Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unveiling the origin of the efficient photocatalytic degradation of nitazoxanide over bismuth (oxy)iodide crystalline phases†
Mirabbos
Hojamberdiev
 *a,
Ronald
Vargas
*a,
Ronald
Vargas
 bc,
Lorean
Madriz
bc,
Lorean
Madriz
 bc,
Kunio
Yubuta
d,
Zukhra C.
Kadirova
bc,
Kunio
Yubuta
d,
Zukhra C.
Kadirova
 ef,
Ulugbek
Shaislamov
ef,
Ulugbek
Shaislamov
 g,
Lokesh Koodlur
Sannegowda
g,
Lokesh Koodlur
Sannegowda
 h,
Katarzyna
Jędruchniewicz
i,
Rafał
Typek
j,
Katsuya
Teshima
h,
Katarzyna
Jędruchniewicz
i,
Rafał
Typek
j,
Katsuya
Teshima
 k and
Bożena
Czech
*i
k and
Bożena
Czech
*i
aInstitut für Chemie, Technische Universität Berlin, Straße des 17. Juni 135, 10623 Berlin, Germany. E-mail: hmirabbos@gmail.com
bInstituto Tecnológico de Chascomús (INTECH), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional de San Martín (UNSAM), Avenida Intendente Marino, Km 8,2, (B7130IWA), Chascomús, Provincia de Buenos Aires, Argentina
cEscuela de Bio y Nanotecnologías, Universidad Nacional de San Martín (UNSAM), Avenida Intendente Marino, Km 8,2, (B7130IWA), Chascomús, Provincia de Buenos Aires, Argentina
dDepartment of Applied Quantum Physics and Nuclear Engineering, Kyushu University, Fukuoka 819-0395, Japan
eDepartment of General and Inorganic Chemistry, Faculty of Chemistry, National University of Uzbekistan, University Street 4, 100174 Tashkent, Uzbekistan
fUzbekistan-Japan Innovation Center of Youth, University Street 2B, 100095 Tashkent, Uzbekistan
gCenter for Development of Nanotechnology at the National University of Uzbekistan, Tashkent, Uzbekistan, University str. 4, 100174 Tashkent, Uzbekistan
hDepartment of Studies in Chemistry, Vijayanagara Sri Krishnadevaraya University, Cantonment, Vinayakanagara, Ballari, 583105, India
iDepartment of Radiochemistry and Environmental Chemistry, Institute of Chemical Sciences, Faculty of Chemistry, Maria Curie-Skłodowska University in Lublin, 3 Maria Curie-Skłodowska Sq., 20-031 Lublin, Poland. E-mail: bozena.czech@mail.umcs.pl
jDepartment of Chromatography, Institute of Chemical Sciences, Faculty of Chemistry, Maria Curie-Skłodowska University in Lublin, 3 Maria Curie-Skłodowska Sq., 20-031 Lublin, Poland
kDepartment of Materials Chemistry, Shinshu University, 4-17-1 Wakasato, Nagano 380-8553, Japan
First published on 5th December 2023
Abstract
Conventional wastewater treatment has been found to be ineffective at fully removing various antiviral drugs and emerging pollutants from wastewater. As an advanced oxidation process, heterogeneous photocatalysis is promising for detoxifying such water pollutants due to its mild operating conditions and efficiency. In this study, we explore the oxidative phase transition from bismuth iodide to bismuth oxyiodides by altering the temperature and time of thermal treatment. The influence of the temperature change from 350 °C to 450 °C on the phase transition from bismuth iodide to bismuth oxyiodides is more pronounced compared with the impact of time. This results in the formation of different bismuth oxyiodide crystalline phases with varying optoelectronic properties and photocatalytic activity. The effect of the bismuth iodide-to-bismuth oxyiodide phase transition on the efficiency of the photocatalytic removal of nitazoxanide is investigated in this study. The significant role of the BiOI/Bi4O5I2 heterostructure is established in facilitating the rapid photocatalytic degradation of nitazoxanide, with respective rate constants of k1 (0.051 min−1) and k2 (4.225 mg g−1 min−1) obtained for the photocatalyst sample thermally treated at 375 °C for 1 h. Trapping experiments provide evidence that photoexcited holes and hydroxyl radicals play a crucial role in the photocatalytic degradation of nitazoxanide. The photodegradation of nitazoxanide in aqueous solution over crystalline bismuth (oxy)iodide proceeds via hydrolysis into acetylsalicylic acid and the respective aminonitrothiazol, followed by the deacetylation and decarboxylation processes. Molecular dynamics simulation confirms that the high photocatalytic activity of BiOI/Bi4O5I2 is correlated to the higher adsorption energy due to the formation of a network of close contacts (<3.5 Å) between nitazoxanide molecules and iodine atoms.
Environmental significanceNitazoxanide is categorized as a pharmaceutical compound possessing both antiparasitic and antibacterial attributes. Despite its cost-effectiveness, the extensive use of nitazoxanide for treating various diseases has resulted in the detection of its traces in wastewater due to its poor permeability and solubility. Conventional wastewater treatment methods have proven to be ineffective in fully eliminating various antiviral drugs and emerging pollutants from wastewater. Heterogeneous photocatalysis, an advanced oxidation process, is promising for the decontamination of such waterborne pollutants due to its efficient performance under mild operational conditions. This study investigates the impact of thermal treatment on the transition from bismuth iodide to bismuth oxyiodides and the efficacy of photocatalytic removal of nitazoxanide. The BiOI/Bi4O5I2 heterostructure exhibits the highest efficiency in photocatalytic removal of nitazoxanide. Assessment of toxicity using Aliivibrio fischeri demonstrates that the BiOI/Bi4O5I2 heterostructure is effective not only in photocatalytic removal of nitazoxanide but also in wastewater detoxification. This underscores its potential for practical application within wastewater treatment processes. |
1. Introduction
The increasing world population and the surge in various viral infections causing either an epidemic or a pandemic are leading to the development and usage of new types of antiviral drugs. As an emerging class of anthropogenic pollutants, these antiviral drugs are reaching the environment and affecting human health.1 Recent studies have revealed that antiviral drugs can travel through wastewater treatment plants with varying degrees of biotransformation, and residues of antiviral drugs have been observed in wastewater effluent,2 surface water,3 and also groundwater.4 Despite their relatively low environmental concentrations (ng L−1 to μg L−1) and natural attenuation by photolysis, hydrolysis, sorption, and biodegradation, the continuous discharge of these antiviral drugs poses a significant risk to bio-organisms and could lead to the formation of antiviral-resistant strains.5,6 Unfortunately, conventional wastewater treatment plants do not effectively remove antiviral drugs by existing water treatment technologies.7 Therefore, advanced water treatment processes must be developed to completely remove these antiviral drugs.Among the advanced oxidation processes, heterogeneous photocatalysis holds promise for effectively removing antiviral drugs and other emerging water pollutants thanks to its in situ generation of reactive species, efficiency, stability, recoverability, reusability, low energy consumption, mild operating conditions, and cost-effectiveness.8 Previously, we used SnO2@ZnS,9 elsmoreite/tungsten oxide@ZnS,10 and industrial waste-derived multiphase photocatalysts11 to effectively remove various selected pharmaceuticals and antiviral drugs from the contaminated model and real wastewater and explored the toxicity of the photocatalytically treated lopinavir- and ritonavir-containing water and wastewater.12 Wang et al.13 studied the photodegradation of oseltamivir phosphate using TiO2-P25 and found that although >95% was removed after 80 min of UV-A irradiation, the removal efficiency of total organic carbon was only 45.6–67.0%. Various α-Fe2O3-based heterostructures favor the photodegradation of sulfamethoxazole, bisphenol-A, tetracycline hydrochloride, norfloxacin, salicylic acid, carbamazepine, metronidazole, tetracycline, and lomefloxacin with efficiencies in the range of >80% to 100%.14
As photocatalytic materials, layered compounds have the potential for solar energy conversion and environmental remediation because of their unique layered crystal structure, favorable electronic band structure, diverse composition, and rich atomic coordination.15 Having a layered structure comprising [Bi2O2]2+ layers interleaved by slabs of iodine atoms, bismuth oxyiodides (BixOyIz) are visible-light-active photocatalysts with optical bandgap energies in the range of 1.8–2.9 eV.16 The open, layered structure of BixOyIz not only favors the separation and transfer of photoexcited charge carriers due to the internal electrostatic field vertical to the layer direction but also reduces the surface trapping of photoexcited charge carriers.17–19 The substitution of more I atoms by O atoms in the BixOyIz lattice leads to the formation of other members: BiOI, Bi4O5I2, α-Bi5O7I, β-Bi5O7I, and Bi7O9I3, which exhibit different optoelectronic properties and photocatalytic activities.20 BiOI single crystal nanosheets with dominant exposed {001} facets were synthesized by annealing BiI3 at 280 °C for 16 h, and the {001} facet-dependent photocatalytic activity for the degradation of rhodamine B was seven times higher than that of irregular BiOI.21 The spherical microstructures of orthorhombic-Bi5O7I were synthesized by thermal conversion of BiOI microspheres in air, and the sample thermally treated at 450 °C exhibited high photocatalytic activity for the degradation of rhodamine B due to its high crystallinity, high surface area and porous microstructure.16 Similarly, Bi4O5I2 nanosheets synthesized by thermal treatment of BiI3 at 450 °C showed high photocatalytic activity for the degradation of rhodamine B and salicylic acid.20 Despite their high photocatalytic activities for the degradation of organic dyes, bismuth oxyiodides (BixOyIz) have not been studied for the degradation of antiviral drugs.
Nitazoxanide [2-acetyloxy-N-(5-nitro-2-thiazolyl)benzamide] (C12H9N3O5S, CAS no. 55981-09-4) is classified as a pharmaceutical substance with antiparasitic and antibacterial activity. During the COVID-19 pandemic, nitazoxanide was used as a low-cost drug, and its active metabolite can inhibit the replication of both RNA and DNA, exhibiting action against various viruses, including SARS-CoV-2 (severe acute respiratory syndrome coronavirus type 2).22 The extensive use of nitazoxanide for treating various diseases, including cryptosporidiosis, resulted in the detection of its traces in wastewater matrices due to its persistence, which stems from its poor permeability and solubility (7.55 mg L−1).23 Therefore, it is important to find a novel approach to efficiently remove it from contaminated water and wastewater. So far, no studies have been conducted to investigate the photocatalytic removal of nitazoxanide except for via photolysis.24 Therefore, the effect of the bismuth iodide-to-bismuth oxyiodide phase transition on the efficiency of the photocatalytic removal of nitazoxanide is investigated in this study. The phase transition from bismuth iodide to bismuth oxyiodides is explored by changing the temperature and time of thermal treatment. The kinetics and mechanism behind the photocatalytic removal of nitazoxanide are also presented.
2. Experimental
2.1. Preparation and characterization
Bismuth(III) iodide (99%, Merck) was thermally treated in air at temperatures in the range of 350–450 °C for 1 h and also at 350 °C for different times (1, 3, 5, 7, and 9 h). The X-ray diffraction (XRD) patterns were acquired with a PANalytical X'Pert PRO diffractometer with Cu Kα radiation. The bright-field and lattice images and selected-area electron diffraction (SAED) patterns were obtained using an EM-002B (TOPCON) high-resolution transmission electron microscope (TEM) at an accelerating voltage of 200 kV. The microstructures were observed by a Carl Zeiss Gemini 982 scanning electron microscope (SEM). The surface chemical states of elements were analyzed by X-ray photoelectron spectroscopy (XPS, JPS-9010MX, JEOL) using non-monochromatic Mg-Kα radiation. The UV-vis diffuse reflectance spectra were recorded on an Evolution 220 UV/vis spectrometer (Thermo Fisher Scientific).The photoanodes were fabricated by depositing the synthesized bismuth (oxy)iodide powders, and the photoelectrochemical (PEC) measurements were carried out according to a protocol reported elsewhere.25 The geometric area of the working electrode was 1.54 cm2, the counter electrode was a Pt wire, a reference hydrogen electrode (RHE) was used, and 0.1 M Na2SO4 (50 mL) was employed as the supporting electrolyte. The chronoamperometry (CA) results at 1.8 V vs. RHE and open-circuit potential decay (OCPD) data were obtained (Princeton Applied Research PAR263 Potentiostat). Moreover, the lifetime (τ) of the carriers was calculated according to the following equation: τ = |kBT/e0 (dEOC/dt)−1|, where EOC is the open-circuit potential, t is the time, kB is the Boltzmann potential constant, T is the absolute temperature, and e0 is the elemental charge.26 Irradiation was provided by a Newport solar simulator (150 W Xe lamp), and the light photon flux was set to 100 mW cm−2.
2.2. Photocatalytic activity tests
The photocatalytic activity of the photocatalyst samples for the degradation of nitazoxanide was evaluated. First, 0.4 mg L−1 of the photocatalyst sample was dispersed in an aqueous solution of nitazoxanide (10 mg L−1) in a photochemical reactor (0.7 L, Heraeus) in the dark for 30 min to achieve adsorption–desorption equilibrium. Then, a 150 W mercury lamp, which was vertically placed in the center of the photoreactor, with emitting radiation centered at 500–550 nm, an intensity of 7.31–7.53 mW cm−2, and a photon flux of 20.83 × 1019 m2 s−1, was turned on. Aliquots were collected at various times before and during the photocatalytic reaction (−30, 0, 5, 15, 30, 45, 60, and 120 min) and filtered. The collected aliquots were analyzed using an Agilent 1260 Infinity II LC-DAD chromatograph equipped with an InfinityLab Poroshell 120 EC-C18 column (3.0 × 150 mm, 2.7 μm), using H2O + CH3COONH4 (pH = 6)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN (v/v 60
ACN (v/v 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) as the mobile phase (flow 0.5 mL min−1, temperature 30 °C), and analyzed at λNTZ = 240 nm, RT = 3.99 min. The fitting of the calibration curve was R2 = 0.9998. The ratio of the nitazoxanide concentrations before and after the photocatalytic reactions, C/C0, was used to indicate the amount of nitazoxanide that had been photocatalytically removed from the aqueous solution.
40) as the mobile phase (flow 0.5 mL min−1, temperature 30 °C), and analyzed at λNTZ = 240 nm, RT = 3.99 min. The fitting of the calibration curve was R2 = 0.9998. The ratio of the nitazoxanide concentrations before and after the photocatalytic reactions, C/C0, was used to indicate the amount of nitazoxanide that had been photocatalytically removed from the aqueous solution.
The chromatographic analysis of by-products was performed using an LC/MS system consisting of an ultra-high-performance liquid chromatograph (UltiMate 3000, Thermo Fisher Scientific) connected with a linear trap quadrupole-Orbitrap mass spectrometer (LTQ-Orbitrap Velos, Thermo Fisher Scientific) equipped with an ESI source. In all analyses, a Gemini C18 column (4.6 × 100 mm, 3 μm) (Phenomenex) was used for the chromatographic separation. During the chromatographic process, gradient elution was used. Mobile phase A was 25 mM formic acid in water, and mobile phase B was 25 mM formic acid in acetonitrile. The gradient program started at 5% B, increasing to 85% by 40 min, followed by isocratic elution at 85% B for 5 min. The total run time was 45 min at the flow rate of 0.5 mL min−1. During each run, the MS spectra in the m/z range of 50–500 were continuously collected.
The effects of various parameters, such as competing ions (Cl−, NO3−, and H2PO4− from respective sodium salts with a concentration of 5 × 10−3 mol L−1) and water matrices (distilled water [DW], tap water [TW], and treated wastewater [TWW]), on the efficiency of the photocatalytic degradation of nitazoxanide over the prepared samples were studied. TWW was collected from the “Hajdów” municipal wastewater treatment plant in Lublin, which is a mechanical–biological wastewater treatment facility with increased removal of biogenic compounds (nitrogen and phosphorus) from wastewater. TWW was characterized by significantly reduced biological (5.7 mg L−1) and chemical (35.1 mg L−1) oxygen demands, total suspended solids (up to 6.4 mg L−1), and total nitrogen (10.72 mg L−1) and phosphorous (0.27 mg L−1) contents. TW was characterized by total organic carbon (<3 mg L−1), chlorides (35 mg L−1), nitrates (<2 μg L−1), sulfates (41 mg L−1) contents, and pH of 7.2. The reusability test was performed using the sample thermally treated at 375 °C for three repetitive cycles with an intermediate washing with ethanol and distilled water and drying at 105 °C.
To gain insights into the possible reaction mechanism for the photodegradation of nitazoxanide over the prepared samples, the trapping experiments were conducted using isopropyl alcohol (IPA, 1000 mg L−1), p-benzoquinone (PBQ, 1 mg L−1), and ethylenediaminetetraacetic acid disodium salt dihydrate (Na2EDTA, 100 mg L−1) as scavengers of ˙OH, O2˙−, and h+, respectively. Toxicity towards marine bacteria (Aliivibrio fischeri) was evaluated based on the inhibition of bioluminescence using the Microtox® test.27 The luminescence inhibition was determined after 5 min and 15 min of exposure of Aliivibrio fischeri to the water samples before and after the photocatalytic reaction according to the standard protocol (Microtox®, 1995) in a Microtox M500 analyzer using Omni software.
3. Results and discussion
3.1. Characterization of materials
The phase transition from bismuth iodide to bismuth oxyiodides was studied by changing the temperature and time of thermal treatment in air. Fig. 1a shows the XRD patterns of samples thermally treated at temperatures ranging from 350 °C to 450 °C for 1 h. The XRD pattern of the as-received bismuth iodide indicates major reflections assignable to trigonal BiI3 with space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) and lattice parameters of a = 7.5250 Å and c = 20.7030 Å (ICSD# 98-007-8791). In addition, minor reflections belonging to tetragonal BiOI with space group P4/nmm and lattice parameters of a = 3.9950 Å and c = 9.1510 Å (ICSD# 98-039-1354) are also observed. At 350 °C, tetragonal BiOI becomes a dominating crystalline phase as a result of the evaporation of iodine and oxidation. Monoclinic Bi4O5I2 with space group P1211 and lattice parameters of a = 11.2630 Å, b = 5.6980 Å, c = 14.9440 Å, and β = 99.81° (ICSD# 98-041-2590) accompanies tetragonal BiOI in the sample thermally treated at 375 °C and turns into a dominating crystalline phase at 400 °C. At 425 °C and 450 °C, the reflections associated with monoclinic Bi4O5I2 disappear altogether, and new reflections referable to orthorhombic Bi5O7I with space group Ibca and lattice parameters of a = 5.3440 Å, b = 16.2650 Å, and c = 23.0200 Å (ICSD# 98-041-1666). The XRD patterns in Fig. 1b confirm that extending the time of thermal treatment at 350 °C from 1 h to 9 h led to the phase transition from trigonal BiI3 to tetragonal BiOI first and then to monoclinic Bi4O5I2. Evidently, the change in the temperature of the thermal treatment had a stronger impact on the phase transition than the shift in the time of the thermal treatment. Previous studies found that the oxidative conversion of BiI3 to Bi2O3 occurred either through the formation of Bi4O5I2 (and BiOI)20 or Bi5O7I (and BiOI),21 but not both. Here, by controlling the temperature of the thermal treatment, the oxidative conversion of BiI3 proceeded according to the following stages:
and lattice parameters of a = 7.5250 Å and c = 20.7030 Å (ICSD# 98-007-8791). In addition, minor reflections belonging to tetragonal BiOI with space group P4/nmm and lattice parameters of a = 3.9950 Å and c = 9.1510 Å (ICSD# 98-039-1354) are also observed. At 350 °C, tetragonal BiOI becomes a dominating crystalline phase as a result of the evaporation of iodine and oxidation. Monoclinic Bi4O5I2 with space group P1211 and lattice parameters of a = 11.2630 Å, b = 5.6980 Å, c = 14.9440 Å, and β = 99.81° (ICSD# 98-041-2590) accompanies tetragonal BiOI in the sample thermally treated at 375 °C and turns into a dominating crystalline phase at 400 °C. At 425 °C and 450 °C, the reflections associated with monoclinic Bi4O5I2 disappear altogether, and new reflections referable to orthorhombic Bi5O7I with space group Ibca and lattice parameters of a = 5.3440 Å, b = 16.2650 Å, and c = 23.0200 Å (ICSD# 98-041-1666). The XRD patterns in Fig. 1b confirm that extending the time of thermal treatment at 350 °C from 1 h to 9 h led to the phase transition from trigonal BiI3 to tetragonal BiOI first and then to monoclinic Bi4O5I2. Evidently, the change in the temperature of the thermal treatment had a stronger impact on the phase transition than the shift in the time of the thermal treatment. Previous studies found that the oxidative conversion of BiI3 to Bi2O3 occurred either through the formation of Bi4O5I2 (and BiOI)20 or Bi5O7I (and BiOI),21 but not both. Here, by controlling the temperature of the thermal treatment, the oxidative conversion of BiI3 proceeded according to the following stages:| 2BiI3 + O2 → 2BiOI + 2I2↑ | (1) |
| 8BiOI + O2 → 2Bi4O5I2 + 2I2↑ | (2) |
| 10Bi4O5I2 + 3O2 → 8Bi5O7I + 6I2↑ | (3) |
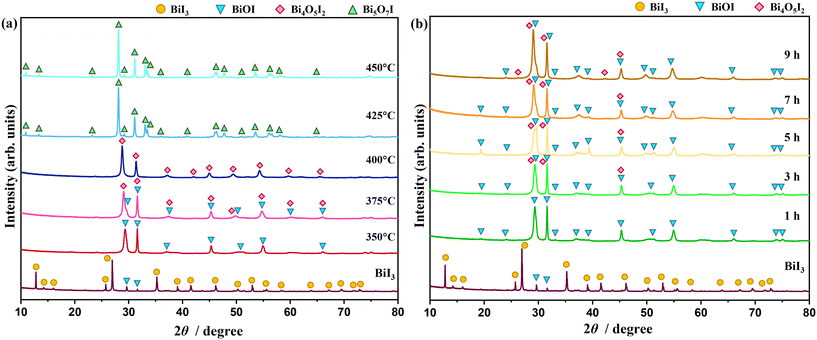 | ||
| Fig. 1 XRD patterns of samples thermally treated at temperatures ranging from 350 °C to 450 °C for 1 h (a) and at 350 °C for 1–9 h (b) in air. | ||
The morphological change as a consequence of the oxidative conversion of bismuth iodide was selectively observed by means of scanning electron microscopy (SEM). Fig. 2 shows the SEM images of samples before and after thermal treatment at 350 °C and 450 °C. As shown in Fig. 2a, bismuth iodide has an irregular morphology in different sizes. In contrast, the sample thermally treated at 350 °C contains plate-like structures of BiOI as a result of the oxidation of bismuth iodide and evaporation of I2 (Fig. 2b). Interestingly, the thickness and shape of the plate-like structures of BiOI are different from those reported previously.20,21 Presumably, such discrepancy arises from the difference in internally accumulated I2 vapor pressure, which leads to peeling off the surface shells of BiOI layer by layer and forming ultrathin nanosheets. A further increase in the thermal treatment temperature to 450 °C resulted in the formation of layered structures of Bi5O7I with pores because of the accelerated release of I2 vapor (Fig. 2c). The corresponding energy-dispersive X-ray spectroscopy (EDX) data indicate a lessening of the peak intensity of iodine due to its evaporation during thermal treatment. The surface chemical states of samples were analyzed by XPS. The XPS results for the samples thermally treated at different temperatures are shown in Fig. 3. Note that BiI3 was not stable during the XPS analysis. In Fig. 3a, two peaks corresponding to the Bi 4f7/2 and Bi 4f5/2 states are observed at 159.4 eV and 164.7 eV, respectively, indicating the +3 oxidation state of bismuth. In the I 3d XPS spectra in Fig. 3b, two peaks at 619.4 eV and 630.8 eV can be assigned to the I 3d5/2 and I 3d3/2 states, respectively, implying the −1 oxidation state of iodine. The O 1s XPS spectra in Fig. 3c can be deconvoluted into two peaks at 530.1 eV and 528.4 eV, which can be ascribed to the lattice oxygen and surface chemisorbed oxygen, respectively. Although no significant difference can be seen in the XPS spectra, the results of quantitative XPS analysis in atomic percentage confirm that the contents of bismuth and oxygen increased and the bismuth content decreased with increasing thermal treatment temperature.
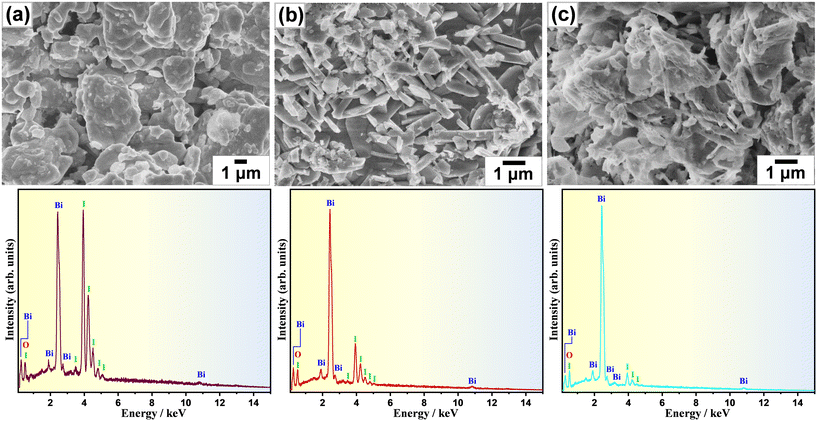 | ||
| Fig. 2 SEM images and EDS spectra of samples (a) before and after thermal treatment at (b) 350 °C and (c) 450 °C for 1 h in air. | ||
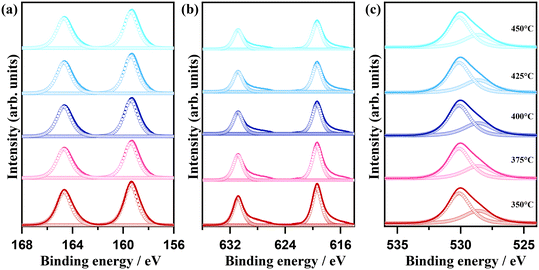 | ||
| Fig. 3 (a) Bi 4f, (b) I 3d, and (c) O 1s XPS spectra of samples thermally treated at temperatures ranging from 350 °C to 450 °C for 1 h in air. | ||
Next, the nanostructural transformation as a result of the oxidative conversion of bismuth iodide was analyzed by means of transmission electron microscopy (TEM). Fig. 4 shows the bright-field TEM and HRTEM images and corresponding selected area electron diffraction (SAED) patterns of samples before and after thermal treatment at 350 °C, 400 °C, and 450 °C. The bright-field TEM images clearly indicate a smooth surface with hexagonal nanosheets for the bismuth iodide before thermal treatment, a rough surface with plate-like nanostructures for the samples thermally treated at 350 °C and 400 °C due to the phase transition, crystallization, and inception of pore formation (average pore size is about 30 nm), and plate-like nanoporous nanostructures for the sample thermally treated at 450 °C because of the increased emission of I2 vapor. In the HRTEM images, the lattice fringes observed in the regions of the plate-like nanostructures show the d-spacing values of 0.303, 0.285, 0.946, and 0.832 nm, revealing the exposed (113), (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0), (
0), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01), and (200) crystal planes of BiI3, BiOI, Bi4O5I2, and Bi5O7I, respectively. This corroborates the oxidative phase transition from bismuth iodide to bismuth oxyiodides at the nanoscale. No significant defects were observed in the analyzed areas, implying their high crystallinity. The highly ordered SAED patterns confirm the single-crystalline nature of BiI3, BiOI, Bi4O5I2, and Bi5O7I. By indexing the diffraction spots in the SAED patterns, the trigonal, tetragonal, monoclinic, and orthorhombic crystal structures of BiI3, BiOI, Bi4O5I2, and Bi5O7I were confirmed. In addition, the SEM image of the sample thermally treated at 375 °C is shown in Fig. S1 in the ESI,† indicating the formation of BiOI/Bi4O5I2 heterostructures.
01), and (200) crystal planes of BiI3, BiOI, Bi4O5I2, and Bi5O7I, respectively. This corroborates the oxidative phase transition from bismuth iodide to bismuth oxyiodides at the nanoscale. No significant defects were observed in the analyzed areas, implying their high crystallinity. The highly ordered SAED patterns confirm the single-crystalline nature of BiI3, BiOI, Bi4O5I2, and Bi5O7I. By indexing the diffraction spots in the SAED patterns, the trigonal, tetragonal, monoclinic, and orthorhombic crystal structures of BiI3, BiOI, Bi4O5I2, and Bi5O7I were confirmed. In addition, the SEM image of the sample thermally treated at 375 °C is shown in Fig. S1 in the ESI,† indicating the formation of BiOI/Bi4O5I2 heterostructures.
