Direct observation of the influence of chirality on the microstructure of regioregular poly(3-alkylthiophene)s at the liquid/solid interface†
Hai
Cao
a,
Marie-Paule
Van Den Eede
b,
Guy
Koeckelberghs
b,
Kunal S.
Mali
a and
Steven
De Feyter
 *a
*a
aKU Leuven-University of Leuven, Department of Chemistry, Division of Molecular Imaging and Photonics, Celestijnenlaan 200F, B-3001, Leuven, Belgium. E-mail: steven.defeyter@kuleuven.be
bKU Leuven-University of Leuven, Department of Chemistry, Division of Polymer Chemistry and Materials, Celestijnenlaan 200F, B-3001 Leuven, Belgium
First published on 14th November 2016
Abstract
The degree of order of poly(3-alkylthiophene)s on atomically flat surfaces is strongly influenced by interchain interactions. Regularly ordered, disordered and amorphous microstructures are observed for achiral, homochiral and meso poly(3-alkylthiophene)s, respectively, as revealed by scanning tunneling microscopy.
π-Conjugated conductive polymers are of interest because they are active materials in for instance organic field-effect transistors (OFET).1,2 Compared to inorganic semiconductors, polymers possess higher mechanical flexibility and better solution processability, but less crystallinity.3 The structural order of conjugated polymers, from chemical structure to micro- and mesoscopic scale organization, determines their carrier mobilities, which is an important parameter in plastic electronics.4 Numerous studies have been devoted to reveal the relation between the degree of order and electronic properties of these polymeric materials.5–8 Side chain engineering is widely utilized to improve solubility and achieve optimum charge transport by tuning the interchain interactions and π–π stacking distance.9 Different aspects such as the role of chain length,10 chain branching11 and chain branching position12 have been considered. Yet much less attention has been paid to the impact of chiral centers in the side chains.13,14 This is somehow surprising given the significant role of molecular chirality in molecular self-assembly,15–17 which motivated us to investigate the impact of chiral centers in the side chains on the microstructure of conductive polymers when adsorbed on atomically flat surfaces. On the one hand, the structural characteristics of these conjugated polymers at interfaces are relevant in relation to organic electronics. On the other hand, scanning probe microscopies may give structural information at the nanoscale when the conjugated polymers are adsorbed on a solid substrate.18
Among the many possible polymeric systems, poly(3-alkylthiophene)s (P3ATs) are an important class that exhibit unique chemical, optical and electronic properties. In addition, its combination with C60 represents the workhorse in the field of polymer solar cells.19 To achieve optimum charge transport and therewith desirable performance of P3ATs systems, morphological control is the key. The adsorption behavior of various mainly achiral20–24,27,28 and few chiral poly(3-alkylthiophene)s (P3ATs)25,26 has been studied using scanning tunneling microscopy (STM) as visualization tool. Most often on the basal plane of graphite,20–26 but rarely on metallic substrates.27,28 The latter are relevant substrates though as noble metals are widely used as electrode materials for OFET. So far the only example showing regular structures of a long chain achiral P3AT on a metallic surface was demonstrated by Liu et al., by taking advantage of an iodine adlayer to decrease the adsorbate–substrate interaction.28
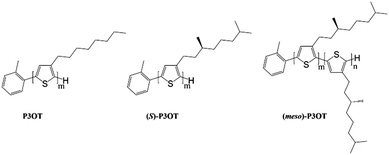
Herein we investigate the adsorption of three different P3ATs at the liquid/metal interface, aiming at bringing insight in the influence of the chirality of side chains on the degree of order of P3AT microstructures. In addition we also probe the adsorption of P3ATs/C60 mixtures. The three polythiophenes, termed as P3OT, (S)-P3OT and (meso)-P3OT according to the chirality of the lateral chains, have the same polythiophene backbone (Scheme 1).
P3OT, (S)-P3OT and (meso)-P3OT were dissolved in 1,2,4-trichlorobenzene (TCB) at low concentrations to avoid, or at least minimize, their self-aggregation in solution phase. The solutions were then deposited on Au(111) surface at ambient conditions via dropcasting and characterized by using STM with submolecular resolution.
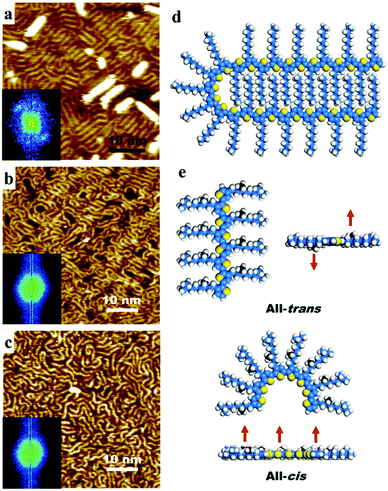
In the STM topography images (Fig. 1), the conjugated polythiophene units can be recognized as the bright sometimes curved lines. The brightest features in Fig. 1a are most likely individual polymer chains on top of the first layer. Individual alkyl chains are not visible but their presence typically leads to a less bright periphery. The occasional black features in Fig. 1b are probably the gold surface. When comparing the STM images that are typical for these compounds, a clear difference in morphology is revealed. P3OT furnishes an ordered linear structure, as shown in Fig. 1a and Fig. S1 (ESI†). Parallel aligned strands, corresponding to the aromatic backbones of P3OT lie parallel to the surface, and are mostly oriented along the three main crystallographic axes of the Au(111) surface, as confirmed by the two-dimensional fast Fourier transformation (2D-FFT) images. Individual polymer chains are either straight or curved, and there is no visible grain boundary. The distance between adjacent parallel polythiophene chains is determined by the alkyl chains. An average value of about 1.6 nm (based on 8 observations in 5 images) is measured, which is smaller than the expected distance (1.78 nm) if the octyl chains are fully stretched and interdigitated (Fig. S1, ESI†). In addition, P3OT exhibits tendency to aggregate into a multiple-layered stacking. Similar structures of P3ATs were reported on HOPG.21–26 It has been suggested that the linear part of the conjugated backbone consists of all-trans conformations of repeated thiophene units while cis conformations of thiophene units are prerequisite for chain folding. A structural model for a hairpin arrangement of P3OT is shown in Fig. 1d.
In contrast, homochiral (S)-P3OT merely forms disordered structures (Fig. 1b and Fig. S2, ESI†). Though small well-aligned regions can occasionally be observed, the surface is dominated with spaghetti-like structures. The 2D-FFT pattern exhibits a hexagon-like shape but without scattering points, indicating that the conjugated backbones are not always aligned parallel to the axes of the underlying substrate. Chain bending and folding are of frequent occurrence. Interchain distance was found between 1.1 nm and 2.1 nm.
When it comes to (meso)-P3OT, barely any organized regions can be observed. The surface is fully covered with what appears as close-packed (Fig. 1c) or randomly adsorbed (meso)-P3OT chains (Fig. S3, ESI†) with no specific orientation. Bends and kinks are frequently observed in the curved polymer chains, especially in low density areas, probably because of the different bending directions of S- and R-segments. Curved chains are widely spread but hardly cross each other, in line with the nearly hexagonal or disk shape 2D-FFT patterns that are obtained. There is quite a spread in the distance between adjacent parallel polythiophene strands, ranging from 1.0 nm to 2.4 nm.
We hypothesize that the decrease in the degree of ordering in going from P3OT to (S)-P3OT and (meso)-P3OT is due to a less efficient interdigitation of the more bulky chiral side chains. The presence of two extra methyl groups hinders interdigitation interactions. Nevertheless, there are two ways to avoid the repulsion, one is by deforming the side chains (Fig. S5–S7, ESI†) and the other one is by trans-to-cis conformational isomerization of the oligothiophene backbone so that all the methyl groups are facing the same direction. Fig. 1e depicts tentative structural models for all-trans and all-cis conformations of a homochiral segment. If the all-trans conformation is adopted on surface, the methyl groups attached to the stereocenters are facing up on one side of the backbone, but pointing down on the other side, causing repulsion with the substrate. The former way to avoid repulsion results in loose and irregular interchain interdigitations, while the latter case favours the formation of curved chains.21 The disorder of polymers is therefore a manifestation of cis–trans conformational isomerization of thiophene units.
However, STM is not very helpful to determine the exact conformation of thiophene units here. The trans/cis ratio, to a certain extent, determines the disorder. Contrariwise, the degree of organization can be used to reflect the changes in chain conformation. In order to reveal the influence of chiral side chains on chain conformation, we have performed a statistical analysis of the surface coverage of well-organized regions, in which the regular interchain interaction occurs (Fig. 2a). P3OT exhibits an excellent degree of order, and we find that 63% of the surface area is well-organized. It decreases sharply to 27% for (S)-P3OT, and 17% for (meso)-P3OT. As the disordered area is not necessary comprised of chains of all-cis conformations of thiophene units, we cannot tell the exact ratio of trans and cis conformations on the surface, but these values surely reflect the trend of trans-to-cis conformational isomerization from P3OT to (S)-P3OT to (meso)-P3OT.
