Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advanced and personalized healthcare through integrated wearable sensors (versatile)
Mayank
Garg†
 *a,
Arpana
Parihar†
*a,
Arpana
Parihar†
 *b and
Md. Saifur
Rahman
*b and
Md. Saifur
Rahman
 a
a
aDepartment of Biomedical Engineering, Texas A&M University, College Station, Texas, USA. E-mail: mayankgarg93@tamu.edu
bIndustrial Waste Utilization, Nano and Biomaterials, CSIR-Advanced Materials and Processes Research Institute (AMPRI), Hoshangabad Road, Bhopal, Madhya Pradesh, India. E-mail: arpana_parihar@yahoo.com
First published on 11th December 2023
Abstract
Due to people's hectic and intense working schedules, health as a priority is often pushed to the sidelines. This is more problematic for people with some health conditions, which require regular monitoring of vitals. Therefore, the development of smart diagnostics is a clinical challenge towards which, a lot of research is being carried out. This primarily includes the development of wearable sensors. These sensors can be worn by individuals for the monitoring of various biomarkers in different bio-fluids. Primary research is being carried out to make them sensitive, reliable, robust, and non/minimally invasive. Since the intended wearable sensor is to be used for continuous monitoring of the individuals, all the components required for the sensing must be on the same platform. This generally includes the sensing platform, microfluidic channels, and its electronic or optical components. The goal of the current review is to highlight the state-of-the-art integrated wearable sensors, describing their important components, methods of manufacturing, and applications of these sensors and discuss the challenges being faced while designing and scaling up these sensors. The contents of this review will help design and develop novel integrated wearable sensors that are expected to revolutionize the field of personalized healthcare.
1. Introduction
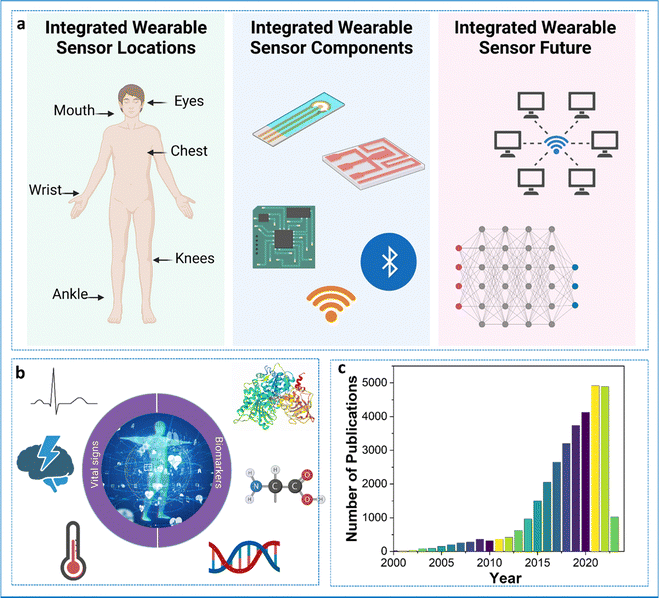
Busy schedules and long working hours have negatively impacted a lot of working professionals. As such, most individuals do not have sufficient time for simple routine check-ups. This is a serious issue since lot of diseases and conditions go unnoticed, creating problems at a later stage. Conditions such as strokes, coronary diseases, elevated glucose or other metabolite levels, cancer, etc., can be fatal if left untreated. For instance, heart attacks are the leading cause of death worldwide, taking around 18 million lives a year.1 The precious lives of people can be saved if the underlying situations or conditions are treated promptly. Since most people do not have access to standard or advanced healthcare settings, the pressing issue becomes aggravated. Taking healthcare solutions to patients in need becomes the most suitable option.In this regard, wearable sensors become the key players.2 Wearable sensors are non/minimally invasive analytical devices capable of providing point-of-care systems for the continuous and regular monitoring of individuals’ physiological as well as biological markers.3 Unlike conventional point-of-care devices, wearable sensors can utilize easy-to-sample bio-fluids such as saliva, tears, and sweat for the detection of clinically important biomarkers. This combined with continuous or regular monitoring can help in timely intervention and preventative measures. Since these devices are autonomous, lightweight, and compact, their impact is barely noticed by the user in their daily life. As the data is transmitted wirelessly, this provides mobility and accessibility to the users/patients wearing these devices. This is particularly useful when monitoring neonates and elderly people. The wearable devices can be modified to provide personalized healthcare. The large amount of data obtained through these devices can be used to train machine learning algorithms, which can be helpful for decision-making through artificial intelligence. Though expensive currently, over time, these wearable devices will prove to be cost-effective. The concept of a wearable device is not new and has been in existence for a long time. The wearables used during the 1960s provided the foundation for today's next-generation wearable sensors. Fig. 1 shows the components of the wearable sensor, the biomarkers for which sensors are being developed, and the tremendous jump in the number of publications related to the development of wearable sensors over the years. The current wearables are capable of reliable continuous monitoring of an individual's vitals such as temperature, humidity, blood oxygen level, blood pressure, EEG, ECG, and many biomarkers of clinical relevance.4–9 Apart from the sensing aspect, wearables also play an important role in the thermal management of the human body.10,11 The electrochemistry-based transducer is the most reliable and flexible option available for the design of wearable sensors because of its sensitivity, ease of fabrication, no moving parts, mechanical robustness, and resistance to other conditions.12 This transducer is the most widely used and trusted option for a biosensor.
Most of the research involving the development of wearable biosensors has been primarily limited to the fabrication and testing of the sensing platform i.e., the development of a sensing surface for a particular physiological or biological marker. For instance, though a wearable sensor for cortisol has been developed, it still requires the use of an external potentiostat for the read-out.13–17 There are many such examples. This essentially is against the concept of a wearable sensor. An ideal integrated wearable sensor should have the sensing platform and the associated electronics in a small and compact form that can be easily worn by the user.
The current review article is an attempt to stress the need for integration by briefly discussing the fabrication aspects of integrated wearable sensors using additive manufacturing technologies, the design and fabrication of multiplexed wearable sensors, and various microfluidic-based applications of wearable sensors. Other than these, the review article will also discuss wearable multifunctional flexible sensors, strain sensors, pressure, and thermal sensors along with some discussion on the implantable sensors. This review will highlight the commercially available integrated wearable sensors and report the challenges encountered while designing and fabricating integrated wearable sensors. It will also provide future insights into the field of integrated wearable sensors.
2. Components of integrated wearable sensors
In order to understand the concept of integrated wearable sensors, it is important to know the different components that make up the integrated wearable sensors. The main parts of wearable sensors are sensors, which detect signals, signal conditioning circuits, which improve the signals, analog-to-digital converters, which convert analogue signals to digital format, microprocessors or microcontrollers, which process the data, memory, which stores the data, wireless communication modules, which transmit the data, power sources,18–20 which supply energy, enclosures, which protect the sensors, and user interfaces, which allow for interaction. Together, these elements make it possible to track and analyze diverse physiological and environmental data, enabling applications for personalized healthcare and well-being.Interactions between a target and a bio-recognition element are the guiding principle while designing a sensing platform. The targets present in the bio-fluid are transported to the sensing surface, where the interactions with the bio-recognition element cause a change in the electrical signal, thereby enabling their detection. The electrochemistry-based transducer is the most reliable and flexible option available for the design of a wearable sensor because of its sensitivity, ease of fabrication, no moving parts, mechanical robustness, and resistance to other conditions.12 This transducer is the most widely used and trusted option for a biosensor. The electrical changes can be amperometric (change in current), voltammetric (change in potential), or impedimetric (change in impedance or resistance). For a typical biosensing surface, the surface of the electrode is modified with a bio-recognition element, which can be an antibody, aptamer, enzyme, peptide, or molecularly imprinted polymers (MIPs).21–24 Using bio-recognition elements is challenging since these need to adapt to the extreme conditions that are generally faced by wearable sensors. Of these elements, the use of an antibody or enzyme is less encouraged since these are the most vulnerable entities outside their physiological conditions. Nowadays, the use of peptides and MIPs is quite prominent since these are almost resistant to physiological conditions. As wearable sensors are intended for continuous monitoring of various biomarkers, the regeneration capability and biocompatibility of these recognition elements also need to be considered. It has been observed that biosensors targeting redox-active molecules are easily regenerated compared to non-redox-active molecules, such as proteins.25 As a wearable biosensor is intended for continuous and long-term usage, the regeneration of the sensing element also needs attention. In an ideal situation, the sensing elements do not need to be replaced. The prolonged exposure of the complex media to the bio-recognition elements reduces their efficiency and therefore they need to be replaced. Since the regeneration of these elements is not possible in wearable conditions, this becomes a bottleneck. MIPs are an attractive alternative to solve this problem since these can be easily regenerated.26 For instance, Gao's group showed the regeneration of a wearable metabolite and nutrient sensor based on MIPs. The sensor was regenerated by the application of an electrical potential for a very short time (less than 30 seconds). During the regeneration step, the electrode's surface gets altered so that it releases the bound analyte and becomes refreshed.27
For the fabrication of a sensor, a substrate is first modified with one of the above-mentioned bio-recognition elements. Since most of the wearable sensors developed are electrochemical transduction-based, the substrate is an electrode.25,28,29 The successful modification of the substrate is a critical part of biosensor design. The electrodes chosen should allow efficient and easy modification with the bio-recognition element, should not get fouled, be bio-compatible, and provide excellent electron mobility for the detection of various biomarkers of interest. These electrode surfaces are then functionalized to immobilize the bio-recognition elements. The use of nanomaterials is quite prominent for the development of electrode materials. Nanomaterials have proven their potential over a long time. They allow for a high surface-to-volume ratio, good electrical and optical properties, and allow efficient immobilization of the bio-recognition elements.30–35 In some cases, the catalytic activity of the nanomaterials has been utilized for the signal generation of electrically active metabolites such as amino acids, ions, or other analytes.
Another component of the sensing platform is the substrate or the platform itself. The wearable sensor should be conformal to the human skin and also the platform on which the sensor is to be fabricated must be flexible and soft. In this regard, many different materials have been explored. For instance, the use of PDMS is quite prominent since it is easy to handle, can be molded into any shape, microfluidic channels can be built on it and is soft on the skin and flexible. For instance, Singh et al. used a PDMS-based platform for the fabrication of a sweat sensor for cortisol. The gold electrode was deposited on the PDMS and microfluidic channels were also created in the PDMS to allow for the transport of sweat to the sensing chamber.36 Polyimide is another low-cost substrate for sensor fabrication. Though this material is not as soft as PDMS, a modified polyimide (PI) surface can be used directly as an electrode material. For instance, the Gao group has demonstrated that the PI surface can be altered using lasers to produce laser-induced graphene, which can then be used as an electrode material. The work reported the use of this Laser Induced Graphene (LIG) electrode for the detection of cortisol in sweat and serum.37
Various electrical components are required to make a wearable sensor work. The foremost is the battery to power up the sensing surface, integrated circuits, and read-out devices. For a typical sweat-based sensor, the induction of sweat through iontophoresis also requires power. The other important component is the integrated circuits (ICs), which manage the power delivery, potentiostat, data acquisition, and transmission.38,39 Various filters are also used in an integrated wearable sensor to eliminate any noise and unwanted signals coming due to external factors or motion artifacts. The electrical components are also responsible for the data transmission onto a smartphone using Bluetooth. The electronic component of a wearable biosensor is fabricated on a solid PCB or sometimes on flexible substrates depending on the intended application.40
The limiting factor for a wearable biosensor is the power supply. Since these sensors are intended for continuous measurements and data transmission, they consume a lot of power. To date, most of the devices developed have an external battery to power them. However, an idealistic situation is that the sensors can power themselves. Self-powered wearable biosensors are next-generation wearable sensors, wherein the sensors rely on energy harvesting from bio-fluids.41 Interested readers can refer to the detailed analysis of various self-powered devices by different researchers.41–45 A notable work is the development of a battery-free powered wearable sensor based on zinc oxide nanowires.46 The work reported the modification of zinc oxide nanowires with lactate oxidase. The flow of sweat on the nanoarrays caused a generation of DC electrical signal which corresponds to the lactate concentration in the sweat. The generation of an electrical double layer by the nanowires creates a potential difference.
The healthcare data generated by the wearable sensor is generally continuously transmitted to a smartphone a laptop or a server for processing. Since the amount of data generated is quite complex, various mathematical models are required to apprehend the data, so that the user can easily understand it. To achieve fast and accurate processing of the data, various models, and cloud computing have been utilized.47–51
3. Additive manufactured wearable microfluidic devices
The use of wearable electrochemical biosensors based on microfluidic systems has attracted a great deal of interest in non-invasive monitoring. It is critical to developing an efficient, flexible, robust, and inexpensive microfluidic system fabrication technique. Additive manufacturing or three-dimensional (3D)-printing emerges as a lucrative technology for fabricating novel devices with complex geometries and high precision for various applications, including microfluidics systems, food, healthcare, and environment monitoring.52 3D printing obtained ample attention for developing innovative microfluidic systems because of its advantages over conventional fabrication. Conventional techniques for manufacturing microfluidic sensors are generally expensive, and a few materials are suitable, but a great variety of reproducible geometries can be prepared using 3D printing.53 Also, polydimethylsiloxane (PDMS)-based epidermal microfluidic devices manufactured using the soft-lithography method require special facilities, such as a clean room with a UV lithography machine, and multi-step processing, which is lengthy and expensive.54 3D printing offers a high throughput and high-resolution fabrication of microfluidic devices.55 Mainly the process of 3D printing involves solidifying materials with computer control to create objects in 3D.56 However, several types of 3D printing such as including inkjet 3D printing (i3DP), fused deposition modeling (FDM), stereolithography (SLA), and two-photon polymerization (2PP) have been demonstrated to fabricate the microfluidic electrochemical sensor.57The i3DP technique has been found to be the most commercially viable among the 3D printing methods.58 To fabricate the microfluidic channel on a submillimeter scale, the i3DP approach has been widely taken. Typically, the i3DP approach relies on the continuous or drop-on-demand (DoD) mode.59 For fabricating the micrometer scale of microfluidic devices, i3DP has become very demanding. Gowers et al. (2015) demonstrated a 3D-printed microfluidic device that can continuously monitor human tissue metabolite levels.60 A needle-type biosensor integrated with regulatory-certified (FDA) clinical microdialysis probes through the use of a probe outlet holder, is shown in Fig. 2.60 This novel and innovative structure minimizes the extra connection tubing and adaptors, thus miniaturizing the device size. Typically, to substitute the micro vial, the microfluidic device is inserted into the probe outlet holder. To mimic the configuration of the micro vial, an L-shaped 3D-printed microfluidic chip was fabricated. The L-shaped design renders a miniaturized microfluidic chip.
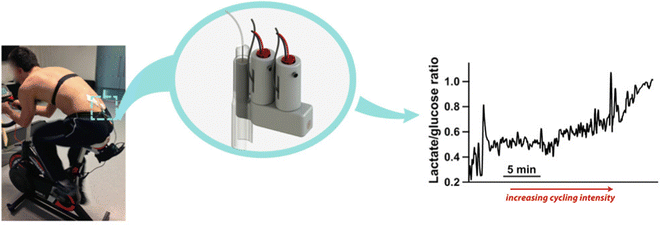 | ||
| Fig. 2 A microfluidic system that measures lactate and glucose levels in dialysate during cycling.60 Copyright 2015 American Chemical Society. | ||
Furthermore, fused deposition modeling (FDM) 3D printing has been widely used to fabricate elastomer-based microfluidic devices due to its low cost.61 The FDM involves feeding and melting polymer filaments into a heated metal cylinder that ends with a nozzle and extrudes them.62 Wei et al. (2022) demonstrated a novel precision-controlled 3D-printed tailored microfluidic channel using malt syrup (Fig. 3a).54 The fabricated flexible microfluidic model combined with an encapsulated microfluidic channel layer for collecting sweat and a pH sweat sensor for sensing pH, as shown in Fig. 3a. The design involves several steps starting with the 3D printing of the sugar filament mold, encapsulation of the fabricated microfluidic channels, incorporation of the pH sensor, and encapsulation of the system. Microfluidic devices were mounted on the skin to collect sweat and analyze it as a proof of concept (Fig. 3b). As shown in Fig. 3c, the microfluidic device showed a good performance as its measured pH values matched the pH values of the commercial pH meter.
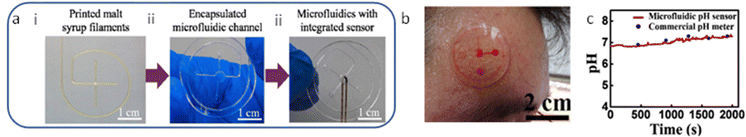 | ||
| Fig. 3 (a) Steps (i, ii, and iii) involving manufacturing the microfluidic device and integration, (b) Photograph of the microfluidic chip onto the forehead for sweat collection, and (c) comparison of the real-time results between the commercial pH meter and the developed sweat pH sensor. Adapted with permission from ref. 54. Copyright 2022 Elsevier. | ||
Though 3D printed microfluidic devices have many advantages still, their resolution is yet to be comparable with the devices made with the conventional lithography technique.58 In addition to the device's resolution, some other issues include a lack of compatible materials, the roughness of the device's surface, layer-by-layer finish, and conformability with the skin.63
4. Design and fabrication of multiplexed integrated wearable sensors
In recent years, there has been a sharp increase in demand for wearable sensors. The sensors that can be worn and able to track body temperature, bodily movements, blood pressure, and heart rate are already often utilized in daily life and are currently commercially accessible has revolutionized the field of health care.3 However, to enhance the capabilities of wearable sensor efficiency and subsequently obtain more detailed data about the health of a wearer and physiological body condition, novel non-invasive biometric sensors are very much needed.64 Recently, several research studies have tried to fill this gap by developing wearable sensors that can track body metabolites like lactate, glucose, or uric acid as well as blood electrolytes like sodium and potassium as well as body pH. The routine status of these analytes would help in the prediction of an individual's health and to manage personalized disease care in times of emergencies.65 Notably, a wearable sensor should have a quick response time, be cost-effective, be selective, have a repeatable response, and be sensitive. Besides, the sensor should be able to detect multiple analytes or parameters in a real-time manner that will reflect more precise health updates of an individual, since the human body is a complex system where one parameter is associated with more than one disease condition.66However, most available wearable sensors are incapable of detecting and integrating the signals from several analytes. There is a need for multiplexed sensors to monitor glucose, sodium lactate, potassium, and other body metabolites simultaneously in a real-time manner. There is undoubtedly a pressing need to create innovative, multi-analyte detection ability, simple-to-use wearable sensor devices. In this context, a completely integrated wireless multiplexed wearable sensing technology was fabricated by Semionatto et al. in 2017 which is capable of monitoring sweat electrolytes and metabolites in a real-time manner.67 This electrochemical sensor was screen-printed with PET stickers attached to the nose pads on each side of the eyeglasses. The wearable sensor was developed by combining a potentiometric potassium ion-selective electrode and an amperometric lactate biosensor into the two nose-bridge pads of the spectacles and connecting them to an electronic backbone mounted on the arms of the spectacles (Fig. 4IA and IB). This amperometric and potentiometric sensor was printed on two circuit boards (PCBs) mounted on the arms of the eyeglass. The potentiometric and amperometric measurement capabilities of the lactate and potassium sensors, as well as wireless data transfer, were connected to each of the PCBs. This sensor can be connected wirelessly to a host device, such as a smartphone, smartwatch, or laptop, using this controller's built-in Bluetooth low energy (BLE) feature. The lactate eyeglasses biosensor is based on the amperometric monitoring of the hydrogen peroxide product and the lactate oxidase-mediated oxidation of lactate to pyruvate and H2O2 (Fig. 4(IC)). Fig. 4(ID) shows the construction and functionalization of the lactate biosensor for the nose-bridge pad. A limit of detection (LOD) of 0.39 mM (CLk = 3) and a linear range of detection up to 10 mM lactate were obtained. Open-circuit potential measurements were used to assess the analytical performance of the potassium sensor, which provides a LOD of 10−3.9 M. The lactate and potassium electrodes were each linked to a PCB that was positioned on each of the arms of the eyeglass to form the integrated MAG multiplex device (Fig. 4(IIA)). Using the wireless boards, in vitro data calibration curves were produced through an experimental arrangement which is depicted in Fig. 4(IIB). The potassium sensor's potential-time response to an increase in concentration of potassium chloride from 0.01 to 10 mM as shown in Fig. 4(IIC). The response of the lactate sensor while increasing the concentration of lactate from 0 to 10 mM is shown in Fig. 4(IID). Before on-body tests, each sensor was calibrated. During the body test, the sweat's potassium and lactate levels were monitored using a sensor as shown in Fig. 4(IIIAa,a′) and (IIIAb,b′). While exercising, potassium and lactate levels in sweat were detected at the same time, the lactate PCB was repeatedly turned on and off, and simultaneously the potassium level was continuously measured. This ON/OFF process of the lactate sensor caused no interference in the potassium response, suggesting low cross-talk and effective integration of a multiplex wireless sensor into the spectacles. Also, the cross-talk experiment indicates a quick return to the formerly recorded signal and a quick and reliable response. The body glucose level in sweat concerning the time profile is shown in Fig. 4(IIICa,a′). The glucose sensor calibration curve was established by measuring the blood glucose (126 mg dl−1) using a commercial glucose meter for comparison purposes (Fig. 4(IIE)). This led to an estimate of the sweat glucose level of 0.2 mM (Fig. 4C), which follows the anticipated sweat-blood glucose association. Wireless signal transduction was demonstrated coupled with sweat lactate and potassium levels measurement simultaneously with no potential cross-talk. Expanding the complete integrated “Lab-on-a-Glass” wireless multiplexed wearable sensor device to simultaneously monitor more sweat electrolytes and metabolites is a simple approach and this system could be further enhanced by adding temperature and pH microsensors integrated with fluidics to regulate the flow of sweat. This non-invasive wearable device can bridge current gaps in wearable sensor platforms, which is needed for continuous health care monitoring and assessable remote diagnostics, and offers an appealing alternative to other sweat-sensing devices.
