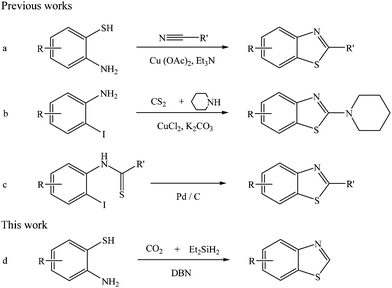
Hydrosilane-promoted cyclization of 2-aminothiophenols by CO2 to benzothiazoles†
Xiang Gao,
Bo Yu,
Yanfei Zhao,
Leiduan Hao and
Zhimin Liu*
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: liuzm@iccas.ac.cn; Fax: +86-10-62562821; Tel: +86-10-62562852
First published on 27th October 2014
Abstract
A new route is presented to synthesize benzothiazoles via cyclization of 2-aminothiophenols by CO2 in the presence of diethylsilane catalyzed by 1,5-diazabicyclo[4.3.0]non-5-ene, and a series of benzothiazoles were obtained in good yields.
Benzothiazole and its derivatives are very important biological and chemical intermediates due to their broad biological and pharmaceutical properties.1 The traditional methods to synthesize benzothiazoles are based on the following reactions as illustrated in Scheme 1: (I) condensation of 2-aminothiophenol with carbonyl or cyano containing compounds like aryl ketones,2 aromatic aldehydes,3 N-acylated benzotriazoles4 or nitriles (Scheme 1a);5 (II) reactions of 2-haloanilides with isothiocyanates,6 carbon disulfide and piperidine (Scheme 1b),7 aldehydes and sulfur,8 carbon disulfide and thiols,9 acid chlorides and Lawesson's reagent;10 (III) cyclization of thioformanilides,11 o-haloarylthioureas12 or o-iodothiobenzanilides (Scheme 1c)13 catalyzed by transition metals. These approaches suffer from their inherent limitations, such as low yields, poor selectivity, requiring harsh reaction conditions and harmful reagents, and using metal catalysts, etc. Therefore, it is highly desirable to develop metal-free and environmentally benign routes by using renewable and relatively green reagents for the synthesis of benzothiazoles.
As a safe, abundant, cheap, and renewable C1 building block,14 CO2 has been used in organic synthesis for the construction of C–N, C–C, C–O, C–H chemical bonds, and value-added chemicals such as ureas,15 amides,16 carbonates,17 methanol,18 formic acid19 have been synthesized. However, due to its inherent thermodynamic stability and kinetic inertness, the activation of CO2 is difficult. Therefore, much work has been focused on the activation of CO2. It was reported that organic bases with tertiary nitrogen could react with CO2 to form the carbamate species, resulting in the activation of CO2.20 Recently, hydrosilylation of CO2 with hydrosilanes has made the CO2 conversion proceed under mild conditions, showing that CO2 could be activated by hydrosilanes.21 The combination of organic base and hydrosilane may provide new possibilities for efficient CO2 chemical transformation.
As a part of our continual investigation on the chemical conversion of CO2 into value-added chemicals via construction of C–N, C–C bonds,22 we herein presented the hydrosilane-promoted cyclization of 2-aminothiophenols with CO2 to benzothiazoles catalyzed by organic base (Scheme 1d). In this protocol, 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) served as a catalyst for activating CO2 to react with hydrosilane to form silyl formate, which further reacted with 2-aminothiophenols, producing benzothiazoles. The reported reactions were successfully applied to the substituted 2-aminothiophenols, affording corresponding benzothiazoles in moderate to excellent yields. To the best of our knowledge, this is the first work on the synthesis of benzothiazoles via the cyclization of 2-aminothiophenols using CO2 as a C1 resource.
