Biotemplated synthesis of catalytic Au–Pd nanoparticles
Simon R.
Hall
*a,
Andrew M.
Collins
a,
Natalie J.
Wood
a,
Wataru
Ogasawara
b,
Moataz
Morad
c,
Peter J.
Miedziak
c,
Meenakshisundaram
Sankar
c,
David W.
Knight
c and
Graham J.
Hutchings
c
aSchool of Chemistry, University of Bristol, Bristol, BS81TS, UK. E-mail: simon.hall@bristol.ac.uk; Tel: +44 (0) 11733 16797
bDepartment of Bioengineering, Nagaoka University of Technology, 1603-1 Kamitomioka, Nagaoka, Niigata 940-2188, Japan. E-mail: owataru@vos.nagaokaut.ac.jp; Fax: 81-258-47-9429
cCardiff Catalysis Institute, School of Chemistry, Cardiff University, Cardiff, UK. E-mail: Hutch@cardiff.ac.uk; Tel: +44 (0) 2920874059
First published on 22nd December 2011
Abstract
In this paper, we demonstrate a facile route to carbon-supported nanoparticles of a gold/palladium alloy which exhibit catalytic activity for the oxidation of benzylic alcohols, using the biopolymer chitosan. The biomolecule acts triply as a structure-directing agent, anti-sintering matrix and in situ source of carbon in which the catalytically active nanoparticles are dispersed. The catalyst works without the need for environmentally harmful bases and solvents.
Due to the size dependent properties of nanoparticles, there is a great deal of interest in ensuring that synthetic routes to them give the lowest polydispersity possible.1 A good example of how this can be achieved is with subtle adaptations to the Brüst synthesis of thiolate-monolayer protected nanoparticulate gold.2,3 These improvements include; etching the outer layer of the gold nanoparticles,4annealing the nanoparticles resulting in the formation of optimum sized clusters of 140–145 Au atoms5 and digestive ripening of the colloid suspension.6 Capping is also an effective restriction on nanoparticle growth and has been reported for silver with a polymer acting as the capping agent.7,8 Owing to the burgeoning interest in environmental issues, there is now a greater emphasis on ‘greener’ routes to functional nanomaterials, with a concomitant rejection of the more environmentally harmful capping agents such as surfactants and other products derived from the petrochemical industry. In terms of fine control over crystal phase and morhpology, it is often to Nature that scientists turn in order to produce materials with high specificity and low environmental impact. This has resulted in the employment of a range of biological entities from microorganisms to their constituent biopolymers in the fabrication of functional nanomaterials.9–11 In the case of the latter, the biopolymer chitin and its derrivative chitosan are the ones which have found most common usage in the synthesis of various metal and metal oxide nanoparticles. Chitin (poly[β-(1→4)-2-amino-2-deoxy-D-glucopyranose]) is one of the most abundant naturally-occurring polysaccharides, present mainly in animals such as shrimp and crab, where it is a major part of the exoskeleton. It is also found in certain fungi as the main structural polymer in the cell wall. Owing to the recalitrance of chitin, for synthetic use it is deacetylated to form chitosan, which is able to be dissolved easily in weakly acidic solutions. Typical commercial chitosan has approximately 85% deacetylation.12 The strong interest in chitin and chitosan is due to the presence of aliphatic amino groups, which make chitosan a particularly strong chelating agent.13,14 Previous research has shown that varying the molecular weight and concentration of chitosan in a synthesis can have a marked effect on the size and polydispersty of nanoparticles produced.15,16
Until relatively recently, gold was thought of as too un-reactive to be of interest chemically, but this perception changed with the discovery of its catalytic capabilities when reduced to the nano-scale.17–20 In a study by Hutchings and co-workers, calcined carbon-supported Au–Pd catalysts were found to be the most active and stable of any metal oxide supported Au–Pd catalyst for the direct synthesis of hydrogen peroxide from hydrogen and oxygen,21 for the aerobic oxidation of alcohols22 and polyols, for example glycerol.23 It was observed that the activity and stability peaked when the metals were added to the supports in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio.24Calcination of the supported Au–Pd nanoparticles resulted in an increase in stability of the catalyst by stronger interaction between the metal and the support thus increasing their re-usability.
1 molar ratio.24Calcination of the supported Au–Pd nanoparticles resulted in an increase in stability of the catalyst by stronger interaction between the metal and the support thus increasing their re-usability.
In this work, we use chitosan as a novel template for the generation of nanoparticulate gold/palladium. By providing an effective cocoon against sintering and uncontrolled nanoparticle growth, we are able to produce nanoparticles of low polydispersity with an average diameter of 3 nm. Furthermore, the nanoparticles show catalytic activity in the solvent-less, aerobic oxidation of benzyl alcohol to benzaldehyde.
The oxidation of alcohols to aldehydes is of immense importance in the fine chemicals industry particularly for the synthesis of intermediates.25 Even today oxidations of this type are carried out at a commercial scale using non-green oxygen donors such as chromate or permanganate, but current environmental concerns are forcing the replacement of these routes. Recently, gold-based catalysts have been found to be very effective for the oxidation of alcohols. Rossi, Prati and co-workers26–28 were the first to clearly demonstrate that supported gold nanoparticles can be very effective catalysts for the oxidation of alcohols. The presence of base (typically NaOH) was found to be essential for activity and consequently sodium salts of the acids were formed as products. Corma and co-workers29–31 have shown that an Au/CeO2 catalyst is active for the selective oxidation of alcohols to aldehydes and ketones and the oxidation of aldehydes to acids in the absence of base and under solvent-free conditions. Subsequently, we showed that the combination of Au and Pd as alloy nanoparticles significantly enhanced the catalytic activity for the oxidation of alcohols in the absence of base.32
In this work, a typical synthesis was as follows: an aqueous acetic acid solution (1%) was formed by the addition of acetic acid (1 ml, Aldrich) to distilled water (99 ml). Medium weight chitosan (2 g, Sigma) was added to this solution and left to stir for 24 h yielding a 2% chitosan solution. Gold/palladium precursor solutions were made by dissolving sodium tetrachloropalladate(II) and gold(III) chloride trihydrate, (both from Sigma) separately in concentrations of 0.001 M.
Chitosan solutions were cast on slides and left over night in a 40 °C oven, forming a solid film. The films were then neutralised with NaOH (0.1 M) for 1 h before being rinsed thoroughly with distilled water (18.2 MΩ) and soaked in an excess of gold/palladium solution, the metals in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The films were then subsequently placed into a NaBH4 solution (1 M) for 5 min to affect the reduction of the metal species. An immediate colour change was observed. Once complete, the reduced films were then washed with deionized water and allowed to dry in an oven (40 °C, 24 h). Carbonization of the Au/Pd chitosan composite films was performed at 10 °C min−1 from room temperature to 400 °C in an argon atmosphere.
1 ratio. The films were then subsequently placed into a NaBH4 solution (1 M) for 5 min to affect the reduction of the metal species. An immediate colour change was observed. Once complete, the reduced films were then washed with deionized water and allowed to dry in an oven (40 °C, 24 h). Carbonization of the Au/Pd chitosan composite films was performed at 10 °C min−1 from room temperature to 400 °C in an argon atmosphere.
The benzyl alcohol oxidation reactions were carried out in a stirred flask with 1 bar oxygen pressure (relative). Benzyl alcohol (2 g) was heated to 120 °C and was stirred at 1000 rpm with catalyst (0.02 g). The reaction was stopped at the desired time, the flask was cooled in an ice bath for 10 min and the reaction mixture centrifuged to remove the solid catalyst. The supernatant liquid mixture was analysed. Analysis of the products was carried out using GC-MS and GC (a Varian star 3400 CX with a 30 m CP-Wax 52 CB column). The products were identified by comparison with known standard samples. For quantification of the amounts of reactants consumed and products generated, an external calibration method was used.
Samples for transmission electron microscopy (TEM) were prepared by dispersing the product in ethanol and drop casting onto a carbon-coated nickel grid. TEM analyses were carried out using a JEOL JEM 1200EX microscope equipped with an Oxford energy dispersive X-ray (EDX) detector. Samples for scanning electron microscopy (SEM) were coated with platinum/palladium and analysed using a JEOL JSM 6330F Field Emission SEM.
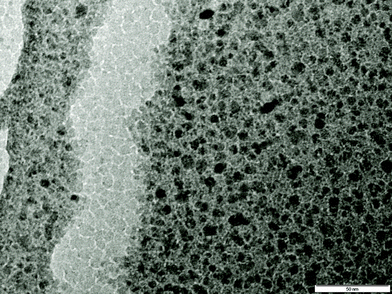
A representative TEM image of the chitosan/nanoparticle composite is shown in Fig. 1. After calcination, all TEM investigations revealed an even distribution of nanoparticles (particle size 4.6 nm ± 1.2 nm) throughout the carbon support.
It is evident from the particle size data that this new synthetic method results in smaller nanoparticles with a lower polydispersity than has previously been reported.33–35 This low average size could be due to the fact that the metal cations are reduced rapidly and as the 2% medium molecular weight chitosan solution is of low viscosity, BH4− ions can diffuse more easily through the film and therefore improve the rate of reaction before aggregation has had a chance to occur.
An SEM image of the surface of a film resulting from the 2% medium molecular weight chitosan solution is shown in Fig. 2a. The ridges in the film are a result of the removal of the film from the glass surface on which it was originally cast. EDX mapping of the area represented by this image shows a homogeneous distribution of both metals throughout the film (Fig. 2c and d). The EDX spectrum of the same film post calcination (Fig. 2b) shows the presence of gold, palladium and carbon, indicating calcination of the film did not result in the loss of the metal species.
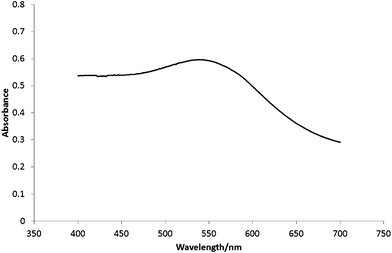
Solid state ultra-violet/visible spectroscopy was carried out on the solid films of chitosan containing gold–palladium nanoparticles (Fig. 3). The shape of the spectra correlates well to the spectrum of a gold/palladium bimetallic colloidal suspension in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Au/Pd ratio as determined by Nagata et al.36
1 Au/Pd ratio as determined by Nagata et al.36
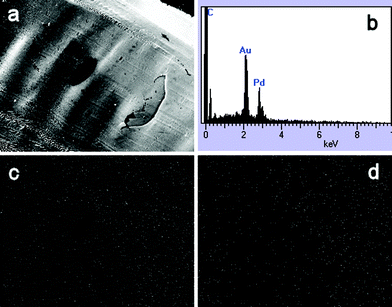 | ||
| Fig. 2 (a) SEM image (×70) of chitosan/Au–Pd film. EDX spectrum (b) and elemental maps for Au (c) and Pd (d) for the image in (a) are shown. | ||
Finally, the carbon-supported Au/Pd composite films derived from the 2 wt% medium molecular weight chitosan were investigated for the oxidation of benzyl alcohol and the results are shown in Table 1. The material was observed to give very high selectivities to benzaldehyde with only a minor by-product of toluene being observed.
| Catalyst | Substrate | TON (h−1)b | Selectivity (%)d | ||
|---|---|---|---|---|---|
| R1 | R2 | A | B | ||
| a Temperature: 393 K; O2 pressure: 1 bar g; substrate: 2 g; stirring: 1000 rpm, catalyst: 0.02 g. b Calculated after 4 h. c Mesitylene (2 g) was used as the solvent. d The only other product detected is benzene. | |||||
| AuPd/C | H | H | 151 | 87 | 11 |
| AuPd/Cc | H | H | 94 | 87 | 8 |
| AuPd/C | OCH3 | H | 17 | 85 | 15 |
| AuPd/C | Cl | H | 10 | 97 | 3 |
| AuPd/C | H | OCH3 | 7 | 76 | 24 |
Recently, ourselves and others have reported a similar system37,38 for the aerobic oxidation of benzylic alcohols using gold palladium nanoparticles supported on chitosan. In the case of the latter, they achieved a turnover frequency of 6 h−1 for benzyl alcohol oxidation using a chitosan-supported gold palladium catalyst, but using a three times stoichiometric excess of K2CO3 as the soluble base in a dilute aqueous medium at 300 K after 8 h. Another interesting feature of this report is that the product mixture contained mostly benzoic acid (97% selectivity) and the more important benzaldehyde product was not detected at all. This shows that in this earlier work there was no control over the selective oxidation of benzyl alcohol to benzaldehyde. Our catalyst is active under very different reaction conditions. One of the most important aspects of green chemistry is the need to avoid using soluble bases and solvents for the catalytic oxidation,17,27–29 however, this necessitates the use of dilute reaction conditions using a solvent. In accordance with the green chemistry principles, we have performed this reaction under solvent-less and base-free conditions. Under our experimental conditions, we have achieved a turnover frequency of 151 h−1 with a selectivity of 87% for the more important benzalehyde, which is the more challenging material to produce. Hence, our findings represent a significant improvement over the catalytic system reported by Sakurai and co-workers.38 Most importantly our catalyst is 25 times more active and we obtain benzaldehyde rather than the overoxidation product benzoic acid. Indeed, use of mesitylene as a solvent showed that a decreased activity was observed (Table 1). We next tested this catalyst for different substituted benzyl alcohols and the results are presented in Table 1. From the results it is clear that substituted benzyl alcohols are less reactive compared to the parent alcohol. In addition, the selectivity to the aldehyde is higher for substituted alcohols. At this time we note that the amount of gold and palladium in the catalyst is very high (ca. 22 wt%) and much of this gold and palladium will be inactive as it will be effectively ‘locked away’ by the carbon cocoon provided by the carbonized chitosan. We have shown previously that often only a fraction of the metal present in a catalyst is indeed active in any case39 and consequently in future studies we will concentrate on devising preparation strategies that improve the utilisation of the active metals.
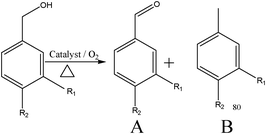
To further understand the applicability of these catalysts for other substrates, cinnamyl alcohol oxidation was performed using the most active gold–palladium catalyst. Cinnamyl alcohol is a more complex substrate compared to benzyl alcohol because of the many reactions possible as given in Scheme 1. Since cinnamyl alcohol is a solid, we used toluene and mesitylene as solvents for these oxidation reactions. The results are presented in Table 2. The catalyst showed excellent activity for the aerobic oxidation of cinnamyl alcohol. For example, when mesitylene was used as the solvent, at 393 K, using 4 h reaction time a conversion level of 90% was achieved. This activity appears to be dependent on the solvent of the reaction, as when toluene was used as the solvent, the activity decreases from 90% to 65%. The selectivity for cinnamaldehyde was not high unfortunately, as a number of products are observed. Trace amount of products are not given here for clarity. We consider that by further optimizing the reaction conditions, a higher selectivity towards cinnmaldehyde could be achieved and this is currently in progress.
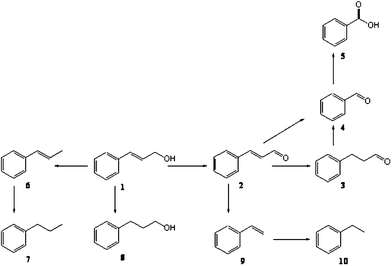 | ||
| Scheme 1 Reaction networks observed in the aerobic oxidation of cinnamyl alcohol using supported gold palladium catalysts. 1: cinnamyl alcohol; 2: cinnamyl aldehyde; 3: 3-phenylpropanal; 4: benzaldehyde; 5: benzoic acid; 6: 1-phenylprop-1-ene; 7: propylbenzene; 8: 3-phenylpropanol; 9: styrene and 10: ethylbenzene. | ||
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Solvent | Conversion (%) | Selectivity (%) | TON (h−1)b | ||||
| Cinn. ald. (2) | Benz. ald. (5) | Benz. acid (6) | Cinn. Acid (3) | Othersc | |||
| a Temperature: 393 K; O2 pressure: 1 bar g; substrate: 2 g; stirring: 1000 rpm, catalyst: 0.02 g: substrate: 2 g; solvent: 2 g. b Calculated after 4 h; cinn. ald. = cinnamaldehyde, benz. ald. = benzyl alcohol, benz. acid = benzoic acid, cin. acid = cinnamic acid. c Others include 3-phenylpropanal, benzaldehyde, benzoic acid, 1-phenylprop-1-ene, propylbenzene, 3-phenylpropanol, styrene, α-methyl styrene, 3-phenylpropanol and ethylbenzene. | |||||||
| Toluene | 65 | 27 | 43 | 5 | 1 | 24 | 650 |
| Mesitylene | 90 | 29 | 33 | 6 | 1 | 31 | 945 |
Conclusions
In conclusion, the simple synthesis of gold/palladium alloy nanoparticles in a chitosan matrix has resulted in fine control over particle size and polydispersity. Once the composite material has been calcined, the chitosan is transformed into a carbon support for the metal nanoparticles, which remain homogeneously dispersed throughout the matrix. Catalytic studies on the efficacy of the carbonized film in the oxidation of aromatic alcohols show that the composite is active and highly selective especially for cinnamyl alcohol oxidation. Future work will involve selectively ‘etching’ the carbon cocoon in order to provide greater accessibility to the gold/palladium nanoparticles, with a concomitant increase in catalytic activity.Acknowledgements
The authors acknowledge the UK EPSRC, (AMC), the Royal Society (SRH, University Research Fellowship), the EPSRC (MS) and the Umm Al-Qura Univeristy (MM) for financial support.References
- J. Park, K. An, Y. Hwang, J. Park, H. Noh, J. Kim, J. Park, N. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891 CrossRef CAS.
- G. Mpourmpakis, S. Caratzoulas and D. G. Vlachos, Bioconjugate Chem., 2010, 21, 214 CrossRef.
- M. Brust, J. Fink, D. Bethell, D. J Schiffrin and C. J. Kiely, J. Chem. Soc., Chem. Commun., 1995, 1655 RSC.
- T. G. Schaaff and R. L. Whetton, J. Phys. Chem. B, 1999, 103, 9394 CrossRef CAS.
- J. F. Hicks, D. T. Miles and R. W. Murray, J. Am. Chem. Soc., 2002, 124, 13322 CrossRef CAS.
- B. L. V. Prasad, S. I. Stoeva, C. M. Sorensen and K. J. Klabunde, Langmuir, 2002, 18, 7515 CrossRef CAS.
- S. T. Dubas and V. Pimpan, Talanta, 2008, 76, 29 CrossRef CAS.
- C. Sabatini, R. V. Pereira and M. H. Gehlen, J. Fluoresc., 2007, 17, 377 CrossRef CAS.
- D. Mandal, M. E. Bolander, D. Mukhopadhyay, G. Sarkar and P. Mukherjee, Appl. Microbiol. Biotechnol., 2006, 69, 485 CrossRef CAS.
- N. Vigneshwaran, N. M. Ashtaputre, K. M. Paralikar, R. P. Nachane, R. H. Balasubramanya and P. V. Varadarajan, Mater. Lett., 2007, 61, 1413 CrossRef CAS.
- N. Vigneshwaran, R. P. Nachane, R. H. Balasubramanya and P. V. Varadarajan, Carbohydr. Res., 2006, 341, 2012 CrossRef CAS.
- G. Roberts, Chitin Chemistry, MacMillan, 1992 Search PubMed.
- R. A. A. Muzzarelli, Natural chelating polymers, First Ed., 1973, p. 144 Search PubMed.
- M. N. V. Ravi Kumar, React. Funct. Polym., 2000, 46, 1 CrossRef.
- S. R. Hall, Adv. Mater., 2006, 18, 487 CrossRef CAS.
- A. M. Collins, S. Mann and S. R. Hall, Nanoscale, 2010, 2, 2370 RSC.
- G. J. Hutchings, Dalton Trans., 2008, 5523 RSC.
- M. Haruta, T. Kobayashi, H. Sano and N. Yamada, Chem. Lett., 1987, 405 CrossRef CAS.
- M. Haruta, N. Yamada, T. Kobayashi and S. Iijima, J. Catal., 1989, 115, 301 CrossRef CAS.
- P. Landon, P. Collier, A. Papworth, C. Kiely and G. J. Hutchings, Chem. Commun., 2002, 2058 RSC.
- J. K. Edwards, B. Solsona, E. Ntainjua N, A. F. Carley, A. A. Herzing, C. J. Kiely and G. J. Hutchings, Science, 2009, 323, 1037 CrossRef CAS.
- D. I. Enache, D. Barker, J. K. Edwards, S. H. Taylor, D. W. Knight, A. F. Carley and G. J. Hutchings, Catal. Today, 2007, 122, 407 CrossRef CAS.
- M. Sankar, N. Dimitratos, D. Knight, A. Carley, R. Tiruvalam, C. Kiely, D. Thomas and G. Hutchings, ChemSusChem, 2009, 2, 1145 CrossRef CAS.
- J. Edwards and G. J. Hutchings, Angew. Chem., Int. Ed., 2008, 47, 9192 CrossRef CAS.
- R. A. Sheldon and J. K. Kochi, Metal-Catalyzed Oxidations of Organic Compounds, Academic Press, New York, 1981 Search PubMed.
- L. Prati and M. Rossi, J. Catal., 1998, 176, 552 CrossRef CAS.
- F. Porta, L. Prati, M. Rossi, S. Colluccia and G. Martra, Catal. Today, 2000, 61, 165 CrossRef CAS.
- L. Prati, Gold Bull., 1999, 32, 96 CrossRef CAS.
- A. Abad, P. Conception, A. Corma and H. Garcia, Angew. Chem., Int. Ed., 2005, 44, 4066 CrossRef CAS.
- A. Corma and M. E. Domine, Chem. Commun., 2005, 4042 RSC.
- A. Abad, A. Corma and H. Garcia, Chem.–Eur. J., 2008, 14, 212 CrossRef CAS.
- D. I Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362 CrossRef.
- T. Azzam, L. Bronstein and A. Eisenberg, Langmuir, 2008, 24, 6521 CrossRef CAS.
- M. Yoonessi, E. Seikel and M. J. Pender, Langmuir, 2009, 25, 3369 CrossRef CAS.
- I. Sevonkaev, I. Halaciuga, D. V. Goia and E. Matijevi, Colloids Surf., A, 2010, 354, 16 CrossRef CAS.
- Y. Mizukoshi, K. Okitsu, Y. Maeda, T. A. Yamamoto, R. Oshima and R. and Y. Nagata, J. Phys. Chem. B, 1997, 101, 7033 CrossRef CAS.
- S. Meenakshisundaram, E. Nowicka, P. Miedziak, G. L. Brett, R. L. Jenkins, N. Dimitratos, S. H. Taylor, D. W. Knight, D. Bethell and G. J. Hutchings, Faraday Discuss., 2010, 145, 341 RSC.
- A. Murugadoss and H. Sakurai, J. Mol. Cat. A., 2011, 341, 1 CrossRef CAS.
- A. A. Herzing, C. J. Kiely, A. F. Carley, P. Landon and G. J. Hutchings, Science, 2008, 321, 1331 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |