Open Access Article
Open Access ArticleNatural products as antivibrio agents: insight into the chemistry and biological activity
Noer Kasanah *a,
Maria Ulfah
*a,
Maria Ulfah b and
David C. Rowleyc
b and
David C. Rowleyc
aDepartment of Fisheries, Faculty of Agriculture, Universitas Gadjah Mada, Indonesia. E-mail: noer.kasanah@ugm.ac.id
bIntegrated Lab. Agrocomplex, Faculty of Agriculture, Universitas Gadjah Mada, Indonesia
cDepartment of Biomedical and Pharmaceutical Sciences, College of Pharmacy, The University of Rhode Island, USA
First published on 1st December 2022
Abstract
Vibriosis causes serious problems and economic loss in aquaculture and human health. Investigating natural products as antivibrio agents has gained more attention to combat vibriosis. The present review highlights the chemical diversity of antivibrio isolated from bacteria, fungi, plants, and marine organisms. Based on the study covering the literature from 1985–2021, the chemical diversity ranges from alkaloids, terpenoids, polyketides, sterols, and peptides. The mechanisms of action are included inhibiting growth, interfering with biofilm formation, and disrupting of quorum sensing. Relevant summaries focusing on the source organisms and the associated bioactivity of different chemical classes are also provided. Further research on in vivo studies, toxicity, and clinical is required for the application in aquaculture and human health.
1. Introduction
The genus Vibrio is Gram-negative, curved-rod shape bacteria, halophilic, fermentative, motile by polar flagella, catalase, and oxidase-positive. The genus inhabits aquatic environment, freshwater, water column, sediment, and is associated with marine organisms.1,2 Vibrio spp. play roles as nutrient cyclers in aquatic ecosystems, take up organic material, produce polyunsaturated fatty acids to the aquatic food web, and degrade chitin.3 These groups of bacteria are responsible for several serious infections and opportunistic pathogens to aquatic animals and humans.1,4,5Studies about the effects of increasing sea surface temperature on the biology and ecology of Vibrio showed that there are correlations between the escalation of the emergence of Vibrio infections and global warming. Climate change induces global warming and as a result, the rising sea surface temperature corresponds to the number and distribution of Vibrio as reported in many places worldwide. Salinity less than 25 ppt contributes to Vibrio prevalence and infection in the marine system.5–8
The term vibriosis is used to refer to infections by the member family of Vibrionaceae both in aquatic animals and humans.9 Vibriosis is one of the primary problems in aquaculture that causes severe economic losses and large-scale mortality of shrimp, fish, and shellfish.10 Comprehensive reviews are available focusing on vibriosis in fish,11–13 shrimps,14 crustaceans,15–17 and mollusks.18
More than a hundred Vibrio species have been identified and caused infections in humans. About 14 species of Vibrio reported as causative agents of human vibriosis cause foodborne and non-foodborne Vibrio infections such as V. cholerae, V. parahaemolyticus, V. alginolyticus, and V. vulnificus.9,19 Vibrio spp. infect humans worldwide and is responsible for gastroenteritis, septicemia, and invasive skin and soft tissue infection (SSTI).9,20 Non-foodborne Vibrio infections, caused by V. vulnificus, V. alginolyticus, and V. parahaemolyticus are fatal and often leads to the amputation or death of immunocompromised patients suffering from liver disease, alcohol abuse, or diabetes.20–22
A single or combinational antibiotic is the treatment for curative against vibriosis both in aquatic animals and humans.20 Most Vibrio spp. are susceptible to most antibiotics for animals or humans. Overuse and unregulated antibiotic used in aquaculture are contributing to growing problems and concerns in antimicrobial resistance that impacts human health. Antimicrobial resistance may reduce the effectiveness of treatment options for fish and human health management.23,24 Multiple-antibiotic resistance of V. vulnificus and V. parahaemolyticus were reported in countries such as the United States, Italy, Brazil, Philippines, Malaysia, Indonesia, Thailand, China, India, Iran, South Africa, and Australia.24–30
Antibiotic resistance and the restricted choices of available antivibrio agents are the reasons for searching natural products as new antivibrio agents. The increase in the emergence of antibiotic-resistant bacterial pathogens, including Vibrio spp. is a major public health concern. Therefore, it has intensified the interest in research on the search for effective alternatives to cope with the issue of antibiotic-resistant bacteria. Attempts have been done on screening, isolation, and structure determination of antivibrio compounds from natural products. This review intends to deliver the exploration of natural products for new antivibrio compounds.
2. Targets for antivibrio
Antibiotic resistance is becoming an important issue in the world of medicine. Newly developed antibiotics also starting to lose their effectiveness against some bacterial strains. As a result, it is critical to look for novel antimicrobial agents that are both effective against resistant microbes and long-lasting.Quorum sensing (QS) is a small diffusible signaling molecule that trigger the expression of multiple genes that govern a wide range of activities including bioluminescence, virulence control, sporulation, host colonization, biofilm development, defense against competition, and environmental adaptability. Vibrio fischeri, V. harveyi, V. cholerae, V. anguillarum, and V. vulnificus use QS to regulate their pathogenicity.31 Biofilm is a complex structure of microbiome having different bacterial colonies or single type of cells in a group; adhere to the surface that are embedded in a membrane structure of the extracellular polymeric substances (EPS) composed of eDNA, proteins and polysaccharides. The matrix complex are attached to the biotic or abiotic surface, showed high resistance to antibiotics.32,33 Biofilms formation is the key factor for accelerating Vibrio spp. to grow and survive by providing access to nutrients, protecting from the host immune system, defending from the predator, and antimicrobial compounds. Studies showed that biofilm is important for survival, virulence and stress resistance of Vibrio sp.34,35 The formation and maintenance of biofilms, as well as their resistance to antimicrobials and the host's innate immune system, are controlled by QS-regulated gene expression.35–37
In the aquaculture system, QS regulates virulence factors and the formation of biofilm. Thus, disruption of QS is a potential strategy for preventing disease in aquaculture systems. Quorum sensing inhibitors (QSIs) or quorum quenchers inhibit both the expression of virulence-associated genes and attenuate the virulence of aquaculture pathogens.32 Quorum sensing plays a role in the formation of biofilms. Thus, fighting Vibrio spp. by interrupting quorum sensing and biofilms formation are the right strategies to combat vibriosis.38,39 Inhibiting growth, interrupting quorum sensing, and interfering biofilms formation are targets for antagonistic effects in searching for antivibrio.
3. Antivibrio from bacteria
3.1. Actinobacteria
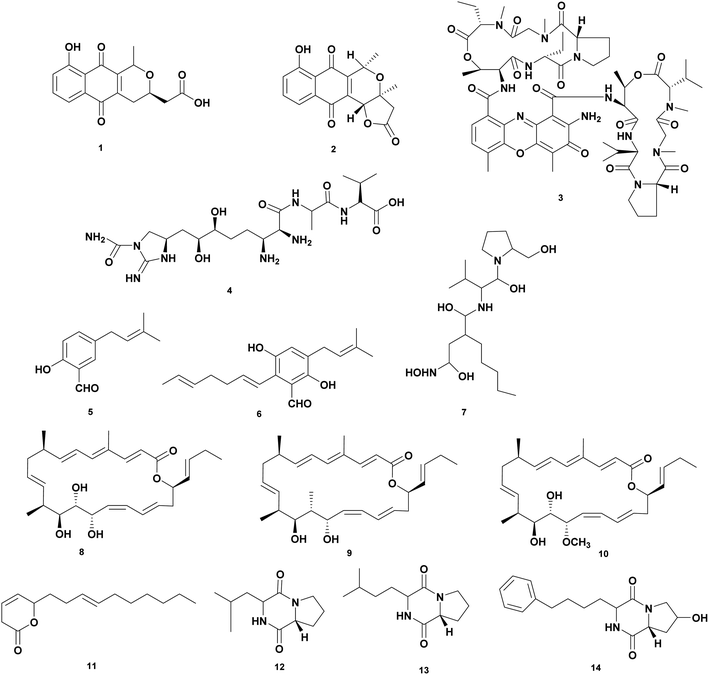
Actinobacteria are important assets for microbial natural products with therapeutic properties for medicinal, agricultural, veterinary, and aquaculture applications including chloramphenicol, tetracycline, erythromycin, rifamycin, rapamycin, vancomycin, bleomycin, and avermectin. Actinobacteria are known to produce 70% of the antibiotics used today.40,41Screening have been carried out to obtain isolates that produce antivibrio compounds. Actinobacteria mainly Streptomyces spp. from different sources were tested for the antagonistic effect against Vibrio spp.42–47 A comprehensive review showed a list of 128 strains of Streptomyces isolated from terrestrial and marine environments that are active against Vibrio spp.48 Most of the studies have focused on the preliminary screening bioactivity of crude extract fermentation. To date, only a limited number of structure elucidations and identified the bioactive compounds that displayed potent antibacterial activity against Vibrio spp. Herein, we collected data on antivibrio compounds isolated from Actinobacteria presented in Fig. 1 and Table 1.
| No. | Compounds | Sources | Antivibrio activities | Mechanism of action | Ref. |
|---|---|---|---|---|---|
| 1 | Nanaomycin A (1) | Streptomyces rosa var. notoensis OS-3966 | V. alginolyticus 138-2, MIC 6.3 μg mL−1, V. parahaemolyticus K-1, MIC 3.1 μg mL−1 | Inhibition of inhibiting biosyntheses of protein, DNA, RNA, and cell-wall peptidoglycan | 50 |
| 2 | Nanaomycin D (2) | Streptomyces rosa var. notoensis OS-3966 | V. alginolyticus 138-2, MIC < 0.05 μg mL−1, V. parahaemolyticus K-1, MIC < 0.05 μg mL−1 | Affect respiratory chain-linked flavin dehydrogenases of a Vibrio alginolyticus | 50 |
| 3 | Munumbicin B (3) | Streptomyces NRRL 3052 | V. harveyi PIC 345, dose 10 μg | Inhibition of the growth | 51 |
| 4 | Guadinomine B (4) | Streptomyces sp. K01-0509 | Vibrio sp. IC50 14 nM | Inhibition of type III secretion system (TTSS) in Vibrio spp. | 52 |
| 5 | 2-Hydroxy-5-(3-methylbut-2-enyl) benzaldehyde (5) | Streptomyces atrovirens PK288-21 | V. anguillarum, MIC 65 μg mL−1, V. harveyi, MIC 20 μg mL−1 | Inhibition of the growth | 54 |
| 6 | 2-Hepta-1,5-dienyl-3,6-dihydroxy-5-(3-methylbut-2-enyl) benzaldehyde (6) | Streptomyces atrovirens PK288-21 | V. anguillarum, MIC 65 μg mL−1, V. harveyi, MIC 32 μg mL−1 | Inhibition of the growth | 54 |
| 7 | Actionin (7) | Streptomyces sp. NHF 165 | V. anguillarum, IC50 2.85 μM | Inhibition of peptide deformylase (PDF) of V. anguillarum | 55 |
| 8 | Chaxalactin A (8) | S. leeuweenhoekii strain C38 | V. parahaemolyticus, MIC 12.5 μg mL−1 | Inhibition of the growth | 56 |
| 9 | Chaxalactin B (9) | S. leeuweenhoekii strain C38 | V. parahaemolyticus, MIC 12.5 μg mL−1 | Inhibition of the growth | 56 |
| 10 | Chaxalactin C (10) | S. leeuweenhoekii strain C38 | V. parahaemolyticus, MIC 20 μg mL−1 | Inhibition of the growth | 56 |
| 11 | Pyranosesquiterpene (11) | Streptomyces sp. SCSIO 01689 | V. anguillarum, MIC > 100 μg mL−1 | Inhibition of the growth | 45 |
| 12 | Cyclo(D)-Pro-(D)-Ile (12) | Streptomyces sp. SCSIO 01689 | V. anguillarum, MIC 0.05 μg mL−1 | Inhibition of the growth | 45 |
| 13 | Cyclo(D)-Pro-(D)-Leu (13) | Streptomyces sp. SCSIO 01689 | V. anguillarum, MIC 0.04 μg mL−1 | Inhibition of the growth | 45 |
| 14 | Cyclo(D)-trans-4-OH-Pro-(D)-Phe (14) | Streptomyces sp. SCSIO 01689 | V. anguillarum, MIC 0.07 μg mL−1 | Inhibition of the growth | 45 |
Brevibacterium casei MSI04 associated with a marine sponge Dendrilla nigra produces poly-hydroxy butyrate with the activity as antiadhesive. The inhibition activity was tested again on pathogen Vibrio spp. from shrimp aquaculture. The compound inhibited V. vulnificus and V. fischeri (96%), V. parahaemolyticus and V. alginolyticus (92%), and V. harveyi (88%).49
Actinobacteria produce wide type of antibiotics such as nanaomycins, munumbicins and guadinomine active against Vibrio spp. Nanaomycins are quinone antibiotics produced by Streptomyces rosa var notoensis OS-3966. Nanaomycin A (1) showed bioactivity against V. parahaemolyticus K-1 and V. alginolyticus 138–2 at MIC 3.1 μg mL−1 and 6.3 μg mL−1, respectively. Nanaomycin D (2) has the greater activity against V. parahaemolyticus K-1 and V. alginolyticus 138–2 at MIC less than 0.05 μg mL−1. The mechanism of action is inhibiting biosynthesis of protein, DNA, RNA, and cell-wall peptidoglycan.50 Munumbicins are antibiotic peptides with broad spectrum activity against Gram-positive and negative bacteria. The peptides were isolated from endophytic Streptomyces NRRL 3052. Munumbicins A–D were tested against V. fischeri PIC 345 at a concentration of 10 μg. Munumbicin A was inactive, while munumbicins B (3), C, and D showed zone inhibition of 16, 9, and 12 cm, respectively.51 Guadinomine B (4) is an antibiotic peptide produced by Streptomyces sp. K01-0509. The compound works as an antivirulence at IC50 14 nM with a novel mechanism of action as an inhibitor of the type III secretion system (TTSS) of Gram-negative bacteria including Vibrio sp.52,53
Streptomyces atrovirens PK288-21 associated with seaweed Undaria pinnatifida produces two compounds 2-hydroxy-5-(3-methylbut-2-enyl) benzal-dehyde (5) and 2-hepta-1,5-dienyl-3,6-dihydroxy-5-(3-methylbut-2-enyl) benzaldehyde (6) were isolated from. Compound (5) inhibited V. anguillarum and V. harveyi at MIC 65 and 20 μg mL−1, respectively. While compound (6) was active against V. anguillarum and V. harveyi at MIC 65 and 32 μg mL−1, respectively.54
High throughput screening of crude extract of marine Actinobacteria was examined targeting peptide deformylase (PDF) of V. anguillarum that catalyzes the removal of N-formyl group from N-terminal methionine following translation in prokaryotes. Extraction of fermentation broth of Streptomyces sp. NHF 165 yielded Actionin (7) that inhibited peptide deformylase (PDF) of V. anguillarum at IC 50 was 2.85 μM.55
Streptomyces leeuweenhoekii strain C34 isolated from the Chilean hyper-arid Atacama Desert soil produces a new type of antibiotic ansamycin which is active as antivibrio. Using the OSMAC approach led to isolating new 22-membered macro lactone-type polyketides called Chaxalactin A-C (8–10). Chaxalactins A (8), B (9), and C (10) inhibited V. parahaemolyticus at MIC 12.5; 20; and 12.5 μg mL−1, respectively.56,57 Streptomyces sp. SCSIO 01689 produces antivibrio compounds pyranosesquiterpene (11) and cyclic peptides Cyclo(D)-Pro-(D)-Ile (12), Cyclo(D)-Pro-(D)-Leu (13), and Cyclo(D)-trans-4-OH-Pro-(D)-Phe (14). The compound 11 inhibited V. anguillarum at MIC at > 100 μg mL−1 while the cyclic peptides showed potency at concentrations of 0.05, 0.04, and 0.07 μg mL−1 for 12, 13, and 14, respectively.48
3.2. Pseudoalteromonas
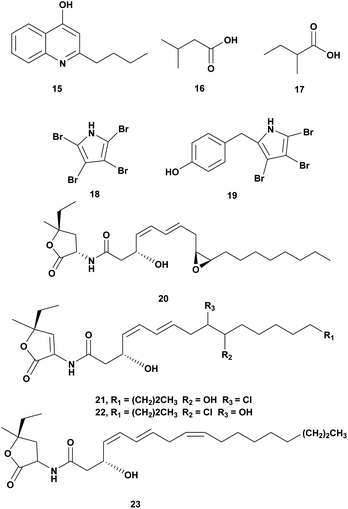
The genus Pseudoalteromonas is Gram-negative bacteria, heterotrophic, aerobic, and belongs to the γ-Proteobacteria class. This genus attracts attention due to its wide array of metabolites and ecological role in the ocean. The metabolites of Pseudoalteromonas have bioactivity including antimicrobial agents.58,59 Antivibrio compounds isolated from Pseudoalteromonas spp. are presented in Fig. 2 and Table 2.| No. | Compounds | Sources | Antivibrio activities | Mechanism of action | Ref. |
|---|---|---|---|---|---|
| 1 | 2-n-Pentyl-4-quinolinol (15) | Pseudoalteromonas A1-J11 | V. harveyi ATCC 14126, V. harveyi ATCC 35084, V. fischeri VF-74, V. harveyi, Dose 10 μg per disk | Inhibition of the growth | 60 |
| 2 | • Isovaleric acid (16), • 2-methyl butyric acid (17) | Pseudoalteromonas haloplanktis INH | V ordalii ATCC 33509, V. alginolyticus ATCC 17749, V. anguillarum IFO 13266, dose 1 mg mL−1 | Inhibition of the growth | 61 |
| 3 | • Tetrabromopyrrole (18), • 4′-((3,4,5-tribromo-1H-pyrrol-2-yl)methyl) phenol (19), • korormicin G-I (20–22), • korormicin K (23) | Pseudoalteromonas J010 | V. campbelii, V. vulnificus, V. coralliilyticus, V. harveyi, Dose 200 μg mL−1 | • Inhibition of the growth, • disrupting of quorum sensing | 62 |
Pseudoalteromonas A1-J11 from the coastal Kagoshima Bay, Japan produced bioactive quilinolinol derivatives 2-n-pentyl-4-quinolinol (15). Disk diffusion assay of the compounds was conducted against V. harveyi ATCC 14126, V. harveyi ATCC 35084, V. alginolyticus ATCC 17749, Vibrio sp. 9M-P5-1, V. fischeri VF-74, V. parahaemolyticus IFO 12711. Based on the bioassay compound 15 was active against V. harveyi ATCC 14126, V. harveyi ATCC 35084, and V. fischeri VF-74 at a concentration of 10 μg.60
Crude extract of Pseudomonas haloplanktis INH from scallop hatchery was tested against V. ordalii ATCC 33509, V. algiynolyticus ATCC 17749, V. anguillarum IFO 13266, and V. anguillarum (VAR). The inhibition of V. ordalii ATCC 33509 was observed at a concentration of 1 mg mL−1. Antibacterial compounds from the ethyl acetate extract were identified as isovaleric acid (16) and 2-methyl butyric acid (17).61
Pseudoalteromonas strain J010 associated with the surface of the crustose coralline alga Neogoniolithon fosliei, produced bioactive compounds antivibrio tetrabromopyrrole (18), 4′-((3,4,5-tribromo-1H-pyrrol-2-yl)methyl)phenol (19), and korormicins G–I (20–22) and K (23). The compounds were tested at a concentration of 200 μg mL−1 using disk diffusion assay and showed antagonistic effects to Vibrio campbellii, V. coralliilyticus, V. harveyi, and V. vulnificus. The korormicins may play a role in disrupting quorum sensing.62
