Open Access Article
Open Access ArticleSynthesis and application of sustainable vegetable oil-based polymers in 3D printing
Rahul
Saraswat
a,
Shagun
b,
Abhimanew
Dhir
b,
A. S. S.
Balan
c,
Satvasheel
Powar
 a and
Mrityunjay
Doddamani
a and
Mrityunjay
Doddamani
 *a
*a
aSchool of Mechanical and Materials Engineering, Indian Institute of Technology Mandi, Mandi, Himachal Pradesh 175005, India. E-mail: mrityunjay@iitmandi.ac.in
bSchool of Chemical Sciences, Indian Institute of Technology Mandi, Mandi, Himachal Pradesh 175005, India
cDepartment of Mechanical Engineering, National Institute of Technology, Surathkal, Karnataka, India
First published on 23rd April 2024
Abstract
In the past ten years, there has been significant growth in the global 3D printing market, particularly in the development of natural and bio-based polymers. However, a major challenge is the limited availability of sustainable 3D printable resins capable of matching the performance of synthetic materials. This underscores the urgent need for the development of innovative and environmentally friendly resin materials. Herein, we introduce bio-based polymers, highlighting their recent advancements and offering a comprehensive overview of their diverse applications across various fields, including 3D printing. An area that has received less attention in this domain is polymers derived from vegetable oil (VO) or plant-based oil. Specifically, we thoroughly investigate the acrylation of epoxidized VOs and the subsequent formation of resins from these acrylates, which are essential materials for digital light processing (DLP), stereolithography (SLA), and extrusion-based 3D printing. The chemical modification of VOs, such as epoxidation and acrylation, is extensively explored, together with their respective types and applications. Furthermore, we delve deeply into the suitability of acrylate resins for 3D printing purposes. In conclusion, this review offers insights into the potential applications of 3D printed products utilizing materials derived from VOs.
Sustainability spotlightIn the last decade, the global 3D printing material market has seen significant growth, encompassing thermoplastics, metals, ceramics, and biomaterials. Notably, there is increasing focus on biodegradable and biobased polymers owing to environmental concerns regarding plastic waste and carbon emissions from fossil fuel-based materials. This study specifically delves into the advancements in 3D printable biopolymers, emphasizing the conversion of plant-based oils (such as vegetable oil) into UV-compatible polymeric materials. Crucially, this research aligns with three United Nations Sustainable Development Goals: responsible consumption and production, life below water, and life on land, addressing key environmental and sustainability objectives in the field of 3D printing materials. |
1. Introduction
Three primary manufacturing methods are widely employed, i.e., subtractive, formative, and additive manufacturing.1 Subtractive manufacturing involves selectively removing materials from raw materials to achieve the intended shape and dimensions by utilizing processes such as drilling, machining, and milling. Alternatively, formative manufacturing involves shaping raw materials by applying forces to attain a desired form. Examples of this method include extrusion, rolling, bending, and similar techniques. In contrast, additive manufacturing (AM) differs from the above-mentioned two methods as it builds the product layer by layer through a computer-controlled process, commonly known as 3D printing. This approach allows for the development of three-dimensional objects, hence the name.2 This technology emerged a couple of years ago and has since experienced rapid growth. Its popularity stems from its distinctive ability to fabricate intricate forms and extensively personalized products, while minimizing the generation of scrap. Consequently, AM has become a more favourable choice compared to traditional processing routes. It enables the application of geometric designs tailored to specific requirements and has already been employed in numerous applications in various industries, including aerospace, construction, biomedical, and automotive industries.3–6The range of materials accessible for 3D printing varies significantly depending on the technology employed and the targeted features of the final product. These materials include thermoplastics, metals, ceramics, biomaterials, and more.4 Nonetheless, there has recently been a marked surge in attention towards biodegradable and bio-based polymers. This interest is driven by concerns about the environmental consequences of plastic waste and the significant carbon emissions associated with fossil fuel-based materials. The European Union (EU) has acknowledged bio-based polymers as a viable substitute for chemically modified plastics to address concerns linked to the limited fossil resources, climate change, and environmental health.7 Thus, the research community is actively exploring the manufacturing and utilization of biodegradable plastics, focusing on technological advancements, environmental considerations, and sustainability impacts.8 Consequently, certain bio-based polymers are experiencing an increase in usage in particular instances because of their beneficial features, including biological compatibility, biodegradability, and non-toxicity. The life cycle assessment technique has also been developed to thoroughly compute the ecological influence of products or systems. This method seeks to evaluate the complete lifecycle of a product, encompassing everything from extracting raw materials to disposal, while considering its environmental impact.9
Presently, an area receiving little attention is the 3D printing of bio-based polymers derived from VOs. Plant-derived VOs are acknowledged as highly readily available sustainable sources and their incorporation in creating environmentally conscious and biodegradable materials is steadily gaining traction.10 However, the current utilization of 3D printing machines is restricted to specific oils such as soybean. This restriction can be attributed to the absence of standardized processes for chemically modifying VOs to make them compatible with these machines. For example, a resin containing acrylate epoxidized oil and a photoinitiator suitable for a particular wavelength range is employed in DLP printing. Therefore, VOs need to undergo sequential processes of epoxidation and acrylate formation to become suitable for the final product. Recent technological advancements that alter the chemical structure of VOs have significantly enhanced their commercial and economic viability across various sectors, including coatings, elastomers, and vitrimers. Recently, epoxidized soybean oil (ESO) has been produced commercially. Furthermore, the acrylation of ESO is suitable for creating UV-curable coatings with exceptional characteristics. Notably, this acrylate has been employed to effectively produce shape memory scaffolds via 3D printing.11 Additionally, different types of acrylates, such as polyethylene glycol diacrylate (PEGDA), are incorporated in VO-based acrylates to develop hybrid resins, adjusting their viscosity to match the requirements of specific 3D printers.12 Furthermore, to replicate the mechanical properties of synthetic resins, composite resins containing fillers as reinforcements have also been introduced in the 3D printing materials market.13 However, despite these developments, the 3D printing of bio-based polymers derived from plant-based oils remains unexplored and necessitates comprehensive scientific research.
This review aims to provide a comprehensive overview of bio-based polymers, focusing on those derived from VOs, and their applications in 3D printing. The objective is to summarize the latest developments in this specific area. Initially, we present a brief introduction on biopolymers and bio-based polymers, highlighting their diverse range of applications. We explore various chemical modifications commonly used to transform VOs into polymers suitable for 3D printing, including epoxidation and acrylation. Moreover, we explore 3D printing by categorizing the different 3D printing methods and exploring how VO-based neat, hybrid, and composite resins are integrated into various 3D printing methods, particularly focusing on DLP/SLA. Additionally, we elaborate and discuss the potential applications of 3D-printed objects made using bio-based polymers derived from VOs. Finally, we address the environmental considerations and pursuit of sustainable development within this context. A schematic of the scope of this article is presented in Fig. 1.
2. Biopolymers and bio-based polymers
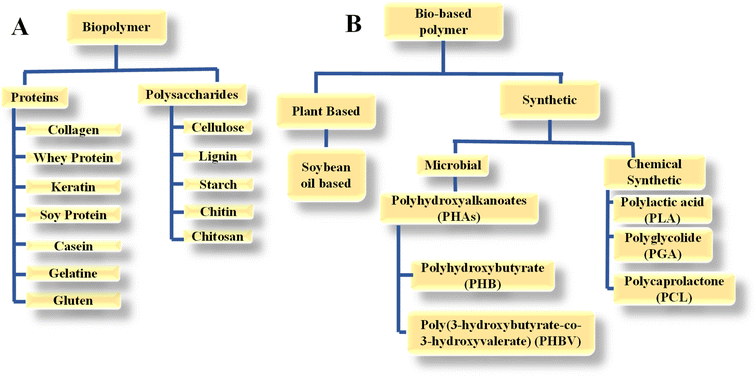
Biopolymers are naturally occurring polymers and synthesized by living organisms, which can be found in various biological systems, including plants, animals, and microorganisms. Examples of biopolymers include proteins, nucleic acids (DNA and RNA), polysaccharides (such as cellulose, starch, and chitin), and some types of lipids. Similar to various polymers, biopolymers are comprised of monomeric units that undergo chemical bonding to create larger molecular structures. Here, biodegradability refers to the ability of microorganisms to break down biopolymers into other matter, for example, carbon dioxide and water.14 Bio-based polymers, often referred to as bio-polymers, are polymers made from biomass, which is renewable and derived from microbes, plants, and animals. These polymers may or may not be naturally occurring and can be produced through various processes, including fermentation, chemical synthesis using bio-derived monomers, and modification of natural polymers. They come in various forms such as polylactic acid and polyhydroxybutyrate, which are predominantly utilized in packaging. However, they encounter difficulties in more rigorous applications due to their drawbacks such as inadequate durability, the need for process adjustments, increased cost, and inferior performance compared to oil-based alternatives.15,16Biopolymers and bio-based polymers are categorized as follows:10,17
(a) Biopolymers from natural origins, including proteins and polysaccharides.
(b) Bio-based polymers are further categorised into following two categories.
• Bio-based polymers that are derived from plants such as soybean oil-based polymer.
• Synthetic bio-based polymer that are manufactured through chemical modifications using bio-derived monomers, for example, polyhydroxyalkanoates (PHA) in the microbial category and polylactic acid (PLA) in the chemically synthetic category.
Fig. 2 provides a more detailed illustration of biopolymers and bio-based polymer classification.
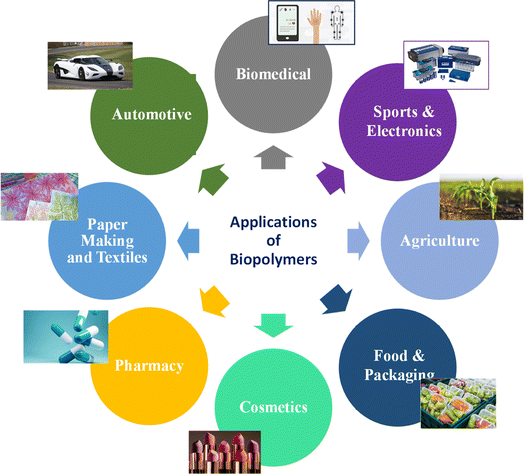
Biopolymers originating from natural sources exhibit noteworthy positive impacts similar to drugs in living organisms, the capability to interact effectively with a suitable host response in certain circumstances, and the capacity for biodegradation. Nevertheless, they often lack processability and sufficient mechanical properties. In contrast, bio-based polymers synthesized artificially are not initially found in the human body. They offer advantages such as ease of processing, ability to shape them into various forms, and potential for scalable production. Additionally, their composition and properties, such as mechanical and rheological characteristics, can be precisely adjusted to meet specific application requirements, such as bio-degradable scaffolds and controlled-release drug delivery devices.18 However, it is essential to note that bio-based polymers tend to exhibit bioinert properties, leading to challenges in cellular adhesion and growth on their surfaces compared to naturally occurring biopolymers such as proteins and saccharides. This can pose difficulties at the interface between cells and the material.19 Nevertheless, to their distinctive characteristics, biopolymers and bio-based polymers are utilized in various industries (Fig. 3), including biomedical uses such as tissue science, drug release systems, and bioprinting to create living tissues and organs. Customized medical devices, sustainable products, food and nutrition solutions, drug delivery systems, and environmental applications are among the many other areas where bio-based polymers and 3D printing intersect.19
Therefore, a variety of biopolymers and bio-based polymeric materials has been employed in 3D printing machines to manufacture a wide range of products for various applications. However, priority should be given to designing 3D-printed products that are biodegradable and recyclable and converting plant-based polymers to replace current materials, supporting the establishment of a cyclic plastic ecosystem that produces no waste. This review focuses on providing a comprehensive description of VO-based polymers, starting with their chemical modification into useful acrylates and the creation of sustainable resin for 3D printing, concluding with their applications across diverse fields.
3. Additive manufacturing for biopolymer and bio-based polymers
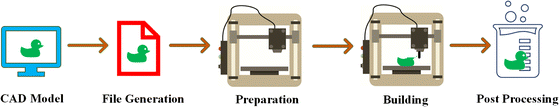
Recently, there has been significant progress in developing additive manufacturing (AM) techniques designed explicitly for biopolymers and bio-based polymers. AM is widely used for the creation of biopolymer and bio-based polymer products for diverse applications. Ongoing research continues to expand their potential in various fields. Fig. 4 demonstrates the steps for the fabrication of a product through AM, which typically involves five key steps,20 as follows:(1) Developing a model generated through Computer-Aided Design (CAD), which involves designing a digital model using specialized software.
(2) Converting the CAD file into a file compatible with 3D printing: CAD model is transformed into a file format known as .stl, which is commonly used in stereolithography CAD software, such as 3D systems.
(3) Verifying the compatibility of the file and configuring the build parameters: .stl file is examined to ensure its integrity and compatibility with the AM system. Build parameters, such as layer thickness and printing settings, are also determined during this stage.
(4) Slicing and printing: compatible file is sliced into layers, and the biopolymer material is progressively deposited in a layered fashion to develop the final object or structure.
(5) Post-processing: additional steps may be required, such as removing support structures, cleaning, curing, and surface finishing, to achieve the final desired quality of the printed biopolymer object.
