A concise asymmetric total synthesis for structure elucidation of 5,6-secoiridoid from Incarvillea argute†
Jian-Jun Fu‡
a,
Zhi-Qian Liu‡b,
Hui-Zi Jinc,
Shou-De Zhanga,
Qing-Yan Sun*d and
Wei-Dong Zhang*abd
aShanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, Shanghai 200237, PR China. E-mail: wdzhangy@hotmail.com; Fax: +86-21-81871244; Tel: +86-21-81871244
bSchool of Pharmacy, Second Military Medical University, Shanghai 200433, PR China
cSchool of Pharmacy, Shanghai Jiao Tong University, Shanghai 200240, PR China
dShanghai Institute of Pharmaceutical Industry, Shanghai 200040, PR China. E-mail: sqy_2000@163.com; Fax: +86-21-20572000-2028; Tel: +86-21-20572000-2028
First published on 21st July 2016
Abstract
A phytochemical investigation of the roots of Incarvillea arguta led to the isolation of secoarguterin (1), which is the first example of 5,6-secoiridoid. Its structure and absolute configuration was elucidated by extensive spectral analysis and total synthesis. Compound 1 showed moderate inhibition against human colon cancer cells HCT116 with an IC50 value of 28.72 μM.
The iridoids comprise a large family of distinctive bicyclic monoterpenes that possess a wide range of pharmacological activities, including anticancer, anti-inflammatory, antifungal and antibacterial activities.1 A bicyclic H-5/H-9β, β-cis-fused cyclopentanopyran ring system is the most common structural feature of these compounds, however several enantiomeric iridoids also exist in nature.2 Secoiridoids are monoterpenoids based on the 7,8-secocyclopenta[c]-pyranoid skeleton which are possibly derived in plants from iridoids (Scheme 1).3 7,8-Secoiridoid is well-known as the unique carbon skeleton of secoiridoids listed in a large number of references and phytochemistry textbooks.1–5 Over the past years, numerous imaginative routes have been delineated for the asymmetric synthesis and biosynthesis of iridoids and secoiridoids, many of which are enlightened by a wide range of biological activities.1a,4a,5
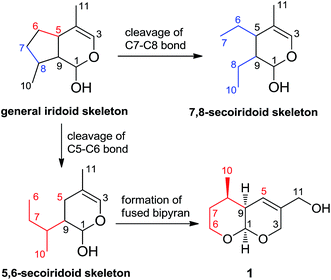 | ||
| Scheme 1 The general skeleton of 7,8-secoiridoid and 5,6-secoiridoid derived from general iridoid skeleton, and the structure of secoarguterin 1. | ||
The genus Incarvillea is notable for being a temperate and herbaceous genus of the primarily tropical and woody family Bignoniaceae. It is composed of 16 species, which mainly occurred in the Himalayas and southwest China.6 Incarvillea arguta has been widely used as a herbal medicine of Yi nationality (known as “Wabuyou”) to treat hepatitis and diarrhea in China.7 Recently, we have reported the isolation and structure elucidation of several new compounds from the roots of I. arguta.8 Further investigation of this plant led us to isolate secoarguterin (1), a new class of secoiridoids with an unprecedented carbon skeleton, which is the first example of 5,6-secoiridoid. Different from the reported 7,8-secoiridoids, compound 1 was derived by cleavage of cyclopentane ring of iridoid at C5–C6 bond, and formation of two cis-pyran ring system fused at C1–C9 bond (Scheme 1). The fused bipyran skeleton of 1 was constructed via the hetero-Diels–Alder cycloaddition. This concise strategy provided further confirmation of the absolute configuration and the relative configuration of 1 using single-crystal X-ray diffraction as well as other chemical methods. Herein, we have described the structural elucidation, total synthesis and in vitro antitumor activity of secoarguterin (1).
