 Open Access Article
Open Access ArticleReview of MXene synthesis and applications in electromagnetic shielding
Yao Gan
and
Yuzhu Xiong
 *
*
Department of Materials and Metallurgy, Guizhou University, Guiyang 550025, China. E-mail: xyzhu789@126.com
First published on 28th March 2025
Abstract
With the ongoing advancements in wireless communication and electronic technology, the issue of electromagnetic radiation (EMR) pollution has become increasingly significant. Consequently, developing materials to mitigate EMR pollution is essential. The utility of 2D MXene (Ti3C2Tx) for electromagnetic interference (EMI) shielding was initially reported in 2016. Since then, MXenes have garnered substantial interest from the scientific community owing to their excellent metallic conductivity, low density, expansive specific surface area, and tunable interlayer spacing. In recent years, MXenes have demonstrated considerable promise in EMI shielding applications. This paper aims to examine the structural and chemical properties of MXenes, the methodologies for their synthesis, and summarize the advancements in MXene-based EMI shielding composites, highlighting their performance benefits. Additionally, this review will discuss the prospective developments in MXene-based materials for EMI shielding.
 Yao Gan | Yao Gan is studying in the Department of Materials and Chemical Engineering, College of Materials and Metallurgy, Guizhou University. Her main research area is the structure and properties of polymer matrix composites. Email address: E-mail: gyao0311@163.com. |
 Yuzhu Xiong | Yuzhu Xiong, a professor at Guizhou University, focuses on research into the structure and properties of polymer matrix composites. Email address: E-mail: xyzhu789@126.com. |
1 Introduction
Electromagnetic waves serve as a critical medium for information transmission, possessing the unique ability to propagate through vacuous space, thereby facilitating long-distance communication.1–3 With the advancement of technology and the integration of 5G communications into daily life, our reliance on electromagnetic applications, such as wireless communication, television, computers, global positioning systems, and radar detectors, continues to grow.3–7 However, excessive exposure to electromagnetic waves can lead to significant electromagnetic radiation pollution, posing unseen and silent threats.8 In the era of electronic information, electromagnetic radiation can disrupt the function of electronic devices in military, industrial, and residential settings, potentially posing severe risks to human health and the biosphere.1,5,7,9–14 Consequently, developing effective EMI shielding materials is crucial to safeguard both the functionality of electronic devices and public health.EMI shielding is a critical strategy for mitigating EMR pollution. This process involves the attenuation or complete shielding of electromagnetic waves by specific materials, thereby ensuring the smooth operation of sensitive equipment and protecting humans from EMR exposure.15–17 Although traditional metal shielding materials, characterized by high conductivity, are effective in reflecting electromagnetic waves, this attribute also leads to secondary reflections of these waves, contributing to contamination. Moreover, these materials often suffer from high density, corrosion susceptibility, and complex manufacturing processes, which restrict their broader application.18,19 Consequently, researchers have been developing alternative shielding materials, such as graphene, carbon nanotubes, and MXene, which offer corrosion resistance, reduced weight, and enhanced performance.
Since the introduction of graphene in 2004, two-dimensional materials have garnered significant interest, as illustrated in Fig. 1. The family of transition metal carbides, nitrides, and carbonitrides, collectively known as MXenes, has particularly drawn global research attention since its first discovery in 2011. These materials are prized for their metallic conductivity, optical properties, and surfaces enriched with functional groups and hydrophilic sites.20–27 A landmark achievement was realized in 2016 when Yury Gogotsi's team advanced from theoretical concepts to practical applications in EMI shielding using 2D materials. They developed a highly flexible MXene film with a thickness of 45 μm, which demonstrated the ability to block 99.99999999999994% of incident radiation, achieving a shielding effectiveness of 92 dB. This performance is on par with that of traditional metal shields.28
MXene is composed of transition metal elements, along with carbon and nitrogen, and is characterized by high electrical and thermal conductivity, as well as a multitude of surface functional groups that enhance its surface reactivity.29 The precursor to MXene, known as the MAX phase, belongs to the hexagonal crystalline system. In this system, ‘M’ denotes the transition metal, ‘A’ represents an element from either the III or IV main group, and ‘X’ indicates an element of carbon or nitrogen. The atomic layers of “M” and “X” are interleaved, and both are bonded by a mixture of ionic and covalent bonds. In contrast, the ‘A’ atomic layer is interspersed through metallic bonding. This configuration hinders the mechanical delamination of MXene due to the strong M–X bond energy compared to the M–A bond, which exhibits greater chemical activity.30 Consequently, selective etching can preferentially disrupt the active M–A bonds to produce 2D MXene materials.31–33 Notably, MXene's excellent electrical conductivity facilitates the improvement of material impedance mismatch, enhancing the reflection of electromagnetic waves or boosting electromagnetic loss capabilities for effective absorption. This leads to outstanding electromagnetic shielding performance.34,35
This paper provides a comprehensive review of the structure, properties, and preparation methods of MXene, as well as the applications of MXene-based composites in the field of electromagnetic shielding. It also summarizes the challenges associated with the current preparation methods and yields of MXene. Furthermore, the paper explores future prospects for the application of MXene in electromagnetic shielding.
2 Studies on the structure, properties and preparation of MXene
2.1 Structure of MXene
MXene is an innovative two-dimensional material characterized by a unique layered structure reminiscent of graphene's hexagonal honeycomb pattern. Each layer is composed of hexagonal transition metal carbides, nitrides, or carbonitrides. The layers of MXene are interconnected and self-stacked through van der Waals forces, electrostatic interactions, and hydrogen bonds, permitting some degree of relative sliding.36 However, the self-stacking of MXene layers, predominantly driven by van der Waals forces, often leads to reduced electronic pathways, thereby diminishing the material's electrochemical performance.37–40 The chemical composition of MXene is typically denoted by the formula Mn+1XnTx, where ‘M’ represents transition metal elements, ‘X’ denotes mainly elements carbon and nitrogen, ‘n’ indicates the number of elements in the interlayer voids, and ‘T’ refers to surface functional groups, including –OH, –F, –O, or –Cl41–43 (Fig. 2).Recent advancements in MXene research have led to its evolution from the initial accordion-like structure to contemporary forms such as nanoribbons, hollow spheres, and porous structures. Zeng et al. synthesized lamellar MXene, networked MXene nanoribbons, and porous lamellar MXene by employing strategies involving hydrofluoric acid etching, KOH shear, and high-temperature molten salts.44 These modifications enhance surface states and microstructures, significantly boosting the absorption of electromagnetic waves. Notably, the networked MXene nanoribbons exhibited reflection loss values as high as −63.01 dB. Moreover, the network structure of MXene nanosheets with low defects and large dimensions is more effective in increasing the conductivity of the material, thus further improving its electromagnetic shielding properties.45 The surface functional groups (e.g., –OH, –F) of the conductive MXene induce local dipoles under electric and magnetic fields, leading to polarization losses of electromagnetic waves. When electromagnetic waves transit through multiple layers of MXene nanosheets, they undergo multiple reflections and dissipations, similar to the described energy loss mechanisms, ultimately resulting in substantial electromagnetic wave attenuation,46,47 as depicted in Fig. 3.
2.2 Properties of MXene
The structural features of MXene fundamentally determine its properties, which in turn facilitate a broad spectrum of applications across various potential fields.Kathleen Maleski et al. produced Ti3C2Tx nanosheets of varying sizes using acoustic wave treatment and density gradient centrifugation.49 They discovered that films made from larger MXene nanosheets exhibited a peak conductivity of approximately 5000 S cm−1. In contrast, films from smaller MXene sheets demonstrated a significantly reduced conductivity of only 1000 S cm−1. This conductivity is lower than that of carbon-based MXene, which was initially synthesized using the in situ generation of hydrofluoric acid method.50 Utilizing the density-functional tight-binding (DFTB) method, Khanal et al. explored the influence of MXene surface functional groups and their ratios on electrical conductivity.51 Notably, unmodified MXene surfaces exhibited higher electrical conductivity compared to those modified by –OH, which in turn showed higher conductivity than MXene surfaces altered by –O. Furthermore, conductivity varied depending on the ratios of –O to –OH. Eom et al. annealed Ti3C2Tx-MXene monolayer nanosheets under argon and ammonia atmospheres, revealing that nitridation in ammonia not only preserved the electron-rich structure of Ti3C2 but also doubled the conductivity compared to the argon-treated MXene films.52 Mathis et al. synthesized MAX powders with a high aluminum content and prepared monolayer MXene sheets with minimal defects and large dimensions (maximum size > 25 μm) by etching, washing, and centrifugation of the precursor.53 These sheets achieved an electrical conductivity as high as 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S cm−1, attributable to their large layer sizes and fewer defects, which enhanced in-sheet electron transfer capability and chemical stability. Such high conductivity positions MXene favorably for development in electromagnetic shielding applications.
000 S cm−1, attributable to their large layer sizes and fewer defects, which enhanced in-sheet electron transfer capability and chemical stability. Such high conductivity positions MXene favorably for development in electromagnetic shielding applications.
Zhang et al. enhanced the magnetic properties of MXene by annealing a monolayer of Ti3C2Tx-MXene to induce the formation of amorphous carbon on the sample surface.54 Liu et al. coated Ti3C2-MXene with superparamagnetic iron oxide nanoparticles, successfully creating magnetic MXene composites for highly efficient cancer therapy diagnostics.55 This material is not only beneficial for enhancing magnetic resonance imaging of tumors but also effective in the photothermal eradication of cancer cells and tumor tissue ablation. Building on the platform of 3D MXene induced by ethylenediamine, Zhang et al. synthesized a magnetic aerogel with a porous structure by the in situ co-precipitation of iron tetraoxide on 3D MXene.56 This unique structure demonstrated the capability to remove up to 2385 mg g−1 of silver from water through adsorption.
2.3 Preparation method of MXene
MXene, a novel two-dimensional material, has experienced rapid development across various fields due to its advantageous properties, including high electrical conductivity, high elastic modulus, large specific surface area, and adjustable hydrophilic surface. It shows particularly strong potential in microwave absorption and electromagnetic shielding.62,63 In the crystal structure of the MAX phase, the M–X bonding energy, which can be covalent or ionic, is significantly higher than the M–A bonding energy, which is metallic. Under certain conditions, the A layer in the MAX phase can be exfoliated to produce MXene.64 The preparation method of MXene influences its elemental composition, morphology, and surface functional groups, subsequently affecting its properties. Therefore, MXenes are selected based on the specific requirements of different practical applications. Currently, several established methods are employed for MXene preparation, including: hydrofluoric acid (HF) etching, in situ HF generation etching, fluoride molten salt etching, ultrasound-assisted etching, alkali solution-assisted etching, electrochemical anodic etching, Lewis acidic molten salt etching, and gas-phase dry etching.To further examine the characteristics of the in situ generation of hydrofluoric acid (HFA) method, Zhang et al. assessed the impact of time and concentration of hydrofluoric acid on etching efficiency.67 They compared the efficiency and electrochemical properties of Ti3C2Tx-MXene prepared by both the direct use of hydrofluoric acid and the in situ generation of HFA. Although both methods effectively removed the Al layer, significant differences were observed in the products: (1) MXene etched directly with hydrofluoric acid displayed an accordion-like morphology under microscopic examination, while products from the in situ generation method exhibited a pleated structure; (2) MXene prepared via the in situ generation of HFA at 10 mV S−1 demonstrated a higher specific capacitance and superior electrochemical performance compared to those prepared by the direct hydrofluoric acid method. This enhanced performance is primarily attributed to the use of an intercalation agent in the in situ generation process, which contains lithium ions that expand the lattice space of the sample, thereby improving the ion transport environment.
 | ||
| Fig. 4 (a) Schematic diagram of conditions and equipment for HIUE method preparation, (b) schematic diagram of etchant mechanism for HIUE method, (c) etching speed of HIUE method is ahead of other etching methods, (d) comparison of time between HIUE method and other etching methods, (e–g) SEM images of the rapid preparation of MXene by HIUE using NaF and HF as etchant, (h–j) AFM, HRTEM and TEM images of the products of this rapid preparation method (RPT), (k) SEM-EDS images of RPT with respect to the elements Ti, C and O, (l and m) MoS2 and MoS2/TiO2/RPT SEM images. Reproduced from ref. 70 with permission from [Elsevier], copyright [2024]. | ||
The majority of studies concerning the stability of MXene have indicated that the introduction of surface termination groups is influenced by the etching method utilized, which subsequently impacts the stability of MXene.76 Thus, Xue et al. employed a tetramethylammonium hydroxide (TMAOH) solution for the etching of MAX, leading to the formation of oxygen-terminated O-MXene.73 In contrast to MXene with fluorine terminations, which were produced through the in situ generation of hydrofluoric acid (HF), O-MXene exhibited superior antioxidant properties and demonstrated a reduced degradation rate in aqueous dispersions. This enhanced stability may be attributed to the greater electron-absorbing capacity of oxygen compared to fluorine, which renders titanium more favorable towards oxygen, resulting in a higher oxidation state of titanium. Additionally, it is plausible that the bonding energy of the titanium–oxygen bond surpasses that of the titanium–fluorine bond.73,77
 | ||
| Fig. 5 (a and b) Mechanism of gas-phase selective dry etching, (c) CV curves of Ti3C2Ix, Ti3C2Br2, and Ti3C2Cl2 at a scan rate of 20 mV s−1, (d) EIS curves of Ti3C2Ix, Ti3C2Br2, and Ti3C2Cl2; (e) CV curves of Ti3C2Ix, Ti3C2Br2, and Ti3C2Cl2 at 50, 100, 200, 500 and 1000 mV s−1 scan rates, (f) cycling stability at a current density of 5 mA cm−2. Reproduced from ref. 81 with permission from [Elsevier], copyright [2024]. | ||
3 MXene in the field of EMI shielding applications
3.1 Comparison of MXene with other EMI shielding materials
The electromagnetic shielding performance of materials is primarily influenced by factors such as electrical conductivity and thickness. However, in recent years, the requirements for electromagnetic shielding materials have expanded beyond high SE to include lightweight properties, impedance matching to minimize reflection and excellent stability in operational environments. Although MXenes are prone to oxidation, their surface functional groups can be effectively modulated through fluorine-free etching and supramolecular self-assembly, thereby enhancing their performance for practical applications.MXene exhibits electrical conductivity comparable to graphene and carbon nanotubes, while demonstrating lower density compared to conventional metallic electromagnetic interference (EMI) shielding materials, as shown in Table 1. This unique combination of properties renders MXene an ideal candidate for lightweight applications, particularly in weight-sensitive aerospace engineering.53,88 More importantly, the synergistic integration of controllable interlayer spacing and tunable surface functional groups enables unprecedented flexibility in engineering the absorption/reflection balance of electromagnetic waves through dipole polarization and interfacial charge transfer mechanisms.50,89 Such tunability facilitates superior EMI shielding performance across multiple spectral frequency ranges (RF region, X-band, Ku-band, Ka-band, V-band, and W-band), outperforming most existing two-dimensional nanomaterials.90
| Materials | Conductivity (S cm−1) | Density | Synthesis methods | Advantages | Limitations |
|---|---|---|---|---|---|
