Understanding the noble metal modifying effect on In2O3 nanowires: highly sensitive and selective gas sensors for potential early screening of multiple diseases†
Wei
Liu‡
a,
Jiao
Sun‡
b,
Lin
Xu
*a,
Shidong
Zhu
a,
Xiangyu
Zhou
a,
Shuo
Yang
a,
Biao
Dong
 a,
Xue
Bai
a,
Xue
Bai
 a,
Geyu
Lu
a,
Geyu
Lu
 a and
Hongwei
Song
a and
Hongwei
Song
 *a
*a
aState Key Laboratory of Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun, 130012, People's Republic of China. E-mail: linxu@jlu.edu.cn; songhw@jlu.edu.cn
bDepartment of Cell Biology, College of Basic Medical Sciences, Jilin University, Changchun, 130021, People's Republic of China
First published on 25th July 2019
Abstract
Detection of multiple volatile organic compounds in human exhaled breath using semiconductor oxide-based sensing devices has attracted increasing attention for the early diagnosis of diseases. However, detecting trace levels of exhaled biomarkers with high sensitivity and selectivity remains a challenge and the corresponding mechanism for the selectivity is still unclear. Herein, ultrafine In2O3 nanowires (NWs) modified with Au, Ag, and Pt noble metal nanoparticles (NMNPs) are synthesized using the electrospinning method and are used to build semiconductor sensors. It is exciting to observe that the introduction of NMNPs can not only enhance the sensing performance, but also effectively adjust the selectivity. The Au-, Ag- and Pt-modified In2O3 NW sensors exhibit excellent selectivity to hydrogen sulfide, formaldehyde, and acetone biomarkers, respectively. To completely understand the mechanism, theoretical simulation is performed based on density functional theory, which demonstrated that the specific absorption energies between the modified In2O3 NWs and the target gases along with the “spillover effect” resulted in excellent specific selectivity. Importantly, the NMNP modified In2O3 NW sensors are successfully used to build a sensor array for effective simultaneous detection of simulated exhaled breath. The present work provides a simple, low-cost, effective tool for early screening of halitosis, breast cancer, and diabetes at the same time and an in-depth understanding of the enhanced sensing performance by the introduction of NMNPs.
New conceptsAnalyzing disease biomarkers in human breath is a very attractive diagnostic method considering the painlessness and convenience of analyte collection. Previous efforts have mostly been focused on the enhancement of sensor performance by modifying with noble metals, known as the “spillover effect”. However, detecting trace levels of volatile organic compounds (VOCs) in a highly selective way and understanding the selectivity mechanism remain challenging. This report is the first attempt of using a uniform and ultrafine In2O3 based sensor array for exhaled biomarker analysis, which can selectively detect trace biomarkers in the exhaled environment. The corresponding sensing mechanisms have been carefully simulated and discussed to give a deeper understanding about the noble metal modifying effect. Furthermore, we show that the as-prepared sensor array can accurately distinguish different trace VOC biomarkers in simulated exhaled breath using principal component analysis. Moreover, each sensor in the array has the same optimal working temperature, which may greatly decrease the complexity of the array circuit design. |
Introduction
Human breath contains thousands of gas species, including numerous volatile organic compounds (VOCs), which are linked to physiological changes of the involved individual.1 Thus, tracing of VOC information in exhaled breath is an efficient, non-invasive biological method for the early diagnosis and monitoring of human diseases.2 As an alternative method to the conventional approaches, such as blood analysis, exhaled breath measurement can reduce feelings of discomfort and avoid additional psychological pain for the patient.3,4 Among the different gas biomarkers in exhaled breath, it is reported that hydrogen sulfide (H2S), formaldehyde (HCHO), acetone, nitrogen dioxide (NO2), and ethanol (CH3CH2OH) can be used to evaluate halitosis, breast cancer, diabetes, kidney malfunction, and lung cancer, respectively.5–9 The exhaled biomarker of the patients generally exhibits a higher concentration than in a healthy person. For example, an excessive concentration of 2 parts per million (ppm) H2S in exhaled breath is a known biomarker for halitosis; exhaled HCHO concentrations from a healthy person are 0.3–0.6 ppm and from breast cancer patients are 1.2 ppm.7,10 In addition, higher acetone concentrations were observed in the exhaled breath of diabetic patients (over 1.8 ppm) as compared to 0.3–0.9 ppm for healthy people.11 To achieve accurate diagnosis of specific diseases by breath analysis, the breath sensors should be sensitive and selective enough to trace concentrations of the breath biomarkers down to sub-ppm or even low parts per billion (ppb), which is a challenge for the conventional gas sensors.Sensors based on the semiconducting metal oxides (SMOs) have been considered to be the most promising sensing devices in the field of exhaled breath biomarker detection, because of their advantages of high sensitivity, practical convenience, low cost, and ready miniaturization.12–14 Generally, the separate SMOs without any modification or regulation show limited sensitivity and selectivity due to the insufficient amount of surface active oxygen ions.15 Morphological control, composition adjustment, and catalyst introduction are the main techniques adopted to improve the sensing performance of SMO based sensors.16–18 In these methods, the noble metal catalysts are very effective additives to largely improve the sensing properties of SMOs. With only a very small amount introduced, the noble metal catalysts can generate more surface active oxygen species on the sensing layers via the “spillover effect” which thus provides more interaction sites with the target gases.19–21 For example, Kim et al. fabricated a WO3 nanofiber gas sensor functionalized with Pt nanoparticles (NPs), which exhibited a response of 834.2 toward 5 ppm of H2S and noticeable selectivity in a humid ambient atmosphere. This response value was over 32-fold improved as compared with that of the pristine WO3 nanofibers.22 Lee et al. reported a Pd@In2O3 yolk–shell sensor which showed selective ability toward ethanol, and the response was approximately 14 times higher (toward 5 ppm ethanol) than that of the pure In2O3 hollow nanospheres.23
However, in spite of the synthesis of noble metal sensitized SMO gas sensors as well as their advanced applications being widely reported,24,25 the adopted “spillover effect” introduced by the noble metal NPs (NMNPs) cannot well explain the reason for the enhanced selectivity along with the increased response. In particular, in the complex breath environment, to the best of our knowledge, limited works are focused on systematically controlling the noble metal catalysts to adjust the sensitivity and even selectivity toward different exhaled breath biomarkers. Furthermore, an in-depth study to reveal the essential causes of the sensitivity improvement after the introduction of NMNPs is missing, which is significant to understand the reason for NMNPs’ enhanced gas-sensing properties beyond the “spillover effect”.