2-Aminothiophenol as a model substrate was investigated to react with CO2 in the presence of hydrosilane under different conditions, and the results are summarized in Table 1. It was demonstrated that the reaction did not occur in the absence of any organic bases (Table 1, entry 1). To our delight, the reaction proceeded to form benzothiazole catalysed by organic bases including 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and DBN (Table 1, entries 2–4). Especially, benzothiazole was obtained in a yield of >90% catalysed by DBN in the presence of diethylsilane (Et2SiH2) in 1-methyl-2-pyrrolidinone (NMP) at 150 °C (Table 1, entry 4). A trace amount of benzothiazolone as byproduct was detected, which may result from the reaction of CO2 with 2-aminothiophenol under the experimental conditions. Compared to DBN, TBD and DBU afforded lower yields of product, which may be ascribed to the joint effects of the basicity (TBD > DBU > DBN) and steric hindrance (DBN < TBD < DBU),23 and the organic base with a relatively less steric hindrance was more active for catalysing this reaction. Hydrosilanes had considerable influences on the cyclization reaction of 2-aminothiophenol with CO2 catalyzed by DBN. As PMHS (poly(methylhydrosiloxane)) was used, both the conversion of 2-aminothiophenol and the yield of benzothiazole decreased dramatically (Table 1, entry 5); while triethylsilane (Et3SiH) afforded a product yield of 29% with 2-aminothiophenol conversion of 96% (Table 1, entry 6). These results suggested that Et2SiH2 was superior to other alkylsilanes for the synthesis of benzothiazole under the tested conditions. Consequently, DBN and Et2SiH2 were, respectively, chosen as catalyst and hydride donor to investigate the synthesis of benzothiazole under various conditions.
| Entry | Base | Hydrosilane | P/MPa | T/°C | Conv.b/% | Yieldc/% |
|---|---|---|---|---|---|---|
| a Reaction conditions: 2-aminothiophenol, 2 mmol; base, 1 equiv.; hydrosilane (Si–H 10 equiv.); NMP, 2 mL; 24 h.b The loss of starting material as determined by 1H NMR (DMSO-d6, 400 MHz) using 4-nitroacetophenone as an internal standard.c The conversion to product as determined by 1H NMR (DMSO-d6, 400 MHz) using 4-nitroacetophenone as an internal standard. | ||||||
| 1 | — | Et2SiH2 | 5 | 150 | 0 | 0 |
| 2 | TBD | Et2SiH2 | 5 | 150 | 78 | 73 |
| 3 | DBU | Et2SiH2 | 5 | 150 | 88 | 70 |
| 4 | DBN | Et2SiH2 | 5 | 150 | 92 | 90 |
| 5 | DBN | PMHS | 5 | 150 | 69 | 57 |
| 6 | DBN | Et3SiH | 5 | 150 | 96 | 29 |
| 7 | DBN | Et2SiH2 | 7 | 150 | 91 | 81 |
| 8 | DBN | Et2SiH2 | 5 | 130 | 90 | 81 |
The influences of the CO2 pressure and temperature on this reaction were studied. As the CO2 pressure was increased from 5 to 7 MPa, the conversion of 2-aminothiophenol was almost unchanged, while the benzothiazole yield decreased from 90% to 81% (Table 1, entries 4 and 7), accompanied with the detectable benzothiazolone. This indicates that higher pressure promoted the reaction of CO2 with 2-aminothiophenol, leading to the formation of benzothiazolone as byproduct. Temperature also had an impact on this reaction. As temperature was lowered from 150 to 130 °C, the yield of benzothiazole decreased to 81% (Table 1, entries 4 vs. 8), and more benzothiazolone was produced. Therefore, a CO2 pressure of 5 MPa and temperature at 150 °C were selected for the synthesis of benzothiazole.
In the above experiments, benzothiazolone was detected as a byproduct, which was formed by the reaction of 2-aminothiophenol and CO2. To suppress the formation of this byproduct, the influence of hydrosilane amount on the formation of benzothiazole was investigated. Fig. 1 shows the dependence of the yields of the benzothiazole and benzothiazolone on the molar ratio of Et2SiH2 to 2-aminothiophenol. It was obvious that benzothiazole was the main product under the tested conditions, and its yield was considerably affected by the amount of Et2SiH2. Increasing the hydrosilane amount from 1.5 to 5 equiv., the yield of benzothiazole increased from 70% to 90%, and the yield of benzothiazolone decreased from 22% to trace amount. These findings indicated that the formation of the byproduct could be restrained by enough hydrosilane.
 | ||
| Fig. 1 Influence of Et2SiH2 amount on the reaction. Reaction conditions: 2-aminothiophenol, 2 mmol; DBN, 1 equiv.; NMP, 2 mL; CO2, 5 MPa; 150 °C; 24 h. | ||
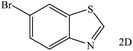
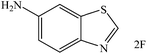
Encouraged by the above results, five substituted 2-aminothiophenols with electron-donating or electron-withdrawing groups were tested to react with CO2 in the presence of Et2SiH2 using DBN as the catalyst in NMP (1-methyl-2-pyrrolidinone), and the results are listed in Table 2. To our delight, all the substrates could be transformed to the corresponding benzothiazoles. The reaction of 1a with CO2 provided an isolated 2A yield of 82% under the selected reaction conditions (Table 2, entry 1). The substrate with electron-donating group (e.g., methyl) showed poor reactivity, affording a 2B yield of 55% even prolonging the reaction time to 72 h (Table 2, entry 2). In the case of 2-amino-5-methoxybenzenethiol as the substrate, the product yield reached 71% (Table 2, entry 3). The reactivity of the substrates with electron withdrawing groups was also investigated. Experimental results showed that 2-amino-5-bromobenzenethiol (1d) and 2-amino-4-chlorothiophenol (1e) could react smoothly with CO2, giving isolated product yields of 78% and 72%, respectively (Table 2, entries 4 and 5). As for 5-aminolbenzothiazole (1f) as the substrate, the nitro group was further reduced to amino group, producing corresponding 5-aminolbenzothiazole (Table 2, entry 6) with a yield of 35%. It was worth noticing that only trace amount of substituted benzothiazolones as byproducts were detected in these reaction processes.
In order to explore the reaction pathway and better understand the reaction mechanism, a control reaction of benzothiazolone with Et2SiH2 was performed in the presence of DBN under the same other conditions, and no benzothiazole was detectable. This indicated that benzothiazolone was not an intermediate for the formation of benzothiazole. 1H NMR analysis was employed to identify the possible intermediates formed during the reaction process. As illustrated in Fig. 2, a new signal appeared at δ = 8.12 ppm when Et2SiH2 reacted with CO2 in the presence of DBN comparing with 1H NMR spectra of pure Et2SiH2 and DBN. This signal was ascribed to silyl formate produced from the reaction of Et2SiH2 with CO2, suggesting that it was an intermediate for the synthesis of benzothiazole. It was reported that formylation reaction could accessibly proceed between amines and silyl formate due to the relatively low Gibbs free energy confirmed by the theoretic calculations.16a
Based on the experimental results and the previous reports,16a,24 a possible reaction pathway was proposed, as illustrated in Scheme 2. Firstly, silyl formate was formed via the reaction of Et2SiH2 with CO2 catalyzed by DBN, which was the key step for the synthesis of benzothiazole. Then the nucleophilic NH2 group in 2-aminothiophenol would easily attack the silyl formate, leading to the formation of the formamide intermediate 1. Subsequently, intermediate 2 was obtained through the intramolecular nucleophilic attack of SH group to the carbon atom of intermediate 1. Finally, the dehydration reaction of intermediate 2 took place, producing benzothiazole. It was worth noticing that the byproduct benzothiazolone was inevitably formed due to the competitive side reaction of 2-aminothiophenol and CO2 under the experimental conditions.
