Open Access Article
Open Access ArticleResearch on the mechanism of mechanochemical activation for enhanced leaching of vanadium-bearing shale: activation kinetics and fluorine adsorption
Xuxia Zhao abcd,
Yimin Zhang*abcd,
Nannan Xue*abcd and
Pengcheng Huabcd
abcd,
Yimin Zhang*abcd,
Nannan Xue*abcd and
Pengcheng Huabcd
aSchool of Resource and Environmental Engineering, Wuhan University of Science and Technology, Wuhan 430081, Hubei Province, China. E-mail: zym126135@126.com; cbdis@aliyun.com
bState Environmental Protection Key Laboratory of Mineral Metallurgical Resources Utilization and Pollution Control, Wuhan 430081, Hubei Province, China
cCollaborative Innovation Center of Strategic Vanadium Resources Utilization, Wuhan 430081, Hubei Province, China
dHubei Provincial Engineering Technology Research Center of High Efficient Cleaning Utilization for Shale Vanadium Resource, Wuhan 430081, Hubei Province, China
First published on 16th July 2025
Abstract
Vanadium-bearing shale as a strategic resource is an important raw material for extracting vanadium, and the mechanochemical activation can realize the vanadium extraction by full-wet leaching with green, low-carbon and high efficiency. Based on mineralogical research on mineral composition and distribution, mineral embedded grain size distribution, we employ a graded activation process. The mechanism of mechanochemical activation-enhanced dissolution of vanadium-bearing shale is revealed through the relationship between activation kinetics and vanadium leaching as well as the fluorine adsorption process on different minerals surfaces of vanadium-bearing shale. Vanadium-bearing shale is mainly composed of quartz, muscovite, calcite, pyrite, feldspar and apatite. Vanadium with 94.24% exists in muscovite, while the remaining 5.76% exists in oxide. Muscovite is predominantly closely associated with quartz, calcite and organic carbonaceous and tends to be enriched in fine grained, displaying fine disseminated granularity with 0.005–0.06 mm. The grindability order of vanadium-bearing shale is observed as follows: −3 to +2.5 mm < −2.5 to +2 mm < −2 to +1 mm < −1 to +0.6 mm. The activation process of different particle sizes were well evaluated by kinetic equations (R2 = 0.99). The vanadium leaching efficiency has a positive linear relationship with the activation yield at the optimal leaching particle size with −0.6 mm. The vanadium leaching efficiency and activation time can be expressed by equation of η = γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ktn)μ + ν. The mineral surface of vanadium-bearing shale has a good adsorption of F− (23.89 mg g−1) undergoing amorphous phenomena. The order of F− adsorption capacity is calcite, pyrite, dolomite, muscovite, feldspar, and quartz. The adsorption process of F− alters the surface potential on the vanadium-bearing shale, and the negative charge on the of muscovite surface increases, while that of pyrite and calcite decreases, which is conducive to the diffusion of H+ to the surface of muscovite and away from pyrite and calcite. F− generates CaF2 with the surface of calcite and FeF3 with Fe(III)–S on the surface of pyrite, hindering and slowing down the dissolution of calcite and pyrite. F− forms Al–F and Si–F bonds with Si and Al on the surface of muscovite, promoting the dissolution of muscovite.
exp(−ktn)μ + ν. The mineral surface of vanadium-bearing shale has a good adsorption of F− (23.89 mg g−1) undergoing amorphous phenomena. The order of F− adsorption capacity is calcite, pyrite, dolomite, muscovite, feldspar, and quartz. The adsorption process of F− alters the surface potential on the vanadium-bearing shale, and the negative charge on the of muscovite surface increases, while that of pyrite and calcite decreases, which is conducive to the diffusion of H+ to the surface of muscovite and away from pyrite and calcite. F− generates CaF2 with the surface of calcite and FeF3 with Fe(III)–S on the surface of pyrite, hindering and slowing down the dissolution of calcite and pyrite. F− forms Al–F and Si–F bonds with Si and Al on the surface of muscovite, promoting the dissolution of muscovite.
1. Introduction
Vanadium is included in the strategic metal lists of China, America, and the European Union and is widely used in the aerospace, metallurgy, chemical, medicine, and new energy industries.1 Vanadium-bearing shale is a polymetallic ore with vanadium grades ranging from 0.1 to 1.2%.2,3 The gross reserves of V2O5 in vanadium-bearing shale are approximately 118 million tons, accounting for more than 87% of the domestic reserves of vanadium,4,5 which is the crucial raw material for the extraction of vanadium. Primary vanadium-bearing shale is a sedimentary rock rich in organic carbon, argillaceous materials, and sulfides, mainly composed of quartz, mica, calcite and pyrite. Vanadium in vanadium-bearing shale mainly exists in silicate minerals in the form of low-valent isomorphism and is difficult to be released.Except thermal activation, mechanochemical activation is another pretreatment method of enhancing mineral leaching.6–8 The mechanochemical activation refers to the changes in the physical and chemical properties and crystal structure of solids under the action of external forces such as shearing, friction, impact and extrusion.9 Through plastic deformation and defect formation, part of the mechanical energy is converted into the internal energy of the solid, putting it in a high-energy unstable state and causing an increase in the chemical reactivity of the solid, thus inducing physicochemical reactions process.10 Mechanical force disordered the solid crystal structure, resulting in dislocation formation and flow of atoms within solid particles, thereby causing the amorphous transformation of the particles.11,12 During the amorphous process, the internal energy stored in solid particles is much greater than that stored in simple dislocations, and they are in a high-energy unstable state.13 Therefore, this is also the reason for the enhanced reactivity and accelerated reaction rate of solid particles.14 Unlike ordinary thermochemical reactions, mechanochemical reactions is mechanical energy rather than thermal energy, the reactions can be completed without harsh conditions such as high temperature and high pressure.15 Its advantages of good reaction safety and low energy consumption are also major advantages of mechanochemical technology over high-temperature heat treatment.16,17
Mechanical activation is the process of reducing ore particle size under the action of mechanical force.18 Under the action of mechanical force, mechanical energy is directly transferred from the ball mill to the solid system, causing the minerals to be crushed and the particle size of large solid particles to decrease.19 Due to differences in mineral characteristics, during the process of activation, the parameters of ore particle size, grindability, and crushing energy are particularly important to the activation performance of ore.18,20 Therefore, it is necessary to study the activation kinetics and particle size characteristics of these materials.21,22 One study compared the crushing processes of quartz and chlorite by grinding kinetics during wet ball milling.23 At the same time, the time required to crush the particles to obtain the appropriate particle size is a necessary step in designing the activation process.24,25 In our previous studies,26 the mechanochemical activation pretreated the vanadium-bearing shale to effectively improve the leaching efficiency of vanadium and achieves a full hydro-metallurgical process. It was discovered that the activation time is an important factor of affecting the leaching and the particle size and monomer dissociation of vanadium-bearing shale is closely related to activation time. Meanwhile, during the ball milling process, an activator is added, the reactivity of activation is enhanced through the collision, friction and shearing of the spheres, promoting the interaction between the activator and the minerals. Inorganic reagents are utilized to adsorb the mineral solids, thereby altering the physical and chemical properties of the mineral surface.27
In this study, we investigated the process mineralogy of vanadium-bearing shale, examining mineral composition, dissemination relationships, and vanadium occurrence. Furthermore, we analyzed the impacts of graded activation processes on vanadium leaching. We established activation kinetics and a vanadium leaching efficiency model for activation efficiency for vanadium-bearing shale, laying a theoretical foundation for enhancing vanadium leaching efficiency and optimizing activation conditions and performance. Meanwhile, the fluorine adsorption on surfaces of vanadium-bearing shale was revealed using pure minerals under mechanical chemical activation process.
2. Experimental
2.1 Materials
The vanadium-bearing shale examined in this study originated from a mine in Hubei Province, Central China, and underwent pulverization to achieve a grain size of 0–3 mm prior to analysis. Chemical compositions of the raw ore, detailed in Table 1, reveal a V2O5 grade of 0.72%, with silicon, aluminum, calcium, and iron as the primary non-target elements. Specifically, SiO2, Al2O3, CaO, Fe2O3, MgO, and K2O contents were 60.69%, 8.99%, 11.14%, 3.52%, 1.71%, and 1.88%, respectively, indicating a multi-calcium vanadium-bearing shale. Identified mineral phases in the vanadium-bearing shale include mica, quartz, calcite, and apatite (Fig. 1), with calcite predominating as the gangue mineral with high acid consumption. Furthermore, Fig. 2 demonstrates a significant correlation between vanadium and silicon, aluminum, potassium, and magnesium, suggesting muscovite as the primary host for vanadium. Therefore, the vanadium-bearing shale utilized in this study serves as a representative example of mica-type vanadium-bearing shale.| Composition | V2O5 | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | K2O | S | BaO | P | ZnO | Na2O | TiO |
| Content/% | 0.72 | 60.69 | 8.99 | 11.14 | 3.52 | 1.71 | 1.88 | 1.39 | 0.58 | 0.52 | 0.29 | 0.22 | 0.21 |
2.2 Experiments
Particle size screening experiments were conducted using a top strike type vibrating screen machine (HLSDOB-Φ200, Wuhan Hengle Mineral Engineering Equipment Co., Ltd, China). In each trial, 100 g of vanadium-bearing shale was introduced into the vibrating screen and subjected to screening for a duration of 5 min.Under the specified activation conditions—such as a pulp concentration of 50%, a ball-to-pulp ratio of 50, and a 5 wt% NaF activator—vanadium-bearing shale samples with different particle sizes (500) underwent testing at varying activation durations. This was achieved through conical ball milling (HLXMQ-Φ150 × 50, Wuhan Hengle Mineral Engineering Equipment Co., Ltd, China) and rod milling (HLXMB-Φ200 × 240). Following activation, the product was filtered, dried, and subjected to particle size analysis using both a vibrating screen and a laser particle size analyzer.
The leaching efficiency of vanadium was obtained through an acid leaching experiment. The vanadium-bearing shale (50 g) and 30 wt% sulfuric acid were put into a 250 mL conical bottle, oscillated and leached for 12 h in a thermostatic oscillator with a vibration velocity of 200 rpm and a temperature of 95 °C (SHA-2, Jiangsu Jintan Yitong Electronics Co., Ltd). After the leaching was completed, the leaching residue and vanadium-containing leachate were obtained by solid–liquid separation, and the vanadium concentration in the leachate was determined by ammonium ferric sulfate titration.
2.3 Characterization
The chemical composition of the vanadium-bearing shale was detected by an inductively coupled plasma optical emission spectrometer (ICP, Horiba Ultima Expert: Agilent ICP-OES 730, US). The mineral phase compositions of the vanadium-bearing shale were tested by X-ray diffraction (XRD, D/MAX-RB, Rigaku, Japan). The micromorphology and elemental distribution of the vanadium-bearing shale were analyzed by scanning electron microscopy (SEM, JSM-IT300, JEOL, Tokyo, Japan) on an instrument equipped with an X-square energy dispersive spectrometer (EDS, OXFORD, Britain). The mineral dissemination relationship of vanadium-bearing shale was tested by a polarizing microscope (Nikon, LV100POL). The particle size of the activated vanadium-bearing shale was tested by a laser particle sizer (ZEN2600, Malvern Instruments Ltd). The dissemination patterns of the V element in the vanadium-bearing shale obtained by the Advanced Mineral Identification and Characterization System (MLA, Sigma 300, Quantax 400 and AMICS).3. Results and discussion
3.1 Characteristics analysis of vanadium-bearing shale
| Element | Muscovite | V-oxide | Total |
|---|---|---|---|
| V | 94.24 | 5.76 | 100 |
| Mineral | Quartz | Muscovite | Calcite | Pyrite | Potassium feldspar | Apatite |
| Content | 62.04 | 12.95 | 6.5 | 3.07 | 2.63 | 1.98 |
| Mineral | Dolomite | Akermanite | Magnetite | Barite | Anhydrite | Gedrite |
| Content | 0.72 | 0.7 | 0.33 | 0.34 | 0.22 | 0.23 |
| Mineral | V-oxide | Szomolnokite | Hematite | Rutile | Organic carbon | — |
| Content | 0.19 | 0.03 | 0.05 | 0.03 | 8 | — |
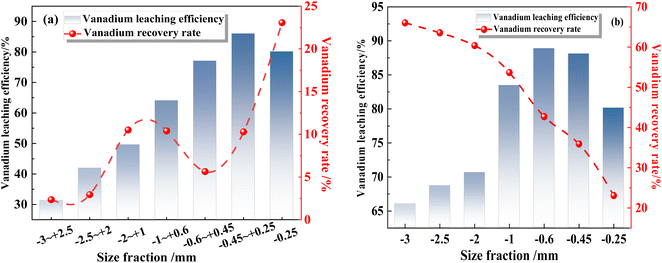
The particle size distribution of the raw vanadium-bearing shale was examined, and the distribution of vanadium across different particle sizes was investigated. Standard screens with mesh sizes of 2.5 mm, 2 mm, 1 mm, 0.6 mm, 0.45 mm, and 0.25 mm were utilized to categorize the raw vanadium-bearing shale into seven size ranges, including particle sizes of −3 to +2.5 mm, −2.5 to +2 mm, −2 to +1 mm, −1 to +0.6 mm, −0.6 to +0.45 mm, −0.45 to +0.25 mm, and −0.25 mm, respectively. The particle size and vanadium distribution of the vanadium-bearing shale are presented in Table 4, the yields of vanadium-bearing shale with different particle sizes are different. The V2O5 grade is different for the various vanadium-bearing shale particle sizes, and the vanadium grade increases with decreasing particle size.
| Particle size (mm) | Yield (%) | V2O5 grade (%) | Distribution rate of V2O5 (%) | Mohs hardness |
|---|---|---|---|---|
| −3 to +2.5 | 12.16 | 0.44 | 7.50 | 6 |
| −2.5 to +2 | 9.64 | 0.52 | 7.01 | 6 |
| −2 to +1 | 25.83 | 0.59 | 22.91 | 6 |
| −1 to +0.6 | 16.07 | 0.73 | 16.22 | 6 |
| −0.6 to +0.45 | 6.52 | 0.81 | 7.31 | 5.5 |
| −0.45 to +0.25 | 9.71 | 0.89 | 12.00 | |
| −0.25 | 20.07 | 1.03 | 28.76 | |
| Total | 100.00 | 0.72 | 100.00 |
The phase analysis of vanadium-bearing shales with different particle sizes were conducted on, as shown in Fig. 4. In Fig. 4(a), the main phases of each granulometric class of vanadium shale is composed of muscovite, quartz, apatite, calcite, pyrite and potassium feldspar. The XRD characteristic peaks of muscovite, apatite and calcite increase with the decrease of particle size in Fig. 4(b) and (d), indicating that muscovite, apatite and calcite are gradually enriched in the fine-grained grade. Specially, muscovite is consistent with the distribution of vanadium grade. On the contrary, the characteristic peaks of quartz weakens as the particle size decreases in Fig. 4(c), indicating quartz is enriched in the coarse-grained grade. According to the characteristic peaks of pyrite and potassium feldspar in Fig. 4(e) and (f), pyrite and potassium feldspar are distributed relatively evenly in each grain size. The minerals distribution of vanadium-bearing shale with different particle sizes are shown in Table 5. With the particle size of vanadium-bearing shale decreasing, the content of quartz reduce from 72.28 to 50.92 wt%, while muscovite, calcite, apatite and carbonaceous increase from 11.42 to 14.36 wt%, 4.04 to 9.07 wt%, 0.52 to 3.25 wt% and 5.05 to 11.41 wt%, respectively.
| Minerals | Particle size (mm) | ||||
|---|---|---|---|---|---|
| −3 to +2.5 | −2.5 to +2 | −2 to +1 | −1 to +0.6 | −0.6 | |
| Quartz | 72.28 | 70.88 | 67.79 | 64.89 | 50.92 |
| Muscovite | 11.42 | 11.78 | 12.27 | 12.72 | 14.36 |
| Calcite | 4.04 | 4.19 | 5.11 | 6.15 | 9.07 |
| Pyrite | 2.51 | 2.43 | 2.46 | 2.28 | 4.21 |
| Potassium feldspar | 2.35 | 2.44 | 2.43 | 2.36 | 3.04 |
| Apatite | 0.52 | 0.83 | 1.36 | 1.91 | 3.25 |
| Dolomite | 0.24 | 0.97 | 0.48 | 1.06 | 0.84 |
| Barite | 0.78 | 0.34 | 0.32 | 0.12 | 0.32 |
| Gedrite | 0.19 | 0.21 | 0.23 | 0.25 | 0.23 |
| Akermanite | 0.28 | 0.3 | 0.41 | 0.57 | 1.22 |
| Anhydrite | 0.11 | 0.21 | 0.23 | 0.23 | 0.24 |
| Vanadium oxide | 0.2 | 0.16 | 0.1 | 0.18 | 0.34 |
| Magnetite | 0.03 | 0.07 | 0.11 | 1.05 | 0.33 |
| Hematite | 0 | 0 | 0 | 0 | 0.14 |
| Szomolnokite | 0 | 0 | 0 | 0 | 0.08 |
| Carbonaceous + clay | 5.05 | 5.19 | 6.7 | 6.23 | 11.41 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
The distribution characteristics of various minerals in vanadium-bearing shale are related to disseminated grain size and hardness of the minerals. The Mohs hardness of main minerals are shown in Table 6. The order of mineral hardness from high to low is as follows: quartz, pyrite, potassium feldspar, calcite and muscovite. During the coarse crushing process of vanadium-bearing shale, due to the different hardness of minerals, the particle size changes to varying degrees. The particle size change of quartz with high hardness is relatively small, while the particle size of minerals with lower hardness, such as mica and calcite, are finer. Meanwhile, the finer the embedding particle size, the more enriched towards the fine-grained. Additionally, the larger the dissemination particle size, the more enriched towards the coarse-grained. Hence, the quartz in vanadium-bearing shale is not only hard but also has a large dissemination particle size, so it is enriched in the coarse-grained grade. While the muscovite, calcite and apatite vanadium-bearing shale are enriched in the fine-grained grade.
| Minerals | Quartz | Pyrite | Potassium feldspar | Apatite | Calcite | Muscovite |
| Mohs hardness | 7 | 6–6.5 | 6 | 5 | 3 | 2–3 |
As shown in Fig. 6, according to the correlation between vanadium leaching efficiency and mineral distribution in vanadium-bearing shale, the vanadium leaching efficiency shows a good positive correlation with the content distribution of muscovite (R2 = 0.98). In addition, the distribution of quartz, muscovite and calcite is relatively good correlation (R2 = 0.99). Among which quartz is negatively correlated, while muscovite and calcite are positively correlated.
3.2 Effect of mechanochemical activation on particle size distribution
 | ||
| Fig. 7 Effect of activation time on the yields of vanadium-bearing shale ((a): yield; (b): cumulative yield). | ||
Fig. 7(a) indicated that with the increase in activation time, the yield of coarse fraction with particle size of −3 to +0.6 mm decreased rapidly and the yield of fine fraction with −0.6 mm increased gradually. Fig. 7(b) illustrated that the negative cumulative yield of finer fraction increased rapidly with the gradual increase in activation time. The cumulative curves of particle size distribution showed that the curve was convex, and the breakage products were mainly coarse particles. In addition, the proportion of fine-grained powder is high, the particle size distribution is wide, and the coarse particle content is reduced from 12.16% to 4.74% and not wholly broken. There were two possibilities for this phenomenon, one is that the crushing rate of fine particle size is larger than that of coarse ones, and the other is that the crushing process of vanadium-bearing shale belongs to surface crushing.
As seen in Fig. 8(a)–(d), there is a swift decline in the content of coarse particle sizes (−3 to +2.5 mm, −2.5 to +2 mm, −2 to +1 mm, and −1 to +0.6 mm) with activation time, while the proportion of −0.6 mm particles increases during activation. The yields of vanadium-bearing shale vary notably across different particle sizes, with smaller particles demonstrating superior activation efficiency. In addition, intermediate particle-grade products are generated during the activation process, as shown in Fig. 8(a)–(c). With increasing activation time, these intermediate particles are gradually crushed into fine particles, which indicates that the whole particle is destroyed by volume destruction; thus, producing intermediate particles. Moreover, the variation in the yield of intermediate-sized particles has no obvious trend of increasing or decreasing with increasing activation time, which shows that the activation process is relatively uniform for each particle size of vanadium-bearing shale.
As depicted in Fig. 8(e)–(h), the negative cumulative yield of vanadium-bearing shale with various particle sizes increased with activation time, though the curve variations exhibited slight differences. The negative cumulative yield curve of vanadium-bearing shale with a particle size of −3 to +2.5 mm displayed an S-shaped pattern, indicating that during activation, particles were mainly distributed in the fine size range of −0.6 mm and the coarse size range of −3 to +2.5 mm, with the intermediate size range showing minimal but uniform distribution (Fig. 8(e)). With prolonged activation time, the curves of the other three particle sizes gradually transitioned from convex to concave, suggesting a gradual enrichment of coarse particles into finer ones (Fig. 8(f)–(h)).
γ = γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ktn) exp(−ktn)
| (1) |
The linear equation can be obtained by mathematical transformation of eqn (1):
 | (2) |
The functional relationship between kinetic parameters n, k and particle size d can be expressed as follows:
| k(d) = A0 + A1dx1 | (3) |
| n(d) = C0 + C1dx2 | (4) |
Activation kinetics experiments of vanadium-bearing shale were conducted to characterize the effect of different particle sizes on ore breakage. Based on the kinetic equations, the kinetic parameters n and k were obtained by fitting. Fig. 9(a) shows the grinding kinetics results for particle sizes of −3 to +0.6 mm, and Fig. 9(b)–(e) show the grinding kinetics for feed sizes of −3 to +2.5 mm, −2.5 to +2 mm, −2 to +1 mm and −1 to +0.6 mm, respectively. The grinding kinetic parameters n and k are shown in Table 3. Fig. 10(a)–(d) showed the curves of the relationships between the activation kinetics parameters n and k and the particle size d.
 | ||
| Fig. 9 Fitting curve of the grinding kinetics of vanadium-bearing shale (feed particle size: (a): −3 to +0.6 mm; (b): −3 to +2.5 mm; (c): −2.5 to +2 mm; (d): −2 to +1 mm; (e): −1 to +0.6 mm). | ||
Fig. 10 illustrates the quantitative description of vanadium-bearing shale through activation kinetic equations, achieving a fitting accuracy of 0.99. The parameters n and k are determined by the material's characteristics and activation conditions. The n value primarily reflects the material's homogeneity and strength, indicating its grindability. A higher n suggests that the ore can be activated easily,31 and a lower n implies greater difficulty in activation. The activation fineness determines the k value. When activation time (t) is less than e1/k, k predominantly influences the cumulative yield on sieve (γ). A higher k corresponds to a smaller γ, resulting in a greater reduction of γ and faster activation efficiency.29
As depicted in Table 7, vanadium-bearing shale exhibits improved grindability with finer particle sizes, making it easier to grind. During the activation process, the material often forms a multiparticle layer between the activation media. The force exerted by the media on the material can be transmitted through these particles, potentially without direct contact, leading to breakage. During mixed crushing, the particle size of the fragile material is smaller than that during single crushing, and the particle size during difficult crushing is larger than that during single crushing. With graded activation, the crushing yield of coarse particles is greater than that with mixed activation. Thus, graded activation is exceedingly necessary to improve the activation efficiency and prevent the overactivation of fine particles.
| Kinetic equation | n order kinetic model | |||
|---|---|---|---|---|
| Feed size (mm) | Particle size (mm) | n | k | R2 |
| −3 | −3 to +2.5 | 0.52 | 0.23 | 0.994 |
| −2.5 to +2 | 0.76 | 0.21 | 0.998 | |
| −2 to +1 | 0.67 | 0.25 | 0.998 | |
| −1 to +0.6 | 0.48 | 0.30 | 0.997 | |
| −3 to +2.5 | −3 to +2.5 | 0.50 | 0.40 | 0.987 |
| −2.5 to +2 | 0.57 | 0.23 | 0.988 | |
| −2 to +1 | 0.75 | 0.11 | 0.995 | |
| −1 to +0.6 | 0.90 | 0.07 | 0.994 | |
| −2.5 to +2 | −2.5 to +2 | 0.50 | 0.59 | 0.995 |
| −2 to +1 | 0.82 | 0.16 | 0.998 | |
| −1 to +0.6 | 0.95 | 0.09 | 0.999 | |
| −2 to +1 | −2 to +1 | 0.77 | 0.36 | 0.997 |
| −1 to +0.6 | 1.04 | 0.14 | 0.995 | |
| −1 to +0.6 | −1 to +0.6 | 1.06 | 0.44 | 0.994 |
The activation kinetic parameters n and k for different particle sizes were obtained by fitting the data in Table 3. Thus, the optimal activation kinetic equation of vanadium-bearing shale of different sizes is obtained, as shown in eqn (5)–(9).
−3 mm: γ = γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(0.336d2 − 1.07d + 0.05)t(0.056d2−0.211d+0.406)] exp[(0.336d2 − 1.07d + 0.05)t(0.056d2−0.211d+0.406)]
| (5) |
−3 to +2.5 mm: γ = γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(−0.029d2 + 0.12d − 0.12)t(0.068d2−0.412d+1.11)] exp[(−0.029d2 + 0.12d − 0.12)t(0.068d2−0.412d+1.11)]
| (6) |
−2.5 to +2 mm: γ = γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(−0.181d2 + 0.115d − 0.094)t(0.018d2−0.367d+1.16)] exp[(−0.181d2 + 0.115d − 0.094)t(0.018d2−0.367d+1.16)]
| (7) |
−2 to +1 mm: γ = γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(−0.19 + 0.55d)t(1.44−0.68d)] exp[(−0.19 + 0.55d)t(1.44−0.68d)]
| (8) |
−1 to +0.6 mm: γ = γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(0.44t1.06) exp(0.44t1.06)
| (9) |
3.3 The relationship between activation yield and vanadium leaching efficiency
| Particle size/mm | Linear equation | R2 |
|---|---|---|
| −3 to +2.5 | y = 28.285 + 0.588x | 0.981 |
| −2.5 to +2 | y = 44.229 + 0.745x | 0.987 |
| −2 to +1 | y = 48.361 + 0.462x | 0.982 |
| −1 to +0.6 | y = 65.639 + 0.279x | 0.997 |
According to the fitting equation and correlation coefficient of the relationship between the graded activation yield of vanadium-bearing shale and the vanadium leaching efficiency with the fitting accuracy reaching 0.99 in Table 8. Hence, the relationship between the activation yield and the vanadium leaching efficiency can be expressed by linear eqn (10).
| η = μγ + ν | (10) |
The eqn (1) was substituted into eqn (10) to obtain the relationship model between vanadium leaching efficiency and activation time, as shown in eqn (11).
η = γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ktn)μ + ν exp(−ktn)μ + ν
| (11) |
According to the activation kinetics equation of vanadium-bearing shale, the equations among the specific particle size, activation time and vanadium leaching rate of different particle sizes can be obtained.
(1) −3 to +2.5 mm:
η = 0.588 × γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(−0.02 exp[(−0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(d/0.79) + 0.05)t(0.80 exp(d/0.79) + 0.05)t(0.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−d/1.31)+0.39)] + 28.285, 2 ≤ t ≤ 10 exp(−d/1.31)+0.39)] + 28.285, 2 ≤ t ≤ 10
| (12) |
(2) −2.5 to +2 mm:
η = 0.745 × γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(0.05 exp[(0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(d/0.78) − 0.01)t(1.14−0.32d)] + 44.229, 0 ≤ t ≤ 10 exp(d/0.78) − 0.01)t(1.14−0.32d)] + 44.229, 0 ≤ t ≤ 10
| (13) |
(3) −2 to +1 mm:
η = 0.462 × γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(−0.19 + 0.05d)t(1.14−0.68d)] + 48.361, 0 ≤ t ≤ 10 exp[(−0.19 + 0.05d)t(1.14−0.68d)] + 48.361, 0 ≤ t ≤ 10
| (14) |
(4) −1 to +0.6 mm:
η = 0.279 × γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(0.44t1.06) + 65.639, 0 ≤ t ≤ 10 exp(0.44t1.06) + 65.639, 0 ≤ t ≤ 10
| (15) |
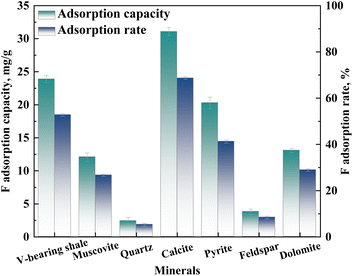
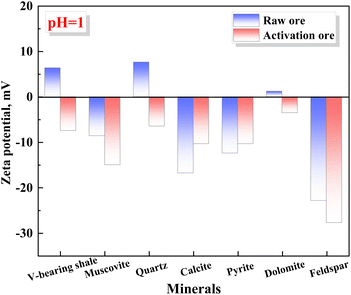
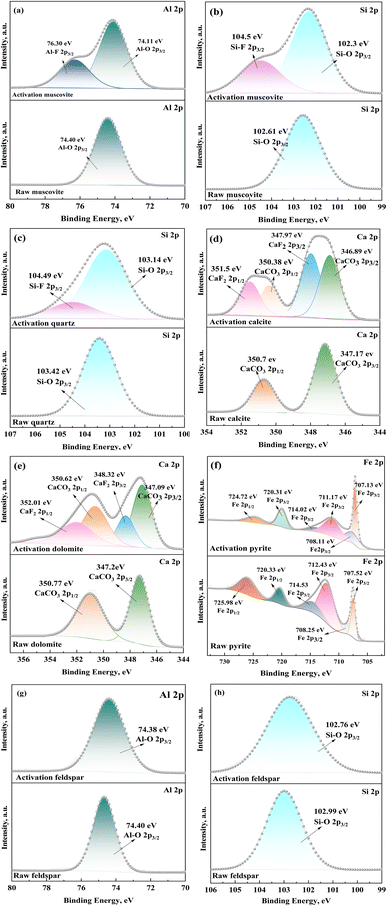
3.4 The adsorption mechanism of vanadium-bearing shale in mechanochemical process
During the mechanochemical activation process, the vanadium-bearing shale will adsorb the activator simultaneously as the particle size decreases. Under the action of mechanical force, the mineral surface of vanadium-bearing shale undergoes amorphous phenomena. The atoms with unsaturated surface charges specifically adsorb F− in the activator, reducing the surface potential of vanadium-bearing shale and increasing its hydrophilicity, promoting the adsorption and diffusion of H+ on the mineral surface. Meanwhile, F− is adsorbed at the central atomic positions (Si, Al) on the surface of muscovite, weakening the adjacent Si–O and Al–O bonds on the surface. During the leaching process, the weakened Si–O and Al–O bonds are prone to break during the dehydroxylation process of H+, thereby accelerating the dissolution of muscovite during the leaching process and ultimately increasing the vanadium leaching efficiency. In order to reveal the adsorption mechanism of F− by vanadium-bearing shale during the mechanochemical activation process, the main minerals in vanadium-bearing shale undergo mechanochemical activation using pure minerals.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, indicating that the adsorption of F by calcite may result in the formation of CaF2 adsorbed on the surface of calcite. In Fig. 15(d), the content of F− at the adsorption points G and H on the surface of pyrite are very high, reaching more than 10%, while the content of Na+ is zero, indicating that pyrite has a good adsorption on F−. In Fig. 15(e), the adsorption of F− varies greatly at I and J points of potassium feldspar. The F− content at I point reaches 11%, while that at J point is only about 2.6%. In Fig. 15(f), the adsorption correlation of fluorine on the dolomite surface is better, but the adsorption amount is different at different locations.
2, indicating that the adsorption of F by calcite may result in the formation of CaF2 adsorbed on the surface of calcite. In Fig. 15(d), the content of F− at the adsorption points G and H on the surface of pyrite are very high, reaching more than 10%, while the content of Na+ is zero, indicating that pyrite has a good adsorption on F−. In Fig. 15(e), the adsorption of F− varies greatly at I and J points of potassium feldspar. The F− content at I point reaches 11%, while that at J point is only about 2.6%. In Fig. 15(f), the adsorption correlation of fluorine on the dolomite surface is better, but the adsorption amount is different at different locations.
4. Conclusion
This study systematically discusses process mineralogy, the optimal leaching particle size, and the kinetics of activation processes of vanadium-bearing shale. Based on the mineral characteristics of vanadium-bearing shale, a classification mechanochemical activation method was proposed. The adsorption mechanism of vanadium-bearing shale on F− was revealed through pure minerals during the mechanochemical activation process.(1) Vanadium-bearing shale is a complex low-grade ore, which is mainly composed of quartz, muscovite, calcite, pyrite, feldspar and apatite. Vanadium with 94.24% exists in muscovite, while the remaining 5.76% exists in oxide. Muscovite is predominantly closely associated with quartz, calcite and organic carbonaceous and tends to be enriched in fine grained, displaying fine disseminated granularity with 0.005–0.06 mm.
(2) The mineral distribution of vanadium-bearing shale of different particle sizes leads to differences in activation efficiency. The grindability order of vanadium-bearing shale is observed as follows: −3 to +2.5 mm < −2.5 to +2 mm < −2 to +1 mm < −1 to +0.6 mm. The activation process of different particle sizes were well evaluated by kinetic equations (R2 = 0.99). The vanadium leaching efficiency has a positive linear relationship with the activation yield at the optimal leaching particle size with −0.6 mm. The vanadium leaching efficiency and activation time can be expressed by equation of η = γ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ktn)μ + ν.
exp(−ktn)μ + ν.
(3) During the mechanochemical activation process, the mineral surface of vanadium-bearing shale undergoes amorphous phenomena, then has a good adsorption of F− (23.89 mg g−1). The order of F− adsorption capacity is calcite, pyrite, dolomite, muscovite, feldspar, and quartz. The adsorption process of F− alters the surface potential on the vanadium-bearing shale, especially, the negative charge on the of muscovite surface increases, while that of pyrite and calcite decreases, which is conducive to the diffusion of H+ to the surface of muscovite and away from pyrite and calcite. F− forms Al–F and Si–F bonds with Si and Al on the surface of muscovite, promoting the dissolution of muscovite. While F− generates CaF2 with the surface of calcite and FeF3 with Fe(III)–S on the surface of pyrite, hindering and slowing down the dissolution of calcite and pyrite.
(4) The mechanochemical activation can realize the vanadium extraction by full-wet leaching with green, low-carbon and high efficiency. However, the impact of mechanochemical activation on the subsequent purification and enrichment of vanadium are further studied, especially the improvement of vanadium leaching efficiency and the accompanying leaching of impurity elements such as iron and aluminum in muscovite.
Data availability
All data included in this study are available upon request by contact with the corresponding author.Author contributions
Xuxia Zhao: conceptualization, investigation, methodology, writing – original draft, writing – review & editing. Yimin Zhang: writing – review & editing, supervision. Nannan Xue: writing – review & editing, supervision. Pengcheng Hu: writing – review & editing, supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was financially supported by National Key Research and Development Program of China (2023YFC3903900), Science and Technology Innovation Talent Program of Hubei Province (2022EJD002), National Natural Science Foundation of China (52004187 and 52174260).References
- W. Bo, Y. Zhang and H. Liu, et al., Optimization of precursor structure by dispersants to promote nitrogen reduction process to prepare high-quality VN, J. Alloys Compd., 2024, 1006, 176285 CrossRef CAS.
- S. Dai, X. Zheng and X. Wang, et al., Stone coal in China: a review, Int. Geol. Rev., 2018, 60(5–6), 736–753 CrossRef.
- Y. Zhang, S. Bao and T. Liu, et al., The technology of extracting vanadium from stone coal in China: History, current status and future prospects, Hydrometallurgy, 2011, 109(1–2), 116–124 CrossRef CAS.
- Z. Tang, Z. Zhou and J. Jin, et al., Vanadium extraction from stone coal using a novel two-stage roasting technology, Fuel, 2022, 321, 124031 CrossRef CAS.
- B. Chen, S. Bao and Y. Zhang, et al., A high-efficiency and sustainable leaching process of vanadium from shale in sulfuric acid systems enhanced by ultrasound, Sep. Purif. Technol., 2020, 240, 116624 CrossRef CAS.
- R. K. Asamoah, W. Skinner and J. Addai-Mensah, Leaching behaviour of mechano-chemically activated bio-oxidised refractory flotation gold concentrates, Powder Technol., 2018, 331, 258–269 CrossRef CAS.
- P. Baláž, Mechanical activation in hydrometallurgy, Int. J. Miner. Process., 2003, 72(1–4), 341–354 CrossRef.
- M. Khezri, B. Rezai and A. A. Abdollahzadeh, et al., Investigation into the effect of mechanical activation on the leaching of chalcopyrite in a glycine medium, Hydrometallurgy, 2021, 203, 105492 CrossRef CAS.
- H. Pálková, M. Barlog and J. Madejová, et al., Structural changes in smectites subjected to mechanochemical activation: The effect of the occupancy of the octahedral sites, Appl. Clay Sci., 2021, 213, 106214 CrossRef.
- K. Liu, Q. Tan and L. Liu, et al., Acid-Free and Selective Extraction of Lithium from Spent Lithium Iron Phosphate Batteries via a Mechanochemically Induced Isomorphic Substitution, Environ. Sci. Technol., 2019, 53(16), 9781–9788 CrossRef CAS PubMed.
- X. Geng, Y. Duan and S. Zhao, et al., Mechanism study of mechanochemical bromination on fly ash mercury removal adsorbent, Chemosphere, 2021, 274, 129637 CrossRef CAS PubMed.
- H. Niu, P. Kinnunen and H. Sreenivasan, et al., Structural collapse in phlogopite mica-rich mine tailings induced by mechanochemical treatment and implications to alkali activation potential, Miner. Eng., 2020, 151, 106331 CrossRef CAS.
- G. Yao, T. Cui and Z. Jia, et al., Effect of anhydrite on hydration properties of mechanically activated muscovite in the presence of calcium oxide, Appl. Clay Sci., 2020, 196, 105742 CrossRef CAS.
- J. Li and M. Hitch, Mechanical activation of magnesium silicates for mineral carbonation, a review, Miner. Eng., 2018, 128, 69–83 CrossRef CAS.
- A. G. Patil and S. Anandhan, Influence of planetary ball milling parameters on the mechano-chemical activation of fly ash, Powder Technol., 2015, 281, 151–158 CrossRef CAS.
- Y. Wang, X. He and Y. Su, et al., Efficiency of wet-grinding on the mechano-chemical activation of granulated blast furnace slag (GBFS), Constr. Build. Mater., 2019, 199, 185–193 CrossRef CAS.
- T. C. Alex, R. Kumar and S. K. Roy, et al., Mechanically induced reactivity of gibbsite: Part 1. Planetary milling, Powder Technol., 2014, 264, 105–113 CrossRef CAS.
- W. Zhou, Y. Han and Y. Sun, et al., Multi-scale impact crushing characteristics of polymetallic sulphide ores, Trans. Nonferrous Met. Soc. China, 2019, 29(9), 1929–1938 CrossRef CAS.
- Y. P. Singh, H. Tanvar and G. Kumar, et al., Investigation of planetary ball milling of sericite for potash recovery, Powder Technol., 2019, 351, 115–121 CrossRef CAS.
- J. Yu, Y. Qin and P. Gao, et al., An innovative approach for determining the grinding media system of ball mill based on grinding kinetics and linear superposition principle, Powder Technol., 2021, 378, 172–181 CrossRef CAS.
- L. Wang, H. Wang and J. Zhu, et al., Experimental study on particle size distribution of impact crushed coal containing gas, Fuel, 2022, 325, 124745 CrossRef CAS.
- N. A. Miele, A. Borriello and M. Fidaleo, et al., Modeling grinding kinetics of fat based anhydrous pastes, J. Food Eng., 2020, 268, 109732 CrossRef.
- R. Zhao, Y. Han and M. He, et al., Grinding kinetics of quartz and chlorite in wet ball milling, Powder Technol., 2017, 305, 418–425 CrossRef CAS.
- P. Li, Z. Cao and R. Zhao, et al., The kinetics and efficiency of batch ball grinding with cemented tungsten carbide balls, Adv. Powder Technol., 2020, 31(6), 2412–2420 CrossRef CAS.
- V. K. Gupta, Effect of particulate environment on the grinding kinetics of mixtures of minerals in ball mills, Powder Technol., 2020, 375, 549–558 CrossRef CAS.
- X. Zhao, Y. Zhang and N. Xue, et al., Effect of mechano-chemical activation with NaF on improved acid leaching of vanadium-bearing shale, Hydrometallurgy, 2023, 221, 106126 CrossRef CAS.
- Z. H. Wang, F. J. Yu and S. Cai, et al., Effect of Grinding Aids on the Fracture Energy of Mica, Adv. Mater. Res., 2011, 402, 503–509 Search PubMed.
- J. Duroudier, Appendix 1-Mohs Scale, Divided Solids Transport, 2016, pp. 153–155 Search PubMed.
- H. Wang, C. Ju and M. Zhou, et al., Grinding kinetics of lead–zinc tailing powders and its optimal particle size as a pozzolanic admixture in cement mortar, Adv. Powder Technol., 2022, 33(9), 103730 CrossRef CAS.
- Y. Qin, Y. Han and P. Gao, et al., Characterization of chalcopyrite ore under high voltage pulse discharge: Particle size distribution, fractal dimension, specific energy consumption, grinding kinetics, Miner. Eng., 2022, 184, 107631 CrossRef CAS.
- H. Choi, J. Lee and H. Hong, et al., New evaluation method for the kinetic analysis of the grinding rate constant via the uniformity of particle size distribution during a grinding process, Powder Technol., 2013, 247, 44–46 CrossRef CAS.
- Q. Xu, Y. Sun and L. Yang, et al., Leaching mechanism of ion-adsorption rare earth by mono valence cation electrolytes and the corresponding environmental impact, J. Cleaner Prod., 2019, 211, 566–573 CrossRef CAS.
- W. de Poel, S. L. Vaessen and J. Drnec, et al., Metal ion-exchange on the muscovite mica surface, Surf. Sci., 2017, 665, 56–61 CrossRef CAS.
- P. Frank, G. Hlawacek and O. Lengyel, et al., Influence of surface temperature and surface modifications on the initial layer growth of para-hexaphenyl on mica (001), Surf. Sci., 2007, 601(10), 2152–2160 CrossRef CAS.
- D. Bhowmik and P. Karmakar, Tailoring and investigation of surface chemical nature of virgin and ion beam modified muscovite mica, Surf. Interface Anal., 2019, 51(6), 667–673 CrossRef CAS.
- C. Elmi, M. F. Brigatti and S. Guggenheim, et al., Sodian muscovite-2M1: Crystal chemistry and surface features, Can. Mineral., 2013, 51(1), 5–14 CrossRef CAS.
- G. Yao, T. Cui and Y. Su, et al., Hydration properties of mechanically activated muscovite in the presence of calcium oxide, Clays Clay Miner., 2020, 68(6), 580–587 CrossRef CAS.
- C. Elmi, S. Guggenheim and R. Gieré, Surface crystal chemistry of phyllosilicates using X-ray photoelectron spectroscopy; a review, Clays Clay Miner., 2016, 64(5), 537–551 CrossRef CAS.
- S. A. Saslow Gomez and F. M. Geiger, Precipitates of Al(III), Sc(III), and La(III) at the Muscovite–Water Interface, J. Phys. Chem. A, 2014, 118(46), 10974–10981 CrossRef CAS PubMed.
- NIST XPS Database, http://srdata.nist.gov/xps/selEnergyType.aspx, 2025.
- Z. Ding, J. Li and J. Yuan, et al., Insights into the influence of calcium ions on the adsorption behavior of sodium oleate and its response to flotation of quartz: FT-IR, XPS and AMF studies, Miner. Eng., 2023, 204, 108437 CrossRef CAS.
- X. Yang, Y. Li and J. Chen, et al., Enhanced flotation separation of fluorite and F−-activated calcite by sodium gluconate and sodium silicate, Miner. Eng., 2025, 229, 109375 CrossRef CAS.
- P. Hu, Y. Zhang and T. Liu, et al., Source separation of vanadium over iron from roasted vanadium-bearing shale during acid leaching via ferric fluoride surface coating, J. Cleaner Prod., 2018, 181, 399–407 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |