Zhang, X.-S. Yin, S. Zhao, S.-L. Fang and B.-F. Shi, Chem. Commun., 2014, 50, 8353 RSC; (d) Y. Wei, H. Tang, X. Cong, B. Rao, C. Wu and X. Zeng, Org. Lett., 2014, 16, 2248 CrossRef CAS PubMed.
- F. Pan, P.-X. Shen, L.-S. Zhang, X. Wang and Z.-J. Shi, Org. Lett., 2013, 15, 4758 CrossRef CAS PubMed.
- Y. Aihara and N. Chatani, Chem. Sci., 2013, 4, 664 RSC.
- (a) M. Nishino, K. Hirano, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2013, 52, 4457 CrossRef CAS PubMed; (b) R. Odani, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2013, 78, 11045 CrossRef CAS PubMed.
- R. Shang, L. Ilies, A. Matsumoto and E. Nakamura, J. Am. Chem. Soc., 2013, 135, 6030 CrossRef CAS PubMed.
- (a) Z. Li, L. Cao and C.-J. Li, Angew. Chem., Int. Ed., 2007, 46, 6505 CrossRef CAS PubMed; (b) Y. Zhang and C.-J. Li, Eur. J. Org. Chem., 2007, 4654 CrossRef CAS PubMed; (c) Z. Li, R. Yu and H. Li, Angew. Chem., Int. Ed., 2008, 47, 7497 CrossRef CAS PubMed; (d) Y.-Z. Li, B.-J. Li, X.-Y. Lu, S. Lin and Z.-J. Shi, Angew. Chem., Int. Ed., 2009, 48, 3817 CrossRef CAS PubMed; (e) C. M. R. Volla and P. Vogel, Org. Lett., 2009, 11, 1701 CrossRef CAS PubMed; (f) P. P. Singh, S. Gudup, S. Ambala, U. Singh, S. Dadhwal, B. Singh, S. D. Sawant and R. A. Vishwakarma, Chem. Commun., 2011, 47, 5852 RSC.
- Q. Gu, H. H. Al Mamari, K. Graczyk, E. Diers and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 3868 CrossRef CAS PubMed.
- Y. Aihara and N. Chatani, J. Am. Chem. Soc., 2014, 136, 898 CrossRef CAS PubMed.
- M. Li, J. Dong, X. Huang, K. Li, Q. Wu, F. Song and J. You, Chem. Commun., 2014, 50, 3944 RSC.
- (a) S. I. Kozhushkov and L. Ackermann, Chem. Sci., 2013, 4, 886 RSC; (b) C. Feng, D. Feng and T.-P. Loh, Org. Lett., 2013, 15, 3670 CrossRef CAS PubMed; (c) N. P. Grimster, C. Gauntlett, C. R. A. Godfrey and M. J. Gaunt, Angew. Chem., Int. Ed., 2005, 44, 3125 CrossRef CAS PubMed; (d) S. H. Cho, S. J. Hwang and S. Chang, J. Am. Chem. Soc., 2008, 130, 9254 CrossRef CAS PubMed; (e) Y.-H. Zhang, B.-F. Shi and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 5072 CrossRef CAS PubMed; (f) X. Ye and X. Shi, Org. Lett., 2014, 16, 4448 CrossRef CAS PubMed.
- A. Deb, S. Bag, R. Kancherla and D. Maiti, J. Am. Chem. Soc., 2014, 136, 13602 CrossRef CAS PubMed.
- R. Shang, L. Ilies, S. Asako and E. Nakamura, J. Am. Chem. Soc., 2014, 136, 14349 CrossRef CAS PubMed.
- (a) Y. Zhao and G. Chen, Org. Lett., 2011, 13, 4850 CrossRef CAS PubMed; (b) S.-Y. Zhang, G. He, W. A. Nack, Y. Zhao, Q. Li and G. Chen, J. Am. Chem. Soc., 2013, 135, 2124 CrossRef CAS PubMed.
- S.-Y. Zhang, Q. Li, G. He, W. A. Nack and G. Chen, J. Am. Chem. Soc., 2013, 135, 12135 CrossRef CAS PubMed.
- K. Chen and B.-F. Shi, Angew. Chem., Int. Ed., 2014, 53, 11950 CrossRef CAS PubMed.
- Y. Aihara and N. Chatani, J. Am. Chem. Soc., 2013, 135, 5308 CrossRef CAS PubMed.
- W. Song, S. Lackner and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 2477 CrossRef CAS PubMed.
- X. Wu, Y. Zhao and H. Ge, J. Am. Chem. Soc., 2014, 136, 1789 CrossRef CAS PubMed.
- (a) G. Rouquet and N. Chatani, Chem. Sci., 2013, 4, 2201 RSC; (b) K. Shibata and N. Chatani, Org. Lett., 2014, 16, 5148 CrossRef CAS PubMed.
- B. M. Monks, E. R. Fruchey and S. P. Cook, Angew. Chem., Int. Ed., 2014, 53, 11065 CrossRef CAS PubMed.
- (a) Acetylene Chemistry, ed. F. Diederich, P. J. Stang and R. R. Tykwinski, Wiley-VCH, Weinheim, Germany, 2005 Search PubMed; (b) M. G. Finn and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1231 RSC; (c) W. Zhang and J. S. Moore, Adv. Synth. Catal., 2007, 349, 93 CrossRef CAS PubMed; (d) S. I. Lee and N. Chatani, Chem. Commun., 2009, 371 RSC.
- (a) A. S. Dudnik and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2096 CrossRef CAS PubMed; (b) S. Messaoudi, J.-D. Brion and M. Alami, Eur. J. Org. Chem., 2010, 6495 CrossRef CAS PubMed; (c) J. P. Brand, J. Charpentier and J. Waser, Angew. Chem., Int. Ed., 2009, 48, 9346 CrossRef CAS PubMed; (d) F. Besselièvre and S. Piguel, Angew. Chem., Int. Ed., 2009, 48, 9553 CrossRef PubMed; (e) J. P. Brand and J. Waser, Angew. Chem., Int. Ed., 2010, 49, 7304 CrossRef CAS PubMed; (f) J. P. Brand, D. F. Gonzalez, S. Nicolai and J. Waser, Chem. Commun., 2011, 47, 102 RSC; (g) S. H. Kim, J. Yoon and S. Chang, Org. Lett., 2011, 13, 1474 CrossRef CAS PubMed and the references cited therein; (h) S. Murata, K. Teramoto, M. Miura and M. Nomura, J. Chem. Res., Synop., 1993, 434 CAS; (i) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2004, 126, 11810 CrossRef CAS PubMed; (j) Z. Li, D. S. Bohle and C.-J. Li, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8928 CrossRef CAS PubMed; (k) C. A. Correia and C.-J. Li, Adv. Synth. Catal., 2010, 352, 1446 CrossRef CAS PubMed; (l) D. F. González, J. P. Brand and J. Waser, Chem. – Eur. J., 2010, 16, 9457 CrossRef PubMed.
- (a) Y. Ano, M. Tobisu and N. Chatani, J. Am. Chem. Soc., 2011, 133, 12984 CrossRef CAS PubMed; (b) M. Al-Amin, M. Arisawa, S. Shuto, Y. Ano, M. Tobisu and N. Chatani, Adv. Synth. Catal., 2014, 356, 1631 CrossRef CAS PubMed.
- Y. Ano, M. Tobisu and N. Chatani, Org. Lett., 2012, 14, 354 CrossRef CAS PubMed.
- M. Shang, H.-L. Wang, S.-Z. Sun, H.-X. Dai and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 11590 CrossRef CAS PubMed.
- (a) S. Allu and K. C. K. Swamy, J. Org. Chem., 2014, 79, 3963 CrossRef CAS PubMed; (b) J. Dong, F. Wang and J. You, Org. Lett., 2014, 16, 2884 CrossRef CAS PubMed; (c) W. Zhu, D. Zhang, N. Yang and H. Liu, Chem. Commun., 2014, 50, 10634 RSC.
- (a) S. Inoue, H. Shiota, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2009, 131, 6898 CrossRef CAS PubMed; (b) N. Hasegawa, V. Charra, S. Inoue, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2011, 133, 8070 CrossRef CAS PubMed; (c) H. Shiota, Y. Ano, Y. Aihara, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2011, 133, 14952 CrossRef CAS PubMed.
- L. Grigorjeva and O. Daugulis, Angew. Chem., Int. Ed., 2014, 53, 10209 CrossRef CAS PubMed.
- (a) L. Grigorjeva and O. Daugulis, Org. Lett., 2014, 16, 4684 CrossRef CAS PubMed; (b) L. Grigorjeva and O. Daugulis, Org. Lett., 2014, 16, 4688 CrossRef CAS PubMed.
- L. D. Tran, I. Popov and O. Daugulis, J. Am. Chem. Soc., 2012, 134, 18237 CrossRef CAS PubMed.
- D. Katayev, K. F. Pfister, T. Wendling and L. J. Gooßen, Chem. – Eur. J., 2014, 20, 9902 CrossRef CAS PubMed.
- (a) G. He, Y. Zhao, S.-Y. Zhang, C. Lu and G. Chen, J. Am. Chem. Soc., 2012, 134, 3 CrossRef CAS PubMed; (b) G. He, C. Lu, Y. Zhao, W. A. Nack and G. Chen, Org. Lett., 2012, 14, 2944 CrossRef CAS PubMed; (c) E. T. Nadres and O. Daugulis, J. Am. Chem. Soc., 2012, 134, 7 CrossRef CAS PubMed; (d) X. Ye, Z. He, T. Ahmed, K. Weise, N. G. Akhmedov, J. L. Petersen and X. Shi, Chem. Sci., 2013, 4, 3712 RSC.
- G. He, S.-Y. Zhang, W. A. Nack, Q. Li and G. Chen, Angew. Chem., Int. Ed., 2013, 52, 11124 CrossRef CAS PubMed.
- Q. Zhang, K. Chen, W. Rao, Y. Zhang, F.-J. Chen and B.-F. Shi, Angew. Chem., Int. Ed., 2013, 52, 13588 CrossRef CAS PubMed.
- (a) Z. Wang, J. Ni, Y. Kuninobu and M. Kanai, Angew. Chem., Int. Ed., 2014, 53, 3496 CrossRef CAS PubMed; (b) X. Wu, Y. Zhao, G. Zhang and H. Ge, Angew. Chem., Int. Ed., 2014, 53, 3706 CrossRef CAS PubMed; (c) X. Wu, Y. Zhao and H. Ge, Chem. – Eur. J., 2014, 20, 9530 CrossRef CAS PubMed.
- C. Wang, C. Chen, J. Zhang, J. Han, Q. Wang, K. Guo, P. Liu, M. Guan, Y. Yao and Y. Zhao, Angew. Chem., Int. Ed., 2014, 53, 9884 CrossRef CAS PubMed.
- (a) L. D. Tran, J. Roane and O. Daugulis, Angew. Chem., Int. Ed., 2013, 52, 6043 CrossRef CAS PubMed; (b) M. Shang, S.-Z. Sun, H.-X. Dai and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 3354 CrossRef CAS PubMed.
- S.-Y. Zhang, G. He, Y. Zhao, K. Wright, W. A. Nack and G. Chen, J. Am. Chem. Soc., 2012, 134, 7313 CrossRef CAS PubMed.
- L. Ju, J. Yao, Z. Wu, Z. Liu and Y. Zhang, J. Org. Chem., 2013, 78, 10821 CrossRef CAS PubMed; R. K. Rit, M. R. Yadav and A. K. Sahoo, Org. Lett., 2012, 14, 3724 CrossRef PubMed.
- (a) G. Shan, X. Yang, Y. Zong and Y. Rao, Angew. Chem., Int. Ed., 2013, 52, 13606 CrossRef CAS PubMed; (b) F.-J. Chen, S. Zhao, F. Hu, K. Chen, Q. Zhang, S.-Q. Zhang and B.-F. Shi, Chem. Sci., 2013, 4, 4187 RSC.
- Y. Zong and Y. Rao, Org. Lett., 2014, 16, 5278 CrossRef CAS PubMed.
- R. K. Rit, M. R. Yadav, K. Ghosh, M. Shankar and A. K. Sahoo, Org. Lett., 2014, 16, 5258 CrossRef CAS PubMed.
- X. Li, Y.-H. Liu, W.-J. Gu, B. Li, F.-J. Chen and B.-F. Shi, Org. Lett., 2014, 16, 3904 CrossRef CAS PubMed.
- F.-J. Chen, G. Liao, X. Li, J. Wu and B.-F. Shi, Org. Lett., 2014, 16, 5644 CrossRef CAS PubMed.
- (a) Y. Kuninobu, K. Yamaguchi, N. Tamura, T. Seiki and K. Takai, Angew. Chem., Int. Ed., 2013, 52, 1520 CrossRef CAS PubMed; (b) A. M. Lapointe, F. C. Rix and M. Brookhart, J. Am. Chem. Soc., 1997, 119, 906 CrossRef CAS; (c) B. Lu and J. R. Falck, J. Org. Chem., 2010, 75, 1701 CrossRef CAS PubMed; (d) J. M. Larsson, T. S. N. Zhao and K. J. Szabó, Org. Lett., 2011, 13, 1888 CrossRef CAS PubMed; (e) E. M. Simmons and J. F. Hartwig, Nature, 2012, 483, 70 CrossRef CAS PubMed; (f) Y. Kuninobu, T. Nakahara, H. Takeshima and K. Takai, Org. Lett., 2013, 15, 426 CrossRef CAS PubMed.
- K. S. Kanyiva, Y. Kuninobu and M. Kanai, Org. Lett., 2014, 16, 1968 CrossRef CAS PubMed.
- (a) X. Mu, S. Chen, X. Zhen and G. Liu, Chem. – Eur. J., 2011, 17, 6039 CrossRef CAS PubMed; (b) L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2012, 134, 1298 CrossRef CAS PubMed.
- (a) A. Casitas, M. Canta, M. Solá, M. Costas and X. Ribas, J. Am. Chem. Soc., 2011, 133, 19386 CrossRef CAS PubMed; (b) A. M. Suess, M. Z. Ertem, C. J. Cramer and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 9797 CrossRef CAS PubMed.
- D. Leow, G. Li, T.-S. Mei and J.-Q. Yu, Nature, 2012, 486, 518 CrossRef CAS PubMed.
- L. Wan, N. Dastbaravardeh, G. Li and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 18056 CrossRef CAS PubMed.
- S. Lee, H. Lee and K. L. Tan, J. Am. Chem. Soc., 2013, 135, 18778 CrossRef CAS PubMed.
- R.-Y. Tang, G. Li and J.-Q. Yu, Nature, 2014, 507, 215 CrossRef CAS PubMed.
- G. Yang, P. Lindovska, D. Zhu, J. Kim, P. Wang, R.-Y. Tang, M. Movassaghi and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 10807 CrossRef CAS PubMed.
- M. Bera, A. Modak, T. Patra, A. Maji and D. Maiti, Org. Lett., 2014, 16, 5760 CrossRef CAS PubMed.
- F. J. Hernández, M. Simonetti and I. Larrosa, Angew. Chem., Int. Ed., 2013, 52, 11458 CrossRef PubMed.
- R. J. Phipps and M. J. Gaunt, Science, 2009, 323, 1953 CrossRef PubMed.
- B. Chen, X.-L. Hou, Y.-X. Li and Y.-D. Wu, J. Am. Chem. Soc., 2011, 133, 7668 CrossRef CAS PubMed.
- H. A. Duong, R. E. Gilligan, M. L. Cooke, R. J. Phipps and M. J. Gaunt, Angew. Chem., Int. Ed., 2011, 50, 463 CrossRef CAS PubMed.
- O. Saidi, J. Marafie, A. E. W. Ledger, P. M. Liu, M. F. Mahon, G. Kociok-Köhn, M. K. Whittlesey and C. G. Frost, J. Am. Chem. Soc., 2011, 133, 19298 CrossRef CAS PubMed.
- N. Hofmann and L. Ackermann, J. Am. Chem. Soc., 2013, 135, 5877 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2015 |