Secoarguterin§ (1) was obtained as yellow oil. The molecular formula was determined as C10H16O3 by HRESIMS at m/z 207.0999 [M + Na]+ (calcd for C10H16O3Na, 207.0992) in conjunction with the 13C NMR spectrum, which required three degrees of unsaturation. Analysis of the 1H NMR data of 1 indicated the presence of an olefinic proton at δH 5.88 (1H, d, J = 3.9 Hz), one oxygenated methylene singlet at 4.05 (2H), four oxygenated methylene protons at δH 3.97 (1H, t, J = 11.0 Hz), 3.73 (1H, m), 4.36 (1H, J = 16 Hz), and 4.41 (1H, J = 16 Hz), together with one methyl doublet at δH 1.02 (3H, d, J = 6.1 Hz). Four additional hydrogens were observed at δH 1.39, 1.54, 1.76, and 1.80. The 13C NMR and DEPT spectra (Table 1) established that 1 possessed one methyl (δC 19.3), four methylenes (δC 33.1, 61.7, 63.6, and 66.7), four methines (δC 32.0, 41.7, 95.3, and 123.5), one quaternary carbon (δC 136.9), that is, a total of 15 protons attached 10 carbons. One more proton was inferred from IR spectra showing that a 3410 cm−1 band was attributed to a hydroxyl group.
| No. | δC mult.b,c | δH mult.a (J in Hz) |
|---|---|---|
| a Recorded at 500 MHz (in CDCl3; δ in ppm).b Recorded at 125 MHz.c Multiplicities inferred from by DEPT and HMQC experiments. | ||
| 1 | 95.3d | 4.88 (d, 3.0) |
| 3a | 66.7t | 4.41 (d, 16.0) |
| 3b | 4.36 (d, 16.0) | |
| 4 | 136.9s | |
| 5 | 123.5d | 5.88 (d, 3.9) |
| 6a | 61.7t | 3.97 (d, 11.0) |
| 6b | 3.73 (m) | |
| 7a | 33.1t | 1.54 (d, 13.1) |
| 7b | 1.39 (m) | |
| 8 | 32.0d | 1.80 (m) |
| 9 | 41.7d | 1.76 (m) |
| 10 | 19.3q | 1.02 (d, 6.1) |
| 11 | 63.6t | 4.05 (s) |
The two-dimensional NMR experiments (HMQC, 1H–1H COSY, HMBC, and NOESY measurements) were thus performed to furnish the skeleton of 1. HMQC data allowed the assignment of all the protons to their bonding carbons. Two hydrogenated pyran subunits (C-1, C-3 to C-5; C-9) and (C1, C-6 to C-9) (Fig. 1), drawn with bold bonds, were established on the basis of 1H–1H COSY data. The HMBC correlations (Fig. 1) enabled assembly of the tetrahydropyran and the dihydropyran with the two tertiary carbons (C-1 and C-9) and other functionalities. In the HMBC spectra of 1 (Fig. 1), The HMBC correlations of H3-10 to C-7, 8, and 9 implied that the methyl C-10 was attached to methine C-8. The hydroxymethyl group C-11 was assigned to the quaternary carbon C-4 due to the HMBC correlations of H2-11 with the tri-substituted olefinic bond (C-4/C-5), and another oxygenated methylene C-3. Key correlations of a ketal resonance at δC 95.3 (C-1) with H2-3 and H2-6 and correlations from H-1 to two oxygenated methylenes C-3 and C-6 established the linkage of the two hydrogenated pyrans. The above analysis suggested that 1 was a mono-substituted tetrahydropyran fused with another mono-substituted dihyropyran at C-1 and C-9. The planar structure of secoarguterin (1) was, therefore, determined as depicted in Scheme 1.
The relative stereochemistry of 1 was determined by a NOESY experiment (Fig. 2). The key correlations of H-1/H-9, H-1/H-8, and H-8/H-9 indicated that H-1, H-8, and H-9 were co-facial, and were arbitrarily assigned as the α-configuration. Subsequently, the Me-10 was assigned to β-oriented on the basis of the absence of NOESY correlations of Me-10/H-1. The small coupling constant (J = 3.0 Hz) between H-1 and H-9 indicated a cis-configuration for these protons, which is consistent with NOESY correlations.
To identify the stereochemistry of compound 1, compounds 1 and 9 were synthesized for the first time according to the route shown in Scheme 2. The single crystal X-ray structure of 9 was obtained as shown in Fig. 3. Starting material unsaturated ester 2 and enol ether 3 was prepared according to the reported methods.
The unsaturated ester 2 (ref. 9) was treated with enol ether 3 (ref. 10) in the presence of [Cu((S,S)-t-Bu-box)](SbF6)2, then hetero Diels–Alder reaction11 selectively occurred to give the hetero [4 + 2] adduct 4 (dr = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The direct transformation 4 to compound 5 was achieved by using DIBAL-H at −78 °C. Decarbonylation product 6 was obtained by using RhCl(PPh3)3 catalyst.12 Cleavage of the benzyl group and reducing vinyl by hydrogenolysis, followed by Swern's oxidation10 afforded compound 7. Sequential treatment of 7 with KHDMS and PhNTf2 afforded enol triflate.13 Palladium-catalyzed methoxycarbonylation of enol triflate provided ester 8.14 Reduction of 8 with an excess of diisobutylaluminum hydride (DIBAL-H) gave the target molecule 1. Finally, treatment of 7 with 2,4-dinitrophenylhydrazine provided compound 9.
1). The direct transformation 4 to compound 5 was achieved by using DIBAL-H at −78 °C. Decarbonylation product 6 was obtained by using RhCl(PPh3)3 catalyst.12 Cleavage of the benzyl group and reducing vinyl by hydrogenolysis, followed by Swern's oxidation10 afforded compound 7. Sequential treatment of 7 with KHDMS and PhNTf2 afforded enol triflate.13 Palladium-catalyzed methoxycarbonylation of enol triflate provided ester 8.14 Reduction of 8 with an excess of diisobutylaluminum hydride (DIBAL-H) gave the target molecule 1. Finally, treatment of 7 with 2,4-dinitrophenylhydrazine provided compound 9.
The absolute configuration of target molecule 1 was established as 1R,8R,9S by analysis of the single crystal X-ray diffraction (Cu-Kα) of the derived compound 9 (Fig. 3).
The antitumor activity of secoarguterin (1) against four tumor cell lines (A549, HCT116, BT474, and K562), were determined by the MTT assay,15 with doxorubicin as a positive control. It displayed significant dose-dependent inhibition against human colon cancer cells HCT116 with an IC50 value of 28.72 μM. At the highest concentration (100 μM) tested, this compound did not show any cytotoxicity to A549, BT474, and K562 cell lines, almost consistent with the other iridoid derivatives.16
Acknowledgements
The work was supported by Professor of Chang Jiang Scholars Program, National Natural Science Foundation of China (81520108030, 81573318, 81373301, 1302658), Shanghai Leading Academic Discipline Project (B906), Shanghai Engineering Research Center for the Preparation of Bioactive Natural Products (10DZ2251300), and the Fundamental Research Funds for the Central Universities of China.Notes and references
- (a) F. Geu-Flores, N. H. Sherden, V. Courdavault, V. Burlat, W. S. Glenn, C. Wu, E. Nims, Y. H. Cui and S. E. O'Connor, Nature, 2012, 492, 138–142 CrossRef CAS PubMed; (b) B. Dinda, D. R. Chowdhury and B. C. Mohanta, Chem. Pharm. Bull., 2009, 57, 765–796 CrossRef CAS PubMed; (c) B. Dinda, S. Debnath and R. Banik, Chem. Pharm. Bull., 2011, 59, 803–833 CrossRef CAS PubMed.
- B. Dinda, S. Debnath and Y. Harigaya, Chem. Pharm. Bull., 2007, 55, 159–222 CrossRef CAS PubMed.
- (a) H. K. Obied, P. D. Prenzler, D. Ryan, M. Servili, A. Taticchi, S. Esposto and K. Robards, Nat. Prod. Rep., 2008, 25, 1167–1179 RSC; (b) B. Dinda, S. Debnath and Y. Harigaya, Chem. Pharm. Bull., 2007, 55, 689–728 CrossRef CAS PubMed.
- Selected examples: (a) K. Miettinen, L. Dong, N. Navrot, T. Schneider, V. Burlat, J. Pollier, L. Woittiez, S. Krol, R. Lugan, T. Ilc, R. Verpoorte, K. M. Oksman-Caldentey, E. Martinoia, H. Bouwmeester, A. Goossens, J. Memelink and D. Werck-Reichhart, Nat. Commun., 2014, 5, 3606, DOI:10.1038/ncomms4606; (b) K. Konno, C. Hirayama, H. Yasui and M. Nakamura, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 9159–9164 CrossRef CAS PubMed; (c) P. M. Dewick, Medicinal Natural Products: a biosynthetic approach, John Wiley & Sons Ltd, Chichester, 2nd edn, 2002, ch. 5, pp. 187–191 Search PubMed; (d) R. S. Xu, Y. Ye and W. M. Zhao, Natural Products Chemistry, Science Press, Beijing, 2nd edn, 2004, ch. 5, pp. 183–190 Search PubMed.
- Selected examples: (a) P. Piccinini, G. Vidari and G. Zanoni, J. Am. Chem. Soc., 2004, 126, 5088–5089 CrossRef CAS PubMed; (b) H. M. Liu, F. Y. Zhang and D. P. Zou, Chem. Commun., 2003, 2044–2045 RSC; (c) R. A. Jones and M. J. Krische, Org. Lett., 2009, 11, 1849–1851 CrossRef CAS PubMed; (d) S. Hanessian, E. Mainetti and F. Lecomte, Org. Lett., 2006, 8, 4047–4049 CrossRef CAS PubMed; (e) H. Yamamoto, N. Katano, A. Ooi and K. Inoue, Phytochemistry, 2000, 53, 7–12 CrossRef CAS PubMed.
- (a) S. T. Chen, K. Y. Guan, Z. K. Zhou, R. Olmstead and Q. Cronk, Am. J. Bot., 2005, 92, 625–633 CrossRef CAS PubMed; (b) S. T. Chen, K. Y. Guan, Z. K. Zhou and T. Fujiki, Acta Bot. Yunnanica, 2003, 25, 458–464 Search PubMed.
- J. J. Fu, H. Z. Jin, Y. H. Shen, J. J. Qin, Y. Wang, Y. Huang, Q. Zeng and W. D. Zhang, Chem. Biodiversity, 2009, 6, 818–826 CAS.
- (a) J. J. Fu, H. Z. Jin, Y. H. Shen, W. D. Zhang, W. Z. Xu, Q. Zeng and S. K. Yan, Helv. Chim. Acta, 2007, 90, 2151–2155 CrossRef CAS; (b) J. J. Fu, H. Z. Jin, Y. H. Shen, J. J. Qin, Y. Wang, Y. Huang, Q. Zeng, S. K. Yan and W. D. Zhang, Helv. Chim. Acta, 2009, 92, 491–494 CrossRef CAS; (c) J. J. Fu, J. J. Qin, Q. Zeng, Y. Huang, H. Z. Jin and W. D. Zhang, Chem. Pharm. Bull., 2010, 58, 1263–1266 CrossRef CAS PubMed; (d) J. J. Fu, J. J. Qin, Q. Zeng, Y. Huang, H. Z. Jin and W. D. Zhang, Arch. Pharmacal Res., 2011, 34, 199–202 CrossRef CAS PubMed; (e) J. J. Fu, L. Y. Wang, H. L. Li, J. J. Qin, Y. H. Shen, J. G. Cheng, H. Z. Jin and W. D. Zhang, J. Asian Nat. Prod. Res., 2012, 14, 496–502 CrossRef CAS PubMed.
- L. Gremaud and A. Alexakis, Angew. Chem., Int. Ed., 2012, 51, 794–797 CrossRef CAS PubMed.
- M. G. Beaver, S. B. Billings and K. A. Woerpel, J. Am. Chem. Soc., 2008, 130, 1520–5126 CrossRef PubMed.
- D. A. Evans, J. S. Johnson and E. J. Olhava, J. Am. Chem. Soc., 2000, 122, 1635–1649 CrossRef CAS.
- M. Tanaka, T. Ohshima, H. Mitsuhashi, M. Maruno and T. Wakamatsu, Tetrahedron, 1995, 51, 11693–11702 CrossRef CAS.
- B. B. Snider, N. H. Vo, S. V. O'Neil and B. M. Foxman, J. Am. Chem. Soc., 1996, 118, 7644–7645 CrossRef CAS.
- S. K. Thompsonl and C. H. Heathcock, J. Org. Chem., 1992, 57, 5979–5989 CrossRef.
- F. Denizot and R. Lang, J. Immunol. Methods, 1986, 89, 271–277 CrossRef CAS PubMed.
- S. Pandeti, K. Sharma, S. R. Bathula and N. Tadigoppula, Phytomedicine, 2014, 21, 333–339 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experiment procedures and NMR spectra for all compounds. CCDC 1402281. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra15153a |
| ‡ These authors contributed equally to this work. |
| § Secoarguterin (1): C10H16O3; yellow oil; [α]20D −97.0° (c 0.300, CHCl3); IR (KBr) νmax 3410, 2960, 2926, 2883, 1707, 1630, 1458, 1394, 1261, 1165, 1150, 1108, 1076, 1020, 960, 935, 870 cm−1; ESI-MS: m/z 207 [M + Na]+; HR-ESI-MS: m/z 207.0999 [M + Na]+ (calcd for C10H16O3Na, 207.0992); for 1H-NMR and 13C-NMR see Table 1. |
| This journal is © The Royal Society of Chemistry 2016 |