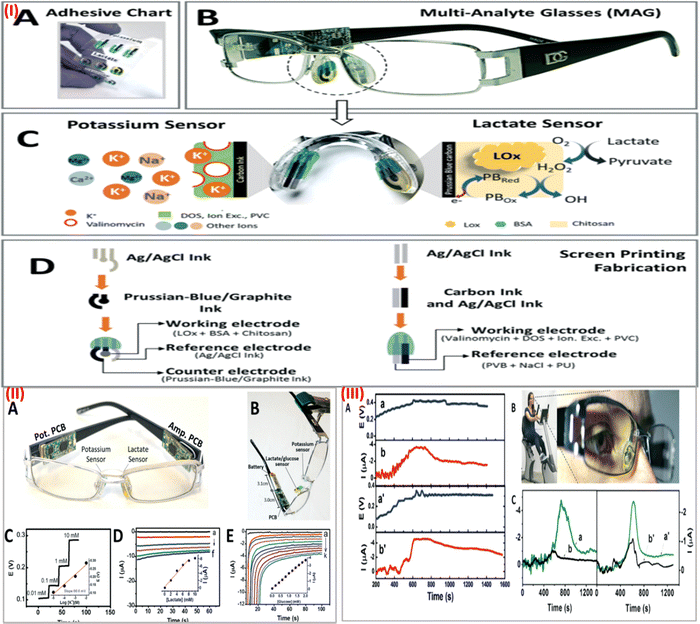 | ||
| Fig. 4 (I) (A) screen-printed interchangeable sticker sensors. (B) Printed MAG eyeglasses combined with a wireless circuit board. (C) Electrochemical sensor on a nose pad with a potassium and lactate sensor associated with recognition and transduction steps. (D) Screen printing process steps for lactate and potassium sensor. (II) (A) MAG device with amperometric and potentiometric PCBs. (B) In vitro measurement setup of the device. (C) Potassium sensor response. (D) Lactate sensor response curve. (E) Glucose sensor response curve. Inset: Calibration plot for lactate, potassium, and glucose respectively. (III) (A) Wireless measurement of sweat potassium (a,a′) and lactate (b,b′) while cycling. (B) Stationary cycling bike with volunteer wearing the MAG device. (C) Measurement of glucose (a,a′) and control with no sensor (b,b′) during exercise using the device. Adapted with permission from ref. 67. Copyright 2017 Royal Society of Chemistry. | ||
Wearable strain sensors
Ultrasensitive stretchable strain sensors for human motion detection are currently receiving a lot of attention.68 In a recent study, spray-coated graphite nanoflakes were used for developing a wearable sensor.69 This sensor was attached to highly elastic nitrile elastomers. The wearable strain sensor is easy to manufacture and exhibits an ultra-high strain sensitivity much higher than the other available stretchable strain wearable sensors. The development of the graphite nanoflake/nitrile rubber nanocomposite-based wearable sensors is depicted in Fig. 5Ia. The wearable and skin-mountable strain sensors with noticeable stretchability are illustrated in Fig. 5Ib. The SEM picture and cross-section of the graphite nanoflake/nitrile rubber nanocomposite are shown in Fig. 5Ic–e, highlighting its outstanding adhesion characteristics and conspicuous uniformity. By placing the strain sensor on the leaf of a plant, as shown in Fig. 5IIa, the lightweight quality of the device was demonstrated. For various applications, graphite nanoflake/nitrile elastomer nanocomposites can be easily synthesized in various shapes, such as triangles, hearts, and stars, as shown in Fig. 5IIb. Additionally, the nanocomposites withstand considerable stretching while holding, bending, and twisting, demonstrating their notable flexibility (Fig. 5IIc). The stretchable strain sensors can sense up to 30% strain and exhibit a highly sensitive gauge factor (GF) of 868.12 ± 56.90, which is noteworthy. The electrical resistance variation, which is a key factor in electromechanical performance, is significantly changed by microstructural percolative network cracks with strain during mechanical distortions. This sensor can be used as an electronic skin for health monitoring applications which helps in monitoring full-range human activity while projecting reversibility, fast response time and recovery speeds (7.5 ms and 5 ms, respectively), and admirable robustness. This sensor can be employed for human activity monitoring in healthcare diagnostics, soft robotics, and other biomedical applications.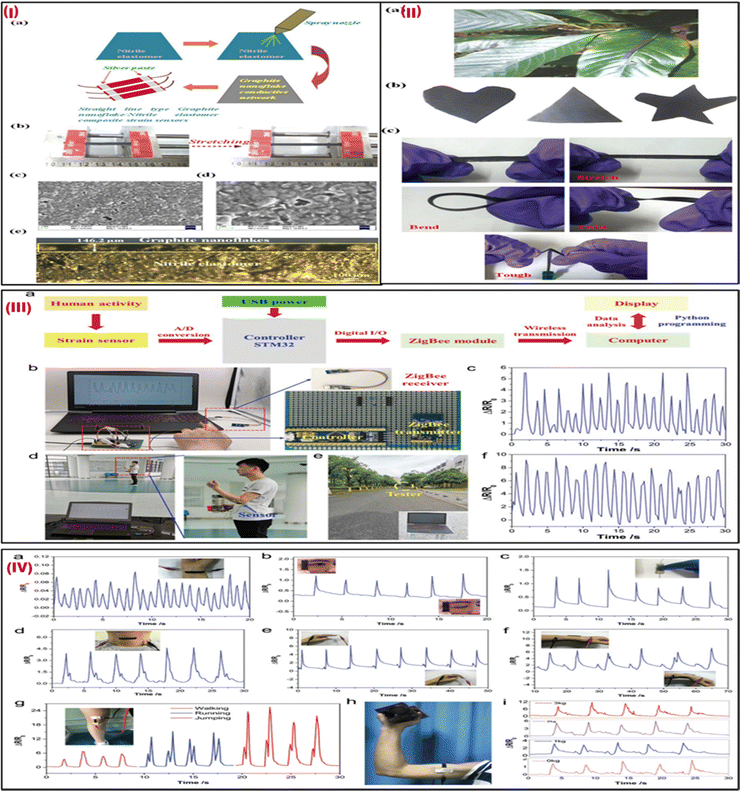 | ||
| Fig. 5 (I) (a) The manufacturing procedure for strain sensors made of an ultrasensitive graphite nanoflake/nitrile elastomer composite. (b) The tensile strains revealed the good stretchability of the strain sensor. (c) An SEM picture of the conductive network made up of graphite nanoflakes. (d) A magnified SEM picture of (c). (e) A cross-sectional optical image of the graphite nanoflake/nitrile elastomer nanocomposite strain sensors. (II) (a) Strain sensors are robust and resilient, and lightweight and can be placed on leaves. (b) Various shapes of a graphite nanoflake/nitrile elastomer nanocomposite. (c) Graphite nanoflake/nitrile elastomer nanocomposite strain sensors are tough and can be stretched, bent, and twisted. Adapted with permission from ref. 69 Copyright 2022 Royal Society of Chemistry. (III) (a) A setup of the wireless strain sensor. (b) Images of the sensor comprise a portable power source, controller, with a ZigBee receiver, and strain sensor. (c) Signal responses of finger-bending were captured using the sensor system. (d) A tester holding a circuit board and a power source while wearing a sensor on their elbow joint and observing a computer display of electrical signals. (e) A tester who was moving outdoors 50 meters to the ZigBee module. (f) Response signals from the arm bending sensor. (IV) the G/CB/Ni-2 strain sensor monitors motion in a real-time manner. Reaction signals include (a) wrist pulse, (b) blinking, (c) tensing, (d) swallowing, (e) bending of fingers, and (f) wrist movement. A picture of the sensor that is fastened to the wrist shown in insets (b)–(f). Photos of the matching human gestures are included as (g) walking, running, and jumping reaction signals. An image of the sensor mounted over the knee joint is shown in the inset in (g). (h) A picture of a sensor that was fastened to the tester's biceps muscle. (i) Reaction signals when lifting dumbbells of various weights. Adapted with permission from ref. 70 Copyright 2020 Royal Society of Chemistry. | ||
In another study, Sun et al. (2020) fabricated a flexible, waterproof, and extremely sensitive Internet of Things (IoT) integrated strain sensor by drop-coating graphene (G), carbon black (CB), and polydimethylsiloxane (PDMS) into the 3D framework of a commercial Ni sponge.70 The sensor comprises a G/CB/Ni-2 strain sensor, a ZigBee module, an STM32 controller, and a portable power source as shown in Fig. 5IIIa. The STM32 controller was used to interpret the electrical data from the G/CB/Ni-2 strain sensor before transmitting it in real-time to a computer via the ZigBee module. This allowed the device to monitor human motions wirelessly and remotely. The self-compiled Python program was then used to further store and analyze the data before finally displaying the results on the computer screen. A 5 V portable power source supplied power to the wireless strain sensor via a USB interface. Fig. 5IIIb depicts a picture of the entire system. The ZigBee transmitter and controller were mounted on the circuit board, the controller and strain sensor were attached to the tester's forefinger using 3M tape, and the ZigBee receiver was linked to the PC. The sensor system's electrical response was captured (Fig. 5IIIc). The subject wearing the sensor and holding the circuit board and portable power supply was roughly 10–15 m distant from the PC as depicted in Fig. 5IIId. The distance between the tester and the computer, as shown in Fig. 5IIIIe, was roughly 50 meters. The electrical response seen on the PC screen remained stable and exhibited nearly no change when the subject moved slowly away from the ZigBee (Fig. 5IIIf). Fig. 5(IV) shows the real-time response of motions using this strain sensor. The GF of 138 at 16% strain and prolonged stability of the sensor revealed that it is very sensitive, flexible, and stable. The G/CB/Ni sensor can measure muscular strength as well as accurately track minute human movements like blinking, pulse, and swallowing. This strain sensor also has good waterproof properties, making it safe to use for tracking human motions in humid environments and on water. The flexibility, high sensitivity, and waterproofness of the G/CB/Ni strain sensor make it ideal for use in outdoor motion monitoring, personal rehabilitation training, and healthcare management.
Wearable thermal sensors
Wearable thermal sensors are small, portable tools that track body temperature in real-time for health surveillance. They offer important data for early disease identification, performance optimization, and general well-being when used in smart clothes and fitness wearables. Bio-inspired colour-changing and self-healing hybrid hydrogel-based wearable sensors with adaptive camouflage were synthesized by Liu et al. in 2023.71 In this study, multi-branched polyacrylate, zinc sulphate, thermochromic dye, and photochromic dye were blended to fabricate a colour-changing and self-healing hybrid hydrogel. A flowchart showing the steps of the TDM/PDM/polyacrylate hybrid hydrogel preparation process is shown in Fig. 6I. When the TDM/PDM/polyacrylate hybrid hydrogel was stuck to the body, the signal observed was instantly transferred to a PC or mobile phone as a pre-warning, allowing the issue to be resolved promptly (Fig. 6IIa). The hydrogel was applied to the palm to measure hand temperature changes via a thermal camera, and the results indicate that the TDM/PDM/polyacrylate hybrid hydrogel was found to be more responsive at a temperature of 38 °C (Fig. 6IIb). Additionally, when exposed to various temperature conditions (such as 25, 38, 65, and 95 °C), the sensor displayed distinct resistance change ratios (ΔR/R0) (Fig. 6IIc). The resistance change ratio (ΔR/R0) of the sensor was highly variable after five cycles of heating and chilling between 25 and 38 °C (Fig. 6IId). But at 25 °C, its resistance change ratio (ΔR/R0) altered just a little bit. Additionally, the sensor still demonstrated a good sensing property that responded swiftly to a tilted finger at 38 °C (Fig. 6IIe). A chameleon's skin can heal when it is damaged and regain its ability to colour change (Fig. 6IIIa). This wearable sensor follows a similar kind of self-healing process. After heating from 25 °C at a rate of 2 °C min−1, the two halves of the cut TDM/PDM/polyacrylate hybrid hydrogel came into contact with one another, and the hydrogel repaired itself (Fig. 6IIIb). The hybrid hydrogel's colour changed to blue after being mended, and it was able to withstand twisting and stretching without cracking when it was put back into the 25 °C environment (Fig. 6IIIc and d). The repaired hybrid hydrogel can also quickly change colour (Fig. 6IIIe). Additionally, the stained hybrid hydrogel displayed good deformation and twisting capabilities (Fig. 6IIIf). The healed hybrid hydrogel was able to relight the LED lamp (Fig. 6IIIg), indicating that its electrical conductivity had also been restored. It is expected that this self-healing, colour-changing hybrid hydrogel can be successfully employed for human activity monitoring, health monitoring, and safety warning. Wearable thermal sensors have the potential to be used in personalized medicine, ongoing health assessment, early illness identification, and sophisticated health monitoring in the future. By enabling proactive health management through integration with AI-driven analytics, preventive care might be revolutionized, and the healthcare ecosystem could become more connected and knowledgeable overall.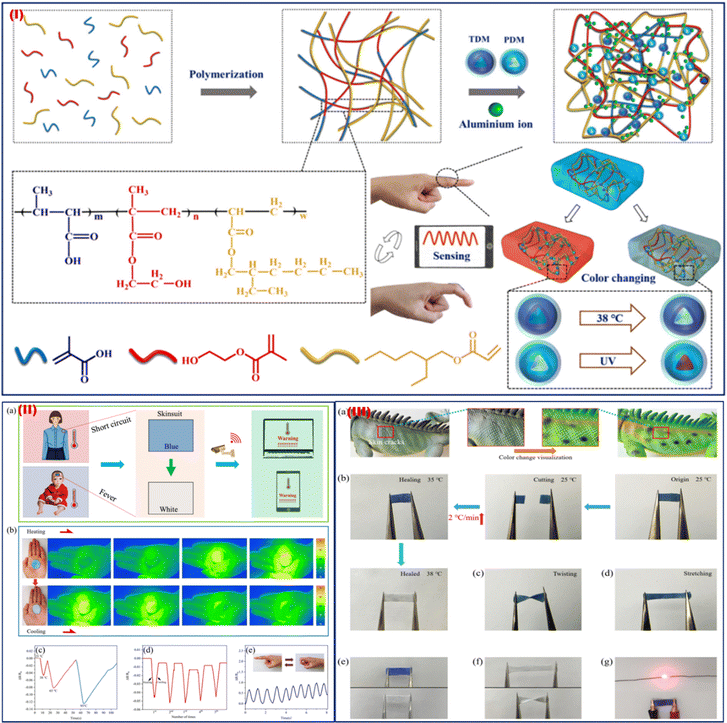 | ||
| Fig. 6 (I) Illustration of the TDM/PDM/polyacrylate hybrid hydrogel preparation process. (II) Early warning system to track the human temperature. (b) Thermal images were captured using an infrared thermal camera while the hybrid hydrogel was being heated. (c) The hybrid hydrogel's resistance change ratio (DR/R0) at 25, 38, 65, and 95 °C. (d) During heating and chilling cycles, the resistance change ratio (ΔR/R0) of the TDM/PDM/polyacrylate hybrid hydrogel. (e) The hybrid hydrogel's sensing capabilities at 38 °C. (III) (a) Chameleons in various forms mending their discolored damaged skin. (b) The hybrid hydrogel's visible self-healing mechanism. (c) and (d) Properties of the repaired hybrid hydrogel in terms of twisting and stretching. (e) The hybrid hydrogel's stain-resistance characteristics. (f) The hybrid hydrogel's stretching and twisting characteristics following repair. (g) A picture of the fixed TCP/PCP/polyacrylate hybrid hydrogel shining an LED bulb. Adapted with permission from ref. 71 Copyright 2023 Royal Society of Chemistry. | ||
Wearable pressure sensors
Wearable pressure sensors are devices that are incorporated into clothing or accessories to track and examine changes in body pressure. They are used in sports performance analysis, healthcare for patients with limited mobility, and ergonomic design to enhance posture. The technology optimises physical activity and rehabilitation while improving personalised health insights. Recently, a Ti3C2Tx MXene/bamboo cellulose fiber (BCF)/poly(dimethylsiloxane) (PDMS) composite-based pressure wearable sensor with a wide linear range (up to 2 MPa), a high linearity (R2 = 0.966), and demonstrated longer stability was fabricated by Zhu et al. in 2022.72 The MXene/BCF (MB) foam was first created using a productive freeze-drying technique, which resulted in foam with optimized porosity and connection. Then, the MB foams were directly incorporated into PDMS elastomers to create the MB-based piezoresistive composite (PMB). Fig. 7Ia provides a schematic of the steps of the PMB composite pressure sensor and MXene nanosheet fabrication and characterization of the fabricated sensor. The AFM, TEM, SAED pattern, MXene solution, and developed foam over the flower is shown in Fig. 7I(b)–(j). This PMB pressure sensor demonstrates great pressure sensing capability, good biocompatibility, and noteworthy work trustworthiness to resist temperature variation, moisture/water, and UV irradiation—in stark contrast to earlier MXene composite-based pressure sensors (Fig. 8II(a)–(i)). Furthermore, to demonstrate the PMB pressure sensor's enormous potential for robotic tactile sense, it was also efficaciously combined with soft robotic hands. A soft robotic gripper hand equipped with the PMB pressure wearable sensor was used to grab several things of varying weights, including a beaker, a plastic box, and a table tennis ball. Various human actions under normal and challenging environmental conditions can also be monitored using a PMB pressure sensor. Beyond their present uses, wearable pressure sensors have a bright future ahead. Improvements in these devices could result in better medical diagnostics, such as tracking cardiovascular health or anticipating respiratory problems. These sensors could improve performance analysis in sports. Furthermore, integrating AI with IoT could transform sectors like ergonomics, fitness, and healthcare by promoting a more responsive and connected world.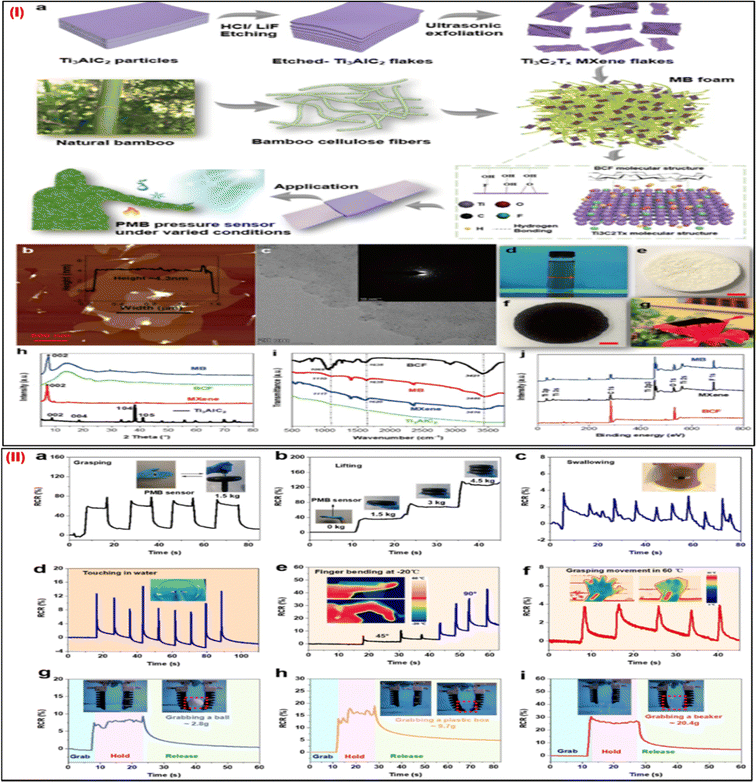 | ||
| Fig. 7 (I) (a) Steps of fabrication of PMB pressure wearable sensors. (b) Images of the MXene nanosheets taken with an AFM and (c) TEM images; the inset displays the SAED pattern. (d) The MXene solution, (e) the BCF foam, (f) the MB foam, and (g) the MB foam on a flower. (h) The XRD spectra of Ti3AlC2 powders, MXene nanosheets, BCF foam, and MB foam, and (i) FTIR. (j) MXene nanosheets, MB foam, and BCF XPS spectra. (II) The use of the PMB pressure sensor in soft robotics and the sensing of human movement. RCR responses of the sensors to detect significant motions such as (a) gripping and (b) lifting heavy objects. (c) RCR sensor responses are used to track minute movements. RCR sensor responses in challenging conditions, such as (d) detecting finger touches in water, (e) observing finger bending at a low temperature of 20 °C, and (f) sensing gripping motion at a high temperature of 60 °C. Adapted with permission from ref. 72. Copyright 2022 American Chemical Society. | ||
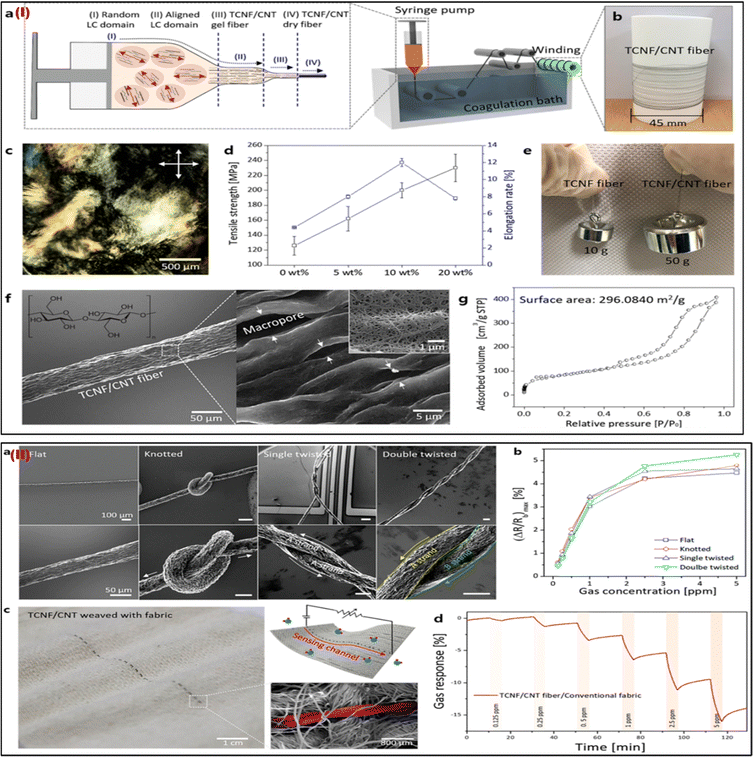 | ||
| Fig. 8 (I) Characterizations of the TCNF/CNT fibers and their scalable production. (a) Diagrams for the hybrid LC spinning technique-based continuous manufacture of TCNF/CNT fiber. (b) Picture of the bobbin-collected TCNF/CNT (20) fiber. (c) The image shows the hybrid schlieren LC texture of TCNF/CNT (20) at 30 mg mL−1 taken with polarised optical microscopy (POM). (d) The relationship between the TCNF/CNT fiber's mechanical characteristics and the CNT content. (e) A picture of the weights that the single fibers were able to lift. (f) TCNF/CNT (20) fiber SEM pictures at low and high magnifications. (g) The TCNF/CNT (20) fiber's isothermal adsorption/desorption plot and BET surface area. (II) TCNF/CNT fibers have good mechanical flexibility, making them ideal for wearable sensors. (a) SEM pictures of the TCNF/CNT (20) fibers that have been merged with sensing apparatus in knotted, flat, single-, and double-twisted forms. (b) The maximum (ΔR/Rb) of the flat, knotted, single- and double-twisted TCNF/CNT (20) wearable sensors in the NO2 concentration range of 0.125–5 ppm. (c) Images of a TCNF/CNT fiber woven into standard fabrics, including photos, schematics, and SEM pictures. (d) The woven TCNF/CNT fiber's real-time NO2-sensing performance with common wearable textiles. Adapted with permission from ref. 73. Copyright 2019 American Chemical Society. | ||
Integrating carbon nanotubes (CNTs) into nano cellulose enabled Cho et al. (2019) to mass-produce sensing fibers with high mechanical qualities and sensing capabilities without requiring extra effort.73 To facilitate liquid-crystal characteristics, nano cellulose obtained from tunicate (TCNF) was homogeneously mixed with single-walled CNTs. Composite fibers (TCNF/CNT) are continuously created by wet spinning in aligned directions. The TCNF/CNT can be adequately concentrated during the LC phase to use the common wet-spinning method for constant fiber synthesis (Fig. 8Ia). The mechanical characteristics of the TCNF/CNT fiber, the weights lifted by single fibers, the TCNF/CNT image, the polarized optical microscopic image, the SEM image, and the isothermal adsorption/desorption plot are shown in Fig. 8Ib–g. As shown by SEM pictures and the time-dependent NO2 sensing response of the intertwined TCNF/CNT fiber, TCNF/CNT fibers have high mechanical flexibilities and good sensing abilities as wearable sensors (Fig. 8IIa–d). Excellent gas (NO2) sensing efficiency with great selectivity and high sensitivity is confirmed by the TCNF/CNT fibers. The TCNF/CNT fibers can also withstand difficult distortions while preserving their inherent sensing qualities, and they can be seamlessly incorporated with traditional fabrics utilizing a direct weaving process. The ability to run this wearable sensor fiber manufacturing approach at extremely low process costs is also advantageous. For the manufacturing of one meter of TCNF/CNT fiber, the process cost of synthesis is around 0.00403 $ per meter. This scalable, meter-scale production of functional composite fibers is anticipated to offer a framework for the mass manufacture of adaptable wearable sensors.
5. Microfluidic-based platform for multiplexed integrated wearable sensors
Recently, novel microfluidics approaches have sparked the development of miniature devices that can be employed for carrying out a variety of tasks, including the trapping of particles and cells, size-based segregation of particles and cells, the production of nanomaterials, the formation of droplets, and testing of drugs.74 Sample collection, treatment, and analysis have all been carried out using soft microfluidic technology. These tools are used to monitor electrolyte loss and compositions while also measuring biomarkers in real-time. Numerous applications in the field of diagnostics, such as food quality testing, environmental monitoring, and health diagnostics, have employed paper-based microfluidic analytical devices. However, most of these platforms are unusable and unfit for continuous monitoring applications. Moreover, flexible microfluidic patches are fabricated using traditional photolithographic procedures, which are costly and time-consuming and obstruct the mass manufacturing of electrochemical multianalyte sensing.Real-time continuous sweat biomarker investigations are a prerequisite for health care and for this purpose the development of cost-effective microfluidic devices with quick sample collection and multiplexed analyte identification capabilities is crucial.75 Vinoth et al. (2021) recently fabricated a printed microfluidic-based wearable device for multiplexed high-throughput electrochemical analysis of sweat samples.76 For this, an array of electrodes printed over a flexible polyimide layer and an insulator film to create leakage-free closed, fluid paths to direct sweat samples to the sensing electrodes allows for the one-step production of numerous identical patterns on affordable substrates (Fig. 9(IA)). The fabrication of roll-to-roll several uniform patterns using the screen-printing technique is used to create a carbon-based master mould. After the carbon ink is generated using a thermoplastic elastomer called SBS binder, it is screen-printed with the appropriate fluidic channel arrangement onto a clear polyester substrate (Fig. 9(IB)). The application of a relatively high weight percentage (45%) of SBS binder increases the pattern strength, and ink adhesion, and evades the development of cracks or delamination when multiple fluidic channels are peeled off. By employing carbon black as a filler, the ink's viscosity can be controlled and used to create printed tracks with excellent resolution. These printed carbon tracks that are tightly glued to the substrate enable the recycling and repeated fabrication of a silicone elastomer-based microfluidic multichannel. Additionally, because of the binder's capacity to stretch, it has the constructive flexibility needed to withstand the mechanical stress involved in removing the preserved channel layer from the mould surface. A variety of master moulds created on the polyester layer and a variety of printed sensing electrodes on a polyimide substrate are shown in Fig. 9(IC) and (D), respectively. When the silicone elastomer layer covering the master is peeled off, the cured layer has carved microfluidic channels on the inner surface due to the mould design. These flexible, microfluidic tubes are incorporated into the flexible electrodes to flow the samples to the surface of the transducer and to catch sweat in real-time while exercising.
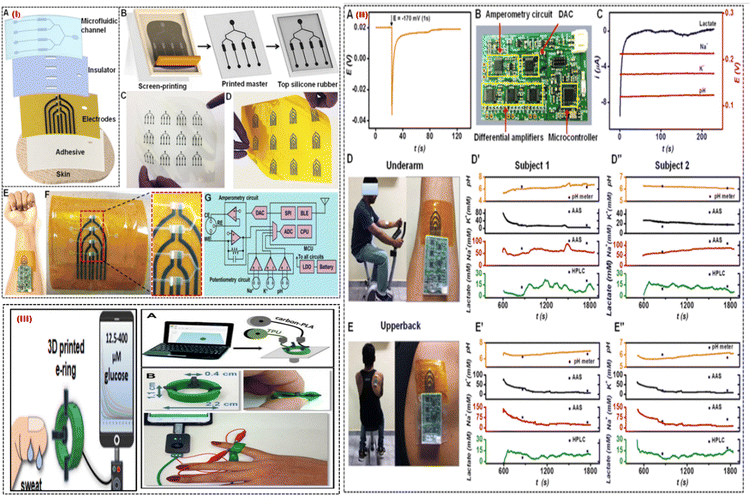 | ||
| Fig. 9 (I) Process of a printed microfluidic device. (A) The microfluidic patch's several layers, and (B) the silicone rubber-based microfluidic channels from the screen-printed carbon master is shown schematically. (C) The optical pictures display a variety of printed carbon masters and (D) multiarray electrodes. (E) Pictures of a skin-interfaced, completely integrated microfluidic sensor and (F) a multiarray electrode with four sensing chambers in close-up. (G) Diagram of the design of a tiny PCB with multiplexed analysis and wireless mode of data transmission capabilities. (II) Analysing sweat on the body. (A) The OCP responses for Na-ISE are shown when a nearby amperometric sensor is subjected to a constant voltage of about 170 mV for one second. (B) An optical picture of the portable circuit board. (C) Using the tiny circuit board, measurements of the potentiometric and amperometric responses are simultaneous. (D) Optical pictures displaying the integrated microfluidic wearable sensor on the underarm and (E) upper back areas. For two participants, multiplexed sensor responses were recorded in the (D′, D′′) underarm and (E′, E′′) upper back regions. Adapted with permission from ref. 76. Copyright 2021 American Chemical Society. (III) (A) The stages involved in making the 3D-printed e-ring. (B) Images of the 3D-printed e-ring that display its flexibility, dimensions, and connection to the mobile and small potentiostat. Adapted with permission from ref. 77. Copyright 2021 American Chemical Society. | ||
Fig. 9(IE) shows a flexible sensor patch that can easily integrate onto human skin, collect sweat samples in real-time, and conduct electrochemical analysis. The electrochemically active region is exposed to the sweat samples through the microfluidic channels placed on the printed electrode Fig. 9(IF). The tiny PCB can fully remove cross-signal interference from nearby electrodes for multiplexed sweat biomarker monitoring. The key components on the PCB for supporting synchronized measurements on one channel of amperometry and 3 channels of potentiometry, are shown in Fig. 9(IG). It is noted that the quick potential deactivation also introduces noise into OCP signals, and retrieving the start OCP levels requires about 60 s. Fig. 9(IIA). This issue was solved by a mini potentiostat which collected interference-free real-time responses from sweat biomarkers Fig. 9(IIB). Fig. 9IIC shows that amperometric lactate signals were recorded at a fixed polarization potential of 170 mV, and the Na+, K+, and pH sensors displayed stable OCP responses. By analyzing sweat from the underarm Fig. 9(IID) and upper back Fig. 9(IIE) regions, it is possible to better understand the geographical dependence on sweat secretion and its biomarker makeup. Fig. 9(IID′,D′′) displays the outcomes of the on-body test performed at the underarm for 2 subjects. Fig. 9(IIE′,E′′) shows the on-body evaluation results for both subjects in the upper back position. The technology enables recurrent pattern transfer from the same master and provides low-cost simple patterning of the master for building soft microfluidic devices. This wearable platform made use of surface functionalization and surface treatment advantages to achieve leakage-free fluidic system construction with an increased sampling rate. The fluidic chamber's easy integration into the printed active sensor region reduces the need for sample volume. Additionally, the physically separated individual sensors prevent electrode components and reaction product interferents from combining. They are excellent candidates for wearable sensing applications due to their high levels of selectivity, sensitivity, long-term stability, carryover efficiency, and reproducibility. Pumpless fluidic systems are made possible by the autonomous fluid flow through capillary action, which also allows real-time fluid flow and assessment. Additionally, a miniaturized electronic circuit board with an in situ signal recorder and wireless components was interfaced with the printed microfluidic patch to enable real-time interpretation of sweat biomarkers.
A smartphone-addressable 3D-printed electrochemical ring for nonenzymatic self-monitoring of glucose in sweat sample perspiration was developed by Katseli et al. in 2021.77 The wearable component, an electrochemical ring (e-ring), was created using a dual extruder 3D printer in a single step (Fig. 9III). The 3D-printed e-ring is made up of three integrated printed electrodes from the conductive carbon-loaded filament and connected to the inside of the green cylindrical holder made of TPU (Fig. 9IIIA). TPU is a material that is often used in the medical sector and has great mechanical characteristics including durability and flexibility. It was chosen to print the holder so that the e-ring would have the right amount of flexibility for optimal finger-fitting (Fig. 9IIIB). The tool allows non-invasive detection of glucose in sweat without interference from other electroactive molecules in the concentration range of 12.5–400 mM and is mechanically robust during bending. The 3D-printed e-ring can be used as a model for internally produced wearable sensors since it fills the gap between current wearable fabrication/sensing technologies and the much-needed real-time glucose self-measurement. The e-ring has an appealing electroanalytical efficiency for sweat glucose measurement because of the utilization of catalytic glucose sensing on gold which is different to the enzymatic sensing approach, in terms of having good sensitivity, selectivity, repeatability, and stability. The e-ring can be used in future research to conduct a more thorough analysis of the relationship between human perspiration and blood glucose levels.
One of the pioneering works in the field of wearable sensors is the development of fully integrated wearable sensors for the simultaneous detection of glucose, lactate, sodium, potassium ions, and skin temperature (to calibrate the response of the sensors).4 The work demonstrated the use of a flexible sensor array for the above-mentioned analytes connected with a wireless circuit board transmitting the data directly to the smartphone. The developed sensor was used for the on-body real-time sweat analysis of subjects during stationary cycling. Gao group reported in 2022 the development of an integrated wearable sensor for the detection of essential amino acids and vitamins.27 The work reported the use of a MIP towards the analyte creating a highly selective electrode surface. The sensor platform also had electrodes for the in-situ induction of sweat, which can also be analyzed for stationary subjects. The integrated system also had a flexible potentiostat and accompanied circuitry for data analysis and wireless transmission. The sensor was also made in a smartwatch configuration wherein the sensor was placed inside a watch with an opening for the sweat through the wrist. After sweat analysis, the screen on the watch gave a read-out corresponding to the analyte concentration. The same group recently reported the development of a flexible wearable bioelectronic system to monitor the analytes during wound healing and release drugs to the infected wound.78 The multiplexed sensor was developed for uric acid, lactate, glucose, ammonium, pH, and temperature sensing.
The microneedle-based wearable sensor is another wearable platform for the continuous monitoring of biomarkers in the interstitial fluid.79 Since it samples the bio-fluid inside the body, it gives a more detailed account of the biomarkers of interest.80 Tehrani et al, demonstrated the development of a wearable microneedle-based sensor for the sensing of glucose, lactate, and alcohol content in the interstitial fluid.81 Continuous monitoring of these biomarkers is of significant interest since these are related to various illnesses. Fig. 10I shows the developed wearable sensor along with the exploded view of the sensor construction. The sensor construction shows the presence of three working electrodes. The electrodes were modified with glucose oxidase, lactate oxidase, and alcohol oxidase. The in vivo testing of the sensor showed its sensing performance during cycling, eating, and drinking. Cheng et al., reported the development of a touch-based and wearable microneedle-based glucose sensor.82 The authors in this work tried to address the issue of low flux and inconsistency during the reverse iontophoresis approach that is generally used for glucose sensing. The authors proposed using a microneedles-based skin penetration system, which was then used for the reverse iontophoresis-based extraction. The authors showed that this system improved the glucose extraction flux by 1.6 times. Fig. 10II(a) and (b) show the sensor construction. The sensor shows the presence of microneedles for the sample extraction. The electrodes were modified with glucose oxidase for glucose sensing and the data generated was transmitted wirelessly to a smartphone. The determination of ions also helps in the determination of various illnesses in the human body. In this regard, Huang et al. developed a stainless steel-based microneedle patch for the simultaneous sensing of calcium, potassium, and sodium ions.83 The stainless-steel microneedles were modified differently for the detection of different ions as shown in Fig. 10III(a) and (b). A layer of gold was first deposited electrochemically on the microneedles, followed by the deposition of PEDOT:PSS polymer. These microneedles were then modified with calcium, potassium, and sodium selective membranes for the detection of calcium, potassium, and sodium respectively.
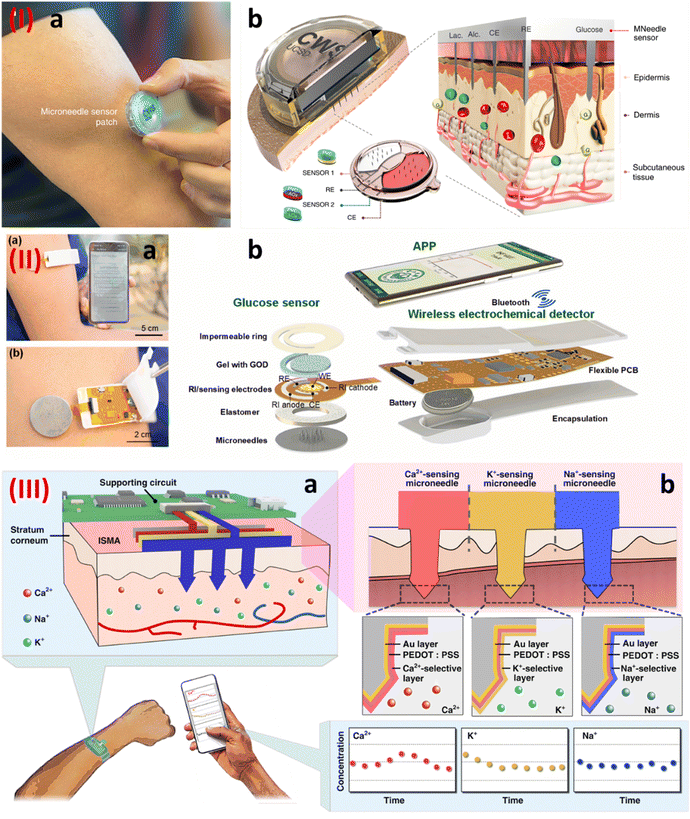 | ||
| Fig. 10 (I) (a) A wearable integrated microneedle-based sensor, and (b) an enlarged view of the sensor construction.81 Copyright 2022 Nature Biomedical Engineering. (II) (a) An integrated microneedle-based sensor transmitting data wirelessly, and (b) an enlarged view of the various components of the wearable sensor.82 Copyright 2022 Elsevier. (III) (a) and (b) A stainless steel-based microneedle patch for the simultaneous detection of calcium, potassium, and sodium ions.83 Copyright 2023 Nature. | ||
Multifunctional flexible sensors
Wearable technology relies on multifunctional flexible sensors, which can be used for various purposes, including health monitoring, motion and activity tracking, environmental and contextual sensing, human-machine interfaces, pain management and rehabilitation, agriculture and sports, sports performance monitoring, and wearable energy harvesting. The long-term monitoring of physiological parameters with precise pulse monitoring could provide much information non-invasively, which demands a versatile pressure sensor with high sensitivity over a wide linear range (up to 10 kPa). Still, the combination of linearity region and sensitivity could be challenging. He et al. in 2019 demonstrated a highly conductive inter-facially self-assembled graphene-based high-sensitivity and high-accuracy wearable pulse monitoring system. The authors showed highly accurate pulse signal monitoring in real time, even during exercise with high sensitivity (1875.53 kPa−1) and a wide linear detection range (0–40 kPa).84 Next-generation high-performance flexible or wearable electronics rely on flexible human–machine interfaces, which require high-performance novel materials, augmented structures, and design methodologies. A metal oxide (zinc oxide) based on a flexible acoustic platform has been demoted by Zhang et al. in 2022 and could be utilized in a wide range of applications, including as a loudspeaker, a microphone, or an ambient sensor based on its excitation frequencies. The authors exhibited respiratory monitoring using their zinc oxide-based flexible sensor with a high-temperature coefficient of frequency of −289![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ppm K−1.85 The future bioelectronics for accurately monitoring human physiological health requires integrated flexible and stretchable multifunctional sensors. Many challenges remain, including the crosstalk among the integrated multifunctional sensors into a common substrate intended for the simultaneous monitoring of health. Zhang et al. in 2022 demonstrated multifunctional sensors integrated into a stretchable substrate for the real-time measurement of strain, temperature, and ECG without interfering with each other. In-situ polymerization self-assembly of 3,4-ethylenedioxythiophene (EDOT) on MXene-Ti3C2Tx was achieved in an aqueous solution in the absence of an oxidant to develop a single composite material. The composite material showed high strain sensitivity (2075; >22% strain), a good temperature coefficient of resistance (0.52% K−1), and a low skin-electrode impedance (51.08 kΩ at 10
ppm K−1.85 The future bioelectronics for accurately monitoring human physiological health requires integrated flexible and stretchable multifunctional sensors. Many challenges remain, including the crosstalk among the integrated multifunctional sensors into a common substrate intended for the simultaneous monitoring of health. Zhang et al. in 2022 demonstrated multifunctional sensors integrated into a stretchable substrate for the real-time measurement of strain, temperature, and ECG without interfering with each other. In-situ polymerization self-assembly of 3,4-ethylenedioxythiophene (EDOT) on MXene-Ti3C2Tx was achieved in an aqueous solution in the absence of an oxidant to develop a single composite material. The composite material showed high strain sensitivity (2075; >22% strain), a good temperature coefficient of resistance (0.52% K−1), and a low skin-electrode impedance (51.08 kΩ at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hz). No oxidant and facile transfer of functional materials into thermoplastic elastomer (ultrathin and stretchable) makes this method more feasible for fabricating high-performance multifunctional sensors.86 A sensor with a stable electrical output is highly desired for developing next-generation wearable health monitors. Zhu et al. in 2018 reported stretchable carbon nanotube transistor-based robust temperature sensors by adopting circuit design strategies. Inaccuracy, strain-dependent errors, and sensor calibration of any sensor are the major challenges. However, the authors reported inaccuracy of only ±1 °C within a uniaxial strain range of 0–60% by employing the static and dynamic differential readout techniques.87
Hz). No oxidant and facile transfer of functional materials into thermoplastic elastomer (ultrathin and stretchable) makes this method more feasible for fabricating high-performance multifunctional sensors.86 A sensor with a stable electrical output is highly desired for developing next-generation wearable health monitors. Zhu et al. in 2018 reported stretchable carbon nanotube transistor-based robust temperature sensors by adopting circuit design strategies. Inaccuracy, strain-dependent errors, and sensor calibration of any sensor are the major challenges. However, the authors reported inaccuracy of only ±1 °C within a uniaxial strain range of 0–60% by employing the static and dynamic differential readout techniques.87
Implantable sensors
Implantable sensors are biomedical devices capable of providing continuous biomarker information once implanted in the human body.88 These devices provide wireless, real-time, and continuous data without the need for repeated surgical interventions.89,90 The most important biomarker that is of significant interest to clinicians and researchers is glucose and hence, a lot of research has been conducted for continuous glucose monitoring systems. Kim et al. in 2022 developed an electromagnetic biosensor for continuous glucose monitoring, which was subcutaneously implanted. The sensor was able to track the changing dielectric permittivity as the blood glucose levels changed. The authors demonstrated the sensors working in swine and beagle through intravenous glucose tolerance tests.91 Another example of an application of an implantable sensor is for tactile pressure sensing. Here the sensing system is implanted subdermally, which was demonstrated by Du et al. in 2023. The sensing system was based on a capacitive pressure sensor, which was supported by other electronic parts. The sensor was tested by implanting it in the fingertip and was capable of measuring the skin forces at a resolution of 4.3 mN.92 Having mentioned a few examples of implantable sensors, it is important to mention one of the key challenges associated with them. Since these devices provide continuous and real-time data wirelessly, they require significant power consumption,93 and hence, research is ongoing for self-powered biosensing systems.94The development of goods that are simple to incorporate into a person's lifestyle is necessary for wearable sensor technology to gain widespread acceptance. Sweat analysis has been carried out using most wearable chemical sensors in conjunction with flexible, durable, temporary tattoo-like devices, skin patches, headbands, spectacles, or wristbands. Recent research has also investigated smart bandages for wound healing, mouthguards, diagnostic devices for saliva monitoring, and eyeglasses, and contact lenses for tear assays as additional devices and accessories for human body biomarker sensing applications.95 Sensors integrated into the textiles allow for efficient sweat collection and can present unique opportunities for real-time biomarker monitoring.96 These not only provide a platform for sensing analytes but can also be used for the generation of human motion-induced triboelectric charges, which in turn block microbes.97 Combining the strengths of the fashion trend and wearable sensors to create wearable fashion accessories will result in a wider adoption of wearable sensor technology that can urgently address the need for disease diagnostics and real-time health monitoring. Some of the commercially available wearable sensors are listed in Table 1.
| S. No | Product name | Company | Analyte, Sample | Wearable Platform | Monitoring Technique | Price | FDA Approval | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Lingo sensor | Abbott | Track both glucose and ketone, lactate, and alcohol levels | Skin | Optical colorimetery | $420 | Yes | 98 |
| 2 | Biosensor BX100 | Philips | Deterioration detection solution | Patch | Image-guided therapy | Yes | 99 | |
| 3 | Lifetemp | Isansys lifecare | Body temperature | Armpit | Silicone gel adhesive | 100 | ||
| 4 | Paediatric sensors | Isansys lifecare | Heart rate, pulse rate and respiratory rate | Patches | Remote accessibility | 100 | ||
| 5 | 3150 WristOx | Isansys lifecare | Robustness and reliability | Wrist-worn | PPG waveform | $739 | 100 | |
| 6 | Sensit smart | PalmSens | Capable of EIS up to 200 kHz | Skin | Screen printed electrodes | 101 | ||
| 8 | Fitbit smart watch | Fitbit versa | Heart rate monitor | Wrist watch | Electrochemical device | $179.95 | Yes | 102 |
| 9 | Fingertip SpO2 sensor AMD-DS-NM0002–4 | Amydi-med Electronics | SpO2 sensor | Fingertip, multi-position, foot, toe | Transflectance, reflectance | $3 | Yes | 103 |
| 10 | Vios chest sensor | Vios | ECG, heart rate, respiratory rate, SpO2, pulse rate, posture, and activity | Chest | Medico electrode | Yes | 104 |
6. Challenges and future scope
Wearable sensor development and commercialization encounter several difficulties. The precision and dependability of sensors are essential for obtaining useful data. First, sensor performance may be impacted by elements like motion artifacts and ambient factors, necessitating rigorous calibration and testing. Second, integration and miniaturization represent significant obstacles. Wearable sensors must be functional while remaining small and unobtrusive. It is technically difficult to balance power usage, computational power, and data transfer in a small area. Third, for wearable sensors, power management is crucial. This is a challenging undertaking that calls for effective power management strategies and low-power components to maximize battery life while satisfying the power requirements of sensors, CPUs, and wireless connectivity. Moreover, data analysis and interpretation present difficulties. It is essential to create algorithms that can handle and analyse enormous amounts of sensor data and extract valuable insights. Accuracy, miniaturization, power management, data analytics, and user experience will all see advancements as a result of ongoing research and development. Wearable sensors can revolutionize personalized healthcare and promote greater well-being by overcoming these difficulties. Devices like smartwatches and fitness trackers enable sensors to monitor blood pressure, body temperature, blood glucose levels, heart rate, activity levels, sleep patterns, and blood pressure and have revolutionized the field of diagnostics (Fig. 11). | ||
| Fig. 11 Futuristic applications of integrated wearable sensors in personalized healthcare [Figure created using icons from Biorender and MS Office]. | ||
7. Conclusion
Keeping up with the fast pace of life is significantly taking a toll on people's heath. Access to affordable healthcare for all is also a bottleneck to improving the health of the population. Wearable sensors are a promising candidate to solve this critical issue. These sensors can detect biomarkers and metabolites of interest non-invasively in sweat, saliva, and tears. Though promising, the mass availability of these sensors is still very far from reality. The main reasons are cost, comfort levels, and a lack of integrated systems. This review highlighted the recent efforts in the development of complete integrated prototypes. In a typical integrated system, the sensors, powering system, electronics, and communication systems are packaged tightly in a compact manner, which can be comforting and can be worn by the user without any issue. In this review, we have discussed various physio-chemical sensors, which have the potential for healthcare improvement. We also delved into wearable strain, pressure, thermal, and multifunctional flexible sensors. The challenges being faced while designing and scaling up these sensors are also discussed. This review will be helpful in the design and development of novel integrated wearable sensors which are expected to start a new era in the field of personalized healthcare.Conflicts of interest
The authors do not have any conflicts of interest to declare.Acknowledgements
A. P. would like to thank DST for providing the fellowship (DST/WOS-B/HN-4/2021) under the DST-WOS-B scheme. M. G. would like to thank the Department of Biomedical Engineering, Texas A&M University, College Station, USA for providing the Postdoctoral Fellowship.References
- [cited 2023 02/19/2023]; Available from: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1.
- S. Cheng, et al., Recent Progress in Intelligent Wearable Sensors for Health Monitoring and Wound Healing Based on Biofluids, Front. Bioeng. Biotechnol., 2021, 9, 765987 CrossRef PubMed.
- H. C. Ates, et al., End-to-end design of wearable sensors, Nat. Rev. Mater., 2022, 7(11), 887–907 CrossRef PubMed.
- W. Gao, et al., Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis, Nature, 2016, 529(7587), 509–514 CrossRef CAS PubMed.
- X. Zhang, et al., Integrated Wearable Sensors for Sensing Physiological Pressure Signals and β-Hydroxybutyrate in Physiological Fluids, Anal. Chem., 2022, 94(2), 993–1002 CrossRef CAS PubMed.
- S. Bi, L. Hou and Y. Lu, An integrated wearable strain, temperature and humidity sensor for multifunctional monitoring, Composites, Part A, 2021, 149, 106504 CrossRef CAS.
- Y. Lu, et al., Multifunctional Flexible Humidity Sensor Systems Towards Noncontact Wearable Electronics, Nano-Micro Lett., 2022, 14(1), 150 CrossRef CAS PubMed.
- Y. Luo, et al., Functionalized Hydrogel-Based Wearable Gas and Humidity Sensors, Nano-Micro Lett., 2023, 15(1), 136 CrossRef CAS PubMed.
- W. Wu, et al., Extensible Integrated System for Real-Time Monitoring of Cardiovascular Physiological Signals and Limb Health, Adv. Mater., 2023, 2304596 CrossRef CAS PubMed.
- J. Dong, et al., Superelastic Radiative Cooling Metafabric for Comfortable Epidermal Electrophysiological Monitoring, Nano-Micro Lett., 2023, 15(1), 181 CrossRef PubMed.
- Y. Jung, et al., Functional Materials and Innovative Strategies for Wearable Thermal Management Applications, Nano-Micro Lett., 2023, 15(1), 160 CrossRef PubMed.
- H. Teymourian, et al., Wearable Electrochemical Sensors for the Monitoring and Screening of Drugs, ACS Sens., 2020, 5(9), 2679–2700 CrossRef CAS PubMed.
- S. M. Mugo and J. Alberkant, Flexible molecularly imprinted electrochemical sensor for cortisol monitoring in sweat, Anal. Bioanal. Chem., 2020, 412(8), 1825–1833 CrossRef CAS PubMed.
- S. Demuru, et al., Antibody-Coated Wearable Organic Electrochemical Transistors for Cortisol Detection in Human Sweat, ACS Sens., 2022, 7(9), 2721–2731 CrossRef CAS PubMed.
- S. M. Mugo, et al., Wearable microneedle dual electrochemical sensor for simultaneous pH and cortisol detection in sweat, Electrochem. Sci. Adv., 2022, 2(1), e2100039 CrossRef CAS.
- S. M. Mugo, et al., Flexible electrochemical aptasensor for cortisol detection in human sweat, Anal. Methods, 2021, 13(36), 4169–4173 RSC.
- S. M. Mugo, W. Lu and S. Robertson, A Wearable, Textile-Based Polyacrylate Imprinted Electrochemical Sensor for Cortisol Detection in Sweat, Biosensors, 2022, 12(10), 854 CrossRef CAS PubMed.
- Y. Yan, Z. Li and Z. Lou, Photodetector based on Ruddlesden-Popper perovskite microwires with broader band detection, J. Semiconductors, 2023, 44(8), 082201 CrossRef.
- S. Yao, et al., A Two-in-One Annealing Enables Dopant Free Block Copolymer Based Organic Solar Cells with over 16% Efficiency, Chin. J. Chem., 2023, 41(6), 672–678 CrossRef CAS.
- T. Liu, et al., Multifunctional all-polymer photovoltaic blend with simultaneously improved efficiency (18.04%), stability and mechanical durability, Aggregate, 2023, 4(3), e308 CrossRef CAS.
- M. Janghorban, et al., Recent Advances, Opportunities, and Challenges in Developing Nucleic Acid Integrated Wearable Biosensors for Expanding the Capabilities of Wearable Technologies in Health Monitoring, Biosensors, 2022, 12(11), 986 CrossRef CAS PubMed.
- D. J. Saldanha, A. Cai and N.-M. Dorval Courchesne, The Evolving Role of Proteins in Wearable Sweat Biosensors, ACS Biomater. Sci. Eng., 2021, 2020–2047 Search PubMed.
- Q. Zhang, et al., Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat, Sens. Actuators, B, 2020, 320, 128325 CrossRef CAS.
- Aparna, et al., Molecularly imprinted conducting polymer based sensor for Salmonella typhimurium detection, Bioelectrochemistry, 2022, 147, 108211 CrossRef CAS PubMed.
- H. Park, W. Park and C. H. Lee, Electrochemically active materials and wearable biosensors for the in situ analysis of body fluids for human healthcare, npg Asia Mater., 2021, 13(1), 23 CrossRef CAS.
- O. Parlak, et al., Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing, Sci. Adv., 2018, 4(7), eaar2904 CrossRef CAS PubMed.
- M. Wang, et al., A wearable electrochemical biosensor for the monitoring of metabolites and nutrients, Nat. Biomed. Eng., 2022, 6(11), 1225–1235 CrossRef CAS PubMed.
- D. Pérez and J. Orozco, Wearable electrochemical biosensors to measure biomarkers with complex blood-to-sweat partition such as proteins and hormones, Microchim. Acta, 2022, 189(3), 127 CrossRef PubMed.
- J. Min, et al., Wearable electrochemical biosensors in North America, Biosens. Bioelectron., 2021, 172, 112750 CrossRef CAS PubMed.
- M. Garg, et al., White graphene quantum dots as electrochemical sensing platform for ferritin, Faraday Discuss., 2021, 227(0), 204–212 RSC.
- M. Garg, et al., Amine-Functionalized Graphene Quantum Dots for Fluorescence-Based Immunosensing of Ferritin, ACS Appl. Nano Mater., 2021, 4(7), 7416–7425 CrossRef CAS.
- M. Garg, et al., Label-free approach for electrochemical ferritin sensing using biosurfactant stabilized tungsten disulfide quantum dots, Biosens. Bioelectron., 2020, 151, 111979 CrossRef CAS PubMed.
- M. Garg, et al., Microfluidic-Based Electrochemical Immunosensing of Ferritin, Biosensors, 2020, 10(8), 91 CrossRef CAS PubMed.
- M. Garg, et al., Lysine-Functionalized Tungsten Disulfide Quantum Dots as Artificial Enzyme Mimics for Oxidative Stress Biomarker Sensing, ACS Omega, 2020, 5(4), 1927–1937 CrossRef CAS PubMed.
- A. Gupta, et al., Highly Sensitive Optical Detection of Escherichia coli Using Terbium-Based Metal–Organic Framework, ACS Appl. Mater. Interfaces, 2020, 12(42), 48198–48205 CrossRef CAS PubMed.
- N. K. Singh, et al., A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer, Biosens. Bioelectron., 2023, 227, 115097 CrossRef CAS PubMed.
- R. M. Torrente-Rodríguez, et al., Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system, Matter, 2020, 2(4), 921–937 CrossRef PubMed.
- S. Ha, et al., Low-Power Integrated Circuit Design for Wearable Biopotential Sensing, in Wearable Sensors, ed. E. Sazonov and M. R. Neuman, Academic Press, Oxford, 2014, ch. 4.3, pp. 323–352 Search PubMed.
- S. Ha, et al., Low-power integrated circuits for wearable electrophysiology, in Wearable Sensors, ed. E. Sazonov, Academic Press, Oxford, 2nd edn, 2021, pp. 163–199, ch. 6 Search PubMed.
- H. Chen, et al., Design of an Integrated Wearable Multi-Sensor Platform Based on Flexible Materials for Neonatal Monitoring, IEEE Access, 2020, 8, 23732–23747 Search PubMed.
- K. Veenuttranon, K. Kaewpradub and I. Jeerapan, Screen-Printable Functional Nanomaterials for Flexible and Wearable Single-Enzyme-Based Energy-Harvesting and Self-Powered Biosensing Devices, Nano-Micro Lett., 2023, 15(1), 85 CrossRef CAS PubMed.
- Y. Song, et al., Self-Powered Wearable Biosensors, Acc. Mater. Res., 2021, 2(3), 184–197 CrossRef CAS.
- C. Xu, et al., Portable and wearable self-powered systems based on emerging energy harvesting technology, Microsyst. Nanoeng., 2021, 7(1), 25 CrossRef PubMed.
- A. Rayegani, et al., Recent Advances in Self-Powered Wearable Sensors Based on Piezoelectric and Triboelectric Nanogenerators, Biosensors, 2023, 13(1), 37 CrossRef CAS PubMed.
- Y. Gai, et al., A Self-Powered Wearable Sensor for Continuous Wireless Sweat Monitoring, Small Methods, 2022, 6(10), 2200653 CrossRef CAS PubMed.
- W. Zhang, et al., Wearable Battery-Free Perspiration Analyzing Sites Based on Sweat Flowing on ZnO Nanoarrays, Nano-Micro Lett., 2020, 12(1), 105 CrossRef CAS PubMed.
- M. Uddin and S. Syed-Abdul, Data Analytics and Applications of the Wearable Sensors in Healthcare: An Overview, Sensors, 2020, 20(5), 1379 CrossRef PubMed.
- G. Manogaran, et al., CDP-UA: Cognitive Data Processing Method Wearable Sensor Data Uncertainty Analysis in the Internet of Things Assisted Smart Medical Healthcare Systems, IEEE J. Biomed. Health Inform, 2021, 25(10), 3691–3699 Search PubMed.
- P. Ajay and R. Huang, Wearable Sensor Data for Classification and Analysis of Functional Fitness Exercises Using Unsupervised Deep Learning Methodologies, Security Commun. Networks, 2022, 2022, 8706784 CrossRef.
- C. Doukas and I. Maglogiannis. Managing Wearable Sensor Data through Cloud Computing. in 2011 IEEE Third International Conference on Cloud Computing Technology and Science. 2011.
- M. S. Mahmud, Healthcare data analytics for wearable sensors, in Wearable Physical, Chemical and Biological Sensors, ed. E. Morales-Narvaez and C. Dincer, Elsevier, 2022, pp. 169–182, ch. 6 Search PubMed.
- M. Garg, Significance of 3D printing for a sustainable environment, Mater. Today Sustainability, 2023, 23, 100419 CrossRef.
- B. Carnero, et al., Microfluidic devices manufacturing with a stereolithographic printer for biological applications, Mater. Sci. Eng., C, 2021, 129, 112388 CrossRef CAS PubMed.
- L. Wei, et al., 3D-printed low-cost fabrication and facile integration of flexible epidermal microfluidics platform, Sens. Actuators, B, 2022, 353, 131085 CrossRef CAS.
- A. Waldbaur, et al., Let there be chip—towards rapid prototyping of microfluidic devices: one-step manufacturing processes, Anal. Methods, 2011, 3(12), 2681–2716 RSC.
- J. Ruiz-Morales, et al., Three dimensional printing of components and functional devices for energy and environmental applications, Energy Environ. Sci., 2017, 10(4), 846–859 RSC.
- Mayank Garg, Significance of 3D printing for a sustainable environment, Materials Today Sustainability, 2023, 23, 100419, DOI:10.1016/j.mtsust.2023.10041.
- S. Waheed, et al., 3D printed microfluidic devices: enablers and barriers, Lab Chip, 2016, 16(11), 1993–2013 RSC.
- G. Gonzalez, et al., Current and emerging trends in polymeric 3D printed microfluidic devices, Addit. Manuf., 2022, 102867 CAS.
- S. A. Gowers, et al., 3D printed microfluidic device with integrated biosensors for online analysis of subcutaneous human microdialysate, Anal. Chem., 2015, 87(15), 7763–7770 CrossRef CAS PubMed.
- G. I. J. Salentijn, et al., Fused Deposition Modeling 3D Printing for (Bio)analytical Device Fabrication: Procedures, Materials, and Applications, Anal. Chem., 2017, 89(13), 7053–7061 CrossRef CAS PubMed.
- M. Rafiee, R. D. Farahani and D. Therriault, Multi-Material 3D and 4D Printing: A Survey, Adv. Sci., 2020, 7(12), 1902307 CrossRef CAS PubMed.
- M. Padash, C. Enz and S. Carrara, Microfluidics by additive manufacturing for wearable biosensors: a review, Sensors, 2020, 20(15), 4236 CrossRef CAS PubMed.
- X. Mei, et al., Wearable, nanofiber-based microfluidic systems with integrated electrochemical and colorimetric sensing arrays for multiplex sweat analysis, Chem. Eng. J., 2023, 454, 140248 CrossRef CAS.
- J. R. Sempionatto, et al., Wearable chemical sensors for biomarker discovery in the omics era, Nat. Rev. Chem., 2022, 6(12), 899–915 CrossRef PubMed.
- Y. Huang, et al., Multi-analyte sensing strategies towards wearable and intelligent devices, Chem. Sci., 2022, 13(42), 12309–12325 RSC.
- J. R. Sempionatto, et al., Eyeglasses based wireless electrolyte and metabolite sensor platform, Lab Chip, 2017, 17(10), 1834–1842 RSC.
- Z. Liu, et al., Functionalized Fiber-Based Strain Sensors: Pathway to Next-Generation Wearable Electronics, Nano-Micro Lett., 2022, 14(1), 61 CrossRef CAS PubMed.
- R. Madhavan, Nanocrack-based ultrasensitive wearable and skin-mountable strain sensors for human motion detection, Mater. Adv., 2022, 3(23), 8665–8676 RSC.
- S. Sun, et al., A wearable, waterproof, and highly sensitive strain sensor based on three-dimensional graphene/carbon black/Ni sponge for wirelessly monitoring human motions, J. Mater. Chem. C, 2020, 8(6), 2074–2085 RSC.
- H. Liu, et al., Bio-inspired color-changing and self-healing hybrid hydrogels for wearable sensors and adaptive camouflage, J. Mater. Chem. C, 2023, 11(1), 285–298 RSC.
- W.-B. Zhu, et al., Ti3C2Tx MXene/Bamboo Fiber/PDMS Pressure Sensor with Simultaneous Ultrawide Linear Sensing Range, Superb Environmental Stability, and Excellent Biocompatibility, ACS Sustainable Chem. Eng., 2022, 10(11), 3546–3556 CrossRef CAS.
- S.-Y. Cho, et al., Continuous Meter-Scale Synthesis of Weavable Tunicate Cellulose/Carbon Nanotube Fibers for High-Performance Wearable Sensors, ACS Nano, 2019, 13(8), 9332–9341 CrossRef CAS PubMed.
- A. Kumar, et al., Microfluidics-Based Point-of-Care Testing (POCT) Devices in Dealing with Waves of COVID-19 Pandemic: The Emerging Solution, ACS Appl. Bio Mater., 2022, 5(5), 2046–2068 CrossRef CAS PubMed.
- J. Li, et al., Engineering Smart Composite Hydrogels for Wearable Disease Monitoring, Nano-Micro Lett., 2023, 15(1), 105 CrossRef CAS PubMed.
- R. Vinoth, et al., Fully Printed Wearable Microfluidic Devices for High-Throughput Sweat Sampling and Multiplexed Electrochemical Analysis, ACS Sens., 2021, 6(3), 1174–1186 CrossRef CAS PubMed.
- V. Katseli, A. Economou and C. Kokkinos, Smartphone-Addressable 3D-Printed Electrochemical Ring for Nonenzymatic Self-Monitoring of Glucose in Human Sweat, Anal. Chem., 2021, 93(7), 3331–3336 CrossRef CAS PubMed.
- E. Shirzaei Sani, et al., A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds, Sci. Adv., 2023, 9(12), eadf7388 CrossRef PubMed.
- Z. Xie, et al., Wearable microneedle-integrated sensors for household health monitoring, Eng. Regener., 2022, 3(4), 420–426 Search PubMed.
- W. Lee, et al., Conformable microneedle pH sensors via the integration of two different siloxane polymers for mapping peripheral artery disease, Sci. Adv., 2021, 7(48), eabi6290 CrossRef CAS PubMed.
- F. Tehrani, et al., An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid, Nat. Biomed. Eng., 2022, 6(11), 1214–1224 CrossRef CAS PubMed.
- Y. Cheng, et al., A touch-actuated glucose sensor fully integrated with microneedle array and reverse iontophoresis for diabetes monitoring, Biosens. Bioelectron., 2022, 203, 114026 CrossRef CAS PubMed.
- X. Huang, et al., 3D-assembled microneedle ion sensor-based wearable system for the transdermal monitoring of physiological ion fluctuations, Microsyst. Nanoeng., 2023, 9(1), 25 CrossRef CAS PubMed.
- J. He, et al., A Universal high accuracy wearable pulse monitoring system via high sensitivity and large linearity graphene pressure sensor, Nano Energy, 2019, 59, 422–433 CrossRef CAS.
- Q. Zhang, et al., Flexible multifunctional platform based on piezoelectric acoustics for human–machine interaction and environmental perception, Microsyst. Nanoeng., 2022, 8(1), 99 CrossRef PubMed.
- S. Zhang, et al., On-skin ultrathin and stretchable multifunctional sensor for smart healthcare wearables, npj Flexible Electron., 2022, 6(1), 11 CrossRef CAS.
- C. Zhu, et al., Stretchable temperature-sensing circuits with strain suppression based on carbon nanotube transistors, Nat. Electron., 2018, 1(3), 183–190 CrossRef.
- H. C. Koydemir and A. Ozcan, Wearable and Implantable Sensors for Biomedical Applications, Annu. Rev. Anal. Chem., 2018, 11(1), 127–146 CrossRef CAS PubMed.
- M. Veletić, et al., Implants with Sensing Capabilities, Chem. Rev., 2022, 122(21), 16329–16363 CrossRef PubMed.
- B. D. Nelson, et al., Wireless Technologies for Implantable Devices, Sensors, 2020, 20(16), 4604 CrossRef PubMed.
- S. Kim, et al., Subcutaneously implantable electromagnetic biosensor system for continuous glucose monitoring, Sci. Rep., 2022, 12(1), 17395 CrossRef CAS PubMed.
- L. Du, et al., An implantable, wireless, battery-free system for tactile pressure sensing, Microsyst. Nanoeng., 2023, 9(1), 130 CrossRef CAS PubMed.
- D. Yogev, et al., Current state of the art and future directions for implantable sensors in medical technology: Clinical needs and engineering challenges, APL Bioeng., 2023, 7(3) DOI:10.1063/5.0152290.
- Z. Xue, et al., Self-Powered Biosensors for Monitoring Human Physiological Changes, Biosensors, 2023, 13(2), 236 CrossRef CAS PubMed.
- Y. Bi, et al., Universal Fully Integrated Wearable Sensor Arrays for the Multiple Electrolyte and Metabolite Monitoring in Raw Sweat, Saliva, or Urine, Anal. Chem., 2023, 95(16), 6690–6699 CrossRef CAS PubMed.
- Y. Shi, et al., Wearable sweat biosensors on textiles for health monitoring, J. Semiconductors, 2023, 44(2), 021601 CrossRef.
- I.-Y. Suh, et al., Self-powered microbial blocking textile driven by triboelectric charges, Nano Energy, 2023, 110, 108343 CrossRef CAS.
- [20230113]; Available from: https://abbott.mediaroom.com/2022-01-06-Abbott-Announces-Future-of-Biowearables-at-Consumer-Electronics-Show13-01-23.
- [20230409]; Available from: https://www.philips.com/a-w/about/news/media-library/20210223-philips-biosensor-bx100.html.
- [cited 2023 20230408]; Available from: https://www.isansys.com/en/Wearable-Sensors.
- [cited 2023 20230407]; Available from: https://www.palmsens.com/product/sensit-smart/.
- [cited 2023 20230410]; Available from: https://www.fitbit.com/global/us/technology/irregular-rhythm.
- [cited 2023 20230406]; Available from: https://www.medicalexpo.com/prod/shenzhen-amydi-med-electronics-tech/product-130960-1010724.html.
- [cited 2023 20230405]; Available from: https://usermanual.wiki/Vios-Medical/CS2050.
Footnote |
| † Equal contribution for the first authorship. |
| This journal is © The Royal Society of Chemistry 2024 |