Open Access Article
Open Access ArticleSalazinic acid attenuates male sexual dysfunction and testicular oxidative damage in streptozotocin-induced diabetic albino rats†
Kishore Naidu Killaria,
Haritha Polimati a,
D. S. N. B. K. Prasanthb,
Gagandeep Singh
a,
D. S. N. B. K. Prasanthb,
Gagandeep Singh cd,
Siva Prasad Pandae,
Girija Sastry Vedulaa and
Vinay Bharadwaj Tatipamula
cd,
Siva Prasad Pandae,
Girija Sastry Vedulaa and
Vinay Bharadwaj Tatipamula *fg
*fg
aDepartment of Pharmaceutical Sciences, AU College of Pharmaceutical Sciences, Andhra University, Visakhapatnam-530 003, India
bDepartment of Pharmacognosy, KVSR Siddhartha College of Pharmaceutical Sciences, Vijayawada, AP 520010, India
cSection of Microbiology, Central Ayurveda Research Institute, Jhansi, Uttar Pradesh 284003, India
dKusuma School of Biological Sciences, Indian Institute of Technology Delhi, New Delhi, India
eInstitute Pharmacology Research Division, Institute of Pharmaceutical Research, GLA University, 281406, Mathura, Uttar Pradesh, India
fCenter for Molecular Biology, College of Medicine and Pharmacy, Duy Tan University, Danang 550000, Vietnam
gInstitute of Research and Development, Duy Tan University, Da Nang 550000, Vietnam. E-mail: vinaybharadwajtatipamula@duytan.edu.vn
First published on 26th April 2023
Abstract
Male sexual dysfunctions such as infertility and impotence are recognized as the consequences of diabetes. Salazinic acid (Sa) is a depsidone found in lichen genera of Lobaria, Parmelia, and Usnea, which has prominent free radical and α-glucosidase inhibitory actions. The present study establishes the beneficial role of salazinic acid (Sa) to combat the deleterious effects of streptozotocin-induced diabetes on the male reproductive system of rats. In a dose-dependent manner, Sa significantly restored the reproductive organs weight, sperm characteristics, and testicular histoarchitecture in diabetic rats. Further, a significant recovery of insulin, follicle-stimulating hormone, luteinizing hormone and testosterone levels in serum was recorded in Sa-treated diabetic rats. The malondialdehyde levels were significantly lowered, and the activities of glutathione, superoxide dismutase, glutathione peroxidase and catalase, markedly elevated in the blood serum, as well as testicular tissue after Sa-supplementation. Sa also suppressed the protein expression levels of tumor necrosis factor-α in serum. The high dose of Sa showed significant improvement in glycemia and testicular protection, similar to sildenafil citrate. Moreover, the docking results showed that both Sa and sildenafil have a high affinity toward the target protein, PDE5 with binding affinity values found to be −9.5 and −9.2 kcal mol−1, respectively. Molecularly, both Sa and sildenafil share similar hydrogen bonding patterns with PDE5. Hence, our study clearly showed the protective role of Sa against diabetic-induced spermatogenic dysfunction in rats, possibly by competing with cGMP to bind to the catalytic domain of PDE5 and thereby controlling the oxidative impairment of testes.
Introduction
Diabetes mellitus is a chronic lifelong metabolic disorder characterized by raised plasma glucose levels, which is also known as hyperglycemia, due to impairment in secretion and/or action of insulin. According to the World Health Organization (WHO), around 422 million people have diabetes worldwide with 1.5 million deaths recorded annually.1 Long-term hyperglycemia causes diabetes and its complications in multiple organs including cardiovascular, foot ulcers, nephropathy, neuropathy, retinopathy, and sexual dysfunctions.2 Among these, sexual dysfunction is recognized as a common complication of diabetes that affects nearly 90% of male diabetic patients.3Male sexual dysfunction is a complex biological process that includes erectile dysfunction, hormonal pathway, neurogenic and hemodynamic factors.4,5 Of these, erectile dysfunction is one of the most common consequences that results from smooth muscle relaxation and impairment of penile vascular in diabetes conditions.6 It is well-documented that the risk of erectile dysfunction is three-fold higher in diabetic men as compared to non-diabetic men.6–8 It is estimated that 45% of men with diabetes develop erectile dysfunction within 10 years of the onset.9 The prevalence of erectile dysfunction among diabetes conditions is strongly related to the duration of diabetes, micro- and macro-vascular complications, and degree of obesity.10 Besides, the male sexual disorders such as abnormal spermatogenesis, decrease in reproductive organ weight, sperm deformities, rapid or retard ejaculation, and retrograde ejaculation or anejaculation in diabetic men remain often underdiagnosed and undertreated. Although many clinical studies have been performed on the sexual dysfunction in diabetic patients, only a few clinical therapeutic drugs like phosphodiesterase-5 (PDE5) inhibitors (sildenafil) are presently available for the diagnosis and treatment of diabetes-related sexual dysfunction.11 These medications work by inhibiting the PDE5 enzyme, increasing cyclic guanosine monophosphate (cGMP) in the penis and relaxing smooth muscles. There are eleven families of phosphodiesterases in mammals, of which, only PDE5 has been identified in smooth muscle cells and platelets.12 Hence, the search for clinically proved PDE5 inhibitor drugs is a core topic in the diagnosis of diabetic sexual dysfunction.
In recent times, herbal formulations are attaining popularity in the management and control of diabetes and its complications.13 For instance, herbal compounds such as baicalein (flavonoid)14 and epigallocatechin gallate (polyphenol)15 have shown to be able to protect diabetes-induced male sexual dysfunction in rodents by attenuating antioxidant and anti-inflammatory enzymes. A lichen depsidone, Salazinic acid (Sa) has been found to be significantly expressed in the genus of Lobaria,16 Parmelia,17 and Usnea,18,19 has been acknowledged for its various biological activities including antioxidant, antidiabetic, antimicrobial, antimycobacterial, cytotoxicity, wound healing, anti-inflammatory, and xanthine oxidase inhibitory actions.17–21 Its remarkable free radical scavenging activity, as well as potent antidiabetic effects have been linked to the competitive inhibition towards α-glucosidase enzyme.21 In the present study, we intended to assess the ameliorative effects of Sa on the reproductive dysfunction in streptozotocin (STZ)-induced diabetic albino male rats.
Results and discussion
In vitro antioxidant and antidiabetic activities
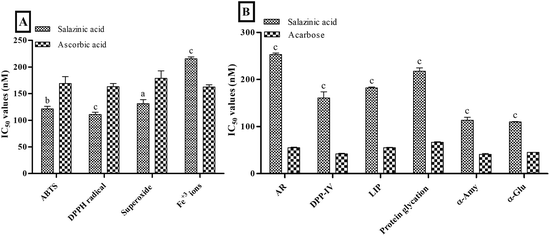
The in vitro screening of Sa against free radicals and metal ions revealed the prominent antioxidant activity compared to ascorbic acid. The antioxidant activity results of Sa showed a significant low IC50 values of 121.47 ± 4.53, 110.79 ± 4.32 and 131.17 ± 7.60 nM against ABTS+· (P < 0.001), DPPH· (P < 0.0001) and superoxide (P < 0.05) free radicals, respectively, compared to ascorbic acid (168.99 ± 12.86, 163.10 ± 5.94 and 178.63 ± 14.33 nM, respectively) (Fig. 1A). While Sa showed moderate antioxidant activity against ferric (Fe3+) ions (P < 0.0001) with an IC50 value of 215.51 ± 3.24 nM compared to ascorbic acid (162.36 ± 4.30 nM) (Fig. 1A). Therefore, the IC50 values obtained from the in vitro screening of Sa against various free radicals (ABTS+·, DPPH· and superoxide) and Fe3+ ions revealed its more specificity towards free radicals, compared to metal ions. Based on the antioxidant screening it could be postulated that the Sa acts via radical scavenging activity (Fig. 1A).On the other hand, Sa significantly (P < 0.0001) lowered antidiabetic activity against enzymes: aldose reductase (AR, IC50 values = 253.30 ± 3.12 nM), dipeptidyl peptidase IV (DPP-IV, IC50 values = 160.75 ± 13.24 nM), lipase (LIP, IC50 values = 182.08 ± 1.96 nM), α-amylase (α-Amy, IC50 values = 113.63 ± 6.12 nM), and α-glucosidase (α-Glu, IC50 values = 109.99 ± 0.84 nM) and protein glycation (IC50 values = 217.60 ± 7.25 nM) compared to acarbose (Fig. 1B). Furthermore, the IC50 values obtained from the enzymes (AR, DPP-IV, LIP, α-Amy and α-Glu) and protein glycation inhibitory assays of Sa revealed its better inhibition of digestive enzymes namely α-Amy and α-Glu (Fig. 1B). In another study, Sa was found to have remarkable free radical scavenging activity, as well as potent antidiabetic effects that are linked to the competitive type of suppression towards α-Glu enzyme.21 Both our antioxidant and antidiabetic activity results were consistent with this previous study.21
Acute toxicity study
The acute toxicity study on male albino rats has exposed the non-toxic aptitude of Sa up to 100 mg kg−1 body weight (b.w) dosage and the median lethal dose (LD50) value of Sa could not be estimated due to no mortality. Thus, 1/10 and 1/20 of determined tolerated dose of Sa were selected as high dose (10 mg kg−1) and low dose (5 mg kg−1) for the current study.Body weight and relative reproductive organs weight
At the beginning of the study, there was no significant change in the bodyweight of albino rats was observed between normal and all treated groups. At the end of 5th and 9th week, the body weight of diabetic control group rats was significantly lowered (P < 0.0001) than the normal control group (Table 1). However, Sa (5 and 10 mg kg−1 b.w) and sildenafil citrate (a clinically unapproved anti-diabetic drug) administration had significantly improved the body weight of animals compared to the diabetic control group at the end of 5th and 9th week (P < 0.0001) (Table 1).| Groups | Body weight in g (% changed) | Relative reproductive organs weight in g (% changed) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 week | 5 weeks | 9 weeks | Testis | Penile | Seminal vesicle | Prostate gland | Cauda epididymis | |
| a Values are expressed as mean ± standard deviation (n = 6). aP < 0.05, bP < 0.001, and cP < 0.0001 statistically significant from the normal control group. xP < 0.05, yP < 0.001, and zP < 0.0001, statistically significant from the diabetic control group. Multiple group comparisons were performed by one-way analysis of variance followed by Tukey's multiple comparison post hoc test at P ≤ 0.05. | ||||||||
| Normal control | 192.33 ± 6.65 | 205.17 ± 5.00 (6.73 ± 2.63) | 212.67 ± 6.56 (10.60 ± 2.45) | 1.05 ± 0.03 | 0.58 ± 0.03 | 0.31 ± 0.03 | 0.18 ± 0.01 | 0.37 ± 0.02 |
| Diabetic control | 191.50 ± 5.39 | 151.17 ± 5.00c (−21.06 ± 1.44c) | 132.50 ± 2.88c (−30.76 ± 2.74c) | 0.53 ± 0.04c (−49.76 ± 3.55) | 0.37 ± 0.04b (−34.87 ± 9.56) | 0.09 ± 0.01c (−71.92 ± 3.14) | 0.06 ± 0.01c (−67.48 ± 6.81) | 0.13 ± 0.02c (−64.26 ± 4.15) |
| Sa-5 | 198.17 ± 7.91 | 162.50 ± 7.40c (−17.99 ± 2.06c) | 171.83 ± 5.91cz (−13.23 ± 3.17cy) | 0.63 ± 0.03c (−39.59 ± 2.33) | 0.39 ± 0.03b (−32.90 ± 8.35) | 0.13 ± 0.01c (−56.70 ± 4.33) | 0.11 ± 0.01cx (−36.71 ± 6.68y) | 0.21 ± 0.02c (−44.79 ± 6.91) |
| Sa-10 | 193.33 ± 5.43 | 172.67 ± 5.50b (−10.65 ± 3.05c) | 178.83 ± 5.91bz (−7.46 ± 3.24bz) | 0.93 ± 0.01z (−11.37 ± 3.39) | 0.53 ± 0.04y (−7.74 ± 5.42z) | 0.22 ± 0.01bz (−26.95 ± 5.11z) | 0.16 ± 0.01z (−13.90 ± 2.85z) | 0.27 ± 0.02az (−28.08 ± 3.65z) |
| Sildenafil citrate | 200.33 ± 6.68 | 170.83 ± 6.79b (−14.69 ± 3.27c) | 179.50 ± 5.05bz (−10.32 ± 3.18cz) | 0.95 ± 0.03z (−9.49 ± 3.90) | 0.53 ± 0.03y (−8.02 ± 4.67z) | 0.18 ± 0.01cy (−40.11 ± 4.95z) | 0.13 ± 0.01az (−25.86 ± 3.28z) | 0.24 ± 0.02az (−36.12 ± 4.44y) |
In association to normal control rats, a significant decrease in the relative weight of reproductive organs, namely testis (P < 0.001), penile (P < 0.0001), seminal vesicles (P < 0.0001), prostate gland (P < 0.0001), and cauda epididymis (P < 0.0001), were detected in the diabetic control rats (Table 1). The oral administration of Sa and sildenafil citrate prevented a significant reduction in the relative weight of reproductive organs compared to the diabetic control rats (Table 1). Overall, sildenafil citrate-treated group showed almost equivalent recovery of body weight and relative reproductive organ weights as that of the Sa-treated group at 10 mg kg−1 b.w (Table 1).
Biochemical parameters
From week 0th to 9th, a gradual rise of fasting plasma glucose (FPG) levels (P < 0.0001) were noticed in the diabetic control group compared to the normal control group. Contrarily, Sa-treated groups significantly (P < 0.0001) reduced FPG levels compared to the diabetic rats in a dose-dependent way. In contrast to normal control, the serum level of insulin from week 0th to 9th was sharply decreased (P < 0.0001) in diabetic control rats. Sa administration steadily improved (P < 0.0001) the serum insulin levels compared to diabetic control rats in a dose-dependent way. However, the high dose of Sa treatment showed more potency in controlling serum FPG and insulin levels than sildenafil citrate (5 mg kg−1 b.w) (Table 2). This finding confirms the ability of Sa to increase insulin concentration to lower plasma glucose, proving its antidiabetic activity.| Groups | Fasting plasma glucose in mg dL−1 (% reduced) | Insulin in mIU L−1 (% increased) | ||||
|---|---|---|---|---|---|---|
| 0 week | 5 weeks | 9 weeks | 0 week | 5 weeks | 9 weeks | |
| a Values are expressed as mean ± standard deviation (n = 6). aP < 0.05, bP < 0.001, and cP < 0.0001 statistically significant from the normal control group. zP < 0.0001, statistically significant from the diabetic control group. Multiple group comparisons were performed by one-way analysis of variance followed by Tukey's multiple comparison post hoc test at P ≤ 0.05. | ||||||
| Normal control | 94.83 ± 5.53 | 99.67 ± 6.77 (−5.08 ± 2.86) | 103.33 ± 5.13 (−10.91 ± 3.27) | 8.30 ± 0.18 | 8.57 ± 0.11 (3.25 ± 2.64) | 8.67 ± 0.16 (4.50 ± 3.13) |
| Diabetic control | 334.17 ± 6.59c | 390.33 ± 7.20c (−16.83 ± 2.59a) | 434.67 ± 8.45c (−30.13 ± 4.11b) | 3.41 ± 0.32c | 3.24 ± 0.23c (−4.87 ± 2.43) | 2.97 ± 0.19c (−12.85 ± 3.18) |
| Sa-5 | 331.67 ± 5.43c | 243.33 ± 6.44cz (26.61 ± 2.72cz) | 206.83 ± 5.85cz (37.64 ± 1.55cz) | 3.39 ± 0.13c | 5.06 ± 0.26cz (49.27 ± 6.08cz) | 5.98 ± 0.13cz (76.52 ± 7.74cz) |
| Sa-10 | 326.00 ± 4.73c | 190.00 ± 7.29cz (41.73 ± 1.64cz) | 141.00 ± 7.90az (56.72 ± 2.94cz) | 3.43 ± 0.20c | 6.27 ± 0.16cz (88.47 ± 6.49cz) | 7.41 ± 0.20cz (116.64 ± 8.47cz) |
| Sildenafil citrate | 348.67 ± 6.80c | 219.17 ± 5.38cz (37.12 ± 2.15cz) | 190.67 ± 5.79cz (45.28 ± 2.37cz) | 3.43 ± 021c | 6.00 ± 0.29cz (75.37 ± 3.54cz) | 6.82 ± 0.14cz (99.58 ± 9.18cz) |
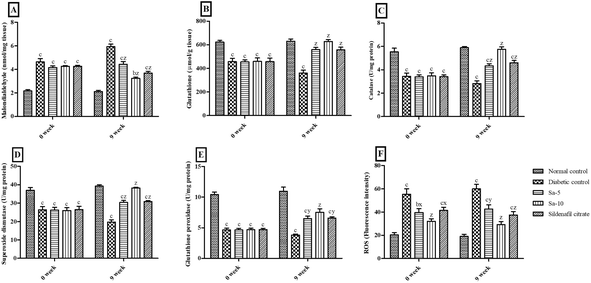
At the end of 0 week, significant (P < 0.0001) variations of the serum concentrations of malondialdehyde (MDA), glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) and reactive oxygen species (ROS) were observed in STZ-induced diabetic rats compared to the normal control group (Fig. 2). Simultaneously, treatment with Sa for 9 weeks significantly reduced MDA (P < 0.0001) and ROS (P < 0.001) concentrations with significant improvement in GSH (P < 0.0001), CAT (P < 0.0001), SOD (P < 0.0001) and GPx (P < 0.001–0.0001) concentrations in serum compared with those of the diabetic control rats in a dose-dependent way. At the end of week 9th, all the serum oxidative stress parameters were almost similar in normal control, Sa-treated (10 mg kg−1 b.w), and sildenafil citrate-treated groups (Fig. 2).
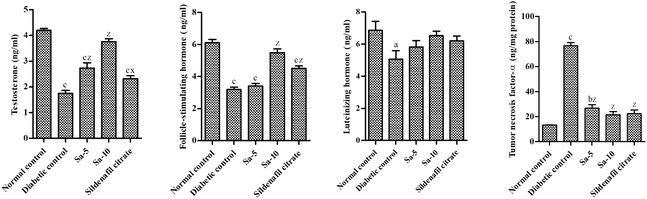
On the end of week 9th, a significant reduction of serum hormones such as testosterone (T) (P < 0.0001), follicle-stimulating hormone (FSH) (P < 0.0001) and luteinizing hormone (LH) (P < 0.05) levels and significantly higher levels of tumor necrosis factor (TNF)-α (P < 0.0001) was observed in diabetic control group compared to normal control group (Fig. 3). In a dose-dependent manner, the oral treatment of Sa for 9 weeks significantly raised the above specified hormonal levels and significantly lowered TNF-α levels compared to the diabetic control group. The higher concentration of Sa-treated group was more potent than sildenafil citrate-treated group in elevating the serum hormonal levels (T, FSH and LH). As the serum T concentration is inversely proportional with the plasma glucose levels, the elevated T levels could be associated with androgen releasing activity, as well as antidiabetic effects of Sa. Moreover, the serum TNF-α levels were almost similar in both Sa-treated (10 mg kg−1 b.w) and sildenafil citrate-treated groups (Fig. 3), suggest that Sa could inhibit inflammation to alleviate diabetic male reproduction dysfunction.
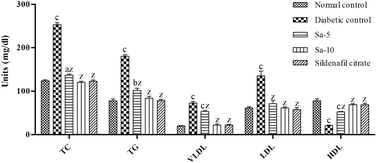
Similarly, an abnormal regulation (P < 0.0001) of lipid parameters including total cholesterol (TC), triglyceride (TG), and very-low-, low- and high-density lipoprotein (VLDL, LDL and HDL) content were observed in the diabetic control group compared to normal control (Fig. 4). In contrast to diabetic control group, Sa-treated groups showed a significant (P < 0.0001) reduction of TC, TG, VLDL and LDL contents with a significant (P < 0.0001) rise in HDL content in a dose-dependent way. At the end of week 9th, the content of lipid parameters was almost similar in normal control, Sa-treated (10 mg kg−1 b.w), and sildenafil citrate-treated groups (Fig. 4).
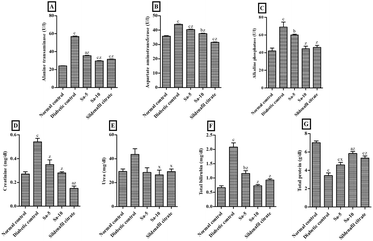
As compared to the normal control group, significantly (P < 0.0001) elevated levels of liver enzymes namely alanine transferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), were observed in the diabetic control group (Fig. 5A–C). Both, low and high doses of Sa-treated groups showed a significant (P < 0.0001) reduction of ALT, AST and ALP activities compared to the diabetic control group, in a dose-dependent way. In contrast to sildenafil citrate-treated group, a highest reduction in the ALT and ALP activities were noticed in high dose of Sa (Fig. 5A–C).
Also, the levels of the liver biomarkers, including creatinine (Cr) (P < 0.0001), urea (U) and total bilirubin (TBIL) (P < 0.0001), were significantly increased in the diabetic control group, and a remarkable reduction was observed in Sa-treated (P < 0.05–0.0001) and sildenafil citrate-treated (P < 0.0001) groups (Fig. 5D–F). The highest reduction in the U and TBIL levels were observed in the high dose of Sa-treated group, compared to the sildenafil citrate-treated group (Fig. 5D–F). On the other hand, a significant reduction of total protein (TP) levels was noticed in diabetic control group (P < 0.0001) and a significant recovery was observed in Sa-treated (P < 0.05–0.0001) and sildenafil citrate-treated (P < 0.0001) groups. However, a highest decrease in the TP level was observed in the sildenafil citrate-treated group, compared to the sildenafil citrate-treated group (Fig. 5G).
Testicular oxidative stress markers
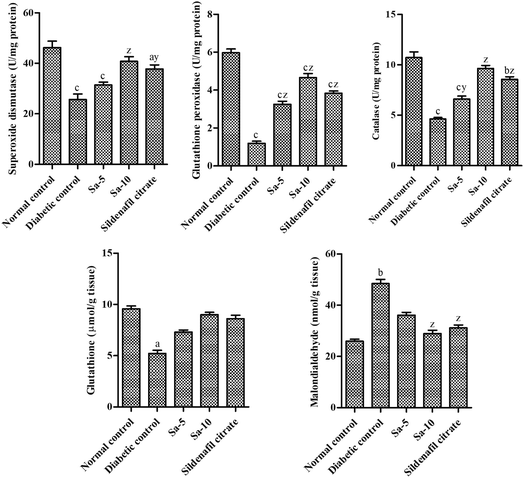
The diabetic control group rats showed a significant (P < 0.0001) reduction of testicular concentrations of SOD, GPx, CAT and GSH with elevated MDA concentrations in testicular homogenate than the normal control group (Fig. 6). The oral supplementation of Sa significantly restored the levels of SOD (P < 0.0001), GPx (P < 0.0001), CAT (P < 0.001–0.0001), GSH and MDA (P < 0.0001) in the testicular tissues compared to the diabetic control group. However, treatment with Sa at 10 mg kg−1 b.w showed more potent effects in controlling the antioxidant profile in the testicular tissues of diabetic rats compared to the sildenafil citrate-treated group (Fig. 6). This finding reflects that the elevated antioxidant enzymes convert free radicals into neutralized substances via scavenging ability in diabetic male rats and ultimately reduce oxidative damages. Therefore, as an effective antioxidant agent, Sa can defend the integrity of sperm membranes against oxidative stress conditions.Sexual behavior of Sa-treated diabetic albino rats
The chronic diabetic conditions in the disease control group had significantly (P < 0.001–0.0001) altered the sexual function in male diabetic rats compared to the normal control group (Table 3). Our findings indicate that the oral supplementation of Sa and sildenafil citrate had significantly decreased mount latency (ML) (P < 0.001–0.0001), ejaculation latency (EL) (P < 0.05), intromission latency (IL) (P < 0.05–0.001), and post-ejaculatory interval (PEI), and significant improvement in mount frequency (MF) (P < 0.001–0.0001) and intromission frequency (IF) (P < 0.001–0.0001) compared to diabetic control group. The 10 mg kg−1 b.w of Sa-treated group had shown more prominent results in improving male sexual activities compared to the sildenafil citrate group (Table 3), which specifies the restoration of T levels in rats.| Groups | Mount latency (sec) | Mount frequency (sec) | Ejaculatory latency (sec) | Intromission latency (sec) | Intromission frequency (no.) | Post ejaculatory interval (sec) |
|---|---|---|---|---|---|---|
| a Values are expressed as mean ± standard deviation (n = 6). aP < 0.05, bP < 0.001, and cP < 0.0001 statistically significant from the normal control group. xP < 0.05, yP < 0.001, and zP < 0.0001, statistically significant from the diabetic control group. Multiple group comparisons were performed by one-way analysis of variance followed by Tukey's multiple comparison post hoc test at P ≤ 0.05. Mount latency: time from the introduction of female rat into the cage of the male rat up to the first mount; mount frequency: number of mounts before ejaculation; ejaculation latency: time from the first intromission of a series up to the ejaculation; intromission latency: time from the introduction of the female rat up to the first intromission by the male rat; intromission frequency: number of intromission before ejaculation; post-ejaculatory interval: time from the first ejaculation up to the next intromission by the male rat. | ||||||
| Normal control | 401.17 ± 30.27 | 881.67 ± 28.23 | 597.33 ± 29.38 | 512.50 ± 29.41 | 12.67 ± 0.82 | 1055.67 ± 86.24 |
| Diabetic control | 695.33 ± 47.54c | 399.83 ± 19.42c | 1227.00 ± 140.16c | 1063.33 ± 98.68c | 4.83 ± 0.75c | 1702.33 ± 121.66b |
| Sa-5 | 560.17 ± 29.53a | 607.67 ± 30.53cz | 999.17 ± 94.20a | 895.33 ± 32.92c | 6.33 ± 0.52c | 1399.67 ± 120.17 |
| Sa-10 | 480.00 ± 35.25y | 754.83 ± 29.99az | 828.00 ± 58.45x | 736.00 ± 34.48ay | 10.67 ± 0.82z | 1268.17 ± 154.05 |
| Sildenafil citrate | 539.67 ± 27.06x | 578.50 ± 38.01cy | 948.83 ± 32.29a | 805.33 ± 23.84bx | 8.20 ± 0.55by | 1311.67 ± 95.19 |
The observations of the mating test revealed that the diabetic control group animals had remarkably reduced fertilizing capabilities such as number of females mated (4/12), mating success (33.34%), number of females pregnant (3/12), number of foetuses (5.0 ± 1.0; P < 0.0001) and corpora lutea (11.67 ± 0.58) formed, and male fertility (42.68 ± 6.87%; P < 0.0001) compared to the normal control group (Table 4). In a dose-dependent manner, Sa (low and high doses) treatment had improved the mating success (58.34 and 83.34%) by the number of females mated (7/12 and 10/12) and the number of females pregnant (6/12 and 10/12) compared to the diabetic control group. Also, the number of foetuses (8.00 ± 0.89 and 10.50 ± 0.71 (P < 0.0001)) and corpora lutea (12.33 ± 0.82 and 12.30 ± 0.48) formed per female rat were improved with Sa at 5 and 10 mg kg−1 b.w doses, respectively, compared to the diabetic control group (Table 4). The male fertility percentage of Sa-treated groups (low and high doses) significantly increased to 64.99 ± 7.20 and 85.38 ± 5.17% (P < 0.0001), respectively, compared to the diabetic control group. On the other hand, sildenafil citrate-treated group (5 mg kg−1 b.w) showed almost equivalent fertilizing capability as that of a high dose of Sa in STZ-induced diabetic male albino rats (Table 4).
| Groups | No. of females mated | Mating success (%) | No. of females pregnant | No. of fetuses/rat | No. of corpora lutea/rat | Male fertility (%) |
|---|---|---|---|---|---|---|
| a Values are expressed as mean ± standard deviation (n = 6). bP < 0.001 and cP < 0.0001 statistically significant from the normal control group. zP < 0.0001, statistically significant from the diabetic control group. Multiple group comparisons were performed by one-way analysis of variance followed by Tukey's multiple comparison post hoc test at P ≤ 0.05. Mating success is the ratio of the total number of female animals mated to the total number of female animals paired. Male fertility (expressed in % fertility) is the ratio of the total number of fetuses to the total number of corpora lutea. | ||||||
| Normal control | 12 | 100.00 | 12 | 12.25 ± 0.75 | 12.58 ± 1.08 | 97.57 ± 3.59 |
| Diabetic control | 4 | 33.34 | 3 | 5.00 ± 1.00c | 11.67 ± 0.58 | 42.68 ± 6.87c |
| Sa-5 | 7 | 58.34 | 6 | 8.00 ± 0.89b | 12.33 ± 0.82 | 64.99 ± 7.20b |
| Sa-10 | 10 | 83.34 | 10 | 10.50 ± 0.71z | 12.30 ± 0.48 | 85.38 ± 5.17z |
| Sildenafil citrate | 10 | 83.34 | 10 | 10.40 ± 0.70z | 12.40 ± 0.52 | 83.85 ± 3.98z |
Epididymal sperm characteristics
A significant (P < 0.0001) drop in sperm count (36.08 ± 2.51 million cells per mL), motility (48.33 ± 2.07%) and viability (45.67 ± 2.80%) with significantly (P < 0.0001) more abnormalities (18.17 ± 2.14%) and detached head from sperm (41.00 ± 3.22%) in sperm morphology were detected in the diabetic control rats (Video S1†) compared to normal control rats (Table 5). In contrast to the diabetic control group, Sa-treated groups counteracted the reduction in all the above sperm parameters in a dose-dependent way. However, animals that received high dose of Sa showed the best reduction effects in sperm parameters than the sildenafil citrate-treated group (Table 5; Videos S2 and S3†), which clearly justifies its ability to improve the quality of sperm, gonadotropin levels and spermatogenesis.| Groups | Sperm count in million cells per mL (% reduced) | Sperm motility (%) | Sperm viability (%) | Sperm abnormalities (%) | Detached head from sperm (%) |
|---|---|---|---|---|---|
| a Values are expressed as mean ± standard deviation (n = 6). aP < 0.05, bP < 0.001, and cP < 0.0001 statistically significant from the normal control group. xP < 0.05, yP < 0.001, and zP < 0.0001, statistically significant from the diabetic control group. Multiple group comparisons were performed by one-way analysis of variance followed by Tukey's multiple comparison post hoc test at P ≤ 0.05. | |||||
| Normal control | 74.92 ± 2.60 | 86.50 ± 2.88 | 92.50 ± 2.88 | 3.67 ± 0.82 | 5.17 ± 1.47 |
| Diabetic control | 36.08 ± 2.51c (51.83 ± 3.03) | 48.33 ± 2.07c | 45.67 ± 2.80c | 18.17 ± 2.14c | 41.00 ± 3.22c |
| Sa-5 | 53.80 ± 3.52cz (28.15 ± 4.90y) | 61.33 ± 1.03c | 56.33 ± 2.34c | 13.17 ± 1.47c | 26.50 ± 4.85cx |
| Sa-10 | 63.13 ± 1.30az (15.64 ± 3.67z) | 80.00 ± 5.25z | 79.83 ± 2.40az | 6.33 ± 0.82z | 5.83 ± 0.75z |
| Sildenafil citrate | 56.69 ± 1.37cz (24.24 ± 3.66z) | 68.67 ± 3.14by | 68.17 ± 2.56cz | 9.83 ± 0.75ay | 9.67 ± 2.73z |
Histopathological examination
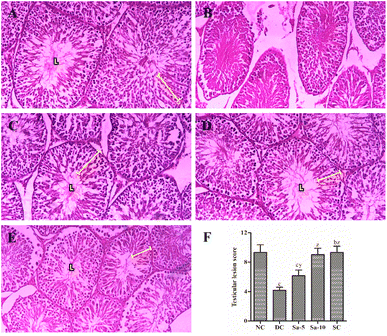
The slides of testicular tissues in the normal control group visualized an intact basal membrane (no interstitial space) lined with Leydig cells and healthy matured seminiferous tubules containing various stages of spermatogonial cells in the germinal epithelium zone (Fig. 7A). In the diseased control group, huge interstitial spaces were noticed between the seminiferous tubules with a great irregularity and/or degeneration in the order of spermatogenic cells, and no Leydig cells (Fig. 7B). On the other hand, oral treatment of Sa or sildenafil citrate exhibited marked development in the testicular structure and restoration in the series of spermatogenic cells (Fig. 7C–E). However, the recovery in Sa-treated groups was proportional to their dosages. Furthermore, a significant (P < 0.0001) reduction of testicular lesion score (TLS), mean Johnson's score (MJS), mean seminiferous tubule diameter (MSTD) and mean seminiferous tubule diameter (MSTD) were detected in the diabetic control group compared to normal control rats (Table 6). Over 9 weeks of treatment with Sa at 5 (P < 0.05) and 10 (P < 0.0001) mg kg−1 b.w significantly improved the early-mentioned factors compared with the diabetic control group in a dose-dependent manner. Besides, Sa at 10 mg kg−1 b.w showed almost equivalent improvement in the TLS, MJS, MSTD and HGE as that of a sildenafil citrate-treated group (Table 6).| Groups | Mean Johnson's score | Mean seminiferous tubule diameter (μm) | Height of seminiferous epithelium (μm) |
|---|---|---|---|
| a Values are expressed as mean ± standard deviation (n = 6). aP < 0.05 and cP < 0.0001 statistically significant from the normal control group. xP < 0.05, yP < 0.001, and zP < 0.0001, statistically significant from the diabetic control group. Multiple group comparisons were performed by one-way analysis of variance followed by Tukey's multiple comparison post hoc test at P ≤ 0.05. | |||
| Normal control | 9.40 ± 0.52 | 255.30 ± 11.11 | 75.70 ± 4.06 |
| Diabetic control | 4.30 ± 0.48c | 150.00 ± 13.96c | 38.70 ± 4.83c |
| Sa-5 | 6.50 ± 0.71a | 193.60 ± 12.36a | 56.20 ± 3.36ax |
| Sa-10 | 9.10 ± 0.74z | 235.00 ± 10.45z | 68.90 ± 4.93z |
| Sildenafil citrate | 9.30 ± 0.48z | 217.00 ± 15.14y | 70.60 ± 3.10z |
In silico studies
| Ligands | Binding affinity, ΔG (Kcal mol−1) | Amino acids involved and distance (Å) | |
|---|---|---|---|
| Hydrogen-bond interactions | Hydrophobic interactions | ||
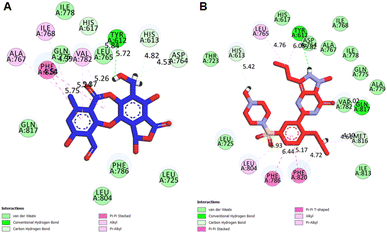
| Salazinic acid | −9.5 | TYR A:612 (5.72) | PHE A:820 (5.75), ALA A:767 (4.54), ILE A:768 (4.39), VAL A: 782 (5.23, 5.26), ASP A:764 (4.53), HIS A:613 (4.82), HIS A:617 (5.84) |
| Sildenafil | −9.2 | TYR A:612 (5.82), GLN A:817 (5.02) | PHE A:786 (6.44), LEU A:804 (5.93), PHE A:820 (4.72, 5.17), HIS A:613 (5.42), LEU A:765 (4.76), TYR A:612 (6.08), MET A:816 (4.10, 4.61) |
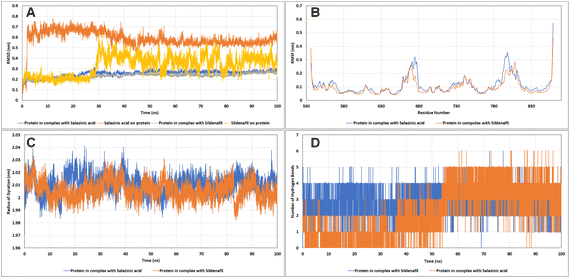 | ||
| Fig. 10 The analysis of the molecular dynamics simulation trajectories (A) RMSD plot, (B) RMSF plot, (C) radius of gyration, and (D) hydrogen bonds of PDE5 with salazinic acid and sildenafil. | ||
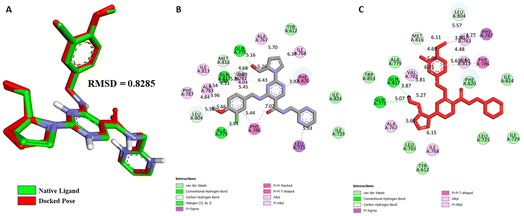
The root mean square fluctuation (RMSF) analysis showed the most or least fluctuating residues with respect to the mean protein structure on ligand binding. Both C- and N-terminal residues exhibited higher fluctuations. All the residues of the protein showed RMSF less than 2 Å except for residues 666–678 of the H-loop (RMSF up to 3.192 Å) and residues 792–805 of the M-loop (RMSF up to 3.254 Å), wherein these residues showed higher fluctuations in the case of Sa than sildenafil (Fig. 10B). These kinds of movements have been observed previously in recent articles where the H-loop moves inward as a result of binding of sildenafil to the Q-pocket,25 similar pattern was observed in case of Sa. The effect of ligand binding on protein shape (contraction/expansion) and structure was observed by calculating the radius of gyration (Rg) of the protein over the trajectories. No significant changes in protein structure were observed as a result of Sa and sildenafil binding (Fig. 10C). We also assessed the number of hydrogen bonds formed between the active site residues of PDE5 and sildenafil and Sa (Fig. 10D). Sa stably formed up to five hydrogen bonds in decreasing order of occupancy with the residues Gln817, Gln775, Val782, Leu804 and Met816. Sildenafil formed up to four hydrogen bonds with the catalytic site of PDE5, with the highest occupancy of hydrogen bonds formed with Gln817, followed by Tyr612, Gln775 and Phe820. Hence, both Sa and sildenafil share similar hydrogen bonding patterns. Sildenafil and Sa showed similar binding affinities and interactions with PDE5. Both were stably interacted with a hydrophobic clamp (Phe786 and Phe820) and glutamate switch (Gln775 and Gln817), which play crucial roles in high-affinity substrate selectivity and binding (Table 7). Both the molecules also interacted with Gln775 which along with Gln817 and Trp853, are indirectly involved in cGMP selectivity by promoting Gln775 in a conformation favourable to bind cGMP.26 Interestingly, the metal binding site residues Asp654, Asp764 and the proton donor residue His613 negatively contributed to the binding energy of Sa and sildenafil.
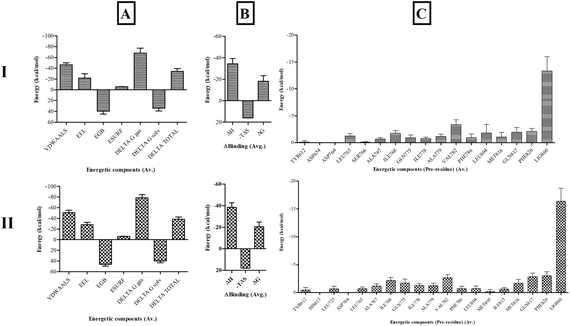 | ||
| Fig. 11 The (A) enthalpic energy, (B) binding free energy and (C) per-residue energy contribution of active site residues of PDE5 with (I) salazinic acid and (II) sildenafil. | ||
Hence, based on the molecular docking and MD simulation results, it is proposed that Sa follows a similar pattern of binding mode and interactions with the substrate binding site of PDE5 and can possibly compete with cGMP to bind to the catalytic domain of PDE5.
Drug likeliness
The physicochemical properties of the Sa were studied using DruLiTo software. A new chemical entity, that is intended to be used as a drug should follow Lipinski's rule. The rule states that a drug should have molecular weight ≤500, hydrogen bond donor (HBD) ≤5, hydrogen bond acceptor (HBA) ≤10, and calculated logarithm of the partition coefficient (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) ≤5. DruLiTo also provides a convenient method of predicting other molecular properties that a drug should possess. Based on the Lipinski's rule, the Sa follows and has no violations. The details of drug likeliness of Sa were furnished in Table 8.
P) ≤5. DruLiTo also provides a convenient method of predicting other molecular properties that a drug should possess. Based on the Lipinski's rule, the Sa follows and has no violations. The details of drug likeliness of Sa were furnished in Table 8.
| Prediction | Parameters | Salazinic acid |
|---|---|---|
| Drug likeliness | Molecular weight | 388.04 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p p |
0.338 | |
A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p p |
−1.497 | |
| HBA | 10 | |
| HBD | 4 | |
| TPSA | 159.82 | |
| AMR | 94.8 | |
| No of violations | No | |
| SwissADME | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P o/w P o/w |
1.66 |
| Water solubility | Moderately soluble | |
| GI absorption | Low | |
| Lipinski rule | Yes | |
| Veber's rule | No | |
| PAINS alert | 0 | |
| TPSA | 159.82 | |
| Lead likeliness | No | |
| admetSAR | HIA | 0.9687 |
| CaCO2 | 0.7129 | |
| BBB | 0.6091 | |
| CYP1A2 | 0.7605 | |
| CYP2C19 | 0.6566 | |
| CYP2D6 | 0.7062 | |
| ProTox-II | LD50 (mg kg−1) | 0.9549 |
| Hepatotoxicity | 962 | |
| Carcinogenicity | Inactive | |
| Immunotoxicity | Inactive | |
| Mutagenicity | Active | |
| Cytotoxicity | Inactive |
Absorption, distribution, metabolism, excretion, and toxicity (ADMET) studies
The ADMET profile of a molecule should be evaluated during the early stages of drug discovery and design to avoid drug withdrawal from the market.27 Using these descriptors, it is possible to determine whether a compound is absorbed, distributed, metabolized, excreted, as well as if it is toxic. Although there are different in vitro methods for establishing ADMET profiles, in silico determination is a faster, cheaper, and life-saving method of determining ADMET profiles.28In addition to being nontoxic, ideal drug candidates should exhibit acceptable ADME characteristics. Based on SwissADME, ProTox-II and admetSAR we examined the ADME profiles, including drug-likeness, partition coefficients, solubility, human gastrointestinal absorption (HIA), blood–brain barrier (BBB), and cytochrome P450 inhibition, of the identified molecules (Table 8).29
One of the most important properties of ADMET is its ability to absorb drugs from the human gut. HIA plays a pivotal role in drug transport to the target cells.30 A higher HIA resulted in improved intestinal absorption of the compound. In this context, Sa showed HIA values greater than 0.9, indicating good membrane permeation. A Pan-Assay Interference Structural (PAINS) alert was used to determine the toxicity of compounds with desirable physicochemical properties. This assay is also referred to as a toxicophore test because of the presence of group elements that affect biological processes by interfering with DNA or proteins, which can cause fatal conditions such as cancer and hepatotoxicity.31 PAINS analysis provides information on the potential toxicity of a molecule. However, the Sa had 0 PAINS structural alerts, indicating their non-toxic nature (Table 8). The results from ProTox server indicated Sa (with LD50 of 962 mg kg−1) have toxicity class 4 (1 is for the most and 6 is for the least toxicity level). It exhibits inactive towards hepatotoxicity, carcinogenicity, immunotoxicity and cytotoxicity as mentioned in the Table 8.
These obtained predictions reveal acceptable properties od Sa, in terms of intestinal adsorption, distribution, permeability and toxicity across the blood–brain barrier, and therefore an interesting candidate for further studies.
In vitro PDE5 inhibitory activity
Table 9 displays the in vitro PDE5 inhibitory activity of Sa in comparison to sildenafil citrate. The IC50 value for Sa was 8.16 ± 0.62 nM, which was almost nearer than that of sildenafil citrate (5.77 ± 0.37 nM). These results are consistent with the in silico studies and indicate that Sa is significantly effective at inhibiting PDE5, compared to sildenafil citrate.| Compound | Concentration (μg mL−1) | % Inhibition | IC50 value (nM) |
|---|---|---|---|
| a Values are expressed as mean ± standard deviation (n = 3). aP < 0.05 statistically significant from the sildenafil citrate. Statistical analyses were performed by one-way analysis of variance followed by Tukey's test at P ≤ 0.05. | |||
| Salazinic acid | 2.5 | 45.79 ± 2.95 | 8.16 ± 0.62 |
| 5.0 | 60.43 ± 2.22 a | ||
| 7.5 | 73.55 ± 1.38 a | ||
| 10.0 | 85.30 ± 0.60 a | ||
| Sildenafil citrate | 2.5 | 48.06 ± 1.48 | 5.77 ± 0.37 |
| 5.0 | 67.84 ± 1.21 | ||
| 7.5 | 84.08 ± 0.86 | ||
| 10.0 | 97.30 ± 1.64 | ||
Experimental
Materials
Sa was previously isolated from Usnea subfloridana Stirton18 and Usnea laevis Nyl.19 in good yields and reported by our group. STZ was obtained from Himedia Laboratories Pvt. Ltd. (Mumbai, India) and amylase HR reagent was obtained from Pro Lab Marketing Pvt. Ltd. (New Delhi, India). Recombinant human (rH) AR, rH DPP-IV, LIP from porcine pancreas, α-Amy from porcine pancreas, α-Glu from Saccharomyces cerevisiae, 2,2-diphenyl-1-picrylhydrazyl (DPPH·), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS+·) and intestinal acetone powders from rats were purchased from Sigma Aldrich (Mumbai, India). Sildenafil citrate and rat feed were procured from Sun Pharma Ltd. (Mumbai, India) and Hindustan Lever Ltd. (Mumbai, India), respectively. All other chemicals used in the present study were at analytical grade.In vitro antioxidant activity
Sa was respectively exposed to ABTS+·,32 DPPH·,32 superoxide radical,33 Fe3+ ion reducing power33 assays previously described. Each experiment was performed in triplicate and results were reported as IC50 values. Particularly, to each known concentration (25–100 μg mL−1) of Sa and ascorbic acid (standard drug) added either 1 mL of ABTS+· solution, 0.004% DPPH solution, nicotinamide adenine dinucleotide (73 μM) and nitroblue tetrazolium (50 μM), or 1% potassium ferricyanide and 0.1% ferric chloride. The mixture was incubated at appropriate temperature and time duration and absorbance at the specific wavelength (ABTS+·: 37 °C, 6 min, 750 nm; DPPH·: 37 °C, 30 min, 517 nm; superoxide: 37 °C, 30 min, 562 nm; Fe3+ ion: 37 °C, 30 min, 700 nm) was assessed against the blank.In vitro antidiabetic activity
Sa was respectively exposed to each of the seven inhibitory assays, namely AR,33 DPP-IV,34 LIP,35 protein glycation,36 α-amylase and α-glucosidase33 as previously described. Each experiment was performed in triplicate and results were reported as IC50 values. To each known concentration (25–100 μg mL−1) of Sa and acarbose (standard drug), added specific volume/amount of the corresponding enzyme and the substrate. The mixture was then incubated at a certain temperature and time duration, followed by measuring absorbance at particular wavelength against the blank.Animals
Forty male and 120 female albino rats weighing 190–200 g were employed for the present experiment. Seven days before the experiment, all animals were fed under standard diet and adapted to the laboratory environment. The present experimental protocol was designed and executed as per OECD regulations and approved by the Institutional Animal Ethics Committee (No. 516/PO/c/01/IAEC) of the University College of Pharmaceutical Sciences, Andhra University, Visakhapatnam, India.Acute toxicity study
The standardized normal male albino rats were orally dosed with 100 mg kg−1 body weight (b.w) of Sa and carefully observed for 14 days. During this time, the behavioral parameters and mortality rate were recorded to determine the LD50.32Induction of diabetes
Overnight fasted male albino rats were intraperitoneally (ip) induced with 55 mg kg−1 b.w of STZ and allowed them to have free access to drink glucose solution (5% v/w). After 72 h, the animals having FPG levels of above 250 mg dL−1 were considered as diabetic and used for the subsequent experiment.32Experimental design
The diabetic male albino rats were randomly categorized into groups (n = 6) and six other normal male albino rats were also included as the normal control group. The normal control and diabetic control groups were assigned only 0.5% sodium carboxymethyl cellulose. The standard group was administered with sildenafil citrate (5 mg kg−1 b.w), while sample groups treated with Sa at 5 and 10 mg kg−1 b.w for 9 successive weeks, respectively. The FPG levels and body weights of all groups were measured at the end of the 0th (first day), 5th and 9th week.Mating and copulatory behavior tests
On the 8th week of the experiment, each treated diabetic male albino rat was paired with two normal virgin female albino rats in a separate cage for 5 days. Every morning, vaginal smear analysis was conducted on each female rat to identify the spermatozoa, which is used as a successful mating indicator. The positive (with the presence of spermatozoa) female rats were separated for 10 days, and their pregnancy was confirmed by euthanizing with an overdose of diethyl ether (ip), and the total number of foetuses and corpora lutea were ascertained.6On day 63rd, the mating behaviour test was conducted on all animals after being treated with the test compound at certain doses. Initially, female albino rats were ovariectomized and subcutaneously injected 0.1 mL of 3-benzoyloxy-17b-estrol (48 h prior copulatory behaviour experiment) and 500 μg progesterone (4 h prior test). Each treated male albino rat was permitted 10 min of adaptation time to experimental conditions (cages in dim red light) and later paired with sexually receptive rats on a one-to-one basis. Finally, male mating behaviours such as ML, MF, EL, IL, IF, and PEI, were assessed. The copulatory behaviour test was terminated only in the cases where the male or female rats were unsuccessful to commence sexual behaviour.6,13
Biochemical estimation
By piercing of retro-orbital plexus under diethyl ether, fasting blood samples were collected from treated male albino rats at the end of the 0th, 5th and 9th week, and serum was separated by centrifugation of these blood samples for 15 min at 3500 rpm. The FPG levels were determined by using commercially available kits (Accu-Chek Glucometer, Roche Diabetes Care, India), and serum insulin levels, T, gonadotropins (FSH and LH) and TNF-α were measured by using ELISA kits (Novatec, India) according to the manufacturer's guidelines. The levels of MDA and GSH and antioxidant enzyme activities such as CAT, SOD, GPx and ROS in serum were assessed using the corresponding assay kits purchased from Biochrome Scientific (India) according to the manufacturer's information. In addition, ALT, AST, ALP, Cr, U, TP, TBIL, serum levels of TC, TG, VLDL, LDL and HDL, were assessed by using the corresponding commercially available kits (Biochrome Scientific, India) as per the manufacturer's guidelines. Lastly, male albino rats were sacrificed with an overdose of diethyl ether (ip), and reproductive organs were detached and stored in 10% formalin.Weights of reproductive organs
The dissected reproductive organs (testis, penile, cauda epididymis, seminal vesicle and prostate) were weighed (Shimadzu ATX224 Analytical Balance, Japan; capacity: 0.1 mg to 220 g) and the relative weight of each organ [(individual organ weight/body weight of the particular animal) × 100] was calculated.Assessment of epididymal sperm characteristics
From the cauda epididymis, semen was gently sucked by using a red blood pipette (0.5 mark) and diluted up to 101 mark with normal saline and used for below sperm characteristics.13A drop of stained solution from the Eppendorf tube was taken and spread over a clean slide by using a spreader and air-dried. The dried smear was observed for morphological features, namely, sperm head, sperm tail and sperm acrosome, of around 200 sperm cells under a microscope (x40, Optscopes Classic IS 500, Germany) and results of sperm abnormalities and detached head from sperm were expressed in percentage incidence.13
Assessment of testicular oxidative stress markers
In ice-cold phosphate buffer solution (4 °C), the testis specimens were homogenized (Heidolph homogenizer Diax 900, Germany), centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min (4 °C), and the obtained supernatants were stored at −80 °C. The antioxidant enzyme activities such as SOD, GPx and CAT, and levels of GSH and MDA in testicular homogenates were assessed using the corresponding assay kits purchased from Biochrome Scientific (India) according to the manufacturer's information.
000 rpm for 15 min (4 °C), and the obtained supernatants were stored at −80 °C. The antioxidant enzyme activities such as SOD, GPx and CAT, and levels of GSH and MDA in testicular homogenates were assessed using the corresponding assay kits purchased from Biochrome Scientific (India) according to the manufacturer's information.
Histopathological examination
The testes stored in 10% formalin were embedded in paraffin and subjected to microtome (5 μm). The obtained slices were stained with hematoxylin-eosin and examined microscopically (x400, Optscopes Classic IS 500, Germany). The MSTD and the HGE were calculated from 20 cross-sections of seminiferous tubules (mostly or nearly circular in shape) from three best slides from the lower, middle and upper parts of testes. Similarly, the MJS (range: 1–10) was evaluated to identify the spermatogenesis (testicular injury) in seminiferous tubules (50 in number).In silico study
End-state MMGBSA binding free-energy calculations were performed using gmx_MMPBSA tool42 on equally spaced 1000 frames extracted from the MD trajectories. The solute dielectric constant of 1 was used to calculate the enthalpic contributions, and the entropic contributions were estimated using the interaction entropy method.
Prediction of drug likeliness
To predict drug likeliness, DruLiTo was used. Sa was subjected to DruLiTo that runs the algorithm to predict whether the molecules possess drug likeliness or not. DruLiTo is a java enabled application that is available at http://www.niper.gov.in/pi_dev_tools/DruLiToWeb/DruLiTo_index. html, which aids in easy prediction about the drug likeliness of molecule.In vitro PDE5 inhibitory activity
The inhibitory activity of Sa and sildenafil citrate on PDE5 was tested in vitro by utilizing a commercially available purified human PDE5A active (Cat No. P93-31G, Signalchem, Canada) expressed by baculovirus in sf9 insect cells and PDE Glo Phosphodiesterase Assay kit (Cat No. V1361) from Promega, UK. The assay was run in triplicate and the IC50 of Sa and sildenafil citrate was determined against the PDE5A.Conclusions
To the best of our knowledge, this is the first study representing Sa as a protective agent on male spermatogenic dysfunction in STZ-induced diabetic albino rats. In the present study, the in vitro antioxidant and antidiabetic assays of Sa revealed the stronger inhibitory properties of free radicals and digestive enzymes. Further, low and high doses of Sa were administered to diabetic rats for 9 successive weeks. In contrast to the diabetic control group, a significant restoration in the body weight, reproductive organs weight, hyperglycemia (FPG), hyperlipidemia (TC, TG, VLDL, LDL and HDL), insulin resistance, and sperm parameters were noticed in Sa-treated groups. Also, oral supplementation of Sa significantly restored male reproductive dysfunction by elevating the serum hormone (FSH, LH and T) levels, with a significant decrease in liver biomarkers (ALT, AST and ALP; Cr, U and TBIL). Sa also improved the activity of antioxidants (SOD, GPx, CAT and GSH) and reduced MDA content in both serum and testicular homogenate, and significantly downregulated the TNF-α expression in blood serum. Furthermore, Sa significantly restored the mating parameters, sperm parameters and histological changes in testicular tissues of diabetic rats and preserved the structural integrity of the testis. Also, the in silico studies results were in concordance with the pharmacological data. Overall, the data support the spermatogenic protective efficacy of Sa through the competition with cGMP to bind to the catalytic domain of PDE5 and thereby lead to the control in the oxidative impairment of testis by its free radical scavenging, anti-inflammatory and antidiabetic properties. However, further experiments on Sa are required to clarify Sa as a clinical drug to treat male sexual dysfunction in diabetic patients.Ethical statement
The present experimental protocol was designed and executed as per OECD regulations and approved by the Institutional Animal Ethics Committee (No. 516/PO/c/01/IAEC) of the University College of Pharmaceutical Sciences, Andhra University, Visakhapatnam, India.Author contributions
KNK: data curation, formal analysis, visualization, investigation and methodology of in vivo studies. HP and GSV: formal analysis and investigation of in vivo studies. DSNBKP, GS and SPP: investigation, methodology and writing – original draft of in silico studies. VBT: supervision, investigation, conceptualization, formal analysis, writing – original draft and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
GS are thankful to the HPC facility of IIT Delhi for providing computational resources for this study.References
- World Health Organization, Diabetes, Genève, 2021 Search PubMed.
- American Diabetes Association, Diabetes Care, 2014, 37, S81–S90 CrossRef PubMed.
- A. A. Choudhury and V. D. Rajeswari, Biomed. Pharmacother., 2021, 143, 112183 CrossRef CAS PubMed.
- L. Chen, G. R. Shi, D. D. Huang, Y. Li, C. C. Ma, M. Shi, B. X. Su and G. J. Shi, Biomed. Pharmacother., 2019, 112, 108585 CrossRef CAS PubMed.
- T. Mostafa and I. A. Abdel-Hamid, J. Diabetes, 2021, 12, 954 Search PubMed.
- N. Minaz, R. Razdan, B. D. Hammock, S. Mujwar and S. K. Goswami, Biomed. Pharmacother., 2019, 115, 108897 CrossRef CAS PubMed.
- D. F. Penson, D. M. Latini, D. P. Lubeck, K. L. Wallace, J. M. Henning and T. F. Lue, Diabetes Care, 2003, 26, 1093–1099 CrossRef PubMed.
- H. A. Feldman, I. Goldstein, D. G. Hatzichristou, R. J. Krane and J. B. McKinlay, J. Urol., 1994, 151, 54–61 CrossRef CAS PubMed.
- L. S. Malavige and J. C. Levy, J. Sex. Med., 2009, 6, 1232–1247 CrossRef PubMed.
- G. Corona, C. B. Giorda, D. Cucinotta, P. Guida and E. Nada, J. Sex. Med., 2014, 11, 2065–2073 CrossRef PubMed.
- G. Corona, A. M. Isidori, A. Aversa, M. Bonomi, A. Ferlin, C. Foresta, S. L. Vignera, M. Maggi, R. Pivonello, L. Vignozzi and F. Lombardo, Rev. Endocr. Metab. Disord., 2019, 21, 57–65 CrossRef PubMed.
- R. C. Kukreja, F. Salloum, A. Das, R. Ockaili, C. Yin, Y. A. Bremer, P. W. Fisher, M. Wittkamp, J. Hawkins, E. Chou, A. K. Kukreja, X. Wang, V. R. Marwaha and L. Xi, Vasc. Pharmacol., 2005, 42, 219–232 CrossRef CAS PubMed.
- G. A. Soliman, A. S. Saeedan, R. F. Abdel-Rahman, H. A. Ogaly, R. M. Abd-Elsalam and M. S. Abdel-Kader, Saudi Pharm. J., 2019, 27, 326–340 CrossRef PubMed.
- Y. Chen, B. Zhou, Z. Yu, P. Yuan, T. Sun, J. Gong, Y. Zhang, T. Wang, S. Wang, K. Liu and J. H. Liu, J. Sex. Med., 2020, 17, 1434–1447 CrossRef CAS PubMed.
- A. C. Huang, T.-C. Yeh, N.-C. Wu, C.-Y. Yeh, P.-H. Lin, K.-Y. Yeh, A. Durazzo, M. Lucarini, G. Lombardi-Boccia, A. C. Huang, T.-C. Yeh, N.-C. Wu, C.-Y. Yeh, P.-H. Lin and K.-Y. Yeh, J. Mol. Sci., 2022, 23, 9759 CrossRef CAS PubMed.
- T. J. Nolan and J. Keane, Nature, 1933, 132, 281 CrossRef CAS.
- M. Candan, M. Yılmaz, T. Tay, M. Erdem, A. Ö. Türk and C. Z. Naturforsch, J. Biosci., 2007, 62, 619–621 CAS.
- T.-T. Nguyen, S. Nallapaty, G. S. N. K. Rao, S. T. Koneru, S. S. P. Annam and V. B. Tatipamula, Pharm Sci, 2021, 27, 291–296 CrossRef CAS.
- V. B. Tatipamula and S. S. P. Annam, J. Ethnopharmacol., 2022, 282, 114641 CrossRef CAS PubMed.
- B. Burlando, E. Ranzato, A. Volante, G. Appendino, F. Pollastro and L. Verotta, Planta Med., 2009, 75, 607–613 CrossRef CAS PubMed.
- N. Verma, B. C. Behera and B. O. Sharma, J. Biol. Chem., 2012, 40, 7–21 Search PubMed.
- H. Wang, Y. Liu, Q. Huai, J. Cai, R. Zoraghi, S. H. Francis, J. D. Corbin, H. Robinson, Z. Xin, G. Lin and H. Ke, J. Biol. Chem., 2006, 281, 21469–21479 CrossRef CAS PubMed.
- B. J. Sung, K. Y. Hwang, Y. H. Jeon, J. il Lee, Y. S. Heo, J. H. Kim, J. Moon, J. M. Yoon, Y. L. Hyun, E. Kim, S. J. Eum, S. Y. Park, J. O. Lee, T. G. Lee, S. Ro and J. M. Cho, Nature, 2003, 425, 98–102 CrossRef CAS PubMed.
- K. Y. J. Zhang, G. L. Card, Y. Suzuki, D. R. Artis, D. Fong, S. Gillette, D. Hsieh, J. Neiman, B. L. West, C. Zhang, M. V. Milburn, S. H. Kim, J. Schlessinger and G. Bollag, Mol. Cell, 2004, 15, 279–286 CrossRef CAS PubMed.
- C. M. Hsieh, C. Y. Chen, J. W. Chern and N. L. Chan, J. Med. Chem., 2020, 63, 8485–8494 CrossRef CAS PubMed.
- W. S. Ahmed, A. M. Geethakumari and K. H. Biswas, Biomed. Pharmacother., 2021, 134, 111128 CrossRef CAS PubMed.
- L. L. Ferreira and A. D. Andricopulo, Drug Discovery Today, 2019, 24, 1157–1165 CrossRef CAS PubMed.
- U. Norinder and C. A. Bergström, ChemMedChem, 2006, 1, 920–937 CrossRef CAS PubMed.
- G. R. Bickerton, G. V. Paolini, J. Besnard, S. Muresan and A. L. Hopkins, Nat. Chem., 2012, 4, 90–98 CrossRef CAS PubMed.
- S. Ejeh, A. Uzairu, G. A. Shallangwa and S. E. Abechi, Chem. Afr., 2021, 4, 563–574 CrossRef CAS.
- J. B. Baell and G. A. Holloway, J. Med. Chem., 2010, 53, 2719–2740 CrossRef CAS PubMed.
- V. B. Tatipamula and B. Kukavica, Drug Chem. Toxicol., 2020, 45, 680–687 CrossRef PubMed.
- V. B. Tatipamula, S. S. P. Annam, H. T. Nguyen, H. Polimati and R. P. Yejella, Nat. Prod. Res., 2021, 35, 5420–5424 CrossRef CAS PubMed.
- I. J. Sagbo, M. van de Venter, T. Koekemoer and G. Bradley, J. Evidence-Based Integr. Med., 2018, 2018, 4170372 Search PubMed.
- A. B. Justino, N. C. Miranda, R. R. Franco, M. M. Martins, N. M. da Silva and F. S. Espindola, Biomed. Pharmacother., 2018, 100, 83–92 CrossRef CAS PubMed.
- H. Y. Kim and K. Kim, J. Agric. Food Chem., 2003, 51, 1586–1591 CrossRef CAS PubMed.
- X. X. Han, Y. P. Jiang, N. Liu, J. Wu, J. M. Yang, Y. X. Li, M. Sun, T. Sun, P. Zheng and J.-Q. Yu, Biomed. Pharmacother., 2019, 110, 561–570 CrossRef CAS PubMed.
- C. Palanichamy, P. Pavadai, T. Panneerselvam, S. Arunachalam, E. Babkiewicz, S. R. K. Pandian, K. S. Jeyarajaguru, D. N. Ammunje, S. Kannan, J. Chandrasekaran, K. Sundar, P. Maszczyk and S. Kunjiappan, Molecules, 2022, 27, 3799 CrossRef CAS PubMed.
- D. S. N. B. K. Prasanth, M. Murahari, V. Chandramohan, S. P. Panda, L. R. Atmakuri and C. Guntupalli, J. Biomol. Struct. Dyn., 2021, 39, 4618–4632 CrossRef CAS PubMed.
- L. P. Kagami, G. M. das Neves, A. W. S. da Silva, R. A. Caceres, D. F. Kawano and V. L. Eifler-Lima, J. Mol. Model., 2017, 23, 304 CrossRef PubMed.
- M. J. Abraham, D. van der Spoel, E. Lindahl and B. Hess, Development Team GROMACS User Manual Version 5.1. 5, https://manual.gromacs.org/documentation/5.1/user-guide/index.html, accessed 14 November 2022 Search PubMed.
- M. S. Valdés-Tresanco, M. E. Valdés-Tresanco, P. A. Valiente and E. Moreno, J. Chem. Theory Comput., 2021, 17, 6281–6291 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01542d |
| This journal is © The Royal Society of Chemistry 2023 |