MOF derived metal oxide nanohybrids with in situ grown rGO: a smart material for simultaneous electrochemical sensing of HQ and RS†
Tayyaba
Iftikhar‡
a,
Muhammad Irfan
Majeed
 b,
Ayesha
Aziz
c,
Anees A.
Khadom
d,
Zhuo
Huang
e,
Ghazala
Ashraf
c,
Guangfang
Li
a,
Muhammad
Asif§
*a,
Fei
Xiao
*a and
Hongfang
Liu
b,
Ayesha
Aziz
c,
Anees A.
Khadom
d,
Zhuo
Huang
e,
Ghazala
Ashraf
c,
Guangfang
Li
a,
Muhammad
Asif§
*a,
Fei
Xiao
*a and
Hongfang
Liu
 *a
*a
aKey Laboratory of Material Chemistry for Energy Conversion and Storage, Ministry of Education, Hubei Key Laboratory of Material Chemistry and Service Failure, Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan 430074, P. R. China. E-mail: asif83chemist@gmail.com; xiaofei@hust.edu.cn; liuhf@hust.edu.cn
bUniversity of Agriculture, Faisalabad, Punjab, Pakistan
cCollege of Life Science and Technology, Huazhong University of Science and Technology (HUST), Wuhan, 430074, P. R. China
dDepartment of Chemical Engineering, College of Engineering, University of Diyala, Baquba City 32001, Diyala Governorate, Iraq
eChangjiang River Scientific Research Institute of Changjiang Water Resources Commission, 289 Huangpu Street, Wuhan, Hubei, P. R. China
First published on 12th December 2023
Abstract
The untapped potential of electrochemical sensors based on metal–organic framework (MOF) derived metal oxides is still challenging in this globalization era to sense environmental pollutants. Herein, HKUST-1/rGO/CuO/α-Fe2O3 nanocomposites (NCs) have been fabricated via a single facile step of the hydrothermal process and utilized to modify the glassy carbon electrode (GCE) surface for simultaneous hydroquinone (HQ) and resorcinol (RS) detection. Electrochemical sensing is nevertheless hampered by the fact that MOFs have limited electrical conductivity, and their framework normally collapses upon calcination, which limits their applicability. MOFs can be used as a template and combined with other conductive materials to evade these pitfalls. The high electrocatalytic activity, increased surface area, abundant nanoscale interactions, and superb conductivity of these hybrids have efficiently increased redox reactions through their synergistic effect at the electrode surface. With a working potential of +0.39 V and +0.72 V (vs. Ag/AgCl electrode), the modified GCE exhibits great electro-oxidation for HQ and RS. The respective limits of detection (LODs) are 50 nM and 80 nM (S/N = 3) with 0.05–10 μM and 0.08–12 μM linear ranges, respectively. The sensing podium based on HKUST-1/rGO/CuO/α-Fe2O3 has also been employed to detect HQ and RS in skin whitening creams and hair toners to assess its practicability. Thus, we believe that this structural integration technique has much potential in material synthesis, energy storage, catalysis, and sensing.
Environmental significanceThe study on HKUST-1/rGO/CuO/α-Fe2O3 nanocomposites (NCs) for electrochemical sensing of hydroquinone (HQ) and resorcinol (RS) has significant implications for environmental pollution control. These environmental pollutants can contaminate waterways and soil, leading to toxic effects on aquatic life, wildlife, humans and the environment. Likewise, the presence of HQ and RS in skin whitening creams and hair toners highlights the potential for environmental contamination from personal care products. The successful individual and simultaneous detection of these pollutants is crucial for effective environmental pollution control and management. These sensors could be used in industrial processes and personal care product manufacturing to prevent contamination. In summary, this study presents a promising approach to detecting and managing the impact of environmental pollutants, contributing to the goal of protecting human health and the environment. |
I. Introduction
The swift evolution of modern industry has been found to contaminate the environment which is becoming more threatening to the survival of animals as well as humans. Phenolic compounds such as hydroquinone (1,4-dihydroxybenzene/benzene-1,4-diol/quinol indicated as HQ) and resorcinol (benzene-1,3-diol/1,3-dihydroxybenzene, depicted as RS) have been reported by the International Agency for Research on Cancer (IARC) as group 2B and 3B carcinogens because of their extremely i.e., high or low noxious degradation level in the ecological environment, respectively.1,2 These two major dihydroxybenzene isomers are frequently used in engineering technologies, including drugs, insecticides, bronzing, colorants, polymers, flavoring agents, beauty products, etc. They have alike structures and behaviors that can cause interference with each other during their simultaneous detection.3 Consequently, developing a simple, fast, selective, sensitive, and accurate analytical tool for simultaneous and individual detection of these isomers is mandatory.Detecting phenolic compounds, including HQ and RS, presents challenges in analytical chemistry. Various approaches have been explored, such as high-performance liquid chromatography (HPLC),4 spectrophotometry,5 fluorescence,6 chemiluminescence,7 capillary electrochromatography,8 gas chromatography/mass spectrometry,9 and electroanalytical methods, among others. Despite their utility, these techniques are often marred by complexities in sample preparation, time-intensive procedures, and high costs.10 As a more favorable alternative, electrochemical analysis has garnered considerable attention in recent years due to its simplicity, cost-effectiveness, ease of operation, robotic compatibility, portability, and rapid response times.11–13 It is important to note that the efficacy of electrochemical analysis is closely linked to the specific composition of the electrode material used.
Porous coordination polymers like metal–organic frameworks (MOFs) have gained prominence in analytical chemistry for electrochemically detecting dihydroxybenzene isomers (HQ and RS) in ecological and commercialization specimens.14 These compounds belong to an emerging category of hybrid organic–inorganic functional materials, combining metal ions or clusters with organic bridging linkers.15–17 An essential focus in MOF sensing research is the efficient transduction of electrochemical signals. Attaining this objective relies on the precise preparation of MOFs while addressing their inherent limitation of low electrical conductivity, as MOFs tend to demonstrate insulating properties.18,19
Distinguished by their intricate structural composition and unique morphological features, MOFs have drawn significant attention within the scientific community. For instance, Abrori et al. recently demonstrated the effectiveness of MOF-71, a cobalt-based MOF, in detecting uric acid within a neutral three-electrode system. Moreover, the combination of MOF-71 with the DPV technique exhibited a notable sensitivity, with a detection range of 50.0–1000 μM and a LOD of 15.61 μM.20 Hu et al. illustrated the successful utilization of Cr-MOF nanoparticles synthesized through a hydrothermal approach for effective electrochemical detection of p-nitrophenol (p-NP) on a Cr-MOF/GCE. Notably, the sensor exhibited a 0.7 μM detection limit for p-NP within a comprehensive 2–500 μM range, demonstrating exceptional stability in various interfering media. Moreover, its sensitivity was superior or equivalent to previously reported sensors.21
Additionally, to address the limited electrical conductivity of MOFs, researchers have explored synergistic strategies by integrating MOFs with other functional materials known for their high electrical conductivity.22 This method not only boosts the electrochemical stability of MOFs but also retains their distinctive porous structure, facilitating the development of efficient sensors.23 Nevertheless, Cu-based MOFs (HKUST-1/Cu-MOFs) have become more popular due to their economical and easy preparation. Additionally, they have a large porous volume, wide surface area, exceptional structural tunability, and excellent chemical stability.24 In this regard, Arul et al. addressed the low conductivity issue of Cu-MOFs by fabricating a Cu-MOF on a GC electrode via electrodeposition. Optimizing the CuO electrodeposition, they successfully detected glucose.25
To further improve the overall conductivity, MOFs can be coupled with highly conductive materials, i.e., graphene, graphene oxide (GO), reduced graphene oxide (rGO), and mixed-transition metal oxides (MTMOs). Thus, they may perform better electrochemistry than their single-metal or non-metal oxide material counterparts.26–28 Furthermore, nanostructured metal oxides, particularly CuO, are vital in the electrochemical detection of various analytes.29,30 Likewise, Chen et al. developed a CuO-histidine functionalized graphene quantum dot hybrid with an open-porous structure, enhancing the detection of HQ compared with the pure CuO/GCE. On the other hand, Jahani et al. introduced a protocol to synthesize CuO NPs on N-doped rGO NCs, leveraging the sp2 hybridized carbon framework provided by rGO. The CuO/N-rGO-modified CPE displayed high sensitivity, a wide detection range, and a low LOD for HQ detection.31 Also, Senthil et al. established the α-Fe2O3–GO/GCE for detecting HQ, highlighting its enhanced performance over the pure α-Fe2O3/GCE.32
MTMOs have gained popularity as an alternative to single transition metal oxides (STMOs) due to their superior electrochemical properties.33 A typical transition metal oxide, iron oxide (Fe2O3), has excellent potential among other transition metal oxides.34 Additionally, α-Fe2O3, β-Fe2O3 (hematite), γ-Fe2O3 (maghemite), and ε-Fe2O3 are four crystalline phases of Fe2O3. Among all, α-Fe2O3 is the most stable under ambient conditions with n-type semiconductor properties, is abundant in nature, has a suitable working window, and has a favorable bandgap of 2.1–2.2 eV.35 Moreover, the synergy influence of these MTMOs, such as CuO and α-Fe2O3, along with a carbon conductive material (rGO), is currently expected to make them effective sensing elements for ensuring environmental protection against hazards, which researchers are pursuing.36 There is a variety of NCs based on transition metals that have been recommended for HQ and RS monitoring. However, these NCs have some drawbacks, including low stability and sensitivity, overpotential, and less current response.37 They still need precise control over size and distribution.