3.3. Pseudomonas spp.
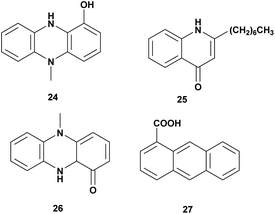
Pseudomonas aeruginosa is Gram-negative bacteria, widespread in the terrestrial and marine environment. It has been reported that Pseudomonas aeruginosa exhibited antagonistic activity to aquaculture and agriculture pathogens. Some antivibrio compounds have been identified from P. aeruginosa as seen in Fig. 3 and Table 3.| No | Compounds | Sources | Antivibrio activities | Mechanism of action | Ref. |
|---|---|---|---|---|---|
| 1 | N-Methyl-1-hydroxyphenazine (24) | Pseudomonas MCCB 102 and 103 | V. harveyi, dose 0.5 mg L−1 | Bacteriostatic | 63 |
| 2 | 2-Heptyl-4-quinolone (25) | Pseudomonas aeruginosa sp. W3 | V. harveyi, MIC: 225–450 μg mL−1 | Inhibition of the growth | 64 |
| 3 | Pyocyanin (26) | Pseudomonas aeruginosa | V. parahaemolyticus, V. vulnificus, V. alginolyticus, V. fluvialis, V. mediteranii, V. nereis, V. harveyi, Dose 30 mg L−1 | Inhibition of the growth | 65 |
| 4 | Phenazine-1-carboxylic acid (27) | Pseudomonas aeruginosa PA31X | V. anguillarum C312, dose 3 μg mL−1 | Inhibition of the growth | 66 |
Pseudomonas MCCB 102 and 103A produces phenazine antibiotic, N-methyl-1-hydroxyphenazine (24). The compound has bacteriostatic activity against V. harveyi at the dose of 0.5 mg L−1. The toxicity in Penaeus monodon haemocyte at IC50 was 1.4 ± 0.31 mg L−1.63 Investigation of bioactivities and toxicities of ethyl acetate extract of Pseudomonas aeruginosa sp. W3 led to the isolation of 2-heptyl-4-quinolone (HHQ) (25) that was active against 18 strains of shrimp pathogenic of V. harveyi. The compound was active at MIC value 225–450 μg mL−1.64
Antagonistic activity of Pseudomonas aeruginosa was tested against V. parahaemolyticus, V. vulnificus, V. alginolyticus, V. fluvialis, V. mediteranii, V. nereis, and V. harveyi. Isolation of extract fermentation led to identify pyocyanin (26) as the bioactive compound responsible for the antagonistic effect at a concentration of more than 30 mg L−1.65 Pseudomonas aeruginosa PA31X produces phenazine-1-carboxylic acid (27) that is active against V. anguillarum C312 at 3 μg mL−1.66
3.4. Miscellaneous bacteria
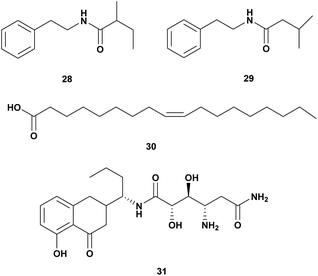
A Gram-positive marine bacterium Halobacillus salinus produced two phenetylamide metabolites: N-(2′-phenylethyl)-isobutyramide (28) and 3-methyl-N-(2′-phenylethyl)-butyramid (29). The compounds are anti-quorum sensing and bioluminescence of V. harveyi at a concentration below 200 μg mL−1.67Oleic acid (30) isolated from Vibrio sp. from North Chile inhibited the growth of V. parahaemolyticus. Long-chain fatty acids such as oleic, linoleic, and linolenic have antibacterial activity through inhibition of fatty acid synthesis (Table 4).68
| No | Compounds | Sources | Antivibrio Activities | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| 1 | • N-(2′-Phenyl ethyl)-iso butyramide (28), • 3-methyl-N-(2′-phenyl ethyl)-butyramid (29) | Halobacillus salinus | V. harveyi, dose 500 μg per disk | Quorum sensing inhibitor | 67 |
| 2 | Oleic Acid (30) | Vibrio sp. | V. parahaemolyticus. | Inhibition of fatty acid biosynthesis | 68 |
| 3 | Amicoumacin A (31) | Bacillus pumilus H2 | V. natriegens, V. vulnificus, V. alginolyticus, V. harveyi, V. azareus, V. campbelli, V. fischeri, MIC 0.5–64 μg mL−1 | 69 |
Bacillus pumilus H2 produces an antibacterial compound amicoumacin A (31) (Fig. 4) inhibited broad range species of Vibro V. natriegens, V. vulnificus, V. alginolyticus, V. harveyi, V. azareus, V. campbelli, V. fischeri.69
4. Antivibrio from marine fungi
Since the discovery of penicillin from Penicillium chrysogenum in the twentieth century, the fungus is an important source of natural products for drug discovery. Marine fungi have been explored for their bioactive secondary metabolites and potential for anti-microbial agents.70–72 To date, 38% of 22.000 bioactive microbial metabolites are from fungi.73 Among those metabolites, there are only a few antivibrio agents derived from marine fungi as presented in Fig. 5 and Table 5.| No. | Compounds | Sources | Antivibrio activities | Mechanism of action | Ref. |
|---|---|---|---|---|---|
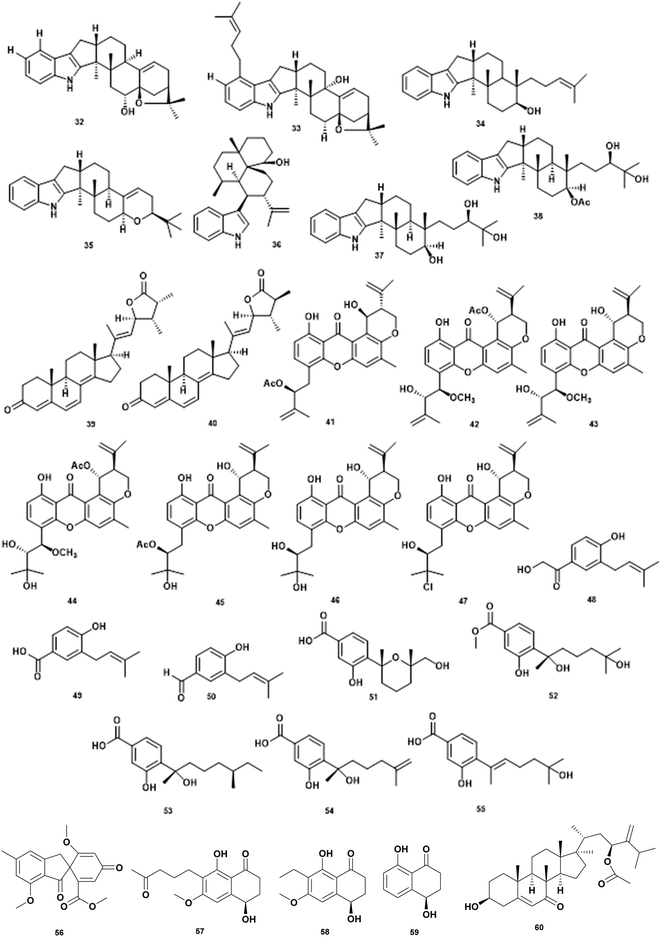
| 1 | 6-Hydroxylpaspaline (32) | Penicillium sp. AS-79 | V. parahaemolyticus MIC 64.0 μg mL−1 | Inhibition of the growth | 74 |
| 2 | Paspalitrem C (33) | Penicillium sp. AS-79 | V. parahaemolyticus MIC 8.0 μg mL−1, V. alginolyticus MIC 4 μg mL−1 | Inhibition of the growth | 74 |
| 3 | Emindole SB (34) | Penicillium sp. AS-79 | V. parahaemolyticus MIC 2.0 μg mL−1, V. alginolyticus MIC 1 μg mL−1 | Inhibition of the growth | 74 |
| 4 | 3-Deoxo-4b-deoxypaxilline (35) | Penicillium sp. AS-79 | V. parahaemolyticus MIC 16.0 μg mL−1 | Inhibition of the growth | 74 |
| 5 | 10,23 Dihydro-24,25-dehydroaflavinine (36) | Penicillium sp. AS-79 | V. parahaemolyticus MIC 0.5 μg mL−1, V. alginolyticus MIC 0.5 μg mL−1 | Inhibition of the growth | 74 |
| 6 | Penijanthine C (37) | Penicillium janthinellum | V. anguillarum MIC 3.1 μM, V. parahaemolyticus MIC 6.3 μM, V. alginolyticus MIC 3.1 μM | Inhibition of the growth | 75 |
| 7 | Penijanthine D (38) | Penicillium janthinellum | V. anguillarum MIC 12.5 μM, V. parahaemolyticus MIC 12.5 μM, V. alginolyticus MIC 12.5 μM | Inhibition of the growth | 75 |
| 8 | Penijanthoid A (39) | Penicillium janthinellum | Vibrio spp. MICs 25.0–50.0 μM | Inhibition of the growth | 75 |
| 9 | Penijanthoid B (40) | Penicillium janthinellum | Vibrio spp. MICs 25.0–50.0 μM | Inhibition of the growth | 75 |
| 10 | Aspergixanthone I (41) | Aspergillus sp. ZA-01 | V. parahaemolyticus MIC 1.56 μM, V. anguillarum MIC 1.56 μM, V. alginolyticum MIC 3.12 μM | Inhibition of the growth | 76 |
| 11 | Aspergixanthone J (42) | Aspergillus sp. ZA-01 | V. parahaemolyticus MIC 6.25 μM, V. anguillarum MIC 25 μM, V. alginolyticum MIC 25 μM | Inhibition of the growth | 76 |
| 12 | Aspergixanthone K (43) | Aspergillus sp. ZA-01 | V. parahaemolyticus MIC 3.12 μM, V. anguillarum MIC 25 μM, V. alginolyticum MIC 12.5 μM | Inhibition of the growth | 76 |
| 13 | Aspergixanthone A (44) | Aspergillus sp. ZA-01 | V. parahaemolyticus MIC 25 μM, V. anguillarum MIC 25 μM, V. alginolyticum MIC 25 μM | Inhibition of the growth | 76 |
| 14 | 15-Acetyl tajixanthone hydrate (45) | Aspergillus sp. ZA-01 | V. parahaemolyticus MIC 12.5 μM, V. anguillarum MIC 25 μM, V. alginolyticum MIC 12.5 μM | Inhibition of the growth | 76 |
| 15 | Tajixanthone hydrate (46) | Aspergillus sp. ZA-01 | V. parahaemolyticus MIC 6.25 μM, V. anguillarum MIC 6.25 μM, V. alginolyticum MIC 12.5 μM | Inhibition of the growth | 76 |
| 16 | 16-Chlorotajixanthon (47) | Aspergillus sp. ZA-01 | V. parahaemolyticus MIC 25 μM, V. anguillarum MIC 6.25 μM, V. alginolyticum MIC 25 μM | Inhibition of the growth | 76 |
| 17 | Terreprenphenol A (48) | Aspergillus terreus EN-539 | V. harveyi MIC 4 μg mL−1, V. parahemolyticus MIC 8 μg mL−1, V. vulnificus MIC 32 μg mL−1 | Inhibition of the growth | 77 |
| 18 | 4-Hydroxy-3-prenybenzoic acid (49) | Aspergillus terreus EN-539 | V. harveyi MIC 32 μg mL−1, V. parahemolyticus MIC 8 μg mL−1 | Inhibition of the growth | 77 |
| 19 | 4-Hydroxy-3-(3-methyl-but-2-enyl)-benzaldehyde (50) | Aspergillus terreus EN-539 | V. harveyi MIC 8 μg mL−1, V. parahemolyticus MIC 8 μg mL−1, V. vulnificus MIC 64 μg mL−1 | Inhibition of the growth | 77 |
| 20 | Bisabolene sesquiterpenoid (51) | Aspergillus versicolor SD-330 | V. harveyi MIC 4 μg mL−1, V. parahemolyticus MIC 16 μg mL−1 | Inhibition of the growth | 78 |
| 21 | Aspergoterpenin C (52) | Aspergillus versicolor SD-330 | V. harveyi MIC 8 μg mL−1, V. parahemolyticus MIC 8 μg mL−1 | Inhibition of the growth | 78 |
| 22 | (7S,11S)-(þ)-12-Hydroxysydonic acid (53) | Aspergillus versicolor SD-330 | V. anguillarum MIC 32 μg mL−1, V. harveyi MIC 16 μg mL−1, V. parahemolyticus MIC 32 μg mL−1 | Inhibition of the growth | 78 |
| 23 | (S)-(þ)-11-Dehydrosydonic acid (54) | Aspergillus versicolor SD-330 | V. anguillarum MIC 32 μg mL−1, V. harveyi MIC 32 μg mL−1 | Inhibition of the growth | 78 |
| 24 | Engyodontiumone I (55) | Aspergillus versicolor SD-330 | V. harveyi MIC 4 μg mL−1, V. parahemolyticus MIC 8 μg mL−1 | Inhibition of the growth | 78 |
| 25 | Trypacidin (56) | Aspergillus fumigatus HX-1 | V. harveyi MIC 31.25 μg mL−1 | Inhibition of the growth | 79 |
| 26 | Paraconthone A (57) | Paraconiothyrium sp. | V. anguillarum & V. parahemolyticus, MIC 30 μg mL−1 | Inhibition of the growth | 80 |
| 27 | Botryosphaerone (58) | Paraconiothyrium sp. | V. anguillarum & V. parahemolyticus, MIC 30 μg mL−1 | Inhibition of the growth | 80 |
| 28 | O-Methylaspmenone (59) | Paraconiothyrium sp. | V. anguillarum & V. parahemolyticus, MIC 30 μg mL−1 | Inhibition of the growth | 80 |
| 29 | Acremocholone (60) | Acremonium sp. NBUF150 | V. scophthalmi MIC 8 μg mL−1, V. shiloni MIC 8 μg mL−1, V. brasiliensis MIC 8 μg mL−1 | Inhibition of the growth | 81 |
The genera of Penicillium contributes diverse of antivibrio compounds. Extraction of culture Penicillium sp. AS-79 associated with sea anemone Haliplanella luciae yielded indole diterpenoids that are active against V. parahaemolyticus and V. alginolyticus. The various compounds: 6-hydroxylpaspalinine (32), paspalitrem C (33), emindole SB (34), 3-deoxo-4b-deoxypaxilline (35), and 10,23-dihydro-24,25-dehydroaflavinine (36) exhibited activity against the aquatic pathogen V. parahaemolyticus. In addition, compounds 33, 34, 36 showed inhibition activity against V. alginolyticus.74 Chemical investigation of ethyl acetate extract of culture Penicillium janthinellum yielded two indole diterpenoid penijanthine C (37) and D (38), two new steroids penijanthoid A (39) and B (40) along with two known analogs PC-M6 and 7-hydoxy-13-dehydroxypaxilline. The compounds 37–40 were active against V. anguillarum, V. parahaemolyticus, and V. alginolyticus. Indole diterpenoid is a new class of antivibrio agents.75
The genera of Aspergillus produce flourishing classes of antivibrio compounds. Deep investigation of marine-derived fungus Aspergillus sp. ZA-01 led to the isolation of new antivibrio compounds prenylxanthone derivate aspergixanthones I–K (41–43) along with known compounds (44–47). The compounds were tested against V. parahaemolyticus, V. anguillarum, and V. alginolyticus.76 Marine fungi Aspergillus terreus EN-539 associated red algae Laurencia okamurai, produced new prenylated phenol derivative terreprenphenol A (48) along with 4-hydroxy-3-prenybenzoic acid (49), and 4-hydroxy-3-(3-methyl-but-2-enyl)-benzaldehyde (50). Evaluation of antivibrio activity against V. harveyi, V. parahemolyticus, and V. vulnificus showed inhibitory activity at MIC values ranging from 4 to 64 μg mL−1.77 The deep-sea sediment-derived fungus Aspergillus versicolor SD-330 yielded one new aromatic bisabolene-type sesquiterpenoid (51) along with four known analogs, aspergoterpenin C (52), (7S,11S)-(þ)-12-hydroxysydonic acid (53), (S)-(þ)-11-dehydrosydonic acid (54), and engyodontiumone I (55). All compounds exhibited inhibitory activities against V. anguillarum, V. harveyi, and V. parahaemolyticus with the MIC values ranging from 4 to >32 μg mL−1.78 Bioassay-guided isolation has identified the bioactive compound trypacidin (56) from a marine symbiotic fungi Aspergillus fumigatus HX-1. In vitro bacteriostatic assay confirms the MIC value at 31.25 μg mL−1.79 The MIC of each compound is presented in Table 5.
Marine fungi associated with crab, Paraconiothyrium sp., produced a new polyketide, paraconthone A (57) together with botryosphaerone (58) and O-methylaspmenone (59). The compounds showed moderate inhibitory effects against V. anguillarum and V. parahaemolyticus at 30 μg mL−1.80
A new steroid acremocholone (60) was produced by sponge-associated fungi Acremonium sp. NBUF150. Acremocholone exhibited antivibrio activity against V. scophthalmi, V. shilonii and V. brasiliensis at MIC of 8 μg mL−1 .81
5. Antivibrio from sponges
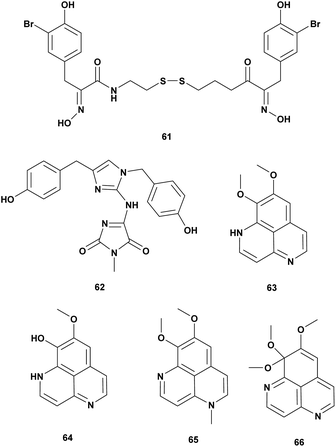
Sponges are the oldest metazoan and have been investigated extensively for bioactive metabolites. Three new alkaloids isonaamide D, di-isonaamide A, and leucettamine D, and two known compounds isonaamine A and isonaanidine from a sponge Leucetta chagosensis Dendy, 1863 from French Polynesia. The compounds were screened for quorum sensing (QS) inhibitor of V. harveyi. The result showed that Isonaamidine A (61) inhibited the QS pathway at IC50 1 μg mL−1. None of the compounds affected bacterial growth at 50 μg mL−1.82In the searching for antimicrobial agents against V. vulnificus twelve pure marine compounds from a variety of sponges were screened for inhibition effect. Psammaplin A (62), a bromotyrosine derivative from the sponge Poecillastra sp., Jaspis sp., and Psammaplin aplysilla inhibited V. vulnificus in vitro and in vivo assay at 5–50 μg (Table 6).83
| No. | Compounds | Sources | Antivibrio activities | Mechanism of action | Ref. |
|---|---|---|---|---|---|
| 1 | Isonaamidin A (61) | Leucetta chagosensis | V. harveyi, quorum sensing, dose 1 μg mL−1 | Altering of quorum sensing | 82 |
| 2 | Psammaplin A (62) | Poecillastra sp., Jaspis sp., Psammaplinaplysilla | V. vulnificus dose 5–50 μg | Inhibition of the growth | 83 |
| 3 | Aaptamine (63), 9-demethyl aaptamine (64), 4-N-methyl aaptamine (65), 9-methoxy aaptamine (66) | Aaptos aaptos | V. harveyi dose 1 mg mL−1 | Inhibition of the growth | 84 |
Alkaloid aaptamin and derivates from sponge Aaptos aaptos were tested against Vibrio spp. and V. harveyi. Aaptamine (63), 9-demethylaaptamine (64), 4-N-methylaaptamine (65), 9-methoxyaaptamine (66) were active at concentration 1 mg mL−1 (Fig. 6).84
6. Antivibrio from coral
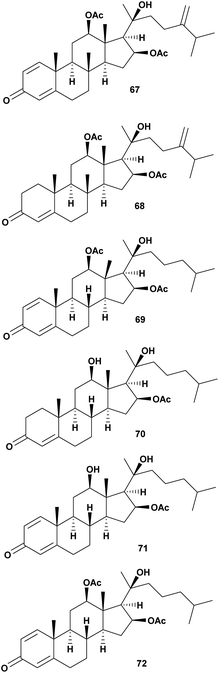
Four new steroids, dendronecholones A–D (67–70), and two known analogues, 12β,16β,20-trihydroxycholesta-1,4-dien-3-one 16-acetate (71) and nanjiol A (72) were identified from soft coral Dendronephthya collected in waters off Zhejiang Province, China. Antivibrio assay was conducted against V. parahaemolyticus, V. scophthalmi, and V. harveyi. The MIC range from 8–>32 μg mL−1 is presented in Table 7.85| No. | Compounds | Sources | Antivibrio Activities | Mechanism of action | Ref. |
|---|---|---|---|---|---|
| 1 | Dendronecholone A (67) | Dendronephthya | V. scophthalmi MIC 32 μg mL−1, V. parahemolyticus MIC >32 μg mL−1, V. harveyi MIC 32 μg mL−1 | Inhibition of the growth | 85 |
| 2 | Dendronecholone B (68) | Dendronephthya | V. scophthalmi MIC 8 μg mL−1, V. parahemolyticus MIC >32 μg mL−1, V. harveyi MIC 8 μg mL−1 | Inhibition of the growth | 85 |
| 3 | Dendronecholone C (69) | Dendronephthya | V. scophthalmi MIC 32 μg mL−1, V. parahemolyticus MIC 8 μg mL−1, V. harveyi MIC >32 μg mL−1 | Inhibition of the growth | 85 |
| 4 | Dendronecholone D (70) | Dendronephthya | V. scophthalmi MIC 16 μg mL−1, V. parahemolyticus MIC >32 μg mL−1, V. harveyi MIC >32 μg mL−1 | Inhibition of the growth | 85 |
| 5 | 12β,16β,20-Trihydroxycholesta-1,4-dien-3-one 16-acetate (71) | Dendronephthya | V. scophthalmi MIC 8 μg mL−1, V. parahemolyticus MIC >32 μg mL−1, V. harveyi MIC >32 μg mL−1 | Inhibition of the growth | 85 |
| 6 | Nanjiol A (72) | Dendronephthya | V. scophthalmi MIC 8 μg mL−1, V. parahemolyticus MIC 8 μg mL−1, V. harveyi MIC 8 μg mL−1 | Inhibition of the growth | 85 |
7. Antivibrio from seaweeds
Seaweeds are well known as rich sources of primary and secondary metabolites with diverse applications for food, feed, agriculture, pharmaceutical, and cosmetics.86,87 Numerous substances were isolated from seaweed such as halogenated compounds,88,89 polyether,90 phenolic compounds,91 and polyunsaturated fatty acid.92 Antimicrobial activity testing of seaweed extracts support the possibility of using seaweeds as a source of antimicrobial agents or as a health-promoting feed for aquaculture.93 Bioactive compounds from seaweed can be applied in aquaculture health and disease management to control bacterial infection.94–96 Seaweeds are rich in fatty acid and the mechanism of action of fatty acid as an antibacterial agent through inhibition of the electron transport chain and normal oxidative phosphorylation in bacterial cell membranes.97 Polysaccharides from seaweed have been examined for the purpose as prebiotic or immunostimulant in aquaculture98 while red seaweed (Rhodophyta) are good source of antibacterial agents (Table 8).99| No. | Compounds | Sources | Antivibrio Activities | Mechanism of action | Ref. |
|---|---|---|---|---|---|
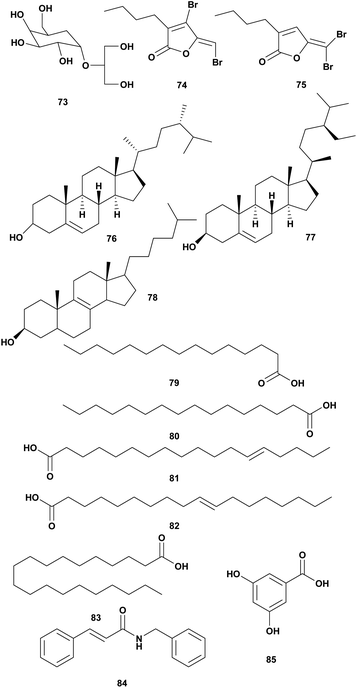
| 1 | Floridosid (73) | Palmaria palmata | V. harveyi | Inhibition of the growth | 16 |
| 2 | Fimbrolide A and B (74–75) | Delisea pulchra | V. harveyi, V. campbelli | Altering of quorum sensing | 101 |
| 3 | Hexadecanoic acid, Ergost-5-en-3-ol (76), Stigmast-5-en-3.β.-ol (77) | Gracilaria arcuata | Vibrio spp. MIC 1.25 μg mL−1 | Inhibition of the growth | 102 |
| 4 | Cholest-8-en-3-ol (78), 9-hexadecenoic acid (79) hexadecanoic acid (80), 13-octadecenoic acid (81), 10-octadecenoic acid (82) eicosanoic acid (83) | Gracilaria edulis | V. fluvialis MIC 2.5 μg mL−1 | Inhibition of the growth | 103 |
| 5 | N-Benzyl cinnamamide (84), α-resorcylic acid (85) | Gracilaria fischeri | V. harveyi 1114 MIC 11.27 mg mL−1, V. harveyi 1114 MIC 1.66 mg mL−1 | Altering of quorum sensing | 105 |
Water-soluble fractions of red algae Palmaria paltata and Grateloupia turuturu were examined for the activity against V. harveyi. The NMR data suggested that the active water fraction of Palmaria paltata contains floridoside (73) (Fig. 7).16 Further structure elucidation should be done to identify principal compounds responsible for an antivibrio agent.16
Red algae Delisea pulchra produced halogenated furanones called fimbrolide (Fig. 8).101 Brominated furanones from marine algae inhibited biofilm formation and quorum sensing (QS) Gram-negative without affecting their growth. The structure is similar to bacterial acyl homoserine lactones (AHL).100 Some marine algae produced halogenated furanones as AHL antagonists as a response to the negative impact of bacterial colonization. Fimbrolide 1 (74) and Fimbrolide 2 (75) were tested for inhibiting bioluminescence in V. harveyi and V. campbellii with the target on LuxS, PhaB, and uncharacterized IMPD protein.101
Extracts of Indonesian red seaweeds have been screened for bioactivity against fish pathogens including Vibrio spp. Extract of Gracilaria arcuata was active against Vibrio sp. at a concentration of 2.5 μg μL−1. The active fraction contained hexadecanoic acid and sterol compounds such as Ergost-5-en-3-ol (76), Stigmast-5-en-3β-ol (77). The MIC of the active fraction was 1.25 μg μL−1.102 Extract of Indonesian seaweed Gracilaria edulis showed inhibition against V. fluvialis and V. compbelii. Further analysis showed that the active fraction contained sterol cholest-8-en-3-ol (78) and long-chain fatty acids such as pentadecanoic acid (79), hexadecanoic acid (80), 13-octadecenoic acid (81), 10-octadecenoic acid (82), eicosanoic acid (83). The active fraction showed inhibition against V. fluvialis at MIC 2.5 μg mL−1.103
Ethanolic extract of Gracilaria fischeri exhibited anti-quorum sensing activity in V. harveyi and V. parahaemolyticus at concentrations of 5, 10, and 100 μg mL−1. The extract also reduced the luminescence of V. harveyi.104 Further investigation showed G. fisheri contains N-benzyl cinnamamide (84) and α-resorcylic acid (85) and which are responsible for antivibrio activity.105
8. Antivibrio from plants
Plants are well known as a source of bioactive compounds and are used in traditional medicine. Various plant extracts containing phenolic, alkaloid, flavonoid, and polysaccharide have been tested and used in aquaculture as an immunostimulant, antioxidant, prebiotic, antibacterial, and antifungal.106,107 Plant extracts have been screened as sources for antivibrio agents.108,109 Phytochemicals can be used to interfere with bacterial quorum sensing to counteract the biofilm resistance. Medicinal plants are rich resources for screening bioactive QS.110 Antivibrio compounds identified from plants are shown in Table 9.| No. | Compounds | Sources | Antivibrio Activities | Mechanism of action | Ref. |
|---|---|---|---|---|---|
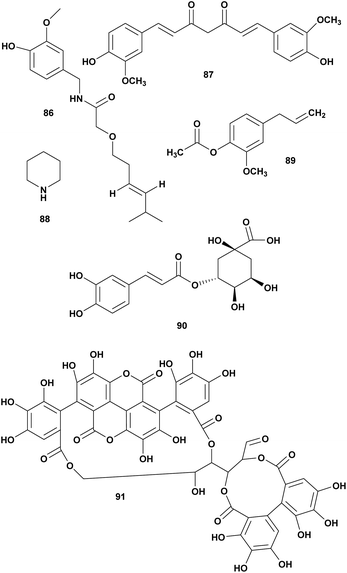
| 1 | Capsaicin (86) | Capsicum annum | V. chloreae | Inhibition of toxin | 112 |
| 2 | Curcumin (87) | Curcuma longa | V. harveyi reduce bioluminescence 88% | Interfere the production of QS-dependent virulence factors in Vibrio spp., inhibition of bacterial adhesion and RTX toxin binding | 113 |
| 3 | Piperidine (88) | Piper bettle | Vibrio spp., MIC90 2–6 mg mL−1 | Inhibition of the growth | 114 |
| 4 | Chlogenic acid (89) | Piper bettle | Vibrio spp. MIC90 5–16 mg mL−1 | Inhibition of the growth | 114 |
| 5 | Eugenyl acetate (90) | Piper bettle | Vibrio spp. MIC90 5–20 mg mL−1 | Inhibition of the growth | 114 |
| 6 | Punicalagin (91) | Punica granatum Linn | V. anguillarum MIC 25 mg mL−1 | Inhibition of the growth | 115 |
The essential oil from aromatic plants Mentha longifolia, M. pulegium, Eugenia caryophyllata, Thymus vulgaris, and Rosmarinus officinalis were tested against V. alginolyticus, V. parahaemolyticus, V. vulnificus, and V. fluvialis strains. Results showed variable activity and essential oils of T. vulgaris yielded the highest zone of growth inhibition against V. parahaemolyticus.111
One of the approaches in the screening of natural products as antivibrio is targeting the production of virulence factors such as capsaicin and curcumin. Extract methanol of Capsicum annum containing capsaicin was reported to inhibit CT (cholera toxin) production in V. cholerae. The transcriptions of ctxA, tcpA, and toxT genes were repressed by capsaicin (86). On the contrary, capsaicin significantly enhanced the transcription of the hns gene, the product of which is known to regulate negatively the transcription of ctxAB, tcpA, and toxT genes. These results suggest that capsaicin might act as a potent repressor for CT production possibly by enhancing the transcription of hns.112 Curcumin (87) from Curcuma longa reduced 88% of bioluminescence of V. harveyi and inhibited components of biofilms and virulence factor in V. parahaemolyticus, V. vulnificus, V. harveyi.113
Three compounds piperidine (88), chlorogenic acid (89), and eugenyl acetate (90) isolated from Piper bettle were reported as bactericidal against several pathogenic Vibrio spp. The MIC range 0.6 to 16 mg mL−1. Piperidine has the strongest inhibition effect on Vibrio spp. compare to chlorogenic acid and eugenyl acetate (Fig. 9).114
Punicalagin (91) from pomegranate (Punica granatum Linn.) was reported against V. anguillarum at MIC 25 μg mL−1.115
9. Conclusions and perspective
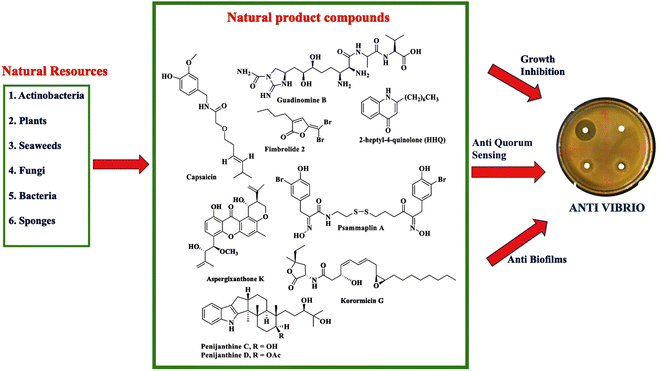
Climate change and global warming will impact increasing cases of vibriosis in the future. Vibrio spp. cause serious problems in aquaculture with consequent huge economic losses. Moreover, vibriosis threatens human health through seafood contamination and contact with seawater during wound events. To date, an effective vaccine to prevent vibriosis has not been available yet. Efforts have been done to prevent vibriosis in aquaculture with probiotics, prebiotics, and immunostimulants. The rising incidence of Vibrio resistance to antimicrobial agents and the limited option of antibiotics have driven the search for new antivibrio agents.Different stages of work have been performed ranging from the preliminary screening to an in-depth characterization of antivibrio compounds. This review provides proof that natural products are promising as a source of antivibrio agents. Screening of natural products from different sources has been carried out to discover antivibrio agents. Fig. 10 summarizes the exploration of natural resources to discover antivibrio agents. Natural product compounds exhibit bioactivity against Vibrio spp. through mechanism of action inhibiting the growth, disrupting quorum sensing, and interfering with biofilm formation.
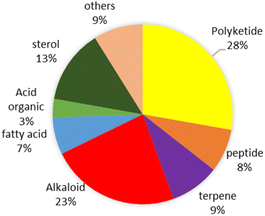
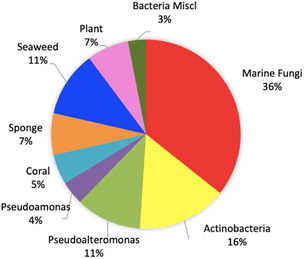
This review shows that natural products as antivibrio are produced by prokaryotes and eukaryotes living in terrestrial and marine environments (Fig. 11). Based on data on this review, marine fungi demonstrated prolific sources of antivibrio and contribute 36% of bioactive antivibrio. Actinobacteria and sponges are well-known as sources of bioactive compounds for decades, but their compounds account for only 16% and 7%, respectively for antivibrio. The type classes of natural antivibrio derived from natural product compounds are alkaloid, polyketide, peptide, sterol, terpene, organic acid, and fatty acid. Polyketide and alkaloid are the major class of antivibrio compounds and count about 28% and 23%, respectively presented in this review (Fig. 12). The alkaloids are produced by fungi and sponges, while polyketides were produced by mostly all organisms except coral. Antivibrio from coral and seaweed are mostly sterol.
This review summarizes that nature has provided a plethora of natural products with extraordinary chemistry and bioactivity against Vibrio spp. Further research and development of promising compounds are necessary for application in aquaculture and human health. Future efforts are necessary to evaluate the biological activities in vivo, toxicity, and mechanisms of action. Biofilms is the leading cause of multidrug resistance among microorganisms including Vibrio spp. Thus, study and examination of antivibrio compounds as inhibitor of biofilm formation is needed. The clinical study of antivibrio compounds has not been reported yet.
Author contribution
NK and MU wrote the manuscript, DCR helped supervise the project and reviewed the manuscript, MU contributed to collect data and references. NK received the funding and DCR provided lab access to do research on antivibrio.Conflicts of interest
The authors report there are no competing interests to declare.Acknowledgements
This work is a part of Research Antivibrio for Human Health and Aquaculture. N. K. would like to thank DIKTI-Fulbright Visiting Scholar Program 2019 (PS 00285337) for a scholarship to conduct part of the study in collaboration with David C. Rowley, Department of Pharmaceutical and Biomedical Sciences, College of Pharmacy, University of Rhode Island.References
- B. Austin, Vet. Microbiol., 2010, 140(3), 310–317, DOI:10.1016/j.vetmic.2009.03.015.
- C. N. Johnson, Microb. Ecol., 2013, 65(4), 826–851, DOI:10.1007/s00248-012-0168-x.
- F. L. Thompson, T. Iida and J. Swings, Microbiol. Mol. Biol. Rev., 2004, 68(3), 403–431, DOI:10.1128/MMBR.68.3.403-431.2004.
- A. Newton, M. Kendall, D. J. Vugia, O. L. Henao and B. E. Mahon, Clin. Infect. Dis., 2012, 54, 391–395, DOI:10.1093/cid/cis243.
- L. Vezzulli, R. R. Colwell and C. Pruzzo, Microb. Ecol., 2013, 65(4), 817–825, DOI:10.1007/s00248-012-0163-2.
- L. Vezzulli, I. Brettar, E. Pezzati, P. C. Reid, R. R. Colwell, M. G. Hofle and C. Pruzzo, ISME J., 2012, 6, 21–30, DOI:10.1038/ismej.2011.89.
- C. Baker-Austin, J. A. Trinanes, N. G. H. Taylor, R. Hartnell, A. Siitonen and J. Martinez-Urtaza, Nat. Clim. Change, 2013, 3, 73–77, DOI:10.1038/nclimate1628.
- L. Vezzulli, E. Pezzati, I. Brettar, M. Hofle and C. Pruzzo, Microbiol. Spectrum, 2014, 3, 1, DOI:10.1128/microbiolspec.VE-0004-2014.
- J. M. Janda, A. E. Newton and C. A. Bopp, Clin. Lab. Med., 2015, 35(2), 273–288, DOI:10.1016/j.cll.2015.02.007.
- M. Y. Ina-Salwany, N. Al-Saari, A. Mohamad, F.-A. Mursidi, A. Mohd-Aris, M. N. A. Amal, H. Kasai and M. Zamri-Saad, J. Aquat. Anim. Health, 2019, 31, 3–22, DOI:10.1002/aah.10045.
- A. E. Toranzo, B. Magarinos and J. L. Romalde, Aquaculture, 2005, 246, 37–61, DOI:10.1016/j.aquaculture.2005.01.002.
- I. Frans, C. W. Michiels, P. Bossier, K. A. Willems, B. Lievens and H. Rediers, J. Fish Dis., 2011, 34, 643–661, DOI:10.1111/j.1365-2761.2011.01279.x.
- N. Mohamad, M. N. A. Amala, I. S. Yasin, M. Z. Saad, N. S. Nasruddin, N. Al-Saarif, S. Minog and T. Sawab, Aquaculture, 2019, 512, 734289, DOI:10.1016/j.aquaculture.2019.734289.
- U. Khimmakthong and P. Sukkarun, Microb. Pathog., 2017, 113, 107–112, DOI:10.1016/j.micpath.2017.10.028.
- B. Austin and X. H. Zhang, Lett. Appl. Microbiol., 2006, 43(2), 119–124, DOI:10.1111/j.1472-765X.2006.01989.x.
- N. Garcia-Bueno, P. Decottignies, V. Turpin, J. Dumay, C. Paillard, V. Stiger-Pouvreau, N. Kervarec, Y.-F. Pouchus, A. A. Marŕin-Atucha and J. Fleurence, Aquat. Living Resour., 2014, 27, 83–89, DOI:10.1051/alr/2014009.
- J. Dubert, J. L. Barja and J. L. Romalde, Front. Microbiol., 2017, 8, 762, DOI:10.3389/fmicb.2017.00762.
- R. Beaz-Hidalgo, S. Balboa, J. L. Romalde and M. J. Figueras, Environ. Microbiol. Rep., 2010, 2(1), 34–43, DOI:10.1111/j.1758-2229.2010.00135.
- C. Baker-Austin, J. D. Oliver, M. Alam, A. Ali, M. K. Waldor, F. Qadri and J. Martinez-Urtaza, Nat. Rev. Dis. Prim., 2018, 4, 8, DOI:10.1038/s41572-018-0005-8.
- R. Finkelstein and I. Oren, Curr. Infect. Dis. Rep., 2011, 13, 470–477, DOI:10.1007/s11908-011-0199-3.
- A. M. Dechet, A. Y. Patricia, N. Koram and J. Painter, Clin. Infect. Dis., 2008, 46, 970–976, DOI:10.1086/529148.
- S.-P. Heng, V. Letchumanan, C.-Y. Deng, N.-S. S. Mutalib, T. M. Khan, L.-H. Chuah, K.-G. Chan, B.-H. Goh, P. Pusparajah and L.-H. Lee, Front. Microbiol., 2017, 8, 997, DOI:10.3389/fmicb.2017.00997.
- S. M. Aly and A. Albutti, J. Aquacult. Res. Dev., 2014, 5, 4, DOI:10.4172/2155-9546.1000247.
- J. E. M. Watts, H. J. Schreier, L. Lanska and M. S. Hale, Mar. Drugs, 2017, 5, 158, DOI:10.3390/md15060158.
- R. H. Rebouças, O. V. Sousa, A. S. Lima, F. R. Vasconcelos, P. B. Carvalho and R. H. S. F. Viera, Environ. Res., 2011, 11, 21–24, DOI:10.1016/j.envres.2010.09.012.
- C. Scarano, C. Spanu, G. Ziino, F. Pedonese, A. Dalmasso, V. Spanu, S. Virdis and E. P. De Santis, New Microbiol., 2014, 37(3), 329–337 Search PubMed.
- R. X. Wang, J. Y. Wang, Y. C. Sun, B. L. Yang and A. L. Wang, Mar. Pollut. Bull., 2015, 101(2), 701–706, DOI:10.1016/j.marpolbul.2015.10.027.
- Q. Yu, M. Niu, M. Yu, Y. Liu, D. Wang and X. Shi, Food Control, 2016, 60, 263–268, DOI:10.1016/j.foodcont.2015.08.005.
- S. Elmahdi, L. V. DaSilva and S. Parveen, Food Microbiol., 2016, 57, 128–134, DOI:10.1016/j.fm.2016.02.008.
- C. H. Kang, Y. Shin, S. Jang, Y. Jung and J. S. So, Environ. Sci. Pollut. Res., 2016, 23(20), 21106–21112, DOI:10.1007/s11356-016-7426-2.
- D. L. Milton, Int. J. Med. Microbiol., 2006, 296(2), 61–71, DOI:10.1016/j.ijmm.2006.01.044.
- Y. Deng, Y. Liu, J. Li, X. Wang, S. He, X. Yan, Y. Shi, W. Zhang and L. Ding, Eur. J. Med. Chem., 2022, 239, 114513, DOI:10.1016/j.ejmech.2022.114513.
- J. Zhao, M. Chen, C. S. Quan and S. D. Fan, J. Fish Dis., 2015, 38(9), 771–786, DOI:10.1111/jfd.12299.
- F. H. Yildiz and K. L. Visick, Trends Microbiol., 2009, 17(3), 109–118, DOI:10.1016/j.tim.2008.12.004.
- L. A. Hawver, S. A. Jung and W. L. Ng, FEMS Microbiol. Rev., 2016, 40, 738–752, DOI:10.1093/femsre/fuw014.
- K. Papenfort and B. L. Bassler, Nat. Rev. Microbiol., 2016, 14(9), 576–588, DOI:10.1038/nrmicro.2016.89.
- B. Vu, M. Chen, R. J. Crawford and E. P. Ivanova, Molecules, 2009, 14(7), 2535–2554, DOI:10.3390/molecules14072535.
- S. E. Rossiter, M. H. Fletcher and W. M. Wuest, Chem. Rev., 2017, 117, 12415–12474, DOI:10.1021/acs.chemrev.7b00283.
- J. F. M. Ong, H. C. Goh, S. C. Lim, L. M. Pang, J. S. F. Chin, K. S. Tan, Z.-X. Liang, L. Yang, E. Glukhov, W. H. Gerwick and L. T. Tan, Mar. Drugs, 2019, 17, 72, DOI:10.3390/md17010072.
- O. Geniloud, Nat. Prod. Rep., 2017, 34, 1203–1232, 10.1039/c7np00026j.
- S. W. Behie, B. Bonet, V. M. Zacharia, D. J. McClung and M. F. Traxler, Front. Microbiol., 2017, 7, 2149, DOI:10.3389/fmicb.2016.02149.
- J. L. You, L. X. Cao, G. F. Liu, S. N. Zhou, H. M. Tan and Y. C. Lin, World J. Microbiol. Biotechnol., 2005, 21(5), 679–682, DOI:10.1007/s11274-004-3851-3.
- J. L. You, X. L. Xue, L. X. Cao, X. Lu, J. Wang, L. Zhang and S. Zhou, Appl. Microbiol. Biotechnol., 2007, 76(5), 1137–1144, DOI:10.1007/s00253-007-1074-x.
- N. Augustine, S. Kerkar and S. Thomas, Curr. Microbiol., 2012, 64(4), 338–342, DOI:10.1007/s00284-011-0073-4.
- L. T.-H. Tan, K.-G. Chan, L.-H. Lee and B.-H. Goh, Front. Microbiol., 2016, 7, 79, DOI:10.3389/fmicb.2016.00079.
- M. Bodhaguru, S. Prakash, R. Ramasubburayan, N. K. Ahila, L. Mariselvam, G. Immanuel, A. Palavesa and E. Kannapiran, Microb. Pathog., 2019, 134, 103597, DOI:10.1016/j.micpath.2019.103597.
- G. Raissa, D. E. Waturangi and D. Wahjuningrum, BMC Microbiol., 2020, 20, 343, DOI:10.1186/s12866-020-02022-z.
- L. T.-H. Tan, L.-H. Lee and B.-H. Goh, Prog. Microbes. Mol. Biol., 2019, 2, 1 Search PubMed.
- G. S. Kiran, A. N. Lipton, S. Priyadharshini, K. Anitha, L. E. Suárez, M. V. Arasu, K. C. Choi, J. Selvin and N. A. Al-Dhabi, Microb. Cell Fact., 2014, 13, 114, DOI:10.1186/s12934-014-0114-3.
- M. Hayashi, T. Unemoto, S. Minami-Kakinuma, H. Tanaka and S. Omura, J. Antibiot., 1982, 35, 1078–1085, DOI:10.7164/antibiotics.35.1078.
- U. F. Castillo, G. A. Strobel, E. J. Ford, W. M. Hess, H. Porter, J. B. Jensen, H. Albert, R. Robinson, M. A. M. Condron, D. B. Teplow, D. Stevens and D. Yaver, Microbiology, 2002, 148(9), 2675–2685, DOI:10.1099/00221287-148-9-2675.
- M. Iwatsuki, R. Uchida, H. Yoshijima, H. Ui, K. Shiomi, A. Matsumoto, Y. Takahashi, A. Abe, H. Tomoda and S. Omura, J. Antibiot., 2008, 61, 222–229, DOI:10.1038/ja.2008.32.
- T. C. Holmes, A. E. May, K. Zaleta-Rivera, J. G. Ruby, P. Skewes-Cox, M. A. Fischbach, J. L. DeRisi, M. Iwatsuki, S. Omura and C. Khosla, J. Am. Chem. Soc., 2012, 134(42), 17797–17806, DOI:10.1021/ja308622d.
- J. Y. Cho and M. S. Kim, Fish. Sci., 2012, 78(5), 1065–1073, DOI:10.1007/s12562-012-0531-3.
- N. Yang and C. Sun, Front. Microbiol., 2016, 7, 1467, DOI:10.3389/fmicb.2016.01467.
- M. E. Rateb, W. E. Houssen, W. T. A. Harrison, H. Deng, C. K. Okoro, J. A. Asenjo, B. A. Andrews, A. T. Bull, M. Goodfellow, R. Ebel and M. Jaspars, J. Nat. Prod., 2011, 74(9), 1965–1971, DOI:10.1021/np200470u.
- J. F. Castro, V. Razmilic, J. P. Gomez-Escribano, B. Andrews, J. Asenjo and M. Bibb, Antonie van Leeuwenhoek, 2018, 111(8), 1433–1448, DOI:10.1007/s10482-018-1034-8.
- J. P. Bowman, Mar. Drugs, 2007, 5, 220–241, DOI:10.3390/md504220.
- C. Offret, F. Desriac, P. L. Chevalier, J. Mounier, C. Jegou and Y. Fleury, Mar. Drugs, 2016, 14, 129, DOI:10.3390/md14070129.
- C. S. Castillo, M. I. Wahid, T. Yoshikawa and T. Sakata, Fish. Sci., 2008, 74, 174–179, DOI:10.1111/j.1444-2906.2007.01507.x.
- G. Hayashida-Soiza, A. Uchida, N. Mori, Y. Kuwahara and Y. Ishida, J. Appl. Microbiol., 2008, 105(5), 1672–1677, DOI:10.1111/j.1365-2672.2008.03878.x.
- J. Tebben, C. Motti, D. Tapiolas, P. Thomas-Hall and T. Harder, Mar. Drugs, 2014, 12, 2802–2815, DOI:10.3390/md12052802.
- R. Preeta, S. Jose, S. Prathapan and K. K. Vijayan, Aquacult. Res., 2010, 41(10), 1452–1461, DOI:10.1111/j.1365-2109.2009.02436.x.
- P. Rattanachuay, D. Kantachote, M. Tantirungkij, T. Nitoda and H. Kanzak, World J. Microbiol. Biotechnol., 2011, 27, 869–880, DOI:10.1007/s11274-010-0529-x.
- P. Priyaja, P. Jayesh, N. S. Correya, B. Sreelakshmi, N. S. Sudheer, R. Philip and I. S. B. Singh, J. Coastal Life Med., 2014, 2(1), 76–84, DOI:10.12980/JCLM.2.2014J30.
- L. Zhang, X. Tian, S. Kuang, G. Liu, C. Zhang and C. Sun, Front. Microbiol., 2017, 8, 289, DOI:10.3389/fmicb.2017.00289.
- M. E. Teasdale, J. Liu, J. Wallace, F. Akhlaghi and D. C. Rowley, Appl. Environ. Microbiol., 2009, 75(3), 567–572, DOI:10.1128/AEM.00632-08.
- Y. Leyton, J. Borquez, J. Darias, M. Cueto, A. R. Díaz-Marrer and C. Riquelme, Mar. Drugs, 2011, 9(10), 2155–2163, DOI:10.3390/md9102155.
- X. Y. Gao, Y. Liu, L. L. Miao, E. W. Li, T. T. Hou and Z. P. Liu, AMB Express, 2017, 7, 23, DOI:10.1186/s13568-017-0323-3.
- P. Bhadury, B. Mohammad and P. C. Wright, J. Ind. Microbiol. Biotechnol., 2006, 33(5), 325–337, DOI:10.1007/s10295-005-0070-3.
- J. F. Imhof, Mar. Drugs, 2016, 14, 19, DOI:10.3390/md14010019.
- D. Tasdemir, Fungal Biol. Biotechnol., 2017, 4, 5, DOI:10.1186/s40694-017-0034-1.
- H. J. Shin, Mar. Drugs, 2020, 18(5), 230, DOI:10.3390/md18050230.
- X.-Y. Hu, L.-M. Meng, X. Li, S.-Q. Yang, X.-M. Li and B.-G. Wang, Mar. Drugs, 2017, 15, 137, DOI:10.3390/md15050137.
- X.-C. Guo, L.-L. Xu, R.-Y. Yang, M.-Y. Yang, L.-D. Hu, H.-J. Zhu and F. Cao, Front. Chem., 2019, 7, 80, DOI:10.3389/fchem.2019.00080.
- A. Zhu, X.-W. Zhang, M. Zhang, W. Li, Z.-Y. Ma and H.-J. Zhu, Mar. Drugs, 2018, 16(9), 312, DOI:10.3390/md16090312.
- H. L. Li, X. M. Li, S. Q. Yang, L. H. Meng, X. Li and B. G. Wang, Mar. Drugs, 2019, 17(11), 605, DOI:10.3390/md17110605.
- X. D. Li, X. Li, X. M. Li, X. L. Yin and B. G. Wang, Nat. Prod. Res., 2021, 35(22), 4265–4271, DOI:10.1080/14786419.2019.1696792.
- X. Xu, S. Guo, H. Chen, Z. Zhang, X. Li, W. Wang and L. Guo, 3 Biotech, 2021, 11(4), 1–7, DOI:10.1007/s13205-021-02754-3.
- X. Wei, Y. Ding and F. An, Nat. Prod. Commun., 2021, 17(3), 1–5, DOI:10.1177/1934578X221075986.
- Y.-P. Feng, H.-K. Wang, J.-L. Wu, P. Shao, W.-L. Zhou, Q.-L. Lai, H.-W. Lin, B. Naman, T.-T. Wang and S. He, Chem. Biodiversity, 2022, 19(4), e202200028, DOI:10.1002/cbdv.202200028.
- T. Mai, F. Tintillier, A. Lucasson, C. Moriou, E. Bonno, S. Petek, K. Magre, A. A. Mourabit, D. Saulnier and C. Debitus, Lett. Appl. Microbiol., 2015, 61, 31–317, DOI:10.1111/lam.12461.
- B. C. Lee, A. Lee, J. H. Jung, S. H. Choi and T. S. Kim, Mol. Med. Rep., 2016, 14, 2691–2696, DOI:10.3892/mmr.2016.5522.
- H. Mohamad, R. Rosmiati, T. S. T. Muhammad, Y. Andriani, K. Bakar, N. Ismail, J. Saidin, J. Latip, N. Musa and A. Parenrengi, Nat. Prod. Commun., 2017, 12(8), 1227–1230, DOI:10.1177/1934578X1701200819.
- T. Wang, J. Zou, T. Li, P. Shao, W. Zhou, Q. Lai, Y. Feng, C. B. Naman, X. Yan and S. He, Aquaculture, 2022, 549, 737727, DOI:10.1016/j.aquaculture.2021.737727.
- S. L. Holdt and S. Kraan, J. Phycol., 2011, 23, 543–597, DOI:10.1007/s10811-010-9632-5.
- A. Leandro, L. Pereira and A. M. M. Gonçalves, Mar. Drugs, 2019, 18(1), 17, DOI:10.3390/md18010017.
- M. Kladi, C. Vagias and V. Roussis, Phytochem. Rev., 2004, 3, 337–366, DOI:10.1007/s11101-004-4155-9.
- M. T. Cabrita, C. Vale and A. P. Rauter, Mar. Drugs, 2010, 8, 2301–2317, DOI:10.3390/md8082301.
- F. C. Pacheco, Mar. Drugs, 2010, 8, 1178–1188, DOI:10.3390/md8041178.
- J. Cotas, A. Leandro, P. Monteiro, D. Pacheco, A. Figueirinha, A. M. M. Gonçalves, G. J. da Silva and J. Pereira, Mar. Drugs, 2020, 18, 384, DOI:10.3390/md18080384.
- H. Pereira, L. Barreira, F. Figueiredo, L. Custódio, C. V. Duarte, C. Polo, E. Rešek, A. Engelen and J. Varela, Mar. Drugs, 2012, 10, 1920–1935, DOI:10.3390/md10091920.
- S. Thanigaivel, N. Chadrasekaran, A. Mukherjee and J. Thomas, Aquaculture, 2015, 448, 82–86, DOI:10.1016/j.aquaculture.2015.05.039.
- I. N. Vatsos and C. Rebours, J. Appl. Phycol., 2015, 27, 2017–2035, DOI:10.1007/s10811-014-0506-0.
- S. Thanigaivel, N. Chadrasekaran, A. Mukherjee and J. Thomas, Aquaculture, 2016, 464, 529–536, DOI:10.1016/j.aquaculture.2016.08.001.
- V. Zammuto, M. G. Rizzo, A. Spanò, D. Spagnuolo, A. Di Martino, M. Morabito, A. Manghisi, G. Genovese, S. Guglielmino, G. Calabrese, F. Capparucci, C. Gervasi, M. S. Nicolo and C. Gugliandolo, Algal Res., 2022, 63, 102646, DOI:10.1016/j.algal.2022.102646.
- E. Shannon and N. Abu-Ghannam, Mar. Drugs, 2016, 14, 81, DOI:10.3390/md14040081.
- K. Wongprasert, T. Rudtanatip and J. Praiboon, Fish Shellfish Immunol., 2014, 36, 52–60, DOI:10.1016/j.fsi.2013.10.010.
- N. Kasanah, T. Triyanto, D. S. Seto, W. Amelia and A. Isnansetyo, Indones. J. Chem., 2015, 15(2), 201–209, DOI:10.22146/ijc.21215.
- G. Brackman and T. Coenye, Curr. Pharm. Des., 2015, 21(1), 5–11, DOI:10.2174/1381612820666140905114627.
- W. Zhao, N. Lorenz, K. Jung and S. A. Sieber, Angew. Chem., Int. Ed., 2016, 55(3), 1187–1191, DOI:10.1002/anie.201508052.
- A. P. A. Wijnana, N. Kasanah and T. Triyanto, Nat. Prod. J., 2018, 8, 1–6, DOI:10.2174/1573401313666170925161408.
- N. Kasanah, W. Amelia, A. Mukminin, T. Triyanto and A. Isnansetyo, Nat. Prod. Res., 2019, 33(22), 3303–3307, DOI:10.1080/14786419.2018.1471079.
- K. Karnjana, C. Soowannayan and K. Wongprasert, Fish Shellfish Immunol., 2019, 88, 91–101, DOI:10.1016/j.fsi.2019.01.058.
- K. Karnjana, S. Nobsathian, C. Soowannayan, W. Zhao, Y. J. Tang and K. Wongprasert, Mar. Drugs, 2020, 18(2), 80, DOI:10.3390/md18020080.
- M. Reverter, N. Bontemps, D. Lecchini, B. Banaigs and P. Sasal, Aquaculture, 2014, 433, 50–61, DOI:10.1016/j.aquaculture.2014.05.048.
- P. Elumalai, A. Kurian, S. Lakshmi, C. Faggio, M. A. Esteban and E. Ringø, Rev. Fish. Sci., 2021, 29(1), 33–35, DOI:10.1080/23308249.2020.1779651.
- E. Sanchez, S. Garcia and N. Heredia, Appl. Environ. Microbiol., 2010, 76, 6888–6894, DOI:10.1128/AEM.03052-09.
- M. Aminzare, M. Hashemi, Z. Abbasi, Z. M. Mohseni and E. Amiri, J. Appl. Pharm. Sci., 2018, 8, 170–177, DOI:10.7324/JAPS.2018.8126.
- A. Borges, A. C. Abreu, C. Dias, M. J. Saavedra, F. Borges and M. Simões, Molecules, 2016, 21(7), 877, DOI:10.3390/molecules21070877.
- M. Snoussi, H. Hajlaoui, E. Noumi, D. Usai, L. A. Sechi, S. Zanetti and A. Bakhrouf, World J. Microbiol. Biotechnol., 2008, 24(12), 3071–3076, DOI:10.1007/s11274-008-9848-6.
- S. Chatterjee, M. Asakura, N. Chowdury, S. B. Neogi, N. Sugimoto, S. Haldar, S. P. Awasthi, A. Hinenoya, S. Aoki and S. Yamasaki, FEMS Microbiol. Lett., 2010, 306(1), 54–60, DOI:10.1111/j.1574-6968.2010.01931.x.
- I. A. S. V. Packiavathy, P. Sasikumar, S. K. Pandian and A. V. Ravi, Appl. Microbiol. Biotechnol., 2013, 97, 10177–10187, DOI:10.1007/s00253-013-4704-5.
- E. Acosta-Smith, N. Leon-Sicairos, S. Tiwari, H. Flores-Villasensor, A. Canizalez-Roman, R. Kumavath, P. Ghosh, V. Azevedo and D. Barh, Pathogen, 2019, 8, 64, DOI:10.3390/pathogens8020064.
- Z. Shan, N. Guan, Y. Yang, T. Jin, X. Xia and W. Liu, Aquaculture, 2021, 533, 736109, DOI:10.1016/j.aquaculture.2020.736109.
| This journal is © The Royal Society of Chemistry 2022 |