In this work, we successfully fabricated In2O3 NWs loaded with different NMNPs (Au, Ag and Pt) which were specifically sensitive to certain exhaled biomarkers, and the corresponding sensing mechanisms regarding the enhanced sensing performance introduced by different NMNPs were carefully studied and discussed based on theoretical simulation. In2O3 is a typical n-type SMO with oxygen vacancies acting as electron donors, which has been widely applied as a gas sensing material for effectively detecting various gases.26 Similar to most of the nanomaterials, the sensing properties of In2O3 have been proven to be morphology and component dependent.27 Among different structures, the ultrafine NWs fabricated via electrospinning have been demonstrated to possess great potential in the application of gas sensors due to their high specific surface area, high interfacial charge-transfer efficiency and the mesopore structure for gas diffusion.28 In our case, NMNP modified ultra-long In2O3 NWs (Au-, Ag-, and Pt-In2O3 NWs) were fabricated via a simple electrospinning method, followed by a controlled thermal treatment. The obtained In2O3 NWs possessed an ultrafine NW diameter (<60 nm) with a uniform distribution of NMNPs. The designed sensing material not only facilitated the diffusion of gas molecules, but also provided more active sites to react with target gases. Interestingly, we found that different NMNP modified In2O3 NW sensors enabled sensitive permanence and highly improved selectivity toward different biomarker gases (such as H2S, HCHO and acetone). The simulated results obtained from density functional theory (DFT) revealed that the enhanced sensitivity and selectivity toward H2S, HCHO, and acetone of NMNP modified In2O3 NW sensors could be assigned to the strong adsorption energies between the target gases and the loaded NPs. Furthermore, we built a sensor array using different NMNP modified In2O3 NW sensors for the first time, which could effectively and simultaneously distinguish different exhaled breath biomarkers. The results demonstrated that the as-proposed sensing array has high potential for early detection of multiple diseases in a noninvasive manner.
Experimental sections
Materials
Polyvinylpyrrolidone (PVP, Mw ∼ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), chloroauric acid (HAuCl4), silver nitrate (AgNO3), and chloroplatinic acid (H2PtCl6) were purchased from Sigma-Aldrich. Indium nitrate (In(NO3)3·4.5H2O, 99.5%), N,N-dimethylformamide (DMF, 99.5%), ethanol (99.9%), formaldehyde (99.9%), and absolute acetone (99.9%) were purchased from Sinopharm Group, China. All reagents were of analytical grade and used as purchased without further purification.
000 g mol−1), chloroauric acid (HAuCl4), silver nitrate (AgNO3), and chloroplatinic acid (H2PtCl6) were purchased from Sigma-Aldrich. Indium nitrate (In(NO3)3·4.5H2O, 99.5%), N,N-dimethylformamide (DMF, 99.5%), ethanol (99.9%), formaldehyde (99.9%), and absolute acetone (99.9%) were purchased from Sinopharm Group, China. All reagents were of analytical grade and used as purchased without further purification.
Preparation of pristine In2O3 NWs
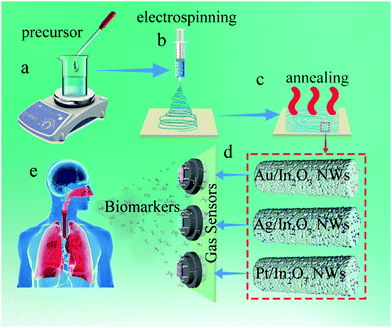
Pristine In2O3 NWs were prepared by electrospinning followed by a calcination step. For the preparation of the precursor solution, 0.72 g of In(NO3)3·4.5H2O was dissolved into a mixture of 8 mL of DMF and 2 mL of absolute ethanol (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 4
v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After being stirred for 30 min at room temperature, 2 g of PVP was added and the solution was further vigorously stirred for 6 h (Scheme 1a). Electrospinning was processed using the prepared solution with a feeding rate of 3 µL min−1 by using a syringe pump at room temperature. During the electrospinning, a voltage of 15 kV was applied from a high-voltage direct current (DC) power supply to the tip of the stainless steel needle and the aluminum foil collector (Scheme 1b), and the distance between the tip of the needle and the collector was fixed as 15 cm. The as-spun In(NO3)3·4.5H2O/PVP composite NWs were calcined at 500 °C for 3 h in an air atmosphere with a temperature ramping rate of 1 °C min−1 (Scheme 1c).
1). After being stirred for 30 min at room temperature, 2 g of PVP was added and the solution was further vigorously stirred for 6 h (Scheme 1a). Electrospinning was processed using the prepared solution with a feeding rate of 3 µL min−1 by using a syringe pump at room temperature. During the electrospinning, a voltage of 15 kV was applied from a high-voltage direct current (DC) power supply to the tip of the stainless steel needle and the aluminum foil collector (Scheme 1b), and the distance between the tip of the needle and the collector was fixed as 15 cm. The as-spun In(NO3)3·4.5H2O/PVP composite NWs were calcined at 500 °C for 3 h in an air atmosphere with a temperature ramping rate of 1 °C min−1 (Scheme 1c).
 | ||
| Scheme 1 Schematic diagram of the electrospinning process for In2O3 NWs and the preparation of the sensor array. | ||
Preparation of Au-In2O3, Ag-In2O3 and Pt-In2O3 NWs
Au-In2O3, Ag-In2O3 and Pt-In2O3 NWs were fabricated by using similar processes as in the case of the pristine In2O3 NWs with a little modification, as shown in Scheme 1a–c. For the preparation of the precursor solution, 0.72 g of In(NO3)3·4.5H2O and 150 µL HAuCl4, 200 µL AgNO3, and 100 µL H2PtCl6 were dissolved respectively into a mixture with 8 mL of DMF and 2 mL of absolute ethanol (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 4
v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The following parts were the same as the fabrication process of the pristine In2O3 NWs.
1). The following parts were the same as the fabrication process of the pristine In2O3 NWs.
Fabrication and measurement of gas sensors
At the beginning, a pair of Au electrodes was previously printed on the surface of a ceramic tube substrate, and platinum–lead wires attached to the Au electrodes were used as electrical contacts. The as-obtained NWs were mixed with appropriate ethanol to form a paste, which was then coated on the surface of the ceramic tube as the sensing layers. The heater of the sensors was Ni–Cr alloy wires, which were inserted into the interior of the above-mentioned ceramic tube to control the working temperature. Subsequently, the as-prepared sensors were sintered at 350 °C for 3 h to improve the stability of the sensing layers. Then, the gas sensors were thermally aged at a heating voltage of 5 V in aging equipment for 48 h before the first measurement (Scheme 1d). The glass chamber was pumped to a vacuum state to avoid the interference of impurities. In order to seal the glass chamber well, the mouth of the glass chamber and the corresponding rubber stopper were uniformly painted with petroleum jelly. During testing, clean air was used as the background gas and a certain amount of the standard gas (the gas source was 200 ppm of the standard gas mixed with N2) was injected into the glass chamber (approximately 2.5 L in volume) using a microsyringe. In this process, the sensor was placed into the glass chamber beforehand. When the response reached the final equilibrium value, the sensors were transferred into another chamber filled with clean air to recover. The gas response was designated as the resistance ratio of Rair/Rgas, where Rair is the sensor resistance in air and Rgas is the resistance in the target gas. The response and recovery times were defined as the times taken by the sensor to reach 90% of the total resistance change in the tested gas and air.Computational methods
The calculations were carried out using the periodic DFT method in the generalized gradient approximation (GGA) of Perdew, Burke and Ernzerhof (PBE) with a plane wave basis set in CASTEP.29 The plane wave cutoff energy was set to be 340 eV, and the Vanderbilt ultrasoft pseudopotentials were used for the atomic core regions.30 To simulate the In2O3 surfaces, the body-centered cubic bixbyite structure with a space group of Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) was employed. A grid of 3 × 3 × 3 Monkhorst–Pack k-points was used to optimize the bulk unit cell. For all the calculations, the convergence threshold was set as 2 × 10−6 eV per atom. Maximum force magnitude that remained on each atom was limited to 0.05 eV Å−1. The calculated cell parameter was 9.0796 Å, in agreement with the experimental data of 10.117 Å. The In2O3(111) surface was modeled as a slab of (14.8 × 14.8 × 17.2 Å) with a vacuum region of 12 Å. Zhang et al. reported that the (111) facet presented the lowest surface free energy under oxygen-rich conditions, while the indium-terminated (100) facet was the most stable one under oxygen-lean conditions.31 Our experiment was carried out under an air environment. We calculated the absorption of HCHO, H2S and CH3COCH3 on the In2O3(111) surface according to the fact of oxygen-rich conditions.