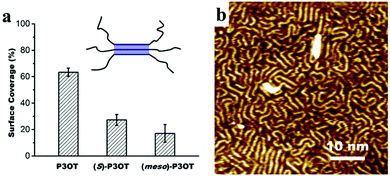 | ||
| Fig. 2 (a) Statistics of the surface coverage of well-organized regions (blue shadowed area in the upright scheme, see ESI† for detailed information) for different polymers. (b) STM image of a mixture of P3OT and (meso)-P3OT at the TCB/Au(111) interface. Concentration: P3OT, 1.4 × 10−2 mg mL−1; (meso)-P3OT, 1.5 × 10−2 mg mL−1. | ||
Despite the huge difference in the morphology of P3OT and (meso)-P3OT, these two polymers mix well and form a disordered monolayer that shows a degree of order in between that of the pure polymers (Fig. 2b and Fig. S8, ESI†). As such, the degree of order of polymers can be modulated, which may provide an opportunity for future studies on revealing the relationship between the degree of order and performance of conductive polymers.
Polythiophene is well-known as a class of p-type polymer. Its combination with an n-type material, C60, has been widely used in organic photovoltaic devices. The nature of the polymer–fullerene complexation at the interface, which is largely dependent on molecular arrangement, is critical to its performance.19 Therefore, in a further attempt, we tried to study the polythiophene–fullerene interface on gold and explored the influence of polythiophene microstructure on polymer–fullerene interaction.
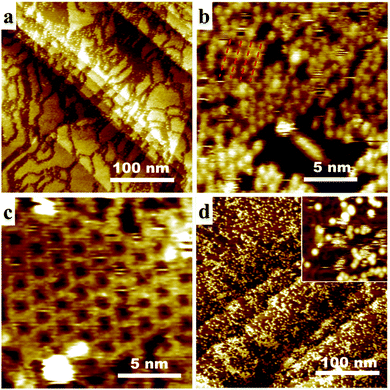
As a result of the totally different organization of P3OT and (meso)-P3OT, the mixing behavior of C60 with these two polymers exhibits distinct features, as shown in Fig. 3. Upon deposition a premixed solution of P3OT and C60 on gold surface, two layers can be clearly identified: a regular layer of P3OT with large C60 islands on top (Fig. 3a and Fig. S9, ESI†). The addition of C60 has no obvious impact on the self-assembled P3OT microstructures: well-organized monolayer can be easily identified under the C60 islands. The arrangement of C60 in the islands, however, shows no large scale periodicity (Fig. 3b). Nevertheless, the influence of the P3OT layer underneath can still be identified. From the high-resolution STM image of C60 island in Fig. 3b, we found that C60 arrays exist at some small regions (marked with red dash lines). A distance of about 1.5 nm is measured between these arrays, which happens to be the interchain distance of P3OT. In addition, small domains of a hexagonal network of C60 is also observed (Fig. 3c). Both C60 patterns are different from the spontaneously formed C60 arrays on bare gold, suggesting that the arrangement of C60 islands is being guided by the P3OT layer. The mixing of (meso)-P3OT with C60, on the other hand, results in randomly adsorbed C60 molecules on top of the (meso)-P3OT monolayer (Fig. 3c and Fig. S10, ESI†). Since (meso)-P3OTs barely assembles into well-organized structures, small aggregates instead of large regions of C60 are formed. The microstructure of the polymer layer is therefore manifested at the polymer/C60 interface.
To conclude, we have demonstrated the first example of the impact of the chiral nature of the alkyl side chains on P3ATs microstructures on gold. The chirality of alkyl side chains has a significant impact on the chain conformation of P3ATs and therewith influences the microstructures. Our results also show the possibility of using chiral side chains to tune the degree of order, which can be further applied to control the macroscopic properties of conductive polymers.
This work is supported by the Fund of Scientific Research–Flanders (FWO), Internal Funds KU Leuven and Belgian Federal Science Policy Office (IAP-7/05). This research has also received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007–2013)/ERC Grant Agreement No. 340324. H. C. is a FWO Pegasus Marie Curie Fellow.
Notes and references
- H. Koezuka, A. Tsumura and T. Ando, Synth. Met., 1987, 18, 699 CrossRef CAS.
- H. Sirringhaus, Adv. Mater., 2014, 26, 1319 CrossRef CAS PubMed.
- A. J. Heeger, Chem. Soc. Rev., 2010, 39, 2354 RSC.
- R. A. Street, Science, 2013, 341, 1072–1073 CrossRef CAS PubMed.
- J. Rivnay, S. C. B. Mannsfeld, C. E. Miller, A. Salleo and M. F. Toney, Chem. Rev., 2012, 112, 5488 CrossRef CAS PubMed.
- Y. Huang, E. J. Kramer, A. J. Heeger and G. C. Bazan, Chem. Rev., 2014, 114, 7006 CrossRef CAS PubMed.
- R. Noriega, J. Rivnay, K. Vandewal, F. P. V. Koch, N. Stingelin, P. Smith, M. F. Toney and A. Salleo, Nat. Mater., 2013, 12, 1038 CrossRef CAS PubMed.
- P. M. Beaujuge and J. M. J. Fréchet, J. Am. Chem. Soc., 2011, 133, 20009 CrossRef CAS PubMed.
- J. G. Mei and Z. N. Bao, Chem. Mater., 2014, 26, 604 CrossRef CAS.
- Y. D. Park, D. H. Kim, Y. Jang, J. H. Cho, M. Hwang, H. S. Lee, J. A. Lim and K. Cho, Org. Electron., 2006, 7, 514 CrossRef CAS.
- F. Zhang, Y. Hu, T. Schuettfort, C. A. Di, X. Gao, C. R. McNeill, L. Thomsen, S. C. Mannsfeld, W. Yuan, H. Sirringhaus and D. Zhu, J. Am. Chem. Soc., 2013, 135, 2338 CrossRef CAS PubMed.
- T. Lei, J. H. Dou and J. Pei, Adv. Mater., 2012, 24, 6457 CrossRef CAS PubMed.
- L. A. P. Kane-Maguire and G. G. Wallace, Chem. Soc. Rev., 2010, 39, 2545 RSC.
- M. Verswyvel and G. Koeckelberghs, Polym. Chem., 2012, 3, 3203 RSC.
- M. H. Liu, L. Zhang and T. Y. Wang, Chem. Rev., 2015, 115, 7304 CrossRef CAS PubMed.
- D. B. Amabilino, Chem. Soc. Rev., 2009, 38, 669 RSC.
- J. A. A. W. Elemans, I. De Cat, H. Xu and S. De Feyter, Chem. Soc. Rev., 2009, 38, 722 RSC.
- S. S. Sheiko and M. Möller, Chem. Rev., 2001, 101, 4099 CrossRef CAS PubMed.
- B. C. Thompson and J. M. J. Frechet, Angew. Chem., Int. Ed., 2008, 47, 58 CrossRef CAS PubMed.
- L. R. Xu, L. Yang and S. B. Lei, Nanoscale, 2012, 4, 4399 RSC.
- E. Mena-Osteritz, A. Meyer, B. M. W. Langeveld-Voss, R. A. J. Janssen, E. W. Meijer and P. Bäuerle, Angew. Chem., Int. Ed., 2000, 39, 2679 CrossRef PubMed.
- R. Payerne, M. Brun, P. Rannou, R. Baptist and B. Grévin, Synth. Met., 2004, 146, 311 CrossRef CAS.
- B. Grévin, P. Rannou, R. Payerne, A. Pron and J. P. Travers, J. Chem. Phys., 2003, 118, 7097 CrossRef.
- P. Willot, J. Teyssandier, W. Dujardin, J. Adisoejoso, S. De Feyter, D. Moerman, P. Leclère, R. Lazzaroni and G. Koeckelberghs, RSC Adv., 2015, 5, 8721 RSC.
- G. Koeckelberghs, C. Samyn, A. Miura, S. De Feyter, F. De Schryver, S. Sioncke, T. Verbiest, G. de Schaetzen and A. Persoons, Adv. Mater., 2005, 17, 708 CrossRef CAS.
- H. Peeters, P. Couturon, S. Vandeleene, D. Moerman, P. Leclère, R. Lazzaroni, I. De Cat, S. De Feyter and G. Koeckelberghs, RSC Adv., 2013, 3, 3342 RSC.
- H. Kasai, H. Tanaka, S. Okada, H. Oikawa, T. Kawai and H. Nakanishi, Chem. Lett., 2002, 696 CrossRef CAS.
- Y. F. Liu, K. Krug and Y. L. Lee, Nanoscale, 2013, 5, 7936 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, additional experimental data and synthesis of P3OTs. See DOI: 10.1039/c6cc08074j |
| This journal is © The Royal Society of Chemistry 2017 |

![[M with combining overline]](https://www.rsc.org/images/entities/i_char_004d_0305.gif)