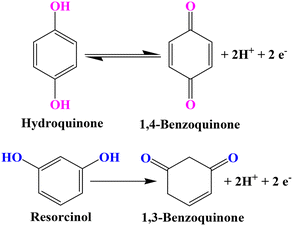
Herein, we report a novel, simple strategy to synthesize HKUST-1/rGO/CuO/α-Fe2O3 NCs through a single-step hydrothermal route and use it to develop an electrochemical sensor. The incorporation of electrochemically active α-Fe2O3 nano-flake flowers into HKUST-1/rGO/CuO NCs has a synergistic effect with the conductive backbone and robust support provided by the HKUST-1/rGO/CuO that can be a driving force behind the superb electrocatalytic aptitude of HKUST-1/rGO/CuO/α-Fe2O3. However, the prepared NCs are simple to fabricate, cheap, ecologically friendly, and sensitive enough. They can monitor HQ and RS in real samples without pre-concentration stages or chemical separation. HQ and RS quantification in cosmetics such as whitening cream and hair toner samples has been carried out through a GCE modified with HKUST-1/rGO/CuO/α-Fe2O3 NCs. In addition, the modified electrode is an excellent choice for real sample analysis due to its low LOD, high selectivity, and reliability. The redox reaction mechanism of HQ and RS is also depicted in Scheme 1.38
II. Experimental setup
1. Materials and reagents
Various reagents like lauric acid (C12H24O2, 98%), copper nitrate trihydrate (Cu(NO3)2·3H2O, 99%), ethanol (C2H5OH, 99%), 1,3,5-benzenetricarboxylic acid (BTC, 99%), methanol (CH3OH, 99.8%), acetone (99%), n-butyl alcohol (99.8%), hydroquinone (C6H6O2, 99%), resorcinol (C6H6O2, 99%), isopropanol (98.8%), ferric nitrate hexahydrate (Fe(NO3)3·6H2O, 99%), and hexamethylenetetramine (HMT, 99.9%) were bought from Chemical Reagent Limited Subsidiary Company of Sino Pharm Group based in Shanghai, China. All the chemicals, as mentioned above, were pure and of analytical grade. Throughout the studies, double-distilled (DD) H2O was employed to prepare all solutions. During experiments, KH2PO4 (>99%) and K2HPO4·3H2O (>99%) were used in a suitable amount to make 0.1 M PBS (7.2 pH) as a supporting electrolyte solution.2. Instrumentation
X-ray diffraction patterns (XRD) recorded on a Rigaku D/max-r A diffractometer (Japan manufactured) operating with Cu Kα radiation (40 kV, 200 mA) and a Ni filter were used to analyze the structural features of the synthesized materials. A DLD-600 W-X spectrometer (AXIS-ULTRA) from Shimadzu Company performed XPS in order to determine the valence state of the constituent composition. With an acceleration voltage of 200 kV, a G220 U-Twin instrument from TECNAI, The Netherlands was used to perform transmission electron microscopy (TEM) as well as high transmission electron microscopy (HRTEM), and scanning electron microscopy (SEM) was carried out on a HITACHI X-650, Hitachi Company based in Japan for morphological analysis. The determination of pore size, pore volume, and specific surface area through Brunauer–Emmett–Teller (BET) analysis was conducted using Micro Active for ASAP 2460 (Version 2.01) obtained from Shanghai, China. A CHI760E electrochemical workstation from CH Instruments, Shanghai, China was used to perform all the electrochemical experiments like electron impedance spectroscopy (EIS), CV, and DPV in 0.1 M PBS (10 mL, pH = 7.2) deoxygenated in the conventional system of three electrodes such as counter (platinum, Pt wire), reference (silver/silver chloride/saturated KCl), and working (GCE) electrodes at ambient temperature to conduct the experiments. In CV and DPV, −0.6 V to 1.2 V (working potentials) were used. For EIS, a 0.1–106 Hz frequency range and sinusoidal potential amplitude (0.005 V) were accomplished.3. Synthesis of HKUST-1/rGO/CuO/α-Fe2O3 nanocomposites
Hummers' modified method was used to synthesize graphene oxide (GO) from pure graphite. The GO (1 mg mL−1) was reduced by N2H2 (4 mL) overnight to synthesize reduced graphene oxide (rGO) at 80 °C. After achieving a rGO dispersion concentration of 0.5 mg mL−1, 2 mL of this rGO dispersion was mixed with GO solution. Then, this solution was further sonicated and stirred overnight.HKUST-1/rGO was fabricated through a solvothermal synthesis route in which 1.8 g of Cu(NO3)2·3H2O and BTC (2 g) were dissolved separately in CH3OH (15 mL), with these two solutions being designated as A and B, respectively. A clear form of solution C is produced after mixing solution A with solution B, and 2.55 g of lauric acid was added into solution C while being vigorously stirred. After half an hour, 2 mL of rGO, prepared from the above procedure, was introduced to solution C. At room temperature, it was stirred under persistent mechanical stirring for two hours. In a Teflon-lined stainless steel autoclave, the suspension was heated to 120 °C for 24 hours. For the next step, the powder was extracted and washed with CH3OH multiple times before drying it up to 12 hours at 60 °C. Finally, HKUST-1/rGO was prepared and further transformed into HKUST-1/rGO/CuO at 300 °C for two hours in a muffle furnace. Similar steps were followed, but no rGO was employed to prepare HKUST-1/CuO.
For the synthesis of HKUST-1/rGO/CuO/α-Fe2O3 NCs, 0.12 g of HKUST-1/rGO/CuO, 1.41 g of Fe(NO3)3·6H2O, and 0.45 g of HMT were dispersed in 40 mL of deionized H2O at room temperature to form a turbid solution. The above solution was then kept under constant stirring until it became clear. Next, the solution was autoclaved for 6 hours at 160 °C in a Teflon-lined hydrothermal autoclave (50 mL). At 80 °C, the resultant products were washed, rinsed, and dried several times with CH3OH for 12 hours. After cooling to room temperature, the final products were calcined at 500 °C with 3.5 °C min−1 (ramping rate). Thus, the HKUST-1/rGO/CuO/α-Fe2O3 NCs were obtained by placing the sample in flowing air for one hour. Fig. 1 shows the formation of HKUST-1/rGO/CuO/α-Fe2O3 NCs.
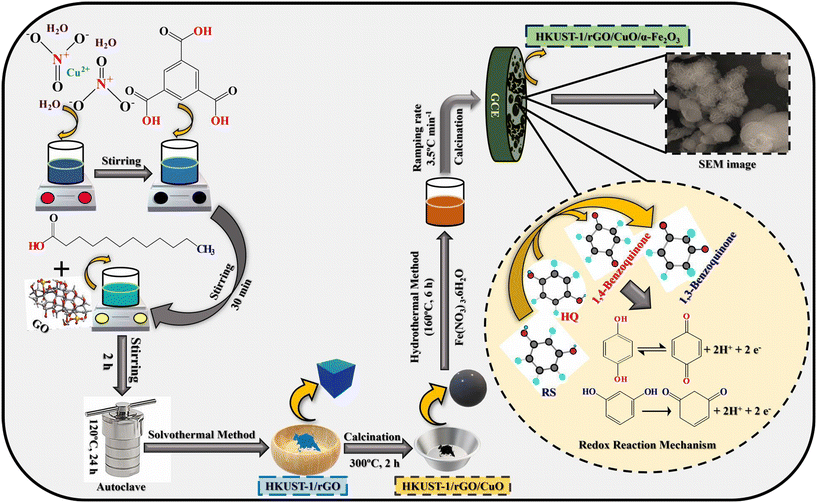 | ||
| Fig. 1 Schematic diagram of numerous stages involved in the fabrication route of HKUST-1/rGO/CuO/α-Fe2O3 NCs and the electrochemical sensing mechanism. | ||
4. Working electrode preparation
Alumina slurry of 0.30 and 0.050 μm on micron-sized cloth pads was used to clean and modify the GCE surface (3 mm in diameter). After that, double-distilled H2O, as well as C2H5OH, was further utilized to rinse the GCE surface ultrasonically for a minute. N2 gas was purged to remove the moisture altogether. Finally, 2 mg mL−1 of each suspension, including all prepared NCs of HKUST-1, HKUST-1/CuO, HKUST-1/rGO/CuO, and HKUST-1/rGO/CuO/α-Fe2O3, was put into a vial. At ambient temperature, 5 μL of these suspensions (freshly prepared) were painted individually onto the polished surface of the GCE and dried in air.5. Whitening cream sample preparation
Two commercial whitening cream products with HQ were examined. For each sample, 0.10 g of each was mixed with 5 mL of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (60
water (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40). Then, filter paper was used to filter the mixture. The filtrate was maintained at 4 °C until the proposed sensor was used to analyze it. Afterward, the voltammetric cell was filled with 10 mL of PBS (7.2 pH), and 1 mL of that prepared solution was added.
40). Then, filter paper was used to filter the mixture. The filtrate was maintained at 4 °C until the proposed sensor was used to analyze it. Afterward, the voltammetric cell was filled with 10 mL of PBS (7.2 pH), and 1 mL of that prepared solution was added.
6. Sample preparation of hair toner
Two commercial products of hair toner containing RS were bought to test RS. Each product (1 g) was added to deionized water with continuous stirring (30 min.). This was followed by adding 1 mL of the freshly made solution straight into the voltammetric cell along with 10 mL of PBS (pH = 7.2).III. Results and discussion
1. HKUST-1/rGO/CuO/α-Fe2O3 NC morphology and characterization
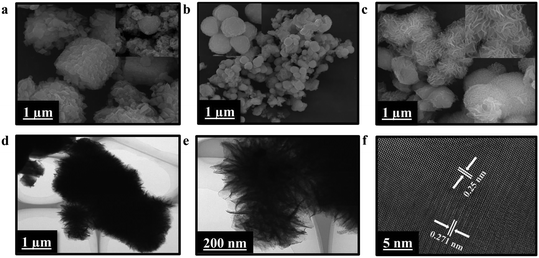
The surface morphology and structure of HKUST-1/rGO/CuO/α-Fe2O3 NCs at different magnifications have been analyzed via SEM and TEM techniques. HKUST-1, HKUST-1/CuO (inset of Fig. 2a), HKUST-1/rGO/CuO, and HKUST-1/rGO/CuO/α-Fe2O3 SEM images are presented correspondingly in Fig. 2a–c. Fig. 2a, inset of Fig. 2ab, and 2 show the effective formation of porous cubic (HKUST-1) and spherical (CuO) particles along with in situ development of rGO (2D nanosheets), respectively. The spherical form of CuO NPs has a diameter ranging from 4–4.626 μm. It can be seen in Fig. 2c that the nano-flake flowers of α-Fe2O3 in HKUST-1/rGO/CuO/α-Fe2O3 possess a uniform and smooth distribution with no agglomeration, resulting in loose porous nanostructures with a large number of open spaces and surface active sites.The morphologies of HKUST-1/rGO/CuO/α-Fe2O3 NCs following calcination are shown by typical images of TEM and HRTEM (Fig. 2d–f). The rGO dispersion restricts the aggregation of HKUST-1, CuO, and α-Fe2O3 in HKUST-1/rGO/CuO/α-Fe2O3 NCs. The HRTEM image is depicted in Fig. 2f, displaying the crystallinity of CuO and α-Fe2O3 with 0.25 nm and 0.271 nm lattice parameters. Additionally, this refers to CuO monoclinic (111) and α-Fe2O3 rhombohedral (104) planes, following XRD analysis. Therefore, Fig. 2 illustrates the augmented charge transfer mobility attributable to the crystal structure and porosity of the material and its expansive surface area, narrow band gap, and distinctive cubic, spherical, and nano-flake flower-like morphology of the NCs.
The Brunauer–Emmett–Teller (BET) method has been used for analyzing the surface area of HKUST-1, HKUST-1/CuO, HKUST-1/rGO/CuO, and HKUST-1/rGO/CuO/α-Fe2O3 NCs. The pore volume and pore size have been calculated using the Barrett–Joyner–Halenda (BJH) method. In the domain of nanomaterials, the correlation between surface area and performance is a well-established phenomenon, notably observed in sorbents and catalysts. The possibility exists that the introduction of new materials or synthetic modifications can induce alterations in the surface area, consequently influencing the overall performance of the material. Employing BET with gas adsorption facilitates the identification of these changes. In our study, the surface area of HKUST-1/rGO/CuO/α-Fe2O3 is notably larger, measuring 60.018 m2 g−1, compared to HKUST-1/rGO/CuO, HKUST-1/CuO, and HKUST-1. This increase is attributed to the synergistic effects arising from the distinctive characteristics of HKUST-1/rGO/CuO and α-Fe2O3 nanostructures. The physical properties, including pore size, pore volume, and surface area, have been comprehensively calculated and listed in Table S1.† Their comprehensive evaluation provides valuable insights into it.
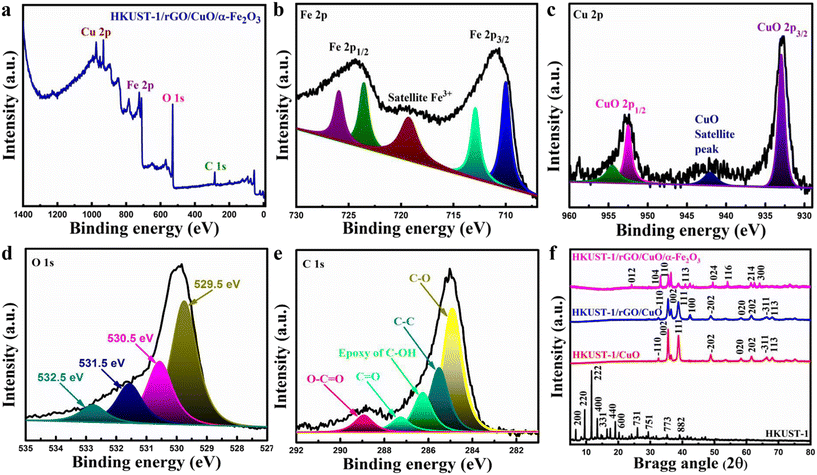
The XPS technique is applied to inspect the elemental chemical state and surface composition of HKUST-1/rGO/CuO/α-Fe2O3 NCs. The full XPS spectra of HKUST-1/rGO/CuO/α-Fe2O3 NCs can be seen in Fig. 3a, which proves the existence of elements like Fe, O, Cu, and C. In Fig. 3b, the Fe 2p deconvolution spectrum produces four distinct peaks. The core-level fitting of Fe 2p3/2 and Fe 2p1/2 specifies binding energies (BEs) at 709.5 eV and 723 eV, respectively, as signatures of the Fe2+ oxidation state. In comparison, the BEs at 712.8 eV and 725.3 eV of the Fe 2p3/2 and Fe 2p1/2 core-level spectra confirm the presence of Fe3+ moieties, respectively. Moreover, a satellite peak at 719.21 eV is assigned to Fe3+ in α-Fe2O3. Furthermore, the Fe 2p3/2 and Fe 2p1/2 core-level spectra show oxygen vacancies on the α-Fe2O3 surface due to the mixed iron system (Fe3+/Fe2+) in the nano-flake flowers.39
Fig. 3c presents the core level spectrum of CuO 2p3/2 in which copper exists as Cu2+ at a BE of 932.7 eV. However, the BEs of core level CuO 2p1/2 at 952.3 eV and 954.53 eV also indicate the presence of a Cu2+ oxidation state. At 942.6 eV, there is a satellite peak of Cu2+ in the 3d9 electronic configuration.40 Likewise, Fig. 3d depicts an O 1s spectrum that has been deconvoluted into four peaks at different BEs of 529.5 eV (metal–O bonds), 530.5 eV (OH groups), 531.5 eV (organic C–O), and 532.5 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O organic), respectively.41 The core-level spectrum of C 1s presents five distinct peaks in Fig. 3e as rGO characteristic at BEs of 288.321 eV, i.e., O–C
O organic), respectively.41 The core-level spectrum of C 1s presents five distinct peaks in Fig. 3e as rGO characteristic at BEs of 288.321 eV, i.e., O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 287.641 eV, i.e., C
O, 287.641 eV, i.e., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 286.316 eV, i.e., epoxy of C–OH, 285.491 eV, i.e., C–C, and 284.721 eV, i.e., C–O, correspondingly.42
O, 286.316 eV, i.e., epoxy of C–OH, 285.491 eV, i.e., C–C, and 284.721 eV, i.e., C–O, correspondingly.42
Fig. 3f illustrates an XRD analysis used to determine the crystallographic planes of the NCs. All the diffraction peaks in HKUST-1, particularly those at 6.6°, 9.4°, 11.5° and 13.3°, correspond to the (200), (220), (222), and (400) crystal planes which are accredited to the successful synthesis of HKUST-1.43 Similarly, all CuO diffraction peaks attributable to the monoclinic system are present in HKUST-1/CuO (JCPDS no. 05-0661).44 A diffraction peak at 42.38° demonstrates the 100 plane of rGO and is assigned to rGO successful intercalation in the HKUST-1/rGO/CuO composite.45,46 The other sharp peaks of CuO indicate its high degree of crystallinity. Additionally, the absence of other pure HKUST-1 and rGO peaks is primarily ascribed to the strong peaks of CuO and significantly less quantity of pure HKUST-1 and rGO than CuO. Besides, it could be discerned in HKUST-1/rGO/CuO/α-Fe2O3 that the different diffraction peaks originating at 24.2°, 33.1°, 35.8°, 40.8°, 49.6°, 54.1°, 61.3° and 64.1° can be well indexed to the rhombohedral structure lattice planes of 012, 104, 110, 113, 024, 116, 214, and 300 in the α-Fe2O3 phase (JCPDS: 79-0007).47
In contrast to these lattice planes, the remaining peaks at 32.6°, 35.4°, 38.6°, 48.9°, 53.4°, 58.3°, 66° and 68.02° in HKUST-1/rGO/CuO/α-Fe2O3 are all derived from the CuO planes that belong to the −110, 002, 111, −202, 020, 202, −311 and 113 crystal planes, respectively. Nevertheless, there are no such α-Fe2O3 peaks in HKUST-1, HKUST-1/CuO, and HKUST-1/rGO/CuO. The presence of α-Fe2O3 peaks in HKUST-1/rGO/CuO/α-Fe2O3 confirms the α-Fe2O3 thick coating. Thus, all these results verify the efficacious HKUST-1/rGO/CuO/α-Fe2O3 NC synthesis via integration of pure and highly crystalline α-Fe2O3 nano-flake flowers.
2. Voltammetric response of the HKUST-1/rGO/CuO/α-Fe2O3 NC electrode
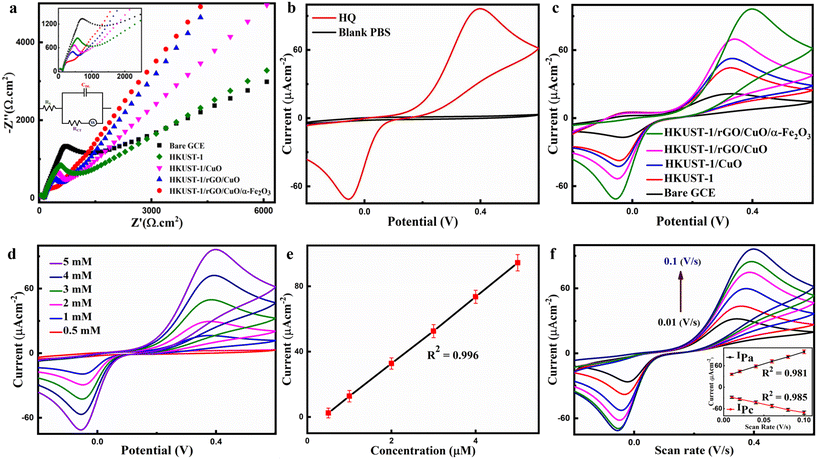
EIS is the most effective method for examining the interfacial characteristics of the unmodified and modified GCEs, i.e., HKUST-1/GCE, HKUST-1/CuO/GCE, HKUST-1/rGO/CuO/GCE, and HKUST-1/rGO/CuO/α-Fe2O3/GCE with an [Fe(CN)6]3−/4− redox probe and KCl (0.1 M) as depicted in Fig. 4a (fitted with Nyquist plots). The solution resistance, double-layer capacitance, charge transfer resistance, and Warburg constant are all presented by the terms such as Rs, Cdl, Rct, and W in the inset of the Randles equivalent circuit. The results reveal that the unmodified GCE displays considerably large resistance with an Rct of 1332.36 Ω. On the other hand, HKUST-1/GCE, HKUST-1/CuO/GCE, and HKUST-1/rGO/CuO/GCE exhibit a decrease in Rct values of 929.11 Ω, 770.32 Ω, and 676.13 Ω because of their conductivity.Furthermore, HKUST-1/rGO/CuO/α-Fe2O3 illustrates a huge decrease in the Rct value up to 461 Ω in comparison to the unmodified and modified GCEs. This decrease might have been attributed to various factors such as (i) incorporation of α-Fe2O3 in the composite of HKUST-1/rGO/CuO, which provides a plethora of electrochemical active sites for the reaction owing to the coordination effect between electrocatalysts of mixed transition metal oxides (CuO and α-Fe2O3), (ii) HKUST-1 might have supplied a large surface volume for the reaction to proceed, (iii) the conductive backbone of rGO might have enhanced the electrochemical conductivity and reduced the solid-state diffusion, and (iv) the synergy effect in HKUST-1/rGO/CuO/α-Fe2O3 NCs might have improved the adequate bonding between the electrode and electrolyte by rapid electron transfer pathways.
Meanwhile, the HKUST-1/rGO/CuO/α-Fe2O3/GCE is considered as the best choice for sensor applications. Also, these modified electrodes have shown similar results while performing CV in [Fe(CN)6]3−/4−, as shown in Fig. S1.† However, the Randles–Sevcik equation is used to figure out the active surface areas of the unmodified and various modified GCEs electrochemically. Moreover, CV measurements have also been recorded to investigate the electrocatalytic efficiency of the modified electrode in PBS (pH = 7.2) with 5 mM HQ. The electrochemical responses of both unmodified and modified GCEs are examined across the potential range of −0.20 V to 0.90 V, utilizing a scan rate of 0.1 V s−1. The cyclic voltammograms in Fig. 4b illustrate the presence of HQ, demonstrating a distinct anodic peak current (Ipa) and cathodic peak current (Ipc) at 0.39 V and −0.05 V, respectively. These peaks signify the quasi-reversible two-electron, two-proton reaction mechanism of HQ, as depicted in Scheme 1. However, in the absence of HQ, no anodic peak can be found. There is a 0.44 V difference between the redox peak potential for HQ. The stability of p-benzoquinone at the electrode surface can be inferred by the nearly unity peak current ratio of Ipc/Ipa.48,49
Meanwhile Fig. 4c shows the CV responses of the unmodified GCE and various modified electrodes (HKUST-1, HKUST-1/CuO, HKUST-1/rGO/CuO, and HKUST-1/rGO/CuO/α-Fe2O3) in 5 mM HQ concentration PBS (pH = 7.2), scanned at a rate of 0.100 V s−1 within the potential window of −0.2 V to 0.90 V. It can be seen in Fig. 4c that with a sluggish response to the electrocatalytic reaction, the bare GCE demonstrates a limited capacity for facilitating the redox reaction of HQ, leading to a relatively lower Ipa value. Next, the surface of the bare GCE modified with HKUST-1 shows improved adsorption capacity, resulting in enhanced redox current peaks of HQ compared to the bare GCE. The increased Ipa value indicates the enhanced electrocatalytic activity facilitated by the strong adsorption capacity of HKUST-1. Later, the bare GCE modified with the HKUST-1/CuO composite further amplifies the electrocatalytic response, leading to a notable increase in the Ipa value. The presence of CuO contributes to efficient electron transfer and redox reactions, thereby enhancing the electrochemical activity of the electrode.
Furthermore, the integration of rGO in the HKUST-1/CuO composite notably enhances the conductivity and accelerates the electron transfer kinetics, ultimately resulting in an evident increase in the Ipa value upon the modification of the bare GCE surface with HKUST-1/rGO/CuO. The synergistic effect of HKUST-1, CuO, and rGO collectively contributes to the enhanced electrocatalytic activity for HQ detection. The addition of α-Fe2O3 to the HKUST-1/rGO/CuO nanocomposite further enhances the electrocatalytic activity, resulting in a remarkable increase in the Ipa value followed by HKUST-1/rGO/CuO/α-Fe2O3/GCE. The synergistic interplay among HKUST-1, rGO, CuO, and α-Fe2O3 contributes to an expanded active surface area, improved conductivity, and facilitated electron transfer, enhancing HQ detection sensitivity.
The composite material enhances the oxidation of HQ by providing an active surface for the electrochemical reaction to occur. The CuO component aids in the electron transfer process, promoting the removal of electrons from the HQ molecules and forming benzoquinone. The rGO and α-Fe2O3 components contribute to the conductivity and structural stability of the composite, further enabling the oxidation process. However, during the reduction of benzoquinone back to HQ, the HKUST-1/CuO/rGO/α-Fe2O3 composite assists by facilitating the transfer of electrons back to the quinone molecules. The composite's synergistic effect enhances the overall electron transfer efficiency, enabling the reduction process to occur more effectively.
In short, the HKUST-1/rGO/CuO/α-Fe2O3/GCE exhibits tremendous electrochemical activity for the HQ redox peak current owing to the synergy effect of the HKUST-1/rGO/CuO composite and α-Fe2O3 nano-flake flowers, superb electrocatalytic activity, an abundance level of nanoscale interfacial associations, wide active surface area, and upgraded conductivity.
Therefore, it is evident from Fig. 4d that Ipa values rise linearly, whilst Ipc values also rise as the HQ concentration increases. Besides that, the Ipa values are plotted against the HQ concentration, as depicted in Fig. 4e, revealing an R2 of 0.996 with a linear relationship. In the following assays, HKUST-1/rGO/CuO/α-Fe2O3/GCE is further employed to monitor HQ electrochemically based on the relative performance of the as-prepared different NCs. The electrochemical behavior of HQ corroborates that the Fe3+/Fe2+ and Cu3+/Cu2+ redox couples are involved in the electrooxidation of HQ during CV scans.50,51 The solid-state diffusion distance is also reduced by rGO. Furthermore, CuO/α-Fe2O3 as MTMOs in the NCs of HKUST-1/rGO/CuO/α-Fe2O3 synergistically improves interfacial surfaces that facilitate the transport of electrons via the α-Fe2O3 nano-flake flower interconnected system with spherical CuO. Based on the literature, two species, i.e., Fe3+ and Cu3+, are expected to serve as electron transfer mediators rather than the other oxidation states.40 The HQ electrochemical oxidation in the presence of O2 and OH− can be observed in an alkaline medium. Cu2+ and Fe2+ ions can be transformed into Cu3+ and Fe3+ ions via electrochemical reactions. Cu3+ and Fe3+ moieties are reduced back to their initial states when HQ is oxidized to 1,4-benzoquinone. In this way, redox transformations (Cu3+/Cu2+ and Fe3+/Fe2+) are provided by the peculiar HKUST-1/rGO/CuO/α-Fe2O3 NCs, which supports the greatest electrocatalytic activity to carry out the oxidation of HQ in an alkaline medium as shown in Fig. S2.†52,53
Here, it is noteworthy that the rapid transfer of electrons between the redox couple at the ternary structural interfaces results in a synergistic enhancement of the electrocatalytic activity. It is proposed that the numerous facets, entailing large specific surface area, higher crystallinity, and porosity, the excellent electron transfer rate network, the superconductivity of doped rGO, and the effective coordination effect of CuO and α-Fe2O3 have been pointed out in contributing to the extraordinary efficiency of HKUST-1/rGO/CuO/α-Fe2O3 in comparison to other control materials.
Likewise, HQ oxidation has also been studied at scan rates of 0.1, 0.08, 0.06, 0.04, and 0.01 V s−1 by HKUST-1/rGO/CuO/α-Fe2O3/GCE to examine the scan rate effect. The highest Ipa and Ipc values are achieved with a scan rate of 0.1 V s−1 (Fig. 4f). There is a drop in both peak current values when the scan rate is reduced from 0.1 to 0.01 V s−1. The calibration curve between the scan rate and peak current values is shown in the inset of Fig. 4f. These findings indicate that HQ redox reactions on the HKUST-1/rGO/CuO/α-Fe2O3/GCE surface appear to be controlled by the mass diffusion process.
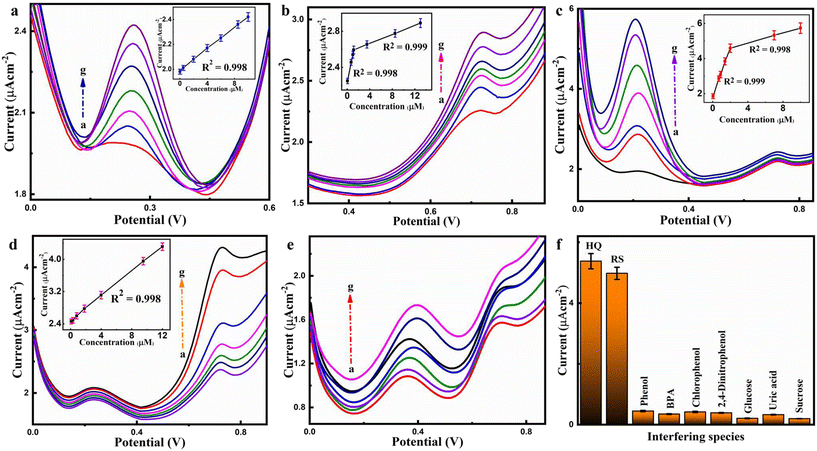
3. Selective and simultaneous detection of HQ and RS
The prepared sensor has been analyzed using DPV for quantitative and qualitative analysis. It has been found that DPV is highly sensitive and eradicates non-faradaic current, producing better analytical signals than using CV. Selective and simultaneous measurements of HQ and RS have been performed using HKUST-1/rGO/CuO/α-Fe2O3/GCE. However, the Ipa values for HQ and RS are recorded at 0.39 V and 0.72 V, correspondingly. Each of these isomers is determined individually by altering the concentrations of each isomer. Thus, Fig. 5a and b demonstrate that the HQ and RS concentrations varied from 0.02–10 μM and 0.06–13 μM with corresponding linear calibration plots in insets where the value of R2 = 0.998 for HQ while in the case of RS, the calibration curve plot expresses two sorts of linear ranges, one is from 0.06–1 μM (R2 = 0.998) and the other from 1–13 μM with R2 = 0.999.The porous and highly crystalline NCs of HKUST-1/rGO/CuO/α-Fe2O3/GCE provide swift reaction time, as seen from the linear increase in Ipa values of HQ and RS with each successive addition. This is due to the rapid electron transport along simple HQ and RS molecule diffusion on the active surface. The LODs of these dihydroxybenzene isomers HQ and RS are 20 nM and 60 nM, respectively, with (signal to noise ratio (S/N) = 3). Moreover, Fig. 5c and d illustrate DPV assessments in which one isomer concentration is changed while the other remains constant. Similarly, the oxidation currents rise as the concentration of each isomer increases, but the peak potentials persist nearly constant. The different concentrations of HQ in 0.1 M PBS having pH = 7.2 with a 10 μM fixed concentration of RS can be seen in Fig. 5c.
The findings display that Ipa is proportional to the increase in HQ concentration, although RS peak currents are relatively constant. The Fig. 5c inset shows two linear ranges from 0.05–2 μM (R2 = 0.999) and 2–10 μM (R2 = 0.998). In our investigation, we noted the presence of two distinct linear regression equations originating from separate linear ranges in the calibration plot. Specifically, as evidenced by the insets of Fig. 5b and c, one linear regression equation is determined to be y = 0.3127x + 2.2324 within the range of 0.06–1 μM (R2 = 0.998), whereas another equation is observed within the range of 1–13 μM with R2 = 0.999, represented by y = 0.0249x + 2.5655. Furthermore, the inset in Fig. 5c elucidates two linear ranges, one spanning 0.05–2 μM (R2 = 0.999) with the equation y = 0.3238x + 2.8801, and the other extending from 2–10 μM (R2 = 0.998) with the equation y = 0.1442x + 4.3054. These findings can be elucidated by the concept that the higher slope of the curve suggests the presence of an ample number of active sites on the electrode surface for the analyte at lower concentrations. Conversely, the lower slope signifies the relatively restricted availability of active sites for the concentrated analytes.
Likewise, Fig. 5d shows the DPV records of RS concentrations ranging from 0.08 μM to 12 μM with a constant concentration of 10 μM HQ, while the Fig. 5d inset presents a linear calibration plot in which the value of R2 is equal to 0.998. Additionally, the LODs for HQ and RS are found to be 50 nM and 80 nM (S/N = 3), respectively.54 The calibration plots in the insets of Fig. 5c and d with a 0.998 determination coefficient are also used to deduce the sensitivity of 4746.4 μA mM−1 cm−2 and 2279.5 μA mM−1 cm−2, respectively.
The calibration plots sometimes may have two sorts of linear ranges because the rate-determining step of the oxidation kinetics is regulated by target analyte adsorption at very low concentration, and activation at extreme concentration. Therefore, it is easy to see an increase in oxidation current density early in the experiment since the electrode surface is still fresh and may adsorb further analyte concentration. A progressive decline in current density occurs as the electrode surface becomes fouled over time.55 According to Fig. 5c and d, it is anticipated that an increase in analyte concentration leads to a steady increase in peak current, indicating that the co-existence of these dihydroxybenzene isomers has no effect on the HQ and RS oxidation at the HKUST-1/rGO/CuO/α-Fe2O3/GCE.
Furthermore, in order to accurately quantify the concentrations of HQ and RS, the concentrations of both analytes are varied simultaneously. Interestingly, these two isomers have clearly distinct anodic peak potentials, their Ipa values grow linearly corresponding to spiked concentrations as reflected in Fig. 5e and their linear calibration plots can be seen in Fig. S3(a and b),† which show the linear range from 0.03–15 μM (R2 = 0.997) for HQ and 0.06 μM to 11 μM (R2 = 0.998) for RS, respectively. There are LODs of 30 nM and 60 nM for HQ and RS, respectively. However, it reveals no significant variations in linear ranges along with LODs between HQ and RS individual and simultaneous electrochemical sensing. Yet, it is recommendable to conduct selective as well as simultaneous determination of HQ and RS in order to avoid any valid interference between them. A probable reason in this aspect is that the as-fabricated EC sensor consists of HKUST-1/rGO/CuO/α-Fe2O3 which holds a plethora of active sites and its synergistic impact enhances the electron transfer rate, making it a good choice as an up-and-coming sensor. Table 1 shows the HKUST-1/rGO/CuO/α-Fe2O3/GCE performance in comparison to other modified electrodes.
| Modified electrodes | Linear range (μM) | Detection limit (μM) | Ref. | ||
|---|---|---|---|---|---|
| HQ | RS | HQ | RS | ||
| a Cobalt iron selenides. b Porous carbon nanofibers-2. c Glassy carbon electrode. d Gold. e Palladium. f Nanofibers. g Reduced graphene oxide. h Copper metal–organic framework. i Chitosan. j Graphitic carbon nitride. k Metal–organic framework. l Carbon paste electrode. m Copper oxide. n Graphene oxide. o Tungsten sulfide. p Graphene. q Zeolitic imidazolate framework-8. r Carbon nanofibers. s Polyaniline. t Zinc oxide. u Graphene. | |||||
| CoFe2Se4a/PCFb-2/GCEc | 0.5–200 | 5–350 | 0.13 | 1.36 | 56 |
| Aud-PdeNFf/rGOg/GCEc | 1.6 – 100 | 2–100 | 0.5 | 0.7 | 57 |
| Cu-MOFh/CSi-ErGOg/GCEc | 5–400 | 1–200 | 0.44 | 0.33 | 58 |
| Aud-gC3N4j-MOFk-CPEl | 0.005 – 5 | 0.005–100 | 0.113 | 0.113 | 59 |
| CuOm/GOn/CPEl | 0.2 – 360 | 0.7–250 | 1 | 1.8 | 60 |
| WS2o-Grp/GCEc | 1–100 | 1–100 | 0.1 | 0.1 | 61 |
| ZIF-8q/CNFr/GCEc | 2–400 | 2–400 | 0.06 | 0.32 | 62 |
| PANIs/MnO2/GCEc | 0.2–100 | 0.2–100 | 0.13 | 0.09 | 63 |
| ZnOt/Gu/GCEc | 0–70 | 0–700 | 0.1 | 1 | 64 |
| HKUST-1/rGO/CuO/α-Fe 2 O 3 /GCE | 0.05–10 | 0.08–12 | 0.05 | 0.08 | This work |
4. Interference study
DPV has been conducted to thoroughly investigate the selectivity of HKUST-1/rGO/CuO/α-Fe2O3/GCE against potential interfering species. For this purpose, a particular amount of interferent is added to 0.1 M PBS with 7.2 pH comprising 10 μM HQ and RS. The data in Fig. 5f show that the oxidation current response of these dihydroxybenzene isomers is unaffected by the presence of 100 μM phenol, bisphenol A (BPA), chlorophenol, 2,4-dinitrophenol, glucose, uric acid, and sucrose at the potential window from −0.2 V–+0.90 V. The limited interference observed from phenol, BPA, chlorophenol, and 2,4-dinitrophenol, despite their classification as phenolic compounds, can be attributed to the distinctive characteristics shared by HQ and RS within the phenolic family. These compounds possess similar reductive groups to those found in HQ and RS, enabling them to exhibit reduction behavior similar to that of HQ and RS. Additionally, their impact remains minimal, particularly at lower concentrations. Notably, even at concentrations ten times higher than those of co-existing species, no significant interference is observed within the potential window of −0.2 V to 0.90 V, confirming the high selectivity of the developed sensor.The exceptional properties of HKUST-1/CuO/α-Fe2O3 NCs, including an efficient electron transfer network, reduced internal resistance, coordination effects, enhanced surface area, good crystallinity, and porosity, are attributed to the synergistic effects of HKUST-1/CuO and α-Fe2O3. As indicated, the modified electrode HKUST-1/CuO/α-Fe2O3/GCE demonstrates excellent catalytic activity for the targeted HQ and RS analytes. Thus, this analysis of the above data reveals that the newly developed sensor HKUST-1/rGO/CuO/α-Fe2O3 offers exceptional selectivity. Further, the reproducibility test is another crucial parameter to detect HQ and RS accurately by DPV at HKUST-1/rGO/CuO/α-Fe2O3/GCE. In this manner, five electrodes are prepared by the same procedure, and the electrochemical responses towards 5 mM HQ and RS (inset of Fig. S4†) are compared with 0.1 M PBS. The results demonstrate that the RSD of Ipa current peaks is 2.61% and 2.82% for HQ and RS, respectively, in triplicate (n = 3). This shows the excellent reproducibility and reliability of the fabrication procedure by which the HKUST-1/rGO/CuO/α-Fe2O3/GCE sensor has been designed.
The stability assessment of the HKUST-1/rGO/CuO/α-Fe2O3/GCE sensor involved a 10-day exposure to room temperature (25 °C) in ambient air. Daily DPV detection has been conducted under consistent conditions throughout this ongoing period. The stability is determined by calculating the percentage (%) of current retention compared to the initial response. The % retention for HQ and RS currently reaches 89.1% and 86.3% of their respective initial values, respectively, demonstrating remarkable stability (Fig. S4†). In the realm of practical electrochemical sensing, electrode fouling remains a critical consideration. The electrochemical oxidation of HQ and RS results in the formation of derivatives of o-benzoquinone through double electron transfer, eventually depositing on the electrode surface. This ongoing deposition significantly hinders the surface redox performance of HQ and RS. Understanding these mechanisms is pivotal for optimizing the long-term stability of the sensor in real-world applications. Thus, these satisfactory results verify the synergistic effects of the electrocatalytic activity between HKUST-1/rGO/CuO and α-Fe2O3 providing elevated electrocatalytic performance in terms of selectivity towards HQ and RS and are helpful to differentiate the compounds.
5. Real sample analysis
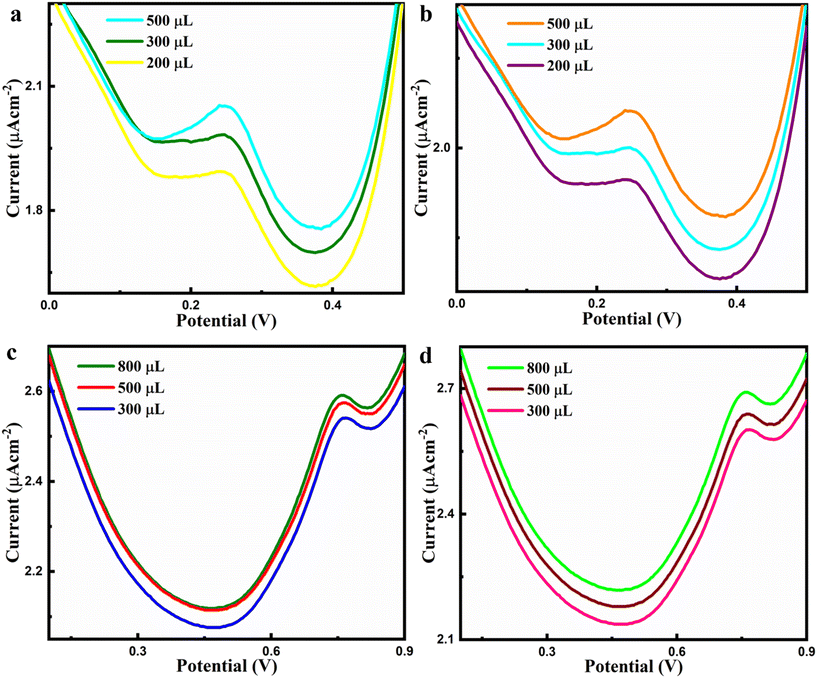
To assess the possible applicability of our prepared sensor for HQ and RS quantitative examination in skin whitening creams and hair toners, HKUST-1/rGO/CuO/α-Fe2O3/GCE has been utilized without any prior pretreatment method via DPV. In addition, skin lightening/whitening creams and hair toners are ubiquitous practices across the globe, and they are more prevalent in women than in men.HQ is considered a highly whitening agent compared to bleaches in whitening creams, and RS, as a coupling agent, is vigorously used in hair toners. As a result, the cosmetic sector is gaining worldwide attention. Fig. 6a and b show skin whitening cream samples 1 and 2 while hair toner samples 1 and 2 can also be viewed in Fig. 6c and d correspondingly (n = 3) as an example of real-time performance of an as-constructed sensor.
The DPV results also show elevated Ipa at around the same potential, specifying the existence of HQ and RS in the aforementioned cosmetic samples. The results are summarized in Table 2. Furthermore, Table 2 also compares the values obtained from the samples using both DPV and HPLC techniques, revealing close agreement between the results. This comparison is crucial as it validates the accuracy and reliability of the DPV technique in measuring the target analytes. The consistency observed between the DPV and HPLC results further strengthens the credibility of the newly developed method. Such agreement emphasizes the potential of the DPV technique as a practical and accessible alternative for precise analysis without compromising accuracy. These findings conclude that the developed sensor can detect HQ and RS reliably and accurately in real samples at low concentrations within the standard error range. With its excellent sensitivity, simplicity, fast response, reliability, and low LOD, the as-constructed electrochemical sensor can be very beneficial in the cosmetic industry for assessing skin whitening cream and hair toner sample quality.
| Samples | Added (μM) | Found (μM) DPV | Found (μM) HPLC | RSD % |
|---|---|---|---|---|
| Skin whitening cream sample 1 | 0 | 1.993 | 1.972 | 0.67 |
| Skin whitening cream sample 2 | 0 | 2.005 | 1.989 | 0.22 |
| Hair toner sample 1 | 0 | 2.568 | 2.614 | 0.51 |
| Hair toner sample 2 | 0 | 2.644 | 2.609 | 0.58 |
IV. Conclusions
In summary, an electrochemical sensor has been developed using HKUST-1/rGO/CuO/α-Fe2O3 NCs for highly selective as well as simultaneous detection of environmental pollutants, including HQ and RS. This research is the pioneer in fabricating HKUST-1/rGO/CuO/α-Fe2O3 NCs utilizing a sacrificial template of HKUST-1. HKUST-1/rGO/CuO/α-Fe2O3 possesses a highly crystalline and porous structure and huge specific surface area with the synergy effect of HKUST-1/rGO/CuO and α-Fe2O3, resulting in improved electrocatalytic performance to detect these dihydroxybenzene isomers simultaneously. This new HKUST-1/rGO/CuO/α-Fe2O3/GCE sensor is more economical, environment friendly, and energy saving in contrast to other frequently used detection methods. These NCs also exhibit outstanding sensing performance such as superb selectivity, low detection limits, superb reproducibility, and wide linear ranges towards HQ and RS determination compared to previously reported HQ and RS electrochemical sensors. Hence, this electrochemical sensing platform has been successfully employed to monitor HQ and RS in skin whitening creams and hair toners with satisfactory results, confirming its applicability and reliability. Moreover, this MOF-derived fabrication of HKUST-1/rGO/CuO/α-Fe2O3 NCs will open a new horizon in synthesis of multi-component hybrids for electrocatalysis, environmental monitoring, and bioanalysis.Author contributions
Tayyaba Iftikhar: conceptualization, data curation, formal analysis, software, validation, writing – original draft, editing. Muhammad Irfan Majeed: investigation, formal analysis, & visualization. Ayesha Aziz: investigation, visualization. Anees A. Khadom: investigation, visualization. Zhuo Huang: supervision, investigation. Ghazala Ashraf: investigation, visualization. Guangfang Li: investigation, visualization. Muhammad Asif: supervision, investigation, formal analysis, writing – review & editing. Fei Xiao: supervision, writing – review & editing. Hongfang Liu: supervision, writing – review & editing.Conflicts of interest
According to the authors, there are not any conflicts of interest (financial or personal) that might have influenced the findings described in this research.Acknowledgements
This work was supported by the National Key Research and Development Program of China Sino-Austrian Intergovernmental Industry-University-Research Cooperation Project (Project No. 2022YFE0117000), the Foundation of Hubei Key Laboratory of Material Chemistry and Service Failure (2020MCF02), and the Key Laboratory of Material Chemistry for Energy Conversion and Storage, Ministry of Education (2018). We also acknowledge the support of the Analytical and Testing Center of the Huazhong University of Science and Technology for the XRD, TEM, SEM and XPS measurements.References
- X. B. Joseph, S. Kogularasu, S.-F. Wang and J.-K. Sheu, Hydrothermal-dependent synthesis of exfoliated nickel cobaltite layers for simultaneous determination of IARC group 2B, 3B carcinogens, ACS Appl. Nano Mater., 2021, 4, 12788–12797 CrossRef CAS.
- L. Sun, H. Guo, Z. Pan, B. Liu, T. Zhang, M. Yang, N. Wu, J. Zhang, F. Yang and W. Yang, In-situ reducing platinum nanoparticles on covalent organic framework as a sensitive electrochemical sensor for simultaneous detection of catechol, hydroquinone and resorcinol, Colloids Surf., A, 2022, 635, 128114 CrossRef CAS.
- T. Iftikhar, M. Asif, A. Aziz, G. Ashraf, S. Jun, G. Li and H. Liu, Topical advances in nanomaterials based electrochemical sensors for resorcinol detection, Trends Environ. Anal. Chem., 2021, 31, e00138 CrossRef CAS.
- P. Gimeno, A.-F. Maggio, M. Bancilhon, N. Lassu, H. Gornes, C. Brenier and L. Lempereur, HPLC–UV method for the identification and screening of hydroquinone, ethers of hydroquinone and corticosteroids possibly used as skin-whitening agents in illicit cosmetic products, J. Chromatogr. Sci., 2016, 3, 343–352 Search PubMed.
- D. H. Mohammed and F. K. Omar, Spectrophotometric determination of catechol and resorcinol by oxidative coupling with 2, 4-dinitrophenyl hydrazine, Egypt. J. Chem., 2021, 9, 5061–5065 Search PubMed.
- W. Zeng, D. Huang, G. Zhu, B. Lv and Y. Yi, 3-Aminobenzeneboronic acid functionalized MoS2 quantum dot as fluorescent nanoprobe for the determination of o-dihydroxybenzene, Anal. Sci., 2020, 1, 20P011 Search PubMed.
- Z. Haixiang, T. Xijuan and S. Zhenghua, Determination of picogram amounts of dihydroxybenzenes in water samples with luminol–lysozyme chemiluminescence system, J. Chin. Chem. Soc., 2012, 12, 1512–1519 CrossRef.
- J. F. He, F. J. Yao, H. Cui, X. J. Li and Z. B. Yuan, Simultaneous determination of dihydroxybenzene positional isomers by capillary electrochromatography using gold nanoparticles as stationary phase, J. Sep. Sci., 2012, 8, 1003–1009 CrossRef.
- K. Cai, W. Gao, Y. Yuan, C. Gao, H. Zhao, Y. Lin, W. Pan and B. Lei, An improved in situ acetylation with dispersive liquid-liquid microextraction followed by gas chromatography–mass spectrometry for the sensitive determination of phenols in mainstream tobacco smoke, J. Chromatogr. A, 2019, 1603, 401–406 CrossRef CAS.
- A. Aziz, M. Asif, G. Ashraf, T. Iftikhar, J. Hu, F. Xiao and S. Wang, Boosting electrocatalytic activity of carbon fiber@fusiform-like copper-nickel LDHs: Sensing of nitrate as biomarker for NOB detection, J. Hazard. Mater., 2022, 422, 126907 CrossRef CAS.
- J. Peng, B. Wang, H. Cheng, R. Yang, Y. Yin, S. Xu and C. Wang, Highly sensitive and superhydrophobic fabric sensor based on AgNPs/polypyrrole composite conductive networks for body movement monitoring, Compos. Sci. Technol., 2022, 227, 109561 CrossRef CAS.
- R. Zeng, M. Qiu, Q. Wan, Z. Huang, X. Liu, D. Tang and D. Knopp, Smartphone-based electrochemical immunoassay for point-of-care detection of SARS-CoV-2 nucleocapsid protein, Anal. Chem., 2022, 94, 15155–15161 CrossRef CAS.
- S. Shahrokhian, M. Ezzati and H. Hosseini, Fabrication of a sensitive and fast response electrochemical glucose sensing platform based on co-based metal-organic frameworks obtained from rapid in situ conversion of electrodeposited cobalt hydroxide intermediates, Talanta, 2020, 210, 120696 CrossRef CAS PubMed.
- T. Iftikhar, A. Aziz, G. Ashraf, Y. Xu, G. Li, T. Zhang, M. Asif, F. Xiao and H. Liu, Engineering MOFs derived metal oxide nanohybrids: Towards electrochemical sensing of catechol in tea samples, Food Chem., 2022, 395, 133642 CrossRef CAS PubMed.
- Y. Wang, Y. Lu, W. Zhan, Z. Xie, Q. Kuang and L. Zheng, Synthesis of porous Cu2O/CuO cages using Cu-based metal–organic frameworks as templates and their gas-sensing properties, J. Mater. Chem. A, 2015, 3, 12796–12803 RSC.
- K. Zhang, A. Xie, M. Sun, W. Jiang, F. Wu and W. Dong, Electromagnetic dissipation on the surface of metal organic framework (MOF)/reduced graphene oxide (RGO) hybrids, Mater. Chem. Phys., 2017, 199, 340–347 CrossRef CAS.
- T. Iftikhar, N. Iftikhar, G. Chi, W. Qiu, Y. Xie, Z. Liang, C. Huang and L. Su, Unlocking the future of brain research: MOFs, TMOs, and MOFs/TMOs for electrochemical NTMs detection and analysis, Talanta, 2024, 267, 125146 CrossRef CAS PubMed.
- H. Hosseini, H. Ahmar, A. Dehghani, A. Bagheri, A. Tadjarodi and A. R. Fakhari, A novel electrochemical sensor based on metal-organic framework for electro-catalytic oxidation of L-cysteine, Biosens. Bioelectron., 2013, 42, 426–429 CrossRef CAS PubMed.
- M. Ezzati, S. Shahrokhian and H. Hosseini, In Situ two-step preparation of 3D NiCo-BTC MOFs on a glassy carbon electrode and a graphitic screen printed electrode as nonenzymatic glucose-sensing platforms, ACS Sustainable Chem. Eng., 2020, 38, 14340–14352 CrossRef.
- S. A. Abrori, N. L. W. Septiani, F. N. Hakim, A. Maulana, I. Anshori and B. Yuliarto, Non-enzymatic electrochemical detection for uric acid based on a glassy carbon electrode modified with MOF-71, IEEE Sens. J., 2021, 21, 170–177 CAS.
- C. Hu, P. Pan, H. Huang and H. Liu, Cr-MOF-based electrochemical sensor for the detection of p-nitrophenol, Biosensors, 2022, 12, 813 CrossRef CAS.
- S. Shahrokhian, E. Khaki Sanati and H. Hosseini, Direct growth of metal-organic frameworks thin film arrays on glassy carbon electrode based on rapid conversion step mediated by copper clusters and hydroxide nanotubes for fabrication of a high performance non-enzymatic glucose sensing platform, Biosens. Bioelectron., 2018, 112, 100–107 CrossRef CAS PubMed.
- W. Liu and X.-B. Yin, Metal–organic frameworks for electrochemical applications, TrAC, Trends Anal. Chem., 2016, 75, 86–96 CrossRef CAS.
- K.-Y. A. Lin and Y.-T. Hsieh, Copper-based metal organic framework (MOF), HKUST-1, as an efficient adsorbent to remove p-nitrophenol from water, J. Taiwan Inst. Chem. Eng., 2015, 50, 223–228 CrossRef.
- P. Arul and S. Abraham John, Electrodeposition of CuO from Cu-MOF on glassy carbon electrode: A non-enzymatic sensor for glucose, J. Electroanal. Chem., 2017, 799, 61–69 CrossRef CAS.
- T. Iftikhar, Y. Xu, A. Aziz, G. Ashraf, G. Li, M. Asif, F. Xiao and H. Liu, Tuning electrocatalytic aptitude by incorporating α-MnO2 nanorods in Cu-MOF/rGO/CuO hybrids: electrochemical sensing of resorcinol for practical applications, ACS Appl. Mater. Interfaces, 2021, 13, 31462–31473 CrossRef CAS.
- Y.-F. Yang, H.-Q. Huang, Z.-Y. Song, H.-Q. Li, X.-Y. Yu, Y.-M. Cui, M. Yang, S.-H. Chen and X.-J. Huang, Increasing reductive Fe(II)/Co(III) sites on P-doped FeCo2O4−x nanosheets to accelerate the valence cycle for the electroanalysis of As(III), Environ. Sci.: Nano, 2023, 10, 800–811 RSC.
- W.-Q. Zheng, Y.-D. Tu, X.-G. Wang, Y. Huang and F. Xia, In situ growth of metal–organic frameworks in nanochannels for highly sensitive microcystin-LR detection, Environ. Sci.: Nano, 2023, 10, 834–842 RSC.
- G. Ashraf, M. Asif, A. Aziz, T. Iftikhar and H. Liu, Rice-spikelet-like copper oxide decorated with platinum stranded in the CNT network for electrochemical in vitro detection of serotonin, ACS Appl. Mater. Interfaces, 2021, 5, 6023–6033 CrossRef.
- R. Wahab, F. Khan, N. Ahmad, M. Alam, J. Ahmad and A. Al-Khedhairy, Rapid sensing response for phenol with CuO nanoparticles, Colloids Surf., A, 2020, 607, 125424 CrossRef CAS.
- P. M. Jahani, F. G. Nejad, Z. Dourandish, M. P. Zarandi, M. M. Safizadeh, S. Tajik and H. Beitollahi, A modified carbon paste electrode with N-rGO/CuO nanocomposite and ionic liquid for the efficient and cheap voltammetric sensing of hydroquinone in water specimens, Chemosphere, 2022, 302, 134712 CrossRef CAS PubMed.
- R. A. Senthil, A. Selvi, P. Arunachalam, L. S. Amudha, J. Madhavan and A. M. Al-Mayouf, A sensitive electrochemical detection of hydroquinone using newly synthesized α-Fe2O3-graphene oxide nanocomposite as an electrode material, J. Mater. Sci., 2017, 28, 10081–10091 CAS.
- M. Asif, A. Aziz, G. Ashraf, Z. Wang, J. Wang, M. Azeem, X. Chen, F. Xiao and H. Liu, Facet-inspired core–shell gold nanoislands on metal oxide octadecahedral heterostructures: high sensing performance toward sulfide in biotic fluids, ACS Appl. Mater. Interfaces, 2018, 10, 36675–36685 CrossRef CAS.
- M. Asif, A. Aziz, H. Wang, Z. Wang, M. Ajmal, F. Xiao and H. Liu, Core-shell iron oxide-layered double hydroxide: high electrochemical sensing performance of H2O2 biomarker in live cancer cells with plasma therapeutics, Biosens. Bioelectron., 2017, 97, 352–359 CrossRef CAS PubMed.
- A. Lassoued, B. Dkhil, A. Gadri and S. Ammar, Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method, Results Phys., 2017, 7, 3007–3015 CrossRef.
- M. Mehdipour Ghazi, M. Ilbeigi and M. Jahangiri, Synthesis, characterization and degradation activity of Methyl orange Azo dye using synthesized CuO/α-Fe2O3 nanocomposite, Adv. Environ. Technol., 2017, 2, 143–151 Search PubMed.
- A. S. Agnihotri, A. Varghese and M. Nidhin, Transition metal oxides in electrochemical and bio sensing: A state-of-art review, Appl. Surf. Sci. Adv., 2021, 4, 100072 CrossRef.
- J. G. Manjunatha, Poly (Adenine) modified graphene-based voltammetric sensor for the electrochemical determination of catechol, hydroquinone and resorcinol, Open Chem. Eng. J., 2020, 1(14), 1–10 Search PubMed.
- T. Han, Y. Wei, X. Jin, H. Jiu, L. Zhang, Y. Sun, J. Tian, R. Shang, D. Hang and R. Zhao, Hydrothermal self-assembly of α-Fe2O3 nanorings@ graphene aerogel composites for enhanced Li storage performance, J. Mater. Sci.: Mater. Electron., 2019, 9, 7119–7130 CrossRef.
- M. Asif, A. Aziz, Z. Wang, G. Ashraf, J. Wang, H. Luo, X. Chen, F. Xiao and H. Liu, Hierarchical CNTs@CuMn layered double hydroxide nanohybrid with enhanced electrochemical performance in H2S detection from live cells, Anal. Chem., 2019, 6, 3912–3920 CrossRef.
- B. B. Nayak, T. Dash and B. K. Mishra, Purple coloured natural ruby: X-ray photoelectron spectroscopy, X-ray diffraction, X-ray tomography and other microstructural characterizations, International Journal of Sciences: Basic and Applied Research, 2016, 25, 94–114 Search PubMed.
- F. T. Johra and W.-G. Jung, Hydrothermally reduced graphene oxide as a supercapacitor, Appl. Surf. Sci. Adv., 2015, 357, 1911–1914 CrossRef CAS.
- A. K. Kar and R. Srivastava, An efficient and sustainable catalytic reduction of carbon–carbon multiple bonds, aldehydes, and ketones using a Cu nanoparticle decorated metal organic framework, New J. Chem., 2018, 12, 9557–9567 RSC.
- Y.-W. Hsu, T.-K. Hsu, C.-L. Sun, Y.-T. Nien, N.-W. Pu and M.-D. Ger, Synthesis of CuO/graphene nanocomposites for nonenzymatic electrochemical glucose biosensor applications, Electrochim. Acta, 2012, 82, 152–157 CrossRef CAS.
- F. Deng, X. Pei, Y. Luo, X. Luo, D. D. Dionysiou, S. Wu and S. Luo, Fabrication of hierarchically porous reduced graphene oxide/SnIn4S8 composites by a low-temperature co-precipitation strategy and their excellent visible-light photocatalytic mineralization performance, Catalysts, 2016, 6, 113 CrossRef.
- M. Asif, A. Aziz, H. Wang, Z. Wang, W. Wang, M. Ajmal, F. Xiao, X. Chen and H. Liu, Superlattice stacking by hybridizing layered double hydroxide nanosheets with layers of reduced graphene oxide for electrochemical simultaneous determination of dopamine, uric acid and ascorbic acid, Microchim. Acta, 2019, 2, 1–11 Search PubMed.
- M. Hjiri, Highly sensitive NO2 gas sensor based on hematite nanoparticles synthesized by sol–gel technique, J. Mater. Sci.: Mater. Electron., 2020, 6, 5025–5031 CrossRef.
- K. Ahmad, P. P. Kumar and S. M. Mobin, A highly sensitive and selective hydroquinone sensor based on a newly designed N-rGO/SrZrO3 composite, Nanoscale Adv., 2020, 1, 502–511 RSC.
- R. Zhang, R. Zhang, R. Jian, L. Zhang, M.-T. Zhang, Y. Xia and S. Luo, Bio-inspired lanthanum-ortho-quinone catalysis for aerobic alcohol oxidation: semi-quinone anionic radical as redox ligand, Nat. Commun., 2022, 1, 428 CrossRef.
- K. Justice Babu, S. Sheet, Y. S. Lee and G. Gnana Kumar, Three-dimensional dendrite Cu–Co/reduced graphene oxide architectures on a disposable pencil graphite electrode as an electrochemical sensor for nonenzymatic glucose detection, ACS Sustainable Chem. Eng., 2018, 2, 1909–1918 CrossRef.
- T.-t. Liu, Y.-h. Zhu, E.-h. Liu, Z.-y. Luo, T.-t. Hu, Z.-p. Li and D. Rui, Fe3+/Fe2+ redox electrolyte for high-performance polyaniline/SnO2 supercapacitors, Trans. Nonferrous Met. Soc. China, 2015, 8, 2661–2665 CrossRef.
- P. Balasubramanian, T. Balamurugan, S.-M. Chen, T.-W. Chen and T. Sathesh, Rational design of Cu@Cu2O nanospheres anchored B, N co-doped mesoporous carbon: a sustainable electrocatalyst to assay eminent neurotransmitters acetylcholine and dopamine, ACS Sustainable Chem. Eng., 2018, 7, 5669–5680 CrossRef.
- Y. Xia, Y. Mo, W. Meng, X. Du and C. Ma, Graphene/carbon paper combined with redox active electrolyte for supercapacitors with high performance, Polymers, 2019, 8, 1355 CrossRef PubMed.
- Z. Yu, H. Gong, Y. Li, J. Xu, J. Zhang, Y. Zeng, X. Liu and D. Tang, Chemiluminescence-derived self-powered photoelectrochemical immunoassay for detecting a low-abundance disease-related protein, Anal. Chem., 2021, 93, 13389–13397 CrossRef CAS.
- H. Wang, Q. Hu, Y. Meng, Z. Jin, Z. Fang, Q. Fu, W. Gao, L. Xu, Y. Song and F. Lu, Efficient detection of hazardous catechol and hydroquinone with MOF-rGO modified carbon paste electrode, J. Hazard. Mater., 2018, 353, 151–157 CrossRef CAS PubMed.
- D. Yin, J. J. Liu, X. Bo and L. Guo, Cobalt-iron selenides embedded in porous carbon nanofibers for simultaneous electrochemical detection of trace of hydroquinone, catechol and resorcinol, Anal. Chim. Acta, 2020, 1093, 35–42 CrossRef CAS PubMed.
- Y. Chen, X. Liu, S. Zhang, L. Yang, M. Liu, Y. Zhang and S. Yao, Ultrasensitive and simultaneous detection of hydroquinone, catechol and resorcinol based on the electrochemical co-reduction prepared Au-Pd nanoflower/reduced graphene oxide nanocomposite, Electrochim. Acta, 2017, 231, 677–685 CrossRef CAS.
- Y. Yang, Q. Wang, W. Qiu, H. Guo and F. Gao, Covalent immobilization of Cu3(btc)2 at chitosan–electroreduced graphene oxide hybrid film and its application for simultaneous detection of dihydroxybenzene isomers, J. Phys. Chem. C, 2016, 18, 9794–9803 CrossRef.
- M. H. Mashhadizadeh, S. M. Kalantarian and A. Azhdeh, A novel electrochemical sensor for simultaneous determination of hydroquinone, catechol, and resorcinol using a carbon paste electrode modified by Zn-MOF, nitrogen-doped graphite, and AuNPs, Electroanalysis, 2021, 33, 160–169 CrossRef CAS.
- M. Kumar, B. K. Swamy, B. Hu, M. Wang, G. Yasin, B. Liang, H. Madhuchandra and W. Zhao, Electrochemical activation of copper oxide decorated graphene oxide modified carbon paste electrode surface for the simultaneous determination of hazardous di-hydroxybenzene isomers, Microchem. J., 2021, 168, 106503 CrossRef CAS.
- K.-J. Huang, L. Wang, Y.-J. Liu, T. Gan, Y.-M. Liu, L.-L. Wang and Y. Fan, Synthesis and electrochemical performances of layered tungsten sulfide-graphene nanocomposite as a sensing platform for catechol, resorcinol and hydroquinone, Electrochim. Acta, 2013, 107, 379–387 CrossRef CAS.
- S. Yang, M. Yang, X. Yao, H. Fa, Y. Wang and C. Hou, A zeolitic imidazolate framework/carbon nanofiber nanocomposite based electrochemical sensor for simultaneous detection of co-existing dihydroxybenzene isomers, Sens. Actuators, B, 2020, 320, 128294 CrossRef CAS.
- M. A. B. Satpati and R. Srivastava, Facile preparation of polyaniline/MnO2 nanofibers and its electrochemical application in the simultaneous determination of catechol, hydroquinone, and resorcinol, Sens. Actuators, B, 2013, 186, 67–77 CrossRef.
- C. Ge, H. Li, M. Li, C. Li, X. Wu and B. Yang, Synthesis of a ZnO nanorod/CVD graphene composite for simultaneous sensing of dihydroxybenzene isomers, Carbon, 2015, 95, 1–9 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3en00745f |
| ‡ Present address: School of Biomedical Engineering, International Health Science Innovation Center, Shenzhen Key Laboratory of Nano-Biosensing Technology, Marshall Laboratory of Biomedical Engineering, Medical School, Shenzhen University, Shenzhen 518055, P.R. China. |
| § Present address: School of Chemistry and Chemical Engineering, Shanxi University, Taiyuan, China. |
| This journal is © The Royal Society of Chemistry 2024 |