Conclusions
In summary, we have demonstrated a new route to synthesize benzothiazoles via the cyclization of 2-aminothiophenols by CO2 in the presence of Et2SiH2 catalyzed by DBN. Hydrosilane played an important role in the formation of benzothiazoles, and suppressed the production of benzothiazolones as the byproducts. This strategy provides an environmental benign approach for the synthesis of benzothiazoles and achieves the construction of C–S chemical bond using CO2 as C1 building block. Further investigation on reducing the amounts of hydrosilanes for the synthesis of benzothiazoles is in progress in our laboratory.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21125314, 21321063) and Chinese Academy of Sciences.Notes and references
-
(a) S. Noel, S. Cadet, E. Gras and C. Hureau, Chem. Soc. Rev., 2013, 42, 7747 RSC
; (b) H. Yao, M.-k. So and J. Rao, Angew. Chem., Int. Ed., 2007, 46, 7031 CrossRef CAS PubMed
.
- Y. Liao, H. Qi, S. Chen, P. Jiang, W. Zhou and G.-J. Deng, Org. Lett., 2012, 14, 6004 CrossRef CAS PubMed
.
- K. Bahrami, M. M. Khodaei and F. Naali, J. Org. Chem., 2008, 73, 6835 CrossRef CAS PubMed
.
- S. S. Panda, M. A. Ibrahim, A. A. Oliferenko, A. M. Asiri and A. R. Katritzky, Green Chem., 2013, 15, 2709 RSC
.
- Y. Sun, H. Jiang, W. Wu, W. Zeng and X. Wu, Org. Lett., 2013, 15, 1598 CrossRef CAS PubMed
.
- R. Xiao, W. Hao, J. Ai and M.-Z. Cai, J. Organomet. Chem., 2012, 705, 44 CrossRef CAS PubMed
.
- D. Ma, X. Lu, L. Shi, H. Zhang, Y. Jiang and X. Liu, Angew. Chem., Int. Ed., 2011, 50, 1118 CrossRef CAS PubMed
.
- H. Deng, Z. Li, F. Ke and X. Zhou, Chem.–Eur. J., 2012, 18, 4840 CrossRef CAS PubMed
.
- L. Shi, X. Liu, H. Zhang, Y. Jiang and D. Ma, J. Org. Chem., 2011, 76, 4200 CrossRef CAS PubMed
.
- Q. Ding, X.-G. Huang and J. Wu, J. Comb. Chem., 2009, 11, 1047 CrossRef CAS PubMed
.
- D. S. Bose and M. Idrees, J. Org. Chem., 2006, 71, 8261 CrossRef CAS PubMed
.
- L. L. Joyce, G. Evindar and R. A. Batey, Chem. Commun., 2004, 446 RSC
.
- Y. Cheng, Q. Peng, W. Fan and P. Li, J. Org. Chem., 2014, 79, 5812 CrossRef CAS PubMed
.
-
(a) M. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann and F. E. Kuhn, Angew. Chem., Int. Ed., 2011, 50, 8510 CrossRef CAS PubMed
; (b) T. Sakakura, J. C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365 CrossRef CAS PubMed
.
- F. Shi, Y. Q. Deng, T. L. SiMa, J. J. Peng, Y. L. Gu and B. T. Qiao, Angew. Chem., Int. Ed., 2003, 42, 3257 CrossRef CAS PubMed
.
-
(a) C. Das Neves Gomes, O. Jacquet, C. Villiers, P. Thuéry, M. Ephritikhine and T. Cantat, Angew. Chem., Int. Ed., 2012, 51, 187 CrossRef CAS PubMed
; (b) O. Jacquet, C. Das Neves Gomes, M. Ephritikhine and T. Cantat, J. Am. Chem. Soc., 2012, 134, 2934 CrossRef CAS PubMed
.
-
(a) K. Kohno, J. C. Choi, Y. Ohshima, H. Yasuda and T. Sakakura, ChemSusChem, 2008, 1, 186 CrossRef CAS PubMed
; (b) J. Ma, J. L. Song, H. Z. Liu, J. L. Liu, Z. F. Zhang, T. Jiang, H. L. Fan and B. X. Han, Green Chem., 2012, 14, 1743 RSC
.
-
(a) S. Wesselbaum, T. Vom Stein, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2012, 51, 7499 CrossRef CAS PubMed
; (b) F. L. Liao, Z. Y. Zeng, C. Eley, Q. Lu, X. L. Hong and S. C. E. Tsang, Angew. Chem., Int. Ed., 2012, 51, 5832 CrossRef CAS PubMed
.
-
(a) Z. F. Zhang, Y. Xie, W. J. Li, S. Q. Hu, J. L. Song, T. Jiang and B. X. Han, Angew. Chem., Int. Ed., 2008, 47, 1127 CrossRef CAS PubMed
; (b) D. Preti, C. Resta, S. Squarcialupi and G. Fachinetti, Angew. Chem., Int. Ed., 2011, 50, 12551 CrossRef CAS PubMed
.
-
(a) D. J. Heldebrant, P. G. Jessop, C. A. Thomas, C. A. Eckert and C. L. Liotta, J. Org. Chem., 2005, 70, 5335 CrossRef CAS PubMed
; (b) Z. Z. Yang, L. N. He, C. X. Miao and S. Chanfreau, Adv. Synth. Catal., 2010, 352, 2233 CrossRef CAS
.
-
(a) B. Marciniec, Coord. Chem. Rev., 2005, 249, 2374 CrossRef CAS PubMed
; (b) D. Addis, S. Das, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 6004 CrossRef CAS PubMed
; (c) L. Zhang, J. Cheng and Z. Hou, Chem. Commun., 2013, 49, 4782 RSC
.
-
(a) Y. F. Zhao, B. Yu, Z. Z. Yang, H. Y. Zhang, L. D. Hao, X. Gao and Z. M. Liu, Angew. Chem., Int. Ed., 2014, 53, 5922 CrossRef CAS PubMed
; (b) B. Yu, Y. F. Zhao, H. Y. Zhang, J. L. Xu, L. D. Hao, X. Gao and Z. M. Liu, Chem. Commun., 2014, 50, 2330 RSC
; (c) L. D. Hao, Y. F. Zhao, B. Yu, H. Y. Zhang, H. J. Xu and Z. M. Liu, Green Chem., 2014, 16, 3039 RSC
; (d) B. Yu, H. Y. Zhang, Y. F. Zhao, S. Chen, J. L. Xu, C. L. Huang and Z. M. Liu, Green Chem., 2013, 15, 95 RSC
; (e) B. Yu, H. Y. Zhang, Y. F. Zhao, S. Chen, J. L. Xu, L. D. Hao and Z. M. Liu, ACS Catal., 2013, 3, 2076 CrossRef CAS
.
-
(a) Z. Z. Yang, L. N. He, Y. N. Zhao, B. Li and B. Yu, Energy Environ. Sci., 2011, 4, 3971 RSC
; (b) Y. N. Zhao, Z. Z. Yang, S. H. Luo and L. N. He, Catal. Today, 2013, 200, 2 CrossRef CAS PubMed
.
-
(a) W. Sattler and G. Parkin, J. Am. Chem. Soc., 2012, 134, 17462 CrossRef CAS PubMed
; (b) S. Park, D. Bézier and M. Brookhart, J. Am. Chem. Soc., 2012, 134, 11404 CrossRef CAS PubMed
; (c) A. Berkefeld, W. E. Piers and M. Parvez, J. Am. Chem. Soc., 2010, 132, 10660 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, experimental details, and characterization. See DOI: 10.1039/c4ra09372k |
| This journal is © The Royal Society of Chemistry 2014 |