The heterogeneous reactions of toluene/O3/NH3 on hematite nanoparticles: the impact of light illumination on organic ammonium salt formation†
Xin
Liu
abc,
Xiang
He
 *abc,
Zhi-Cheng
Ma
abc,
Xi
Xi
abc and
Shuang-Xi
Wang
abc
*abc,
Zhi-Cheng
Ma
abc,
Xi
Xi
abc and
Shuang-Xi
Wang
abc
aCollege of Ecology and Environment, Xinjiang University, Urumqi 830017, PR China. E-mail: xianghe@xju.edu.cn
bKey Laboratory of Oasis Ecology, Ministry of Education, Xinjiang University, Urumqi 830017, PR China
cXinjiang Jinghe Observation and Research Station of Temperate Desert Ecosystem, Ministry of Education, Xinjiang University, Urumqi 830017, PR China
First published on 15th November 2023
Abstract
Organic ammonium salts which are formed from heterogeneous reactions are one of the important components of nitrogen-containing organic compounds (NOCs) in the atmosphere. In order to investigate the formation process of organic ammonium salts, a gas-flow system with the diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) technique was applied to monitor the influence of a simulated illumination on the heterogeneous reactions of toluene/O3/NH3 on hematite nanoparticles. The results revealed that toluene was transformed into benzoic acid under the action of oxidants (O3 and OH radicals). The carboxylic acid was neutralized with NH3 to form ammonium benzoate. The effect of light intensities on the reaction kinetics of ammonium benzoate formation from the heterogeneous reactions was also analyzed. With an increase in the light intensity from dark to 36 mW cm−2, the reaction rates increased from (1.20 ± 0.02) × 1018 ions per g s−1 to (2.30 ± 0.09) × 1018 ions per g s−1. This induced the formation of abundant active radicals, which accelerated the conversion of toluene to ammonium benzoate on hematite nanoparticles. However, the reaction rates decreased to (1.80 ± 0.03) × 1018 ions per g s−1 as the light intensity continued to increase to 100 mW cm−2. The yield of organic ammonium salts might be reduced owing to the volatilization of ammonium benzoate at a high light intensity. Meanwhile, the initial uptake coefficient showed a similar change trend. The values of the uptake coefficient increased by 81.1% when the light intensity increased from 0 mW cm−2 to 36 mW cm−2 but decreased by 21.1% when the light intensity increased from 36 mW cm−2 to 100 mW cm−2. Our results not only propose the heterogeneous reaction kinetics of toluene/O3/NH3 on the nanoscale hematite surface under different light intensity conditions, but also provide a theoretical support for further understanding the conversion process of volatile organic compounds (VOCs) under combined atmospheric pollution.
Environmental significanceNanoscale hematite particles, which have a large specific surface area for the heterogeneous formation of nitrogen-containing organic compounds (NOCs), are widely observed in the atmospheric environment. Heterogeneous reactions are considered of great importance in the formation of NOCs under different environmental conditions. The light intensity and interactions of precursor gases (such as toluene, NH3, and O3) can inevitably affect the formation of NOCs. This experimental study investigates the heterogeneous reaction kinetics of toluene/O3/NH3 on the nanoscale hematite surface under different light intensities, which reveals the important roles of light illumination in the formation processes of NOCs on hematite nanoparticles. This work gives a new perspective for understanding the accelerated NOC formation on nanoparticles and simultaneously provides a theoretical foundation for controlling the high concentration NOC pollution in severe haze events. |
1 Introduction
Nitrogen-containing organic compounds (NOCs) are the important components of secondary organic aerosols in the atmospheric environment.1 The concentration of NOCs can account for 10–20% of the total mass of atmospheric aerosols, which has a huge impact on the atmospheric environment.2,3 During the coal burning process in winter, the concentration of NOCs may increase, accounting for approximately 61–68% of organic aerosols on polluted days in the North China Plain.4 As the crucial components of atmospheric brown carbon,5 NOCs can not only effectively absorb near-ultraviolet light (300–400 nm) and short-wavelength visible light radiation to influence the radiation balance in different regions,6,7 but also act as cloud condensation nuclei to affect the optical properties of aerosols.8 Furthermore, the lifetime of NOCs formed in the atmosphere is much longer than that of NO2.9 Therefore, NOCs may have a great impact on the atmospheric radiation balance, atmospheric visibility, climate change, air quality, and human health.10,11 The main precursors of NOCs in the atmosphere are volatile organic compounds (VOCs) and nitrogen containing inorganic gases, which are emitted from natural and man-made sources.12 The heterogeneous processes on the mineral particle surface, especially heterogeneous acid–base reactions between NH3 and atmospheric VOCs, are increasingly recognized as an important source of NOCs.8,12 Meanwhile, the formation processes of NOCs can exert a significant impact on nitrogen concentrations in ecosystems.13 Therefore, the heterogeneous formation processes of NOCs have become the focus of current atmospheric studies.Benzene series emitted from industrial production and vehicle exhaust are important precursors of NOC formation.12,14 Their derived intermediates can react with NOx and/or NH3 to generate the formation of organic nitrogen compounds.8,12,15 Toluene is the most abundant aromatic compound in the urban atmosphere, which accounted for 20–40% of the total aromatic VOCs.16 Toluene in the troposphere can form highly reactive free radicals through heterogeneous reactions with atmospheric oxidants (like OH, NO3 radicals and O3) to form SOA.10,16 Furthermore, its derived intermediates can, via reaction with NOx and/or NH3, lead to the formation of brown carbon.10,15,17 As the most abundant basic gas existing in the atmosphere, NH3 is mainly derived from agricultural nitrogen fertilizer use and animal husbandry emission with a global land emission of 70–92 Tg a−1.18,19 Due to the large emission from agriculture and animal husbandry in China, the maximum concentration of NH3 exceeds 100 ppbv in the North China Plain.20,21 Therefore, NH3 is becoming an important pollution factor that aggravates the haze problem. Field and laboratory studies found that NH3 was one of the major gaseous precursors of particle-bound NOCs.15,22–24 As presented in recent studies, the heterogeneous acid–base neutralization reaction between NH3 and gaseous organic acid produced by oxidation of toluene could generate condensable organic ammonium salts.12,22 It indicates that NH3 is significantly correlated with the formation of NOCs. To date, the concentration of NOCs in field studies is significantly higher than that in model simulation, which indicated that there are still apparent uncertainties in the identification of the formation processes of NOCs.5 The neglect of formation processes might lead to incorrect predictions of the sources and distribution of SOA in the PM2.5.5 Therefore, further experimental studies are needed to improve the understanding of the association between NH3 and NOC formation processes. Moreover, it is well known that tropospheric ozone (O3) is an important secondary atmospheric pollutant from photochemical smog. Simultaneously, O3 is also the main oxidant in the atmosphere, which can not only directly react with VOCs to form secondary organic aerosols,25 but also indirectly influence the formation of SOA by increasing the content of oxidized species (OH radicals).26 In previous studies, O3 could significantly accelerate the heterogeneous reactions of VOCs with inorganic nitrogenous gases and promote the formation of NOCs.17,27 These indicated that the oxidation of O3 plays a key role during the heterogeneous reactions of NOCs.
Hematite, also known as α-Fe2O3, is the most stable iron oxide under natural conditions and constitutes about 6% of total atmospheric aerosol.16 Hematite nanoparticles exhibit a large specific surface area and can provide reaction sites for trace gases.16,28 In addition, hematite nanoparticles also have relatively strong oxidation and photochemical activity, which can produce hydroxyl radicals with strong reactivity under the condition of light, thus significantly affecting the progress of heterogeneous reactions.29 Furthermore, the environmental factors in the formation of secondary pollution cannot be ignored. The light illumination can affect both the properties of particles and heterogeneous reactions of SOA formation.30,31 In the presence of light, alkenes can react with O3 and hydroxyl radicals to form RO radicals and RO2 radicals and further generate NOC products with nitrogen-containing inorganic gases.32 Similarly, light conditions also contribute to the production of a large number of hydroxyl radicals, which accelerate the transformation of toluene into highly reactive free radicals and further promote the formation of SOA.33 It is obvious that the photoreaction process has an obvious effect on the formation of secondary organic matter in the atmosphere. In addition, field studies proposed that the concentrations of NOCs were varied by diurnal variations of light illumination, increasing from morning hours and peaking at afternoon.34 Moreover, Qiu et al. also found that the production rate of peroxyacetyl nitrate reached its maximum within 4 hours at noon, which indicated that the intensity of light illumination was related to NOCs.35 It may be due to the fact that intense light illumination enhances photochemical activity and increases the atmospheric abundance of oxidants, thus increasing the atmospheric capacity to oxidize organics.36 However, few studies were aimed at investigating the influence of different light intensities on the formation of NOCs in laboratory; it is necessary to study the light intensity effect on the heterogeneous reaction mechanisms and kinetics of formation of nitrogen-containing organic compounds.
In order to explore the formation process of NOCs during heterogeneous reaction, this study used diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) to systematically monitor the reaction of toluene/O3/NH3 on hematite nanoparticles. The influence of different light intensities on the formation of NOCs during the heterogeneous reactions was investigated in situ. This work can be helpful for gaining a better understanding of heterogeneous reactivity of toluene as well as the formation mechanisms and sources of nitrogenous organic compounds under complex air pollution conditions.
