Boryl substituted group 13 metallylenes: complexes with an iron carbonyl fragment†
Deepak
Dange
a,
Christian P.
Sindlinger
b,
Simon
Aldridge
b and
Cameron
Jones
*a
aMonash Centre for Catalysis, School of Chemistry, PO Box 23, Monash University, VIC 3800, Australia. E-mail: cameron.jones@monash.edu
bDepartment of Chemistry, Inorganic Chemistry Laboratory, South Parks Road, Oxford, OX1 3QR, UK
First published on 3rd November 2016
Abstract
The first examples of boryl substituted aluminylene and gallylene complexes, [(DAB)B(THF)Al{Fe(CO)3(μ-CO)}]2 and [(DAB)BGa{μ-Fe(CO)4}]2 (DAB = {(C6H3Pri2-2,6)NCH}2) have been prepared by reduction of MX2(THF){B(DAB)} (M = Al or Ga, X = Cl or Br) with K2[Fe(CO)4]. Spectroscopic and crystallographic analyses of the compounds show them to be structurally distinct dimers, the latter of which possesses a close Ga⋯Ga separation that computational analyses reveal has negligible bonding character.
The chemistry of group 13 metallylenes, RM: (R = alkyl, aryl, silyl, amido etc.), is now well developed, and numerous complexes of these carbene analogues, involving metals from across the periodic table, have been prepared and studied in depth.1,2 While monomeric, uncomplexed examples of such systems are known for all the group 13 metals, these typically incorporate bi- or polydentate anionic ligands (e.g. β-diketiminate (Nacnac), guanidinate or tris(pyrazolyl)borate) at the metal(I) centre.3–5 With that said, a handful of one-coordinate metallylenes (M = Ga, In or Tl) have been kinetically stabilised by extremely bulky, mono-dentate terphenyl or amide ligands, although no one-coordinate aluminylenes have yet been reported.6 Given that two-coordinate aluminylenes (e.g. (Nacnac)Al:) are emerging as powerful reagents for the activation of an array of catalytically relevant bonds (e.g. E–H (E = H, N, B, Si, P) and C–F linkages),7 it would be of considerable interest to access one-coordinate aluminylenes, which should be markedly more electrophilic, and thus more reactive, than their two-coordinate counterparts.
In recent years, we have had significant success in stabilising a variety of low oxidation state/low-coordinate groups 13–15 complexes (e.g.I–III,8Fig. 1), by utilising Yamashita's reagent, (THF)2Li{B(DAB)} (DAB = {(C6H3Pri2-2,6)NCH}2),9 as the source of the boryl ligand in those compounds. In light of the imposing steric profile of this ligand (cf. Power's terphenyls10), its high nucleophilicity, and the fact that a range of aluminium complexes incorporating it have recently come forward,11 we postulated that monomeric boryl-aluminylenes and their heavier analogues, viz. (DAB)BM: (M = group 13 metal), might be isolable species. Although our efforts to achieve this goal have so far proved fruitless, we have prepared the first examples of metal complexes derived from these systems, as detailed below.
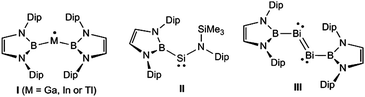 | ||
| Fig. 1 Examples of low oxidation state group 13–15 complexes stabilised by Yamashita's heterocyclic boryl ligand (Dip = 2,6-diisopropylphenyl). | ||
As logical precursors to the target compounds, the boryl aluminium and gallium halides, 1–3 (Scheme 1) were prepared in moderate to good yields via 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reactions of (THF)2Li{B(DAB)} with group 13 metal trihalides in toluene. Attempts to remove the THF of coordination from the complexes by heating them under vacuum were not successful. This is in contrast to the related gallium alkyl complex, GaMe2(THF){B(DAB)}, which does undergo a desolvation process in vacuo.11b It is noteworthy that indium analogues of 1–3 could not be prepared in our hands, as even 1
1 reactions of (THF)2Li{B(DAB)} with group 13 metal trihalides in toluene. Attempts to remove the THF of coordination from the complexes by heating them under vacuum were not successful. This is in contrast to the related gallium alkyl complex, GaMe2(THF){B(DAB)}, which does undergo a desolvation process in vacuo.11b It is noteworthy that indium analogues of 1–3 could not be prepared in our hands, as even 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reactions of (THF)2Li{B(DAB)} with indium halides led to bis(boryl) complexes, InX{B(DAB)}2 (X = Cl8a or Br, see ESI† for full details), and unreacted InX3. Complexes 1–3 represent the first examples of crystallographically characterised boryl group 13 metal dihalides (see ESI† for details), and all are monomeric, possessing distorted tetrahedral metal geometries, as is the case for the isostructural methyl analogues, MMe2(THF){B(DAB)} (M = Al or Ga).11c,12 In line with the planar three-coordinate geometries for the boron centres of the complexes are their 11B{1H} NMR spectra, all of which exhibit broad resonances between δ 27–28 ppm (cf. δ 32.0 ppm for GaMe2(THF){B(DAB)}12).