Generating a CAD model can be achieved through various methods, either by manual creation or redeveloping existing data sources. The most popular file type for 3D printing is .stl. However, newer and more advanced formats such as .3mf and .amf are especially suitable for designs incorporating multiple colors, materials, or structures. These formats offer increased flexibility and possibilities in 3D printing.17
AM provides advantages over conventional manufacturing approaches, including greater design freedom, increased flexibility, improved customization options, and reduced material wastage.21 For instance, PrusaSlicer is a user-friendly platform used to prepare 3D models for printing. It generates G-code for various 3D printers and supports customized print settings and automatic support generation. Alternatively, the nTopology software is focused on advanced generative design, simulation, and optimization. It facilitates the crafting of intricate geometries, lattice structures, and optimized designs that are ideal for AM such as 3D printing.22–24
3.1. 3D printing techniques for biopolymers and bio-based polymers
Over recent years, notable progress has been achieved in refining AM methods specifically designed for producing biopolymers. This section presents an overview of the most prevalent AM techniques utilized for biopolymer and bio-based polymers. Fig. 5 depicts a comprehensive classification of the AM techniques into six distinct groups. Given that each process uses a specific material throughout the printing process, AM is possible in various ways. Below, we provide a concise introduction to each of these AM groups.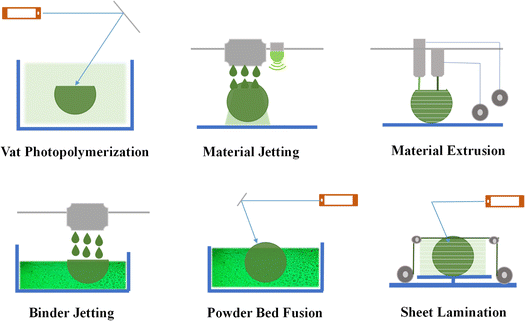 | ||
| Fig. 5 Methods used in biopolymer additive manufacturing (reproduced from ref. 14 with permission from Elsevier, Copyright 2023.). | ||
The frequently employed bio-based polymers for the above-mentioned 3D printing methods include polycaprolactone (PCL), polylactic acid (PLA), and polyethylene glycol diacrylate (PEGDA). Nevertheless, their application significantly depends on the AM method employed. For example, polylactic acid (PLA) can be 3D printed using fused filament fabrication as part of material extrusion, DLP within vat photopolymerization, and selective laser sintering (SLS) within powder bed fusion.27 The astounding biocompatibility, biodegradability, and 3D printing flexibility of PLA have made it very popular in the biomedical industry. Its application is prevalent, ranging from porous scaffolds41 and drug release systems to surgical implants, tissue scaffolds for regenerative medicine, and biodegradable stents.42 Moreover, polycaprolactone (PCL) can be 3D printed using vat photopolymerization, power bed fusion (PBF), and binder jetting (BJ). PCL has also found diverse applications similar to PLA, including manufacturing of scaffolds, drug release systems, nerve conduits, scaffolds for organ-on-a-chip models, surgical implants, and other functional fixtures.43 Besides, PEGDA is a water-soluble polymer cross-linked using UV light during 3D printing, offering control of the mechanical properties, porosity, and degradation rate. Its biocompatibility, biodegradability, and tunable properties make it attractive for use in the bio-medical industry. PEGDA-based hydrogels can deposit living cells in bioprinting, enabling the fabrication of complex tissue constructs with bioactive molecules and promising functional tissue-like structures.44 In addition, hydrogels such as collagen, gelatin, and alginate are frequently employed in bioprinting applications. These hydrogels have unique features that make them excellent for generating bio-inks, which are the printable materials used in bioprinting to deposit living cells and form tissue-like structures.45
Nonetheless, the focus of this review is 3D printed resin made from VO that works with several 3D printing techniques, specifically DLP-, SLA- and UV-based material extrusion systems. UV-based resins, synthesized from acrylates of vegetable oil, are particularly utilized in these processes. The global research community is actively investigating numerous oils in this context. To date, soybean oil,11 castor oil,46 and various combinations of rapeseed, linseed, and grapeseed oils47 are the plant oils that have been successfully 3D printed, leading to the development of products suitable for diverse applications. This advancement has the potential to serve as a significant milestone in the pursuit of a sustainable world. The following section delves into transforming VOs into a 3D-printable resin through chemical modification such as epoxidation, acrylation, and resin formation.
4. Chemical modification of vegetable oils (VOs) into 3D-printable resin
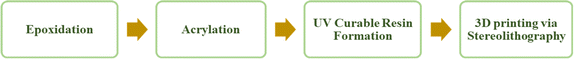
Producing the ultimate 3D product using polymers derived from VO involves four key stages, as illustrated in Fig. 6, including epoxidation, acrylation, formulation of a resin that can be cured with UV light, and the actual 3D printing process.Each stage of the process is interlinked with the other stage. The entire procedure commences with the epoxidation of vegetable oil (VO), which is succeeded by its acrylation. Subsequently, this acrylate is combined with a photoinitiator of appropriate wavelength to create a printable resin. Subsequently, the resin is utilized in a 3D printer to produce the final product. Therefore, each phase is integral to achieving the intended outcome. Thus, the initial step involves converting these VO into the acrylate, with the process of acrylate formation discussed in the following section.
4.1. Chemical pathway for VO-based acrylate
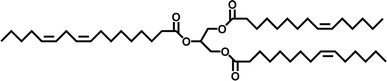
Plant-based oils, also called vegetable or natural oils (VOs), can be obtained from various bio-resources such as plants and trees. These oils are readily accessible in various regions worldwide, making them highly suitable alternatives to chemical feedstocks. These oils primarily consist of molecules known as triglycerides, the structure of which is depicted in Fig. 7. Triglycerides are combinations of three fatty acids and glycerol. Around 95% of their weight is made up of fatty acids and their composition varies depending on the source of the plant oil.48 Different oils have distinct fatty acid compositions, leading to diverse properties and applications. Oleic, linoleic, and linolenic acid are the most often used fatty acids for polymerization, which can have a carbon atom length of 14 to 22 and one to three double bonds in different plant oils.49Triglycerides are adaptable compounds used in two basic methods to produce cross-linked polymeric materials. The first method uses the functional groups already present in triglycerides, such as internal double bonds, epoxides, and alcohols, which can form polymers in various ways. In this method, the inherent reactivity of triglycerides is harnessed to form cross-linked networks. The second strategy involves modifying triglycerides chemically before polymerization. This process is used to overcome the comparatively poor reactivity of organic triglycerides mainly due to the presence of double bonds. Accordingly, the responsiveness of triglycerides is increased by adding easily polymerizable functional molecules by chemical modification, resulting in more efficient and regulated polymerization processes.50 As a result, it broadens the range of synthetic possibilities. Table 1 illustrates the distribution of fatty acids in several commonly used oils.51 However, recent advancements in genetic engineering have enabled the manipulation of the unsaturation levels in plants such as soybeans and corn.52,53
| Fatty acid | Canola | Corn | Cottonseed | Linseed | Olive | Palm | Soybean | C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBa DBa |
|---|---|---|---|---|---|---|---|---|
a No. of carbon atoms and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds/fatty acid.
b Avg. C C double bonds/fatty acid.
b Avg. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds/triglyceride molecule. C double bonds/triglyceride molecule.
|
||||||||
| Oleic | 60.9 | 25.4 | 18.6 | 19.1 | 71.1 | 39.3 | 23.4 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Linoleic | 21.0 | 59.6 | 54.4 | 15.3 | 10.0 | 10.0 | 53.2 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| Linolenic | 8.8 | 1.2 | 0.7 | 56.6 | 0.6 | 0.4 | 7.8 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| Palmitic | 4.1 | 10.9 | 21.6 | 5.5 | 13.7 | 44.4 | 11.0 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| Other fatty acids | 5.2 | 2.9 | 4.7 | 3.5 | 4.6 | 5.9 | 4.6 | — |
| Average DB/triglycerideb | 3.9 | 4.5 | 3.9 | 6.6 | 2.8 | 1.8 | 4.6 | — |
Triglycerides have a reactive location for chemical reactions because they contain a double bond. Similar procedures can be used to add polymerizable units to triglycerides to create polymers from petrochemicals. The crucial objective is to achieve a higher cross-link density and introduce chemical functionalities that enhance the stiffness of the polymer network.48Fig. 8 depicts the various methods employed for functionalizing triglycerides with polymerizable chemical groups.
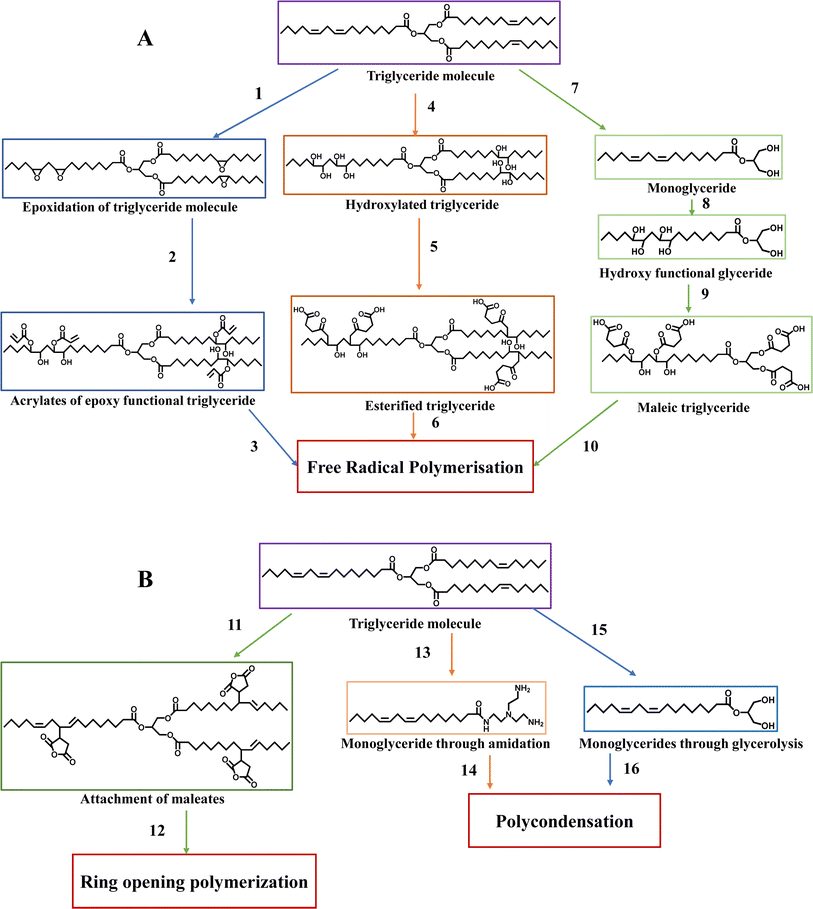 | ||
| Fig. 8 Synthetic pathway leading to polymerization: (A) free-radical polymerization and (B) polycondensation and ring-opening polymerization. | ||
Triglyceride molecules undergo polymerization through three distinct pathways, i.e., free radical polymerization (Fig. 8A), ring opening polymerization, and polycondensation (Fig. 8B). In free radical polymerization, the unsaturated bonds in natural triglycerides are transformed into epoxy groups. This process involves reacting epoxy-functionalized triglycerides with acrylic acid to introduce acrylates (1–3), as seen in Fig. 8.54,55 Alternatively, hydroxyl groups can be attached to natural triglycerides. Subsequently, the hydroxylated triglycerides can react with maleic anhydride, incorporating maleate half-esters and esters onto the triglycerides (4–6).56,57 Another approach involves converting triglycerides into monoglycerides through glycerolysis, followed by attaching maleate half-esters to these monoglycerides, enabling them to undergo free-radical polymerization (7–10).58 An alternative approach is to attach maleate esters, which are derivatives of maleic acid, to the triglycerides. This modification enables the triglyceride to engage in ring-opening polymerization upon reaction (11–12).59,60 In polycondensation, monoglycerides obtained either through a glycerolysis reaction or an amidation reaction can undergo polycondensation reactions with a comonomer through alcohol groups (13–14 and 15–16).58
These modified triglycerides and a reactive diluent can be blended and cured through free-radical polymerization. Epoxidized triglycerides can be made by applying a typical epoxidation process to readily accessible unsaturated oils such as soybean and flaxseed oil or obtained from organic oils such as Vernonia plant oil.61 However, VOs can undergo epoxidation, acrylation, or both. For instance, if a higher epoxy content is desired, commercially available epoxidized linseed oil can be utilized. In contrast, commercially available epoxidized soybean oil contains 4.4 epoxy rings per triglyceride. These triglycerides have been widely employed in various applications. One important application is as a substitute softener to phthalate for polyvinyl chloride.62
Based on the discussed literature, the triglyceride molecules present in the fatty acids of VOs can undergo chemical modifications to transform into polymeric structures. The resin commonly employed for vat photopolymerization is UV-based epoxy or acrylates. Among the routes depicted in Fig. 5, the most suitable path for obtaining 3D printed parts is 1–3 in Fig. 5A, emphasizing polymerization through epoxidation followed by acrylation. The first US patents for UV resins were published in 1990, mostly based on acrylates, which are recognized for their strong reactivity, but frequently produce fragile components because of their contraction and curling defects.63 Epoxy resins demonstrate improved strength, hardness, and precision compared to acrylate resins.64 Furthermore, unlike acrylic formulations, the polymerization of epoxy-based resins is not inhibited by ambient oxygen, enabling lower photoinitiator concentrations and a reduction in residual odour.65 However, epoxy resins have several disadvantages, such as brittle cured pieces and poor photo-speed.66 Also, their susceptibility to moisture, which can obstruct polymerization, is another drawback.65 Therefore, achieving a well-balanced blend of epoxides and acrylates in the resin is crucial to combining the advantages of both curing types.
Hence, the upcoming sections predominantly focus on the elaborate procedures of epoxidation and acrylation, with specific reference to routes 1–3 illustrated in Fig. 8A. Furthermore, it emphasizes the utilization of these epoxides and acrylates across diverse fields.
