Open Access Article
Open Access ArticleA highly efficient tandem [3 + 2] “click” cycloaddition/6-exo-cyclization strategy for the construction of triazole fused pyrazines†
Biswajit Roy,
Debashis Mondal,
Joydev Hatai and
Subhajit Bandyopadhyay*
Indian Institute of Science Education and Research (IISER) Kolkata, Mohanpur, Nadia, WB 741246, India. E-mail: sb1@iiserkol.ac.in
First published on 27th October 2014
Abstract
The pharmaceutically important tetrahydro-[1,2,3]triazolopyrazine heterocyclic architecture has been synthesized via a concise tandem “click”/6-exo-dig cyclization strategy in mixed aqueous–organic media. The generality of this mild method was expanded to various amino acid based substrates. The scopes and limitations of this method are discussed in the paper.
Novel heterocyclic frameworks via concise synthetic routes from easily available starting materials are highly coveted in synthetic chemistry. A myriad of compounds containing the 1,2,3-triazole structural motif possess interesting biological properties.1 Many natural products containing the tetrahydropyrazine framework display a broad spectrum of biological effects including antitumor activity.2 This framework is present in the HIV protease inhibitor crixivan,3 and other drug candidates.4
There are, however, only a few reports of heterocyclic systems with [1,2,3]triazole fused tetrahydropyrazine systems. The 4,5,6,7-tetrahydro [1,2,3]triazolo-[1,5-a]pyrazin-6-ones5 were synthesized by the group of Chandrasekaran,5a Abbott Laboratories5b and Appella5c using multistep synthesis. Likewise, the 4,5,6,7-tetrahydro[1,2,3]triazolo[1,5-a]pyrazines,6 bearing such bicyclic fused rings were synthesized by Gurjar6a and Couty6b in multiple steps. Recently, Shen and coworkers from Merck Research Laboratories have also reported a multistep synthesis of compound containing the same the scaffold as agonists for the G-protein-coupled niacin receptor.7 Schreiber and coworkers have synthesized the fused tetrahydrotriazolopyrazine system in a single step from aziridine through a propargylamine azide intermediate following the ‘build/couple/pair’ strategy of diversity-oriented syntheses.8 A series of triazolo[1,5-a]quinoxaline systems, a variant of the scaffold, has been synthesized by Cai via another tandem approach from N-(2-haloaryl)propiolamides.9 Gulevskaya and coworkers have recently demonstrated the azide mediated tandem cyclization of (Z)-enediynes for the formation of the corresponding [1,2,3]triazolo[1,5-a]pyridines.10
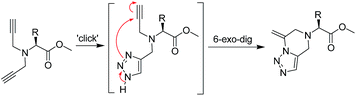
Inspired by the resource efficient tandem reactions,11 we envisioned that a tandem approach combining a 1,3-dipolar [3 + 2] (“click”) cycloaddition12 followed by an intramolecular exo-cyclization13 may offer a versatile route to synthesize this important fused-heterocyclic architecture from readily available synthons (Fig. 1 and Scheme 1). Thus, we have developed a simple synthetic route for the preparation of a series of 1,2,3-triazole-fused pyrazines from easily available primary amines and naturally occurring amino acids. The key step of the reaction is a mechanistically interesting tandem “click”/6-exo-cyclization of the N,N-dipropargylamine precursors as shown in Scheme 1. The reaction proceeds via a triazole–alkyne intermediate tailored for the selective 6-exo cyclization step.
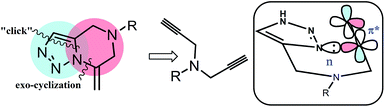 | ||
| Fig. 1 Tandem approach for the synthesis of the triazole fused pyrazine system. (Box) Orbitals involved in the 6-exo-dig cyclization. | ||
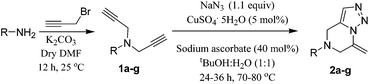
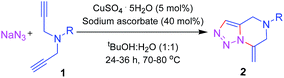
Our preliminary efforts were dedicated towards the synthesis of compound 2a from 4-amino benzophenone. The dipropargyl starting material 1a was synthesized in 93% yield from the 4-amino benzophenone and propargyl bromide in dry DMF at 25 °C using K2CO3 as the proton scavenger (see ESI†). The structure of compound 1a was confirmed by NMR spectroscopy, IR and mass spectrometric analysis.
Compound 1a was heated under reflux at 80 °C for 24 h in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of tBuOH (TBA) and H2O with sodium azide (1.1 equiv.) using CuSO4·5H2O (5 mol%) in presence of sodium ascorbate (40 mol%) as the reducing agent. A 1,3 dipolar cycloaddition reaction between the azide and one of the alkyne moieties formed the incipient 1,2,3 triazole ring. The triazole underwent a constrained intramolecular 6-exo-dig cycloaddition with the another alkyne moiety to furnish the desired 1,2,3 triazole-fused 4,5,6,7 tetrahydropyrazine moiety 2a in 86% yield as the exclusive product (Scheme 1). The reaction was examined under various conditions (Table 1) and it was found that at 80 °C with 1.1 equiv. of NaN3 in TBA–water (1
1 mixture of tBuOH (TBA) and H2O with sodium azide (1.1 equiv.) using CuSO4·5H2O (5 mol%) in presence of sodium ascorbate (40 mol%) as the reducing agent. A 1,3 dipolar cycloaddition reaction between the azide and one of the alkyne moieties formed the incipient 1,2,3 triazole ring. The triazole underwent a constrained intramolecular 6-exo-dig cycloaddition with the another alkyne moiety to furnish the desired 1,2,3 triazole-fused 4,5,6,7 tetrahydropyrazine moiety 2a in 86% yield as the exclusive product (Scheme 1). The reaction was examined under various conditions (Table 1) and it was found that at 80 °C with 1.1 equiv. of NaN3 in TBA–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) the best yields of 2a were obtained from the starting materials.
1 v/v) the best yields of 2a were obtained from the starting materials.
| Entry | Conditions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
NaN3 (equiv.) | °Ca | Yieldb (%) |
|---|---|---|---|---|
| a Time 24 h.b Isolated yield. | ||||
| 1 | THF–H2O | 1.0 | 25 | 23 |
| 2 | THF–H2O | 1.0 | 80 | 41 |
| 3 | THF–H2O | 1.1 | 80 | 46 |
| 4 | TBA–H2O | 1.0 | 25 | 35 |
| 5 | TBA–H2O | 1.0 | 80 | 80 |
| 6 | TBA–H2O | 1.1 | 80 | 92 |
| 7 | TBA–H2O | 1.5 | 80 | 81 |
| 8 | TBA–H2O | 1.7 | 80 | 78 |
| 9 | DMF–H2O | 1.1 | 80 | 55 |
| 10 | Toluene–H2O | 1.1 | 80 | 29 |
| 11 | CH3CN–H2O | 1.1 | 80 | 52 |
| 12 | Ethanol–H2O | 1.1 | 80 | 51 |
| 13 | DME–H2O | 1.1 | 80 | 52 |
| 14 | Dioxane–H2O | 1.1 | 80 | 56 |
To verify its generality, the procedure was tested on a variety of dipropargyl amines. The N,N-dipropargyl precursors 1b–g14 (see ESI†) were synthesized from a series of amines. Compounds 1b–g were subsequently treated with sodium azide under the reaction conditions described earlier. The reactions in all the cases were found to afford the desired 1,2,3-triazole-fused 4,5,6,7-tetrahydropyrazines 2b–g as the exclusive products in moderate to excellent yields as summarized in Table 2. The reaction was effective for a broad range of aromatic amines containing electron donating as well as electron withdrawing aromatic amines.
a Conditions: NaN3 (1.1 equiv.), CuSO4·5H2O (0.05 equiv.), sodium ascorbate (0.4 equiv.), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of tBuOH and H2O; 70–80 °C; 24–36 h. (See ESI for details). 1 mixture of tBuOH and H2O; 70–80 °C; 24–36 h. (See ESI for details). |
|---|
 |
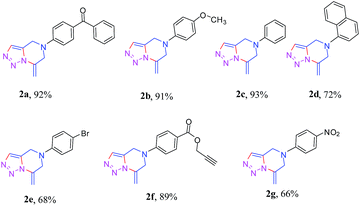 |
For instance, N,N-dipropargyl amines 1a, 1f, and 1g with electron-withdrawing benzophenone, benzoate ester and nitrophenyl moieties furnished the target products 2a, 2f and 2g in good to moderate yields (92%, 89%, and 66% respectively).
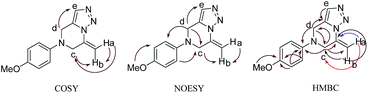
The N,N-dipropargyl amine with an electron donating methoxy group (1b) in the aryl unit afforded the desired product 2b in excellent 91% yield. The case of 2f was interesting since the additional propargyl unit linked to the carboxylate remained untouched in the reaction. The structures of these compounds were established by the 1D and 2D NMR spectroscopy. The key 2D NMR correlations for the product (2b) are presented schematically in Fig. 2 as an example.
The complete set of data for the structural analysis is given in the ESI.† The characteristic resonance observed at δ = 7.58 corresponded to hydrogen atom of the triazole ring and the resonance at δ = 129.6, 130.8, and 46.9 ppm corresponded to olefinic carbon atoms and the methylene carbon adjacent to the double bond of the triazole ring. Additionally, characteristic resonances were observed at δ = 4.13 and 4.48 ppm corresponding to methylene protons of pyrazine ring. The resonances at δ = 4.98 and 6.06 in the 1H NMR spectrum and the resonance at δ = 100.2 in the 13C NMR spectra were attributed to the methylene protons and carbon of the exocyclic double bond of pyrazine ring respectively. The methylene group of the exocyclic double bond of the pyrazine ring was confirmed by 1H–1H COSY, 1H–13C HMBC, and 1H–1H NOESY spectra (Fig. 2 and S28–S31, ESI†).
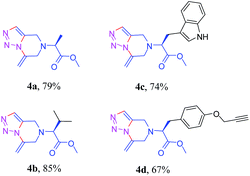
The success of the general strategy on a broad range of substrates for the syntheses of the 1,2,3-triazole-fused tetrahydropyrazines from various aromatic primary amines motivated us to extend this synthetic protocol to naturally abundant L-amino acids. A series of methyl esters of the amino acids – alanine, valine, tryptophan and tyrosine were thus synthesized and subsequently reacted with propargyl bromide in the presence of K2CO3 (5 equiv.) in dry DMF at 25 °C for 12 h to form the dipropargyl precursors 3a–d (see ESI†). It is to be noted that the methyl ester of tyrosine on treatment with propargyl bromide in the presence of K2CO3 afforded a product with three propargyl groups: two attached to the nitrogen, and the third one to the oxygen of the phenolic –OH group of the amino acid. Interestingly, for tryptophan, attachment of the propargyl unit to the indole-N was not observed. When subjected to our tandem “click”/exo-cyclization reaction conditions, substrates 3a–d afforded compounds 4a–d in moderate to good yields (67–85%) as summarized in Table 3.
a Conditions: NaN3 (1.1 equiv.), CuSO4·5H2O (5 mol%), sodium ascorbate (40 mol%), tBuOH–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 80 °C, t = 28 h, for entry 1 and 2; 36 h for entry 3 and 4. 1 v/v, 80 °C, t = 28 h, for entry 1 and 2; 36 h for entry 3 and 4. |
|---|
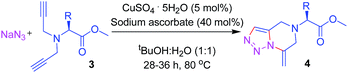 |
 |
A probable mechanism of the reaction for the formation of the triazole-fused pyrazine derivatives (series 2 and 4) is shown in Scheme 2. Initial reaction of the diprop-2-ynylamines (series 1 and 3) with sodium azide allows a copper(I) mediated 1,3-dipolar cycloaddition of one of the terminal alkyne groups15 leading to the formation of a triazole-yne intermediate which subsequently undergoes a tandem intramolecular 6-exo-dig cycloaddition16 through the attack of a non-bonding electron of a N of the triazole to the π* orbital of the alkyne unit (Fig. 1 and S43, ESI†) leading to the products.
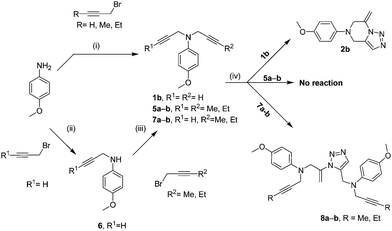
Intramolecular attack of trizoles to internal alkynes in enediene substrates was observed leading to endo-cyclization.10,17 Intramolecular exo-attack on an alkyne by a thiol attached to an imidazole has also recently been reported by Cai and coworkers.11a These served as an inspiration to check if the generality of our method can also be extended to non-terminal alkynes.18 Thus compounds 5a and 5b bearing a methyl and an ethyl group at the alkyne terminals were prepared via the route used for the preparation of the dipropargyl derivatives 1a–g (Scheme 3, ESI†). However, when subjected to our tandem click/exo-dig cyclization conditions, these non-terminal alkynes did not afford any desired product even under a series of varied drastic conditions (microwave, THF–H2O, DMF–H2O, toluene–H2O, CH3CN–H2O, ethanol–H2O, DME–H2O, dioxane–H2O and prolonged heating (up to 150 h) at elevated range of temperatures from 120–180 °C. Although these results were somewhat disheartening, it is not completely unexpected because for the “click” reaction to occur, the substrate requires a C–H bond at the terminal alkyne.19 The classical Huisgen 1,3-dipolar cycloaddition, which occurs in the absence of the Cu-salt at elevated temperatures, also did not work in our case.
This result led us to alter our strategy: it was anticipated that unsymmetrical alkynes (e.g., 7a or 7b, via compound 6) containing both the terminal alkyne and the internal alkyne groups would ensure the formation of the triazoles (Scheme 3, ESI†) that would to undergo the subsequent exo-cyclization to yield the corresponding desired pyrazine products. Surprisingly, for these cases instead of the expected ‘6-exo-dig’ intramolecularly cyclized pyrazines, intermolecular, dimeric acyclic ‘click’ products (8a and 8b) were obtained in trace quantities (Scheme 3, ESI†).
Since severe conditions failed to produce the desired intermolecular products for the substrates with both the alkyne moieties as internal alkynes (as in the case 5a and 5b), and since the substrates with both terminal and internal alkynes (7a and 7b) produced the products 8a and 8b involving only the reactions at the terminal alkynes leaving the internal alkynes intact, we were prompted to speculate that the Cu-salt might have a role for the exo-dig attack.20 A plausible mechanism of the formation of the unexpected products (8a and 8b), as shown in Scheme 4, suggests that the ‘click’ reaction between NaN3 and the unsubstituted propargyl group generates the 1,2,3 triazole ring in the initial step. In contrast to the intramolecular attack, the triazoles in these cases undergo an intermolecular nucleophilic attack on the terminal acetylenic moiety of another molecule, leaving the substituted propargyl group intact, thereby leading to the formation of the unexpected (1H-1,2,3-triazol-1-yl)allyl moiety (in 8a and 8b) as the exclusive products.
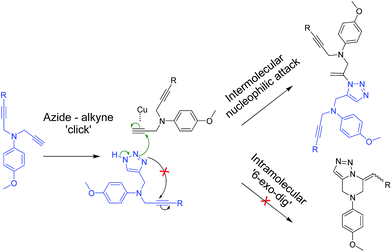 | ||
| Scheme 4 Probable mechanism for the formation of intermolecular acyclic ‘clicked’ product through 1,3-dipolar cycloaddition reaction followed by intermolecular nucleophilic reaction. | ||
In conclusion, we have reported an efficient and facile synthetic approach for the syntheses of [1,2,3]triazolo[1,5-a]pyrazines in excellent yields from several primary amines and amino acids using a novel, modular approach that involves a one pot 1,3-dipolar cycloaddition reaction followed by a tandem intramolecular 6-exo-dig cycloaddition reaction as the key step. The reaction offers high atom economy. The method is limited to the substrates bearing terminal alkynes – for the substrates with one non-terminal alkyne, the reaction yields intermolecular products in low yields. However, for the substrates where both the alkyne moieties are substituted, the reaction does not yield any product. Thus, the two step synthetic protocol permitted the construction of N-heterocyclic compounds from readily available primary amine or amino acid substrates which should open up many possibilities for more such heterocycles with functional diversity.
Acknowledgements
The authors gratefully acknowledge the help of Dr Sushovan Paladhi for the determination of specific rotation, Dr Bhaskar Pramanik for crystallography and Mr Chiranjit Dutta for HPLC. BR is supported by an Int. Ph.D. fellowship, DM by a UGC fellowship and JH by a CSIR SRF. The authors acknowledge CSIR-India for a research grant.Notes and references
- (a) S. Velaquez, R. Alvarez, C. Perez, F. Gogo, C. De, J. Balzarini and M. J. Carmarasa, Antiviral Chem. Chemother., 1998, 9, 481 CAS; (b) D. R. Buckle and C. J. M. Rockell, J. Chem. Soc., Perkin Trans. 1, 1982, 627 RSC; (c) H. Wamhoff, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon, Oxford, 1984, vol. 5, p. 669 Search PubMed.
- (a) R. D. Norcross and I. Paterson, Chem. Rev., 1995, 95, 2041 CrossRef CAS; (b) D. J. Faulkner, Nat. Prod. Rep., 2002, 19, 1 CAS.
- (a) K. Rossen, S. A. Weissman, J. Sagar, A. Reamer, D. A. Askin, R. P. Volante and P. J. Reider, Tetrahedron Lett., 1995, 36, 6419 CrossRef CAS; (b) B. D. Dorsey, R. B. Levin, S. L. McDaniel, J. P. Vacca, J. P. Guare, P. L. Darke, J. A. Zugay, E. A. Emini, W. A. Schleif, J. C. Quintero, J. H. Lin, I.-W. Chen, M. K. Holloway, P. M. D. Fitzgerald, M. G. Axel, D. Ostovic, P. S. Anderson and J. R. Huff, J. Med. Chem., 1994, 37, 3443 CrossRef CAS.
- P. Choudhary, R. Kumar and K. Verma, Bioorg. Med. Chem., 2006, 14, 1819 CrossRef PubMed.
- (a) V. S. Sudhir, R. B. N. Baig and S. Chandrasekaran, Eur. J. Org. Chem., 2008, 2423 CrossRef; (b) I. Arkitopoulou-Zanze, V. Gracias and S. W. Djuric, Tetrahedron Lett., 2004, 45, 8439 CrossRef PubMed; (c) J. K. Pokorski, L. M. M. Jenkins, H. Feng, S. R. Durell, Y. Bal and D. H. Appella, Org. Lett., 2007, 9, 2381 CrossRef CAS PubMed.
- (a) D. K. Mohapatra, P. K. Maity, R. G. Gonnade, M. S. Chorgade and M. K. Gurjar, Synlett, 2007, 1893 CrossRef CAS; (b) F. Couty, F. Durrat and D. Prim, Tetrahedron Lett., 2004, 45, 3725 CrossRef CAS PubMed.
- H. C. Shen, F. X. Ding, Q. Deng, L. C. Wilsie, M. L. Krsmanovic, A. K. Taggart, E. Carballo-Jane, N. Ren, T. Q. Cai, T. J. Wu, K. K. Wu, K. Cheng, Q. Chen, M. S. Wolff, X. Tong, T. G. Holt, M. G. Waters, M. L. Hammond, J. R. Tata and S. L. Colletti, J. Med. Chem., 2009, 52, 2587 CrossRef CAS PubMed.
- A. M. Taylor and S. L. Schreiber, Tetrahedron Lett., 2009, 50, 3230 CrossRef CAS PubMed.
- J. Yan, F. Zhou, D. Qin, T. Cai, K. Ding and Q. Cai, Org. Lett., 2012, 14, 1262 CrossRef CAS PubMed.
- A. V. Gulevskaya, A. S. Tyaglivy, A. F. Pozharskii, J. I. Nelina-Nemtseva and D. V. Steglenko, Org. Lett., 2014, 16, 1582 CrossRef CAS PubMed.
- Recent examples include: (a) W. Hao, J. Zenga and M. Cai, Chem. Commun., 2014, 50, 11686 RSC; (b) W. Hao, Y. Jiang and M. Cai, J. Org. Chem., 2014, 79, 3634 CrossRef CAS PubMed; (c) A. Behr, A. J. Vorholt, K. A. Ostrowskia and T. Seidenstickera, Green Chem., 2014, 16, 982 RSC; (d) K. Cao, F.-M. Zhang, Y.-Q. Tu, X.-T. Zhuo and C.-A. Fan, Chem.–Eur. J., 2009, 15, 6332 CrossRef CAS PubMed; (e) S. Yugandar, N. C. Misra, G. Parameshwarappa, K. Panda and H. Ila, Org. Lett., 2013, 15, 5250 CrossRef CAS PubMed.
- (a) R. Huisgen in 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, Wiley, New York, 1984, pp. 1–176 Search PubMed; (b) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS; (c) L. Ackermann, H. K. Potukuchi, D. Landsberg and R. Vicente, Org. Lett., 2008, 10, 3081 CrossRef CAS PubMed.
- (a) J. E. Baldwin, J. Chem. Soc., Chem. Commun., 1976, 734 RSC; (b) K. Gilmore and I. V. Alabugin, Chem. Rev., 2011, 111, 6513 CrossRef CAS PubMed; (c) I. Alabugin, K. Gilmore and M. Manoharan, J. Am. Chem. Soc., 2011, 133, 12608 CrossRef CAS PubMed; (d) Y. Kwon, H. Cho and S. Kim, Org. Lett., 2013, 15, 920 CrossRef CAS PubMed; (e) C. Bengtsson and F. Almqvist, J. Org. Chem., 2011, 76, 9817 CrossRef CAS PubMed.
- (a) N. G. Kundu and B. Nandi, J. Org. Chem., 2001, 66, 4563 CrossRef CAS PubMed; (b) M. Vedamalai and S.-P. Wu, Eur. J. Org. Chem., 2012, 1158 CrossRef CAS; (c) U. K. Roy and S. Roy, J. Organomet. Chem., 2006, 691, 1525 CrossRef CAS PubMed; (d) A. González-Gómez, L. Añorbe, A. Poblador, G. Domínguez and J. Pérez-Castells, Eur. J. Org. Chem., 2008, 1370 CrossRef; (e) A.-C. Cantet, H. Carreyre, J.-P. Gesson, M.-P. Jouannetaud and B. Renoux, J. Org. Chem., 2008, 73, 2875 CrossRef CAS PubMed; (f) S. A. Vizer, K. B. Yerzhanov, Z. N. Manchuk and A. G. Wieser, Eurasian Chem.–Technol. J., 1999, 1, 1 CAS.
- (a) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (b) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef PubMed.
- (a) K. Gilmore and I. V. Alabugin, Chem. Rev., 2011, 111, 6513 CrossRef CAS PubMed; (b) I. Alabugin, K. Gilmore and M. Manoharan, J. Am. Chem. Soc., 2011, 133, 12608 CrossRef CAS PubMed.
- Z.-Y. Chen and M.-J. Wu, Org. Lett., 2005, 7, 475 CrossRef CAS PubMed.
- This was also suggested by two anonymous reviewers.
- F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210–216 CrossRef CAS PubMed.
- (a) G. Zhang, H. Yi, G. Zhang, Y. Deng, R. Bai, H. Zhang, J. T. Miller, A. J. Kropf, E. E. Bunel and A. Lei, J. Am. Chem. Soc., 2014, 136, 924 CrossRef CAS PubMed; (b) P. Siemsen, R. C. Livingston and F. Diederich, Angew. Chem., Int. Ed., 2000, 39, 2632 CrossRef CAS; (c) H. A. Stefani, A. S. Guarezemini and R. Cella, Tetrahedron, 2010, 66, 7871 CrossRef CAS PubMed; (d) C. He, J. Ke, H. Xu and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 1527 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data for dipropargyl precursors 1a, 3a–d; triazole fused pyrazines compounds 2a–g, 4a–d and compounds 5a and 5b, 7a and 7b and 8a and 8b. CCDC 1026579. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra12489h |
| This journal is © The Royal Society of Chemistry 2014 |