1 reactions of (THF)2Li{B(DAB)} with indium halides led to bis(boryl) complexes, InX{B(DAB)}2 (X = Cl8a or Br, see ESI† for full details), and unreacted InX3. Complexes 1–3 represent the first examples of crystallographically characterised boryl group 13 metal dihalides (see ESI† for details), and all are monomeric, possessing distorted tetrahedral metal geometries, as is the case for the isostructural methyl analogues, MMe2(THF){B(DAB)} (M = Al or Ga).11c,12 In line with the planar three-coordinate geometries for the boron centres of the complexes are their 11B{1H} NMR spectra, all of which exhibit broad resonances between δ 27–28 ppm (cf. δ 32.0 ppm for GaMe2(THF){B(DAB)}12).
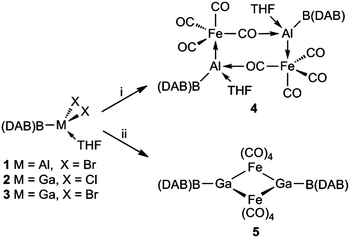 | ||
| Scheme 1 . Preparation of compounds 4 and 5; (i) M = Al, K2[Fe(CO)4], THF, –KBr; (ii) M = Ga, K2[Fe(CO)4], THF, –KCl or –KBr. | ||
With 1–3 in hand, their reductions with a variety of reducing agents (e.g. magnesium(I) dimers,13 KC8 and K(naphthalenide)) were carried out. However, in all cases complex product mixtures resulted, the 11B{1H} NMR spectra of which revealed to include (DAB)BH and (DAB)BX as significant components. It is noteworthy that the apparent instability of the boryl metallylenes, (DAB)BM:, has previously been suggested from the reactions of (THF)2Li{B(DAB)} with heavier group 13 metal(I) halides or amides. These yielded the radical species, I, which were proposed to form via disproportionation of putative metal(I) boryl intermediates.8a
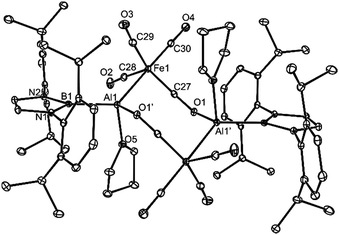
A common strategy used to stabilise group 13 metallylenes (and borylenes) has been to coordinate them to transition metal carbonyl fragments, through their metal-centred lone pair.14 This tactic was adopted in the current study by carrying out reactions of 1–3 with K2[Fe(CO)4] in THF. These led to the formation of boryl metallyne complexes, 4 and 5, in low isolated crystalline yields (Scheme 1). In these reactions the ferrate starting material acts as a reducing agent, in a similar fashion to that previously reported for the preparations of both aluminylene and gallylene–iron carbonyl complexes.14 Although the dimeric structures of 4 and 5 are ostensibly similar, they differ in the fact that the μ-Fe(CO)4 fragments of 4 bridge both Al centres through intermolecular isocarbonyl O–Al bonds, giving rise to an eight membered Fe2Al2C2O2 ring, while the iron centres of 5 symmetrically bridge the galyllene fragments, yielding a four-membered Fe2Ga2 ring. These differences possibly occur due to the higher Lewis acidity of Al over Ga, which leads to THF ligation of the Al centres in 4, thereby suppressing the formation of Al–(μ-Fe)–Al bridges.
Both 4 and 5 are thermally stable in the solid state, and do not show any signs of decomposition in toluene solutions over 1 week. 1H and 13C{1H} NMR spectra are consistent with the compounds retaining their solid state structures in solution, while the 11B{1H} NMR signals (4: δ 30.5 ppm; 5: δ 27.0 ppm) are at fields reminiscent of three-coordinate boron systems. The infrared spectrum of 4 exhibits strong CO stretching bands that were assigned to terminal (ν = 2005, 1937, 1902 cm−1) and bridging (ν = 1653 cm−1) carbonyl ligands. The latter band is at a wavenumber characteristic of bridging isocarbonyl units O-ligated to aluminium centres, (e.g. ν = 1650 cm−1 for {CpW(CO)(μ-CO)2AlMe2}215). In contrast, the infrared spectrum of 5 highlights a terminal CO stretching band pattern (ν = 2036, 1965, 1938, 1920(sh) cm−1) similar to those reported for closely related iron bridged systems (e.g. [(THF)ClGa{μ-Fe(CO)4}]216).
The X-ray crystal structures of 4 and 5 were determined and their molecular structures are depicted in Fig. 2 and 3. As mentioned above, the monomeric units of compound 4 associate through Al–(μ-CO)–Fe isocarbonyl bridges, yielding eight-membered heterocycles. Similar bridging motifs have been observed previously in main group metal-first row transition metal carbonyl systems.15,17 As in those compounds, there is a significant shortening and lengthening of the bridging Fe–C (1.687(3) Å) and C–O bonds (1.232(3) Å), respectively, relative to the means for those bonds in the terminal FeCO fragments (1.782 Å and 1.162 Å). This is consistent with increased Fe(dπ) → CO(π*) back-bonding in the bridging carbonyl unit. The geometry of the Al centres of 4 is distorted tetrahedral, while that of the Fe centres is trigonal bipyramidal. Both unique Al–O bonds, and the Al–Fe linkage, are in the normal ranges for such interactions,18 while the Al–B separation (2.128(3) Å) is similar to all other such bonds involving the same boryl ligand (2.119–2.167 Å, 6 examples18).
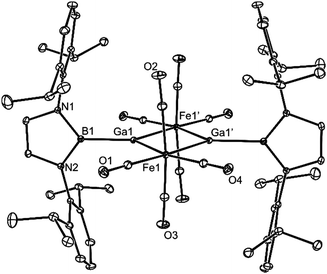
Compound 5 is also dimeric, but unlike 4, its monomeric units associate through bridging octahedral Fe centres, generating a planar Fe2Ga2 four-membered ring, in which the Ga atoms have distorted trigonal planar geometries. Several structurally related compounds have been previously reported (e.g. [(THF)ClGa{μ-Fe(CO)4}]216), though the Ga centres in each are four-coordinate. The central rings of the boryl substituents in 5 are close to orthogonal to the Fe2Ga2 plane, and coordinate the iron centres with B–Ga bond lengths (2.067(2) Å) that are within the narrow range reported for (DAB)B–Ga bonds (2.045–2.121 Å, 11 examples18). Similarly, the Fe–Ga bond lengths in 5 (2.472 Å, mean) are unexceptional (cf. 2.472 Å for [(THF)ClGa{μ-Fe(CO)4}]216), while the trans-annular Ga⋯Ga separation (2.9239(4) Å, cf. 2.881 Å in [(THF)ClGa{μ-Fe(CO)4}]216) is short enough to suggest a possible metal–metal interaction in that compound.
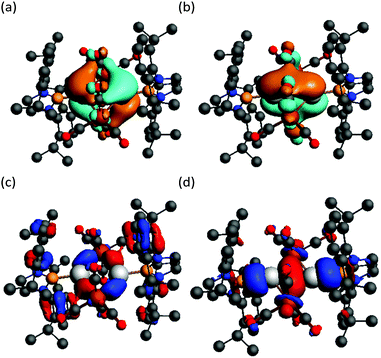
In order to investigate this possibility, DFT calculations (BP86-D3(BJ)/TZ2P) were carried out on 5 in the gas phase (i.e.5′). The geometry of 5′ optimised to be similar to that of 5, but with a slightly shorter Ga⋯Ga separation (2.865 Å). An orbital analysis of 5′ revealed a number of filled MO's that exhibit significant Ga–Fe σ-bonding character (e.g. HOMO−6 and HOMO−11, Fig. 4), while there is what appears to be a small degree of trans-annular Ga–Ga σ-bonding derived from overlap of the two B–Ga σ-bonds (HOMO−21).19 Despite this, a Bader analysis of 5′ did not show a bond critical point between the two Ga centres, so any interaction is likely to be negligible. Character of the empty p-orbitals at the Ga centres is displayed in the LUMO and LUMO+1, which show a small degree of Ga–Ga π-bonding and π*-antibonding character, respectively. In consideration of this, and in an attempt to populate the LUMO of 5, the compound was treated with two equivalents of potassium naphthalenide in THF. However, this led only to an intractable mixture of products.
In summary, the first examples of boryl substituted aluminylene and gallylene complexes have been prepared and analysed by spectroscopic, crystallographic and computational techniques. Both prepared compounds are dimers, though they are structurally distinct in that the aluminylene adduct dimerises through Al–(μ-CO)–Fe isocarbonyl bridges, while the gallylene complex associates through Ga–{μ-Fe(CO)4}–Ga linkages. The latter gives rise to a relatively short Ga⋯Ga separation within the generated Fe2Ga2 four-membered ring, though computational studies do not point to any significant bonding character between the two gallium centres. We continue to explore the ability of the nucleophilic boryl ligand, –B(DAB), to stabilise low oxidation state/low coordinate main group systems, and will report on our efforts in this direction in the due course.
CJ and SA gratefully acknowledge financial support from the Australian Research Council, while CJ acknowledges support from the U.S. Air Force Asian Office of Aerospace Research and Development (FA2386-14-1-4043). CS acknowledges support of a Feodor Lynen Research fellowship from the Alexander von Humboldt Stiftung. Part of this research was undertaken on the MX1 beamline at the Australian Synchrotron, Victoria, Australia.
Notes and references
- C. Jones and A. Stasch, in The Group 13 Metals Aluminium, Gallium, Indium and Thallium; Chemical Patterns and Peculiarities, ed. S. Aldridge, A. J. Downs, Wiley, Sussex, 2011, ch. 5, pp. 285–341 Search PubMed.
- Selected reviews (a) M. Asay, C. Jones and M. Driess, Chem. Rev., 2011, 111, 354 CrossRef CAS PubMed; (b) S. Gonzalez-Gallardo, G. Prabusankar, T. Cadenbach, C. Gemel, M. von Hopffgarten, G. Frenking and R. A. Fischer, Struct. Bonding, 2010, 136, 147 CrossRef CAS; (c) C. Jones, Coord. Chem. Rev., 2010, 254, 1273 CrossRef CAS; (d) R. J. Baker and C. Jones, Coord. Chem. Rev., 2005, 249, 1857 CrossRef CAS; (e) C. Gemel, T. Steinke, M. Cokoja, A. Kempter and R. A. Fischer, Eur. J. Inorg. Chem., 2004, 4161 CrossRef CAS; (f) H. W. Roesky, Inorg. Chem., 2004, 43, 7284 CrossRef CAS PubMed.
- Selected examples of AlI and GaI Nacnac complexes: (a) D. Dange, S. L. Choong, C. Schenk, A. Stasch and C. Jones, Dalton Trans., 2012, 41, 9304 RSC; (b) X. Li, X. Cheng, H. Song and C. Cui, Organometallics, 2007, 26, 1039 CrossRef CAS; (c) C. Cui, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, H. Hao and F. Cimpoesu, Angew. Chem., Int. Ed., 2000, 39, 4274 CrossRef CAS; (d) N. J. Hardman, B. E. Eichler and P. P. Power, Chem. Commun., 2000, 1991 RSC.
- Guanidinato GaI complexes: (a) J. Overgaard, C. Jones, D. Dange and J. A. Platts, Inorg. Chem., 2011, 50, 8418 CrossRef CAS PubMed; (b) G. Jin, C. Jones, P. C. Junk, A. Stasch and W. D. Woodul, New J. Chem., 2008, 32, 835 RSC; (c) C. Jones, P. C. Junk, J. A. Platts and A. Stasch, J. Am. Chem. Soc., 2006, 128, 2206 CrossRef CAS PubMed.
- A GaI tris(pyrazoly)lborate complex: M. C. Kuchta, J. B. Bonanno and G. Parkin, J. Am. Chem. Soc., 1996, 118, 10914 CrossRef CAS.
- Selected examples of one-coordinate group 13 metallylenes: (a) D. Dange, J. Li, C. Schenk, H. Schnöckel and C. Jones, Inorg. Chem., 2012, 51, 13050 CrossRef CAS PubMed; (b) Z. Zhu, R. C. Fischer, B. D. Ellis, E. Rivard, W. A. Merrill, M. M. Olmstead, P. P. Power, J. D. Guo, S. Nagase and L. Pu, Chem. – Eur. J., 2009, 15, 5263 CrossRef CAS PubMed; (c) R. J. Wright, M. Brynda, J. C. Fettinger, A. R. Betzer and P. P. Power, J. Am. Chem. Soc., 2006, 128, 12498 CrossRef CAS PubMed; (d) S. T. Haubrich and P. P. Power, J. Am. Chem. Soc., 1998, 120, 2202 CrossRef CAS.
- See for example: (a) M. R. Crimmin, M. J. Butler and A. J. P. White, Chem. Commun., 2015, 51, 15994 RSC; (b) T. Chu, I. Korobkov and G. I. Nikonov, J. Am. Chem. Soc., 2014, 136, 9195 CrossRef CAS PubMed.
- (a) A. V. Protchenko, D. Dange, J. R. Harmer, C. Y. Tang, A. D. Schwarz, M. J. Kelly, N. Phillips, R. Tirfoin, K. H. Birjkumar, C. Jones, N. Kaltsoyannis, P. Mountford and S. Aldridge, Nat. Chem., 2014, 6, 315 CrossRef CAS PubMed; (b) A. V. Protchenko, K. H. Birjkumar, H. Krishna, D. Dange, A. D. Schwarz, D. Vidovic, C. Jones, N. Kaltsoyannis, P. Mountford and S. Aldridge, J. Am. Chem. Soc., 2012, 134, 6500 CrossRef CAS PubMed; (c) D. Dange, A. Davey, J. A. B. Abdalla, S. Aldridge and C. Jones, Chem. Commun., 2015, 51, 7128 RSC.
- Y. Segawa, M. Yamashita and K. Nozaki, Science, 2006, 314, 113 CrossRef CAS PubMed.
- E. Rivard and P. P. Power, Inorg. Chem., 2007, 46, 10047 CrossRef CAS PubMed.
- (a) N. Dettenrieder, C. O. Hollfelder, L. N. Jende, C. Maichle-Mossmer and R. Anwander, Organometallics, 2014, 33, 1528 CrossRef CAS; (b) N. Dettenrieder, C. Schadle, C. Maichle-Mossmer and R. Anwander, Dalton Trans., 2014, 43, 15760 RSC; (c) N. Dettenrieder, H. M. Dietrich, C. Schadle, C. Maichle-Mossmer, K. W. Tornroos and R. Anwander, Angew. Chem., Int. Ed., 2012, 51, 4461 CrossRef CAS PubMed.
- N. Dettenrieder, C. Schaedle, C. Maichle-Moessmer, P. Sirsch and R. Anwander, J. Am. Chem. Soc., 2014, 136, 886 CrossRef CAS PubMed.
- Reviews: (a) C. Jones and A. Stasch, Top. Organomet. Chem., 2013, 45, 73 CrossRef CAS; (b) A. Stasch and C. Jones, Dalton Trans., 2011, 40, 5659 RSC.
- See for example: (a) G. Tan, T. Szilvasi, S. Inoue, B. Blom and M. Driess, J. Am. Chem. Soc., 2014, 136, 9732 CrossRef CAS PubMed; (b) J. Weiss., D. Stetzkamp, B. Nuber, R. A. Fischer, C. Boehme and G. Frenking, Angew. Chem., Int. Ed. Engl., 1997, 36, 70 CrossRef CAS; (c) A. H. Cowley, V. Lomeli and A. Voight, J. Am. Chem. Soc., 1998, 120, 6401 CrossRef CAS; (d) H. Braunschweig, Q. Ye and K. Radacki, Chem. Commun., 2012, 48, 2701 RSC.
- A. J. Conway, G. J. Gainsford, R. R. Schrieke and J. D. Smith, J. Chem. Soc., Dalton Trans., 1975, 2499 RSC.
- T. Adamczyk, G.-M. Li, G. Linti, H. Pritzkow, A. Seifert and T. Zessin, Eur. J. Inorg. Chem., 2011, 3480 CrossRef CAS.
- See for example: (a) M. P. Blake, N. Kaltsoyannis and P. Mountford, J. Am. Chem. Soc., 2015, 137, 12352 CrossRef CAS PubMed; (b) J. A. B. Abdalla, I. M. Riddlestone, J. Turner, P. A. Kaufman, R. Tirfoin, N. Philips and S. Aldridge, Chem. – Eur. J., 2014, 20, 17624 CrossRef CAS PubMed.
- As determined from a survey of the Cambridge Crystallographic Database, October, 2016.
- N.B. Both the HOMO and HOMO−1 of 5′ largely encompass π-bonding interactions within the boryl heterocycles.
Footnote |
| † Electronic supplementary information (ESI) available: Full details and references for synthetic, crystallographic and computational studies. CCDC 1510775–1510780. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc08449d |
| This journal is © The Royal Society of Chemistry 2017 |