2 Experimental section
2.1 Materials
The hematite nanoparticles (>99.5% purity, Aldrich) used in this experiment were purchased from commercial sources without further processing. N2 (99.999% purity) and O2 (99.999% purity) were introduced as synthetic air (79% N2 and 21% O2). An ozone generator (UV-M2, Beijing Tongling, China) was used to produce O3 by introducing synthetic air with a stable flow through a UV lamp. The gas flow of synthetic air was controlled by using two mass flow meters (M500SLPM, Shanxi Yidu Instrumentation Instrument Co., Ltd.). Calibration toluene (500 ppm in N2) and NH3 (500 ppm in N2) were used as reactant trace gases. Concentrations of toluene and NH3 were adjusted by using special mass flow controllers (M100SLPM, Beijing Measure Instrument Co., Ltd.). All experimental gases were purchased from Xinjiang Sennisai Gas Co., Ltd. A 250 W xenon lamp coupled DRIFTS situ cell via an optical fiber (CEL-TCX250, Beijing) was used for simulating solar irradiation conditions.2.2 Gas supply system
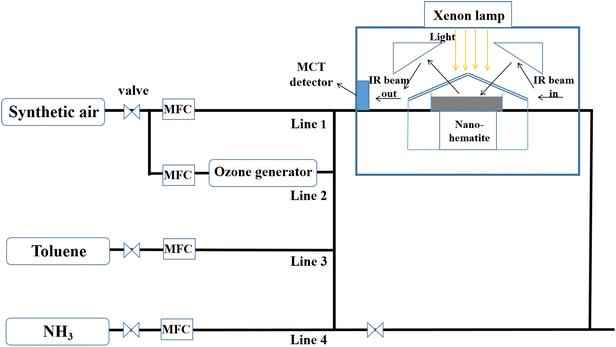
The experiment apparatus is shown in Fig. 1. The gas flow system was composed of four Teflon inlet lines and mass flow controllers (MFCs). Firstly, line 1 was supplied with high purity N2 and O2 (79% N2 and 21% O2) to dilute the gaseous reactants and maintain a stable reactant flux. Line 2 supplied O3 produced by the ozone generator (2.49 × 1013 molecules per cm3 O3). And lines 3 and 4 provided diluted NH3 or toluene by high purity N2 as simulated atmospheric trace pollutants (2.49 × 1013 molecules per cm3 toluene; 2.49 × 1013 molecules per cm3 NH3). Simultaneously, the gas supply system was capable of mixing all types of reactant gases together before they entered into the reaction chamber, with the blending of gases with a total flow of about 300 mL min−1.2.3 In situ DRIFTS method
The heterogeneous reactions of O3/NH3 with toluene on hematite nanoparticles were investigated by the in situ diffuse reflectance infrared Fourier transform spectroscopic (DRIFTS) technique. The spectra were recorded using a VERTEX 70 Fourier transform infrared spectroscope (FTIR) equipped with a highly sensitive mercury cadmium telluride (MCT) detector cooled by liquid-nitrogen. The hematite sample (about 50 mg) was compressed into the sample cup (0.5 mm depth, 10 mm diameter) of an in situ diffuse reflection chamber. Before the reactant gases were introduced, the hematite sample was preprocessed in a stream of zero air for about 30 minutes to wipe off adsorbed substances like water and impurities. At the same time, the spectrum of the unreacted hematite nanoparticles was collected before the heterogeneous reactions as a background spectrum. Then the reactant gas flow was switched to the trace lines to conduct heterogeneous reactions. The FTIR spectra were scanned in the spectral range from 4000 to 800 cm−1 with a resolution of 4 cm−1 for 64 scans and analyzed with the OPUS 6.5 program (Bruker Co.). In simulated sunlight experiments, a 250 W xenon lamp as the experimental simulation light source was used. In this way, the radiation spectrum was closer to that of sunlight and more consistent with the actual atmospheric illumination conditions. As the light intensities of the summer noon could reach 115.5–116.5 mW cm−2,37 the xenon lamp illumination intensities in this study were controlled within the range of 0–100 mW cm−2. All the experiments were conducted under room temperature (∼295 K) under well-controlled central air-conditioning.2.4 Other measurement methods
Transmission electron microscopy (TEM) (JEM-2100, JEOL, Japan) was used to characterize the morphology of the nanoscale hematite particles at an accelerating voltage of 200 kV. As can be seen in Fig. S1a,† pure hematite particles were uniformly distributed with clusters or aggregates. Meanwhile, the scanning electron microscope (SEM) (S-4800, Hitachi, Japan) image showed that the morphology of hematite particles was rough and uneven (Fig. S1b†). Hematite nanoparticles were stacked with each other and mainly in the form of aggregated particles. Our previous study verified that the grain size of hematite particles was in the order of nanometers.17 The surface area of the hematite nanoparticle sample was ascertained to be 153.8 m2 g−1 by nitrogen Brunauer–Emmett–Teller (BET) physisorption (Autosorb-IQ2-MP, Quantachrome Instruments U.S.). The X-ray photoelectron spectroscopy (XPS, ESCALAB250Xi, Thermo Fisher Scientific U.S.) method was applied to obtain the chemical properties of the surface product. The signal intensity of XPS was mainly contributed by the surface layer of the sample particles (depth of about a few nanometers). Binding energies were calibrated using the energy of the C 1s peak at 284.8 eV.3 Results and discussion
3.1 DRIFTS spectra of heterogeneous reactions under dark condition
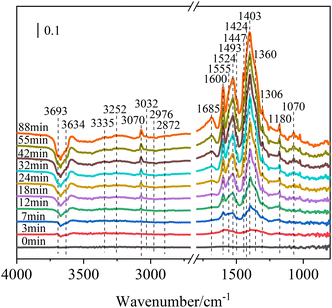
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1685 cm−1)45,46 and COO– (1524, 1447, and 1403 cm−1).46,47 This implied that the existence of O3 might significantly promote the oxidation of toluene to form more oxygen-containing organic products. Two negative peaks at 3693 and 3634 cm−1 were attributed to the free hydroxyl groups on hematite nanoparticles and the peak intensity decreased with reaction time.48–50 The loss of surface hydroxyl groups revealed that surface –OH groups were involved in the reaction of toluene/O3/NH3 on mineral dust under the dark condition. These results indicated that the toluene/O3/NH3 could be oxidized to form organic ammonium salts by consuming surface hydroxyl groups (M–OH) on hematite nanoparticles under the dark condition.12
O (1685 cm−1)45,46 and COO– (1524, 1447, and 1403 cm−1).46,47 This implied that the existence of O3 might significantly promote the oxidation of toluene to form more oxygen-containing organic products. Two negative peaks at 3693 and 3634 cm−1 were attributed to the free hydroxyl groups on hematite nanoparticles and the peak intensity decreased with reaction time.48–50 The loss of surface hydroxyl groups revealed that surface –OH groups were involved in the reaction of toluene/O3/NH3 on mineral dust under the dark condition. These results indicated that the toluene/O3/NH3 could be oxidized to form organic ammonium salts by consuming surface hydroxyl groups (M–OH) on hematite nanoparticles under the dark condition.12
XPS analysis is especially sensitive to light elements such as C and nitrogen (N).51 In order to verify the functional groups of the main products, XPS was used to fit the C and N elements of functional groups after the reaction of toluene/O3/NH3 on hematite nanoparticles. The XPS spectra for the particle surface after reaction are presented in Fig. S2.† Apart from the C–C groups of the aromatic ring skeleton with vibration at 284.8 eV,51,52 the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (286.4 eV) and O
O groups (286.4 eV) and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O groups (288.4 eV) of the organic acid were also observed.51,52 In addition, a high resolution spectrum of N1s can be seen in Fig. S2† and the 401.7 eV excitation was associated with the N atom from the ammonium groups.51 Combined with infrared spectroscopy analysis, the NOCs generated on hematite nanoparticles were ammonium benzoate.
C–O groups (288.4 eV) of the organic acid were also observed.51,52 In addition, a high resolution spectrum of N1s can be seen in Fig. S2† and the 401.7 eV excitation was associated with the N atom from the ammonium groups.51 Combined with infrared spectroscopy analysis, the NOCs generated on hematite nanoparticles were ammonium benzoate.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bands of the aromatic ring were observed at 1600, 1560 and 1497 cm−1.25 In addition, some oxygen-containing functional group peaks also appeared, such as C
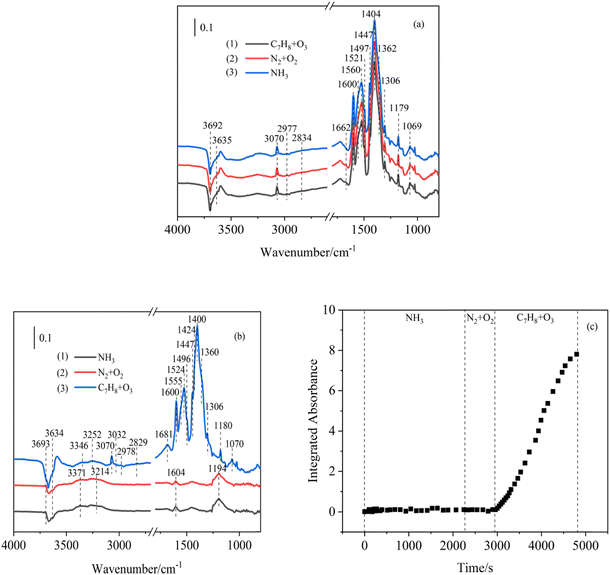
C bands of the aromatic ring were observed at 1600, 1560 and 1497 cm−1.25 In addition, some oxygen-containing functional group peaks also appeared, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1662 cm−1),53 COO− (1521, 1447, and 1404 cm−1)46 and C–O bands (1179 and 1069 cm−1).44,54 This illustrated that toluene and O3 on the surface of hematite nanoparticles undergo oxidation reactions to form benzoic acid. After stopping the flow of toluene/O3 and flushing the cell with synthetic air for 30 minutes, the spectrum was recorded as the blue line, as shown in Fig. 3a. Air purging did not noticeably change the spectra, showing a relatively steady adsorption of these formed organic components. The toluene/O3-preadsorbed hematite nanoparticles were exposed to NH3 in the final step, which is marked by the red line in Fig. 3a. There was also no change in this stage. Based on the above bands, the main product was organic acid and no NOC species formed in this step experiment. The possible reason might be that the formation of oxidation products from toluene/O3 could take up abundant active sites on the nanoparticle surface and significantly suppress the NOC formation processes during the following NH3 exposure.
O (1662 cm−1),53 COO− (1521, 1447, and 1404 cm−1)46 and C–O bands (1179 and 1069 cm−1).44,54 This illustrated that toluene and O3 on the surface of hematite nanoparticles undergo oxidation reactions to form benzoic acid. After stopping the flow of toluene/O3 and flushing the cell with synthetic air for 30 minutes, the spectrum was recorded as the blue line, as shown in Fig. 3a. Air purging did not noticeably change the spectra, showing a relatively steady adsorption of these formed organic components. The toluene/O3-preadsorbed hematite nanoparticles were exposed to NH3 in the final step, which is marked by the red line in Fig. 3a. There was also no change in this stage. Based on the above bands, the main product was organic acid and no NOC species formed in this step experiment. The possible reason might be that the formation of oxidation products from toluene/O3 could take up abundant active sites on the nanoparticle surface and significantly suppress the NOC formation processes during the following NH3 exposure.
In the second part of the step experiments, the exposure sequence was reversed. At the first step of the reaction, NH3 flow was introduced into the hematite nanoparticle surface. As shown in Fig. 3b, several absorption peaks were observed, indicating that NH3 interacted with hematite nanoparticles. The peak at 3371 cm−1 was assigned to the anti-symmetric stretching vibration of NH3, while the bands at 3214 and 1604 cm−1 could be ascribed to the symmetric stretching vibration of NH3. Moreover, peaks at 3693 and 3634 cm−1 were attributed to the anti-symmetric deformation vibration and symmetric deformation vibration of NH3, respectively. It was obvious that NH3 was adsorbed on the surface of hematite nanoparticles.41,42,55 When the NH3 flow was cut off and the nanoparticles were flushed with synthetic air for 30 minutes, the bands remained unchanged. This implied that the presence form of NH3 on the particle surface was chemical adsorption. Finally, when toluene/O3 were introduced into the reaction system, the infrared spectrum changed obviously. The consumption of –OH groups (3693 and 3634 cm−1)38,56 on the sample nanoparticle surface was observed. Meanwhile, the peaks of the aromatic ring appeared during the reaction, including the C–H groups (3070 and 1362 cm−1),25 –CH2 groups (2978, 2829 and 1306 cm−1)38–40 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups (1600, 1555 and 1497 cm−1).25 Some bands that are attributed to carboxylic acids also appeared, such as the C
C groups (1600, 1555 and 1497 cm−1).25 Some bands that are attributed to carboxylic acids also appeared, such as the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (1681 cm−1),53 COO– groups (1524, 1447, and 1400 cm−1)46,47 and C–O groups (1180 and 1070 cm−1).43,44 In addition, the bands of NH4+ of ammonium benzoate were observed at 3032 and 1424 cm−1.41,42 Because the adsorption of NH3 did not occupy all the active sites, the carboxylic acid formed on the surface could react with the adsorbed NH3 to produce organic ammonium salts. Compared with the mixed experiment (Fig. 2), similar characteristic peaks from the ammonium benzoate of heterogeneous reactions of toluene/O3/NH3 were also observed in the step experiment. However, the absorbance of the characteristic peaks in the step-exposure reaction was weakened to a certain extent, indicating that the step reaction showed an inhibitory effect on the generation of NOCs. In order to understand the formation amounts of NOCs, the integrated absorbance of the organic ammonium salt peak (1442–1417 cm−1) is presented in Fig. 3c. When NH3 was introduced to the particle surface first, the peak intensity of NOCs always approached zero. This showed that NH3 was chemically adsorbed on the surface of hematite nanoparticles and no ammonium ion was formed in this system. The peak area also remained unchanged by air purging, implying the absence of any other physical adsorption forms. Finally, after toluene/O3 was introduced, the peak area of organic ammonium obviously increased and the formation amount of organic ammonium salts also rapidly increased. After a period of time, the peak area eventually reached a value of 7.805. With a similar reaction time, when the toluene/O3/NH3 mixture was introduced to hematite nanoparticles, the peak area of organic ammonium salts could reach 11.656 (Fig. 5). It was proved that the stepwise response presented a certain inhibitory effect on the generation of NOCs. This was mainly due to the fact that NH3 might occupy certain active sites on the surface of hematite nanoparticles during the first step. Therefore, heterogeneous reactions of subsequent toluene/O3 on the particle surface were blocked and the production of ammonium benzoate was reduced.28,57
O groups (1681 cm−1),53 COO– groups (1524, 1447, and 1400 cm−1)46,47 and C–O groups (1180 and 1070 cm−1).43,44 In addition, the bands of NH4+ of ammonium benzoate were observed at 3032 and 1424 cm−1.41,42 Because the adsorption of NH3 did not occupy all the active sites, the carboxylic acid formed on the surface could react with the adsorbed NH3 to produce organic ammonium salts. Compared with the mixed experiment (Fig. 2), similar characteristic peaks from the ammonium benzoate of heterogeneous reactions of toluene/O3/NH3 were also observed in the step experiment. However, the absorbance of the characteristic peaks in the step-exposure reaction was weakened to a certain extent, indicating that the step reaction showed an inhibitory effect on the generation of NOCs. In order to understand the formation amounts of NOCs, the integrated absorbance of the organic ammonium salt peak (1442–1417 cm−1) is presented in Fig. 3c. When NH3 was introduced to the particle surface first, the peak intensity of NOCs always approached zero. This showed that NH3 was chemically adsorbed on the surface of hematite nanoparticles and no ammonium ion was formed in this system. The peak area also remained unchanged by air purging, implying the absence of any other physical adsorption forms. Finally, after toluene/O3 was introduced, the peak area of organic ammonium obviously increased and the formation amount of organic ammonium salts also rapidly increased. After a period of time, the peak area eventually reached a value of 7.805. With a similar reaction time, when the toluene/O3/NH3 mixture was introduced to hematite nanoparticles, the peak area of organic ammonium salts could reach 11.656 (Fig. 5). It was proved that the stepwise response presented a certain inhibitory effect on the generation of NOCs. This was mainly due to the fact that NH3 might occupy certain active sites on the surface of hematite nanoparticles during the first step. Therefore, heterogeneous reactions of subsequent toluene/O3 on the particle surface were blocked and the production of ammonium benzoate was reduced.28,57
3.2 Effect of light illumination on heterogeneous reactions of toluene/O3/NH3 with hematite nanoparticles
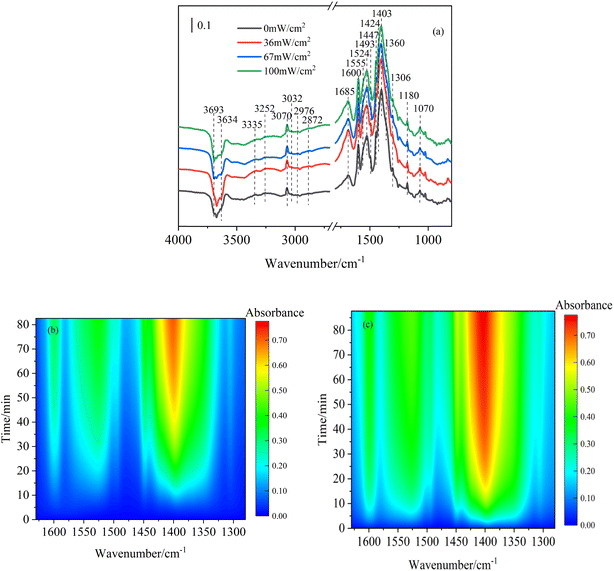
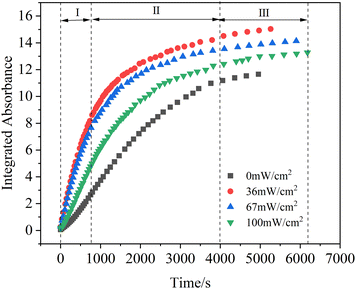
Light illumination plays a vital role in the formation of hydroxyl radical, which is one of the active species on mineral dust. Therefore, it is necessary to study NOC formation processes from the heterogeneous reactions under different light illumination intensities. In order to explore the NOC formation processes during the heterogeneous reactions of toluene under the irradiation condition, the infrared spectra of toluene/O3/NH3 on hematite nanoparticles at the end of the reaction time under different light intensity conditions are shown in Fig. 4a. The positions of the spectra after the reaction were similar, implying that the main products were the same. When the reaction conditions changed from dark to light, the –OH group peaks (3693 and 3634 cm−1) showed an obvious decreasing trend, whereas the characteristic peak intensities of organic functional groups increased and these peaks were all higher than those under the dark condition. This demonstrated that light promoted more –OH groups to participate in the reaction.38 Under simulated light illumination, hematite nanoparticles could be excited by irradiation to form photogenerated electrons and holes, and then those electrons and holes of the nanoparticles could react with O2 to produce additional reactive oxygen species (ROS, such as ·O2− and ·OH).28 These ROS could further promote the conversion of toluene to ammonium benzoate on hematite nanoparticles. Fig. 4b and c show the 2D counter DRIFTS spectra of organic products (1280–1680 cm−1) during heterogeneous reactions under dark and 36 mW cm−2 light conditions, respectively. As presented in Fig. 4c, the absorbance of organic ammonium salts could reach a value of approximately 0.32 within 10 minutes of reaction under the light intensity of 36 mW cm−2. However, the absorbance remained below 0.1 under the dark condition at a similar reaction time (Fig. 4b). In addition, during the initial 10 minutes, a significant dark blue region was observed under the dark condition, suggesting an absorbance below 0.1. However, in the presence of 36 mW cm−2 light illumination, some green regions appeared, which showed that the absorbance of the products reached ∼0.4. The absorbance reached 0.6 at about 20 minutes, which was significantly faster than the dark condition (about 45 minutes). This proved that the organic ammonium products could easily reach higher values of absorbance under the light condition. Moreover, the red area in Fig. 4c below was much larger than in Fig. 4b, which suggested that the response of the characteristic peak of accumulated ammonium benzoate under the light condition was also higher than that under the dark condition. Therefore, light illumination had an obvious promoting effect on the generation of NOCs.To illustrate the promoting effect of simulated light on NOC formation during the heterogeneous reactions of toluene/O3/NH3, the integrated absorbance of organic ammonium salts (1417–1442 cm−1) as a function of reaction time under different light conditions is presented in Fig. 5. Three stages, which were the initial region (I), the transition region (II) and the steady region (III), were considered for the kinetics study of heterogeneous reactions. The initial region I and the steady region III are usually used for the kinetics study of the heterogeneous reaction of trace gas mixtures on the particles.58,59 In the initial stage I, due to the abundant reactive sites on the hematite nanoparticle surface, as soon as the reactant gases were introduced into the particle layers, the NOCs formed quickly and linearly from the heterogeneous processes with reaction time. After a certain time of the reaction, the NOC absorbance increased at a slower speed at the transition region II. Finally, as the reactions proceeded, the active sites on the surface were completely consumed and covered by newly formed products. At this period, the increasing yield of NOCs slowed down and reached a constant rate; the steady region III was confirmed. Comparing the increasing trend of the organic ammonium salt amounts during the heterogeneous processes, it was obvious that light illumination could promote the heterogeneous reactions of toluene/O3/NH3 on the surface of hematite nanoparticles. However, the effect of light illumination on heterogeneous reactions did not continuously increase with increasing light intensity. When the light intensity was 36 mW cm−2, the product growth rate reached its maximum. After that, as the light continued to increase, the production of NOCs showed a decreasing trend. The main reason for this phenomenon was the volatilization of ammonium salts under high light intensities.60 Although the growth trend of spectral peak intensity was lower, the production of organic ammonium salts under the light intensity of 100 mW cm−2 was still stronger than that under the dark condition.
Referring to the method of the linear correlation in the literature, the amount of ammonium benzoate ions formed during the reaction was determined by the DRIFTS calibration curves made by mixing the weighed ammonium acetate (as a reference material, >99.95% purity, Aladdin) uniformly into hematite nanoparticles to a certain concentration.38,61 The organic ammonium salt formation rates were converted from ABU s−1 to NH4+ s−1 by a conversion factor obtained from a calibration plot (Fig. S3†). Because the IR absorbance signal was proportional to the NH4+ concentration over a wide concentration range, the plot presented a conversion factor that could calculate the amount of organic ammonium salts formed during the reaction. The organic ammonium ion concentration was calculated as follows:
| {NH4+} = f × (intergrated absorbance) | (1) |
The corresponding relationship between formation rates and different light illumination intensities is shown in Fig. 6. The formation rates of organic ammonium salts on hematite nanoparticles increased in the range of 0 to 36 mW cm−2, whereas the trend was reversed from 36 mW cm−2 to 100 mW cm−2. From the darkness to the light (36 mW cm−2), the ammonium benzoate formation rate increased from (1.20 ± 0.02) × 1018 ions per g s−1 to (2.30 ± 0.09) × 1018 ions per g s−1. The formation rate increased by nearly 2-fold, which implied that light enhanced NOC formation significantly because hematite nanoparticles could be excited to produce photo-generated electrons and holes, thus inducing the formation of more ROS groups under the light condition. The active groups accelerated the oxidation process from toluene to benzoic acid. The carboxylic acid generated in this process could further react with NH3 and promote the formation rates of organic ammonium salts. However, when the light intensity increased from 36 mW cm−2 to 100 mW cm−2, the reaction rate gradually decreased from (2.30 ± 0.09) × 1018 ions per g s−1 to (1.80 ± 0.03) × 1018 ions per g s−1. This might be due to the instability of ammonium benzoate under the light condition. If the light intensity was too strong, the organic ammonium salts would gradually volatilize away from the surface of hematite nanoparticles. Therefore, the formation rate and yield of NOCs on nanoparticles were relatively reduced.62
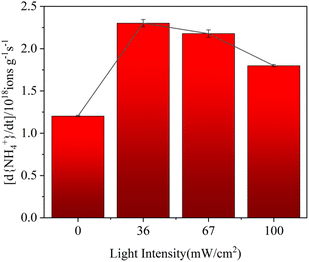 | ||
| Fig. 6 The formation rates of organic ammonium salts as a function of light intensities in the initial stage. | ||
3.3 Influence of light intensity on the uptake coefficient
The efficiency of the heterogeneous reactions on the surfaces of the particles can be described by the uptake coefficient. Combined with the calculation method of uptake coefficient in other literature,63 the reactive uptake coefficients (γ) for the heterogeneous oxidation of toluene/O3/NH3 were deduced from the DRIFTS experiments. The reactive uptake coefficient, γ, is defined as the rate of product formation (d{NH4+}/dt) divided by the rate of surface collisions per unit time (Z).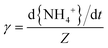 | (2) |
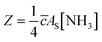 | (3) |
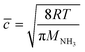 | (4) |
![[c with combining macron]](https://www.rsc.org/images/entities/i_char_0063_0304.gif) is the mean molecular velocity of NH3 in the gas phase (cm s−1), AS is the effective surface of the hematite nanoparticles (m2 g−1), [NH3] is the number of NH3 molecules per unit gas volume (molecules per cm3), T is the temperature (K), R is the gas constant (J mol−1 K−1), and MNH3 is the molar mass (g mol−1). Uptake coefficients were usually determined by the BET specific surface area, which can best represent the reaction surface under atmospheric conditions.59
is the mean molecular velocity of NH3 in the gas phase (cm s−1), AS is the effective surface of the hematite nanoparticles (m2 g−1), [NH3] is the number of NH3 molecules per unit gas volume (molecules per cm3), T is the temperature (K), R is the gas constant (J mol−1 K−1), and MNH3 is the molar mass (g mol−1). Uptake coefficients were usually determined by the BET specific surface area, which can best represent the reaction surface under atmospheric conditions.59
Although the initial stage of uptake coefficient of toluene/O3/NH3 on the surface of hematite nanoparticles was firstly reported in our study, the previous literature studies have studied different gases' uptake coefficients on the surface of mineral dust oxides. The uptake coefficients for different gases reacted with mineral dust by using the BET surface and FTIR under dark or light conditions are listed in Table 1. Combined with the uptake coefficients of different heterogeneous reactions, the values of uptake coefficient varied within a range of about three orders of magnitude (10−6–10−4) for different reaction systems. The uptake coefficient of heterogeneous reactions of toluene/O3/NH3 on hematite nanoparticles was (5.26 ± 0.07) × 10−6 and (1.01 ± 0.04) × 10−5 under dark and light conditions, respectively. The values were within the orders of magnitude range of previous studies. The differences of initial absorption coefficients between our experiments and other systems' results were valid. Firstly, the reason for this discrepancy might be due to the measurement evaluation between different experimental methods. Li et al. and Kebede et al. selected the Knudsen cell and flow-tube reactor as the experimental method, while we used DRIFTS for our study. The Knudsen cell and flow tube reactor primarily measured the loss rate of gaseous species during the heterogeneous reactions of exposure to mineral dust, whereas DRIFTS determined the formation rate of surface products and dismissed the physical adsorption or other possible adsorption forms of gaseous reactants as a result of the detection limit of DRIFTS.55,63 Therefore, the uptake coefficients measured with the above two techniques were slightly higher than those obtained with DRIFTS in our result. On the other hand, the differences in the properties of reactants and mineral dust particles could also induce discrepancies in the reaction mechanism, which in turn can lead to variations in the uptake coefficient. For example, Wang et al. selected a mixture of levoglucosan and kaolin to react with NH3; kaolin had the capacity to initiate the decay of WSOCs and provided new pathways of photochemical products of levoglucosan with NH3. However, hematite nanoparticles mainly provided an oxidation site in our experiment and oxidized toluene to form ammonium benzoate on hematite nanoparticles. It might also lead to the difference between the two experimental results. Furthermore, although the detection techniques used were similar, calibration methods might also cause differences in reaction uptake coefficients. In our study, the conversion of FTIR signals to ion data relied on an external calibration method (eqn (1)), which was different from the Langmuir–Hinshelwood kinetic model calibration used for IC analysis.67 Therefore, in view of the differences in trace gases and mineral dust particles used in different systems, the initial uptake coefficient of toluene/O3/NH3 on hematite nanoparticles under dark or light conditions was different from that of other experiments.
| Reference | Reaction Gas | Particles | Reactor | γ 0 (dark) | γ 0 (light intensity) |
|---|---|---|---|---|---|
| Li et al.64 | Acetaldehyde | α-Fe2O3 | Knudsen cell | 2.9 × 10−6 | — |
| Acetone | 1.6 × 10−4 | — | |||
| Propionaldehyde | 5.1 × 10−5 | — | |||
| Kebede et al.65 | NH3 | TiO2 | Flow tube reactor | — | 1.2 × 10−5 (9.0 × 1016 photons s−1 cm−2) |
| He et al.66 | Toluene/O3/NO2 | α-Fe2O3 | DRIFTS | (1.84 ± 0.06) × 10−5 | — |
| Wang et al.67 | NH3 | Kaolinite | DRIFTS | (3.81 ± 0.27) × 10−6 | (3.24 ± 0.67) × 10−6 (60 mW cm−2) |
| Kaolinite/levoglucosan | (2.06 ± 0.53) × 10−6 | (4.61 ± 0.73) × 10−6 (60 mW cm−2) | |||
| This work | Toluene/O3/NH3 | Hematite | DRIFTS | (5.26 ± 0.07) × 10−6 | (1.01 ± 0.04) × 10−5 (36 mW cm−2) |
The values of uptake coefficient of heterogeneous reactions in the initial stage (γ0) are listed in Table 2. As can be seen from Table 2, when the light intensity increased from 0 mW cm−2 to 36 mW cm−2, the uptake coefficients increased from (5.26 ± 0.07) × 10−6 to (1.01 ± 0.04) × 10−5. However, the values of γ0 decreased from (1.01 ± 0.04) × 10−5 to (7.86 ± 0.11) × 10−6 with the increasing light intensity in a range from 36 mW cm−2 to 100 mW cm−2. This implied that the effects of light intensity on the NOC formation were complex. On the one hand, the chemical activity of hematite nanoparticles was improved under light condition, and more OH radicals were produced to accelerate the oxidation of toluene compared to the dark condition. Then the forming benzoic acid could react with more NH3 to form ammonium benzoate, which facilitated the uptake of NH3. On the other hand, although light illumination promoted the generation of ROS components on the surface and increased NOC production, it could lead to the decomposition of organic ammonium salts on hematite nanoparticles. Additionally, with the increase of light intensity, the formation rate of photogenerated electrons (e−)–holes (h+) on the hematite nanoparticle surface would gradually exceed the generation rate of ROS, resulting in electron–hole recombination.68 Therefore, a further increase in light intensity might correspondingly decrease NH3 uptake and reduce the amounts of NOCs adsorbed on the nanoparticle surface. But the uptake coefficients of NH3 under the light condition were still higher than that under the dark condition, indicating that the conversion of NH3 to NOCs on hematite nanoparticles was obvious under the light condition.
| Light intensity/(mW cm−2) | γ 0 |
|---|---|
| 0 | (5.26 ± 0.07) × 10−6 |
| 36 | (1.01 ± 0.04) × 10−5 |
| 67 | (9.52 ± 0.37) × 10−6 |
| 100 | (7.86 ± 0.11) × 10−6 |
3.4 Possible reaction mechanism under dark and simulated light illumination conditions
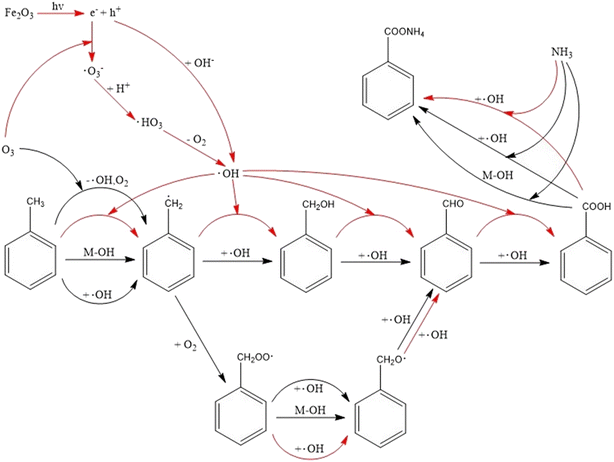
Based on the experimental observations mentioned above, organic ammonium salts were the main product of NOCs. The possible reaction mechanisms for the heterogeneous reactions of toluene with O3/NH3 on hematite nanoparticles under dark (black line) and light conditions (red line) were proposed, shown in Scheme 1.4 Conclusions and atmospheric implications
In our study, heterogeneous reactions of toluene with O3 in the presence of NH3 on the hematite nanoparticle surface were investigated by using a DRIFTS system in order to understand the NOC formation in complex atmospheric pollution. Under the dark condition, the bands at 1658 and 1524 cm−1 were ascribed to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and ν(COO) of benzoic acid formed by the toluene/O3/NH3 reaction, respectively. And the bands at 3032 and 1424 cm−1 were attributed to NH4+ of ammonium benzoate. For the heterogeneous reactions of toluene/O3/NH3 on hematite nanoparticles, the oxidation of toluene could generate organic carboxylic acid products through a chain reaction firstly. Then NH3 molecules could react with carboxylic acid groups to produce ammonium benzoate through an acid–base neutralization. This suggested that acid–base reaction between NH3 molecules and acid groups could lead to the formation of particle-bound organic ammonium salts.8,12 When simulated light illumination was added, the formation amounts of NOCs were higher than that under the dark condition. This was due to the excitation effect of the photoactivity of hematite nanoparticles, which could promote the rapid formation of additional hydroxyl radicals on the surface and enhance the formation of NOCs. In addition, the influence of light intensity on the formation of NOCs was also discussed. With the increase of illumination intensity from 0 mW cm−2 to 36 mW cm−2, the formation rates of organic ammonium salts on the surface of hematite nanoparticles and the uptake coefficients both increased by nearly two-fold at the initial stage. This implied that the illumination had a synergistic effect on the heterogeneous reactions of toluene/O3/NH3 on the hematite nanoparticle surface. However, the NH4+ ion of ammonium benzoate could be volatilized or sublimated under strong sunlight, which led to the loss of NOCs on the particle surface. When the light intensity continued to increase to 100 mW cm−2, the NOC formation rates and initial uptake coefficients would decrease to (1.80 ± 0.03) × 1018 ions per g s−1 and (7.86 ± 0.11) × 10−6, respectively. Therefore, different light intensities exhibited a complex influence on the heterogeneous reactions of toluene/O3/NH3 on the hematite nanoparticle surface.
O) and ν(COO) of benzoic acid formed by the toluene/O3/NH3 reaction, respectively. And the bands at 3032 and 1424 cm−1 were attributed to NH4+ of ammonium benzoate. For the heterogeneous reactions of toluene/O3/NH3 on hematite nanoparticles, the oxidation of toluene could generate organic carboxylic acid products through a chain reaction firstly. Then NH3 molecules could react with carboxylic acid groups to produce ammonium benzoate through an acid–base neutralization. This suggested that acid–base reaction between NH3 molecules and acid groups could lead to the formation of particle-bound organic ammonium salts.8,12 When simulated light illumination was added, the formation amounts of NOCs were higher than that under the dark condition. This was due to the excitation effect of the photoactivity of hematite nanoparticles, which could promote the rapid formation of additional hydroxyl radicals on the surface and enhance the formation of NOCs. In addition, the influence of light intensity on the formation of NOCs was also discussed. With the increase of illumination intensity from 0 mW cm−2 to 36 mW cm−2, the formation rates of organic ammonium salts on the surface of hematite nanoparticles and the uptake coefficients both increased by nearly two-fold at the initial stage. This implied that the illumination had a synergistic effect on the heterogeneous reactions of toluene/O3/NH3 on the hematite nanoparticle surface. However, the NH4+ ion of ammonium benzoate could be volatilized or sublimated under strong sunlight, which led to the loss of NOCs on the particle surface. When the light intensity continued to increase to 100 mW cm−2, the NOC formation rates and initial uptake coefficients would decrease to (1.80 ± 0.03) × 1018 ions per g s−1 and (7.86 ± 0.11) × 10−6, respectively. Therefore, different light intensities exhibited a complex influence on the heterogeneous reactions of toluene/O3/NH3 on the hematite nanoparticle surface.
There was still a large gap between field monitoring and model simulation based on the previous studies. Pye et al. found that the atmospheric concentration and generation rate of simulated NOCs were about 7 times higher than the actual value, which might be related to the lack of identification of the formation mechanism of NOCs. This indicated that the current reaction mechanism of NOC formation might still be unrecognized in laboratory.75 Our study enriched the understanding of NOC formation processes under complex atmospheric conditions. Firstly, the complex gas-phase in the real atmosphere can lead to a wide variety of effects on the heterogeneous formation process of NOCs. The results of this study not only revealed the formation processes of NOCs from the heterogeneous reactions of toluene and hematite nanoparticles, but also demonstrated that the coexistence of NH3 and oxidizing substances (O3) can participate in the complex heterogeneous reaction process in the real atmosphere. Previous field monitoring studies have found that some NOCs were formed from the reaction of organic oxidation products with NH3 by comparing the composition of SOA that was collected in Bakersfield and simulated in the laboratory.76,77 It was confirmed that NH3 was highly correlated with NOC formation and is a potential source of NOCs. Furthermore, O3 in the troposphere is not only a pollutant but also as an oxidant to participate in atmospheric chemical reactions. The increasing O3 concentration has enhanced the atmospheric oxidizing capacity in China; the role of O3 in the formation of atmospheric secondary organic components could not be ignored.26,78 Meanwhile, the influence of gas pre-adsorption order on the heterogeneous transformation of toluene was also explored, further emphasizing the complexity of the reaction between toluene and mineral dust. It showed that the existence of NH3 and O3 plays a key role in the generation of atmospheric NOC pollution, especially in atmospheric environments with high emissions and high pollutant concentration levels. Such complex chemical reactions might occur on the surface of dust particles and significantly affect the chemical reactions and pollutant concentrations in the troposphere. Secondly, as one of the most variable meteorological factors in the atmosphere, the difference of light intensities could significantly affect the atmospheric chemical reaction. The experimental results of this study emphasized the effects of different light intensities on the reaction kinetics of NOCs from the heterogeneous reaction of O3/NH3 with benzene series VOCs on hematite nanoparticles. It showed that the presence of light could accelerate the heterogeneous process generation of SOA benzoic acid on the hematite nanoparticle surface by exciting photo-generated electrons and holes to generate additional ROS. However, the effect of light intensity variation on NOC formation was complex and was influenced by photo-reactivity and product properties. In addition, the different effects of light intensity on the formation rate of NOCs on hematite nanoparticles may provide new ideas for understanding the diurnal variation of NOC concentration in different regions. We demonstrated that excessive simulated light irradiation would lead to the volatilization of ammonium benzoate formed on hematite nanoparticles. Field study proposed that the concentrations of PONs (particulate organic nitrates) were varied by diurnal variations of light illumination.1 At the same time, Zhang et al. also found that atmospheric ammonium had obvious diurnal variation; it reached the peak at 8:00–10:00 am approximately.36 This result was consistent with field observations, implying that the variation of light intensity might influence the complexity of NOC composition at different times of the day and night, which would influence the physicochemical properties of atmospheric components. Finally, the results not only explore the relationship between reaction conditions and reaction kinetic parameters from a microscopic perspective, but also infer a corresponding mechanism of ammonium benzoate formation, which close some gaps of our knowledge on the NOC formation mechanism and reaction kinetics research. The study experimentally supports that the heterogeneous reactions of toluene/O3 with NH3 on nanoparticles can be considered as a new formation pathway of organic ammonium salts, which means that heterogeneous reactions are vital contributors to organic ammonium salt formation and also provide the necessary basis for controlling NOCs. These results highlighted the complexity of NOC formation processes in the atmospheric environment, which could contribute to further optimize the atmospheric chemical reaction model and provide a theoretical foundation for the source of NOCs in the actual environment. In addition, only toluene was investigated in this work, but the heterogeneous oxidation of other VOCs as well as other factors (such as temperature or humidity) could also significantly affect the formation of NOCs in the atmosphere.22,23 In order to well estimate the influence of combined pollution on high concentration NOCs in severe haze events, the effects of other factors should be considered.
Author contributions
Xin Liu: investigation, data curation, writing – original draft. Xiang He: conceptualization, methodology, supervision, writing – review & editing. Zhi-Cheng Ma: investigation, data curation. Xi Xi: investigation, validation. Shuang-Xi Wang: resources.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22366035, 22006124), Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01C387). The authors also thank the extensive help from the Physical and Chemical Testing Center of Xinjiang University.References
- D. Ge, W. Nie, P. Sun, Y. Liu, T. Wang, J. Wang, J. Wang, L. Wang, C. Zhu, R. Wang, T. Liu, X. Chi and A. Ding, Characterization of particulate organic nitrates in the Yangtze River Delta, East China, using the time-of-flight aerosol chemical speciation monitor, Atmos. Environ., 2022, 272, 118927 CrossRef CAS
.
- Q. Zhang, C. Anastasio and M. Jimenez-Cruz, Water-soluble organic nitrogen in atmospheric fine particles (PM2.5) from northern California, J. Geophys. Res.: Atmos., 2002, 107(D11), AAC 3-1-AAC 3-9 CrossRef
.
- A. C. Aiken, B. de Foy, C. Wiedinmyer, P. F. DeCarlo, I. M. Ulbrich, M. N. Wehrli, S. Szidat, A. S. H. Prevot, J. Noda, L. Wacker, R. Volkamer, E. Fortner, J. Wang, A. Laskin, V. Shutthanandan, J. Zheng, R. Zhang, G. Paredes-Miranda, W. P. Arnott, L. T. Molina, G. Sosa, X. Querol and J. L. Jimenez, Mexico-city aerosol analysis during MILAGRO using high resolution aerosol mass spectrometry at the urban supersite (T0) - Part 2: Analysis of the biomass burning contribution and the non-fossil carbon fraction, Atmos. Chem. Phys., 2010, 10(12), 5315–5341 CrossRef CAS
.
- J. Mao, Y. Cheng, Z. Bai, W. Zhang, L. Zhang, H. Chen, L. Wang, L. Li and J. Chen, Molecular characterization of nitrogen-containing organic compounds in the winter North China Plain, Sci. Total Environ., 2022, 838, 156189 CrossRef CAS PubMed
.
- J. R. Horne, S. Zhu, J. Montoya-Aguilera, M. L. Hinks, L. M. Wingen, S. A. Nizkorodov and D. Dabdub, Reactive uptake of ammonia by secondary organic aerosols: Implications for air quality, Atmos. Environ., 2018, 189, 1–8 CrossRef CAS
.
- M. O. Andreae and V. Ramanathan, Climate's Dark Forcings, Science, 2013, 340(6130), 280–281 CrossRef CAS PubMed
.
- A. Laskin, J. Laskin and S. A. Nizkorodov, Chemistry of Atmospheric Brown Carbon, Chem. Rev., 2015, 115(10), 4335–4382 CrossRef CAS PubMed
.
- Y. Liu, J. Liggio, R. Staebler and S. M. Li, Reactive uptake of ammonia to secondary organic aerosols: kinetics of organonitrogen formation, Atmos. Chem. Phys., 2015, 15(23), 13569–13584 CrossRef CAS
.
- F. Xiong, K. M. McAvey, K. A. Pratt, C. J. Groff, M. A. Hostetler, M. A. Lipton, T. K. Starn, J. V. Seeley, S. B. Bertman, A. P. Teng, J. D. Crounse, T. B. Nguyen, P. O. Wennberg, P. K. Misztal, A. H. Goldstein, A. B. Guenther, A. R. Koss, K. F. Olson, J. A. de Gouw, K. Baumann, E. S. Edgerton, P. A. Feiner, L. Zhang, D. O. Miller, W. H. Brune and P. B. Shepson, Observation of isoprene hydroxynitrates in the southeastern United States and implications for the fate of NO, Atmos. Chem. Phys., 2015, 15(19), 11257–11272 CrossRef CAS
.
- M. Huang, J. Xu, S. Cai, X. Liu, W. Zhao, C. Hu, X. Gu, L. Fang and W. Zhang, Characterization of brown carbon constituents of benzene secondary organic aerosol aged with ammonia, J. Atmos. Chem., 2018, 75(2), 205–218 CrossRef CAS
.
- Y. Wang, S. Cui, X. Fu, Y. Zhang, J. Wang, P. Fu, X. Ge, H. Li and X. Wang, Secondary organic aerosol formation from photooxidation of C3H6 under the presence of NH3: Effects of seed particles, Environ. Res., 2022, 211, 113064 CrossRef CAS PubMed
.
- M. Huang, J. Xu, S. Cai, X. Liu, C. Hu, X. Gu, W. Zhao, L. Fang and W. Zhang, Chemical analysis of particulate products of aged 1,3,5-trimethylbenzene secondary organic aerosol in the presence of ammonia, Atmos. Pollut. Res., 2018, 9(1), 146–155 CrossRef CAS
.
- S. E. Cornell, Atmospheric nitrogen deposition: Revisiting the question of the importance of the organic component, Environ. Pollut., 2011, 159(10), 2214–2222 CrossRef CAS PubMed
.
- M. D. Hernández-Alonso, I. Tejedor-Tejedor, J. M. Coronado, M. A. Anderson and J. Soria, Operando FTIR study of the photocatalytic oxidation of acetone in air over TiO2-ZrO2 thin films, Catal. Today, 2009, 143, 364–373 CrossRef
.
- K. M. Updyke, T. B. Nguyen and S. A. Nizkorodov, Formation of brown carbon via reactions of ammonia with secondary organic aerosols from biogenic and anthropogenic precursors, Atmos. Environ., 2012, 63, 22–31 CrossRef CAS
.
- H. Niu, K. Li, B. Chu, W. Su and J. Li, Heterogeneous Reactions between Toluene and NO2 on Mineral Particles under Simulated Atmospheric Conditions, Environ. Sci. Technol., 2017, 51(17), 9596–9604 CrossRef CAS PubMed
.
- X. He, Z. C. Ma, X. Xi, A. Kudesi and J. M. Wang, Heterogeneous reaction of toluene/NO2/O3 on α-Fe2O3 nanoparticles: The impacts of O3, light illumination and relative humidity on the N-containing organic compounds (NOC) formation, Environ. Sci.: Nano, 2022, 9, 3318–3330 RSC
.
- J. X. Warner, R. R. Dickerson, Z. Wei, L. L. Strow, Y. Wang and Q. Liang, Increased atmospheric ammonia over the world's major agricultural areas detected from space, Geophys. Res. Lett., 2017, 44(6), 2875–2884 CrossRef CAS PubMed
.
- Z. Luo, Y. Zhang, W. Chen, M. Van Damme, P. F. Coheur and L. Clarisse, Estimating global ammonia (NH3) emissions based on IASI observations from 2008 to 2018, Atmos. Chem. Phys., 2022, 22(15), 10375–10388 CrossRef CAS
.
- B. Chu, X. Zhang, Y. Liu, H. He, Y. Sun, J. Jiang, J. Li and J. Hao, Synergetic formation of secondary inorganic and organic aerosol: effect of SO2 and NH3 on particle formation and growth, Atmos. Chem. Phys., 2016, 16(22), 14219–14230 CrossRef CAS
.
- Z. Y. Meng, X. B. Xu, W. L. Lin, B. Z. Ge, Y. L. Xie, B. Song, S. H. Jia, R. Zhang, W. Peng, Y. Wang, H. B. Cheng, W. Yang and H. R. Zhao, Role of ambient ammonia in particulate ammonium formation at a rural site in the North China Plain, Atmos. Chem. Phys., 2018, 18(1), 167–184 CrossRef CAS
.
- K. Na, C. Song, C. Switzer and D. R. Cocker, Effect of Ammonia on Secondary Organic Aerosol Formation from α-Pinene Ozonolysis in Dry and Humid Conditions, Environ. Sci. Technol., 2007, 41(17), 6096–6102 CrossRef CAS PubMed
.
- Z. B. Babar, J. H. Park and H. J. Lim, Influence of NH3 on secondary organic aerosols from the ozonolysis and photooxidation of α-pinene in a flow reactor, Atmos. Environ., 2017, 164, 71–84 CrossRef CAS
.
- R. E. O'Brien, T. B. Nguyen, A. Laskin, J. Laskin, P. L. Hayes, S. Liu, J. L. Jimenez, L. M. Russell, S. A. Nizkorodov and A. H. Goldstein, Probing molecular associations of field-collected and laboratory-generated SOA with nano-DESI high-resolution mass spectrometry, J. Geophys. Res.: Atmos., 2013, 118(2), 1042–1051 CrossRef
.
- J. Li, H. Na, X. Zeng, T. Zhu and Z. Liu, In situ DRIFTS investigation for the oxidation of toluene by ozone over Mn/HZSM-5, Ag/HZSM-5 and Mn–Ag/HZSM-5 catalysts, Appl. Surf. Sci., 2014, 311, 690–696 CrossRef CAS
.
- C. Jia, S. Tong, X. Zhang, F. Li, W. Zhang, W. Li, Z. Wang, G. Zhang, G. Tang, Z. Liu and M. Ge, Atmospheric oxidizing capacity in autumn Beijing: Analysis of the O3 and PM2.5 episodes based on observation-based model, J. Environ. Sci., 2023, 124, 557–569 CrossRef CAS PubMed
.
- X. Xi, X. He, Z. C. Ma, H. Q. Ma and P. C. Liao, Formation processes of nitrogen-containing organic compounds from heterogeneous reactions of C3H6/NO2/O3 with α-Fe2O3 particles, Atmos. Environ., 2023, 295, 119567 CrossRef CAS
.
- B. Chu, X. Zhang, Y. Liu, H. He, Y. Sun, J. Jiang, J. Li and J. Hao, Effects of NO2 and C3H6 on the heterogeneous oxidation of SO2 on TiO2 in the presence or absence of UV-is irradiation, Atmos. Chem. Phys., 2019, 19(23), 14777–14790 CrossRef CAS
.
- T. Wang, Y. Liu, H. Deng, H. Cheng, Y. Yang and L. Zhang, Photochemical reaction of NO2 on photoactive mineral dust: Mechanism and irradiation intensity dependence, J. Photochem. Photobiol., A, 2021, 416, 113319 CrossRef CAS
.
- C. E. Nanayakkara, J. Pettibone and V. H. Grassian, Sulfur dioxide adsorption and photooxidation on isotopically-labeled titanium dioxide nanoparticle surfaces: roles of surface hydroxyl groups and adsorbed water in the formation and stability of adsorbed sulfite and sulfate, Phys. Chem. Chem. Phys., 2012, 14(19), 6957–6966 RSC
.
- C. George, M. Ammann, B. D'Anna, D. J. Donaldson and S. A. Nizkorodov, Heterogeneous Photochemistry in the Atmosphere, Chem. Rev., 2015, 115(10), 4218–4258 CrossRef CAS PubMed
.
- K. Li, L. Chen, S. White, H. Yu, X. Wu, X. Gao, M. Azzi and K. Cen, Smog chamber study of the role of NH3 in new particle formation from photo-oxidation of aromatic hydrocarbons, Sci. Total Environ., 2018, 619–620, 927–937 CrossRef CAS PubMed
.
- S. R. Tong, L. Y. Wu, M. F. Ge, W. G. Wang and Z. F. Pu, Heterogeneous chemistry of monocarboxylic acids on α-Al2O3 at different relative humidities, Atmos. Chem. Phys., 2010, 10(16), 7561–7574 CrossRef CAS
.
- G. Zhang, X. Lian, Y. Fu, Q. Lin, L. Li, W. Song, Z. Wang, M. Tang, D. Chen, X. Bi, X. Wang and G. Sheng, High secondary formation of nitrogen-containing organics (NOCs) and its possible link to oxidized organics and ammonium, Atmos. Chem. Phys., 2020, 20(3), 1469–1481 CrossRef CAS
.
- Y. Qiu, Z. Ma, K. Li, W. Lin, Y. Tang, F. Dong and H. Liao, Markedly Enhanced Levels of Peroxyacetyl Nitrate (PAN) During COVID-19 in Beijing, Geophys. Res. Lett., 2020, 47(19), 1–10 CrossRef
.
- J. Zhang, X. Wang, R. Li, S. Dong, J. Chen, Y. Zhang, P. Zheng, M. Li, T. Chen, Y. Liu, L. Xue, X. Zhou, L. Du, Q. Zhang and W. Wang, Significant impacts of anthropogenic activities on monoterpene and oleic acid-derived particulate organic nitrates in the North China Plain, Atmos. Res., 2021, 256, 105585 CrossRef CAS
.
- Y. Ahmed, Z. Yaakob and P. Akhtar, Degradation and mineralization of methylene blue using a heterogeneous photo-Fenton catalyst under visible and solar light irradiation, Catal. Sci. Technol., 2016, 6(4), 1222–1232 RSC
.
- H. Y. Lian, S. F. Pang, X. He, M. Yang, J. B. Ma and Y. H. Zhang, Heterogeneous reactions of isoprene and ozone on α-Al2O3: The suppression effect of relative humidity, Chemosphere, 2020, 240, 124744 CrossRef CAS PubMed
.
- T. Eby, U. Gundusharma, M. Lo, K. Sahagian, C. Marcott and K. Kjoller, Reverse engineering of polymeric multilayers using AFM-based nanoscale IR spectroscopy and thermal analysis, Spectrosc. Eur., 2012, 24, 18–21 CAS
.
- G. Centi, S. Perathoner and S. Tonini, In situ DRIFT study of the reactivity and reaction mechanism of catalysts based on iron-molybdenum oxides encapsulated in Boralite for the selective oxidation of alkylaromatics, Catal. Today, 2000, 61(1), 211–221 CrossRef CAS
.
- W. Yang, H. He, Q. Ma, J. Ma, Y. Liu, P. Liu and Y. Mu, Synergistic formation of sulfate and ammonium resulting from reaction between SO2 and NH3 on typical mineral dust, Phys. Chem. Chem. Phys., 2016, 18(2), 956–964 RSC
.
- W. Yang, Y. Liu, J. Ma, B. Chu, L. Wang and H. He, Role of NH3 in the Heterogeneous Formation of Secondary Inorganic Aerosols on Mineral Oxides, J. Phys. Chem. A, 2018, 122(30), 6311–6320 CrossRef CAS PubMed
.
- L. Jia and Y. Xu, Different roles of water in secondary organic aerosol formation from toluene and isoprene, Atmos. Chem. Phys., 2018, 18(11), 8137–8154 CrossRef CAS
.
- H. Sun, Z. Liu, S. Chen and X. Quan, The role of lattice oxygen on the activity and selectivity of the OMS-2 catalyst for the total oxidation of toluene, Chem. Eng. J., 2015, 270, 58–65 CrossRef CAS
.
- C. Du, L. Kong, A. Zhanzakova, S. Tong, X. Yang, L. Wang, H. Fu, T. Cheng, J. M. Chen and S. Zhang, Impact of heterogeneous uptake of nitrogen dioxide on the conversion of acetaldehyde on gamma-alumina in the absence and presence of simulated solar irradiation, Atmos. Environ., 2018, 187, 282–291 CrossRef CAS
.
- H. Einaga, K. Mochiduki and Y. Teraoka, Photocatalytic Oxidation Processes for Toluene Oxidation over TiO2 Catalysts, Catalysts, 2013, 3, 219–231 CrossRef CAS
.
- Q. Ma, Y. Liu, C. Liu and H. He, Heterogeneous reaction of acetic acid on MgO, α-Al2O3, and CaCO3 and the effect on the hygroscopic behaviour of these particles, Phys. Chem. Chem. Phys., 2012, 14(23), 8403–8409 RSC
.
- T. Wang, Y. Liu, Y. Deng, H. Fu, L. Zhang and J. Chen, Emerging investigator series: heterogeneous reactions of sulfur dioxide on mineral dust nanoparticles: from single component to mixed components, Environ. Sci.: Nano, 2018, 5(8), 1821–1833 RSC
.
- X. Zhao, L. Kong, Z. Sun, X. Ding, T. Cheng, X. Yang and J. Chen, Interactions between Heterogeneous Uptake and Adsorption of Sulfur Dioxide and Acetaldehyde on Hematite, J. Phys. Chem. A, 2015, 119(17), 4001–4008 CrossRef CAS PubMed
.
- H. Chen, L. Kong, J. Chen, R. Zhang and L. Wang, Heterogeneous Uptake of Carbonyl Sulfide on Hematite and Hematite-NaCl Mixtures, Environ. Sci. Technol., 2007, 41(18), 6484–6490 CrossRef CAS PubMed
.
- P. Xu, J. Xu, M. He, L. Song, D. Chen, G. Guo and H. Dai, Morphology and chemical characteristics of micro- and Nano-particles in the haze in Beijing studied by XPS and TEM/EDX, Sci. Total Environ., 2016, 565, 827–832 CrossRef CAS PubMed
.
- Y. J. Kim and C. R. Park, Analysis of Problematic Complexing Behavior of Ferric Chloride with N, N-Dimethylformamide Using Combined Techniques of FT-IR, XPS, and TGA/DTG, Inorg. Chem., 2002, 41(24), 6211–6216 CrossRef CAS PubMed
.
- C. Freitag, S. Besselmann, E. Löffler, W. Grünert, F. Rosowski and M. Muhler, On the role of monomeric vanadyl species in toluene adsorption and oxidation on V2O5/TiO2 catalysts: a Raman and in situ DRIFTS study, J. Mol. Catal. A: Chem., 2000, 162, 401–411 CrossRef
.
- C. Li, Q. He, Z. Fang, S. S. Brown, A. Laskin, S. R. Cohen and Y. Rudich, Laboratory Insights into the Diel Cycle of Optical and Chemical Transformations of Biomass Burning Brown Carbon Aerosols, Environ. Sci. Technol., 2020, 54(19), 11827–11837 CrossRef PubMed
.
- W. Yang, Q. Ma, Y. Liu, J. Ma, B. Chu and H. He, The effect of water on the heterogeneous reactions of SO2 and NH3 on the surfaces of α-Fe2O3 and γ-Al2O3, Environ. Sci.: Nano, 2019, 6, 2749–2758 RSC
.
- Z. Sun, L. Kong, X. Ding, C. Du, X. Zhao, J. Chen, H. Fu, X. Yang and T. Cheng, The effects of acetaldehyde, glyoxal and acetic acid on the heterogeneous reaction of nitrogen dioxide on gamma-alumina, Phys. Chem. Chem. Phys., 2016, 18(14), 9367–9376 RSC
.
- M. Sauerwein and C. K. Chan, Heterogeneous uptake of ammonia and dimethylamine into sulfuric and oxalic acid particles, Atmos. Chem. Phys., 2017, 17(10), 6323–6339 CrossRef CAS
.
- L. Li, Z. M. Chen, Y. H. Zhang, T. Zhu, J. L. Li and J. Ding, Kinetics and mechanism of heterogeneous oxidation of sulfur dioxide by ozone on surface of calcium carbonate, Atmos. Chem. Phys., 2006, 6(9), 2453–2464 CrossRef CAS
.
- L. Y. Wu, S. R. Tong, W. G. Wang and M. F. Ge, Effects of temperature on the heterogeneous oxidation of sulfur dioxide by ozone on calcium carbonate, Atmos. Chem. Phys., 2011, 11(13), 6593–6605 CrossRef CAS
.
- Q. Cao, B. W. Chu, P. Zhang, Q. X. Ma, J. Z. Ma, Y. Liu, J. Liu, Y. Q. Zhao, H. Zhang, Y. H. Wang and H. He, Effects of SO2 on NH4NO3 Photolysis: The Role of Reducibility and Acidic Products, Environ. Sci. Technol., 2023, 57(23), 8671–8679 CrossRef CAS PubMed
.
- C. Han, Y. Liu and H. He, Heterogeneous reaction of NO2 with soot at different relative humidity, Environ. Sci. Pollut. Res., 2017, 24(26), 21248–21255 CrossRef CAS PubMed
.
- Z. Wang, X. Pan, I. Uno, J. Li, Z. Wang, X. Chen, P. Fu, T. Yang, H. Kobayashi, A. Shimizu, N. Sugimoto and S. Yamamoto, Significant impacts of heterogeneous reactions on the chemical composition and mixing state of dust particles: A case study during dust events over northern China, Atmos. Environ., 2017, 159, 83–91 CrossRef CAS
.
- X. He, J. J. Wu, Z. C. Ma, X. Xi and Y. H. Zhang, NH3-promoted heterogeneous reaction of SO2 to sulfate on α-Fe2O3 particles with coexistence of NO2 under different relative humidities, Atmos. Environ., 2021, 262, 118622 CrossRef CAS
.
- P. Li, K. A. Perreau, E. Covington, C. H. Song, G. R. Carmichael and V. H. Grassian, Heterogeneous reactions of volatile organic compounds on oxide particles of the most abundant crustal elements: Surface reactions of acetaldehyde, acetone, and propionaldehyde on SiO2, Al2O3, Fe2O3, TiO2, and CaO, J. Geophys. Res.: Atmos., 2001, 106(D6), 5517–5529 CrossRef CAS
.
- M. A. Kebede, M. E. Varner, N. K. Scharko, R. B. Gerber and J. D. Raff, Photooxidation of Ammonia on TiO2 as a Source of NO and NO2 under Atmospheric Conditions, J. Am. Chem. Soc., 2013, 135(23), 8606–8615 CrossRef CAS PubMed
.
- H. He, A. Kudesi, X. S. Wang, X. Liu and L. Hu, Influence of temperature on the heterogeneous reaction of toluene to N-containing organic compounds using in situ DRIFTS, Atmos. Environ., 2023, 314, 120084 CrossRef
.
- T. Wang, Y. Liu, Y. Deng, H. Cheng, Y. Yang, Y. Feng, L. Zhang, H. Fu and J. M. Chen, Photochemical Oxidation of Water-Soluble Organic Carbon (WSOC) on Mineral Dust and Enhanced Organic Ammonium Formation, Environ.
Sci. Technol., 2020, 54, 15631–15642 CrossRef CAS PubMed
.
- W. H. Ching, M. K. H. Leung and D. Leung, Solar photocatalytic degradation of gaseous formaldehyde by sol-gel TiO2 thin film for enhancement of indoor air quality, Sol. Energy, 2004, 77, 129–135 CrossRef CAS
.
- M. Wu, Y. H. Kwok, Y. Zhang, W. Szeto, H. Huang, D. Y. C. Leung, H. Huang and D. Y. C. Leung, Synergetic effect of vacuum ultraviolet photolysis and ozone catalytic oxidation for toluene degradation over MnO2-rGO composite catalyst, Chem. Eng. Sci., 2021, 231, 116288 CrossRef CAS
.
- H. Einaga, S. Futamura and T. Ibusuki, Heterogeneous photocatalytic oxidation of benzene, toluene, cyclohexene and cyclohexane in humidified air: comparison of decomposition behavior on photoirradiated TiO2 catalyst, Appl. Catal., B, 2002, 38(3), 215–225 CrossRef CAS
.
- K. Barnard, V. R. Bright, R. J. Enright, K. Fahy, A. Liu and P. E. Hoggard, Heterogeneous Catalysis by Tetraethylammonium Tetrachloroferrate of the Photooxidation of Toluene by Visible and Near-UV Light, Catalysts, 2018, 8(2), 79 CrossRef
.
- A. Mills and C. O'Rourke, Photocatalytic oxidation of toluene in an NMR tube, J. Photochem. Photobiol., A, 2012, 233, 34–39 CrossRef CAS
.
- A. J. Jafari, H. Arfaeinia, B. Ramavandi, R. R. Kalantary and A. Esrafily, Ozone-assisted photocatalytic degradation of gaseous toluene from waste air stream using silica-functionalized graphene oxide/ZnO coated on fiberglass: performance, intermediates, and mechanistic pathways, Air Qual., Atmos. Health, 2019, 12(10), 1181–1188 CrossRef CAS
.
- H. Huang and W. Li, Destruction of toluene by ozone-enhanced photocatalysis: Performance and mechanism, Appl. Catal., B, 2011, 102(3), 449–453 CrossRef CAS
.
- H. Pye, D. Luecken, L. Xu, C. Boyd, N. Ng, K. Baker, B. Ayres, J. Bash, K. Baumann, W. Carter, E. Edgerton, J. Fry, W. Hutzell, D. Schwede and P. Shepson, Modeling the Current and Future Roles of Particulate Organic Nitrates in the Southeastern United States, Environ. Sci. Technol., 2015, 49(24), 14195–14203 CrossRef CAS PubMed
.
- R. E. O'Brien, A. Laskin, J. Laskin, S. Liu, R. Weber, L. M. Russell and A. H. Goldstein, Molecular characterization of organic aerosol using nanospray desorption/electrospray ionization mass spectrometry: CalNex 2010 field study, Atmos. Environ., 2013, 68, 265–272 CrossRef
.
- X. Wang, S. Gao, X. Yang, H. Chen, J. M. Chen, G. Zhuang, J. Surratt, M. N. Chan and J. Seinfeld, Evidence for high molecular weight nitrogen-containing organic salts in urban aerosols, Environ. Sci. Technol., 2010, 44(12), 4441–4446 CrossRef CAS PubMed
.
- T. Feng, S. Zhao, B. Hu, N. Bei, X. Zhang, J. Wu, X. Li, L. Liu, R. Wang, X. Tie and G. Li, Assessment of Atmospheric Oxidizing Capacity Over the Beijing-Tianjin-Hebei (BTH) Area, China, J. Geophys. Res.: Atmos., 2021, 126(7), e2020JD033834 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3en00625e |
| This journal is © The Royal Society of Chemistry 2024 |