| a Chemical vapor deposition. | |||||
| MXene53,82 | 1500–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Low | Fluoride etching, non-fluoride etching | High conductivity, lightweight, tunable interlayer spacing | Susceptible to oxidation, high cost, difficult to use for large-scale |
| Graphene83,84 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–20 000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Low | Bottom-up (CVDa), top-down (exfoliation methods) | High conductivity, large surface area | High cost, limited scalability, difficult to use for large-scale |
| Carbon nanotubes85 | 103–104 | Low | CVDa arc discharge | High conductivity, high aspect ratio | High cost, difficult to disperse uniformly |
| Metal (e.g., Cu86,87) | 5.96 × 105 | High | Hydrometallurgy, pyrometallurgy, electrochemical deposition | High SE, low cost, high-thermal conductivity | High density, corrosion susceptibility |
Given the multiple factors affecting electromagnetic shielding performance, we investigated four commonly used EMI shielding materials, with a particular focus on thickness as a representative influencing factor in this study. Liu et al. developed MXene foam through hydrazine-induced foaming, demonstrating a significant enhancement in EMI shielding effectiveness from 53 dB for a 6-μm-thick MXene film to 70 dB for the foamed structure (60 μm).46 Regarding graphene-based materials, due to the challenges in directly foaming graphene films, graphene oxide (GO) is typically employed as a precursor. Following this approach, Shen et al. fabricated graphene foam using a similar hydrazine-induced foaming process with GO films.91 However, despite increasing the thickness from 20 μm to 300 μm, the resulting graphene foam only achieved an EMI SE of 25.2 dB. Reo Yanagi et al. prepared carbon nanotube-based composite aerogels via freeze-drying methodology, exhibiting a shielding effectiveness exceeding 30 dB at 40 wt% CNTs content, though requiring a substantial thickness of 3 mm.92 While bulk metallic materials possess high electrical conductivity, low manufacturing costs, and excellent electromagnetic shielding performance, their practical application in EMI shielding is limited by inherent drawbacks such as high density and susceptibility to corrosion.87,93 Collecitively, MXene emerges as a promising candidate for EMI shielding applications, demonstrating superior performance characteristics among the investigated materials.
3.2 MXene-based composite materials in the field of electromagnetic shielding applications
Due to its exceptional electrical conductivity, MXene and its composite materials have become prominent in the field of EMI shielding research in recent years. MXene-based EMI shielding materials exhibit excellent performance characteristics including wide frequency response, lightweight, high-temperature stability, and corrosion resistance. Consequently, these materials hold significant potential for diverse applications across various sectors such as electronic equipment, medical and military technologies, and aerospace.46,94–99Electromagnetic waves can disrupt the normal operation of electronic equipment, potentially leading to equipment failure or data loss. This interference is particularly critical in wireless communication devices and sensitive electronics, where it can degrade performance and stabilize it.100,101 Therefore, mitigating electromagnetic interference is crucial for enhancing the safety and reliability of electronic devices.
For most electronic products, electromagnetic shielding materials must achieve an effective shielding effectiveness (SE) of at least 20 dB to ensure functionality.102 Flexible MXene-based composite films are promising candidates for stabilizing the operation of wearable electronic devices. Jiao et al. prepared aqueous polyurethane/natural rubber/MXene composite films using vacuum-assisted filtration, achieving an average EM shielding effectiveness of 76.1 dB.103 Additionally, these films exhibited excellent mechanical properties and photo-thermal healing ability, suggesting potential applications in EM shielding devices and flexible wearable electronics. Qu et al. developed heterogeneous multifunctional composite films by integrating MXene with silver nanowires and nano-silver flakes, achieving an EM shielding effectiveness of 70.96 dB.104 These materials also displayed superior thermal conductivity, flame retardance, high temperature resistance, and mechanical properties, indicating significant potential for use in highly integrated advanced flexible electronics. Liu et al. encapsulated MXene materials in shape-stable phase change materials, resulting in MXene-based EM shielding composite phase change materials with an EM shielding effectiveness of 64.7 dB and enhanced thermal management capabilities.105 Du et al. constructed a composite phase change material with 48.1 dB EM shielding effectiveness and thermal management capabilities from a modified MXene-based nanocomposite elastomer.106 This ultra-flexible generator, capable of real-time detection of human body motion and physical signals, was assembled using sensors from this composite. Sang et al. explored a flexible polyvinylidene fluoride (PVDF)/MXene/polyimide (PI) wearable electronic device with a 40 dB EM shielding effectiveness, suitable for body monitoring and thermal therapy.107 Zhuang et al. successfully fabricated a film with high strength and a dense structure by limiting the wet film thickness to less than 60 μm to prevent the formation of a masking effect.108 The film, with a thickness of 1 μm, demonstrated an EMI shielding performance of 48.4 dB in the X-band.
‘Sandwich’ structures have emerged as a highly effective design for EMI shielding due to their unique layered architecture, which combines multiple functional materials to achieve superior performance. Vacuum-assisted filtration (VAF) is instrumental in the creation of multilayer films, particularly ‘sandwich’ films, and in the uniform deposition of functional fillers. Leveraging hydrogen bonding and covalent cross-linking within MXene lamellae, Hu et al. employed vacuum-assisted filtration and impregnation to create MXene-aramid nanofiber composite films with a ‘sandwich’ structure,109 as depicted in Fig. 6(a). This unique layered structure provides excellent mechanical properties and stability in Fig. 6(d), and the composite film achieves an electromagnetic shielding effectiveness of 46.1 dB and a tensile strength of up to 302.1 MPa when the film thickness is approximately 23.3 μm.
 | ||
| Fig. 6 (a) Schematic diagram of the preparation of this composite film, (b) diagram of electromagnetic interference shielding mechanism of this composite film, (c) characterization of the conductivity and electromagnetic shielding properties of this composite film, (d) mechanical properties and electromagnetic shielding properties of this composite film in comparison with other electromagnetic shielding materials under study. Adapted with permission from reference. Reproduced from ref. 109 with permission from [American Chemical Society], copyright [2022]. | ||
Li et al. successfully fabricated sandwich films using VAF, demonstrating exceptional flexibility and high electromagnetic shielding capabilities.110 Their study delved into the impact of varying quantities of ‘sandwich layer’ (MXene) and ‘core layer’ (CNTs/SA) on the electromagnetic shielding efficacy of composite films. The electrical conductivity of the composite films exhibits a proportional increase with the MXene content, owing to the 1D CNTs' dual role in effectively mitigating the self-stacking of the 2D MXene nanosheets and establishing a seamless conductive network with the MXene, ultimately enhancing electron transport. Notably, composite films containing 15 mg of MXene and a total of 6 mg of CNTs/SA (with a mass ratio of CNTs to SA of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) exhibited outstanding electromagnetic shielding performance at a thickness of 12 μm (with a shielding effectiveness of 61.3 dB and specific shielding effectiveness per unit thickness of 2.34 × 105 dB cm2 g−1).110,111 VAF enables the development of multifunctional ‘sandwich’ films, necessitating the incorporation of appropriate functional fillers within the functional layers. In contrast to previously established electromagnetic shielding ‘sandwich’ films, Ding et al. introduced a multifunctional composite film that exhibits superior electromagnetic interference shielding capabilities (nearly 70 dB), as well as optical, electrical, and magnetic-thermal conversion and storage properties, as shown in Fig. 7.112 They optimized the synergistic interaction between one-dimensional silver nanowires and two-dimensional MXene nanosheets. This optimization is attributed to the exceptional electrical conductivity of both materials, which enhances charge transport across their interfaces, thereby minimizing energy loss. Furthermore, the integration of MXene reduces the contact between silver nanowires, effectively lowering their contact resistance and consequently improving the overall electrical conductivity of the composite. Additionally, both materials possess excellent thermal conductivity, and their combination facilitates the formation of an efficient thermal conductive network.
4) exhibited outstanding electromagnetic shielding performance at a thickness of 12 μm (with a shielding effectiveness of 61.3 dB and specific shielding effectiveness per unit thickness of 2.34 × 105 dB cm2 g−1).110,111 VAF enables the development of multifunctional ‘sandwich’ films, necessitating the incorporation of appropriate functional fillers within the functional layers. In contrast to previously established electromagnetic shielding ‘sandwich’ films, Ding et al. introduced a multifunctional composite film that exhibits superior electromagnetic interference shielding capabilities (nearly 70 dB), as well as optical, electrical, and magnetic-thermal conversion and storage properties, as shown in Fig. 7.112 They optimized the synergistic interaction between one-dimensional silver nanowires and two-dimensional MXene nanosheets. This optimization is attributed to the exceptional electrical conductivity of both materials, which enhances charge transport across their interfaces, thereby minimizing energy loss. Furthermore, the integration of MXene reduces the contact between silver nanowires, effectively lowering their contact resistance and consequently improving the overall electrical conductivity of the composite. Additionally, both materials possess excellent thermal conductivity, and their combination facilitates the formation of an efficient thermal conductive network.
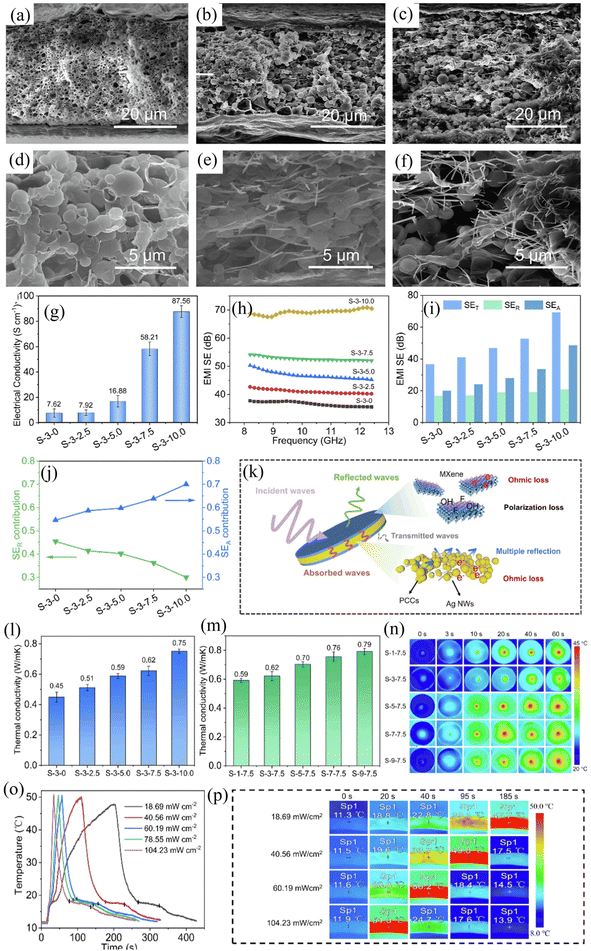 | ||
| Fig. 7 (a–f) Scanning electron microscopy (SEM) schematic of sandwich composite films, (g–j) characterization of conductivity and electromagnetic shielding properties of sandwich composite films, (k) electromagnetic shielding mechanism diagram of sandwich composite films, (l–p) photothermal characterization of sandwich composite films. Reproduced from ref. 112 with permission from [Elsevier], copyright [2024]. | ||
Medical devices frequently generate electromagnetic radiation that can interfere with nearby electronic equipment or adversely affect patients. Utilizing MXene-based electromagnetic shielding materials can effectively mitigate this radiation, thereby reducing its impact on both devices and human health. Additionally, biomedical sensors, which often rely on highly sensitive electrical signals to detect biological indicators, can be compromised by surrounding electromagnetic interference. MXene-based shielding materials can effectively counteract this interference, enhancing the accuracy and reliability of the sensors. Capitalizing on the distinctive properties of MXene materials, alongside thermoelectric effects and self-heating technology, Zhang et al. developed a wearable thermoelectric respiratory sensor based on MXene.113 This sensor dynamically monitors respiration by generating an electrical potential through the temperature difference between respiratory gas and ambient temperature, achieving an impressive electromagnetic shielding effectiveness of 59 dB. This innovative sensing technology holds significant potential for advancing medical monitoring and health management.113–115
MXene nanosheets are recognized for their excellent electrical conductivity and electromagnetic shielding properties, which enable them to absorb, reflect, and scatter electromagnetic waves effectively. These properties make them ideal for manufacturing lightweight, high-strength components for aerospace and military applications, such as aerogels that enhance the safety and multifunctionality of aerospace materials. This is due to the rich porous structure and high specific surface area of the aerogel which facilitates the enhancement of multiple reflections.116
In general, for lightweight electromagnetic shielding materials, it is insufficient to evaluate their capability solely based on the numerical values of electromagnetic shielding effectiveness (SE). It is also essential to consider the density (ρ) and thickness (t) of these porous materials. Consequently, metrics such as specific shielding effectiveness (SSE = SE/ρ) and absolute shielding effectiveness (SSEt = SSE/t) have been introduced to assess the performance of these materials.28 Wu et al. developed an aerogel with a hierarchical pore structure by integrating MXene and chitosan, achieving an electromagnetic shielding effectiveness of approximately 61.4 dB and a specific shielding effectiveness as high as 5155.46 dB cm3 g−1 with only 3 mm thickness.117 Shells exhibit excellent mechanical properties due to their unique ‘sand-brick’ structure, characterized by the alternating arrangement of inorganic calcium carbonate nanosheets (‘bricks’) and organic polymers (‘slurry’). Inspired by this natural design, Liang et al. crafted an aerogel with a wall-like structure using a porous carbon skeleton as the ‘mortar’ and a 3D MXene aerogel as the ‘brick’,118 as depicted in Fig. 8(a–e). This low-density ‘mortar-brick’ structure not only provides the aerogel with an electromagnetic interference shielding efficiency of up to 71.3 dB but also demonstrates exceptional mechanical and thermal insulation properties. Such multifunctional biocarbon-based composite materials hold significant promise for advanced applications in aerospace and defense industries.
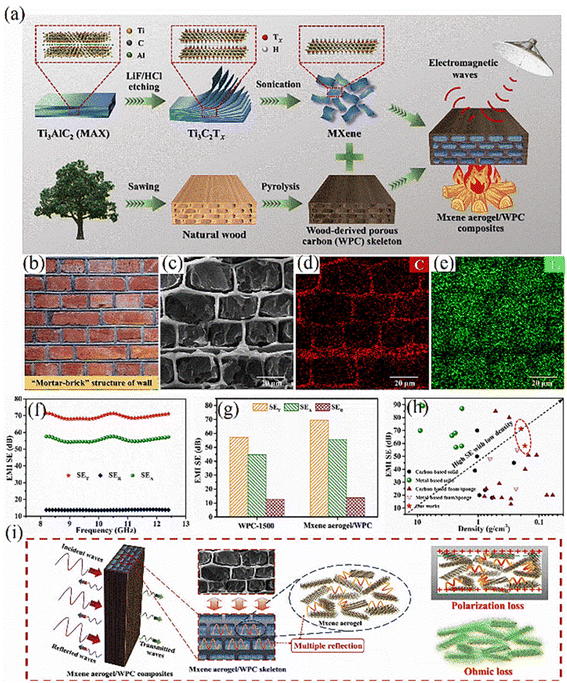 | ||
| Fig. 8 (a) Schematic of the preparation of this biocarbon-based composite; (b–e) SEM images and elemental mapping of C and Ti of the composite; (f–h) EMI SE of the composite; (i) schematic of the electromagnetic shielding of the composite. Reproduced from ref. 118 with permission from [Elsevier], copyright [2024]. | ||
Despite the numerous advantages of MXene, its inferior oxidation stability inevitably leads to a gradual decline in electrical conductivity, thereby compromising long-term EMI shielding performance. Recently, supramolecular self-assembly techniques have been explored to integrate MXene with complementary materials through non-covalent interactions such as hydrogen bonding and electrostatic forces. This strategic approach not only enhances their synergistic performance in practical applications but also effectively prolongs the material's service life by mitigating oxidative degradation pathways.119,120 Zhu et al. utilized metal ions (K+) to induce the self-assembly of MXene nanosheets and vacuum impregnated the MXene-K+ aerogels obtained through freeze-drying technology into molten paraffin to prepare composite phase change materials with electromagnetic shielding properties.121 Thanks to the effective binding between K+ and the hydroxyl groups on the MXene nanosheets, the MXene nanosheets were able to arrange in an orderly manner. When the weight ratio of KCl to MXene reached 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the material exhibited excellent electromagnetic shielding performance, achieving a shielding effectiveness of 57.7 dB. Tang et al. successfully fabricated a porous thin film with exceptional antioxidative properties through electrostatic self-assembly of MXene and GO followed by thermal annealing in an inert atmosphere.122 The resulting composite material demonstrates remarkable electromagnetic shielding performance (47 dB) at an ultra-thin thickness of 15 μm. Notably, the combined process of electrostatic self-assembly and thermal annealing effectively removes hydroxyl groups from both MXene and GO nanosheets, thereby endowing the porous structure with long-term antioxidative stability (12 months duration).
1, the material exhibited excellent electromagnetic shielding performance, achieving a shielding effectiveness of 57.7 dB. Tang et al. successfully fabricated a porous thin film with exceptional antioxidative properties through electrostatic self-assembly of MXene and GO followed by thermal annealing in an inert atmosphere.122 The resulting composite material demonstrates remarkable electromagnetic shielding performance (47 dB) at an ultra-thin thickness of 15 μm. Notably, the combined process of electrostatic self-assembly and thermal annealing effectively removes hydroxyl groups from both MXene and GO nanosheets, thereby endowing the porous structure with long-term antioxidative stability (12 months duration).
MXene-based electromagnetic shielding composites play a pivotal role in electronic science and technology, and their research and development progress and practical application prospects demonstrate an exceptionally broad trajectory. With the continuous advancement of science and technology, as well as the expanding societal demand, this field is poised to experience more in-depth academic research and a wider range of practical applications.
4 Conclusions
In conclusion, this paper reviews the structure, properties, preparation methods, and research progress of MXene-based electromagnetic shielding materials. MXene, as a two-dimensional material with a unique structure and properties, holds significant potential for future developments in the fields of electromagnetic shielding and wave-absorbing materials. Notably, the highly adjustable layer spacing of two-dimensional MXene provides a unique opportunity for its application in electromagnetic shielding and wave absorption. By manipulating the layer spacing of 2D MXene, the absorption or reflection of electromagnetic radiation within specific frequency ranges can be optimized. This capability enables MXene to effectively shield electromagnetic waves, thus offering protection for electronic equipment and communication systems. Furthermore, the excellent electrical conductivity of MXene allows its use as a conductive layer in electromagnetic shielding materials, facilitating the effective guidance and dispersion of electromagnetic radiation. Most importantly, the lightweight structure of two-dimensional MXene enables efficient electromagnetic shielding performance without significantly increasing weight, laying the groundwork for its future integration into lightweight electronic devices.The preparation process of MXene is relatively complex, involving high costs and extended preparation times, which hinder its scalability for industrial applications. Additionally, MXene is vulnerable to morphological and property alterations due to environmental factors such as humidity and temperature. Most critically, prolonged exposure to the environment can lead to a gradual degradation in electrical conductivity due to its oxidation sensitivity, adversely affecting its electromagnetic shielding capabilities. Consequently, it is essential to explore modifications in the preparation of MXene to enhance its oxidation resistance by altering its surface groups. Currently, the fluorine-containing etching method is a prevalent and efficient technique for preparing MXene; however, the environmental impact of the acidic and fluorine-containing compounds used in this process cannot be overlooked. Thus, the development of fluorine-free etching techniques and green preparation methods represents a viable approach for producing environmentally friendly MXene with enhanced performance.
While the high conductivity of MXene contributes to its exceptional electromagnetic shielding performance in composites, it can also cause electromagnetic waves to be reflected due to impedance mismatch induced by the material's highly conductive surface when exposed to air, leading to secondary pollution of electromagnetic waves. Consequently, recent research has increasingly focused on MXene-based electromagnetic shielding composites that exhibit ‘low reflection and high absorption’ characteristics. Specifically, to address the ‘low reflection characteristics’, magnetic particles are often introduced to mitigate the strong electromagnetic reflection caused by MXene's high conductivity and to improve the conditions of impedance matching, though this approach can compromise overall shielding performance. Future studies could investigate how the electrical conductivity of MXene is influenced by the type and shape of magnetic particles. Further exploration could also focus on the synergistic effects of magnetic particles with MXene, particularly in relation to their combined impact on electromagnetic shielding applications in carbon-based materials.
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Author contributions
Yao Gan: writing – original draft, review & editing. Yuzhu Xiong: editing, supervision and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (52063006).References
- M. K. Han and Y. Gogotsi, Carbon, 2023, 204, 17–25 CAS.
- X. Y. Wu, T. X. Tu, Y. Dai, P. P. Tang, Y. Zhang, Z. M. Deng, L. L. Li, H. B. Zhang and Z. Z. Yu, Nano–Micro Lett., 2021, 13, 15 Search PubMed.
- R. K. Amineh, Electronics, 2020, 9, 4 Search PubMed.
- S. H. Ryu, B. Park, Y. K. Han, S. J. Kwon, T. Kim, R. Lamouri, K. H. Kim and S. B. Lee, J. Mater. Chem. A, 2022, 10, 4446–4455 CAS.
- W. Gao, N. Zhao, T. Yu, J. Xi, A. Mao, M. Yuan, H. Bai and C. Gao, Carbon, 2020, 157, 570–577 CrossRef CAS.
- S. T. Gao, Y. C. Zhang, H. L. Xing and H. X. Li, Chem. Eng. J., 2020, 387, 124149 CrossRef CAS.
- B. Jiang, C. Qi, H. Yang, X. Wu, W. Yang, C. Zhang, S. Li, L. Wang and Y. Li, Carbon, 2023, 208, 390–409 CrossRef CAS.
- Y. P. Sun, X. G. Liu, C. Feng, J. C. Fan, Y. H. Lv, Y. R. Wang and C. T. Li, J. Alloys Compd., 2014, 586, 688–692 CrossRef CAS.
- J. C. Lin, IEEE Microwave Mag., 2016, 17, 32–36 Search PubMed.
- P. Song, C. Liang, L. Wang, H. Qiu, H. Gu, J. Kong and J. Gu, Compos. Sci. Technol., 2019, 181, 107698 CrossRef CAS.
- J. Tao, J. Zhou, Z. Yao, Z. Jiao, B. Wei, R. Tan and Z. Li, Carbon, 2021, 172, 542–555 CrossRef CAS.
- S. Wei, C. Zhou and L. Huang, Laser Med. Sci., 2022, 38, 25 Search PubMed.
- Y. Du, Y. Liu, A. Wang and J. Kong, iScience, 2023, 26, 107873 Search PubMed.
- E. Calabrò, Sustainability, 2018, 10, 3326 Search PubMed.
- M. Y. Peng and F. X. Qin, J. Appl. Phys., 2021, 130, 9 Search PubMed.
- G. R. Hu, Z. Q. Cen, Y. Z. Xiong and K. Liang, Nanoscale, 2023, 15, 5579–5597 CAS.
- D. D. L. Chung, Mater. Chem. Phys., 2020, 255, 11 Search PubMed.
- X. X. Wang, J. C. Shu, W. Q. Cao, M. Zhang, J. Yuan and M. S. Cao, Chem. Eng. J., 2019, 369, 1068–1077 CAS.
- C. B. Liang, P. Song, A. J. Ma, X. T. Shi, H. B. Gu, L. Wang, H. Qiu, J. Kong and J. W. Gu, Compos. Sci. Technol., 2019, 181, 7 CrossRef.
- M. Khazaei, A. Ranjbar, M. Arai, T. Sasaki and S. Yunoki, J. Mater. Chem. C, 2017, 5, 2488–2503 RSC.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. Pumera and Z. Sofer, Chem. Soc. Rev., 2017, 46, 4450–4463 RSC.
- M. F. Bhopal, D. W. Lee, A. U. Rehman and S. H. Lee, J. Mater. Chem. C, 2017, 5, 10701–10714 RSC.
- J. Halim, M. R. Lukatskaya, K. M. Cook, J. Lu, C. R. Smith, L. Näslund, S. J. May, L. Hultman, Y. Gogotsi, P. Eklund and M. W. Barsoum, Chem. Mat., 2014, 26, 2374–2381 CrossRef CAS PubMed.
- T. Wu, P. R. C. Kent, Y. Gogotsi and D. E. Jiang, Chem. Mat., 2022, 34, 4975–4982 CrossRef CAS.
- R. Khan and S. Andreescu, Biosens. Bioelectron., 2024, 248, 8 CrossRef.
- Y. Gogotsi, Chem. Mat., 2023, 35, 8767–8770 CAS.
- F. Shahzad, M. Alhabeb, C. B. Hatter, B. Anasori, S. M. Hong, C. M. Koo and Y. Gogotsi, Science, 2016, 353, 1137–1140 CAS.
- A. Lipatov, H. D. Lu, M. Alhabeb, B. Anasori, A. Gruverman, Y. Gogotsi and A. Sinitskii, Sci. Adv., 2018, 4, 7 Search PubMed.
- S. Jin, Y. T. Guo, F. L. Wang and A. G. Zhou, MRS Bull., 2023, 48, 245–252 CAS.
- B. Anasori and M. Naguib, MRS Bull., 2023, 48, 238–244 CAS.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CAS.
- J. G. Sun, B. B. Liu, Q. Zhao, C. H. Kirk and J. Wang, Adv. Mater., 2023, 35, 2306072 CAS.
- S. J. Wu, H. Liu, Q. H. Wang, X. Y. Yin and L. X. Hou, J. Alloys Compd., 2023, 945, 11 Search PubMed.
- W. W. Liu, H. Li, Q. P. Zeng, H. N. Duan, Y. P. Guo, X. F. Liu, C. Y. Sun and H. Z. Liu, J. Mater. Chem. A, 2015, 3, 3739–3747 RSC.
- H. Shin, W. Eom, K. H. Lee, W. Jeong, D. J. Kang and T. H. Han, ACS Nano, 2021, 15, 3320–3329 CrossRef CAS PubMed.
- Z. Y. Tan, W. Wang, M. K. Zhu, Y. C. Liu, Y. X. Yang, X. H. Ji and Z. K. He, Desalination, 2023, 548, 116267 CAS.
- F. T. Ran, T. L. Wang, S. Y. Chen, Y. Y. Liu and L. Shao, Appl. Surf. Sci., 2020, 511, 145627 CAS.
- D. D. Zhao, Z. R. Chen, N. Fu and Z. L. Yang, Mater. Today Sustain., 2023, 24, 100523 Search PubMed.
- Q. Y. Yang, Z. Xu, B. Fang, T. Q. Huang, S. Y. Cai, H. Chen, Y. J. Liu, K. Gopalsamy, W. W. Gao and C. Gao, J. Mater. Chem. A, 2017, 5, 22113–22119 CAS.
- A. Iqbal, P. Sambyal and C. M. Koo, Adv. Funct. Mater., 2020, 30, 2000883 CAS.
- N. Kubitza, C. Büchner, J. Sinclair, R. M. Snyder and C. S. Birkel, ChemPlusChem, 2023, 88, e202300214 CAS.
- Y. Gogotsi and B. Anasori, ACS Nano, 2019, 13, 8491–8494 CAS.
- X. J. Zeng, C. Zhao, X. Jiang, R. H. Yu and R. C. Che, Small, 2023, 19, 10 Search PubMed.
- Q. Q. Wang, W. Q. Cao and M. S. Cao, 2D Mater., 2024, 11, 012001 CAS.
- J. Liu, H. B. Zhang, R. H. Sun, Y. F. Liu, Z. S. Liu, A. G. Zhou and Z. Z. Yu, Adv. Mater., 2017, 29, 6 Search PubMed.
- T. X. Zhou, C. Q. Zhao, Y. H. Liu, J. Huang, H. S. Zhou, Z. D. Nie, M. Fan, T. Y. Zhao, Q. F. Cheng and M. J. Liu, ACS Nano, 2022, 16, 12013–12023 CrossRef CAS PubMed.
- J. Liu, Z. S. Liu, H. B. Zhang, W. Chen, Z. F. Zhao, Q. W. Wang and Z. Z. Yu, Adv. Electron. Mater., 2020, 6, 1901094 CAS.
- K. Maleski, C. E. Ren, M. Q. Zhao, B. Anasori and Y. Gogotsi, ACS Appl. Mater. Interfaces, 2018, 10, 24491–24498 CAS.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–U171 CAS.
- R. Khanal and S. Irle, J. Chem. Phys., 2023, 158, 10 Search PubMed.
- W. Eom, H. Shin, W. Jeong, R. B. Ambade, H. Lee and T. H. Han, Mater. Horiz., 2023, 10, 4892–4902 CAS.
- T. S. Mathis, K. Maleski, A. Goad, A. Sarycheva, M. Anayee, A. C. Foucher, K. Hantanasirisakul, C. E. Shuck, E. A. Stach and Y. Gogotsi, ACS Nano, 2021, 15, 6420–6429 CAS.
- K. Y. Zhang, M. Y. Di, L. Fu, Y. Deng, Y. W. Du and N. J. Tang, Carbon, 2020, 157, 90–96 CrossRef CAS.
- Z. Liu, M. L. Zhao, H. Lin, C. Dai, C. Y. Ren, S. J. Zhang, W. J. Peng and Y. Chen, J. Mater. Chem. B, 2018, 6, 3541–3548 RSC.
- W. J. Zhang, Y. H. Li, Y. X. Wang, Y. L. Wang, M. Kou, X. Y. Yang, Q. Q. Chu and K. Zhao, Mater. Chem. Phys., 2023, 309, 16 Search PubMed.
- X. Q. Qin, Z. G. Wu, J. W. Fang, S. S. Li, S. W. Tang and X. Y. Wang, Appl. Surf. Sci., 2023, 616, 11 Search PubMed.
- L. Li, Y. Lu, Z. T. Qian, Z. Y. Yang, S. F. Zong, Z. Y. Wang and Y. P. Cui, Nanoscale, 2021, 13, 18546–18557 RSC.
- Y. J. Dong, S. S. Li, X. Y. Li and X. Y. Wang, Int. J. Biol. Macromol., 2021, 190, 693–699 CrossRef CAS.
- O. S. Lee, M. E. Madjet and K. A. Mahmoud, Nano Lett., 2021, 21, 8510–8517 CrossRef CAS PubMed.
- X. Z. Wei, G. C. Song, M. H. Li, L. H. Li, Y. X. Lu, L. Zhang, D. Dai, T. Cai, K. Nishimura, C. T. Lin, N. Jiang and J. H. Yu, Compos. Commun., 2023, 43, 101729 CrossRef.
- A. V. Mohammadi, J. Rosen and Y. Gogotsi, Science, 2021, 372, 1165 Search PubMed.
- B. Anasori and Y. Gogotsi, Graphene 2D Mater. Technol., 2022, 7, 75–79 CrossRef.
- M. Kurtoglu, M. Naguib, Y. Gogotsi and M. W. Barsoum, MRS Commun., 2012, 2, 133–137 CrossRef CAS.
- X. Y. Huang, W. N. Mu and C. Chang, Environ. Sci. Pollut. Res., 2023, 30, 52157–52168 CAS.
- B. Soundiraraju and B. K. George, ACS Nano, 2017, 11, 8892–8900 CrossRef CAS PubMed.
- X. Y. Zhang, W. Zhang and H. T. Zhao, Int. J. Energy Res., 2022, 46, 15559–15570 CrossRef CAS.
- Y. B. Wang, B. Zhou, Q. Tang, Y. Yang, B. Pu, J. Bai, J. Xu, Q. G. Feng, Y. Liu and W. Q. Yang, Adv. Mater., 2024, 36, 10 Search PubMed.
- K. Arole, J. W. Blivin, A. M. Bruce, S. Athavale, I. J. Echols, H. X. Cao, Z. Y. Tan, M. Radovic, J. L. Lutkenhaus and M. J. Green, Chem. Commun., 2022, 58, 10202–10205 CAS.
- Y. Yao, Z. F. Wang, W. X. Wang, Y. T. Han and Z. G. Zhu, Chem. Eng. J., 2024, 489, 11 Search PubMed.
- Y. B. Li, H. Shao, Z. F. Lin, J. Lu, L. Y. Liu, B. Duployer, P. Persson, P. Eklund, L. Hultman, M. Li, K. Chen, X. H. Zha, S. Y. Du, P. Rozier, Z. F. Chai, E. Raymundo-Piñero, P. L. Taberna, P. Simon and Q. Huang, Nat. Mater., 2020, 19, 894 CAS.
- S. Ali, S. K. Thakur, A. Sarkar and S. Shekhar, Environ. Chem. Lett., 2016, 14, 291–315 CrossRef CAS.
- N. Xue, X. S. Li, L. Y. Han, H. Zhu, X. Y. Zhao, J. Zhuang, Z. L. Gao and X. T. Tao, J. Mater. Chem. A, 2022, 10, 7960–7967 RSC.
- S. J. Kim, H. J. Koh, C. E. Ren, O. Kwon, K. Maleski, S. Y. Cho, B. Anasori, C. K. Kim, Y. K. Choi, J. Kim, Y. Gogotsi and H. T. Jung, ACS Nano, 2018, 12, 986–993 CAS.
- T. F. Li, L. L. Yao, Q. L. Liu, J. J. Gu, R. C. Luo, J. H. Li, X. D. Yan, W. Q. Wang, P. Liu, B. Chen, W. Zhang, W. Abbas, R. Naz and D. Zhang, Angew. Chem., Int. Ed., 2018, 57, 6115–6119 CAS.
- A. D. Wu, T. H. Wang, L. Zhang, C. Chen, Q. M. Li, X. H. Qu and Y. C. Liu, Int. J. Miner. Metall. Mater., 2024, 31, 1752–1765 CrossRef CAS.
- J. Z. Jiang, S. S. Bai, J. Zou, S. Liu, J. P. Hsu, N. Li, G. Y. Zhu, Z. C. Zhuang, Q. Kang and Y. Z. Zhang, Nano Res., 2022, 15, 6551–6567 CAS.
- M. H. Sheng, X. Q. Bin, Y. W. Yang, Z. Chen and W. X. Que, Adv. Mater. Technol., 2024, 9, 2301694 CrossRef CAS.
- S. Yang, P. P. Zhang, F. X. Wang, A. G. Ricciardulli, M. R. Lohe, P. W. M. Blom and X. L. Feng, Angew. Chem., Int. Ed., 2018, 57, 15491–15495 CrossRef CAS PubMed.
- M. Li, J. Lu, K. Luo, Y. B. Li, K. K. Chang, K. Chen, J. Zhou, J. Rosen, L. Hultman, P. Eklund, P. Persson, S. Y. Du, Z. F. Chai, Z. R. Huang and Q. Huang, J. Am. Chem. Soc., 2019, 141, 4730–4737 CAS.
- J. M. Zhu, S. L. Zhu, Z. D. Cui, Z. Y. Li, S. L. Wu, W. Xu, T. Ba, Y. Q. Liang and H. Jiang, Energy Storage Mater., 2024, 70, 10 Search PubMed.
- B. Anasori and Y. Gogotsi, Graphene 2D Mater., 2022, 7, 75–79 Search PubMed.
- Y. Yu, X. Liu, D. Lu, T. Liu, Y. Li and Z. Wu, Carbon, 2025, 236, 120093 CAS.
- A. Ibrahim, A. Klopocinska, K. Horvat and Z. A. Hamid, Polymers, 2021, 13, 32 Search PubMed.
- M. Syduzzaman, M. S. Islam Saad, M. F. Piam, T. A. Talukdar, T. T. Shobdo and N. M. Pritha, Results Mater., 2025, 25, 100654 CrossRef CAS.
- R. Dang, L. L. Song, W. J. Dong, C. R. Li, X. B. Zhang, G. Wang and X. B. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 622–629 CrossRef CAS.
- S. H. Lee, S. Yu, F. Shahzad, J. P. Hong, W. N. Kim, C. Park, S. M. Hong and C. M. Koo, Compos. Sci. Technol., 2017, 144, 57–62 CAS.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 17 Search PubMed.
- A. Iqbal, F. Shahzad, K. Hantanasirisakul, M. K. Kim, J. Kwon, J. Hong, H. Kim, D. Kim, Y. Gogotsi and C. M. Koo, Science, 2020, 369, 446 CAS.
- A. Iqbal, J. Kwon, T. Hassan, S. W. Park, W. H. Lee, J. M. Oh, J. Hong, J. Lee, S. M. Naqvi, U. Zafar, S. J. Kim, J. H. Park, M. K. Kim and C. M. Koo, Adv. Funct. Mater., 2024, 2409346, DOI:10.1002/adfm.202409346.
- B. Shen, Y. Li, D. Yi, W. T. Zhai, X. C. Wei and W. G. Zheng, Carbon, 2016, 102, 154–160 CrossRef CAS.
- R. Yanagi, T. Segi, A. Ito, T. Ueno and K. Hidaka, Jpn. J. Appl. Phys., 2021, 60, 8 CrossRef.
- H. R. Zhao, J. H. Ding, M. Zhou and H. B. Yu, ACS Appl. Nano Mater., 2021, 4, 3075–3086 CrossRef CAS.
- K. Huang, Z. J. Li, J. Lin, G. Han and P. Huang, Chem. Soc. Rev., 2018, 47, 5109–5124 RSC.
- X. L. Li, X. W. Yin, S. Liang, M. H. Li, L. F. Cheng and L. T. Zhang, Carbon, 2019, 146, 210–217 CAS.
- Y. Li, K. Y. Wu, M. Zhang, X. L. Yang, W. Feng, P. Wang, K. Li, Y. Q. Zhan and Z. W. Zhou, Ceram. Int., 2022, 48, 37032–37038 CrossRef CAS.
- W. J. Ma, W. R. Cai, W. H. Chen, P. J. Liu, J. F. Wang and Z. X. Liu, Chem. Eng. J., 2021, 426, 9 Search PubMed.
- A. Iqbal, J. Kwon, M. K. Kim and C. M. Koo, Mater. Today Adv., 2021, 9, 15 Search PubMed.
- Z. J. Xu, X. Ding, S. K. Li, F. Z. Huang, B. J. Wang, S. P. Wang, X. Zhang, F. H. Liu and H. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 40396–40407 CAS.
- F. Shahzad, A. Iqbal, H. Kim and C. M. Koo, Adv. Mater., 2020, 32, 2002159 CAS.
- Y. H. Zhang, Y. Zhang, H. M. Liu, D. Li, Y. B. Wang, C. C. Xu, Y. P. Tian and H. J. Meng, Int. J. Miner. Metall. Mater., 2024, 31, 2508–2517 CAS.
- D. D. L. Chung, Mater. Chem. Phys., 2020, 255, 123587 CrossRef CAS.
- C. Y. Jiao, Z. M. Deng, P. Min, J. J. Lai, Q. Q. Gou, R. Gao, Z. Z. Yu and H. B. Zhang, Carbon, 2022, 198, 179–187 CrossRef CAS.
- Y. F. Qu, X. Li, X. Wang and H. Q. Dai, Compos. Sci. Technol., 2022, 230, 10 CrossRef.
- H. B. Liu, R. L. Fu, X. Q. Su, B. Y. Wu, H. Wang, Y. Xu and X. H. Liu, Compos. Sci. Technol., 2021, 210, 108835 CrossRef CAS.
- Y. Z. Du, X. D. Wang, X. Y. Dai, W. X. Lu, Y. S. Tang and J. Kong, J. Mater. Sci. Technol., 2022, 100, 1–11 CrossRef CAS.
- M. Sang, S. Liu, W. W. Li, S. Wang, J. Li, J. Li, S. H. Xuan and X. L. Gong, Composites, Part A, 2022, 153, 11 Search PubMed.
- Z. T. Zhuang, H. W. Chen and C. Li, ACS Nano, 2023, 17, 10628–10636 CAS.
- J. A. Hu, C. Y. Liang, J. D. Li, C. W. Lin, Y. J. Liang and D. W. Dong, ACS Appl. Mater. Interfaces, 2022, 14, 33817–33828 CAS.
- X. Y. Li, Z. F. Ma, L. L. Wang, S. K. Li, Y. H. Duan and M. H. Wu, J. Mater. Sci., 2024, 59, 1968–1988 CrossRef CAS.
- H. Chen, L. Y. Yu, Z. F. Lin, Q. Z. Zhu, P. Zhang, N. Qiao and B. Xu, J. Mater. Sci., 2020, 55, 1148–1156 CAS.
- Y. Ding, X. Lu, S. Liu, H. Wu, X. X. Sheng, X. L. Li and J. P. Qu, Composites, Part A, 2022, 163, 12 Search PubMed.
- C. R. Zhang, P. A. Zong, Z. S. Ge, Y. M. Ge, J. Zhang, Y. J. Rao, Z. G. Liu and W. Huang, Nano Energy, 2023, 118, 12 Search PubMed.
- C. C. Jiang, J. Y. Chen, X. J. Lai, H. Q. Li, X. R. Zeng, Y. N. Zhao, Q. T. Zeng, J. F. Gao, Z. Z. Wu and Y. K. Qiu, Chem. Eng. J., 2022, 434, 9 Search PubMed.
- T. S. Peng, S. C. Wang, Z. G. Xu, T. T. Tang and Y. Zhao, ACS Omega, 2022, 7, 29161–29170 CrossRef CAS.
- L. Y. Zhang, H. Y. Jin, H. X. Liao, R. Zhang, B. C. Wang, J. Y. Xiang, C. P. Mu, K. Zhai, T. Y. Xue and F. S. Wen, Int. J. Miner. Metall. Mater., 2024, 31, 1912–1921 CrossRef CAS.
- S. Q. Wu, D. M. Chen, W. B. Han, Y. S. Xie, G. D. Zhao, S. Dong, M. Y. Tan, H. Huang, S. B. Xu, G. Q. Chen, Y. Cheng and X. H. Zhang, Chem. Eng. J., 2022, 446, 137093 CrossRef CAS.
- C. B. Liang, H. Qiu, P. Song, X. T. Shi, J. Kong and J. W. Gu, Sci. Bull., 2020, 65, 616–622 CrossRef CAS PubMed.
- F. Gholamirad, J. Q. Ge, M. Sadati, G. A. Wang and N. Taheri-Qazvini, ACS Appl. Mater. Interfaces, 2022, 49158–44917, DOI:10.1021/acsami.2c14019.
- J. R. Zhao, Z. Wang, H. Wang, Y. Li and C. Q. Fang, Colloids Surf., A, 2023, 659, 130841 CrossRef CAS.
- C. B. Zhu, Y. R. Hao, H. Wu, M. N. Chen, B. Q. Quan, S. Liu, X. P. Hu, S. L. Liu, Q. H. Ji, X. Lu and J. P. Qu, Nano–Micro Lett., 2025, 17, 16 CrossRef.
- X. W. Tang, J. T. Luo, Z. W. Hu, S. J. Lu, X. Y. Liu, S. S. Li, X. Zhao, Z. H. Zhang, Q. Q. Lan, P. M. Ma, Z. C. Wang and T. X. Liu, Nano Res., 2023, 16, 1755–1763 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |