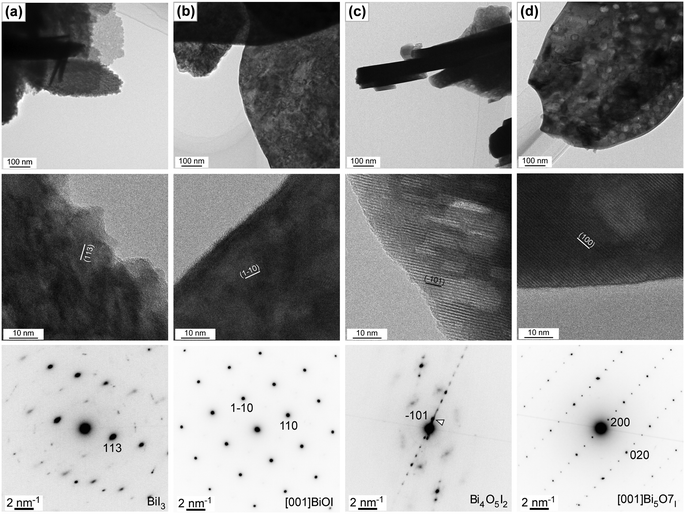 | ||
| Fig. 4 TEM and HRTEM images and SAED patterns of samples (a) before and after thermal treatment at (b) 350 °C, (c) 400 °C, and (d) 450 °C for 1 h in air. | ||
The oxidative phase transition from bismuth iodide to bismuth oxyiodides influences the optical properties. Therefore, the light absorption characteristics of the samples were analyzed by means of UV-vis absorption spectroscopy. Fig. 5 shows the UV-vis diffuse reflectance spectra of the samples. Apparently, the greyish-black color of bismuth iodide powder gradually turned into reddish-orange, orange, yellow, milky white, and white as the thermal treatment temperature increased to 450 °C, whereas the greyish-black color of bismuth iodide powder slowly changed to reddish-orange, vivid orange, and light orange with increasing time of thermal treatment at 350 °C up to 9 h. In the UV-vis diffuse reflectance spectra (Fig. 5a), the absorption edges are observed at the wavelengths of about 715, 635, 615, 520, and 388 nm, which correspond to the optical bandgap energies of 1.73, 1.95, 2.01, 2.38, and 3.19 eV, for the samples before (BiI3) and after thermal treatment at 350 °C (BiOI), 375 °C (BiOI + Bi4O5I2), 400 °C (Bi4O5I2), 425 °C (Bi5O7I), and 450 °C (Bi5O7I), respectively. In contrast, the samples before and after thermal treatment at 350 °C for different times exhibit absorption edges at wavelengths of approximately 715, 639, 636, 628, 623, and 615 nm, which correspond to optical bandgap energies of 1.73, 1.94, 1.95, 1.97, 1.99, and 2.01 eV, respectively (Fig. 5b). As can be seen, most of the synthesized samples are visible light active, harvesting solar energy efficiently. A pronounced increase in the optical bandgap energy to 3.19 eV in the samples thermally treated at different temperatures is consistent with the oxidative phase transition from BiI3 to Bi5O7I. A gradual blueshift in the absorption edges in the UV-vis diffuse reflectance spectra of the samples thermally treated at 350 °C for different times is in good agreement with the oxidative phase transition of BiI3 to BiOI with increased content of Bi4O5I2. This implies that the change in the temperature of the thermal treatment influenced the phase transition more effectively than the time change of the thermal treatment.
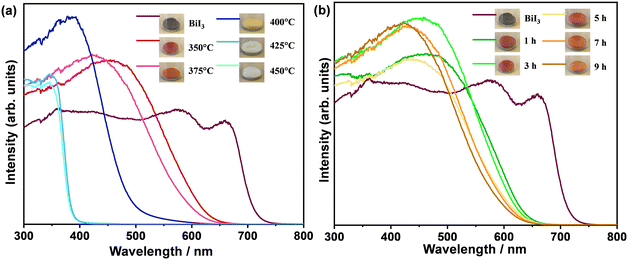 | ||
| Fig. 5 UV-vis diffuse reflectance spectra of samples thermally treated (a) at temperatures ranging from 350 °C to 450 °C for 1 h and (b) at 350 °C for 1–9 h in air. | ||
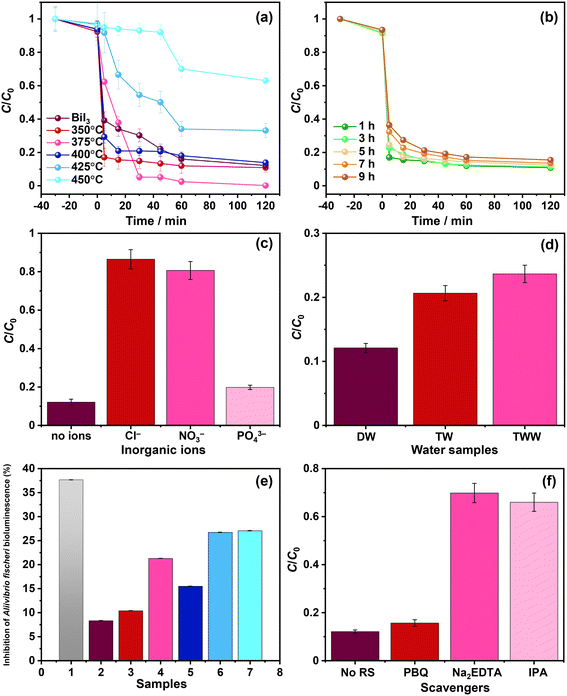
The effect of the oxidative conversion of bismuth iodide to bismuth oxyiodides on the photodegradation of nitazoxanide was studied. Fig. 6a and b show the change in the concentration of nitazoxanide in an aqueous solution during photocatalysis in the presence of the prepared photocatalyst samples. A previous study on the photodegradation kinetics of nitazoxanide revealed that direct UV-assisted photolysis could degrade only 30% of nitazoxanide.24 As shown in Fig. 6a and b, the synthesized photocatalytic materials exhibited a poor adsorption capacity (<10%) for nitazoxanide (C0 = 10 mg L−1) in the dark. In Fig. 6a, even though bismuth oxide and the other two samples thermally treated at 350 °C and 400 °C showed a higher photodegradation rate for nitazoxanide in the initial stage (5–15 min), they did not completely degrade nitazoxanide. In contrast, the sample thermally treated at 375 °C fully mineralized nitazoxanide after 60 min. The samples thermally treated at 425 °C and 450 °C showed the slowest photodegradation rates for nitazoxanide. The discrepancy in the efficiency of photodegradation of nitazoxanide over the samples thermally treated at different temperatures is mainly associated with the oxidative conversion of BiI3 to BiOI, BiOI + Bi4O5I2, Bi4O5I2, and Bi5O7I phases with different optoelectronic properties. The highest photocatalytic activity for the degradation of nitazoxanide was observed for the sample thermally treated at 375 °C due to the formation of a BiOI/Bi4O5I2 heterostructure. Similarly, the BiOI/Bi4O5I2 heterostructure showed a significantly higher photocatalytic activity for the degradation of methylene blue due to the efficient charge transfer and separation.28 Despite its absorption up to 715 nm, bismuth iodide showed lower photocatalytic activity in comparison to BiOI, BiOI + Bi4O5I2, and Bi4O5I2 because of the presence of defects acting as the recombination centers for photoexcited charge carriers. Changing the thermal treatment time from 1 h to 9 h resulted in a less differentiated but reduced photodegradation rate because of the increased content of Bi4O5I2 in the BiOI/Bi4O5I2 heterostructure (Fig. 6b). As shown in Fig. 5a, Bi4O5I2 has limited visible light absorption in comparison to BiOI. The efficiency of the BiOI/Bi4O5I2 heterostructure in the photocatalytic removal of nitazoxanide cannot be compared with other photocatalysts due to the absence of previous studies on the photocatalytic degradation of nitazoxanide in the presence of photocatalysts. Nevertheless, the efficiency of the BiOI/Bi4O5I2 heterostructure was compared with the efficiencies of other bismuth oxyiodides for the photocatalytic degradation of various organic compounds (Table S1†). Apparently, the BiOI/Bi4O5I2 heterostructure exhibits higher efficiency in the photocatalytic degradation of nitazoxanide compared with the other bismuth oxyiodide compounds in Table S1.†
Various BiOI-based photocatalysts have shown high efficiency in the photocatalytic removal of different pharmaceuticals under visible light irradiation. For instance, the BiOI/UiO-66 p–n heterojunction showed nearly 100% removal efficiency for sulfadiazine (5 mg L−1) within 90 min of visible light irradiation.29 Ag-decorated BiOI applied as a counter electrode significantly enhanced the photoelectrocatalytic removal (92%) of diclofenac sodium (10 mg L−1) over an Ag–BiVO4/BiOI photoanode after 2 h due to the formation of a type II p–n heterojunction and a Schottky barrier.30 The ClO2-added BiOI-based system achieved 100% removal of sulfamethoxazole within 30 min because of the activation of chlorite to chlorine dioxide.31 Very recently, an FeIII/BiOIO3 piezo-catalytic self-Fenton system was developed, which can offer exceptional self-recyclable degradation of pollutants with a degradation rate constant for sulfamethoxazole over 3.5 times that of the classic FeII-based system.32
The kinetics of the photocatalytic removal of nitazoxanide by the prepared photocatalysts were estimated by applying two different models: pseudo-first-order (Langmuir–Hinshelwood) and pseudo-second-order, and the results are given in Table 1 and Fig. S2a and b in the ESI.† Quantitatively, the kinetics of the photocatalytic removal of nitazoxanide by the photocatalyst samples thermally treated at different temperatures can be ascribed to both models, while the kinetics of the photocatalytic removal of nitazoxanide by the photocatalyst samples thermally treated for different times follow the pseudo-second-order. In fact, the different kinetic models can all describe the photocatalytic degradation, implying the complex nature of the reaction kinetics. Therefore, the effects of adsorbed states, intermediates, inhibition, and physicochemical phenomena within the photocatalysts must be considered to understand such complex reaction kinetics.33 In addition, exploring the effect of the type of substance to be degraded and its concentration according to proper kinetic models (i.e., Langmuir–Hinshelwood) could help to elucidate these aspects.34,35 In all cases, the changes induced by the synthesis process on the surface of the photocatalysts make it possible to modulate the reaction rate. Among the samples thermally treated at different temperatures and times, the highest k1 (0.051 min−1) and k2 (4.225 mg g−1 min−1) were observed for the sample thermally treated at 375 °C for 1 h, manifesting the important role of the BiOI/Bi4O5I2 heterostructure.
| Photocatalyst | Pseudo-first-order | Pseudo-second-order | |||
|---|---|---|---|---|---|
| k 1 (min−1) | t 1/2 (min) | R 2 | k 2 (mg g−1 min−1) | R 2 | |
| BiI3 | 0.01041 | 42.14307 | 0.95244 | 0.029292 | 0.925258 |
| 350 °C | 0.01645 | 66.54891 | 0.89953 | 0.052519 | 0.956483 |
| 375 °C | 0.05115 | 128.3145 | 0.75580 | 4.225691 | 0.790836 |
| 400 °C | 0.01310 | 49.52926 | 0.84657 | 0.029078 | 0.942764 |
| 425 °C | 0.00540 | 13.55094 | 0.96187 | 0.028774 | 0.914537 |
| 450 °C | 0.00512 | 12.03420 | 0.82674 | 0.026824 | 0.893217 |
| 1 h | 0.05115 | 128.3145 | 0.75580 | 4.225691 | 0.790836 |
| 3 h | 0.01142 | 65.53 | 0.50838 | 0.07649 | 0.66903 |
| 5 h | 0.01135 | 63.45 | 0.43380 | 0.06954 | 0.68673 |
| 7 h | 0.01092 | 61.04 | 0.41180 | 0.05205 | 0.84927 |
| 9 h | 0.01057 | 60.69 | 0.34705 | 0.04790 | 0.87584 |
To substantiate the impact of various competing inorganic ions on the efficiency of the photocatalytic removal of nitazoxanide by the synthesized photocatalysts, additional experiments were conducted with Cl−, NO3−, and PO43− ions and the samples thermally treated at 375 °C. As shown in Fig. 6c, the presence of Cl− and NO3− ions drastically hindered the photocatalytic removal of nitazoxanide. This necessitates the removal of Cl− and NO3− ions before the photocatalytic treatment of water contaminated with nitazoxanide. In contrast, the PO43− ions slightly reduced the efficiency of the photocatalytic removal of nitazoxanide with respect to the absence of inorganic ions. Similar results were noted during the removal of sulfamethoxazole by ClO2-assisted BiOI-based photocatalysis.31 Such a dramatic effect of inorganic ions on the efficiency of the photocatalytic removal of nitazoxanide by the prepared photocatalytic materials may be explained by the change in pH, stemming from the presence of inorganic ions or the scavenging effect of ions and the formation of radicals: HOCl˙− (1.49 V, k = 6.1 ± 0.8 × 109 M−1 s−1), Cl˙ (2.03 V, k = 4.3 × 109 M−1 s−1),36 NO3˙ (k = 1.4 × 108 M−1 s−1),37 and HPO4˙− (k = 8 × 105 M−1 s−1)38 with lower oxidizing power than ˙OH (2.8 V vs. NHE at pH = 0), thus reducing the efficiency of the photocatalytic activity.39 Similarly, the efficiency of the photocatalytic removal of ritonavir was decreased by introducing Cl−, NO3−, and H2PO4−.11Fig. 6d shows the influence of water matrices on the efficiency of the photocatalytic removal of nitazoxanide by the synthesized samples. Apparently, the photocatalytic removal of nitazoxanide was more efficient in distilled water (DW) in comparison to tap water (TW) and treated wastewater (TWW). Although the compositions of TW and TTW significantly differ from that of DW, their efficiency in the photocatalytic removal of nitazoxanide was at least 75%. In the case of TW, the presence of Cl− (35 mg L−1 according to the supplier of the TW samples), not NO3− (<2 mg L−1), was the main reason for hindering the photocatalytic removal of nitazoxanide. The presence of dissolved organic matter and nitrogen in TTW was responsible for lowering the efficiency of the photocatalytic removal of nitazoxanide. The reusability test was conducted for the photocatalytic degradation of nitazoxanide over the BiOI/Bi4O5I2 heterostructure for three consecutive cycles. As shown in Fig. S3,† the BiOI/Bi4O5I2 heterostructure has good stability in the photocatalytic degradation of nitazoxanide for three consecutive cycles.
The toxicity of water samples after the photocatalytic reaction in the presence of the sample thermally treated at 375 °C was evaluated for Aliivibrio fischeri. As shown in Fig. 6e, the initial aqueous solution containing nitazoxanide shows the highest toxicity to Aliivibrio fischeri and >35% bioluminescence is inhibited. Apparently, the photocatalytic treatment reduced the toxicity, and the final aqueous solutions after photocatalytic treatment were slightly toxic, inhibiting the bioluminescence from >10 up to >25%. This confirms that the photocatalytic treatment in the presence of the synthesized photocatalysts is effective not only in the removal of nitazoxanide but also in the detoxification of wastewater. The observed inhibition of bioluminescence for the samples thermally treated at 425 °C and 450 °C possibly resulted from the formation of toxic by-products during the photodegradation of nitazoxanide since no traces of nitazoxanide were detected after the photocatalytic reaction.
In the photocatalytic process, various reactive species are involved, including photoexcited holes, hydroxyl, and superoxide radicals. To verify their impact on the photocatalytic removal of nitazoxanide by the synthesized photocatalysts, the trapping experiments were parallelly conducted using isopropyl alcohol (IPA, 1000 mg L−1), p-benzoquinone (PBQ, 1 mg L−1), and ethylenediaminetetraacetic acid disodium salt dihydrate (Na2EDTA, 100 mg L−1) as the scavengers of ˙OH, O2˙−, and h+, respectively. As shown in Fig. 6f, photoexcited holes and hydroxyl radicals played an important role in the photocatalytic removal of nitazoxanide by the synthesized photocatalyst. Unlike in previous reports,40–43 superoxide radicals were not the main driving force in the photocatalytic reaction, presumably due to the low content of Bi4O5I2, resulting in a smaller number of the BiOI/Bi4O5I2 heterostructures. The photocatalytic performance of the BiOI/Bi4O5I2 heterojunctions was previously studied.41,42 In the context of photocatalytic performance, it is noteworthy that the conduction band (CB) and valence band (VB) potentials reported for BiOI and Bi4O5I2 in previous studies offer valuable information. BiOI exhibits more positive potentials (ECB = 0.07 V vs. NHE and EVB = 1.91 V vs. NHE)42 than Bi4O5I2, where the CB potential is negative (ECB = −0.68 V vs. NHE),42 although with a similar VB potential (EVB = 1.78 V vs. NHE)42 (Fig. 7a). It is important to recognize that, for the crystals synthesized by applying different synthesis routes, these band potential values can vary depending on various factors, including crystal structure, size, crystallinity, defects, and density of electronic states. Therefore, the synthesis method used in this work may influence these potential values. Therefore, it is prudent to consider these reported potentials as initial approximations for our analysis, which allows us to discern relative trends. For example, the relatively positive VB potential of BiOI suggests the feasibility of oxidizing hydroxyl ions to generate hydroxyl radicals (1.9 V vs. NHE at pH = 7 for HO−/HO˙)44,45 within this photocatalyst. Meanwhile, in the CB of Bi4O5I2, the processes, such as oxygen reduction reaction (−0.18 V vs. NHE at pH = 7 for O2/O2˙−)44 and/or redox reactions associated with nitazoxanide photochemistry (Fig. 7b), can potentially complete the charge balance.46,47
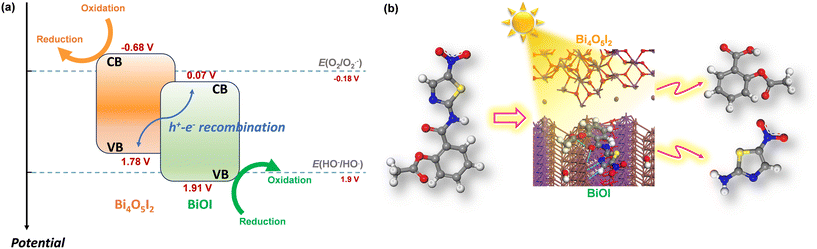 | ||
| Fig. 7 (a) Band structures and redox potentials in BiOI/BI4O5I2 heterostructure (potentials in V vs. NHE at pH = 7) and (b) possible photodegradation pathways52 of nitazoxanide by the synthesized photocatalyst. | ||
Exploring the photoelectrochemical behavior of bismuth (oxy)iodides provides insights into the underlying mechanisms and photocatalytic processes. Fig. 8a shows the chronoamperometric (CA) response at high overpotential in several dark–light cycles. When the light is supplied, the current transiently increases for all samples studied, defining an intense peak or spikes and then decaying to a steady state value. When the light is turned off, the current tends to zero, defining a negative overshoot. This evidence is characteristic of semiconductor–electrolyte interfaces, where the so-called “band edge unpinning” occurs.48 Therefore, surface recombination is important, and there may be some changes in the chemical composition of the interface. The electron transfer reactions are relatively slow, and the concentration of minority carriers increases to levels that modify the potential drop across the Helmholtz layer in the electrolyte.48 The trend observed in the stationary value of the photocurrent at high overpotential follows the order: 375 °C > 350 °C > BiI3 > 400 °C = 425 °C = 450 °C, which is in good agreement with the results of the photocatalytic kinetics study. Fig. 8b shows the lifetime during open-circuit potential decay (OCPD). The OCPD condition manifests as a scenario wherein the PEC response of the interface tends to a low overpotential.25 Therefore, behaviors can be differentiated due to the differences in the recombination processes.26 In fact, the sample thermally treated at 375 °C exhibits higher lifetime values for lower potentials compared to other samples. For all samples studied, the trend of the semilogarithmic graph of lifetime versus open-circuit potential is linear, which is consistent with an electron transfer theory.49 A longer lifetime suggests a slower recombination rate, which infers a greater availability of charge carriers for photocurrent collection.33 Therefore, the PEC results support the idea that electron transfer increases at the expense of recombination, and the BiOI/Bi4O5I2 heterojunction formed in the sample thermally treated at 375 °C results in a higher photocurrent at high overpotential and longer lifetimes during deactivation under OCPD conditions. It is worth noting that the surface photoredox reactions and photocorrosion are the processes that require further investigations through comprehensive PEC studies.50,51
 | ||
| Fig. 8 Results of photoelectrochemical measurements: (a) CA at 1.8 V vs. RHE and (b) lifetime vs. open-circuit potential. | ||
The chemical structure of nitazoxanide contains hydrogen-bonding acceptor sites: the O atom in the ester and –NO2 capable of forming N–O⋯H with the solvent molecules and the N atom in the –NH groups capable of forming N–H⋯O bonds.23 The –NO2 and –NH groups are the principal sites of electrophilic and nucleophilic attacks on the nitazoxanide molecule, respectively.23 The presence of conjugated double bonds and nitro and amide groups being chromophores indicates that the nitazoxanide molecule should be susceptible to light.24 During the photocatalytic process, the nitazoxanide molecule underwent decomposition via hydrolysis into acetylsalicylic acid (1) and the respective aminonitrothiazol (2) (Fig. 7b). In the MS analysis, the appearance of a fragment at m/z = 266 confirmed the deacetylation process, which was followed by the cleavage of the amide according to the fragment at m/z = 121. The fragment at m/z = 163 indicated the cleavage of the amide without the deacetylation process.52 The formed acetylsalicylic acids underwent further oxidation, which led to the formation of the ring-opening products due to the cleavage of a C–C bond (with a cyclopentane-1,3-dione core). Further decarboxylation (ascribed to the photo-Kolbe reaction) that is initiated by hole oxidation of the carboxylic acids resulted in the release of CO2 and radicals.
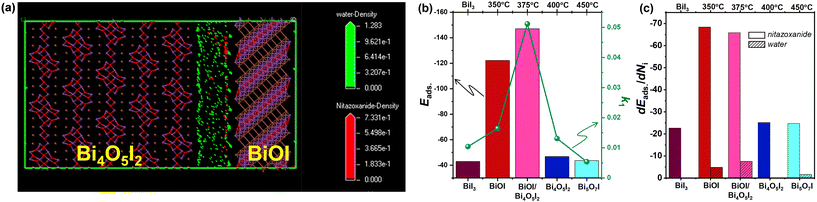
The adsorption of molecules on the photocatalyst surface is an important step for heterogeneous photocatalysis. To gain insights into the photodegradation of nitazoxanide in aqueous solution, its adsorption was studied using numerical molecular dynamics (MD) simulations and stochastic Monte Carlo (MC) simulations.53 In these MD and MC simulations, the interaction among the nitazoxanide and water molecules was taken into account as forces and energy changes related to the random movement of the components, respectively. The MD simulation of nitazoxanide in an aqueous solution was performed by Forcite and Adsorption Locator modules in Materials Studio (BIOVIA). The detailed methodology for the modelling was described elsewhere,54,55 and the crystallographic CIF files for BiI3, BiOI, Bi4O5I2, and Bi5O7I (ref. 56–59) were used. Their predominant crystallographic planes were chosen for modelling according to the XRD and TEM data. The affinities of nitazoxanide and water molecules were evaluated through the distribution field density, adsorption energy (Eads), and differential adsorption dEad/dNi of the components (Table S2†). The Eads values of the nitazoxanide model are much higher on the BiOI surface than the Eads values on the BiI3, Bi4O5I2, and Bi5O7I surfaces in the following order: −138.92 kcal mol−1 for BiOI (1 0 2) > −46.81 kcal mol−1 for Bi4O5I2 (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0 1) > −43.61 kcal mol−1 for Bi5O7I (2 0 0) > −42.94 kcal mol−1 for BiI3 (2
0 1) > −43.61 kcal mol−1 for Bi5O7I (2 0 0) > −42.94 kcal mol−1 for BiI3 (2 ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 3).
3).
According to the data shown in Table S2,† the water molecules have a better affinity for the BiOI surface than for the BiI3, Bi4O5I2, and Bi5O7I surfaces. The calculated Eads values for the crystallographic planes of BiOI are different and significantly exceeded the similar values for BiI3, Bi4O5I2, and Bi5O7I in the following order: −138.92 kcal mol−1 for BiOI (1 0 2) > −124.54 kcal mol−1 for BiOI (1 1 0) > −122.20 kcal mol−1 for BiOI (1 ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0). The experimental data and degradation constants (k1 and k2) showed that the BiOI and Bi4O5I2 has a higher photocatalytic activity for the degradation of nitazoxanide than individual Bi4O5I2 and BiOI (Table 1). Considering the localization of the nitazoxanide and water molecules at the interface formed between two predominant crystallographic planes of BiOI and Bi4O5I2, it was found that the adsorption of nitazoxanide molecules has a preference on the BiOI surface (Fig. 9a) via the formation of a network of close contacts (<3.5 Å) between nitazoxanide molecules and iodine atoms (Fig. 9b). This leads to the re-distribution of field density near BiOI (1 1 0) away from Bi4O5I2 (
0). The experimental data and degradation constants (k1 and k2) showed that the BiOI and Bi4O5I2 has a higher photocatalytic activity for the degradation of nitazoxanide than individual Bi4O5I2 and BiOI (Table 1). Considering the localization of the nitazoxanide and water molecules at the interface formed between two predominant crystallographic planes of BiOI and Bi4O5I2, it was found that the adsorption of nitazoxanide molecules has a preference on the BiOI surface (Fig. 9a) via the formation of a network of close contacts (<3.5 Å) between nitazoxanide molecules and iodine atoms (Fig. 9b). This leads to the re-distribution of field density near BiOI (1 1 0) away from Bi4O5I2 (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0 1). The high photocatalytic activity of BiOI/Bi4O5I2 is correlated to its higher adsorption than that of Bi4O5I2 but is similar to BiOI according to the data for the adsorption energy (Fig. 9b) and differential adsorption energy of nitazoxanide and water molecules (Fig. 9c) on different crystallographic planes (Table S2†). This confirms that the incorporation of BiOI can enhance the photocatalytic activity of Bi4O5I2 for the degradation of nitazoxanide.
0 1). The high photocatalytic activity of BiOI/Bi4O5I2 is correlated to its higher adsorption than that of Bi4O5I2 but is similar to BiOI according to the data for the adsorption energy (Fig. 9b) and differential adsorption energy of nitazoxanide and water molecules (Fig. 9c) on different crystallographic planes (Table S2†). This confirms that the incorporation of BiOI can enhance the photocatalytic activity of Bi4O5I2 for the degradation of nitazoxanide.
4. Conclusions
To sum up, the effect of thermal treatment on the phase transition from bismuth iodide to bismuth oxyiodides was studied at temperatures ranging from 350 °C to 450 °C. Compared with thermal treatment time, the thermal treatment temperature showed a more pronounced effect on phase transition, resulting in the formation of different bismuth oxyiodide crystalline phases with varying optoelectronic properties and photocatalytic activity. Thermal treatment at 375 °C for 1 h led to the formation of the BiOI/Bi4O5I2 heterostructure, which exhibited the highest photocatalytic activity for the degradation of nitazoxanide, with respective rate constants of k1 (0.051 min−1) and k2 (4.225 mg g−1 min−1), highlighting the importance of the presence of multiple phases based on bismuth oxyiodides. Trapping experiments confirmed the involvement of photoexcited holes and hydroxyl radicals as the major reactive species in the photodegradation of nitazoxanide. The results of photoelectrochemical measurements showed that the electron transfer and recombination processes are inversely linked in the sample with the BiOI/Bi4O5I2 heterojunction. The photodegradation of nitazoxanide proceeded via hydrolysis into acetylsalicylic acid and the respective aminonitrothiazol, which were followed by the deacetylation and decarboxylation processes. According to the MD simulation, the high photocatalytic activity of BiOI/Bi4O5I2 is correlated to its higher adsorption. The toxicity test conducted with Aliivibrio fischeri revealed that the BiOI/Bi4O5I2 heterostructure is effective not only in the removal of nitazoxanide but also in the detoxification of wastewater, demonstrating its potential for practical application in the wastewater treatment process.Author contributions
Mirabbos Hojamberdiev: conceptualization, investigation, methodology, validation, visualization, writing – original draft, writing – review & editing; Ronald Vargas: formal analysis, investigation, software, validation, writing – original draft, writing – review & editing; Lorean Madriz: formal analysis, investigation, software, validation, writing – original draft, writing – review & editing; Kunio Yubuta: formal analysis, investigation, software, validation, writing – original draft; Zukhra C. Kadirova – formal analysis, investigation, software, validation, writing – original draft; Ulugbek Shaislamov – formal analysis, investigation, software, validation, writing – original draft; Lokesh Koodlur Sannegowda: investigation, writing – review & editing; Katarzyna Jędruchniewicz: formal analysis, investigation, software, validation, writing – original draft; Rafał Typek: formal analysis, investigation, software, validation, writing – original draft; Katsuya Teshima: formal analysis, investigation, software, validation, writing – original draft; Bożena Czech: conceptualization, investigation, methodology, supervision, validation, visualization, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MPWIK Lublin is acknowledged for providing wastewater samples. The authors gratefully acknowledge the use of services and facilities of The John Paul II Catholic University of Lublin, Poland, co-funded by the European Regional Development Fund (POPW.01.03.00-06-003/09-00). The India-Uzbekistan Collaborative Grants (no. INT/Uzbek/P-21 and UZB-Ind-2021-91) are also acknowledged. The authors thank Ms. Reiko Shiozawa from Shinshu University, Japan, for her kind assistance in the XPS analysis.References
- I. A. Al-Baldawi, A. A. Mohammed, Z. H. Mutar, S. R. S. Abdullah, S. S. Jasim, A. F. Almansoory and N. I. Ismail, Application of phytotechnology in alleviating pharmaceuticals and personal care products (PPCPs) in wastewater: Source, impacts, treatment, mechanisms, fate, and SWOT analysis, J. Cleaner Prod., 2021, 319, 128584 CrossRef.
- L. F. Angeles, R. A. Mullen, I. J. Huang, C. Wilson, W. Khunjar, H. I. Sirotkin, A. E. McElroy and D. S. Aga, Assessing pharmaceutical removal and reduction in toxicity provided by advanced wastewater treatment systems, Environ. Sci.: Water Res. Technol., 2020, 6, 62–77 RSC.
- A. Sengar and A. Vijayanandan, Human health and ecological risk assessment of 98 pharmaceuticals and personal care products (PPCPs) detected in Indian surface and wastewaters, Sci. Total Environ., 2022, 807, 150677 CrossRef PubMed.
- J. He, T. Feng, L. Tao, Y.-E. Peng, L. Tong, X.-W. Zhao, X. Shao, L.-Y. Xu, Y.-L. Yang and Y.-B. Zhao, Distribution and impacts on the geological environment of antiviral drugs in major waters of Wuhan, China, China Geol., 2022, 5, 402–410 Search PubMed.
- L.-A. Phan Thi, S. C. Panchangam, H.-T. Dod and V.-H. Nguyen, Prospects and challenges of photocatalysis for degradation and mineralization of antiviral drugs, in Nanostructured Photocatalysts: From Fundamental to Practical Applications, ed. V.-H. Nguyen, D.-V. N. Vo and S. Nanda, Elsevier, 2021, pp. 489–517 Search PubMed.
- S. Jain, P. Kumar, R. K. Vyas, P. Pandit and A. K. Dalai, Occurrence and Removal of Antiviral Drugs in Environment: A Review, Water, Air, Soil Pollut., 2013, 224, 1410 CrossRef.
- C. Prasse, M. P. Schlüsener, R. Schulz and T. A. Ternes, Antiviral Drugs in Wastewater and Surface Waters: A New Pharmaceutical Class of Environmental Relevance?, Environ. Sci. Technol., 2010, 44, 1728–1735 CrossRef PubMed.
- J. Meijide, G. Lama, M. Pazos, M. A. Sanromán and P. S. M. Dunlop, Ultraviolet-based heterogeneous advanced oxidation processes as technologies to remove pharmaceuticals from wastewater: An overview, J. Environ. Chem. Eng., 2022, 10, 107630 CrossRef CAS.
- M. Hojamberdiev, B. Czech, A. C. Göktaş, K. Yubuta and Z. C. Kadirova, SnO2@ZnS photocatalyst with enhanced photocatalytic activity for the degradation of selected pharmaceuticals and personal care products in model wastewater, J. Alloys Compd., 2020, 827, 154339 CrossRef CAS.
- B. Czech, P. Zygmunt, Z. C. Kadirova, K. Yubuta and M. Hojamberdiev, Effective photocatalytic removal of selected pharmaceuticals and personal care products by elsmoreite/tungsten oxide@ZnS photocatalyst, J. Environ. Manage., 2020, 270, 110870 CrossRef CAS PubMed.
- M. Hojamberdiev, B. Czech, A. Wasilewska, A. Boguszewska-Czubara, K. Yubuta, H. Wagata, S. S. Daminova, Z. C. Kadirova and R. Vargas, Detoxifying SARS-CoV-2 antiviral drugs from model and real wastewaters by industrial waste-derived multiphase photocatalysts, J. Hazard. Mater., 2022, 429, 128300 CrossRef.
- B. Czech, A. Krzyszczak, A. Boguszewska-Czubara, G. Opielak, I. Jośko and M. Hojamberdiev, Revealing the toxicity of lopinavir- and ritonavir-containing water and wastewater treated by photo-induced processes to Danio rerio and Aliivibrio fischeri, Sci. Total Environ., 2022, 824, 153967 CrossRef.
- W.-L. Wang, Q.-Y. Wu, Z.-M. Wang, H.-Y. Hu, N. Negishi and M. Torimura, Photocatalytic degradation of the antiviral drug Tamiflu by UV-A/TiO2: Kinetics and mechanisms, Chemosphere, 2015, 131, 41–47 CrossRef.
- A. H. Asif, S. Wang and H. Sun, Hematite-based nanomaterials for photocatalytic degradation of pharmaceuticals and personal care products (PPCPs): A short review, Curr. Opin. Green Sustainable Chem., 2021, 28, 100447 CrossRef.
- N. Tian, C. Hu, J. Wang, Y. Zhang, T. Ma and H. Huang, Layered bismuth-based photocatalysts, Coord. Chem. Rev., 2022, 463, 214515 CrossRef.
- J. Han, G. Zhu, M. Hojamberdiev, J. Peng and P. Liu, Temperature effect on phase transition and morphological transformation of BiOI microspheres to Bi5O7I microstructures, Mater. Lett., 2016, 169, 122–125 CrossRef.
- L. Zhang, H. Wang, Z. Chen, P. K. Wong and J. Liu, Bi2WO6 micro/nano-structures: Synthesis, modifications and visible-light-driven photocatalytic applications, Appl. Catal., B, 2011, 106, 1–13 Search PubMed.
- M. Hojamberdiev, Z. C. Kadirova, E. Zahedi, D. Onna, M. C. Marchi, G. Zhu, N. Matsushita, M. Hasegawa, S. Aldabe Bilmes and K. Okada, Tuning the morphological structure, light absorption, and photocatalytic activity of Bi2WO6 and Bi2WO6-BiOCl through cerium doping, Arabian J. Chem., 2020, 13, 2844–2857 CrossRef.
- B. Czech and M. Hojamberdiev, UVA- and visible-light-driven photocatalytic activity of three-layer perovskite Dion-Jacobson phase CsBa2M3O10 (M = Ta, Nb) and oxynitride crystals in the removal of caffeine from model wastewater, J. Photochem. Photobiol., A, 2016, 324, 70–80 CrossRef.
- H. Liu, J. Cai, M. Luo, C. Chen and P. Hu, Novel mesoporous bismuth oxyiodide single crystal nanosheets with enhanced catalytic activity, RSC Adv., 2020, 10, 5913–5918 RSC.
- L. Ye, L. Tian, T. Peng and L. Zan, Synthesis of highly symmetrical BiOI single-crystal nanosheets and their {001} facet-dependent photoactivity, J. Mater. Chem., 2011, 21, 12479–12484 RSC.
- N. Chandiwana, C. Kruger, H. Johnstone, M. F. Chughlay, C. Ju, B. Kim, Y. Dineka, S. Arbe-Barnes, R. Miller, A. Owen, A. Hill, D. Windgassen, N. Abla, A. C. Marrast, S. Duparc and W. D. F. Venter, Safety and efficacy of four drug regimens versus standard-of-care for the trea14049tment of symptomatic outpatients with COVID-19: A randomised, open-label, multi-arm, phase 2 clinical trial, EBioMedicine, 2022, 86, 104322 CrossRef.
- Y. Li, C. Li, X. Gao and H. Lv, Nitazoxanide in aqueous co-solvent solutions of isopropanol/DMF/NMP: Solubility, solvation thermodynamics and intermolecular interactions, J. Chem. Thermodyn., 2023, 176, 106928 CrossRef.
- M. D. Malesuik, H. M. L. Gonçalves, C. S. Paim, E. E. S. Schapoval and M. Steppe, LC: Analysis of Photodegradation Kinetics of Nitazoxanide in Pharmaceutical Formulations, J. Chromatogr. Sci., 2009, 47, 745–748 Search PubMed.
- R. Vargas, L. Madriz, V. Márquez, D. Torres, Z. C. Kadirova, K. Yubuta and M. Hojamberdiev, Elucidating the enhanced photoelectrochemical performance of zinc-blende ZnS/wurtzite ZnO heterojunction and adsorption of water molecules by molecular dynamics simulations, Mater. Sci. Semicond. Process., 2022, 142, 106494 CrossRef.
- J. Bisquert, A. Zaban, M. Greenshtein and I. Mora-Seró, Determination of Rate Constants for Charge Transfer and the Distribution of Semiconductor and Electrolyte Electronic Energy Levels in Dye-Sensitized Solar Cells by Open-Circuit Photovoltage Decay Method, J. Am. Chem. Soc., 2004, 126, 13550–13559 CrossRef.
- C. M. Domínguez, P. Ventura, A. Checa-Fernández and A. Santos, Comprehensive study of acute toxicity using Microtox® bioassay in soils contaminated by lindane wastes, Sci. Total Environ., 2023, 856, 159146 CrossRef.
- X. Xiao, Y. Lin, B. Pan, W. Fan and Y. Huang, Photocatalytic degradation of methyl orange by BiOI/Bi4O5I2 microspheres under visible light irradiation, Inorg. Chem. Commun., 2018, 93, 65–68 CrossRef.
- T. Wang, C. Zhao, L. Meng, Y. Li, H. Chu, F. Wang, Y. Tao, W. Liu and C. C. Wang, In-situ-construction of BiOI/UiO-66 heterostructure via nanoplate-on-octahedron: A novel p-n heterojunction photocatalyst for efficient sulfadiazine elimination, Chem. Eng. J., 2023, 451, 138624 CrossRef.
- B. O. Orimolade and O. A. Arotiba, Enhanced photoelectrocatalytic degradation of diclofenac sodium using a system of Ag-BiVO4/BiOI anode and Ag-BiOI cathode, Sci. Rep., 2022, 12, 4214 CrossRef.
- X. Song, R. Su, Y. Wang, Y. Zhang, B. Gao, Y. Wang, D. Ma and Q. Li, Visible light-driven chlorite activation process for enhanced sulfamethoxazole antibiotics degradation, antimicrobial resistance reduction and biotoxicity elimination, Chem. Eng. J., 2023, 452, 139103 CrossRef.
- J. Xu, Q. Zhang, X. Gao, P. Wang, H. Che, C. Tang and Y. Ao, Highly Efficient FeIII-initiated Self-cycled Fenton System in Piezo-catalytic Process for Organic Pollutants Degradation, Angew. Chem., 2023, 135, e202307018 CrossRef.
- O. Núñez and R. Vargas, The interplay between RedOx, photophysics and surface process in Bi2WO6 photocatalyst, in A Closer Look at Chemical Kinetics, ed. V. Martinez-Luaces, Nova Science Publishers, New York, USA, 2023 Search PubMed.
- A. Mills, C. O'Rourke and K. Moore, Powder semiconductor photocatalysis in aqueous solution: An overview of kinetics-based reaction mechanisms, J. Photochem. Photobiol., A, 2015, 310, 66–105 CrossRef.
- R. Vargas, D. Carvajal, L. Madriz and B. R. Scharifker, Chemical kinetics in solar to chemical energy conversion: The photoelectrochemical oxygen transfer reaction, Energy Rep., 2020, 6, 2–12 CrossRef.
- T. Peng, C. Xu, L. Yang, B. Yang, W.-W. Cai, F. Gu and G.-G. Ying, Kinetics and Mechanism of Degradation of Reactive Radical-Mediated Probe Compounds by the UV/Chlorine Process: Theoretical Calculation and Experimental Verification, ACS Omega, 2022, 7, 5053–5063 CrossRef.
- S. P. Mezyk, T. D. Cullen, K. A. Rickman and B. J. Mincher, The Reactivity of the Nitrate Radical (˙NO3) in Aqueous and Organic Solutions, Int. J. Chem. Kinet., 2017, 49, 635–642 CrossRef CAS.
- P. Maruthamuthu and P. Neta, Phosphate radicals. Spectra, acid-base equilibriums, and reactions with inorganic compounds, J. Phys. Chem., 1978, 82, 710–713 CrossRef CAS.
- M. Carballa, F. Omil, T. Ternes and J. M. Lema, Fate of pharmaceutical and personal care products (PPCPs) during anaerobic digestion of sewage sludge, Water Res., 2007, 41, 2139–2150 CrossRef CAS PubMed.
- M. Sun, Q. Wei, Y. Shao, B. Du, T. Yan, L. Yan and D. Li, Engineering composition-tunable 3D hierarchical bismuth oxyiodides heterojunctions: Ionic liquid-assisted fabrication with strong adsorption ability and enhanced photocatalytic properties, Appl. Catal., B, 2018, 233, 250–259 CrossRef.
- H. Ding, A. H. Zahid and Q. Han, Synthesis of 3D flowerlike S-scheme Bi4O5I2/BiOI heterojunction with synergistic effect of adsorption and photocatalysis, Mater. Sci. Eng., B, 2023, 289, 116209 CrossRef.
- H. Cheng, J. Wu, F. Tian, L. Zhao, Z. Ji, F. Li, Q. Li, Z. Guan and T. Zhou, In-situ crystallization for fabrication of BiOI/Bi4O5I2 heterojunction for enhanced visible-light photocatalytic performance, Mater. Lett., 2018, 232, 191–195 CrossRef.
- Y.-H. Cheng, J. Chen, H.-N. Che, Y.-H. Ao and B. Liu, Ultrafast photocatalytic degradation of nitenpyram by 2D ultrathin Bi2WO6: mechanism, pathways and environmental factors, Rare Met., 2022, 41, 2439–2452 CrossRef.
- D. A. Armstrong, R. E. Huie, W. H. Koppenol, S. V. Lymar, G. Merényi, P. Neta, B. Ruscic, D. M. Stanbury, S. Steenken and P. Wardman, Standard electrode potentials involving radicals in aqueous solution: inorganic radicals (IUPAC Technical Report), Pure Appl. Chem., 2015, 87, 1139–1150 CrossRef.
- L. S. Gómez-Velázquez, L. Madriz, M. Rigoletto, E. Laurenti, M. Bizarro, M. L. Dell'Arciprete and M. C. González, Structural and Physicochemical Properties of Carbon Nitride Nanoparticles via Precursor Thermal Treatment: Effect on Methyl Orange Photocatalytic Discoloration, ACS Appl. Nano Mater., 2023, 6, 14049–14062 CrossRef.
- G. X. Castillo-Cabrera, P. J. Espinoza-Montero, P. Alulema-Pullupaxi, J. R. Mora and M. H. Villacís-García, Bismuth Oxyhalide-Based Materials (BiOX: X = Cl, Br, I) and Their Application in Photoelectrocatalytic Degradation of Organic Pollutants in Water: A Review, Front. Chem., 2022, 10, 900622 CrossRef PubMed.
- O. Núñez, L. Madriz, D. Carvajal, J. Tatá and R. Vargas, Unprecedented large solvent (H2O vs D2O) isotope effect in semiconductors photooxidation, J. Phys. Org. Chem., 2019, 32, e3952 CrossRef.
- L. M. Peter, A. B. Walker, T. Bein, A. G. Hufnagel and I. Kondofersky, Interpretation of photocurrent transients at semiconductor electrodes: Effects of band-edge unpinning, J. Electroanal. Chem., 2020, 872, 114234 CrossRef.
- B. Liu, X. Zhao, C. Terashima, A. Fujishima and K. Nakata, Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems, Phys. Chem. Chem. Phys., 2014, 16, 8751–8760 RSC.
- L. M. Peter, E. A. Ponomared and D. J. Fermín, Intensity-modulated photocurrent spectroscopy: reconciliation of phenomenological analysis with multistep electron transfer mechanisms, J. Electroanal. Chem., 1997, 427, 79–96 CrossRef.
- F. E. Bedoya-Lora, I. Holmes-Gentle and A. Hankin, Electrochemical techniques for photoelectrode characterisation, Curr. Opin. Green Sustain. Chem., 2021, 29, 100463 CrossRef.
- F. Barbosa, L. Pezzi, J. Sorrentino, M. Steppe, N. Volpato and A. S. L. Mendez, pH effect on stability and kinetics degradation of nitazoxanide in solution, Drug Anal. Res., 2020, 4, 12–17 CrossRef.
- F. Müller, H. Christiansen, S. Schnabel and W. Janke, Fast, Hierarchical, and Adaptive Algorithm for Metropolis Monte Carlo Simulations of Long-Range Interacting Systems, Phys. Rev. X, 2023, 13, 031006 Search PubMed.
- M. Hojamberdiev, Z. C. Kadirova, Y. Makinose, G. Zhu, N. Matsushita, J. Rodríguez, S. Aldabe Bilmes, M. Hasegawa and K. Okada, Influence of BiOI content on the photocatalytic activity of Bi2WO6/BiOI/allophane composites and molecular modeling studies of acetaldehyde adsorption, J. Taiwan Inst. Chem. Eng., 2017, 81, 258–264 CrossRef.
- M. Hojamberdiev, Z. C. Kadirova, R. V. Gonçalves, K. Yubuta, N. Matsushita, K. Teshima, M. Hasegawa and K. Okada, Reduced graphene oxide-modified Bi2WO6/BiOI composite for the effective photocatalytic removal of organic pollutants and molecular modeling of adsorption, J. Mol. Liq., 2018, 268, 715–727 CrossRef.
- E. Keller and V. Krämer, A Strong Deviation from Vegard's Rule: X-Ray Powder Investigations of the Three Quasi-Binary Phase Systems BiOX–BiOY (X, Y = Cl, Br, I), Z. Naturforsch., B: J. Chem. Sci., 2005, 60, 1255–1263 CrossRef.
- E. Keller, V. Krämer, M. Schmidt and H. Oppermann, The crystal structure of Bi4O5I2 and its relation to the structure of Bi4O5Br2, Z. Kristallogr., 2002, 217, 256–264 Search PubMed.
- U. Eggenweiler, J. Ketterer, E. Keller and V. Krämer, The crystal structure of α-Bi5O7I, Z. Kristallogr., 2001, 216, 230–233 Search PubMed.
- M. Ruck, Darstellung und Kristallstruktur von fehlordnungsfreiem Bismuttriiodid, Z. Kristallogr., 1995, 210, 650–655 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3en00548h |
| This journal is © The Royal Society of Chemistry 2024 |