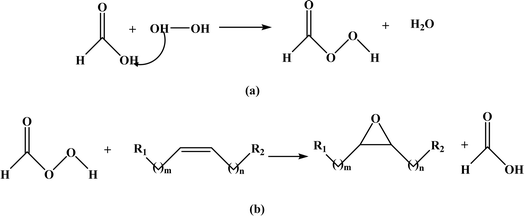 | ||
| Fig. 9 Prileschajew reaction in epoxidation process formation of (a) peracid using hydrogen peroxide and (b) epoxide group using formic acid and peracid. | ||
Considerable efforts have been devoted to finding the most suitable conditions for the Prilezhaev epoxidation of different vegetable oils. The epoxidation process based on the Prileschajew reaction for vegetable oils may vary depending on the specific substrte, catalyst, temperature, and other parameters employed in individual syntheses. Typically, this reaction is performed in a temperature range from room temperature to approximately 75 °C.73 However, in some cases like epoxidation of soybean oil using the methyltrioxorhenium (MTO)-CH2Cl2/H2O2 biphasic system, the reaction was conducted at room temperature without any Lewis base.74
Nonetheless, certain unintended reactions may also occur during the epoxidation process, which have an unfavorable contribution to the overall outcome. This can be attributed to the significant exothermic nature of the epoxidation reaction, resulting in a substantial temperature rise, which can trigger undesirable reactions or even lead to an explosion. However, the existing literature contains numerous studies that specifically explored the thermal effects associated with the epoxidation of VOs.69,75 As a result, various kinetic models have been proposed to analyze and understand the undesirable reactions occurring throughout the process.52,76
Several popular epoxidation techniques are outside the Prileschajew reaction, such as acid ion exchange resin (AIER) and chemoenzymatic epoxidation. Similar to the conventional approach, the AIER method uses carboxylic peracids to EVOs but uses mineral acids or extremely acidic ion-exchanging resins. By slowing down the rate at which the generated oxirane ring is opened, AIER can increase the transformation efficiency into an epoxy compound, especially for glycols and glycolic monoesters. This method is primarily employed for industrial purposes.72 In contrast, chemoenzymatic epoxidation is a recent technique that utilizes minimal free fatty acids. One disadvantage of the chemical technique is the presence of acid-catalyzed reactions that create different byproducts through ring opening. The chemoenzymatic method solves this issue by avoiding these side reactions.77Table 2 presents a concise overview of EVOs, including their molar ratios, catalysts, RPM, and reaction times. The Chinese National Standard provides a formula to determine the epoxidized content of a VO,78 as follows in eqn (1):
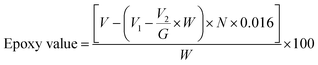 | (1) |
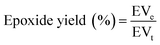 | (2) |
| S. no | VO | Molar ratio | Molar ratio | Catalyst | Temp. (°C) | RPM | Reaction time (h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Acetic acid: double bond | H2O2: double bond | |||||||
| a 1–3% for H2SO4; 1.5% for the other three acids. | ||||||||
| 1 | Soybean | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
H2SO4 | 60 | 500 | 1 | 69 |
| 2 | Neem | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
H2SO4 (0.1 wt%) | 60 | 500 | 1 | 80 |
| 3 | Canola | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Sulfated-SnO2 (10 wt%) | 70 | 600–1000 | 81 | |
| 4 | Cottonseed | 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
H2SO4/HNO3/H3PO4/HCla | 60 | 2400 | 4 | 71 |
| 5 | Rubber seed | Variation | Variation | H2SO4 | 60 | — | — | 82 |
| 6 | Camelina sativa | 0.84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Formic acid | 50 | 10 | 83 | |
| 7 | Soybean | Not given | 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Ti/SiO2 | 150 | — | 2 | 84 |
| 8 | Mahua | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
H2SO4/HNO3 (2 wt%) | — | 1500 | 2 | 70 |
| 9 | Jatropha | 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 60 | — | 4 | 85 |
| 10 | Flaxseed | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (wt) 50 (wt) |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (wt) 50 (wt) |
— | 45 | — | — | 86 |
| 11 | Castor | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 (wt) 5.5 (wt) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.61 (wt) 1.61 (wt) |
Seralite SRC-120 (27 wt%) | 55–60 | — | 8 | 87 |
EVO is a versatile and environmentally friendly compound widely used in various industries, particularly as a stabilizer in polyvinyl chloride (PVC) formulations to prevent thermal degradation by neutralizing hydrogen chloride.88,89 Recent research explored the epoxidation of waste kapok seed oil, indicating its potential as a beneficial co-stabilizer for PVC in industrial applications. The study revealed that the thermal stability of PVC is improved by the addition of epoxidized kapok seed oil, while its mechanical and flow properties remain largely unaffected.90 Similarly, in different studies, epoxidized rubber seed oil was found to act as a primary plasticizer for PVC to improve its migration, extraction, and volatilization characteristics and contribute to its thermal stability.91 Additionally, researchers are becoming increasingly interested in eco-friendly plasticizers that work well with different materials, which fits the worldwide move towards biodegradable polymers.92 Although phthalate esters are widely used, they are harmful to human health, and thus it is essential to find alternative options. In this case, EVOs are an excellent alternatives because they stabilize polymers at higher temperatures.93 Researchers have investigated the use of various oils as plasticizers, such as soybean oil,94 Tung oil,95 cardanol oil,88 waste cooking oil,89Jatropha oil,96 and sunflower oil.97 Moreover, EVO-based vitrimers are also an alternative to non-recyclable petroleum-based thermosets. However, their current properties are not as strong, limiting their use.97 Thus, the EVO selection, covalent adaptable networks (CANs), and material properties play a vital role in improving their properties.98 EVO with a higher epoxy value is recommended for better tensile properties and higher glass transition temperature.99 Exploring more CANs and using a rigid curing agent can enhance the mechanical properties and glass transition temperature.100 Accordingly, with careful selection, EVO-based epoxy vitrimers show potential as a replacement for traditional petroleum-based thermosets.
Besides their use in polymers, EVOs are used as eco-friendly lubricants in diverse industrial operations, such as metalworking fluids. Different oils such as neem oil,101 tilapia oil,102Jatropha oil,103 waste cooking oil,104 and rubber seed oil105 were employed to develop lubricants following their epoxidation. Moreover, EVOs also find utility in adhesives and sealants, offering adhesive strength and biodegradability.106,107 Furthermore, the use of EVOs in polymer nanocomposites has been explored to improve their mechanical and thermal properties, contributing to advanced material development.108 Their potential applications in food packaging underscore their versatility and sustainability, making EVOs valuable resources for environmentally friendly alternatives across diverse sectors.109
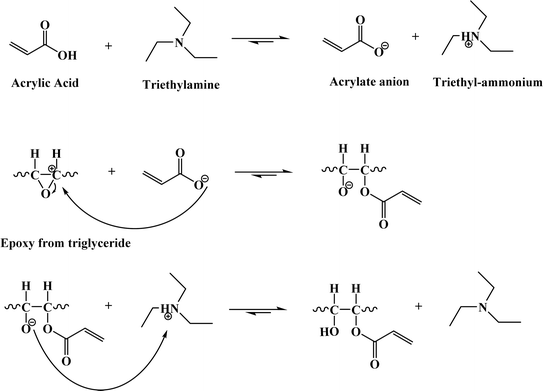
The entire reaction can be broken down into three primary stages to comprehend the chemical processes involved in acrylation, as illustrated in Fig. 11. In the initial phase, the catalyst, typically triethylamine (TEA), plays a crucial role by facilitating the interaction between acrylic acid and TEA, forming an acrylate anion. This anion serves as a nucleophile, seeking an electron-deficient site to react with. In the subsequent phase, the acrylate anion attacks the carbon atom in the epoxy group of the epoxidized oil. This reaction results in the production of an alcoholate anion. Finally, the reaction is completed when the proton of triethyl-ammonium moves to the alcoholate ion in the last phase. The intended outcome of the synthesis is produced by this final step, which results in the production of epoxy acrylates. Notably, this step also regenerates the TEA catalyst, making it available for future reactions and ensuring that its catalytic role can be sustained79 (Fig. 11).
Researchers frequently use catalysts to accelerate the interaction within the epoxy groups in EVOs and the carboxyl groups in acrylic acid, where the two most used catalysts are triethylamine (TEA) and triphenylphosphine oxide. Due to the potential applications of AEVOs in various sectors, such as UV-curable paints, additive manufacturing, and free radical polymerization, numerous investigations have been carried out to investigate their production. The main variables affecting the acrylation process are the molar concentration of EVO to acrylic acid, the rate of reaction, the nature and quantity of EVO, and the catalyst and inhibitor used.110 The primary purpose of an inhibitor is to prevent homo-polymerization during the reaction process. Various inhibitors, such as 4-methoxyphenol,111 hydroquinone,112 and 4-tert-butylcatechol,113 are employed in acrylation reactions. However, hydroquinone is frequently preferred in numerous studies due to its cost-effectiveness. Additionally, the likelihood of homo-polymerization is influenced by reaction conditions, particularly the reaction temperature. Generally, a lower iodine value of the vegetable oil corresponds to a higher reaction temperature, as indicated in the literature. Nonetheless, elevated reaction temperatures may lead to product degradation, necessitating the optimization of the temperature during the process.114,115Table 3 summarizes the acrylation of various EVOs and details the catalysts and inhibitors employed in acrylation processes.
| S. no | Vegetable oil | Molar ratio | Catalyst/inhibitor | Temp. (°C) | Reaction time (h) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Palm | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.52 1.52 |
1% Triethylamine/1% 4-methoxyphenol | 110 | 16 | 111 |
| 2 | Palm | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 2.2 |
Triethylamine/hydroquinone | 80 | 14 | 116 |
| 3 | Soybean | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 1.25 |
1.5% triphenylphosphine oxide/0.15% 4-tert-butylcatechol | 120 | 2.5 | 117 |
| 4 | Jatropha | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2% Triethylamine and acrylic acid 1% 4-methoxyphenol | 110 | 0.5 | 85 |
| 5 | Castor | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
4% Triethylamine/0.1% hydroquinone | 60–70 | 2.5 | 87 |
| 6 | Castor | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
4% Triethylamine/0.5% hydroquinone | 60 | 2 | 118 |
| 7 | Flaxseed | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
— | 75 | 6 | 86 |
| 8 | Flaxseed (linseed) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
8% Triphenylphosphine | 75 | 1.5 | 119 |
| 9 | Rice bran | — | 0.5% N,N-Dimethyl aniline/0.04% hydroquinone | 95 | 10 | 120 |
In addition, researchers have discovered an alternative approach to transform VO directly into its acrylate without the need for prior epoxidation, which is known as a one-step acrylation process. This method employs a catalyst such as the boron trifluoride diethyl ether complex (BF3·Et2O), a widely used Lewis acid, in conjunction with acrylic acid and VO. As a catalyst, BF3·Et2O activates the acylating agent, which is usually acrylic acid, by generating an acylium ion intermediate. Subsequently, this activated species reacts with VO, integrating an acyl group into the oil molecules. The BF3·Et2O catalyst accelerates the reaction rate, enhancing the efficiency and contributing to the selectivity of the process, favouring the desired acrylation pathway.121,122 Incorporating BF3·Et2O in the acylation reaction facilitates the production of acrylate VO with improved yields and controlled properties. Table 4 illustrates various VOs successfully converted into acrylate using this method.
| S. no. | Oil | Catalyst | Reaction time (h) | Additional information | Ref. |
|---|---|---|---|---|---|
| 1 | Linseed | BF3·Et2O | 2 | Bio-based raw ingredient for coatings that resist corrosion | 123 |
| 2 | Soybean | BF3·Et2O | Variable from 2 to 24 | Two different work-up procedures were employed depending on the batch size | 124 |
| 3 | Different oils | BF3·Et2O | 2 | Application in UV-curable coatings | 125 |
| 4 | Soybean | BF3·Et2O | 10 | UV curable resin was formed for DLP | 126 |
| 5 | Rapeseed, grapeseed and linseed | BF3·Et2O | 5 | UV curable resin was formed for a modified syringe-type 3D printer | 47 |
| 6 | Soybean | A range of catalysts used | Variable from 2 to 24 | Lewis acid such as BF3·Et2O demonstrated higher catalytic activity than protic acids | 127 |
Thus, numerous oils described in the literature have undergone epoxidation and acrylation to cater to various applications. For example, neem oil underwent epoxidation to serve as a potential substitute for compounds containing total distillate aromatic extract (TDAE). However, it is crucial to conduct detailed studies on neem oil due to its toxicity, which can lead to contact allergies, lung irritation, and seizures, before considering its application in various uses.80 Cardanol, extracted oil from cashew nut shells, has been modified through acrylation and epoxidation to produce cardanol-based acrylate. These modified compounds have demonstrated the ability to form polymers by incorporating the Darocur 1173 photoinitiator. Their potential for use in UV-curable coatings is promising, given their overall excellent performance as coating materials.128 In another study, cardanol was chemically modified by reacting it with acryloyl chloride, forming cardanyl acrylate. This derivative exhibited desirable flexibility, hydrophobicity, and thermal resistance.129 Castor oil (CO) has become a focal point of research due to its cost-effectiveness and significance as a valuable biomass resource. Researchers have been particularly interested in developing polyurethane acrylate (PUA) from CO without additional modifications. One study reported the synthesis of a CO/pentaerythritol triacrylate-based PUA, utilizing a combination of CO, isophorone diisocyanate, dimethylol butyric acid, and poly(caprolactone diol).130 A different successful effort also created a sustainable polyfunctional PUA using CO.131 Castor oil, which is produced from the kernels of the castor plant, has enormous potential as a biomass resource that is both sustainable and commercially successful.132 Its unique chemical composition, mainly ricinoleic acid, makes it suitable for diverse applications in various industries.133 Researchers have taken a keen interest in exploring its use in producing PUA without requiring complex modifications or chemical treatments.131 Overall, these research efforts demonstrate the potential of castor oil as a valuable and readily available resource for synthesizing PUA, opening opportunities for environmentally friendly and renewable materials in coating applications.
Moreover, these acrylates, sourced from various origins, including vegetable oils, can be employed in creating UV-based resins suitable for a wide range of 3D printers, facilitating the production of valuable bio-based products. The following section explores the formation of acrylate 3D-printable resins that are suitable for DLP, SLA, and UV-based material extrusion systems in detail.
4.2. Acrylate resin formation and its 3D printing
The production of a 3D printable resin begins with acquiring AEVOs. Synthesizing the resin involves combining calculated amounts (according to the molar ratio) of the synthesized AEVO and acetone and thoroughly mixing them using a magnetic stirrer. A precisely measured quantity of photoinitiator is added to the mixture. The resulting solution is put in a vacuum to eliminate the acetone solvent. The final product is a dark-coloured liquid (resin), which can be employed for 3D printing.134 Different photoinitiators correspond to various wavelengths of light used in the 3D printing process, which are specifically compatible with acrylates. Initially, synthetically derived acrylates were employed as materials for 3D printing.There are various approaches to incorporate these eco-friendly resins in 3D printing for the fabrication of product, including utilizing neat acrylate resins, hybrid resins, and composite resins. Some studies chose to employ pure acrylates sourced from EVOs for 3D printing, without any extra comonomers or diluents. For instance, Miao et al. successfully printed bio-scaffolds using AESO with a cutting-edge, in-house stereolithography (SLA) printer that matches or surpasses commercially available systems. They utilized a 355 nm ultraviolet (UV) laser in their printing process.7 Danish et al. used an AESO scaffold and a micro-SLA printer to produce it. In the micro-SLA system, the laser defines the structures and cures the material along the designated pattern by obtaining the pattern from the CAD program, producing a solidified layer. In addition to the micro-SLA parameters, post-curing was required to obtain a better surface finish at roughly 50 µm thickness.134 Y. Liu et al.135 devised a novel approach by designing and creating a versatile photocurable resin for 3D printing. They achieved this by utilizing waste cooking oil as the primary raw material, employing epoxidation and ring-opening esterification methods for the first time. These studies show that VOs can undergo chemical changes to transform into polymers, which can be used for 3D printing in various exciting applications. In a pioneering study, photoactive VO acrylates were also effectively printed through a syringe-based printer equipped with ultra-violet LEDs and a print nozzle with a diameter of 0.8 mm. This approach enables precise material utilization, design adaptability, streamlined processes, and cost efficiency. Consequently, resins were obtained via an optimized one-step acrylation process, enhancing the performance using rapeseed, grapeseed, and linseed oils selected based on their fatty acid composition and agricultural potential, as shown in Fig. 12.47
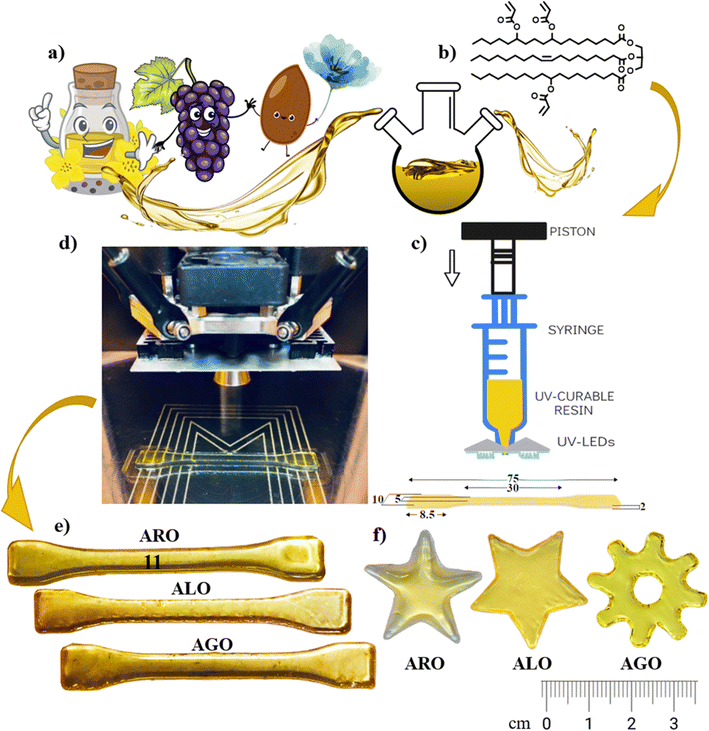 | ||
| Fig. 12 Utilizing vegetable-based oil in syringe-based printing: (a) initial ingredients, (b) structure of acrylated vegetable oil post-synthesis, (c) syringe extruder head, (d) printing procedure, (e) dog-bone-shaped models printed from acrylated vegetable oil, and (f) illustrations of complex geometric forms (reproduced from ref. 47 with permission from The Royal Society of Chemistry, Copyright 2022). | ||
Besides acrylation, another method to eliminate the unsaturation in oils is methacrylation. Typically, acrylate systems are recognized for their fast curing, leading to extensively cross-linked and non-uniform polymer structures that may become brittle and display reduced toughness. Alternatively, methacrylates are less responsive than acrylates and can increase the stiffness and yield strength when added to photocurable resins.136–138
Nonetheless, certain researchers have noted that acrylates obtained from VOs tend to have elevated viscosity at ambient temperature, leading to challenges in flow during the 3D printing process and resulting in frequent printing flaws together with inadequate mechanical characteristics.139 Thus, to address these concerns, a hybrid resin was formulated at a specific temperature by blending thinning agents or co-monomers such as triethylene glycol diacrylate140 and phenoxyethyl acrylate (PHEA)135 with acrylates derived from VO, together with the addition of a photoinitiator. The primary bio-derived acrylates commonly employed in most studies are predominantly sourced from soybean oil due to their easy commercial availability and cost-effectiveness in forming hybrid resins. Nevertheless, the photoinitiators utilized in several investigations mostly consisted of ethyl(2,4,6-trimethylbenzoyl)phenyl phosphinate141–144 and 2,4,6-trimethylbenzoyldiphenylphosphine oxide.145 Several studies have explored the development of hybrid resins through the inclusion of co-monomers to enhance their thermal stability at high temperatures and improve their glass transition temperature. In one investigation, two reactive monomers, namely 1,6-hexanediol diacrylate and trimethylolpropane triacrylate, were chosen to optimize the 3D printing conditions and enhance the thermal properties of the polymer after photocuring. The results indicated that the addition of suitable reactive comonomers could increase the glass transition temperature by 10 °C, increase the thermal degradation temperature by 28 °C, and reduce the required resin photocuring time by nearly half, from 4 s to 2 s.141 In a unique study, different models were printed using green melanized AESO with the addition of 50% of hydroxyethyl methacrylate through a DLP printer, and the printed products are shown in Fig. 13.145 An overview of bio-based hybrid resin formulations, together with their associated 3D printing technique, is provided in Table 5.
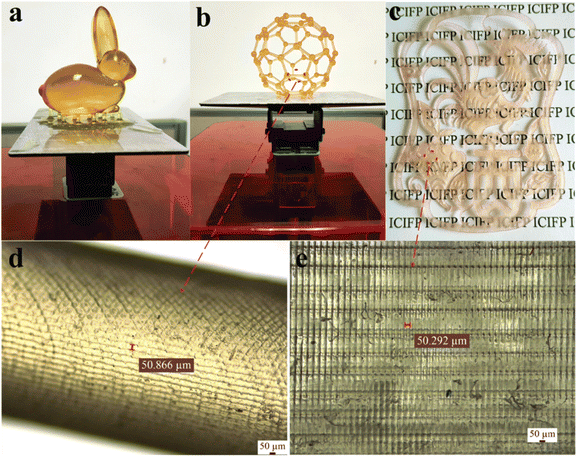 | ||
| Fig. 13 Three different models produced utilizing green melanized AESO-based hybrid resin. These models include (a) rabbit model, (b) footballene model and (c) planar pattern model. Additionally, surface images of both (d) footballene model and (e) planar pattern (reproduced from ref. 145 with permission from The Royal Society of Chemistry, Copyright 2021). | ||
| S. no | Bio-based material | Comonomers | Photo-initiator | Printing technique | Ref. |
|---|---|---|---|---|---|
| a TPO and TPOL refer to ethyl(2,4,6-trimethylbenzoyl) phenyl phosphinate and 2,4,6-trimethylbenzoyldiphenylphosphine oxide, respectively. | |||||
| 1 | Acrylated epoxidized soybean oil | 1,6-Hexanediol diacrylate, trimethylolpropane triacrylate | TPOa | SLA | 141 |
| 2 | Urethane epoxidized soybean oil | Difunctional epoxy acrylate, isobornyl acrylate | TPOa | SLA | 142 |
| 3 | Acrylated epoxidized soybean oil | 2-Hydroxy-2-phenoxypropyl acrylate | TPOLa | DLP | 146 |
| 4 | Acrylated epoxidized soybean oil | Vanillin dimethacrylate, vanillin diacrylate | — | Direct laser writing | 139 |
| 5 | Acrylated epoxidized soybean oil, epoxidized linseed oil | Benzene-1,3-dithiol, pentaerythritol tetra(3-mercaptopropionate) | TPOLa | Direct laser writing | 144 |
| 6 | Acrylated epoxidized soybean oil | Isobornyl methacrylate (IBOMA) | TPOa | SLA | 143 |
| 7 | Acrylated epoxidized soybean oil | 1,6-Hexanediol diacrylate, trimethylolpropane triacrylate | TPOa | SLA | 147 |
| 8 | Rubber seed oil-based polyurethane acrylate | Triethylene glycol diacrylate, trimethylolpropane triacrylate | 2-Hydroxy-2-methyl-phenyl-propane-1-one | DLP | 140 |
| 9 | Waste cooking oil-based acrylate | 2-Phenoxyethyl acrylate | TPOa | LCD 3D printer | 135 |
The utilization of 3D printing with composite resins represents an innovative strategy within this domain aimed at enhancing the overall characteristics of the final product. Composite materials, being heterogeneous in nature, are comprised of multiple phases with distinct physical properties. When amalgamated, these phases result in a material exhibiting enhanced mechanical and chemical–physical attributes compared to its individual constituents. In the context of composite biomaterials derived from VOs, the oils serve as the predominant components forming the matrix, while nanomaterial fillers, added in minor quantities, act as reinforcements to bolster their mechanical properties.12 Various filler materials have been incorporated in matrix materials derived from VOs to improve the properties of the product. These reinforcement materials include Macadamia nut shells,148 hydroxyapatite,149,150 walnut shell,151 calcium silicate hydrate,152 and cellulose crystals.153–155 However, the 3D printing technique has been adapted based on the viscosity of the composite resin and the desired properties of the printed product. In one investigation, formulations containing epoxy linseed oil (ELO) and soybean oil (ESO), as well as different fillers such as walnut shell, tagua, and hemp powder, were examined. The photoinitiator S-BF5 was utilized at 3 parts per hundred resin (phr) in relation to the epoxy resin, while the type of filler varied across different formulations. Encouraging results were achieved in terms of improved rigidity and toughness. Nevertheless, the SEM analysis revealed some imperfections, which was likely attributed to the presence of fillers and the associated scattering and absorption phenomena, potentially compromising the precision of the 3D printed structures (Fig. 14).151Table 6 summarizes the formulated composites, including information on their bio-based matrix material and reinforcement.
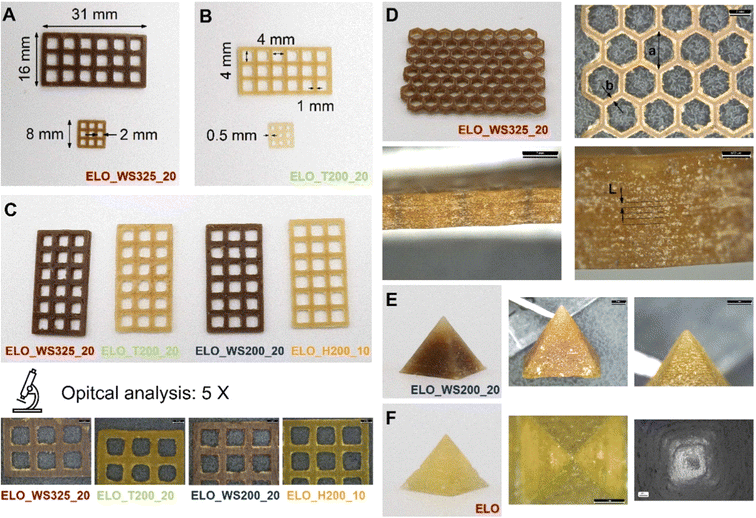 | ||
| Fig. 14 (A–C) Three-dimensional printed lattice structures produced using epoxy linseed oil (ELO) together with various fillers in significant proportions. (D) Honeycomb lattice structure fabricated using ELO mixed with walnut shell filler, with dimensions of 70 × 40 × 4 mm and subjected to optical analysis. (E) Pyramid with a square base, constructed from ELO mixed with walnut shell filler, imaged optically at 5× magnification. (F) ELO-based pyramid examined using optical and scanning electron microscopy (SEM) at magnifications of 10× and 100×, respectively. (reproduced from ref. 151 with permission from Elsevier, Copyright 2024). | ||
| S. no | Matrix material | Reinforcement | 3D printing technique | Additional remarks | Ref. |
|---|---|---|---|---|---|
| 1 | Acrylated soybean oil | Macadamia nut shells | DLP | Increasing glass transition temperature and creating stiffer thermosets with higher Young's modulus | 148 |
| 2 | Acrylated epoxidized soybean oil | Silanized nanohydroxyapatite | DIW | The addition of reinforcement resulted in enhanced mechanical properties | 149 |
| 3 | Epoxy linseed oil and soybean oil | Walnut shell, tagua and hemp | SLA | Hot lithography process boosted the reactivity of epoxy vegetable oils, enabling their use in additive manufacturing | 151 |
| 4 | Acrylated epoxidized soybean oil | Calcium silicate hydrate | DLP | The reinforcement material was derived from aluminium fluoride production waste | 152 |
| 5 | Acrylated soybean oil | Ethyl cellulose | CNC-based 3D extruder | Elevated glass transition temperature and stable thermal properties at high temperatures | 153 |
| 6 | Acrylated soybean oil | Nanocellulose fibrils and crystals | SLA | Enhancing sustainable vat photopolymerization resins with fillers resolves compatibility issues | 154 |
| 7 | Acrylated epoxidized soybean oil | Nanohydroxyapatite | UV-based extrusion printer | Achieved excellent cell viability and proliferation on 3D printed nanocomposite scaffolds | 150 |
DLP and SLA technologies have been applied in product prototyping, modeling, and medical device fabrication. They are used to create accurate prototypes and models for various products during the design and development phase. The ability to develop medical devices such as implants for dentistry and patient-specific scaffolding for regenerating tissues has also given them relevance in the medical field. In product prototyping and modeling, SLA and DLP are preferred due to their ability to produce intricate and highly detailed three-dimensional objects. Manufacturers and designers use these techniques to quickly create prototypes, allowing them to assess the functionality, aesthetics, and ergonomics of the design before its mass production.138,156 Specifically, the DLP technique allows the complete curing of a layer of photocurable resin simultaneously, leading to significantly faster printing than other vat photopolymerization techniques, which utilize a point-by-point curing process. Additionally, due to recent advancements in DLP 3D technology, such as continuous liquid interface production (CLIP), printing speeds up to 100 times faster have been achieved.157
Nevertheless, the parameters employed in DLP/SLA printers, such as the type of UV source,158 exposure time,159 laser frequency (in the case of SLA),134,160 layer thickness (both for the first layer and subsequent layers),161 and post-curing time,162 are crucial in customizing the properties of the end product. Table 7 provides an overview of the parameters utilized in DLP/SLA machines for printing with VOs.
| S. no. | Oil | SLA/DLP | Printing parameters | Post curing time (min) | Additional information | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| UV source | Wavelength (nm) | Layer thickness (mm) | Exposure time | ||||||
| 1 | Soybean | SLA | 25 W LED | 405 | 0.5 | 35 s for first 10 layers, later 7.5 s for each layer | 3 | To reduce irradiation time, suitable functional comonomer trimethylolpropane triacrylate are incorporated | 147 |
| 2 | Soybean | SLA | Laser, 20 µJ at 15 kHz | 355 | Variable | — | — | Laser frequency affects thickness and width of struts | 11 |
| 3 | Soybean | SLA | Laser 150 mW | 405 | 0.1 | 40–60 s | 60 | Samples were printed at a constant 30 °C using an integrated heater to improve mechanical properties | 163 |
| 4 | Soybean | Mask SLA | — | — | 0.05 | 60 s for first six layers, and then 50 s for each layer | — | Polyethylene glycol diacrylate improves resin viscosity and crosslinking density | 164 |
| 5 | Soybean | DLP | 25 mW cm−2 | 405 | 0.05 | The curing depth reached 500 µm as the exposure time was increased in 1 s intervals | — | Isobornyl methacrylate serves as a diluent, while Fe3O4 imparts a shape memory effect to the printed parts | 165 |
| 6 | Soybean | SLA | 15 mW laser power | 405 | — | Variable laser frequencies | — | Printing speeds were set at 60, 80, and 100 mm s−1, and laser power frequencies were adjusted to 140, 160, and 180 pulses/s | 134 |
| 7 | Soybean | DLP | 72 W | 405 | 0.05 | 8–10 s for each layer | 10 | A biobased UV-curable oligomer was developed by combining epoxidized soybean oil with gallic acid | 145 |
| 8 | Soybean | DLP | — | 390–450 | 0.1 | 1 to 12 s, using an interval of 1 s | 30 | 12 different segments were exposed at different intervals | 138 |
| 9 | Palm | DLP | 40 W LCD | 405 | 0.05 | 15 s for each layer | 5 | Isobornyl acrylate was chosen as a comonomer with palm oil-based methacrylate to create photosensitive inks | 166 |
| 10 | Waste cooking | DLP | 12 W LCD | 405 | 0.05 | Bottom exposure: 120 s, normal exposure: 19 s | — | During synthesis, 2-phenoxyethyl acrylate and methacrylic acid were included | 135 |
Nevertheless, transforming raw VOs into a 3D printable resin is complex and time-consuming. However, when the process is standardized, it becomes more accessible even for those with limited experience, allowing the efficient creation of resins. Fig. 15 provides a concise flowchart that visually depicts the entire process, starting from raw VO and culminating in the 3D printed product, which can be particularly helpful in this context.
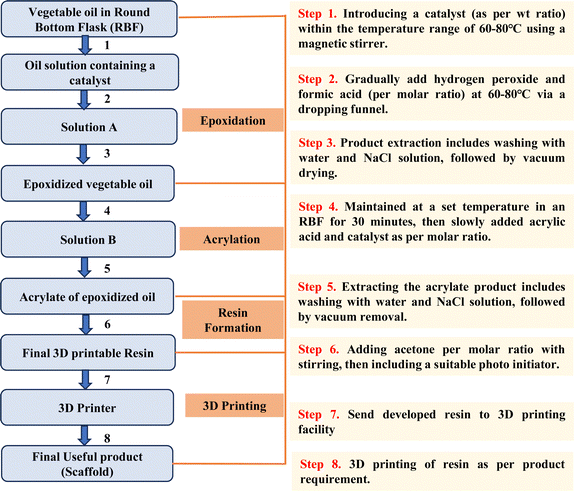 | ||
| Fig. 15 Flowchart illustrating the entire process from raw vegetable oil to the end product developed via 3D printing. | ||
5. Potential application of 3D printed bio-based polymer sourced from VOs
The application of the currently available bio-based polymers has been demonstrated in various fields such as tissue engineering,167 food production,168 drug delivery,169 bioimplants,170 and soft robotics.171,172 In tissue engineering, skin bioprinting is a significant area of advancement for 3D bioprinting, which has been used to manufacture diverse tissue-like constructions.173,174 The exact deposition of various cells and biological materials made possible by 3D bioprinting improves cell communication. It produces more intricate tissue-engineered skin with characteristics such as skin tone,175 hair embryo,176 and sweat glandule.177In pharmaceutical applications, bio-based polymers show great potential for drug delivery due to their ability to design flexible systems that respond dynamically. By incorporating drugs into 3D-printed bio-based polymer structures, it becomes possible to develop drug delivery devices capable of reacting to specific biological cues, releasing medications in a controlled and targeted manner.178 In this case, the concept of 4D printing becomes relevant. 4D printing, an evolution of 3D printing, introduces time as a dimension. Going beyond the capabilities of traditional 3D printing, it enables the creation of objects that can autonomously transform, adapt, or even heal themselves.179,180 This is achieved using materials that undergo changes in size, shape, and properties in response to external stimuli post-initial printing. Exciting biomedical applications, such as ortho and cardiac implants, braces, stents, epidermal dressings, and tissue-engineered scaffolding, are possible when stimuli-responsive bio-based polymers are used in 3D printing.181 Numerous investigations demonstrated the creation of stents for biomedical purposes, with researchers incorporating magnetic-responsive materials such as Fe3O4 and metal wire into bio-based polymers.182,183 This addition enables rapid shape recovery within seconds, achieving a notably high recovery rate. The noteworthy achievement is the successful fusion of 4D shape-changing entities with swift remote activation, showcasing magnetically driven features primarily attributed to the incorporation of Fe3O4. In the study by Zhang et al.,182 a variety of tracheal stents with shape memory poly-lactic acid (Fe3O4) composites featuring diverse structural designs was produced (Fig. 16). The incorporated magnetic particles (Fe3O4) endowed the shape memory PLA/Fe3O4 composite tracheal stent with magnetic driving capabilities. When subjected to a magnetic field, the stent rapidly achieved shape recovery. Moreover, bio-based polymers enable the development of soft actuators, sensors, and structures that mimic natural movements and interactions. Thus, their integration in soft robotics enhances the adaptability, safety, and efficiency of various applications.
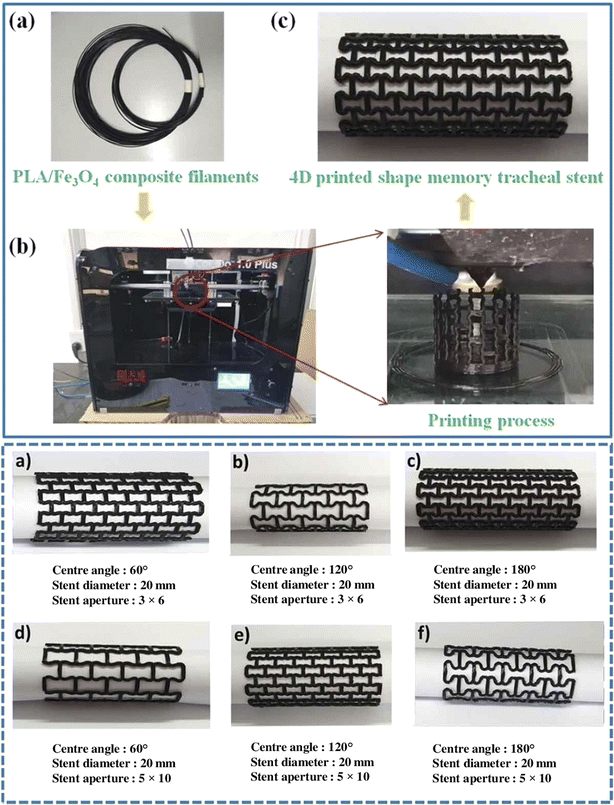 | ||
| Fig. 16 (Top) (a) Filaments composed of shape memory PLA/Fe3O4 composite with memory capabilities. (b) Usage of a standard FDM printer and its printing procedure. (c) Production of a tracheal stent using 4D printing technology from shape memory PLA/Fe3O4 composite material. (Bottom) Tracheal stents with dynamic shape memory properties (reproduced from ref. 182 with permission from Taylor & Francis, Copyright 2021). | ||
In the previously mentioned application, the primary bio-based polymers employed are predominantly synthetic, such as PLA. Thus far, limited research has been documented regarding the use of 3D printed products derived from VOs, despite the vast potential applications of EVOs and their acrylates. Nevertheless, the diverse potential applications of 3D printed VOs have been explored, with reported research focusing on shape memory scaffolds, implants, and stents.135,184 The use of bio-based polymers from VOs and possessing the shape memory attribute offers potential for advancing biomedical devices and fabricating smart materials and responsive structures. In a study, changing the configuration of the printer allowed the seamless construction of porous scaffolds. By adjusting the laser power and print rate, the solidified polymerizing soybean oil epoxidized acrylic exterior configurations were specifically molded. Notably, examinations involving shape memory underscored that the scaffold maintained a defined temporary shape even when exposed to temperatures as low as −18 °C, whereas when the temperature was elevated to 37 °C, it flawlessly restored its original form, as shown in Fig. 17. These findings serve as a compelling indicator of the feasibility of the scaffold in 4D printing, highlighting its potential to dynamically transform its structure over time in response to specific triggers. The cytotoxicity test revealed better adhesion and growth on the printed scaffolds than PEGDA, such as PLA and PCL.11
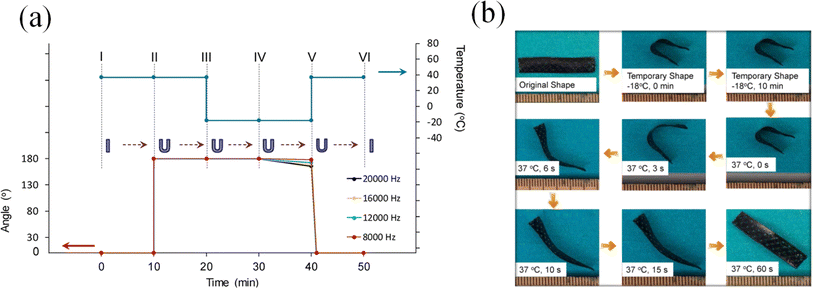 | ||
| Fig. 17 (a) Shape memory loop for the produced scaffolds under varying laser frequencies: (I and II) the scaffold remained at 37 °C for 10 min. (II and III) The scaffold was turned around and maintained at 37 °C for 10 min. (III and IV) The bent scaffold was held at −18 °C for 10 min. (IV and V) The external support was removed, and the scaffold was kept at −18 °C for an additional 10 min to assess its shape fixity. (V and VI) The scaffold was subjected to 37 °C for 10 min to restore its original shape. (b) Shape memory cycle demonstration using a printed sample that was intentionally stained black to enhance the contrast against the background (reproduced from ref. 11 with permission from Springer Nature, Copyright 2016). | ||
Danish et al.134 conducted a similar study with AESO using micro-SLA laser scanning. The objective was to correlate the material characteristics with the 4D printing parameters (laser power and printing rate). AESO showcased a shape memory phenomenon with ideal parameters, resulting in a 1.6 s recuperation period and an 85% fixity rate. This capability allowed the creation of various scaffold configurations. An important research endeavour employed a resin derived from waste cooking oil, specifically combining epoxy waste oil methacrylate, 2-phenoxyethyl acrylate, and methacrylic acid as the monomeric components. This mixture was subjected to 3D printing using light-curing techniques. The resulting material showcased notable traits, including exceptional flexibility, practical pressure-sensitive adhesion characteristics, and the ability to undergo thermally induced shape memory behaviours effectively. These behaviours encompassed deformation and subsequent recovery at room temperature and maintaining a fixed shape at a lower temperature of −12 °C (Fig. 18).135
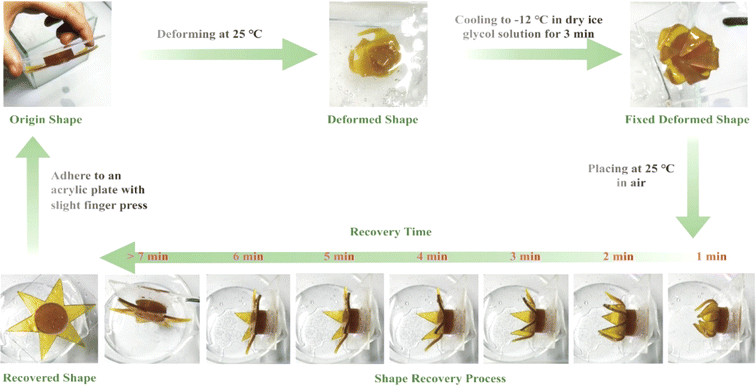 | ||
| Fig. 18 Waste cooking oil (WCO) resin 4D printing star shape-retaining cycle (reproduced from ref. 135 with permission from the American Chemical Society, Copyright 2022). | ||
In a study, nanocomposite scaffolds made from AESO, nano-hydroxyapatite (nHA) rods, and either 2-hydroxyethyl acrylate (HEA) or polyethylene glycol diacrylate (PEGDA), were 3D printed and tested for their mechanical properties and cell growth support. Human bone marrow-derived mesenchymal stem cells (BM-MSCs) were cultured on them for 7, 14, and 21 days. All the scaffold types showed a controlled morphology and supported cell growth and differentiation, but the nature of the ink functional groups affected their mechanical properties and cell compatibility. HEA improved certain aspects despite reducing the nHA dispersion and tensile strength.150Fig. 19 shows digital photographs depicting the nanocomposite scaffolds in their as-printed state.
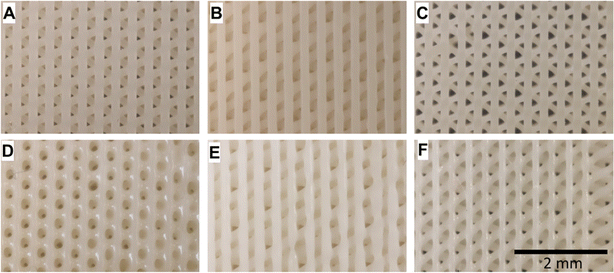 | ||
| Fig. 19 Digital images showing nanocomposite scaffolds in their original printed state. (A–C) Underside (base) and (D–F) upper surfaces of the scaffolds (reproduced from ref. 150 with permission from Elsevier, Copyright 2021). | ||
6. Future directions
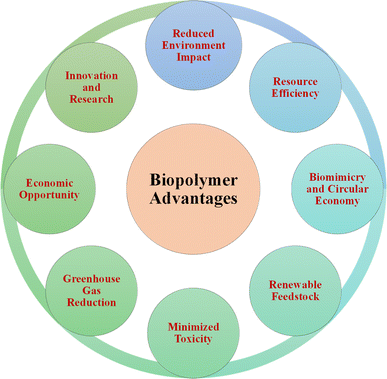
The use of bio-based polymers is essential for sustainable development. Given that they are derived from renewable sources such as plants and microorganisms, these polymers offer benefits that align with sustainability. Specifically, they reduce the reliance on fossil fuels, promote environmental conservation, and minimize the carbon footprint. Also, their biodegradability addresses plastic pollution and supports recycling, while their production requires less energy, reducing their environmental impact. Bio-based polymers enable the creation of innovative, sustainable products such as biodegradable packaging and medical implants, contributing to the sustainability goals in various industries.185 The advantageous aspects of biopolymers promoting sustainable development are illustrated in Fig. 20. The biological cycle is dominated by biopolymers produced by life forms from sustainable biomass. Common natural biopolymers include cellulose, starch, protein, and chitin, which are abundant in nature. The amount of cellulose on Earth is believed to be two teratons each year, making it the most plentiful renewable resource.186 Chitin, hemicellulose, and lignin contribute significantly, with annual volumes of about one hundred billion, sixty billion, and ten billion tons, respectively.187 To put this in perspective, human-produced plastics since 1950 amount to 8.3 billion tons,188 with 367 million tons produced annually in 2020.189Nevertheless, bio-based polymers also exhibit certain drawbacks. For instance, complications have arisen from the fracture of implants and screws made from PLA materials, leading to infections. The representative study involved a 16-year-old patient who had posterior cruciate ligament restoration and developed synovitis two years after implant due to the failure of a PLA screw.190 Similarly, in another investigation, a patient experienced pain and swelling one year after anterior cruciate ligament surgical repair, which was attributed to the fracture of a PLA-based biodegradable screw and the subsequent migration of its head within the joint.191 Similar adverse reactions have also been reported in various other studies.192–194 The exposure of bio-based polymers to the external environment during their production phase can result in contamination by heavy metals and microorganisms. However, using preservatives can effectively prevent this contamination.195 The possible risk of viral infections is a significant issue associated with contamination, as shown by the spread of parvovirus B19 via infected fibrin in the study by Hino et al.196
However, issues such as batch-to-batch inconsistency and delayed processing make it challenging to produce natural polymers on a large scale. In this context, transforming VOs into resins can potentially bring about a revolutionary advancement in sustainable development. These oils are not only biodegradable but also abundantly available worldwide. In Ayurveda, numerous oils with medicinal value are available, presenting versatile applications such as biomedicines, biosensors, paper and textile production, automobiles, and various other fields. For instance, neem oil, which has been recognized for its potent medicinal properties for over two millennia, holds significant promise. Its historical use dates to ancient India and neighboring regions, where the neem tree (Azadirachta indica) was revered as a versatile medicinal plant.197 The therapeutic applications of neem oil encompass a wide range of treatments, including cooling excessive pitta, supporting a healthy blood system (Rasa and Rakta Dhatus), enhancing the natural immunity, particularly in the context of skin health, providing lubrication and soothing effects on the skin, and facilitating the rejuvenation of healthy tissue.198 In addition to neem oil, oils such as castor, olive, sesame, almond, jojoba, sunflower, and flaxseed oil are included not only in Ayurveda but also in the medicinal practices of Unani and Homeopathy.199
In this manner, polymers made from soybean oil, rapeseed oil, linseed oil, and many others have already paved the way for exploring other oil sources for diverse applications in 3D printing. Nevertheless, several significant challenges are associated with the 3D printing of VO-based polymers, including the relatively sluggish building speed and the relatively modest mechanical properties of the printed products. The first difficulty is that these polymers cannot be used in their native forms for 3D printing, demanding considerable efforts to transform them into valuable materials. These efforts often involve physical blending, chemical modification, conversion mechanisms, and incorporating environmentally friendly additives.200 Notably, essential elements, including the amount of soft bio-based polymers, molecular mass, and nature, are crucial in defining the viscosity, gelation behavior, and mechanical characteristics of the final 3D-printed product. Although several VO-based polymers have already achieved successful 3D printing, there are still some hurdles to be overcome as other oil-based polymers are explored for their potential applications. Addressing these challenges is essential to unlock the full potential of 3D printing with VO-based polymers, ultimately contributing to the advancement of sustainable manufacturing practices.
7. Conclusion
Bio-based polymers from natural origins have been employed in diverse applications across various sectors, including healthcare. Advanced manufacturing (AM) techniques tailored for bio-based polymers have evolved, enabling intricate designs and minimizing waste. This collaboration holds significant potential for industries such as tissue engineering and drug delivery. Plant-based oils, also known as vegetable oils (VOs), are renewable resources derived from plants and trees, primarily comprising triglycerides composed of glycerol and multiple fatty acids. Triglycerides can undergo polymerization via inherent functional groups or chemical modifications, developing a diverse array of properties. These chemically modified epoxidized oils have various applications, including plasticizers, coatings, adhesives, and more. Furthermore, to enhance their value, epoxidized oils can be transformed into acrylates through modification, which have applications in polymer additives, biodegradable plastics, paints, composite materials, photocurable polymers, dental materials, electronic encapsulation materials, and more. Among them, UV-based resins for 3D printing stand out as a major application of these acrylates. This is attributed to the ability to produce sustainable 3D printed products of intricate shapes and sizes, meeting market demands. Additionally, their customizable properties facilitate tailored mechanical, thermal, and rheological characteristics, ensuring versatility in various 3D printing applications. These resins readily accommodate additives to create hybrid and composite resins, enhancing their properties and ensuring their strong adhesion to the substrate for superior print quality. However, although soybean oil has made strides in 3D printing, other vegetable oils remain relatively unexplored. Thus, continued research in this domain is crucial for sustainable development. The possible uses of 3D and 4D printed biopolymers show great promise, especially in fields such as tissue engineering. In tissue engineering, 3D printing facilitates the development of intricate tissues featuring vascular networks, while in skin bioprinting, it drives advancements in skin engineering. Additionally, 4D printing, responsive to external stimuli, holds promise in drug delivery, adaptable biomedical devices, and soft robotics. Despite the challenges such as PLA implant fractures and contamination, vegetable oils (VOs) offer sustainable solutions. These bio-based polymers have the potential to revolutionize manufacturing across industries, promoting responsible practices for a better future. Ayurveda utilizes various oils for medicinal purposes, such as neem oil, which is known for its therapeutic benefits. Thus, transforming these VOs into 3D printable polymers represents a significant step towards sustainable development, leveraging their inherent properties and aligning with principles of environmental conservation. Utilizing these bio-based polymers can positively impact society and the planet, fostering responsible and eco-friendly manufacturing practices.Conflicts of interest
There are no conflicts to declare.References
- B. DeBoer, et al., Additive, subtractive, and formative manufacturing of metal components: a life cycle assessment comparison, Int. J. Adv. Des. Manuf. Technol., 2021, 115(1–2), 413–432 CrossRef.
- I. Gibson, D. Rosen, and B. Stucker, Development of Additive Manufacturing Technology, in Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, ed. I. Gibson, D. Rosen, and B. Stucker, Springer New York, New York, NY, 2015, pp. 19–42 Search PubMed.
- M. Despeisse, et al., Unlocking value for a circular economy through 3D printing: A research agenda, Technol. Forecast. Soc. Change, 2017, 115, 75–84 CrossRef.
- J.-Y. Lee, J. An and C. K. Chua, Fundamentals and applications of 3D printing for novel materials, Appl. Mater. Today, 2017, 7, 120–133 CrossRef.
- Z. Liu, et al., 3D printing: Printing precision and application in food sector, Trends Food Sci. Technol., 2017, 69, 83–94 CrossRef CAS.
- N. Shahrubudin, T. C. Lee and R. Ramlan, An overview on 3D printing technology: Technological, materials, and applications, Procedia Manuf., 2019, 35, 1286–1296 CrossRef.
- T. Shevchenko, et al., Promising Developments in Bio-Based Products as Alternatives to Conventional Plastics to Enable Circular Economy in Ukraine, Recycling, 2022, 7(20), 1–15 Search PubMed.
- L. Filiciotto and G. Rothenberg, Biodegradable plastics: Standards, policies, and impacts, ChemSusChem, 2021, 14(1), 56–72 CrossRef CAS PubMed.
- A. Russell, T. Ekvall and H. Baumann, Life cycle assessment – introduction and overview, J. Cleaner Prod., 2005, 13(13), 1207–1210 CrossRef.
- A. C. Lemos de Morais, et al., Chapter 3 – Biopolymers in additive manufacturing, in Additive Manufacturing of Biopolymers, ed. M. Mehrpouya and H. Vahabi, Elsevier, 2023, pp. 39–63 Search PubMed.
- S. Miao, et al., 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate, Sci. Rep., 2016, 6(1), 27226 CrossRef CAS PubMed.
- C. Nurchi, et al., Sustainable vegetable oil-based biomaterials: Synthesis and biomedical applications, Int. J. Mol. Sci., 2023, 24(3), 2153 CrossRef CAS PubMed.
- H. Wang, A. Gupta and B. S. Kim, Photo-crosslinked polymer networks based on graphene-functionalized soybean oil and their properties, Korean J. Chem. Eng., 2019, 36, 591–599 CrossRef CAS.
- M. Mehrpouya and H. Vahabi, Chapter 1 - Additive manufacturing of biopolymers, in Additive Manufacturing of Biopolymers, ed. M. Mehrpouya and H. Vahabi, Elsevier, 2023, pp. 1–10 Search PubMed.
- B. Regubalan, et al., 12 - Starch-based bionanocomposites for food packaging applications, in Bionanocomposites for Food Packaging Applications, ed. S. Ahmed, Woodhead Publishing, 2022, pp. 201–215 Search PubMed.
- V. Koncar, 2 - Composites and hybrid structures, in Smart Textiles for in Situ Monitoring of Composites, ed. V. Koncar, Woodhead Publishing, 2019, pp. 153–215 Search PubMed.
- J. An and K. F. Leong, Chapter 2 - Additive manufacturing and 3D printing techniques for biopolymers, in Additive Manufacturing of Biopolymers, ed. M. Mehrpouya and H. Vahabi, Elsevier, 2023, pp. 11–37 Search PubMed.
- A. Zielińska, et al., Scaffolds for drug delivery and tissue engineering: The role of genetics, J. Controlled Release, 2023, 359, 207–223 CrossRef PubMed.
- M. Mehrpouya, et al., 4D printing of shape memory polylactic acid (PLA), Polymer, 2021, 230, 124080 CrossRef CAS.
- C. K. Chua and K. F. Leong, 3D Printing and Additive Manufacturing: Principles and Applications (With Companion Media Pack)-Of Rapid Prototyping, World Scientific Publishing Company, 2014 Search PubMed.
- M. Mehrpouya, et al., Additive manufacturing of polyhydroxyalkanoates (PHAs) biopolymers: Materials, printing techniques, and applications, Mater. Sci. Eng., C, 2021, 127, 112216 CrossRef CAS PubMed.
- M. P. M. C. Mata, Ntopology Optimization Applied to Additive Manufactured Hydrofoil Wing Components. 2022 Search PubMed.
- S. Rast, Additive Manufacturing: Lattice and Minimal Surface Design Using nTopology. 2021 Search PubMed.
- J. Jafferson and H. Sharma, Design of 3D printable airless tyres using NTopology, Mater. Today: Proc., 2021, 46, 1147–1160 Search PubMed.
- J. P. Kruth, M. C. Leu and T. Nakagawa, Progress in Additive Manufacturing and Rapid Prototyping, CIRP Ann., 1998, 47(2), 525–540 CrossRef.
- W. Schaeffer, UV Curable Materials: Response and its relationship to power levels and lamp spectra, Pigm. Resin Technol., 1990, 19(10), 4–7 CrossRef.
- S. C. Lapin, et al., Stereolithography using vinyl ether-epoxide polymers, US pat., USOO5437964A, 1995 Search PubMed.
- J. P. Fouassier and J. F. Rabek, Radiation Curing in Polymer Science and Technology, Springer, Netherlands, 1993 Search PubMed.
- S. H. Huang, et al., Additive manufacturing and its societal impact: a literature review, J. Adv. Manuf. Technol., 2013, 67, 1191–1203 CrossRef.
- I. G. Ian Gibson, Additive Manufacturing Technologies 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, Springer, 2015 Search PubMed.
- A. Al Rashid, et al., Vat photopolymerization of polymers and polymer composites: Processes and applications, Addit. Manuf., 2021, 47, 102279 CAS.
- H. Gudapati, M. Dey and I. Ozbolat, A comprehensive review on droplet-based bioprinting: Past, present and future, Biomaterials, 2016, 102, 20–42 CrossRef CAS PubMed.
- J. M. Fernández-Pradas and P. Serra, Laser-induced forward transfer: a method for printing functional inks, Crystals, 2020, 10(8), 651 CrossRef.
- S. S. Crump, Apparatus and Method for Creating Three-Dimensional Objects, US pat., US00521329A, 1992 Search PubMed.
- C.-Y. Zhang, et al., Three-dimensional bioprinting of decellularized extracellular matrix-based bioinks for tissue engineering, Molecules, 2022, 27(11), 3442 CrossRef CAS PubMed.
- A. Mostafaei, et al., Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges, Prog. Mater. Sci., 2021, 119, 100707 CrossRef CAS.
- I. Shishkovsky and V. Scherbakov, Selective laser sintering of biopolymers with micro and nano ceramic additives for medicine, Phys. Procedia, 2012, 39, 491–499 CrossRef CAS.
- Y. Zhang, et al., Additive manufacturing of metallic materials: a review, J. Mater. Eng. Perform., 2018, 27, 1–13 CrossRef CAS.
- B. Dermeik and N. Travitzky, Laminated object manufacturing of ceramic‐based materials, Adv. Eng. Mater., 2020, 22(9), 2000256 CrossRef CAS.
- A. Pilipović, Sheet lamination, in Polymers for 3D Printing, Elsevier, 2022, pp. 127–136 Search PubMed.
- F. S. Senatov, et al., Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds, J. Mech. Behav. Biomed. Mater., 2016, 57, 139–148 CrossRef CAS PubMed.
- V. DeStefano, S. Khan and A. Tabada, Applications of PLA in modern medicine, Eng. Regen., 2020, 1, 76–87 Search PubMed.
- X. Yang, et al., The application of polycaprolactone in three-dimensional printing scaffolds for bone tissue engineering, Polymers, 2021, 13(16), 2754 CrossRef CAS PubMed.
- A. C. Dell, et al., 3D Bioprinting Using Hydrogels: Cell Inks and Tissue Engineering Applications, Pharmaceutics, 2022, 14(12), 2596 CrossRef CAS PubMed.
- D. Chimene, et al., Advanced bioinks for 3D printing: a materials science perspective, Ann. Biomed. Eng., 2016, 44, 2090–2102 CrossRef PubMed.
- H. Bhanushali, S. Mestry and S. Mhaske, Castor oil‐based UV‐curable polyurethane acrylate resins for digital light processing (DLP) 3D printing technology, J. Appl. Polym. Sci., 2023, 140(18), e53817 CrossRef CAS.
- S. Briede, et al., State-of-the-art UV-assisted 3D printing via a rapid syringe-extrusion approach for photoactive vegetable oil acrylates produced in one-step synthesis, Mol. Syst. Des. Eng., 2022, 7(11), 1434–1448 RSC.
- S. N. Khot, et al., Development and application of triglyceride‐based polymers and composites, J. Appl. Polym. Sci., 2001, 82(3), 703–723 CrossRef CAS.
- R. P. Wool, Polymers and composite resins from plant oils, Bio-Based Polymers and Composites, 2005, vol. 1: p. 50005 Search PubMed.
- L. Montero de Espinosa and M. A. R. Meier, Plant oils: The perfect renewable resource for polymer science?, Eur. Polym. J., 2011, 47(5), 837–852 CrossRef CAS.
- K. Liu, Soybeans: Chemistry, Technology, and Utilization, Springer, 2012 Search PubMed.
- G. V. Olivieri, J. V. de Quadros Jr and R. Giudici, Epoxidation reaction of soybean oil: experimental study and comprehensive kinetic modeling, Ind. Eng. Chem. Res., 2020, 59(42), 18808–18823 CrossRef CAS.
- T. E. Clemente and E. B. Cahoon, Soybean Oil: Genetic Approaches for Modification of Functionality and Total Content, Plant Physiol., 2009, 151(3), 1030–1040 CrossRef CAS PubMed.
- B. Rangarajan, et al., Kinetic parameters of a two-phase model for in situ epoxidation of soybean oil, J. Am. Oil Chem. Soc., 1995, 72, 1161–1169 CrossRef CAS.
- F. Zaher, M. El-Mallah and M. El-Hefnawy, Kinetics of oxirane cleavage in epoxidized soybean oil, J. Am. Oil Chem. Soc., 1989, 66, 698–700 CrossRef CAS.
- A. Friedman, et al., Hydroxylated milk glycerides, Taiwan patent, TW211523B, 1996 Search PubMed.
- D. Swern, G. N. Billen and J. T. Scanlan, Hydroxylation and epoxidation of some 1-olefins with per-acids, J. Am. Chem. Soc., 1946, 68(8), 1504–1507 CrossRef CAS PubMed.
- D. Swern, Bailey's Industrial Oil and Fat Products. 1982 Search PubMed.
- D. K. Salunkhe, World Oilseeds, Springer Science & Business Media, 1992 Search PubMed.
- G. Bussell, Maleinized fatty acid esters of 9-oxatetracyclo-4.4. 1 O O undecan-4-OL, US pat., US3855163A, 1974 Search PubMed.
- K. Eckwert, et al., Process for the epoxidation of olefinically unsaturated hydrocarbon compounds with peracetic acid, US pat., US4647678A, 1987 Search PubMed.
- K. D. Carlson and S. P. Chang, Chemical epoxidation of a natural unsaturated epoxy seed oil fromVernonia galamensis and a look at epoxy oil markets, J. Am. Oil Chem. Soc., 1985, 62(5), 934–939 CrossRef CAS.
- C. W. Hull, Method for production of three-dimensional objects by stereolithography, US pat., US4999143A, 1990 Search PubMed.
- C. JV, Photoinitiators for free radical cationic and anionic photopolymerization, Surf. Coat. Technol., 1998, 168, 1–586 Search PubMed.
- P. Dufour, State-of-the-art and trends in the radiation-curing market, Radiat. Curing Polym. Sci. Technol., 1993, 1, 2 Search PubMed.
- L. Lu, et al., Origin of shrinkage, distortion and fracture of photopolymerized material, Mater. Res. Bull., 1995, 30(12), 1561–1569 CrossRef CAS.
- P. T. Wai, et al., Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products, RSC Adv., 2019, 9(65), 38119–38136 RSC.
- V. C. Moreno, et al., Thermal risk in semi-batch reactors: The epoxidation of soybean oil, Process Saf. Environ. Prot., 2017, 109, 529–537 CrossRef.
- C. Vianello, et al., Study of soybean oil epoxidation: effects of sulfuric acid and the mixing program, Ind. Eng. Chem. Res., 2018, 57(34), 11517–11525 CrossRef CAS.
- V. V. Goud, A. V. Patwardhan and N. C. Pradhan, Studies on the epoxidation of mahua oil (Madhumica indica) by hydrogen peroxide, Bioresour. Technol., 2006, 97(12), 1365–1371 CrossRef CAS PubMed.
- S. Dinda, et al., Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids, Bioresour. Technol., 2008, 99(9), 3737–3744 CrossRef CAS PubMed.
- E. Milchert, K. Malarczyk-Matusiak and M. Musik, Technological aspects of vegetable oils epoxidation in the presence of ion exchange resins: a review, Pol. J. Chem. Technol., 2016, 18(3), 128–133 CrossRef CAS.
- S. Danov, et al., Recent advances in the field of selective epoxidation of vegetable oils and their derivatives: a review and perspective, Catal. Sci. Technol., 2017, 7(17), 3659–3675 RSC.
- A. E. Gerbase, et al., Epoxidation of soybean oil by the methyltrioxorhenium-CH 2 Cl 2/H 2 O 2 catalytic biphasic system, J. Am. Oil Chem. Soc., 2002, 79, 179–181 CrossRef CAS.
- S. Leveneur, Thermal safety assessment through the concept of structure–reactivity: application to vegetable oil valorization, Org. Process Res. Dev., 2017, 21(4), 543–550 CrossRef CAS.
- S. Leveneur, et al., Interaction of thermal and kinetic parameters for a liquid–liquid reaction system: Application to vegetable oils epoxidation by peroxycarboxylic acid, J. Taiwan Inst. Chem. Eng., 2014, 45(4), 1449–1458 CrossRef CAS.
- M. R. g. Klaas and S. Warwel, Chemoenzymatic epoxidation of unsaturated fatty acid esters and plant oils, J. Am. Oil Chem. Soc., 1996, 73, 1453–1457 CrossRef.
- S. China, Determinating of the Epoxy Value of Plasticizers, Standards Press of China, Beijing, 2008 Search PubMed.
- E. R. Jusoh Taib, et al., Physico-chemical characterisation of epoxy acrylate resin from jatropha seed oil, Pigm. Resin Technol., 2017, 46(6), 485–495 CrossRef.
- R. Koley, et al., Synthesis and characterization of epoxidized neem oil: A bio‐derived natural processing aid for elastomer, J. Appl. Polym. Sci., 2021, 138(20), 50440 CrossRef CAS.
- A. K. Somidi, R. V. Sharma and A. K. Dalai, Synthesis of epoxidized canola oil using a sulfated-SnO2 catalyst, Ind. Eng. Chem. Res., 2014, 53(49), 18668–18677 CrossRef CAS.
- F. Okieimen, O. Bakare and C. Okieimen, Studies on the epoxidation of rubber seed oil, Ind. Crops Prod., 2002, 15(2), 139–144 CrossRef CAS.
- N. Kim, Y. Li and X. S. Sun, Epoxidation of Camelina sativa oil and peel adhesion properties, Ind. Crops Prod., 2015, 64, 1–8 CrossRef CAS.
- A. Campanella, et al., Soybean oil epoxidation with hydrogen peroxide using an amorphous Ti/SiO 2 catalyst, Green Chem., 2004, 6(7), 330–334 RSC.
- J. L. Wong, et al., Spectroscopic Analysis of Epoxidised Jatropha Oil (EJO) and Acrylated Epoxidised Jatropha Oil (AEJO), 2017 Search PubMed.
- A. Rana and R. Evitts, Synthesis and characterization of acrylated epoxidized flaxseed oil for biopolymeric applications, Int. Polym. Process., 2015, 30(3), 331–336 CrossRef CAS.
- G. Sudha, et al., Castor oil modified by epoxidation, transesterification, and acrylation processes: Spectroscopic characteristics, Int. J. Polym. Anal. Charact., 2017, 22(6), 519–525 CrossRef CAS.
- S. Lee, et al., Effect of the individual and combined use of cardanol-based plasticizers and epoxidized soybean oil on the properties of PVC, Polym. Degrad. Stab., 2018, 147, 1–11 CrossRef CAS.
- A. H. Suzuki, et al., Sustainable synthesis of epoxidized waste cooking oil and its application as a plasticizer for polyvinyl chloride films, Eur. Polym. J., 2018, 99, 142–149 CrossRef CAS.
- I. Putrawan, A. Azharuddin and J. Jumrawati, Preparing epoxidized vegetable oil from waste generated by the kapok fiber industry and assessing its thermal stabilization effect as a co-stabilizer for polyvinyl chloride, Heliyon, 2023, 9(9), e19624 CrossRef CAS PubMed.
- N. T. Thuy, V. M. Duc and N. T. Liem, The epoxidized vietnam rubber seed oil as a secondary plasticizer/thermal stabilizer in PVC processing, Int. J. Polym. Sci., 2021, 2021, 1–8 Search PubMed.
- X. Luo, H. Chu and M. Liu, Synthesis of bio-plasticizer from soybean oil and its application in poly (vinyl chloride) films, J. Renewable Mater., 2020, 8(10), 1295–1304 CAS.
- H. Hosney, et al., Epoxidized vegetable oil and bio‐based materials as PVC plasticizer, J. Appl. Polym. Sci., 2018, 135(20), 46270 CrossRef.
- W. He, et al., Green plasticizers derived from epoxidized soybean oil for poly (vinyl chloride): Continuous synthesis and evaluation in PVC films, Chem. Eng. J., 2020, 380, 122532 CrossRef.
- M. Li, et al., Tung oil based plasticizer and auxiliary stabilizer for poly (vinyl chloride), Mater. Des., 2017, 122, 366–375 CrossRef CAS.
- B. W. Chieng, et al., Epoxidized jatropha oil as a sustainable plasticizer to poly (lactic acid), Polymers, 2017, 9(6), 204 CrossRef PubMed.
- M. Bouti, R. Irinislimane and N. Belhaneche-Bensemra, Properties investigation of epoxidized sunflower oil as bioplasticizer for poly (lactic acid), J. Polym. Environ., 2022, 30(1), 232–245 CrossRef CAS.
- M. Guerre, et al., Vitrimers: directing chemical reactivity to control material properties, Chem. Sci., 2020, 11(19), 4855–4870 RSC.
- K. Chong, et al., A review on recent approaches to sustainable bio-based epoxy vitrimer from epoxidized vegetable oils, Ind. Crops Prod., 2022, 189, 115857 CrossRef CAS.
- S. K. Sahoo, V. Khandelwal and G. Manik, Development of completely bio‐based epoxy networks derived from epoxidized linseed and castor oil cured with citric acid, Polym. Adv. Technol., 2018, 29(7), 2080–2090 CrossRef CAS.
- M. Kareemullah, et al., Preparation and physicochemical properties evaluation of epoxidized neem oil-based bio-lubricant, Aust. J. Mech. Eng., 2023, 21(3), 942–951 CrossRef.
- C. P. do Valle, et al., Chemical modification of Tilapia oil for biolubricant applications, J. Cleaner Prod., 2018, 191, 158–166 CrossRef.
- N. Talib, R. Nasir and E. Rahim, Tribological behaviour of modified jatropha oil by mixing hexagonal boron nitride nanoparticles as a bio-based lubricant for machining processes, J. Cleaner Prod., 2017, 147, 360–378 CrossRef CAS.
- M. Sarno, M. Iuliano and C. Cirillo, Optimized procedure for the preparation of an enzymatic nanocatalyst to produce a bio-lubricant from waste cooking oil, Chem. Eng. J., 2019, 377, 120273 CrossRef CAS.
- F. U. Mohammed, I. O. Bakare, and F. E. Okieimen, Characterization of Rubber seed oil modified for biolubricant feedstock application, in TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings, Springer, 2020 Search PubMed.
- Y. Ma, et al., Castor oil-based adhesives: A comprehensive review, Ind. Crops Prod., 2024, 209, 117924 CrossRef CAS.
- S. A. Ab Rahman, et al., The chemistry insight: epoxy sealant as an alternative remedial operation for well integrity, Rev. Chem. Eng., 2023, 39(5), 857–873 CrossRef CAS.
- T. Hassan, et al., Functional nanocomposites and their potential applications: A review, J. Polym. Res., 2021, 28, 1–22 CrossRef.
- A. Zych, et al., Super tough polylactic acid plasticized with epoxidized soybean oil methyl ester for flexible food packaging, ACS Appl. Polym. Mater., 2021, 3(10), 5087–5095 CrossRef CAS.
- Y. H. Ho, et al., Acrylated Biopolymers Derived via Epoxidation and Subsequent Acrylation of Vegetable Oils, Int. J. Polym. Sci., 2022, 2022, 6210128 Search PubMed.
- A. KuShAiri and Y. CHOO, Optimisation on synthesis of acrylated epoxidised palm olein using response surface methodology, J. Oil Palm Res., 2015, 27(4), 366–376 Search PubMed.
- R. Mustapha, et al., Mechanical, thermal and water absorption properties of epoxy/acrylated epoxidized palm oil/montmorillonite nanocomposite, in AIP Conference Proceedings, AIP Publishing, 2018 Search PubMed.
- J. Wong, et al., Spectroscopic Analysis of Epoxidised Jatropha Oil (EJO) and Acrylated Epoxidised Jatropha Oil (AEJO), Pertanika J. Trop. Agric. Sci., 2017, 40(3), 435–448 Search PubMed.
- Y. H. Ho, et al., Acrylated biopolymers derived via epoxidation and subsequent acrylation of vegetable oils, Int. J. Polym. Sci., 2022, 6210128 Search PubMed.
- A. M. Salih, et al., Synthesis of radiation curable palm oil–based epoxy acrylate: NMR and FTIR spectroscopic investigations, Molecules, 2015, 20(8), 14191–14211 CrossRef CAS PubMed.
- R. Mustapha, et al., Mechanical, thermal and water absorption properties of epoxy/acrylated epoxidized palm oil/montmorillonite nanocomposite, AIP Conference Proceedings, 2018, vol. 2030, issue 1 Search PubMed.
- X. M. Chu, S. J. Liu and F. Q. Zhao, Preparation of Acrylated Epoxidized Soybean Oil with Excellent Properties, Appl. Mech. Mater., 2014, 662, 7–10 Search PubMed.
- N. R. Paluvai, S. Mohanty and S. K. Nayak, Synthesis and Characterization of Acrylated Epoxidized Castor Oil Nanocomposites, Int. J. Polym. Anal. Charact., 2015, 20(4), 298–306 CrossRef CAS.
- G. Wuzella, et al., Photocrosslinking of an acrylated epoxidized linseed oil: kinetics and its application for optimized wood coatings, J. Polym. Environ., 2012, 20, 1063–1074 CrossRef CAS.
- N. C. Jadhav and A. C. Jadhav, Synthesis of acrylate epoxidized rice bran oil (AERBO) and its modification using styrene & Shellac to study its properties as a composite material, Polym. Bull., 2023, 80(5), 5023–5045 CrossRef CAS.
- I. Omrani, et al., Synthesis of novel high primary hydroxyl functionality polyol from sunflower oil using thiol-yne reaction and their application in polyurethane coating, Eur. Polym. J., 2016, 82, 220–231 CrossRef CAS.
- S. Anbinder, et al., Structural properties of vegetable oil thermosets: Effect of crosslinkers, modifiers and oxidative aging, Eur. Polym. J., 2020, 124, 109470 CrossRef CAS.
- Y. Su, et al., One-step synthesis of novel renewable multi-functional linseed oil-based acrylate prepolymers and its application in UV-curable coatings, Prog. Org. Coat., 2020, 148, 105820 CrossRef CAS.
- P. Zhang and J. Zhang, One-step acrylation of soybean oil (SO) for the preparation of SO-based macromonomers, Green Chem., 2013, 15(3), 641–645 RSC.
- Y. Su, et al., One-step synthesis of novel renewable vegetable oil-based acrylate prepolymers and their application in UV-curable coatings, Polymers, 2020, 12(5), 1165 CrossRef CAS PubMed.
- C. Mendes-Felipe, et al., One-Step Method for Direct Acrylation of Vegetable Oils: A Biobased Material for 3D Printing, Polymers, 2023, 15(14), 3136 CrossRef CAS PubMed.
- P. Zhang, J. Xin and J. Zhang, Effects of catalyst type and reaction parameters on one-step acrylation of soybean oil, ACS Sustain. Chem. Eng., 2014, 2(2), 181–187 CrossRef CAS.
- Y. Hu, et al., Renewable epoxidized cardanol‐based acrylate as a reactive diluent for UV‐curable resins, Polym. Adv. Technol., 2018, 29(6), 1852–1860 CrossRef CAS.
- Y. Hu, et al., Use of cardanol-based acrylate as reactive diluent in UV-curable castor oil-based polyurethane acrylate resins, Ind. Crops Prod., 2018, 117, 295–302 CrossRef CAS.
- K. Li, et al., Preparation and properties of castor oil/pentaerythritol triacrylate-based UV curable waterborne polyurethane acrylate, Prog. Org. Coat., 2015, 78, 146–154 CrossRef CAS.
- Y. Hu, et al., Synthesis and characterization of novel renewable castor oil-based UV-curable polyfunctional polyurethane acrylate, J. Coat. Technol. Res., 2018, 15, 77–85 CrossRef CAS.
- F. Cheng, et al., Castor oil based high transparent UV cured silicone modified polyurethane acrylate coatings with outstanding tensile strength and good chemical resistance, Prog. Org. Coat., 2022, 163, 106624 CrossRef CAS.
- G. Chen, et al., Synthesis and characterization of UV-curable castor oil-based polyfunctional polyurethane acrylate via photo-click chemistry and isocyanate polyurethane reaction, Prog. Org. Coat., 2016, 93, 11–16 CrossRef CAS.
- M. Danish, et al., 4D printed stereolithography printed plant‐based sustainable polymers: Preliminary investigation and optimization, J. Appl. Polym. Sci., 2021, 138(36), 50903 CrossRef CAS.
- Y. Liu, et al., Four-Dimensional Printing of Multifunctional Photocurable Resin Based on Waste Cooking Oil, ACS Sustain. Chem. Eng., 2022, 10(49), 16344–16358 CrossRef.
- D. Chattopadhyay, S. Panda and R. V. Kothapalli, Thermal and mechanical properties of epoxy acrylate/methacrylates UV cured coatings, Prog. Org. Coat., 2005, 54, 10–19 CrossRef CAS.
- A. R. Kannurpatti, J. W. Anseth and C. N. Bowman, A study of the evolution of mechanical properties and structural heterogeneity of polymer networks formed by photopolymerizations of multifunctional (meth) acrylates, Polymer, 1998, 39(12), 2507–2513 CrossRef CAS.
- J. Guit, et al., Photopolymer resins with biobased methacrylates based on soybean oil for stereolithography, ACS Appl. Polym. Mater., 2020, 2(2), 949–957 CrossRef CAS.
- M. Lebedevaite, et al., Photoinitiator free resins composed of plant-derived monomers for the optical µ-3D printing of thermosets, Polymers, 2019, 11(1), 116 CrossRef PubMed.
- Y. Hu, et al., Rubber seed oil-based UV-curable polyurethane acrylate resins for digital light processing (DLP) 3D printing, Molecules, 2021, 26(18), 5455 CrossRef CAS PubMed.
- A. Barkane, et al., Thermal stability of UV-cured vegetable oil epoxidized acrylate-based polymer system for 3D printing application, Polym. Degrad. Stab., 2020, 181, 109347 CrossRef CAS.
- Y. Cui, et al., 3D printing of a dual-curing resin with cationic curable vegetable oil, Ind. Eng. Chem. Res., 2020, 59(25), 11381–11388 CrossRef CAS.
- R. P. Rosa, et al., Preparation and characterization of a fully biobased resin system for 3d-printing, suitable for replacing fossil-based acrylates, J. Polym. Res., 2023, 30(4), 139 CrossRef CAS.
- S. Grauzeliene, et al., Vegetable oil-based thiol-ene/thiol-epoxy resins for laser direct writing 3D micro-/nano-lithography, Polymers, 2021, 13(6), 872 CrossRef CAS PubMed.
- G. Zhu, et al., High-performance 3D printing UV-curable resins derived from soybean oil and gallic acid, Green Chem., 2021, 23(16), 5911–5923 RSC.
- S. Grauzeliene, et al., Photocuring and digital light processing 3D printing of vitrimer composed of 2-hydroxy-2-phenoxypropyl acrylate and acrylated epoxidized soybean oil, eXPRESS Polym. Lett., 2023, 17(1), 54–68 CrossRef CAS.
- A. Barkane, et al., Uv-light curing of 3d printing inks from vegetable oils for stereolithography, Polymers, 2021, 13(8), 1195 CrossRef CAS PubMed.
- C. Noe, et al., DLP-printable fully biobased soybean oil composites, Polymer, 2022, 247, 124779 CrossRef CAS.
- A. Bahmani, et al., Extrudable hydroxyapatite/plant oil-based biopolymer nanocomposites for biomedical applications: Mechanical testing and modeling, Mater. Des., 2019, 174, 107790 CrossRef CAS.
- D. Mondal, et al., Acrylated epoxidized soybean oil/hydroxyapatite-based nanocomposite scaffolds prepared by additive manufacturing for bone tissue engineering, Mater. Sci. Eng., C, 2021, 118, 111400 CrossRef CAS PubMed.
- L. Pezzana, et al., High temperature vat photopolymerization 3D printing of fully bio-based composites: Green vegetable oil epoxy matrix & bio-derived filler powder, Addit. Manuf., 2024, 79, 103929 CAS.
- M. Lebedevaite, et al., Development and optical 3D printing of acrylated epoxidized soybean oil-based composites with functionalized calcium silicate hydrate filler derived from aluminium fluoride production waste, Composites, Part A, 2022, 157, 106929 CrossRef CAS.
- Z. Liu, et al., 3D printing acrylated epoxidized soybean oil reinforced with functionalized cellulose by UV curing, J. Appl. Polym. Sci., 2022, 139(4), 51561 CrossRef CAS.
- M. Jurinovs, et al., Vat photopolymerization of nanocellulose-reinforced vegetable oil-based resins: synergy in morphology and functionalization, ACS Appl. Polym. Mater., 2023, 5(4), 3104–3118 CrossRef CAS.
- R. Palucci Rosa, et al., Preparation and characterization of 3D-printed biobased composites containing micro-or nanocrystalline cellulose, Polymers, 2022, 14(9), 1886 CrossRef CAS PubMed.
- G.-H. Wu and S.-h. Hsu, polymeric-based 3D printing for tissue engineering, J. Med. Biol. Eng., 2015, 35, 285–292 CrossRef PubMed.
- J. R. Tumbleston, et al., Continuous liquid interface production of 3D objects, Science, 2015, 347(6228), 1349–1352 CrossRef CAS PubMed.
- E. Aznarte, C. Ayranci, and A. Qureshi, Digital light processing (DLP): Anisotropic tensile considerations, in 2017 International Solid Freeform Fabrication Symposium, University of Texas at Austin, 2017 Search PubMed.
- Y. Li, et al., Theoretical prediction and experimental validation of the digital light processing (DLP) working curve for photocurable materials, Addit. Manuf., 2021, 37, 101716 CAS.
- C. Yuan, et al., Ultrafast Three-Dimensional Printing of Optically Smooth Microlens Arrays by Oscillation-Assisted Digital Light Processing, ACS Appl. Mater. Interfaces, 2019, 11(43), 40662–40668 CrossRef CAS PubMed.
- D. Seprianto, R. Sugiantoro and M. Erwin, The effect of rectangular parallel key manufacturing process parameters made with stereolithography DLP 3D printer technology against impact strength, J. Phys.: Conf. Ser., 2020, 012028 CrossRef.
- D. Wu, et al., Mechanics of shape distortion of DLP 3D printed structures during UV post-curing, Soft Matter, 2019, 15(30), 6151–6159 RSC.
- G. Rosace, et al., Photosensitive acrylates containing bio‐based epoxy‐acrylate soybean oil for 3D printing application, J. Appl. Polym. Sci., 2021, 138(44), 51292 CrossRef CAS.
- D. Mondal, et al., mSLA-based 3D printing of acrylated epoxidized soybean oil-nano-hydroxyapatite composites for bone repair, Mater. Sci. Eng., C, 2021, 130, 112456 CrossRef CAS PubMed.
- S. Huang, et al., 4D printing of soybean oil based shape memory polymer and its magnetic-sensitive composite via digital light processing, Polym.-Plast. Technol. Mater., 2022, 61(9), 923–936 CAS.
- Y. Zeng, et al., Photo-Curing 3D Printing of Highly Deformable Palm Oil-Based Thermosets with Soft Fatty Acid Chain Entanglement, ACS Sustain. Chem. Eng., 2023, 11(9), 3780–3788 CrossRef CAS.
- T. Weng, et al., 3D bioprinting for skin tissue engineering: Current status and perspectives, J. Tissue Eng., 2021, 12, 20417314211028574 Search PubMed.
- H. K. Handral, et al., 3D Printing of cultured meat products, Crit. Rev. Food Sci. Nutr., 2022, 62(1), 272–281 CrossRef PubMed.
- S. Zu, et al., 4D printing of core–shell hydrogel capsules for smart controlled drug release, Bio-Des. Manuf., 2022, 5(2), 294–304 CrossRef CAS.
- A. Melocchi, et al., Expandable drug delivery system for gastric retention based on shape memory polymers: Development via 4D printing and extrusion, Int. J. Pharm., 2019, 571, 118700 CrossRef CAS PubMed.
- L. Ouyang, et al., A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo‐crosslinkable inks, Adv. Mater., 2017, 29(8), 1604983 CrossRef PubMed.
- K. Tian, et al., 3D printing of transparent and conductive heterogeneous hydrogel-elastomer systems, Adv. Mater., 2017, 1604827 CrossRef PubMed.
- D. J. Tobin, Biochemistry of human skin—our brain on the outside, Chem. Soc. Rev., 2006, 35(1), 52–67 RSC.
- W. L. Ng, et al., Skin bioprinting: impending reality or fantasy?, Trends Biotechnol., 2016, 34(9), 689–699 CrossRef CAS PubMed.
- W. L. Ng, et al., Proof-of-concept: 3D bioprinting of pigmented human skin constructs, Biofabrication, 2018, 10(2), 025005 CrossRef PubMed.
- W. Zhao, et al., Adaptive multi‐degree‐of‐freedom in situ bioprinting robot for hair‐follicle‐inclusive skin repair: A preliminary study conducted in mice, Bioeng. Transl. Med., 2022, 7(3), e10303 CrossRef CAS PubMed.
- Y. Zhang, et al., Using bioprinting and spheroid culture to create a skin model with sweat glands and hair follicles, Burns Trauma, 2021, 9, tkab013 CrossRef PubMed.
- G. Stoychev, N. Puretskiy and L. Ionov, Self-folding all-polymer thermoresponsive microcapsules, Soft Matter, 2011, 7(7), 3277–3279 RSC.
- J. Choi, et al., 4D Printing Technology: A Review, 3D Printing and Additive Manufacturing, 2015, vol. 2, pp. 159–167 Search PubMed.
- J. M. Taylor, et al., Biomimetic and biologically compliant soft architectures via 3D and 4D assembly methods: a perspective, Adv. Mater., 2022, 34(16), 2108391 CrossRef CAS PubMed.
- W. Zhou, et al., 4D-printed dynamic materials in biomedical applications: chemistry, challenges, and their future perspectives in the clinical sector, J. Med. Chem., 2020, 63(15), 8003–8024 CrossRef CAS.
- F. Zhang, et al., Design of 4D printed shape-changing tracheal stent and remote controlling actuation, Int. J. Smart Nano Mater., 2021, 12(4), 375–389 CrossRef.
- L.-H. Shao, et al., 4D printing composite with electrically controlled local deformation, Extreme Mech. Lett., 2020, 39, 100793 CrossRef.
- M. N. I. Shiblee, et al., 4D printing of shape‐memory hydrogels for soft‐robotic functions, Adv. Mater. Technol., 2019, 4(8), 1900071 CrossRef CAS.
- P. Fratzl and R. Weinkamer, Nature's hierarchical materials, Prog. Mater. Sci., 2007, 52(8), 1263–1334 CrossRef CAS.
- A. S. Ayoub and L. A. Lucia, Fundamental Science and Applications for Biomaterials, Introduction to Renewable Biomaterials: First Principles and Concepts, 2017, pp. 39–62 Search PubMed.
- X. Chen, et al., Base-catalysed, one-step mechanochemical conversion of chitin and shrimp shells into low molecular weight chitosan, Green Chem., 2017, 19(12), 2783–2792 RSC.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), e1700782 CrossRef PubMed.
- Plastics – the Facts 2021, in Plastics Europe, 2021.
- S. Konan and F. Haddad, A clinical review of bioabsorbable interference screws and their adverse effects in anterior cruciate ligament reconstruction surgery, knee, 2009, 16(1), 6–13 CrossRef CAS PubMed.
- O. M. Böstman and H. K. Pihlajamäki, Adverse tissue reactions to bioabsorbable fixation devices, Clin. Orthop. Relat. Res., 2000, 371, 216–227 CrossRef.
- D. S. Mastrokalos and H. H. Paessler, Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone–patellar tendon–bone graft, Arthroscopy, 2008, 24(6), 732–733 CrossRef PubMed.
- J. J. Oppenheimer, et al., Treatment of peanut allergy with rush immunotherapy, J. Allergy Clin. Immunol., 1992, 90(2), 256–262 CrossRef CAS PubMed.
- S. L. Lin and M. O. Christen, Polycaprolactone‐based dermal filler complications: a retrospective study of 1111 treatments, J. Cosmet. Dermatol., 2020, 19(8), 1907–1914 CrossRef PubMed.
- S.-B. Park, et al., Biopolymer-based functional composites for medical applications, Prog. Polym. Sci., 2017, 68, 77–105 CrossRef CAS.
- M. Hino, O. Ishiko, K. Honda, T. Yamane, K. Ohta, T. Takubo and N. Tatsumi, Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery, Br. J. Haematol., 2000, 108, 194–195 CrossRef CAS PubMed.
- K. Biswas, et al., Biological activities and medicinal properties of neem (Azadirachta indica), Curr. Sci., 2002, 1336–1345 CAS.
- L. Vasant, Textbook of Ayurveda: Fundamental Principles, Albuquerque, 2002 Search PubMed.
- R. Subapriya and S. Nagini, Medicinal properties of neem leaves: a review, Curr. Med. Chem.: Anti-Cancer Agents, 2005, 5(2), 149–156 CrossRef CAS PubMed.
- J. Malda, et al., 25th anniversary article: engineering hydrogels for biofabrication, Adv. Mater., 2013, 25(36), 5011–5028 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |