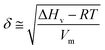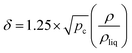Open Access Article
Open Access ArticleSupercritical CO2 technology for the treatment of end-of-life lithium-ion batteries
P. Cattaneo
 a,
F. D'Aprile
b,
V. Kapelyushko
b,
P. Mustarelli
a,
F. D'Aprile
b,
V. Kapelyushko
b,
P. Mustarelli
 c and
E. Quartarone
c and
E. Quartarone
 *a
*a
aDepartment of Chemistry, R2BATT Lab, University of Pavia, Via Taramelli 16, 27100 Pavia, Italy. E-mail: eliana.quartarone@unipv.it
bSyensqo Specialty Polymers, Viale Lombardia, 20, 20021, Bollate, Milan, Italy
cDepartment of Materials Science, R2BATT Lab, University of Milano Bicocca, Via Cozzi 55, 20155 Milan, Italy
First published on 16th April 2024
Abstract
The penetration of Li-ion batteries (LIBs) in the automotive market makes a zero-waste vision for battery recycling urgent. This can play a crucial role in developing a circular economy through the recovery of critical raw materials (CRMs) as well as bringing non-metallic components back to use. In recent years, recycling technologies for LIBs entered a new stage focused on the development of advanced pre-treatment processes to separate all the valuable battery components and more sustainable metallurgical approaches. Compared to common recycling processes, supercritical fluid (SCF) technology has great advantages related to its environmental benignity; chiefly, if CO2 is used as the SCF (scCO2), it is an outstanding solvent for green chemistry approaches. This review aims at providing an overview on the current progresses and open challenges of SCF technology for the treatment of end-of-life LIBs. The fundamentals of SCF technology process are discussed, providing the reader a brief overview of principles, operation procedures and instrumentation. Thereafter, the main applications in the field of battery recycling are reviewed. Successful methods for battery electrolyte recovery via scCO2 are discussed together with pioneering studies on the extraction of critical metals from the cathode that demonstrate promising recovery rates (>60%) for Li, Co, Mn, and Ni. Finally, a specific focus is given on the huge innovation potential of scCO2 to separate and reuse the fluorinated binder from the electrode. At present, the binder is burnt in common recycling processes, leading to hazardous fluorinated gas emissions. This review aims to emphasize the opportunities of the SCF technology in battery waste treatment as a promising approach for resource recovery with significant economy and environmental perspectives.
Sustainability spotlightLi-ion batteries are the key technology for transport and stationary applications to meet the UN's ambitious CO2 goals. Despite their success, LIBs have crucial challenges from a sustainable perspective, including relying on critical materials (Co, Li, Ni, Mn, P, Al, and Cu) and highly complex recycling processes requiring high costs and socio-environmental impacts. There is a need to develop more sustainable recycling technologies, ensuring to recover all the battery components back to use—not only critical materials but also valuable substances (plastics, metal foils, binders, electrolytes, and fluorinated compounds) to the level that causes the lowest sustainability impacts. In this regard, supercritical CO2 technology is in line with several UN sustainable goals, including goal 12 (responsible consumption and production), goal 7 (affordable and clean energy production), and goal 13 (climate action). |
1. Introduction
In 2022, a global LIB manufacturing capacity of 1.5 TW h was estimated. This is expected to increase to 4 TW h by 2025 and reach 6.8 TW h by 2030, with China producing 76% of the global capacity and leading the market, whose total value is projected to increase from 46.2 billion dollars in 2023 to 189.4 billion dollars in 2032.1,2 These figures do not take into account supply shortages, which, nowadays, are increasingly likely and have implications not only on the economic level (causing the increase in LIBs cost of production), but also on the geopolitical level since the majority of material sources usually lie under politically unstable countries.In 2011, the European Commission (EC) made a list of critical raw materials (CRMs), resources mainly used in energy transition and digital technologies, which were defined by Overland as ‘raw materials for which there are no viable substitutes with current technologies, which most consumer countries are dependent on importing, and whose supply is dominated by one or a few producers’.3 The original list has been updated every three years since it was first drawn up; in March 2023, EC proposed the Critical Raw Material Act, which sets benchmarks for the extraction, processing and recycling of CRMs in Europe at 10%, 40% and 15% of EU's annual consumption, respectively.4 Along with the act, the list of CRMs was updated extending the total number to 37, including Al and Cu (widespread in LIBs industry as current collector materials) and Mn and Ni (two more environmentally and economically sustainable alternatives to Co for cathode manufacturing).5
Another relevant issue addressed in the act concerns the diversification of supplies. In fact ‘[the EU] will never be self-sufficient in supplying raw materials and will continue to rely on imports for a majority of its consumption’;4 therefore, the EC has set an import limit of 65% of the EU's annual consumption of each CRM from a single third country in order to diversify sources of supply and cope with possible shortages due to geopolitical implications. In this respect, for example, the war between Russia and Ukraine may have a significant impact on the LIB market. Ukraine plays a crucial role in the global supply chain of CRMs used in battery production. The country is the eighth largest producer of manganese (Mn ore 2 Mt) and also possesses considerable deposits of nickel (containing estimated reserve of about 215 thousand tons) and cobalt (about 8 thousand tons); it also has one of the largest Li reserves in Europe, although these are currently untapped and probably will be unavailable for some time due to the Russian–Ukrainian conflict.6,7
However, LIBs are complex devices and do not only contain metals and graphite but also valuable fluorinated materials. For example, poly(vinylidene fluoride) (PVDF) is the benchmark binder for the state-of-the-art cathodes due to its outstanding properties, such as safety, chemical stability, good adhesion to the current collectors and excellent free-standing performances.8–14 In this specific case, the PVDF market is expected to grow at a Compounded Average Growth Rate (CAGR) of more than 8% by 2026, reaching the value of US$ 826 million by 2026.15 Additionally, fluorinated lithium salts organic solutions (e.g., 1.0 M LiPF6 in EC/DMC) are used as electrolytes.
In addition to the above-mentioned data and reports, the new EU batteries regulation clearly put into evidence the need to recycle LIBs, which in turn must be done in a safe and sustainable way. In this regard, some crucial elements of the directive cover the restrictions of substances, the carbon footprint reduction of the recycling processes, and increased recycled contents (cobalt 16%, lithium 6% and nickel 6% by 2031), recycling efficiency targets (70% for LIBs by 2030) and material recovery targets (95% for Co, Cu, Ni and 80% for Li by 2031).16
Most of the LIBs are recycled through combined pyro-hydrometallurgic or hydrometallurgic processes consisting of several energy-consuming approaches (i.e., pyrolysis, incineration, smelting, and roasting) that lead to the recovery of only the heavier metals present in the battery (Co, Ni, Fe, Mn, Cu).17 In the past years, the lighter metals (Li, Al) were separated as slug used for building purposes; however, due to the high economic value of lithium and the fact that both elements are critical raw materials, they are currently recovered, respectively, as precursors for new cathode active material synthesis (e.g., Li2CO3) or in their native metallic form.18 More recently, also the recycling of electrolytes and binders has been gathering specific attention with the aim to avoid the loss of economic and technological value, in alignment with the European Waste Hierarchy.19 In the last few years, several papers were published on the sustainable recycling of LIBs components, mainly focused on soft solvometallurgical processes with organic acids for the recycling of the cathode active material (CAM), which is the most precious component of the battery and it is often Co-based.20–29
Since 1950s, supercritical fluids (SCFs) gained the interest of the scientific community for their solvating properties, which can be exploited for extractive or chromatographic purposes. The most interesting SCF is CO2, which is eco-friendly, abundant, cheap, reusable and has critical values that can be easily reached also at the industrial level. This work reviews the use of SCFs (especially CO2) used in LIBs recycling: the first part revisits the basic properties of SCFs, and the design of the instruments used in Supercritical Fluid Extraction (SFE). The second part deals with the latest advances of the SFE technology in the extraction processes of metals, electrolytes, and binders with a specific focus on PVDF.
2. Supercritical fluid extraction
Despite being discovered in 1822 by Charles Cagniard de La Tour and being used for the first time as extraction solvents in 1879 by Hannay and Hogarth, SCFs were not given much consideration until the late 1950s, when numerous papers suggested their use as eluents for chromatography in a plethora of applications.30–32In a few years, the interesting properties of SCFs were studied in depth; in particular, they were (and still are) studied for their remarkable extractive properties, which, nowadays, are applied in a lot of fields, such as food industry33–38 (e.g., decaffeination of coffee, extraction of essential oils from spices), petrochemical industry39–41 (e.g., fractionation of oil) and pharmaceutical industry42–44 (e.g., enantiomeric separation, extraction of drugs from plants, milling). Today, SFE is a relevant industrial technology spanning from the extraction of solids and liquids to polymer processing, supercritical drying and cleaning, and chemical and biochemical reactions.45 The reason is that this kind of extraction has several advantages compared to liquid phase extraction, e.g., greater speed, ease of solvent removal, the possibility of choosing the compounds to be extracted according to the pressure applied, and the possibility of using CO2 as the solvent, which is: (i) convenient from an operational point of view; (ii) economical and (iii) non-toxic.46
2.1 Supercritical fluids
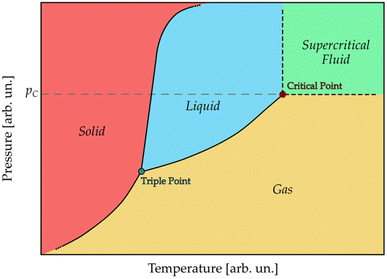
An SCF is a substance that is beyond its critical point, an invariant equilibrium point characterized by a critical temperature and a critical pressure: the critical temperature (Tc) is defined as the temperature above which a substance cannot be liquified, regardless of the pressure applied; the critical pressure (pc) is the pressure required to make a substance liquify at Tc. If both T > Tc and p > pc, a phase transition occurs that leads to a change in the chemical and physical properties of the substance, which will assume characteristics somewhere between those of a liquid and a gas (Table 1);47 this state is called a supercritical state. The dual behavior of SCFs is the reason why, looking at the phase diagram of a substance (Fig. 1), the liquidus line ends in the critical point, beyond which there is only one phase.| Property | Gas | SCF | Liquid |
|---|---|---|---|
| Density [kg m−3] | 0.6–2 | 200–900 | 600–1000 |
| Dynamic viscosity [MPa s] | 0.01–0.3 | 0.1–0.3 | 0.2–3 |
| Diffusivity [106 m2 s−1] | 10–40 | 0.07 | 0.0002–0.002 |
| Surface tension [dyne cm−2] | — | — | 20–40 |
Critical temperature and pressure not only define the critical point but also the critical value of several other variables such as volume, density and compressibility factor: the critical volume (Vc) is the unit volume of a substance at its critical point (for CO2 is 9.4 × 10−2 cm3 mol−1); from Vc, it is possible to determine the critical compressibility factor (Zc), a pure number, which describes the deviation of a real gas from ideal gas behavior, and the critical density (ρc), which can be calculated by means of the gas laws. Table 2 shows the critical parameter values of some of the most used SCFs.48
| Substance | T c [K] | P c [bar] | Z c | ρ c [kg m−3] |
|---|---|---|---|---|
| CO2 | 304 | 74 | 0.274 | 466 |
| H2O | 647 | 221 | 0.235 | 322 |
| C2H6 | 305 | 49 | 0.285 | 203 |
| C2H4 | 282 | 50 | 0.280 | 214 |
| C3H8 | 370 | 43 | 0.281 | 220 |
| NH3 | 406 | 114 | 0.244 | 235 |
| N2O | 310 | 72 | 0.274 | 457 |
| CHF3 | 299 | 49 | 0.259 | 516 |
As the critical point is passed, the meniscus dividing the liquid from the gas disappears and the system switches from two-phases (liquid + gas) to one-phase (fluid). In addition, the phase manifests what is known as critical opalescence; as the critical point is reached, the density of the system takes an intermediate value between that of liquid and that of gas and fluctuate around this value. The continuous density fluctuations have a length comparable to the wavelength of visible light and act as a scattering unit causing critical opalescence due to the Tyndall effect.49
2.2 Principles of SFE
As mentioned above, the field in which SCFs are most widely used is SFE. This technique can be used as a sample preparation step for analytical purposes or, on a larger scale, to remove an unwanted substance from a product or to isolate a desired substance from a matrix.Among the parameters influenced by critical pressure, a crucial one is the so called “solvent strength”, which is related to density. In fact, changes in density are related to changes in solubility and mass transport, and, in turn, to the solvent selectivity. The solvent strength of a SCF could be related to the density by the Hildebrand solubility parameter (δ), which provides a numerical estimate of the degree of interaction between materials and is a good indication of solubility. Materials with similar values of δ are likely to be miscible.50 The Hildebrand parameter is defined as the square root of the cohesive energy density.
where pc is the critical pressure, ρ is the gas density, and ρliq is the liquid density. As the critical pressure is approached, ρ becomes more and more near ρliq, and the solubility parameter increases. Fig. 2 shows the dependence of δ for CO2 from both temperature and pressure in terms of the Span and Wagner equation.53 At pressure lower than 10 MPa, the dependence on temperature is zero or highly non-linear. For pressure higher than 10–15 MPa, the behaviour is nearly linear, and vice versa. This is the key feature for SFE: the solvating power of SCFs can be tuned by small changes in pressure and temperature to favor the dissolution of a target compound.
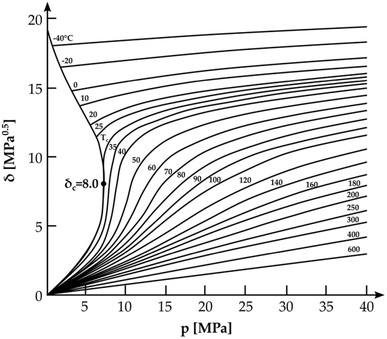 | ||
| Fig. 2 Isothermal curves of the Hildebrand solubility parameter for CO2 as a function of pressure (adapted from ref. 54 with permission of Elsevier). | ||
A competing effect of the density of the solvent is the vapor pressure of the solute, which increases with temperature; depending on which of the two effect is favoured, solubility can ultimately increase or decrease with temperature.55 Given a lower crossover pressure (PL) and an upper crossover pressure (PU), both depending on the components of the system and their ratio, a system shows a positive temperature dependence below PL and above PU (where the vapour pressure effect of the solid is favoured) and a negative temperature dependence between PL and PU (where the density effect of the solvent is favoured). Chimowitz and Pennisi defined mathematically the crossover pressure as the point where the slope of the solubility plot versus temperature changes sign.56 Several efforts have been made through the years to study this behaviour and elaborate a new model to accurately calculate the crossover pressure.57,58
2.3 Solvents
The most used SCF is CO2, which has a critical temperature of 304.4 K and a critical pressure of 73.8 bar; however, there are several other solvents that can be used depending on the desired characteristics, which are gathered in Table 3 for the reader's convenience. Supercritical CO2 has a low polarity; therefore, its efficiency to extract polar compounds is limited; this is the reason why SCFs are often used in combination with co-solvents to improve their extractive properties. Co-solvents, added to the SCF, modify certain characteristics of the supercritical fluid such as polarity, aromaticity, chirality, and the ability to complex metal ions. CO2 is the substance to which co-solvents are most frequently added because they allow this solvent to be used even in circumstances where it would not be the optimal choice; methanol and ethanol are generally added to increase the polarity, aliphatic hydrocarbons to decrease it, toluene to impart aromaticity, [R]-2-butanol to introduce chirality and tributyl phosphate (TBP) to improve the solvation of metal complexes. These co-solvents are usually present in quantities of 5–10 vol%, but 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture can also be used. The effects of co-solvents for scCO2 in Supercritical Fluid Chromatography (SFC) are numerous and were studied in detail by Page;59Table 3 shows the most common co-solvents used for CO2 and their critical parameters.
1 mixture can also be used. The effects of co-solvents for scCO2 in Supercritical Fluid Chromatography (SFC) are numerous and were studied in detail by Page;59Table 3 shows the most common co-solvents used for CO2 and their critical parameters.
| Substance | T c [K] | p c [bar] | ρ c [kg m−3] | δ c [bar0.5] | |
|---|---|---|---|---|---|
| a n.a. = not available. | |||||
| Solvents52 | CO2 | 304.4 | 74 | 470 | 153 |
| H2O | 374.3 | 220 | 322 | 276 | |
| Methanol | 238.8 | 81 | 272 | 182 | |
| Ethene | 283.3 | 51 | 200 | 119 | |
| 1-Butene | 133.3 | 36 | 221 | 106 | |
| 1-Pentane | 196.7 | 34 | 237 | 104 | |
| Hexane | 305.6 | 49 | 200 | 119 | |
| N2O | 309.9 | 73 | 460 | 147 | |
| SF6 | 319.0 | 38 | 730 | 112 | |
| CO2 co-solvents60,61 | Acetone | 508 | 47 | 268 | n.a.a |
| Acetonitrile | 546 | 48 | 240 | n.a.a | |
| Acetic acid | 593 | 58 | 351 | n.a.a | |
| Ethanol | 241 | 61 | 275 | n.a.a | |
| 1-Propanol | 537 | 51 | 273 | n.a.a | |
| 2-Propanol | 508 | 48 | n.a.a | n.a.a | |
| 2-Butanol | 536 | 42 | n.a.a | n.a.a | |
| Diethyl ether | 467 | 36 | 263 | n.a.a | |
| DCM | 510 | 63 | n.a.a | n.a.a | |
| Chloroform | 536 | 5.4 | 516 | n.a.a | |
| Benzene | 562 | 4.9 | 305 | n.a.a | |
| Toluene | 592 | 4.1 | 292 | n.a.a | |
| TBP | 742 | 2.4 | n.a.a | n.a.a | |
The extraction rate of a target component from a matrix can be optimized by studying the best conditions of flow rate and other parameters, such as extraction time and particle size, depending on the parameter controlling the kinetics of the process. A lower flow rate allows the solvent to spend more time around the particles and enhance the rate of extraction in desorption-controlled processes (meaning that static SFE is the best choice); on the other hand, a higher flow rate provide the sample with a large quantity of fresh solvent and enhance the rate of extraction in solubility-controlled processes (meaning that dynamic SFE is the best choice). The difference between static and dynamic extraction will be explained later in this work.
Hawthorne et al. studied the effects of the flow rate on both desorption-controlled and solubility-controlled processes.62 The extraction of fats from potato chips is considered an example of solubility-controlled process; here, desorption kinetics has a little influence, and therefore, the extraction rate is linear until almost 100% of recovery is reached. Fig. 3a shows the curves for the extraction of fat from potato chips at 340 atm and 60 °C (recovery% is referred to the total fat content). The slope of the curves, which is directly linked to the efficiency of extraction, is proportional to the CO2 flow rate and, more precisely, to the total volume of SCF used for the extraction: with a flow rate of 2.6 mL min−1 for 30 min (a total of 78 mL used), 86.9 wt% of fat is extracted; with 1.3 mL min−1 of CO2 for 30 min (39 mL used), the extracted fat is almost exactly the half, 43.8 wt%.62
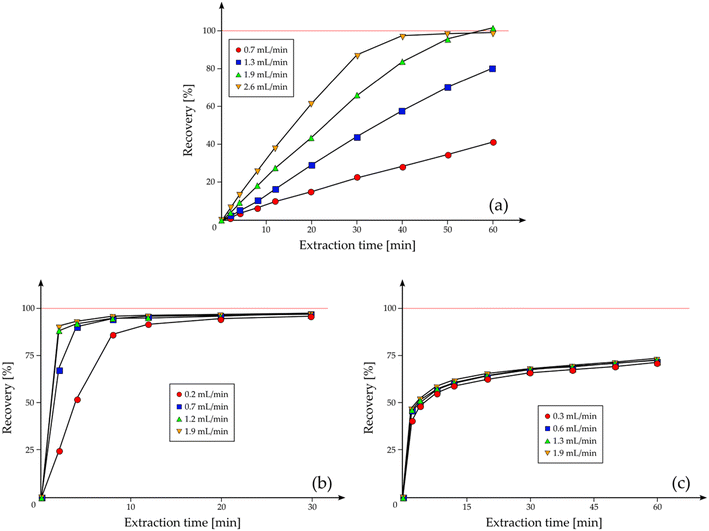 | ||
| Fig. 3 (a) Extraction curves of fats from potato chips as a function of extraction time and flow rates; extraction of fluoranthene from (b) a wood treatment facility soil sample and (c) a railroad bed soil sample showing the strong impact of matrices on the extraction kinetics of the same analyte (adapted from ref. 62 with permission of S. B. Hawthorne, A. B. Galy, V. O. Schmitt, D. J. Miller, Anal. Chem., 1995, 67, 2723. Copyright 2015 American Chemical Society). | ||
In contrast with the behavior of fats in potato chips, several samples show little or no dependence of extraction rates on the CO2 flow rate, and therefore, their kinetics are controlled primarily by the initial desorption step rather than by the solubility step. Hawthorne et al. discussed, as an example, the extraction rates of several PAHs, such as fluoranthene, from a wood treatment facility soil sample (a solubility-controlled process, Fig. 3b) and a railroad bed soil sample (a desorption-controlled process, Fig. 3c), showing how the SFE of the same substance from two different matrices may have different behaviors.62 Concerning the extraction from the wood treatment facility soil sample, the process shows a solubility-controlled process, in which the extraction curves show a trend similar to that found for the extraction of fats from potato chips and, even at low flow rates, they reach 100% recovery if extracted for a sufficiently long period of time; as for the extraction from the railroad bed soil sample, the process is desorption-controlled and the rates decrease smoothly after about 50% recovery. Extending the extraction time has much a less effect on the desorption-controlled processes and, even if the samples are extracted for long periods of time, they may not reach 100% recovery. For these samples, the initial rate of extraction is often fast, followed by a very slow extraction rate for the remaining analytes (around 75% of PAHs separated from the railroad bed soil is extracted in the first 10 min).
2.4 Instrumentation
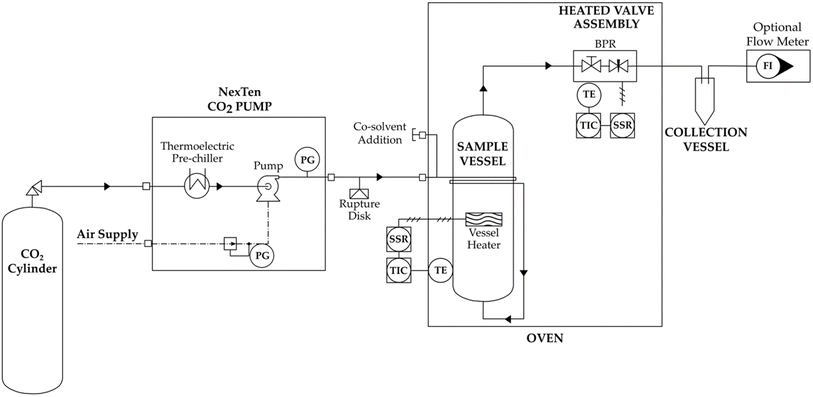
Supercritical fluid extraction systems can be classified, based on their size, into laboratory or industrial scale. Laboratory scale systems produce milligrams to grams of extract using reactors with the volume ranging from 50 to 300 mL; industrial scale systems use reactors with a capacity of several hundred liters and lead to the production of kilos of extract.63Although SFE laboratory-scale instruments (Fig. 4) and industrial plants (Fig. 5) differ substantially in dimensions and components, they have some common elements: a pump, an extraction vessel and a collecting vessel, and pressure control systems incorporated throughout the line (rupture discs, back-pressure regulators, and pressure gauges).
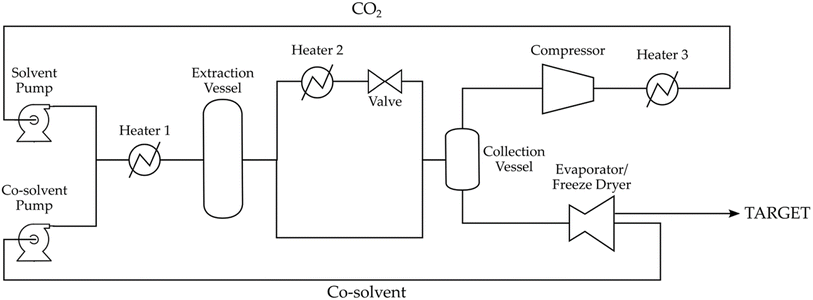 | ||
| Fig. 5 Scheme of an industrial SCF extractor (adapted from ref. 64 with permission from Elsevier). | ||
The pump transfers the pre-chilled CO2 into the extraction vessel and compresses it until it reaches at least pc. For small-scale applications, reciprocating or syringe pumps are used; for larger-scale applications, diaphragm pumps are the best choice. Between the pump and the extraction vessel, the solvent can possibly be mixed with a co-solvent that, as mentioned earlier, improves the extraction properties.
Extraction vessels must be designed to withstand very high pressures since, as mentioned before, the selective extraction of substances relies on the density of the solvent, which is a function of temperature and pressure; so, the actual extraction may require much higher pressures than pc. There is a heating system in the vessel to heat the solvent to at least the critical temperature. The amount of heat supplied by the heating system must also consider the temperature drops due to the adiabatic expansion of CO2 when pumped into the vessel.
The collecting vessel of small-scale systems is like the extraction one, but the pressure is lower so that the solvent density drops and the extract precipitates. In pilot or industrial systems, the collection of the extracted solutes is done either by rapidly reducing the pressure, or by increasing the temperature, or both. Also, traps (e.g., solid trap, liquid trap, and cool trap) can be inserted in the collection vessel to adsorb volatile molecules.
Concerning the extraction step, samples can be extracted in: (i) static mode, consisting in filling the vessel with CO2 to a chosen pressure, then stopping the flow of gas and keeping the system closed for a given time (it is the most frequently used extraction mode on small scale plants); (ii) dynamic mode, which consists of bringing the system to a chosen pressure, then continuously flushing the extract-containing CO2 while pumping fresh solvent inside the extraction vessel to keep the pressure stable (this method is mainly used on medium and large scales); a combination of both can also be used.
Industrial scale SFE generally uses dynamic mode, with the supercritical solvent flowing through the solid until the substrate is depleted; liquid samples on industrial scale SFE and laboratory scale SFE systems are commonly extracted in the static mode.52
3. SFE in LIBs recycling
Nowadays, robust LIBs recycling processes that can cope with the enormous growth in production that these devices will undergo must be designed. It is necessary, on the one hand, to gather ideas about what has been discovered about the recycling of LIBs65 and, on the other hand, to blaze new trails that lead to the recycling of batteries and the production of secondary raw materials comparable to the primary ones in a more sustainable way, both from an environmental and economic point of view.SCFs are gaining the interest of the scientific community because they can be used to recycle different components from LIBs. Main applications in this field are the extractions of the electrolyte, but pioneering studies on the extraction of metals and binder from the cathode can be found.
3.1 Electrolyte
The first proposal for recycling the electrolyte from a LIB with SCF was filed in 2002 by Sloop as a patent application, which was granted in 2007.66 The procedure, which was not fully disclosed in the description of the invention, was intended to be applied to several electrolyte salts (e.g., LiPF6, LiAsF6, LiBF4, LiClO4, lithium pentafluoro-thio-difluoro-methane sulfonated, lithium bis-perfluoro-ethane-sulfonimide, lithium bis-trifluoro-sulfonimide, lithium trifluoro-methane-sulfonate, and lithium trifluoro-methane-sulfonyl-methide) dissolved in a solvent selected from a group consisting of dimethoxy ethane (DME), dimethyl carbonate (DMC), diethyl carbonate (DEC), dipropyl carbonate (DPC), dioxolane, ethyl methyl carbonate (EMC), ethylene carbonate (EC), and propylene carbonate (PC) or a mixture of them. Despite being the first document about electrolyte recycling with SCF, Sloop did not give detailed information about the procedure.Electrolyte extraction via supercritical CO2 is at an early stage and the state-of-the-art is still limited. Some promising results are currently described in the literature, as discussed in the following. However, some discrepancy is found on the recovery rates demonstrated in the available articles, which is likely due to the different operating conditions used in the proposed extraction methods.
In 2014, Grützke et al. reported an electrolyte extraction procedure using Supercritical Helium Head Pressure Carbon Dioxide (scHHPCO2) on a separator soaked with LP30 (LiPF6 1 M in DMC/EC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and LP50 (LiPF6 1 M in EMC/EC 1
1) and LP50 (LiPF6 1 M in EMC/EC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and on a 18650-type LIB.67 They concluded that recovery rates and extract compositions are strongly dependent on the material from which the electrolyte is extracted from and that, if applied to real LIBs, the procedure leads to the recovery of the solvents only, with traces of LiPF6. In a 2015 article from the same author, a solubility-controlled process for the extraction of LIBs electrolyte with scCO2 and liquid CO2 with the addition of co-solvents (e.g., acetonitrile, ACN, and PC) was developed in order to improve the extraction yields.68 The best results (89.1 wt%) were achieved by extracting for 30 minutes with liquid subcritical CO2 (25 °C, 60 bar) and an ACN/PC 3
1) and on a 18650-type LIB.67 They concluded that recovery rates and extract compositions are strongly dependent on the material from which the electrolyte is extracted from and that, if applied to real LIBs, the procedure leads to the recovery of the solvents only, with traces of LiPF6. In a 2015 article from the same author, a solubility-controlled process for the extraction of LIBs electrolyte with scCO2 and liquid CO2 with the addition of co-solvents (e.g., acetonitrile, ACN, and PC) was developed in order to improve the extraction yields.68 The best results (89.1 wt%) were achieved by extracting for 30 minutes with liquid subcritical CO2 (25 °C, 60 bar) and an ACN/PC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture flow-rate of 0.5 mL min−1. Indeed, the addition of co-solvents to the CO2 significantly improved the recovery of LiPF6. Both the extractions with scHHPCO2 (40 °C, 120 bar) and the subcritical CO2/ACN mixture (25 °C, 60 bar) were used by Rothermel and Grützke on a 18650-type LIB anode;69 although the experiment led to the separation of the electrolyte from the electrode, the aim of the work was to purify the anode material, and therefore, no characterization on the electrolyte was carried out.
1 mixture flow-rate of 0.5 mL min−1. Indeed, the addition of co-solvents to the CO2 significantly improved the recovery of LiPF6. Both the extractions with scHHPCO2 (40 °C, 120 bar) and the subcritical CO2/ACN mixture (25 °C, 60 bar) were used by Rothermel and Grützke on a 18650-type LIB anode;69 although the experiment led to the separation of the electrolyte from the electrode, the aim of the work was to purify the anode material, and therefore, no characterization on the electrolyte was carried out.
Simultaneously, Liu et al. reported the extraction of the electrolyte at various conditions: pressure ranging from 150 to 350 bar, temperature between 40 °C and 50 °C, static extraction time within 45 to 75 min.70 The optimal conditions for extraction yield were 230 bar, 40 °C and 45 min. However, the analyses conducted on the recovered product suggested the hydrolyzation of LiPF6 during the extraction process, leading to the formation of several by-products such as POF3, [PO2F2]−, [PO3F]2−, [PO4]3−, [HPO4]2−, [H2PO4]−, H3PO4 and a huge amount of dangerous HF.
Concerning the solvents for electrolyte, Liu et al. studied the composition of the products obtained by extracting a LIB separator imbibed with a carbonate mixture of EC/DMC/EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mimicking the solvent of one of the most widely deployed electrolytes in LIBs.71 Extractions were conducted under pressure ranging from 150 to 350 bar, temperature between 30 and 50 °C and dynamic extraction time of 25–65 min. The samples extracted were analyzed with a gas chromatography flame ionization detector (GC-FID) to study the solvent extraction behavior depending on pressure, temperature, and time. Concerning pressure, most of the extract was obtained in the initial stage when the pressure was low, and it gradually increased with pressure, mainly due to an EC extraction yield enhancement (from 90 wt% to 98 wt%), whereas the DMC and EMC extraction yields remained constant. The trend of overall extraction yield of temperature-resolved experiment was identical to that of the pressure-resolved ones, except for the behavior of EC, whose extraction decreased when temperature rose (from 95 wt% to 89 wt%). Finally, the overall extraction yields clearly improved by prolonging the exposed time, although extending the extraction time may cause unwanted loss of extracts from the collection vessel. Liu et al. concluded that the extraction of carbonates was determined by the polarity of the solvent, suggesting the use of polar co-solvents to modify this SCF property.
1 mimicking the solvent of one of the most widely deployed electrolytes in LIBs.71 Extractions were conducted under pressure ranging from 150 to 350 bar, temperature between 30 and 50 °C and dynamic extraction time of 25–65 min. The samples extracted were analyzed with a gas chromatography flame ionization detector (GC-FID) to study the solvent extraction behavior depending on pressure, temperature, and time. Concerning pressure, most of the extract was obtained in the initial stage when the pressure was low, and it gradually increased with pressure, mainly due to an EC extraction yield enhancement (from 90 wt% to 98 wt%), whereas the DMC and EMC extraction yields remained constant. The trend of overall extraction yield of temperature-resolved experiment was identical to that of the pressure-resolved ones, except for the behavior of EC, whose extraction decreased when temperature rose (from 95 wt% to 89 wt%). Finally, the overall extraction yields clearly improved by prolonging the exposed time, although extending the extraction time may cause unwanted loss of extracts from the collection vessel. Liu et al. concluded that the extraction of carbonates was determined by the polarity of the solvent, suggesting the use of polar co-solvents to modify this SCF property.
In 2017, Liu et al. carried out the first successful extraction of an electrolyte from quality control samples of 40 Ah Li(NixMnyCoz)O2 (NMC) prismatic cells.72 They dismantled the spent LIBs, transferred them in the extraction vessel and extracted the electrolyte with a static extraction at 40 °C and 150 bar for 10 min, followed by dynamic extraction with a constant flow rate of 2.0 L min−1; the best achieved yield was about 88 wt%; the product was characterized and showed ionic conductivity comparable to commercial electrolyte (0.19 mS cm−1 at 20 °C). In a subsequent work, the same authors optimized the conditions by also introducing subcritical CO2 extraction, which proved to be a better extraction solvent than scCO2 (extraction rates about 95 wt% and 85 wt%, respectively).73 Data obtained from several experiments were fitted with a proper model and optimal conditions were found to be 28.86 °C temperature, 88.4 bar pressure and 9.77 min extraction time. Later, Mu et al. combined the electrolyte extraction from spent LIBs using scCO2 with the exfoliation of the cathode material: the optimal conditions for the experiment were found to be 38 °C temperature, 100 bar pressure and 15 min extraction time and led to a delamination efficiency of 99 wt% and a high-purity electrolyte.74
Finally, Niu et al. suggested to implement the use of scCO2 in the industrial treatment of LIB by coupling the extraction of electrolyte with the discharging of the battery.75 This could prevent the use of salt-saturated solutions that can corrode the steel cover of the batteries and cause the leakage of the electrolyte into the solution; this unfortunate event leads to the loss of the recyclable material and, potentially, to the damaging of the instrumentation due to the evolution of HF from the reaction of LiPF6 with water.75 The relevant results obtained in the works mentioned above are summarized in Table 4.
| Year | Electrolyte salt | Co-solvent | T [°C] | P [bar] | T [min] | Yield [wt%] | Extracted compounds | Reference |
|---|---|---|---|---|---|---|---|---|
| a Experiment was carried out using scHHPCO2 technique. b Experiment was carried out using subcritical liquid CO2. c Optimal operating conditions were extrapolated from a model based on the extraction tests conducted. | ||||||||
| 2002 | LiPF6, LiAsF6, LiBF4, LiClO4 | — | Not disclosed | Not disclosed | Not disclosed | Not disclosed | Not disclosed | 66 |
| 2014a | LiPF6 | — | 40 | 120 | 90 | 73.5 | DMC, EC, LiPF6 (traces) | 67 |
| 2014 | LiPF6 | — | 40 | 230 | 45 | 85.1 | DMC, EMC, VC, EC, 1,11-biphenyl, LiPF6 (traces) | 70 |
| 2015b | LiPF6 | ACN/PC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 | 60 | 30 | 89.1 | DEC, PC, EC, LiPF6 (traces) | 68 |
| 2016a | LiPF6 | — | 40 | 120 | 90 | — | — | 69 |
| 2016b | LiPF6 | ACN/PC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 | 60 | 30 | — | — | 69 |
| 2017 | LiPF6 | — | 40 | 150 | 10 | 85 | EMC, DEC, EC, VC, LiPF6 (traces) | 72 |
| 2017a,c | LiPF6 | — | 28.86 | 88.4 | 9.77 | — | PC, EC, EMC, DMC, LiPF6 (traces) | 73 |
| 2022 | LiPF6 | — | 38 | 100 | 15 | — | EMC, VC, DEC, EC | 74 |
3.2 Metals
The first studies on the extraction of metals with SCFs are attributed to Wai et al., who showed how scCO2 can be used as a solvent to extract elements from both solid and liquid matrices when fluorine-substituted organic ligands are used, such as bis-trifluoro-ethyl-dithio-carbamate for transition metals and non-metals, fluorinated β-diketones and TBP for lanthanides and actinides, triazole-containing crown ethers for heavy metals.76–80 Although a huge part of these studies was focused on the recovery of metals from environmental matrices in aqueous solutions, they introduced the use of SCFs in metal recovery.Erkey pointed out the need to develop new ligands to be used as complexing agents in organometallic catalysts to make them more soluble in SCF and help their recovery by means of SFE or to add modifiers to the SCFs to improve their solvation power towards organometallic catalysts.81 Although some advances in finding the best operating conditions and suitable ligands for SFE were reported by Lin et al., the problem became that of finding non-toxic, non-polluting, high-selective chelating agents.82
Basing their work on studies conducted on the recovery of precious metals from printed circuit boards using supercritical water,83–86 Bertuol et al. successfully recovered cobalt from LIBs.87,88 The authors performed acid leaching tests and scCO2 extraction (75 bar, 75 °C) using H2SO4 and H2O2 as co-solvents in both cases. The use of SCFs led to an increase in the Co extraction efficiency from 87 wt% to more than 95 wt%, a reduction of the extraction time from 60 to 5 min, and a reduction in the concentration of H2O2 required from 8 vol% to 4 vol%, compared to the acid leaching at atmospheric pressure.
Although, after 2016, some efforts were made to recover strategic metals from several end-of-life technological devices with SCF (e.g., rare earth elements from NiMH batteries, NdFeB magnets and fluorescent lamps, indium from liquid crystal displays and silver from e-wastes),89–93 their application to LIBs recycling did not progress until 2022, when Zhang and Azimi studied a scCO2 extraction process with TBP–HNO3 adducts as a complexing agent to recover Li, Co, Ni, and Mn from EoL LIBs;94 the reducing effect of H2O2 was also studied in some tests by adding 4 mL of 30% (w/v) H2O2 to the reactor. The extraction results varied between 60% and 80% among 8 tests, and the maximum extraction efficiencies were 70%, 74%, 76% and 67% for Li, Co, Mn, and Ni, respectively, at 40 °C and 310 bar, 60 min extraction time and a ligand concentration of 5 mL g−1 of metal. Zhang and Azimi concluded that: (i) the SFE method was able to improve the extraction efficiency of Li, Ni, Mn and Co compared with conventional leaching processes; (ii) the consumption of chemicals and the extraction time were sensibly lower; (iii) the reducing agent H2O2 can significantly improve the extraction process by reducing Co, Mn, and Ni to +2 oxidation state, which can form stable complexes with TBP–HNO3 adduct that are soluble in scCO2. The relevant results obtained in the works mentioned above are summarized in Table 5.
3.3 Polymers
Nowadays, SCFs are widespread in the field of polymer science because they can be used as a medium for polymerization in order to speed it up or as a medium for foaming processes to obtain products with peculiar morphologies.95–99 Concerning SFE, this technique can be used for the extraction of substances from a polymeric matrix (e.g., plasticizers and dyes) or extraction of a polymer from organic/inorganic matrices.100Some pioneering studies were conducted on the fractionation of polymers in ‘60s and ‘70s; however, the process seemed to be most effective for the fractionation of oligomers and for the removal of low molecular weight species from polymers.101 In 1999, Merz and Muth reported the extraction of thermoplastic polymeric binders from injection molded workpieces composed by 60 vol% of solid matrix (metal or ceramic) and 40 vol% of binder.102 They found that at 50 °C, the extraction efficiency was very low, while between 60 °C and 70 °C, the extraction was more efficient. They concluded that during an isothermal extraction, the variation of pressure had only a small influence on the extraction rate, whereas the variation of temperature during isobaric extractions led to great differences in the extraction efficiency.
When dealing with the SFE of polymers, the operating conditions and the recovery yield of polymers are deeply influenced by the molar mass of the substance to be extracted. Indeed, polymer chains with higher molar mass tend to be less volatile and less prone to be extracted with a SCF than chains with lower molar mass. To fully recover a polymer, regardless of its dispersity, it is necessary to use operating conditions that allows to extract even the longer polymer chains. However, dispersity can also be used as an advantage to fractionate the polymer and recover a fraction of the specific molar mass. This can be done with two different approaches depending on the extraction setup used: isothermal decreasing pressure profiling (patented by Hunter and Richards in 1945) and isothermal increasing pressure profiling.103–105
Isothermal decreasing pressure profiling consists of a sequential pressure reduction to fractionate the polymer. A polymer with a broad molecular weight distribution is placed into the extraction vessel (Fig. 6), and the SCF is pumped inside. The extracted phase exiting the vessel contains the polymer fraction with a molar mass dependent on the solubility characteristics of the polymer chains according to the operating parameters of the extraction vessel. Based on these operating conditions, the heaviest fraction of the polymer can be left undissolved inside the extraction vessel and recovered after the extraction. All the chains with a mass below the heaviest chain are pumped in the first separation vessel, where temperature and pressure are slightly different and cause the second heaviest fraction of the polymer to precipitate. The remaining solution is then transferred in a second separation vessel to precipitate the third heaviest fraction, and so on. The polydispersity of each fraction can be reduced to very low values if small pressure decrements are taken between each pressure level: the smaller the decrement, the less the amount of each fraction recovered (albeit with low polydispersity index).
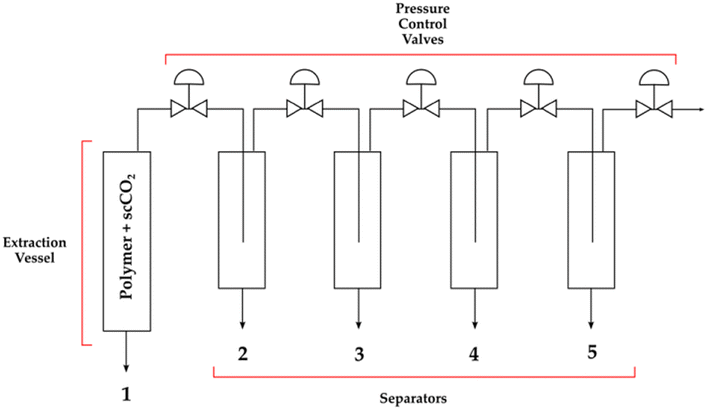 | ||
| Fig. 6 Schematic setup of an extractor for isothermal decreasing pressure profiling of a polymer (the figure is a free adaptation from a generic extractor scheme). | ||
Concerning isothermal increasing pressure profiling, the simple lab-scale apparatus shown in Fig. 4 can be used for this purpose. An amount of polymer is charged to the extraction vessel and the extraction is carried out by increasing pressure stepwise. A low pressure is first used, and the extraction is continued to the highest pressure until no more polymer fraction is collected or until some maximum operating pressure is reached.
As mentioned before, temperature and pressure define solvent density, which is related to the extraction efficiency. Clifford et al. studied the fractionation of polyisobutene and polydimethylsiloxane both at constant density and with a linear density program.106 Concerning polyisobutene, they preliminarily extracted the material at 50 °C and 130 bar to get rid of the lower mass oligomers; then, they proceeded by extracting the polymer with a linear density program from 636 kg m−3 to 1011 kg m−3 at the rate of 6.32 kg m−3 min−1. They extracted eight fractions at equal density intervals of 45 kg m−3 and with a number-averaged molar mass (calculated as the ratio of the oligomer molar mass to the monomer unit molar mass) between 342 and 1528 with a total yield of 52 wt%.
One of the first uses of SFE for recycling purposes was reported in 2017 by Calgaro et al., who successfully experimentally designed the extraction of polymers from PCBs using CO2 and ethanol.107 As expected, the studies showed that temperature and pressure were statistically significant in the extraction process, and the highest extraction percentage (69.5 wt%) was obtained at 70 °C and 75 bar.
PVDF is commonly used as a binder in the cathodes and anodes of LIBs. Its function is to bind together CAM and carbon black particles and make them adhere to the current collector. Due to the several applications mentioned before, PVDF is a technologically relevant material; therefore, industries producing this polymer are becoming more and more interested in recycling it as a part to transition to circular economy.13,108,109
In the first study on the solubility of PVDF in scCO2, Rindfleisch et al. reported the insolubility of the polymer even at 300 °C and 2750 bar;110 the reason for this behavior was found in the high cross-linking degree of the material and the high molar mass of the sample (MW = 5.3 × 105 g mol−1) due to the polymerization process.
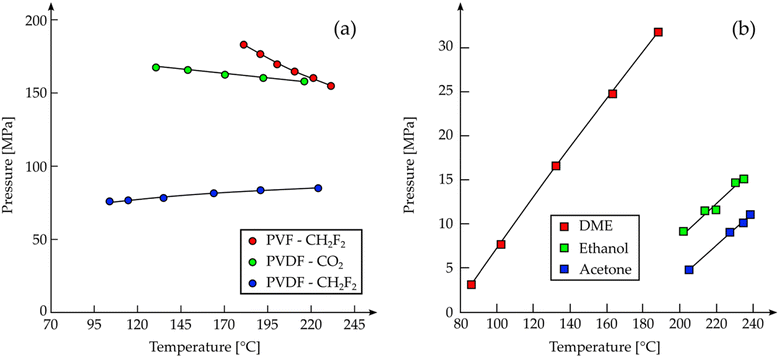
However, based on several evidences111–115 that CO2 can actually dissolve fluoropolymers or co-polymers containing fluorinated groups, Lora et al. re-examined the solubility of PVDF (and polyvinyl fluoride, PVF) in supercritical CH2F2 (or Freon-32™) and scCO2.116 They reported the dissolution of 5 wt% of PVDF (MW = 2.0 × 105 g mol−1) in CH2F2 at a temperature between 100 °C and 225 °C and a pressure between 750 bar and 900 bar, while dissolution in CO2 required pressures above 1600 bar and temperatures between 130 °C and 215 °C. They concluded that a polar solvent is needed to dissolve these polymers, suggesting that this is the reason why the cloud points of PVF and PVDF are lower in CH2F2 than CO2 (the PVDF being more soluble than PVF, as shown in Fig. 7), supporting the considerations of Kazarian et al. regarding the donor–acceptor interaction between the polymer and the solvent.117 In fluoropolymer extraction with SCF, both CH2F2 and CO2 act as electron-acceptors but CH2F2 is a better acceptor than CO2; thus, the resulting interaction is stronger and the cloud point temperature and pressure are lower.
Since CH2F2 does not contain any chlorine atom, it is not dangerous for the ozone layer; it is however a potent greenhouse gas (GWP-20: 2690, GWP-100: 771, GWP-500: 220)118 and it is extremely inflammable when mixed with air. This is one of the reasons why it is preferable to add co-solvents to CO2 to improve its polarity, both from the environmental point of view and the safety one. Lora et al. tested acetone, dimethyl ether, and ethanol as co-solvents in order to improve the CO2 solvating power toward PVDF.116 Among the three investigated co-solvents, they reported the acetone to be the best one, capable of lowering the pressure of the cloud point of PVDF in CO2 of at least one order of magnitude (Fig. 7b).
Dinoia et al. further investigated the solubility of PVDF in several SCFs: CO2, CHF3, CH2F2, CHClF2, CClF3, CH3CHF2, CH2FCF3, CHF2CF3, and CH3CClF2, reporting the cloud point temperature and pressure up to 250 °C and 3000 bar, respectively.119 Concerning scCO2, they concluded that: (i) the weight of the polymers has a low influence on its solubility; (ii) as pointed out by Kazarian et al., scCO2 forms a weak complex with the fluorine atom in the VDF repeat unit, which enhances the solvent power of CO2 (especially at low temperatures);117 (iii) CO2 has a very low polarizability; therefore, it is a poor solvent for PVDF at high temperatures where non-polar dispersion interactions are predominant with respect to polar interactions. The solubility of PVDF was then compared to that of the co-polymer P[VDF78-co-HFP22], which showed lower cloud-point pressures in all solvents. The introduction of a co-monomer into the polymeric backbone of PVDF dramatically lowers the cloud point so that pressures of 400–1000 bar are sufficient to dissolve P[VDF78-co-HFP22].120
Fahmy studied the polymerization and crystallization processes of PVDF in scCO2;121 as a side-result to his work, he found out that a low concentration of the relative monomer VDF (<42 wt%) can decrease the solubility of PVDF in scCO2, while at higher concentration (>52 wt%), the situation is the opposite (Fig. 8).
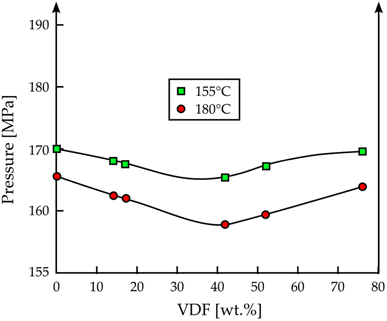 | ||
| Fig. 8 VDF concentration effect on cloud point pressure at 155 °C (green) and 180 °C (red) for PVDF (adapted from S. M. Fahmy, PhD thesis, RWTH Aachen University, 2005 (ref. 121). No permission required). | ||
The studies on the dissolution of PVDF continued in 2021 with Fu et al., who applied the SFE with CO2 to the extraction of the binder from spent LIBs cathodes.122 The experimental conditions were optimized by extracting pure PVDF in the pressure range 40–90 bar and 40–90 °C temperature range for different extraction times (4–17 min); the results indicated that 98.5 wt% of pure PVDF was dissolved using a scCO2-DMSO system under the optimal conditions of 70 °C temperature, 80 bar pressure, and 13 min process time. The same conditions were used to recover PVDF from actual cathodes: Fu et al. characterized the recovered PVDF by means of TGA, FTIR and SEM, which showed no major difference from the raw PVDF used as the reference (Fig. 9).
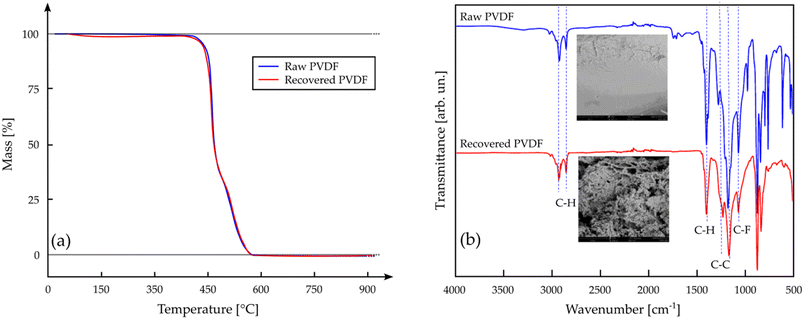 | ||
| Fig. 9 Characterization of the PVDF recovered by Fu et al. (red plots) and comparison with raw PVDF (blue plots) as a reference by means of (a) TGA and (b) FT-IR analyses (adapted from ref. 122. Y. Fu, J. Schuster, M. Petranikova and B. Ebin, Resour., Conserv. Recycl., 2021, 172, 105666. No permission required. License: CC BY 4.0, Elsevier). | ||
SFE was also successful to delaminate the cathode active materials from the current collector. Mu et al. used SFE with CO2 to exfoliate cathodes, extract the electrolyte and free the CAM.74 Mu et al. evaluated the effect of the single parameters on the exfoliation of the material (Fig. 10): both pressure and extraction time showed a peak in the peeling-off efficiency at 100 bar and 15 min, respectively, while temperature did not show any inflection point, at least in the examined range, and the efficiency could increase as temperature rises. However, since the purpose of Mu et al. was also the electrolyte recovery, they stopped at 38 °C because “[we] had reached a conclusion in our published study that the recovered ratio of electrolyte decreased with an increase of temperature”.74 They concluded that the separation of cathode material from the Al current collector was the result of the weakening of the adhesion between the materials of the cathode, which is attributed to the removal of the organic binder.
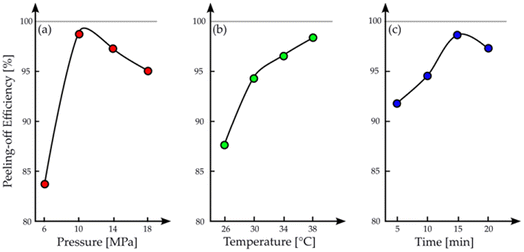 | ||
| Fig. 10 Effect of (a) pressure, (b) temperature and (c) time on the exfoliation efficiency with scCO2. All graphics adapted from the work of Mu et al.74 with permission from John Wiley and Sons. | ||
In 2022, a patent application on the procedure for purifying PVDF with scCO2 was filed.123 The authors claimed a method comprising the washing of a PVDF with a SCF at 100–1000 bar (preferably 200–600 bar) and 20–200 °C (preferably 50–170 °C), a flow-rate of SCF of 1–30 kg h−1 per kg of PVDF and the addition of a co-solvent, if necessary (preferably H2O or EtOH). The whole process takes 1–12 h (preferably 3–10 h). The relevant data on PVDF extraction are reported in Table 6.
| Year | SCF | Co-solvent | T [°C] | P [bar] | T [min] | Reference |
|---|---|---|---|---|---|---|
| a Values in brackets are best mode operating conditions. | ||||||
| 1999 | CO2 | — | 130–215 | 1600 | — | 116 |
| 1999 | CO2 | Acetone, EtOH | 200–250 | 50–150 | — | 116 |
| 1999 | CO2 | DME | 50–300 | 80–200 | — | 116 |
| 1999 | CH2F2 | — | 100–225 | 750–900 | — | 116 |
| 2021 | CO2 | DMSO | 70 | 80 | 13 | 122 |
| 2022 | CO2 | — | 38 | 100 | 15 | 74 |
| 2022a | Any (CO2) | Any (H2O or EtOH) | 20–200 (50–170) | 100–1000 (200–600) | 60–720 (180–600) | 123 |
4. Conclusions
The use of CO2 as a SCF has significant advantages, such as non-toxicity, non-flammability, inertness, and ease of handling. Moreover, CO2 is cheap, abundant, easily removable from the final products, and reusable. Its critical values can also be easily reached, and this makes such technology easily scalable at the industrial level. This last aspect is the major advantage with respect, for example, to H2O, whose supercritical parameters (about 374 °C and 22 MPa) are more difficult to implement at the industrial level. The application of SFE in lithium battery recycling, particularly focusing on binder extraction, presents a promising avenue for sustainable and efficient processes. This innovative approach not only addresses environmental concerns associated with traditional methods but also offers a valuable opportunity to recover and reuse materials. Utilizing SFE, the extraction of binders becomes highly effective, ensuring a cleaner and more resource-efficient recycling process. The removal of the electrolyte increases the safety of the entire recycling process, including the battery deactivation step, since flammable compounds are removed. Furthermore, the extraction of both electrolyte and binder leads to a decrease of the fluorine content in the black mass, which is beneficial in the metallurgical treatment of the black mass itself. Embracing this technology not only contributes to the circular economy but also enhances the viability of lithium batteries as a key component in future energy storage solutions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding from the European Union's Horizon Europe research and innovation program under Grant Agreements No. 101137745 (RENOVATE) and 101104022 (BATTERY2030 CSA3). Additionally, EQ and PM thank Regione Lombardia (IT) for the economic support in the context of the institutional agreement in the establishment of EcoCirc facilities for a “System integrator towards circular economy”.References
- International Energy Agency, https://www.iea.org/data-and-statistics/charts/lithium-ion-battery-manufacturing-capacity-2022-2030, accessed October 2023.
- V. Marpu, J. Prakhar and P. Yerukola, Allied Market Research, Report A01071, 2023 Search PubMed.
- I. Overland, Energy Res. Soc. Sci., 2019, 49, 36 CrossRef.
- European Commission, Proposal for a Regulation of the European Parliament and of the Council Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020 Search PubMed.
- European Commission, Annexes to the Proposal for a Regulation of the European Parliament and of the Council Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020 Search PubMed.
- Ukraine Geological Survey, Investment opportunities in exploration & Production, Strategic and Critical raw Materials, https://www.ageo.gov.ua/en/open-bids, accessed March 2024 Search PubMed.
- G. Garity, https://kleinmanenergy.upenn.edu/news-insights/lithium-the-link-between-the-ukraine-war-and-the-clean-energy-transition/, accessed October 2023.
- K. Aleksandrov, H.-J. Gehrmann, M. Hauser, H. Mätzing, D. Pigeon, D. Stapf and M. Wexler, Chemosphere, 2019, 226, 898e906 CrossRef PubMed.
- S. H. Korzeniowski, R. C. Buck, R. M. Newkold, A. El kassmi, E. Laganis, Y. Matsuoka, B. Dinelli, S. Beauchet, F. Adamsky, K. Weilandt, V. K. Soni, D. Kapoor, P. Gunasekar, M. Malvasi, G. Brinati and S. Musio, Integr. Environ. Assess. Manage., 2022, 19, 326 CrossRef PubMed.
- B. Ameduri and H. Hori, Chem. Soc. Rev., 2023, 52, 4208 RSC.
- B. Ameduri, J. Fluorine Chem., 2023, 267, 110117 CrossRef CAS.
- B. Ameduri, Molecules, 2023, 28, 7564 CrossRef CAS PubMed.
- B. Ameduri, Macromol. Chem. Phys., 2020, 221, 1900573 CrossRef CAS.
- T. Nshizirungu, M. Rana, M. I. H. Khan, Y. T. Jo, S.-J. Park and J.-H. Park, J. Environ. Chem. Eng., 2023, 11, 109160 CrossRef CAS.
- Industry Arc, Report CMR0519, 2023, https://www.industryarc.com/Report/16139/polyvinylidene-fluoride-market.html Search PubMed.
- European Parliament and European Council, Regulation (EU) 2023/1542 of the European Parliament and of the Council of 12 July 2023 concerning batteries and waste batteries, amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and repealing Directive 2006/66/EC, Official Journal of the European Union L191, 2023 Search PubMed.
- Z. J. Baum, R. E. Bird, X. Yu and J. Ma, ACS Energy Lett., 2022, 7, 712 CrossRef CAS.
- N. Wei, Y. He, G. Zhang, Y. Feng, J. Li, Q. Lu and Y. Fu, J. Environ. Manage., 2023, 329, 117107 CrossRef CAS PubMed.
- European Parliament and European Council, Directive 2008/98/EC of the European Parliament and Council of 19 November 2008 on waste and repealing certain Directives, Official Journal of the European Union L312, 2008 Search PubMed.
- X.-X. Zhao, X.-T. Wang, J.-Z. Guo, Z.-Y. Gu, J.-M. Cao, J.-L. Yang, F.-Q. Lu, J.-P. Zhang and X.-L. Wu, Adv. Mater., 2024, 2308927 CrossRef CAS PubMed.
- M. Du, J.-Z. Guo, S.-H. Zheng, Y. Liu, J.-L. Yang, K.-Y. Zhang, Z.-Y. Gu, X.-T. Wang and X.-L. Wu, Chin. Chem. Lett., 2023, 34, 107706 CrossRef CAS.
- Y.-F. Meng, H.-J. Liang, C.-D. Zhao, W.-H. Li, Z.-Y. Gu, M.-X. Yu, B. Zhao, X.-K. Hou and X.-L. Wua, J. Energy Chem., 2022, 64, 166 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, K. J. McKenzie and S. U. Obi, J. Chem. Eng. Data, 2006, 51, 1280 CrossRef CAS.
- D. Pant and T. Dolker, Waste Manage., 2017, 60, 689 CrossRef CAS PubMed.
- L. Li, Y. Bian, X. Zhang, Y. Guan, E. Fan, F. Wu and R. Chen, Waste Manage., 2018, 71, 362 CrossRef CAS PubMed.
- G. Zante, A. Braun, A. Masmoudi, R. Barillon, D. Trebouet and M. Y. Boltoeva, Miner. Eng., 2020, 156, 106512 CrossRef CAS.
- R. Morina, D. Callegari, D. Merli, G. Alberti, P. Mustarelli and E. Quartarone, ChemSusChem, 2022, 15, 202102080 CrossRef PubMed.
- C. Padwal, H. D. Pham, S. Jadhav, T. T. Do, J. Nerkar, L. T. M. Hoang, A. K. Nanjundan, S. G. Mundree and D. P. Dubal, Adv. Energy Sustainability Res., 2022, 3, 2100133 CrossRef CAS.
- P. Cattaneo, D. Callegari, D. Merli, C. Tealdi, D. Vadivel, C. Milanese, V. Kapelyushko, F. D'Aprile and E. Quartarone, Adv. Sustain. Syst., 2023, 7, 2300161 CrossRef CAS.
- B. Berche, M. Henkel and R. Kenna, Adv. Sustainable Syst., 2009, 13, 3001 Search PubMed.
- J. B. Hannay and J. Hogarth, Proc. R. Soc. Lond., 1879, 29, 324 CrossRef.
- D. F. Williams, Chem. Eng. Sci., 1981, 36, 1769 CrossRef CAS.
- T. Fornari, Supercritical CO2 Extraction: Relevance to Food Processing, Reference Module in Food Science, Elsevier, 2016 Search PubMed.
- K. Zosel and Studiengesellschaft Kohle mbH, US Pat., US3806619, Filed: 3rd May 1972.
- K. Ramalakshmi and B. Raghavan, Crit. Rev. Food Sci. Nutr., 1999, 39, 441 CrossRef CAS PubMed.
- P. Hubert and O. G. Vitzthum, Angew. Chem., 1978, 17, 710 CrossRef.
- E. Reverchon, J. Supercrit. Fluids, 1996, 10, 1 CrossRef.
- E. Reverchon and I. De Marco, J. Supercrit. Fluids, 2006, 38, 146 CrossRef CAS.
- T. H. Gouw, R. E. Jentoft and E. J. Gallegos, in High-Pressure Science and Technology, ed. K. D. Timmerhaus and M. S. Barber, Springer, Boston (MA), 1979, pp. 583–592 Search PubMed.
- R. M. Campbell and M. L. Lee, Anal. Chem., 1986, 58, 2247 CrossRef CAS.
- G. Yang and R. A. Wang, J. Pet. Sci. Eng., 1999, 22, 47 CrossRef CAS.
- M. Herrero, J. A. Mendiola, A. Cifuentes and E. Ibáñez, J. Chromatogr. A, 2010, 1217, 2495 CrossRef CAS PubMed.
- C. G. Pereira and M. A. A. Meireles, Food Bioprocess Technol., 2010, 3, 340 CrossRef CAS.
- K. A. Larson and M. L. King, Biotechnol. Prog., 1986, 2, 73 CrossRef CAS PubMed.
- Ž. Knez, E. Markočič, M. Leitgeb, M. Primožič, M. Knez Hrnčič and M. Škerget, Energy, 2014, 77, 235 CrossRef.
- J. Newman, The Wall Street Journal, 2022, https://www.wsj.com/articles/food-beverage-companies-race-to-keep-plants-running-during-wide-co2-shortage-11661527088, accessed October 2023 Search PubMed.
- S. Pereda, S. Bottini and E. Brignole, in Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds, ed. J. L. Martinez, CRC Press, Boca Raton (FL), 2007 Search PubMed.
- B. E. Poling, J. M. Prausnitz and J. P. O'Connell, The Properties of Gases and Liquids, McGraw-Hill, 2000 Search PubMed.
- E. S. R. Gopal, Resonance, 2000, 5, 37 CrossRef.
- A. F. M. Barton, Chem. Rev., 1975, 75, 731 CrossRef CAS.
- W. G. Chapman, K. E. Gubbins, G. Jackson and M. Radosz, Fluid Phase Equilib., 1989, 52, 31 CrossRef CAS.
- J. A. Mendiola, M. Herrero, M. Castro-Puyana and E. Ibáñez, in Natural Product Extraction: Principles and Applications, ed. M. A. Rostagno and J. M. Prado, RSC Publishing, 2013 Search PubMed.
- R. Span and W. Wagner, J. Phys. Chem. Ref. Data, 1996, 25, 1509 CrossRef CAS.
- H. Machida, M. Takesue and R. L. Smith Jr, J. Supercrit. Fluids, 2011, 60, 2 CrossRef CAS.
- N. R. Foster, G. S. Gurdial, J. S. L. Yun, K. K. Liong, K. D. Tilly, S. S. T. Ting, H. Singh and J. H. Lee, Ind. Eng. Chem. Res., 1991, 30, 1955 CrossRef CAS.
- E. H. Chimowitz and K. J. Pennisi, AIChE J., 1986, 32, 1665 CrossRef CAS.
- F. D. Kelley and E. H. Chimowitz, AIChE J., 1989, 35, 981 CrossRef CAS.
- K. P. Johnston, S. E. Barry, N. K. Read and T. R. Holcomb, Ind. Eng. Chem. Res., 1987, 26, 2372 CrossRef CAS.
- S. H. Page, S. R. Sumpter and M. L. Lee, J. Microcolumn Sep., 1992, 4, 91 CrossRef CAS.
- T. Clifford, Fundamentals of Supercritical Fluids, Oxford University Press, 1999 Search PubMed.
- S. W. Benson, J. Phys. Chem., 1948, 52, 1060 CrossRef CAS PubMed.
- S. B. Hawthorne, A. B. Galy, V. O. Schmitt and D. J. Miller, Anal. Chem., 1995, 67, 2723 CrossRef CAS.
- K.-Y. Khaw, M.-O. Parat, P. N. Shaw and J. R. Falconer, Molecules, 2017, 22, 1186 CrossRef PubMed.
- F. Montañés, T. Fornari, A. Olano and E. Ibáñez, J. Chromatogr. A, 2012, 1250, 92 CrossRef PubMed.
- G. D. J. Harper, E. Kendrick, P. A. Anderson, W. Mrozik, P. Christensen, S. Lambert, D. Greenwood, P. K. Das, M. Ahmeid, Z. Milojevic, W. Du, D. J. L. Brett, P. R. Shearing, A. Rastegarpanah, R. Stolkin, R. Sommerville, A. Zorin, J. L. Durham, A. P. Abbott, D. Thompson, N. D. Browning, B. L. Mehdi, M. Bahri, F. Schanider-Tontini, D. Nicholls, C. Stallmeister, B. Friedrich, M. Sommerfeld, L. L. Driscoll, A. Jarvis, E. C. Giles, P. R. Slater, V. Echavarri-Bravo, G. Maddalena, L. E. Horsfall, L. Gaines, Q. Dai, S. J. Jethwa, A. L. Lipson, G. A. Leeke, T. Cowell, J. G. Farthing, G. Mariani, A. Smith, Z. Iqbal, R. Golmohammadzadeh, L. Sweeney, V. Goodship, Z. Li, J. Edge, L. Lander, V. T. Nguyen, R. J. R. Elliot, O. Heidrich, M. Slattery, D. Reed, J. Ahuja, A. Cavoski, R. Lee, E. Driscoll, J. Baker, P. Littlewood, I. Styles, S. Mahanty and F. Boons, JPhys Energy, 2023, 5, 021501 CrossRef.
- S. E. Sloop and Ecobat Resources Indiana LLC, US Pat., US7198865, filed: 9th January 2003.
- M. Grützke, V. Kraft, W. Weber, C. Wendt, A. Friesen, S. Klamor, M. Winter and S. Nowak, J. Supercrit. Fluids, 2014, 94, 216 CrossRef.
- M. Grützke, X. Mönnighoff, F. Horsthemke, V. Kraft, M. Winter and S. Nowak, RSC Adv., 2015, 5, 43209 RSC.
- S. Rothermel, M. Evertz, J. Kasnatscheew, X. Qi, M. Grützke, M. Winter and S. Nowak, ChemSusChem, 2016, 9, 3473 CrossRef CAS PubMed.
- Y. Liu, D. Mu, R. Zheng and C. Dai, RSC Adv., 2014, 4, 54525 RSC.
- Y. Liu, D. Mu, Y. Dai, Q. Ma, R. Zheng and C. Dai, Int. J. Electrochem. Sci., 2016, 11, 7594 CrossRef CAS.
- Y. Liu, D. Mu, R. Li, Q. Ma, R. Zheng and C. Dai, J. Phys. Chem. C, 2017, 121, 4181 CrossRef CAS.
- D. Mu, Y. Liu, R. Li, Q. Ma and C. Dai, New J. Chem., 2017, 41, 7177 RSC.
- D. Mu, J. Liang, J. Zhang, Y. Wang, S. Jin and C. Dai, ChemistrySelect, 2022, 7, e202200841 CrossRef CAS.
- B. Niu, Z. Xu, J. Xiao and Y. Qin, Chem. Rev., 2023, 123, 8718 CrossRef CAS PubMed.
- K. E. Laintz, C. M. Wai, C. R. Yonker and R. D. Smith, J. Supercrit. Fluids, 1991, 4, 194 CrossRef CAS.
- C. M. Wai, Y. Lin, R. Brauer, S. Wang and W. F. Beckert, Talanta, 1993, 40, 1325 CrossRef CAS PubMed.
- C. M. Wai, Anal. Sci., 1995, 11, 165 CrossRef CAS.
- Y. Lin, N. G. Smart and C. M. Wai, TrAC, Trends Anal. Chem., 1995, 14, 123 CAS.
- C. M. Wai and S. Wang, J. Chromatogr. A, 1997, 785, 369 CrossRef CAS.
- C. Erkey, J. Supercrit. Fluids, 2000, 17, 259 CrossRef CAS.
- F. Lin, D. Liu, S. M. Das, N. Prempeh, Y. Hua and J. Lu, Ind. Eng. Chem. Res., 2014, 53, 1866 CrossRef CAS.
- F.-R. Xiu and F.-S. Zhang, J. Hazard. Mater., 2009, 165, 1002 CrossRef CAS PubMed.
- S. Sanyal, Q. Ke, Y. Zhang, T. Ngo, J. Carrell, H. Zhang and L. L. Dai, J. Cleaner Prod., 2013, 41, 174 CrossRef CAS.
- F.-R. Xiu, Y. Qi and F.-S. Zhang, Waste Manage., 2013, 33, 1251 CrossRef CAS PubMed.
- M. Xing and F.-S. Zhang, Chem. Eng. J., 2013, 219, 131 CrossRef CAS.
- C. O. Calgaro, D. F. Schlemmer, M. D. C. R. da Silva, E. V. Maziero, E. H. Tanabe and D. A. Bertuol, Waste Manage., 2015, 45, 289 CrossRef CAS PubMed.
- D. A. Bertuol, C. M. Machado, M. L. Silva, C. O. Calgaro, G. L. Dotto and E. H. Tanabe, Waste Manage., 2016, 51, 245 CrossRef CAS PubMed.
- Y. Yao, N. F. Farac and G. Azimi, ACS Sustainable Chem. Eng., 2018, 6, 1417 CrossRef CAS.
- J. Zhang, J. Anawati, Y. Yao and G. Azimi, ACS Sustainable Chem. Eng., 2018, 6, 16713 CrossRef CAS.
- J. Zhang, J. Anawati and G. Azimi, Waste Manage., 2022, 139, 168 CrossRef CAS PubMed.
- A. B. Argenta, C. M. Reis, G. P. Mello, G. L. Dotto, E. H. Tanabe and D. A. Bertuol, J. Supercrit. Fluids, 2017, 120, 95 CrossRef CAS.
- S. M. Fayaz, M. A. Abdoli, M. Baghdadi and A. Karbasi, Int. J. Environ. Stud., 2021, 78, 459 CrossRef CAS.
- J. Zhang and G. Azimi, Resour., Conserv. Recycl., 2022, 187, 106628 CrossRef CAS.
- A. I. Cooper, J. Mater. Chem., 2000, 10, 207 RSC.
- S.-D. Yeo and E. Kiran, J. Supercrit. Fluids, 2005, 34, 287 CrossRef CAS.
- W.-C. Tsai and Y. Wang, Prog. Polym. Sci., 2019, 98, 101161 CrossRef CAS.
- G. Kravanja, M. K. Hrnčič, M. Škerget and Ž. Knez, J. Supercrit. Fluids, 2016, 108, 45 CrossRef CAS.
- M. Karimi, M. Heuchel, T. Weigel, M. Schossig, D. Hofmann and A. Lendlein, J. Supercrit. Fluids, 2012, 61, 175 CrossRef CAS.
- T. P. Hunt and C. J. Dowle, Analyst, 1991, 116, 1299 RSC.
- I. Yilgör and J. E. McGrath, Polym. Bull., 1984, 12, 491 CrossRef.
- L. Merz and O. Muth, Process Technol. Proc., 1996, 12, 373 CAS.
- Y. Arai, T. Sako and Y. Takebayashi, in Springer Series in Materials Processing, ed. H. Warlimont, E. Weber and W. Michaeli, Springer, 2002 Search PubMed.
- E. Hunter and R. B. Richards and Imperial Chemical Industries Limited, US Pat., US2457238, Filed: 25th October 1945.
- M. A. McHugh and V. J. Krukonis, Supercritical Fluid Extraction: Principles and Practice, Butterworth-Heinemann, 1994 Search PubMed.
- A. A. Clifford, K. D. Bartle, I. Gelebart and S. Zhu, J. Chromatogr. A, 1997, 785, 395 CrossRef CAS.
- C. O. Calgaro, D. F. Schlemmer, M. M. Bassaco, G. L. Dotto, E. H. Tanabe and D. A. Bertuol, J. CO2 Util., 2017, 22, 307 CrossRef CAS.
- Arkema, Virtucycle®, 2022, https://hpp.arkema.com/en/sustainability/virtucycle/, accessed July 2023 Search PubMed.
- F. D'Aprile, V. Kapelyushko, M. Mirenda, G. V. Medeiros, E. Egloff and Solvay Specialty Polymers Italy S.p.A., WO/2023/117971, Filed: 19th December 2021.
- F. Rindfleisch, T. P. DiNoia and M. A. McHugh, J. Phys. Chem., 1996, 100, 15581 CrossRef CAS.
- J. M. DeSimone, Z. Guan and C. S. Elsbernd, Science, 1992, 257, 945 CrossRef CAS PubMed.
- D. A. Newman, T. A. Hoefling, R. R. Beitle, E. J. Beckman and R. M. Enick, J. Supercrit. Fluids, 1993, 6, 205 CrossRef CAS.
- Z. Guan and J. M. DeSimone, Macromolecules, 1994, 27, 5527 CrossRef CAS.
- Y.-L. Hsiao, E. E. Maury, J. M. DeSimone, S. Mawson and K. P. Johnston, Macromolecules, 1995, 28, 8159 CrossRef CAS.
- C. A. Mertdogan, H.-S. Byun, M. A. McHugh and W. H. Tuminello, Macromolecules, 1996, 29, 6548 CrossRef CAS.
- M. Lora, J. S. Lim and M. A. McHugh, J. Phys. Chem. B, 1999, 103, 2818 CrossRef CAS.
- S. G. Kazarian, M. F. Vincent, F. V. Bright, C. L. Liotta and C. A. Eckert, J. Am. Chem. Soc., 1996, 118, 1729 CrossRef CAS.
- C. Smith, Z. R. J. Nicholls, K. Armour, W. Collins, P. Forster, M. Meinshausen, M. D. Palmer and M. Watanabe, in Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. V. Masson-Delmotte, P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu and B. Zhou, Cambridge University Press, 2021 Search PubMed.
- T. P. Dinoia, S. E. Conway, J. S. Lim and M. A. McHugh, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 2832 CrossRef CAS.
- T. A. Hoefling, D. Stoefsky, M. Reid, E. J. Beckman and R. M. Enick, J. Supercrit. Fluids, 1992, 5, 237 CrossRef CAS.
- S. M. Fahmy, PhD thesis, RWTH Aachen University, 2005.
- Y. Fu, J. Schuster, M. Petranikova and B. Ebin, Resour., Conserv. Recycl., 2021, 172, 105666 CrossRef CAS.
- B. Rousselle, B. Allard Breton and Arkema France, WO/2022/018232, Filed: 23rd July 2020.
| This journal is © The Royal Society of Chemistry 2024 |