was employed. A grid of 3 × 3 × 3 Monkhorst–Pack k-points was used to optimize the bulk unit cell. For all the calculations, the convergence threshold was set as 2 × 10−6 eV per atom. Maximum force magnitude that remained on each atom was limited to 0.05 eV Å−1. The calculated cell parameter was 9.0796 Å, in agreement with the experimental data of 10.117 Å. The In2O3(111) surface was modeled as a slab of (14.8 × 14.8 × 17.2 Å) with a vacuum region of 12 Å. Zhang et al. reported that the (111) facet presented the lowest surface free energy under oxygen-rich conditions, while the indium-terminated (100) facet was the most stable one under oxygen-lean conditions.31 Our experiment was carried out under an air environment. We calculated the absorption of HCHO, H2S and CH3COCH3 on the In2O3(111) surface according to the fact of oxygen-rich conditions.
Results and discussion
Morphologies and structural characteristics of different In2O3 NW samples
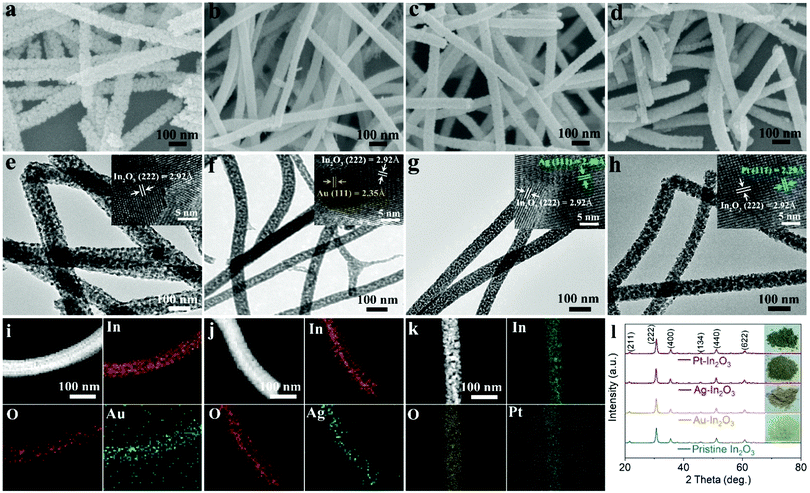
As shown in Fig. 1, the morphologies of the prepared Au-In2O3, Ag-In2O3, and Pt-In2O3 NWs as well as the pristine In2O3 NWs were measured using a scanning electron microscope (SEM). As shown in Fig. 1a, the pristine In2O3 NWs exhibit a diameter distribution of 60–110 nm, with an average diameter of 80 nm (Fig. S1a, ESI†) and a rough surface with some mesopores. A more uniform distribution of the Au-In2O3 NWs can be found in Fig. 1b, and the average diameter is determined to be 56 nm (Fig. S1b, ESI†). The surface of the Au-In2O3 NWs is much smoother than that of the pristine In2O3 NWs, because it is composed of smaller NPs. The Ag-In2O3 (Fig. 1c) and Pt-In2O3 NWs (Fig. 1d) have similar morphologies to those of the Au-In2O3 NWs, and the average diameters are calculated to be 59 and 57 nm, respectively (Fig. S1c and d, ESI†).To obtain detailed information regarding the microstructures of the In2O3 NW samples, transmission electron microscopy (TEM) images of the different NW samples are further examined. In the TEM images of all the In2O3 NWs (Fig. 1e–h), the mesoporous structure of the NWs can be distinguished more clearly, which is beneficial to the interaction between the NWs and gas molecules in the subsequent sensing process. As shown in the high-resolution transmission electron microscopy (HRTEM) in the insets of Fig. 1e–h, lattice fringes with an interplanar spacing of 2.92 Å can be clearly observed, corresponding to the (222) planes of cubic In2O3. In addition, after introducing the NMNPs, the face-centered cubic structure Au(111), face of cubic Ag(111),32 and cubic Pt(111) with interplanar distances of 2.35, 2.40, and 2.29 Å are well distinguished, respectively (in the inset of Fig. 1f–h). To further determine the specific distribution of Au, Ag, and Pt elements, EDX mappings of the samples of Au-In2O3 NWs, Ag-In2O3 NWs, and Pt-In2O3 NWs are conducted, as shown in Fig. 1i–k, respectively. The distributions of Au, Ag, and Pt elements are similar and overlapped with those of In and O elements, demonstrating that the noble metals (Au, Ag and Pt) are well-distributed along the In2O3 NWs.
In the TEM images, the changing trend of the NW diameters is well consistent with that in SEM. To get further information, the grain sizes in all the In2O3 NWs are measured using the HRTEM images (Fig. S2, ESI†). As indicated, the corresponding grain sizes of pristine In2O3 NWs, Au-In2O3 NWs, Ag-In2O3 NWs and Pt-In2O3 NWs are 19, 9.2, 9.5, and 9.8 nm, respectively. After introducing NMNPs into the NWs, the grain size dramatically decreases to 48.4–51.6% of the original size. Similar results were previously observed for NMNP-loaded SMO NWs fabricated via electrospinning.33 It is believed that the NMNPs can effectively prevent the grain growth of semiconductor oxide crystals through the “pinning effect”, which can suppress the semiconductor oxide grain boundary migration and increase the energy barrier in the process of grain growth.34,35 Thus, the introduction of secondary-phase NMNPs may be the main reason responsible for the morphology and size adjustment.
Fig. 1l shows the X-ray diffraction (XRD) patterns of the prepared NWs. For the pristine In2O3 NWs, the diffraction peaks of all the In2O3 NWs are consistent with cubic In2O3, and no trace of any other phases can be detected. This result is consistent with that of the HRTEM characterization. It should be noted that no diffraction peaks of Au, Ag and Pt can be observed due to the small concentration of Au, Ag and Pt.36 However, the different color of the samples revealed that Au, Ag and Pt co-existed in the In2O3 NWs. In addition, the average sizes of the grain can also be obtained from the peak of the XRD pattern using the following Scherrer eqn (1):37
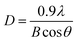 | (1) |
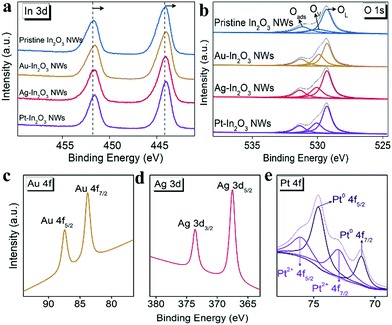
The valence chemistry and binding energy of the constituent elements are further demonstrated via X-ray photoelectron spectroscopy (XPS) analysis. High-resolution XPS spectra of the In 3d binding energy region are shown in Fig. 2a. The binding energies of In 3d3/2 and In 3d5/2 in the pristine In2O3 NWs are determined to be approximately 451.5 and 444.1 eV, respectively. Compared to that of the pristine In2O3 NW sample, the spectra of all the NMNP modified In2O3 NW samples shift slightly to the lower binding energy side, which was attributed to the increase of oxygen vacancies on the surface.38 To further confirm this phenomenon, the O 1s XPS spectra of the various In2O3 NW samples are studied. As presented in Fig. 2b, all the O 1s XPS spectra can be deconvoluted into three peaks at binding energies of around 529.3, 530.1 and 531.4 eV. They are corresponding to the lattice oxygen (OL), oxygen vacancy (OV), and chemisorbed oxygen (Oads), respectively. Compared to the pristine In2O3 NWs (18.6%), the oxygen vacancy ratios (the ratio of the integral area of the oxygen vacancy peak to the whole area of the O 1s peak) of Au-In2O3 NWs, Ag-In2O3 NWs and Pt-In2O3 NWs are determined to be 26.5%, 25.8%, and 27.7%, respectively. Herein, the increased amount of oxygen vacancies can be attributed to the special “spillover effect” of noble metals. This can facilitate the adsorption and desorption of molecular oxygen on the surface of the In2O3 NWs.39
In addition, the XPS peaks of the introduced NMNPs are investigated as shown in Fig. 2c–e. The XPS peaks located at 87.5 and 83.8 eV can be assigned to 4f5/2 and 4f7/2 of Au in the Au-In2O3 NWs (Fig. 2c), and the peaks at around 373.6 and 367.5 eV can be assigned to 3d3/2 and 3d5/2 of Ag derived from Ag-In2O3 NWs (Fig. 2d). In the case of Pt-In2O3 NWs (Fig. 2e), the Pt 4f XPS spectrum of Pt can be deconvoluted into two 4f5/2 and 4f7/2 doublets: one doublet located at 74.6 and 71.3 eV can be assigned to Pt0 from Pt NPs, and the other one at 73.0 and 76.1 eV can be assigned to Pt2+. The existence of Pt2+ is caused by surface oxidation of the Pt NPs during the calcination. In addition, the corresponding peak area ratio between Pt0 and Pt2+ is calculated to be 1.8. The above results further indicate that the NMNPs are successfully introduced into In2O3 NWs.
Gas sensing properties of NMNP modified In2O3 NW sensors
To investigate the feasibility of breath analysis using the NMNP modified In2O3 NWs, the detection capabilities of Au-In2O3 NW, Ag-In2O3 NW, and Pt-In2O3 NW sensors to different target analytes were first evaluated and compared with that of pristine In2O3 NWs (Fig. 3). H2S (for halitosis), HCHO (for breast cancer), nitrogen dioxide (for kidney malfunction), acetone (for diabetes), ethanol (for drunken driving), and the concentrations of all the gases are fixed as 1 ppm. The tests were performed at 300 °C which was the optimum working temperature of the NMNP modified In2O3 NW sensors (Fig. S3, ESI†). Compared to the pristine In2O3 NW sensor, the lowered activation energy for the surface reaction is obtained by introducing NMNPs.40 It should be noted that before testing, the introduced amounts of all the NMNPs used in this study were optimized first. The optimum modified concentrations for Au-In2O3 NW, Ag-In2O3 NW, and Pt-In2O3 NW sensors are determined to be 0.1 wt%, 0.16 wt%, and 0.08 wt%, respectively (Fig. S4, ESI†), and these concentrations are used in all the following investigations.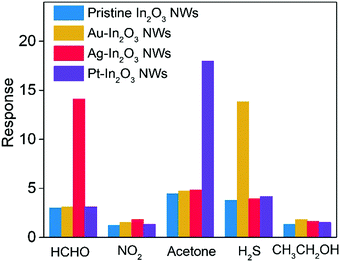 | ||
| Fig. 3 Selective tests of Au-In2O3 NW, Ag-In2O3 NW, and Pt-In2O3 NW gas sensors toward interfering analytes at the corresponding operating temperature compared to pristine In2O3 NWs, respectively. | ||
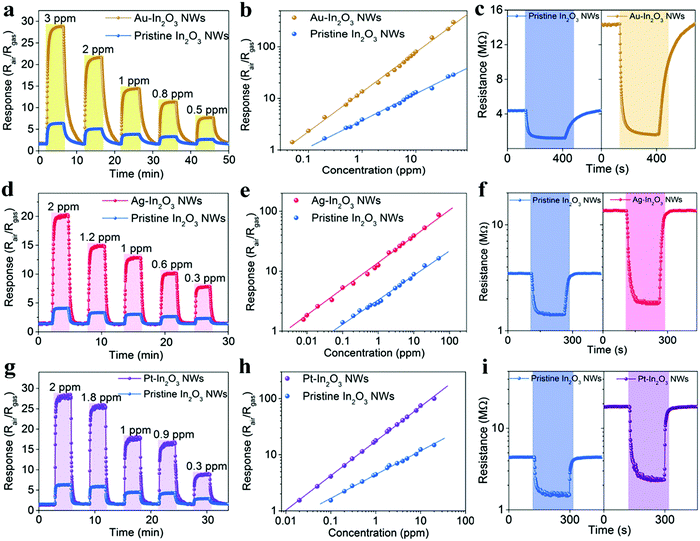
As shown in Fig. 3, the Au-, Ag-, and Pt-In2O3 NW sensors exhibit excellent selectivity toward H2S, formaldehyde, and acetone, respectively. It should be noted that the operating temperature curves for each sensor are studied with respect to the 1 ppm target gas. Each NMNP modified In2O3 NW sensor shows a response value of more than 12.6 to 1 ppm target gases, while it showed only minor responses (Rair/Rgas < 4) toward the other interfering analytes. In addition, the response values of the NMNP modified In2O3 NW sensors are effectively enhanced and the working temperatures are also decreased compared to that of the pristine In2O3 NWs, demonstrating the excellent catalytic activity of the selected NMNPs.
The detailed gas-sensing performance of the NMNP modified In2O3 NW sensors toward target gases was carefully studied and compared with that of the pristine In2O3 NW sensor. First, the dynamic sensing transients of Au-, Ag-, and Pt-In2O3 NW sensors along with the pristine In2O3 NW sensor exposed to various concentrations of H2S, HCHO, and acetone at 300 °C are shown in Fig. 4a, d and g, respectively. All the metal modified In2O3 NW sensors display rapid response and recovery characteristics to their target gases in the concentration range around the health threshold. The baselines are very stable after each cycle, indicating good real-time repeatability. The average response and recovery times of different In2O3 NW sensors are calculated and compared in Table 1. The NMNP modified In2O3 NW sensors exhibit much shorter response and recovery times (1.9–2 folds) compared to the pristine In2O3 NW sensor, which can be attributed to the effectively sensitized performance by introducing NMNPs. Furthermore, information can be obtained through their linear relation curves of response value to gas concentration (Fig. 4b, e and h). It should be noted that those curves are expressed in logarithmic coordinates, indicating the superior sensing abilities of those sensors.41 The actual detection limit (when Rair/Rgas ≥ 1.2 is used as the criterion for reliable gas sensing) and the response values to 1 ppm and 50 ppm target gases are also summarized in Table 1. As can be seen, the actual detection limits of the NMNP modified In2O3 NW sensors toward target gases are 4–12.5 times lower than the pristine In2O3 NW sensors, whereas the response values of different NMNP modified sensors to 1 ppm and 50 ppm of the target gases are 3.5–4.1 times and 5.3–10.5 times higher than that of the pristine In2O3 NW sensor. Taking the Ag-In2O3 NW sensor as an example, the Ag-In2O3 NW sensor exhibits a noticeable response of 8 ppb, which is 12.5 times lower than that of the pristine In2O3 NW based sensors (Rair/Rgas = 1.6). The response value of the Au-In2O3 NW sensor is as high as 301.5 when the response to 50 ppm H2S is 10.5-fold higher than that of the pristine In2O3 NW based sensors (Rair/Rgas = 28.7). The outstanding sensing performances of the as-studied NMNP modified In2O3 NW sensors demonstrates the excellent potential for application in halitosis, breast cancer, and diabetes in quick and painless disease analysis.
| H2S | HCHO | Acetone | ||||
|---|---|---|---|---|---|---|
| Pristine In2O3 NWs | Au-In2O3 NWs | Pristine In2O3 NWs | Ag-In2O3 NWs | Pristine In2O3 NWs | Pt-In2O3 NWs | |
| Response time | 67 s | 35 s | 37 s | 19 s | 41 s | 22 s |
| Recovery time | 214 s | 108 s | 48 s | 24 s | 55 s | 28 s |
| Detection limit | 200 ppb | 50 ppb | 100 ppb | 8 ppb | 100 ppb | 20 ppb |
| Response (1 ppm) | 3.9 | 13.6 | 3.1 | 12.6 | 4.5 | 17.9 |
| Response (50 ppm) | 28.7 | 301.5 | 16.4 | 87.2 | 23.3 | 198.7 |
To verify the enhanced sensing performance of the NMNP modified In2O3 NW sensors, the corresponding resistance changes were further investigated. As shown in Fig. 4c, the baseline resistance of the In2O3 NW sensor (4.31 MΩ) raises to 14.28 MΩ after Au modification. Similar results can be found in the cases of Ag-In2O3 NW sensors (13.74 MΩ in Fig. 4f) and Pt-In2O3 NW sensors (10.89 MΩ in Fig. 4i). Normally, the SMO based gas sensors operate on the basis of surface chemical redox reactions occurring between target gases and oxygen ions, which can change the resistance of the SMOs. The NMNP is a much better oxygen dissociation catalyst than In2O3.42–44 It can spillover more activated oxygen species on the surface of In2O3 (as proved in the O 1s XPS results in Fig. 2b), which can decrease the electron density at the surface of In2O3 NWs and thus increase the baseline resistance of semiconductor conductivity (as shown in Fig. 4c, f and i). Once target gases are injected into the sensor chamber, they react with the activated oxygen species thereby releasing the electrons back into the conduction band of In2O3, leading to improved resistance.
Mechanism of the enhanced response and selectivity of the NMNP modified In2O3 NW sensors
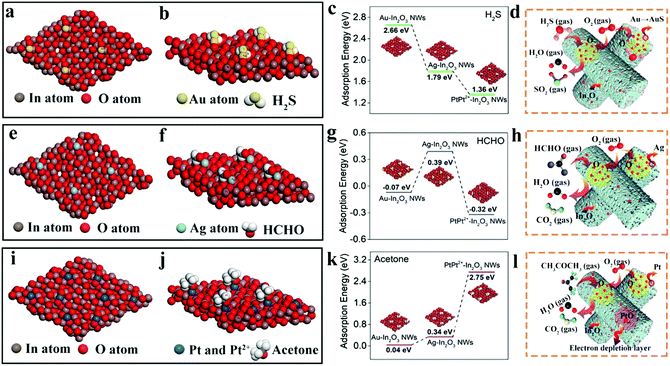
In2O3 is a typical n-type SMO, which can form an electron depletion layer near the surface by chemisorption of oxygen species in the air ambient and lead to the increase of the device resistance. When it is exposed to a reducing gas such as H2S, HCHO or acetone, the reducing gas will react with the adsorbed oxygen molecules and release electrons back into the conductive band of In2O3, thereby decreasing the resistance of the In2O3 sensor.45,46 When further modified by the NMNPs, such as Au, Ag, and Pt, which have much better oxygen dissociated abilities than In2O3, the introduced “spillover effect” can bring much more quantities of active oxygen species, and then improved the sensing performance of In2O3 gas sensors.47,48 It should be pointed out that the morphologies and average diameters of In2O3 NWs after loading with the NMNPs showed some changes compared to those of the pristine In2O3 NWs. To determine the effect of the morphologies and average diameters on the sensor performance, two relative experiments are conducted. First, in order to tune the morphologies and average diameters of NMNP modified In2O3 NWs to be comparable, we controlled all the loading concentration of NMNPs as 0.08 wt%. As shown in Fig. S5 (ESI†), the corresponding SEM and TEM images display the similar morphologies of the different NMNP modified In2O3 NWs. The average diameters of 60 nm are confirmed in the corresponding size distribution diagrams (Fig. S5g–i, ESI†). According to Fig. S4 (ESI†), the response values of Au-, Ag-, and Pt-In2O3 NW (0.08 wt% modified) sensors are estimated to be 11.0, 8.2, and 18.2, respectively. The responses of Au- and Ag-In2O3 NW sensors are decreased compared to those with the optimal modified concentration (13.6 for Au-In2O3 NW sensors and 12.6 for Ag-In2O3 NW sensors, respectively). The decrease of the response values may be caused by the NMNP concentrations and the morphology changes,49 as both factors are changed by adjusting the modified concentration to the optimal condition (0.1 wt% for Au-In2O3 NW sensors and 0.16 wt% for Ag-In2O3 NW sensors, respectively). However, as is known, the variation in the specific surface area caused by a structural change is the main factor that influences the sensing performance in SMO-based sensors. Hence, the specific surface areas of different NMNP modified In2O3 NW samples (under the optimal loading concentration) are further studied using the nitrogen adsorption–desorption isotherm. As shown in Fig. S6 (ESI†), the specific surface areas and the pore sizes of the pristine In2O3 NWs, and Au-, Ag-, and Pt-In2O3 NWs are 41.2, 102.4, 103.2, and 104.7 m2 g−1 and 13, 7, 6, and 8 nm, respectively. After loading with different NMNPs in In2O3 NWs, the specific surface areas and the pore sizes of the NMNPs modified In2O3 NWs show no obvious change. While the corresponding specific surface area values are enlarged about 2.5 times and the pore sizes decrease 0.4–0.5 times compared to that of the pristine In2O3 NWs. These results indicate that the slight variation in the morphologies and average diameter has almost no obvious influence on the specific surface areas of In2O3 NW sensing materials. Thus, we can speculate that the NMNP modified concentrations rather than the changes in morphologies and average diameters are the main factor for the sensing enhancement in our case.The enhanced properties induced by the “spillover effect” of NMNPs normally include the decrease of the working temperature, shortened dynamic process, and the increase of the response.50,51 However, the oxygen species are the open active acceptors without specific selectivity, thus the specific selectivity to different gases after different NMNP loadings cannot be well explained. In order to understand this point in-depth, DFT calculations were implemented to reveal the variation in the adsorption energies and energy band structures of various NMNP modified In2O3 NWs. Fig. 5 shows the optimized configuration of In2O3 embedded with different NMNPs. Under the oxygen-rich condition, the Au and Ag atoms prefer to locate at the center of the four oxygen atoms (Fig. 5a and e). Unlike the cases of Au and Ag, the surface of Pt NPs is composed of Pt0 and Pt2+ in Pt-In2O3, as has been proved in Fig. 2e; the Pt0 atoms tend to be adsorbed in the middle of the O–O bridge and Pt2+ ions incline to locate at the center of the six-membered ring of In and O (Fig. 5i). Since the NMNPs are uniformly dispersed among the In2O3 nanocrystals (as proved in Fig. 2), we can conclude that the enhanced selectivity and response should mainly happen around the NMNPs. Fig. 5b, f, and j show the most stable adsorption configurations when H2S, HCHO, and acetone gas molecules are absorbed on the surface of Au-, Ag-, and Pt-In2O3, respectively. H2S molecules prefer to absorb nearly parallel to the surface of Au-In2O3 in the most stable adsorption configuration (Fig. 5d), in which the S atom combines with the Au atom and the H atoms combine with the O atoms in In2O3. In the case of the HCHO@Ag-In2O3 system, HCHO molecules are likely to attach on the top of Ag-In2O3 (Fig. 5e), and the H atoms won’t bond with O atoms in In2O3 to form hydrogen bonds. Interestingly, in the case of Pt-In2O3 which has been proved to contain Pt0 and Pt2+ (Fig. 5i), the simulated result exhibits that the Pt0 atom cannot absorb the acetone molecules, while the acetone molecules are fond of interacting with Pt2+ ions. When combining, the O atoms of acetone molecules are likely to interact with the Pt2+ ions, and hydrogen bonds will be formed between the H atoms of acetone molecules near to the surface of In2O3 and the O atoms in In2O3.
In addition, the doping adsorption energies (Eads) were calculated using the following eqn (2):
| Eads = (EX-In2O3 + Egas) − Egas/X-In2O3 | (2) |
When exposed to HCHO gas (Fig. 5h), only the Ag-In2O3 NW sensor shows a positive adsorption energy to HCHO (0.39 eV, Fig. 5g). Considering the combination mode and adsorption energy between HCHO and Ag-In2O3 NW, a physical absorption process can be proposed. This is well consistent with the results shown in Fig. 3 and Fig. S7 (ESI†), in which the response of Au- and Pt-In2O3 NW sensors almost remained unchanged compared to that of the pristine In2O3 NW sensor. Regarding the good selectivity toward acetone of the Pt(Pt2+)-In2O3 NW sensor, it displays the highest adsorption energy (2.75 eV) between Pt2+ and In2O3 in all cases of In2O3 for the acetone molecule, while no adsorption occurs between Pt atoms and acetone gas molecules, as exhibited in Fig. S8 (ESI†). In addition, overall considering the value of the adsorption energy and the bonding mode between Pt2+ ions, a strong chemical absorption can be deduced. Weak physical absorptions can be found in the cases of Au- and Ag-In2O3 NW sensors toward acetone molecules, which corresponds to the small lift in sensing responses (as proved in Fig. 3). Besides, from the view point of the density of states (DOS, Fig. S9, ESI†), the existence of Pt2+ ions results in the reconstitution of the surface structure of In2O3 after acetone absorption leading to the shift of DOS toward high energy and the enlargement of the bandgap, whereas this phenomenon won’t happen in the cases of the Au- and Ag-In2O3 NW sensors. In addition, the Pt2+ ions may originate from PtO which is a p-type semiconductor, and the p–n junctions are created at the interfaces of PtO and In2O3. Thus, PtO also helps to enlarge the electron depletion region of In2O3, inducing enhanced resistance variation when the sensors are exposed to target gases (Fig. 5i). Overall, the partial existence of Pt2+ ions produced during the heating treatment plays an important role in enhancing the sensing performance of the Pt(Pt2+)-In2O3 NW sensor.
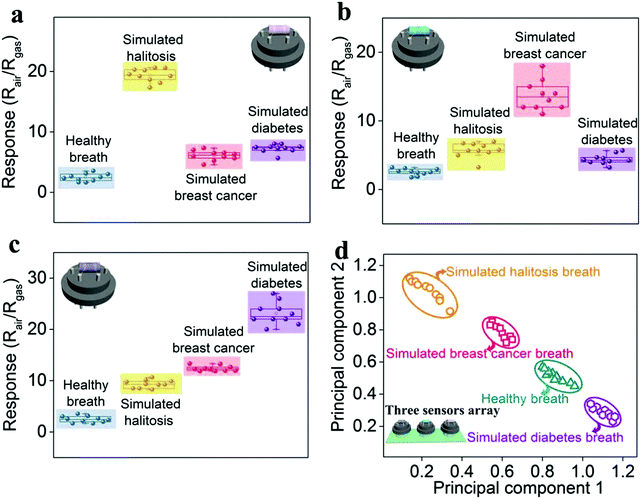
Sensor arrays for the detection of exhaled breath
Based on the above sensing analysis, we investigate the potential feasibility for breath analysis using the sensing array composed of the as-proposed Au-, Ag-, and Pt-In2O3 NW sensors (Scheme 1). Because the optimal working temperatures of all the NMNP modified sensors are the same (300 °C), the sensor array can easily be built without worrying about the attenuation of the sensors’ performance. We performed the tests by exposing the sensor array to the simulated breath of halitosis, breast cancer, and diabetes. As shown in Fig. S10 (ESI†), the exhaled breath samples were collected into the gas collection bags (1 L) with an aluminum coating (De Lin Instrument Company, Dalian) from 10 healthy subjects consisting of 5 male and 5 female nonsmoking individuals. An additional dehumidification device (it was filled with silica gel and molecular sieve desiccants) was used to reduce the effects due to high exhalation humidity.40 As referred to halitosis, breast cancer, and diabetes breath, the collected breath samples were mixed with 2 ppm H2S, 1.2 ppm HCHO, and 1.8 ppm acetone, respectively, since these concentrations represent a recognizable threshold for the diagnosis of halitosis, breast cancer, or diabetes.3,7,10The breath tests were carried out by putting the sensing array into each simulated gas. As shown in Fig. 6a, the given Au-In2O3 NW sensor has a highly sensitive and selective ability toward H2S in the expiratory background. In addition, it can successfully discriminate the healthy breath and simulated halitosis breath through their response values with a large difference of 7.91 times. Furthermore, it's not difficult to find that the Ag- and Pt-In2O3 NW sensors can also well identify the simulated breast cancer and diabetes breath (Fig. 6b and c), respectively. The differences between healthy and simulated breast cancer/diabetes breath are 5.06 and 5.59 times, respectively. All the sensors exhibited a relatively low response value toward the other simulated breath compared to the results obtained in clean air. This is because the exhaled breath contained thousands of gas species including both oxidizing and reducing gases which may occupy some active sites of the sensors. Furthermore, the pattern recognition of the four simulated breath samples (healthy breath, simulated halitosis, breast cancer and diabetes breath) measured by the sensing array was performed using principal component analysis (PCA). As shown in Fig. 6d, all the breath samples were clearly discriminated into four small clusters without any overlap. In addition, we summarized the currently reported semiconductor-based sensor array in Table S1 (ESI†). To the best of our knowledge, this is the first study of an In2O3 based sensor array in exhaled biomarker analysis. Compared to other material systems, the proposed sensor array displays a lower detection limit and a large detection range; moreover, the same optimal working temperature may greatly decrease the complexity of the array circuit design. It should be noted that the response and recovery times are comparable to the works listed in Table S1 (ESI†). Actually, loading with different NMNPs in our present work can improve the response and recovery times to some extent due to the catalysis of the NMNPs. As shown in Table 1, the results illustrate that the response and recovery times can be improved about two folds after loading the NMNPs compared to those of the pristine In2O3 NWs sensors. In addition, besides the modification of the NMNPs, some other factors can also contribute to improving the response and recovery times according to the previous reports. For example, adjusting the surface-to-volume ratio through structural engineering and loading with other materials with high carrier mobility.56,57 Furthermore, some testing parameters, such as the gas flow rate, working temperature, and pressure of the analyte gas, can also influence the dynamic process of the sensing reaction.58 We believe that the future works about developing more facile and effective routes for the improvement of the response and recovery times and investigating their underlying causes are needed. The above results demonstrate that the as-proposed sensing array has high potential for early detection of multiple diseases.
Conclusions
In summary, we have obtained a sensitive and selective sensing array for simultaneous detection of multiple target gases, which has great potential for noninvasive disease diagnosis using exhaled breath biomarkers. The sensing array composed of Au, Ag and Pt NP modified In2O3 NW sensitive layers was synthesized via a simple electrospinning process. The obtained In2O3 NW possessed well-dispersed catalyst NPs with a small NW diameter (<60 nm), and a large amount of surface oxygen vacancies. In order to meet or exceed the detection requirements for the dilute concentration of the biomarker molecule in exhaled breath, the sensing properties of different NMNP modified In2O3 NW sensors were carefully studied with regard to the working temperature, sensitivity, and selectivity. In addition, the DFT results indicated that the strong adsorption energies between the NMNPs and certain target gases should seriously be considered along with the “spillover effect” to explain the enhancement of sensitivity and selectivity. Furthermore, the three NMNP modified In2O3 NW sensors were further used to build the sensor array for the real-time detection of disease biomarkers in simulated exhaled breath. The as-built sensing array exhibited good ability to clearly distinguish the different biomarkers at the same time. This work provides a promising technique for noninvasive diagnosis of different diseases, such as halitosis, breast cancer, and diabetes, at the same time.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 61874049, 61775080, and 61822506), the National Key Research and Development Program (2016YFC0207101), the Jilin Province Natural Science Foundation of China (No. 20180101210JC) and the 13th Five-year Plan on the Science and Technology Project of the Education Department of Jilin Province (No. JJKH20190115KJ), and the Special Project of the Province-University Co-constructing Program of Jilin Province (SXGJXX2017-3).References
- S. Schon, S. J. Theodore and A. T. Güntner, Sens. Actuators, B, 2018, 273, 1780–1785 CrossRef CAS.
- T. Saidi, D. Palmowski, S. Babicz-Kiewlicz, T. G. Welearegay, N. El Bari, R. Ionescu, J. Smulko and B. Bouchikhi, Sens. Actuators, B, 2018, 273, 1719–1729 CrossRef CAS.
- T. Wang, S. Zhang, Q. Yu, S. Wang, P. Sun, H. Lu, F. Liu, X. Yan and G. Lu, ACS Appl. Mater. Interfaces, 2018, 10, 32913–32921 CrossRef CAS PubMed.
- Q.-L. Liao, H. Jiang, X.-W. Zhang, Q.-F. Qiu, Y. Tang, X.-K. Yang, Y.-L. Liu and W.-H. Huang, Nanoscale, 2019, 11, 10702–10708 RSC.
- B. Buszewski, M. Kęsy, T. Ligor and A. Amann, Biomed. Chromatogr., 2007, 21, 553–566 CrossRef CAS PubMed.
- F. L. M. Ricciardolo, Curr. Opin. Pulm. Med., 2014, 20, 53–59 CrossRef CAS PubMed.
- R. M. Matthew, B. Yury, W. Gerard, L. Rafal and K. T. Frank, J. Breath Res., 2007, 1, 014001 CrossRef.
- G. Peng, U. Tisch, O. Adams, M. Hakim, N. Shehada, Y. Y. Broza, S. Billan, R. Abdah-Bortnyak, A. Kuten and H. Haick, Nat. Nanotechnol., 2009, 4, 669 CrossRef CAS PubMed.
- J. Shin, S.-J. Choi, I. Lee, D.-Y. Youn, C. O. Park, J.-H. Lee, H. L. Tuller and I.-D. Kim, Adv. Funct. Mater., 2013, 23, 2357–2367 CrossRef CAS.
- S.-J. Kim, S.-J. Choi, J.-S. Jang, H.-J. Cho, W.-T. Koo, H. L. Tuller and I.-D. Kim, Adv. Mater., 2017, 29, 1700737 CrossRef PubMed.
- S.-J. Choi, F. Fuchs, R. Demadrille, B. Grévin, B.-H. Jang, S.-J. Lee, J.-H. Lee, H. L. Tuller and I.-D. Kim, ACS Appl. Mater. Interfaces, 2014, 6, 9061–9070 CrossRef CAS.
- W. Liu, L. Xu, K. Sheng, C. Chen, X. Zhou, B. Dong, X. Bai, S. Zhang, G. Lu and H. Song, J. Mater. Chem. A, 2018, 6, 10976–10989 RSC.
- W. Y. Li, L. N. Xu and J. Chen, Adv. Funct. Mater., 2005, 15, 851–857 CrossRef CAS.
- Z. Jing and J. Zhan, Adv. Mater., 2008, 20, 4547–4551 CrossRef CAS.
- Q. Li, Y. Cen, J. Huang, X. Li, H. Zhang, Y. Geng, B. I. Yakobson, Y. Du and X. Tian, Nanoscale Horiz., 2018, 3, 525–531 RSC.
- S. A. Vanalakar, V. L. Patil, N. S. Harale, S. A. Vhanalakar, M. G. Gang, J. Y. Kim, P. S. Patil and J. H. Kim, Sens. Actuators, B, 2015, 221, 1195–1201 CrossRef CAS.
- C. Wang, J. Liu, Q. Yang, P. Sun, Y. Gao, F. Liu, J. Zheng and G. Lu, Sens. Actuators, B, 2015, 220, 59–67 CrossRef CAS.
- A. Harley-Trochimczyk, T. Pham, J. Chang, E. Chen, M. A. Worsley, A. Zettl, W. Mickelson and R. Maboudian, Adv. Funct. Mater., 2016, 26, 433–439 CrossRef CAS.
- L. Zhang, R. Dong, Z. Zhu and S. Wang, Sens. Actuators, B, 2017, 245, 112–121 CrossRef CAS.
- J.-g. Kang, J.-S. Park and H.-J. Lee, Sens. Actuators, B, 2017, 248, 1011–1016 CrossRef CAS.
- F. Fan, J. Zhang, J. Li, N. Zhang, R. Hong, X. Deng, P. Tang and D. Li, Sens. Actuators, B, 2017, 241, 895–903 CrossRef CAS.
- S.-J. Choi, K. H. Ku, B. J. Kim and I.-D. Kim, ACS Sens., 2016, 1, 1124–1131 CrossRef CAS.
- P. Rai, J.-W. Yoon, C.-H. Kwak and J.-H. Lee, J. Mater. Chem. A, 2016, 4, 264–269 RSC.
- M. S. Barbosa, P. H. Suman, J. J. Kim, H. L. Tuller, J. A. Varela and M. O. Orlandi, Sens. Actuators, B, 2017, 239, 253–261 CrossRef CAS.
- S.-J. Choi, S.-J. Kim, W.-T. Koo, H.-J. Cho and I.-D. Kim, Chem. Commun., 2015, 51, 2609–2612 RSC.
- X. Zhou, F. Qu, B. Zhang, C. Jiang and M. Yang, Mater. Lett., 2017, 209, 618–621 CrossRef CAS.
- D. R. Miller, S. A. Akbar and P. A. Morris, Sens. Actuators, B, 2014, 204, 250–272 CrossRef CAS.
- X. Xu, P. Zhao, D. Wang, P. Sun, L. You, Y. Sun, X. Liang, F. Liu, H. Chen and G. Lu, Sens. Actuators, B, 2013, 176, 405–412 CrossRef CAS.
- M. Segall, P. J. Lindan, M. A. Probert, C. J. Pickard, P. J. Hasnip, S. Clark and M. Payne, J. Phys.: Condens. Matter, 2002, 14, 2717 CrossRef CAS.
- D. Vanderbilt, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 7892 CrossRef PubMed.
- M. Zhang, W. Wang and Y. Chen, Appl. Surf. Sci., 2018, 434, 1344–1352 CrossRef CAS.
- Y. Ning, Z. Zhang, F. Teng and X. Fang, Small, 2018, 14, 1703754 CrossRef PubMed.
- N.-H. Kim, S.-J. Choi, S.-J. Kim, H.-J. Cho, J.-S. Jang, W.-T. Koo, M. Kim and I.-D. Kim, Sens. Actuators, B, 2016, 224, 185–192 CrossRef CAS.
- D.-J. Yang, I. Kamienchick, D. Y. Youn, A. Rothschild and I.-D. Kim, Adv. Funct. Mater., 2010, 20, 4258–4264 CrossRef CAS.
- N.-L. Wu, S.-Y. Wang and I. Rusakova, Science, 1999, 285, 1375–1377 CrossRef CAS PubMed.
- P. M. Bulemo, H.-J. Cho, D.-H. Kim and I.-D. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 18183–18191 CrossRef CAS PubMed.
- F. Li, T. Zhang, X. Gao, R. Wang and B. Li, Sens. Actuators, B, 2017, 252, 822–830 CrossRef CAS.
- N. Sai Krishna, S. Kaleemulla, G. Amarendra, N. Madhusudhana Rao, C. Krishnamoorthi, I. Omkaram and D. Sreekantha Reddy, J. Mater. Sci.: Mater. Electron., 2015, 26, 8635–8643 CrossRef CAS.
- M. Righettoni, A. Amann and S. E. Pratsinis, Mater. Today, 2015, 18, 163–171 CrossRef CAS.
- R. Xing, Q. Li, L. Xia, J. Song, L. Xu, J. Zhang, Y. Xie and H. Song, Nanoscale, 2015, 7, 13051–13060 RSC.
- R. Xing, K. Sheng, L. Xu, W. Liu, J. Song and H. Song, RSC Adv., 2016, 6, 57389–57395 RSC.
- M. Karmaoui, S. G. Leonardi, M. Latino, D. M. Tobaldi, N. Donato, R. C. Pullar, M. P. Seabra, J. A. Labrincha and G. Neri, Sens. Actuators, B, 2016, 230, 697–705 CrossRef CAS.
- P. Rai, J.-W. Yoon, C.-H. Kwak and J.-H. Lee, J. Mater. Chem. A, 2016, 4, 264–269 RSC.
- W. Liu, L. Xu, K. Sheng, X. Zhou, X. Zhang, C. Chen, B. Dong, X. Bai, L. Geyu and H. Song, Sens. Actuators, B, 2018, 273, 1676–1686 CrossRef CAS.
- N. Du, H. Zhang, B. Chen, X. Ma, Z. Liu, J. Wu and D. Yang, Adv. Mater., 2007, 19, 1641–1645 CrossRef CAS.
- N. Singh, A. Ponzoni, R. K. Gupta, P. S. Lee and E. Comini, Sens. Actuators, B, 2011, 160, 1346–1351 CrossRef CAS.
- D. Degler, S. A. Müller, D. E. Doronkin, D. Wang, J.-D. Grunwaldt, U. Weimar and N. Barsan, J. Mater. Chem. A, 2018, 6, 2034–2046 RSC.
- W. Ouyang, F. Teng, J. H. He and X. Fang, Adv. Funct. Mater., 2019, 29, 1807672 CrossRef.
- W. Ouyang, F. Teng, M. Jiang and X. Fang, Small, 2017, 13, 1702177 CrossRef PubMed.
- A. Kolmakov, D. Klenov, Y. Lilach, S. Stemmer and M. Moskovits, Nano Lett., 2005, 5, 667–673 CrossRef CAS PubMed.
- Y. J. Kwon, H. G. Na, S. Y. Kang, S.-W. Choi, S. S. Kim and H. W. Kim, Sens. Actuators, B, 2016, 227, 157–168 CrossRef CAS.
- P. Rai, J.-W. Yoon, H.-M. Jeong, S.-J. Hwang, C.-H. Kwak and J.-H. Lee, Nanoscale, 2014, 6, 8292–8299 RSC.
- L. T. Lanh, T. T. Hoa, N. D. Cuong, D. Q. Khieu, D. T. Quang, N. Van Duy, N. D. Hoa and N. Van Hieu, J. Alloys Compd., 2015, 635, 265–271 CrossRef CAS.
- J. Geng, M. D. Thomas, D. S. Shephard and B. F. Johnson, Chem. Commun., 2005, 1895–1897 RSC.
- W. Chen, X. Gui, L. Yang, H. Zhu and Z. Tang, Nanoscale Horiz., 2019, 4, 291–320 RSC.
- M. Meyyappan, Small, 2016, 12, 2118–2129 CrossRef CAS PubMed.
- T. Zhai, X. Fang, M. Liao, X. Xu, H. Zeng, B. Yoshio and D. Golberg, Sensors, 2009, 9, 6504–6529 CrossRef CAS PubMed.
- R. Kumar, O. Al-Dossary, G. Kumar and A. Umar, Nano-Micro Lett., 2015, 7, 97–120 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00404a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |