Can Cr(III) substitute for Al(III) in the structure of boehmite?†
Sayandev Chatterjee *a,
Michele A. Conroya,
Frances N. Smitha,
Hee-Joon Junga,
Zheming Wangb,
Reid A. Petersona,
Ashfia Huqc,
David G. Burtta,
Eugene S. Ilton*b and
Edgar C. Buck
*a,
Michele A. Conroya,
Frances N. Smitha,
Hee-Joon Junga,
Zheming Wangb,
Reid A. Petersona,
Ashfia Huqc,
David G. Burtta,
Eugene S. Ilton*b and
Edgar C. Buck *a
*a
aEnergy and Environment Directorate, Pacific Northwest National Laboratory, Richland, WA 99354, USA. E-mail: Sayandev.Chatterjee@pnnl.gov; Edgar.Buck@pnnl.gov
bPhysical and Computational Sciences Directorate, Pacific Northwest National Laboratory, Richland, WA 99354, USA. E-mail: Eugene.Ilton@pnnl.gov
cChemical and Engineering Materials Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831, USA
First published on 4th November 2016
Abstract
The dissolution of boehmite is a technical issue for Al industry because of its recalcitrant nature. In fact, a similar problem exists with boehmite in nuclear waste sludge at the Hanford site in Eastern Washington State, USA. Dissolution of Al phases is required to reduce the waste loadings in the final borosilicate glass waste form. Although not the most common Al-bearing species in the sludge, boehmite may become a rate limiting step in the processing of the wastes. Hanford boehmite is an order of magnitude more resistant to dissolution in hot caustic solutions than expected from surface-normalized rates. We are exploring potential intrinsic and extrinsic effects that may limit boehmite reactivity; one clue comes from microstructural analyses that indicate an association of Cr with Al in the Hanford nuclear waste. Hence, in this first paper, we investigated the potential role of chromium on the reactivity of boehmite in caustic solution. An important finding was that irrespective of the synthesis pathway, amount of Cr(III), or the resultant morphology, there was no evidence for Cr incorporation in the bulk structure, in agreement with QM calculations. In fact, electron microscopic (EM) and spectroscopic analyses showed that Cr was enriched at the (101) edges of the boehmite. However, Cr had no measurable effect on the morphology during the synthesis step. In contrast, comparison of the morphologies of the synthetic Cr-doped and pure boehmite samples after exposure to caustic solutions provided evidence that Cr inhibited the corrosion. TEM showed that Cr was not homogeneously distributed at the surface. Consequently, Cr may have partially passivated the surface by blocking discrete energetic sites on the lateral surfaces of boehmite.
Introduction
Boehmite (orthorhombic γ-AlOOH) is among the most abundant natural ores of aluminum, and occurs as a secondary mineral in weathered surface zones in limestones and clays as well as “silica-poor” igneous rocks. It can be bulk-produced with a wide range of desired functionalities and morphologies,1–3 and has found a broad range of applications in industrial processes.4–6 Boehmite aggregates can have a large surface area with both amphoteric functional groups on lateral faces and a stable protonated basal (010) surface that can be differentially expressed. Consequently, boehmite is a versatile adsorbent over a broad range of pH for both anions and cations.7,8 The Bayer extraction process of alumina from ores utilises caustic leaching at elevated temperatures and pressures. The most common Al-bearing minerals found in bauxite are gibbsite (Al(OH)3) and boehmite. Gibbsite dissolves relatively easily but boehmite is extremely recalcitrant in caustic solutions. This resistance to dissolution can be exacerbated by the presence of other phases, such as anatase (TiO2). Ireland et al., using electron microscopy, demonstrated that the reaction with NaOH resulted in the formation of a mixed Al–Ti oxide layer that inhibited further reaction.3 It is likely that other contaminants might also inhibit boehmite dissolution in a similar manner.Boehmite is also a common material found in the radioactive waste storage tanks at the Department of Energy (DOE) Hanford site in Eastern Washington State, U.S.A.9 Hanford was one of the major U.S. sites of Pu weapons production from 1943 until 1989. As part of the process of extracting Pu from irradiated U metal fuel, large quantities of liquid and solid wastes were produced. These wastes were stored in 177 steel tanks ranging in size from 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 gallons to one million gallons – all awaiting final processing into vitrified borosilicate glass logs which will finally be stored in a geologic waste repository.9
000 gallons to one million gallons – all awaiting final processing into vitrified borosilicate glass logs which will finally be stored in a geologic waste repository.9
The wastes are a highly complex mixture of phases; including Al-oxides, iron oxides, chromium phases, sodium uranium oxides, and other phases and species in a high pH solution.10 The sludge also has a highly variable particle size range. Much of the sludge solids have formed heterogeneous agglomerates.
The Al in these wastes originated from one of three sources: dissolved Al-nuclear fuel cladding, Al(NO3)3 that was added to waste to increase the ionic strength during processing, and Al that was added as a corrosion control agent for the steel tanks.11,12 The solubility limiting phase in the Hanford tanks is believed to be gibbsite. However, temperatures in the tanks briefly became elevated (boiling) due to radioactive decay from 90Sr and 137Cs; this resulted in conversion of some of the gibbsite to the more recalcitrant phase, boehmite. The radioactively hot elements were subsequently removed and since this time, the sludge temperature has not been greater than 45 °C.13 Although necessary for glass formation, too much Al results in a lower quality waste form, so efforts have been concentrated on removing a large fraction of the Al waste in the sludge. Studies have shown that boehmite in Hanford tank waste dissolves by a factor of ∼40× slower than predicted for surface area normalized rates. An outstanding question is whether this is due to extrinsic factors such as agglomeration reducing reactive surface area or intrinsic factors such as incorporation or adsorption of stabilizing elements.
In this study, we examined the potential role of chromium on limiting boehmite reactivity under caustic conditions. Cr(III) is a major species in the tank wastes.14 Indeed, Cr has been observed to be associated with Al in REDOX type wastes; however, there is no direct evidence for incorporation of Al and Cr in the same phase.15,16 Other studies have implied that up to 20% Cr(III) could be incorporated into boehmite nanofibers under hydrothermal conditions.17 Further, increasing Cr concentrations systematically raised the dehydroxylation temperature of boehmite suggesting that Cr might increase boehmite stability, which could account for boehmite's recalcitrant behavior during caustic leaching of Hanford tank waste. In this contribution, we take the first steps towards understanding the interaction of Cr(III) and boehmite under conditions that are relevant to the Hanford tanks. First order questions are: (1) Is Cr(III) incorporated into the bulk structure of boehmite or adsorbed to the surface? (2) Does the morphology of boehmite or synthesis conditions affect the mode of association of Cr(III) with boehmite? (3) Is Cr(III) a determinant of boehmite morphology during synthesis? and (4) Does Cr(III) affect the morphology of boehmite under caustic leaching conditions relevant to tank waste processing conditions? We investigated two different reaction pathways and starting reactants. One pathway is the single step alkaline dehydration of gibbsite to rhombic boehmite plates that are similar to, albeit larger than, boehmite in the Hanford tanks,15,18 (referred to here as synthesis 1). The other pathway is a two-step process that begins with the alkaline hydrolysis of Al(NO3)3 to Al(OH)3, followed by the dehydration step, and yields elongated two-dimensional platelets (i.e., laths; referred to here as synthesis 2). Reaction products were characterized by electron microscopy and various spectroscopic methods. Experimental results were given context by 0 K ab initio calculations of the incorporation energies of Cr(III) substitution for Al(III) in the boehmite structure as a function of Cr–Cr separation and contrasting the bulk to near-surface environments.
Results and discussions
We synthesised two differently shaped boehmite particles; rhombohedral plates and nano-laths, to study the structural effect on Cr incorporation rate. Throughout this article the plates and laths will be referred to as synthesis 1 and 2 respectively, with Cr doping annotated as Cra and Crb for 1 and 10 atomic wt%. As discussed in the experimental section we characterised these particles using electron microscopy (EM), X-ray structural analysis (XRD), neutron diffraction (ND) and various spectroscopic techniques. The experimental data were then compared to theoretical calculations.Diffraction
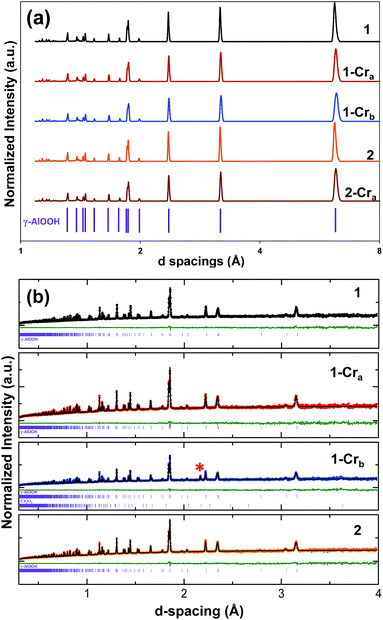
X-ray diffractograms of the undoped and Cr(III) doped boehmite samples are shown in Fig. 1a. The diffractogram of 1 and 2 indexed as pure boehmite with an orthorhombic unit cell (a = 3.700 Å, b = 12.227 Å, c = 2.868 Å, space group symmetry Cmcm, JCPDS PDF no. 21-1307).19 The sharp, intense peaks indicate high crystallinity. No distinct Cr containing phases were detected for the Cr containing samples. Further, XRD did not detect any effect from changing the synthetic conditions and consequent morphologies (compare XRD of 2 and 2-Cra to 1 and 1-Cra).The neutron diffraction (ND) results (Fig. 1b) are consistent with the X-ray diffraction measurements.20 The diffractogram of 1-Cra looks nearly identical to the undoped sample with no separate or distinct Cr phase detected. Further, a Rietveld analysis assuming complete incorporation (i.e., 1%) of Cr in the octahedral Al(III) sites does not match the experimental pattern, strongly suggesting that a portion of Cr must be external to the boehmite structure. In fact, at higher Cr concentration (1-Crb), while boehmite is still the dominant phase, an additional peak appears that can be indexed to Cr2O3. Similar to XRD, no changes are detected as a function of the synthesis pathway (compare patterns for 1 and 2).
In summary, neither XRD nor ND provide compelling evidence for incorporation of Cr(III) into the boehmite structure. In fact, Rietveld refinement of the ND data suggests that Cr concentrations as low as about 0.1% in the boehmite structure should be detectable. This suggests that multiple Cr(III) environments are possible when the Cr concentration exceeds 0.1%, as is the case here. The following spectroscopic analyses largely confirm this finding.
Spectroscopy
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxide ratios for the non-doped boehmite (sample 1) are 2.01 and 1.00 which are consistent with expected stoichiometric ratios. The Al2p and O1s (oxide and hydroxyl) binding energies are higher and lower, respectively, than reported previously by Kloprogge et al.23 However, there is no information given on the boehmite morphology or synthetic route in Kloprogge et al.; given that boehmite crystallographic faces can be differentially expressed by changing the synthesis conditions, it is hard to make comparisons between the two studies. Regardless, our interest is in differences between the Cr and non-Cr syntheses, where the morphologies and pH were identical. In this regard, the binding energy for Al2p drops slightly (−0.37 eV) in 1-Cra relative to the “undoped” sample 1. Conversely, the O1s binding energy increases by +0.87 eV for the oxide peak and +0.89 eV for hydroxyl. A peak at 533.46 eV indicates a minor physi-sorbed H2O component. Therefore, relative to the marginal change in Al binding energy, the change for O is large and in the opposite direction suggesting the increase in O binding energy is real. Whether this variation in O1s binding energy and the increase in the OH/O2− ratio is related to Cr or the sharp increase in the carbon overlayer thickness cannot be determined presently. Whereas Cr(III) is marginally more electronegative than Al(III), which is consistent with a higher O1s binding energy, the increased carbon overlayer thickness decreases the information depth such that more of the signal originates from the very near-surface.
oxide ratios for the non-doped boehmite (sample 1) are 2.01 and 1.00 which are consistent with expected stoichiometric ratios. The Al2p and O1s (oxide and hydroxyl) binding energies are higher and lower, respectively, than reported previously by Kloprogge et al.23 However, there is no information given on the boehmite morphology or synthetic route in Kloprogge et al.; given that boehmite crystallographic faces can be differentially expressed by changing the synthesis conditions, it is hard to make comparisons between the two studies. Regardless, our interest is in differences between the Cr and non-Cr syntheses, where the morphologies and pH were identical. In this regard, the binding energy for Al2p drops slightly (−0.37 eV) in 1-Cra relative to the “undoped” sample 1. Conversely, the O1s binding energy increases by +0.87 eV for the oxide peak and +0.89 eV for hydroxyl. A peak at 533.46 eV indicates a minor physi-sorbed H2O component. Therefore, relative to the marginal change in Al binding energy, the change for O is large and in the opposite direction suggesting the increase in O binding energy is real. Whether this variation in O1s binding energy and the increase in the OH/O2− ratio is related to Cr or the sharp increase in the carbon overlayer thickness cannot be determined presently. Whereas Cr(III) is marginally more electronegative than Al(III), which is consistent with a higher O1s binding energy, the increased carbon overlayer thickness decreases the information depth such that more of the signal originates from the very near-surface.
| Sample | Elemental form | B.E. (eV) | FWHM (eV) | Ratios | ||
|---|---|---|---|---|---|---|
| O/Al | Al/C | Cr/Al | ||||
| a C–O calculated from C1s line.b Chemisorbed water.c Physisorbed water.d There was a significant contribution to the O from C–O species, which was subtracted out. | ||||||
| 1 | Al2p | 74.58 | 1.65 | 2.01 | 3.2 | NA |
| O1s (100%) | 529.85 | 2.74 | ||||
| O2− (47.9%) | 529.43 | |||||
| OH− (52.1%) | 530.71 | |||||
| C–Oa (1.5%) | n.d. | |||||
| 1-Cra | Al2p | 74.21 | 1.43 | 2.07 | 1.9 | 0.020 |
| O1s (100%) | 2.71 | |||||
| O2− (44.8%) | 530.30 | |||||
| OH (53.5%) | 531.60 | |||||
| H2Ob (1.7%) | 533.46 | |||||
| C–Oa (3.4%) | n.d. | |||||
| Cr2p3/2 | 576.34 | 2.65 | ||||
| 1-Crb | Al2p | 74.29 | 1.46 | 1.91(1.73)d | 1.0 | 0.022 |
| O1s | n.a. | 2.73 | ||||
| O2− (36.5%) | 530.43 | |||||
| OH (48.2%) | 531.65 | |||||
| H2Ob (3.8%) | 533.82 | |||||
| H2Oc (3.8%) | 535.20 | |||||
| C–Oa (9.6%) | 532.49 | |||||
| Cr2p3/2 | 576.46 | 2.87 | ||||
The binding energy for Cr2p3/2 is about −0.7 eV lower than expected for Cr(OH)3 but similar to that for Cr2O3.24 The peak shape, however, lacks the prominent multiplet structure characteristic of Cr2O3. Nonetheless, the Cr/Al ratio is twice the expected stoichiometric ratio, which indicates that Cr is not homogeneously incorporated in the bulk boehmite, but rather concentrated towards the near-surface.
The XPS of 1-Crb is similar to 1-Cra with the following exceptions: there is a greater contribution from both chemi- and physi-sorbed H2O, and the O/Al ratio is lower. These differences could be due to the increased Cr over-layer in sample 1-Crb that increases the proportion of signal from the very top surface. Further, it is curious that the Cr/Al ratio is only 10% higher than for 1-Cra, given that the stoichiometric ratio is 10 fold higher. A consideration is that the Cr/Al and O/Al ratios will be affected by the over lay Cr due to the fact that Cr2p and O1s emit lower energy photoelectrons with smaller mean free paths than Al2p. Consequently, for the same absolute Cr and Al concentrations, the thicker the C over-layer the lower the Cr/Al and O/Al ratios. Alternatively, a greater proportion of Cr might be tied up in precipitates where the bulk of the Cr is not detected by XPS due to its surface sensitivity. In this regard, that neutron diffraction (ND) detected a Cr2O3-like phase in sample 1-Crb yet XPS does not provide compelling evidence for this phase, is an indication that XPS is averaging over more than one Cr(III) bonding environment, whereas ND is highlighting the crystalline phase. Evidence for more than one Cr bonding environment is provided in the following section on emission spectroscopy.
The luminescence relaxation profiles of 1-Cra, 1-Crb and 2-Cra were plotted as a function of time (Fig. 4c), where symbols represent experimental emission intensity, and lines are the bi-exponential fit of the emission intensity decay. The bi-exponential decays of the intensities can all be represented by Ae−Bt + Ce−Dt; the decay constants being shown in Table 2. The decay equations for each case are as follows, where IE is emission intensity:
| Boehmite species | Emission decay constants | |||
|---|---|---|---|---|
| A | B | C | D | |
| 1-Cra | 1110 ± 9 | 0.00021 ± 0.00002 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 549 ± 175 549 ± 175 |
0.1289 ± 0.0007 |
| 1-Crb | 1418 ± 67 | 0.003321 ± 0.00059 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 494 ± 319 494 ± 319 |
0.1528 ± 0.0012 |
| 2-Cra | 1210 ± 67 | 0.00152 ± 0.00091 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 658 ± 259 658 ± 259 |
0.1378 ± 0.0001 |
1-Cra:
IE = (1110 ± 9)e−(0.00021±0.00002)t + (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 549 ± 175)e−(0.1289±0.0007)t 549 ± 175)e−(0.1289±0.0007)t
| (1) |
1-Crb:
IE = (1418 ± 67)e−(0.00332±0.00059)t + (52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 494 ± 319)e−(0.1528±0.0012)t 494 ± 319)e−(0.1528±0.0012)t
| (2) |
2-Cra:
IE = (1210 ± 67)e−(0.00152±0.00091)t + (42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 658 ± 259)e−(0.1378±0.0009)t 658 ± 259)e−(0.1378±0.0009)t
| (3) |
Closer inspection of the luminescence relaxation profile of 1-Cra (Fig. 4c) indicates that the transitions at 688 and 701 nm (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 535 and 14
535 and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 265 cm−1 respectively) are very short lived (k = 0.1289 ± 0.0007 μs−1). These are tentatively assigned to the 0, 0 components of the 2E → 4A2 transition (in O symmetry) split by a trigonal field (C3 symmetry).25 The spectra are indicative of isolated Cr species with no Cr–Cr interactions, in O symmetry with an essentially trigonal environment. The second transition is longer lived (k = (2.09 ± 0.23) × 10−4 μs−1) and is tentatively assigned to the symmetry forbidden 4T2 → 4A2 transition. The luminescence relaxation profiles of 1-Crb and 2-Cra (Fig. 4c) show similar bi-exponential decays, suggesting similar presence of multiple Cr(III) coordination environments.
265 cm−1 respectively) are very short lived (k = 0.1289 ± 0.0007 μs−1). These are tentatively assigned to the 0, 0 components of the 2E → 4A2 transition (in O symmetry) split by a trigonal field (C3 symmetry).25 The spectra are indicative of isolated Cr species with no Cr–Cr interactions, in O symmetry with an essentially trigonal environment. The second transition is longer lived (k = (2.09 ± 0.23) × 10−4 μs−1) and is tentatively assigned to the symmetry forbidden 4T2 → 4A2 transition. The luminescence relaxation profiles of 1-Crb and 2-Cra (Fig. 4c) show similar bi-exponential decays, suggesting similar presence of multiple Cr(III) coordination environments.
In the following section, electron microscopy provides direct spatial information on the distribution of Cr associated with both the rhombic and nano-lath boehmite morphologies.
Electron microscopy
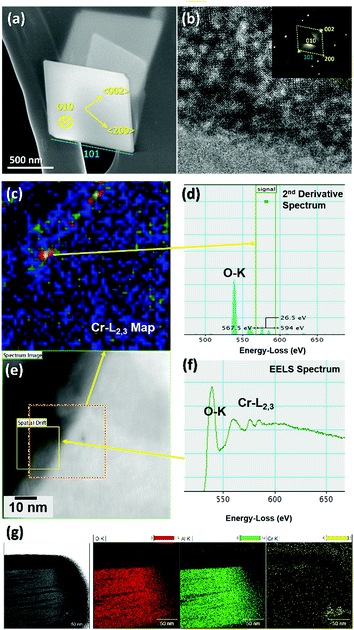
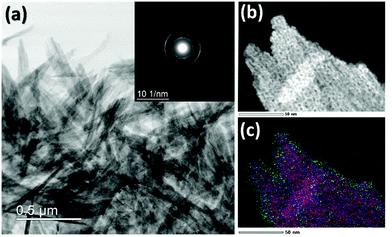
Scanning electron micrographs (SEMs) of 1 show rhombic plates with a uniform size distribution (Fig. 5a). The high resolution transmission electron micrograph (TEM) of Fig. 5b shows the single crystal nature of the plates. The selected area electron diffraction (SAED) inset of Fig. 5b reveal the symmetric sharp diffraction spots that were indexed as orthorhombic γ-AlOOH with basal (010) and lateral (101) faces strongly expressed. The morphologies of 1-Cra and 1-Crb are identical to 1 (Fig. 5a) suggesting that Cr association with boehmite in the 1–10 wt% range does not affect particle morphology. Scanning transmission electron micrographs (STEMs) under high angle annular dark field (HAADF) mode and electron energy loss spectroscopy (EELS) analysis show ∼10 nm islets of Cr on the surface, Fig. 5c–f. Conversely, Cr was not detected in the bulk (detection limit ∼ 1 wt%). This preferential surface enrichment is also verified by focused ion beam (FIB) sections that expose the interior of the boehmite crystals, as shown in the energy-dispersive X-ray spectroscopy (EDX) mapping of Fig. 5g.Representative TEM of 2 and 2-Cra both show lath-like morphology, Fig. 6a. The selected area electron diffraction (SAED) inset of Fig. 6a reveal diffraction indexing as orthorhombic γ-AlOOH.
 | ||
| Fig. 6 Microanalyses of Cr doped boehmite 2-Cra: (a) TEM with SAED inset, (b) STEM HAADF, (c) EDX mapping of (b) (green spots represent Cr and purple spots represent Al). | ||
A magnified section of the laths was examined with STEM under high angle annular dark field (HAADF) (Fig. 6b). The energy-dispersive X-ray spectroscopy (EDX) mapping (Fig. 6c) of the selected region shows the preferential enrichment of Cr on the surface. As in the case of 1-Cra, Cr was not detected in the bulk.
Implications from theory
In order to evaluate the stability of chromium in the boehmite structure, single Cr(III) ions or pairs of Cr(III) ions were substituted for lattice Al(III) ions in a boehmite supercell. Fig. 7a shows the 4 × 1 × 4 boehmite supercell where the substitution of 1 Cr represents 1.38 atomic weight%, and 2 Cr represents 2.76 atomic weight%. In Fig. 7b, calculated incorporation energies (Einc-bulk) are plotted as a function of distance between two Cr(III) ions in the boehmite supercell normalized to the Einc-bulk of 1 Cr(III). For comparison, the Einc-bulk of a single Cr(III) substitution is shown at the minimum spacing between neighboring supercells (∼11 Å). In all cases, the Einc-bulk are positive relative to gas-phase reference ions (eqn (S1) and Table S1; (ESI)†). Additional structural relaxation and hydration energy corrections would serve to help lower this Einc-bulk; however, the strongly positive Einc-bulk suggests that Cr(III) is unlikely to substitute for Al(III) in a bulk-like boehmite environment, or that a significant energy barrier must be overcome in order to stabilize Cr(III) in the structure.Surface slab calculations were performed to test whether Cr(III) incorporation energies vary from the bulk to the (near-)surface. Vacuum-terminated surface energies were calculated for the commonly observed basal (010) and side, (100) and (001) surfaces of boehmite crystals (eqn (S2), ESI†). Due to challenges in stabilizing the commonly expressed (101) surfaces with vacuum terminations, those results are not included here. Single-point energy surface energies increase from the basal plane (010), 0.155 J m−2, to the (001) and (100) surfaces, 1.689 J m−2 and 3.226 J m−2, respectively. These results suggest increasing surface reactivity from the basal planes to the sides under vacuum conditions. Next, chromium incorporation energies (Einc-slab) were calculated as a function of Cr(III) distance from the free surface of the slab for all three terminations of boehmite. In order to avoid the formation of a dipole moment, Cr(III) were substituted for Al(III) in as symmetrically equivalent lattice locations as possible, moving from the center of the slab to the surface (Fig. 7c). While still positive, Einc-slab decrease as the Cr ions move away from the bulk-like center of the slab to the (near-)surface slab environments suggesting that stability is gained by more surface-driven interactions of Cr with boehmite. For the basal (010) surface, this decrease in Einc is only 0.004 eV going from the center of the slab to the top. For the side surfaces, the decrease in Einc becomes more pronounced from the center to the top of the slab, decreasing by 0.603 eV for the (001) and 0.941 eV for the (100) surface slabs. In all cases, Einc-slab for Cr in the center of the slab are consistent with Einc-bulk (e.g., 2.922–2.926 eV), whereas (near-) surface Einc-slab range from 1.981–2.922 eV.
In summary, the calculations are consistent with experiment and show that Cr(III) is incompatible with the boehmite structure at the ∼1% level, likely preferring a (near-)surface environment over a bulk-like environment. Given the large positive energies, it is reasonable to suggest that the solubility of Cr(III) in boehmite is appreciably lower than 1%. This conclusion is also consistent with the lack of Cr(III)–Al(III) phase formation during passivation of aluminum alloys that follow chromate/Al redox pathways.26,27 From a size-based perspective, the crystal radius of high-spin Cr(III) in octahedral coordination is 11.8% larger than the radius of Al(III) (0.675 Å).28 Generally, it is more difficult to substitute a large cation into a structure for a smaller cation;29 however, according to Goldschmidt's rules of substitution, Cr(III) substitution for Al(III) could be plausible as their radii differ by less than 15%. However, if the ions form bonds of different ionic character as is the case for Al(III) versus Cr(III), (e.g., 3p versus 3d bonding orbitals, respectively), then substitution becomes more difficult. In addition, the fact that there is no naturally occurring Cr(III) polymorph equivalent to boehmite suggests that Cr(III) is not stable in the boehmite structure. Future work will focus on lowering the concentration of Cr(III) in the both the experiments and computations; however, there are practical limits to the size of the cell required to explore low Cr(III) concentrations.
Effect of exposure to caustic solutions: relevance to Hanford sludge waste processing
To investigate the possibility of Cr doping/adhesion effect on boehmite corrosion, samples 1 and 1-Cra were exposed to 2 M KOH solutions at 80 °C for 48 hours. The change in morphology due to corrosion was analyzed by SEM as shown in Fig. 8. The distinctive rhombohedral platelet shape is no longer visible for sample 1 as seen in Fig. 8a. The once sharp sides as seen in Fig. 5a, are now rounded with all particles on average significantly smaller in diameter than pre caustic treatment. In contrast 1-Cra shows little to no alteration under identical caustic conditions, Fig. 8b. Additionally the small particles of higher contrast in Fig. 8b, identified as Cr in STEM EDS mapping (ESI†), do not dissolve but instead remained adhered to the boehmite surface.Our preliminary observation is that Cr enrichment at rhombic boehmite surfaces (thus far there is no experimental or theoretical evidence for bulk incorporation of Cr) affects the reactivity of boehmite during caustic leaching. The inhibition or slowing the rate of corrosion by Cr additive has implications for the behavior of boehmite, and possibly other Al phases, in the Hanford tank waste.
For example, microanalyses and oxidative caustic-leaching (i.e., Cr(III) → Cr(VI)) of Cr-rich Hanford tank waste fractions indicate both a close association but poor correlation between Cr and Al during leaching (shown in Fig. 9). In particular, leaching the “group 6” sludge shows that a much greater fraction of Cr was mobilized initially compared to Al (Fig. 9d).15 After approximately 2 hours, Cr release plateaued but Al continued to be gradually released. One plausible interpretation that is bolstered by the present experimental and theoretical work is that the majority of Cr and Al are not forming a solid solution; rather Cr(III) and Al(III) are primarily forming separate phases. In other words, most of the Cr(III) is readily accessible for oxidation to highly soluble Cr(VI). Elsewhere in the tanks, Cr is a minor but not inconsequential constituent. However, further analysis is required to ascertain its relationship to boehmite in the general case. As established here, we predict that any Cr(III) that is associated with boehmite would be concentrated at the near-surface, not in the bulk. If so, oxidative leaching (preferably with a non-metallic oxidant) would indeed be an effective remedy for Cr removal as well as increasing the reactivity of boehmite to caustic leaching. There is also a possibility of some Al being present in the Cr-rich part, which would also support the trend observed in the oxidative leaching experiments.
 | ||
| Fig. 9 (a) TEM images of euhedral boehmite with interdispersed uranium oxide nanoclusters from a mixture of fully radioactive REDOX-type sludge from the Hanford site, (b) EDS analysis showing the potential association of Al and Cr in an amorphous phase from REDOX type wastes and (c) STEM image of the sludge material. (d) Kinetics of oxidative leaching of tank waste sludge from the REDOX waste. Orange circles = Cr(III), blue circles = Al(III) (Data adapted from Fiskum et al., 2008 (ref. 15)). | ||
Conclusions
Irrespective of the starting materials, the synthetic method, morphology, or the stage of Cr introduction, Cr(III) is concentrated at the near-surface of boehmite and not in the bulk. The experimental results are consistent with quantum-mechanical calculations that yielded high positive incorporation energies for Cr(III) substituting for Al in the boehmite structure. We note that adsorption of Cr to surfaces, as opposed to bulk incorporation, could still strongly affect the energetics of boehmite, particularly nano-size variants due to the large contribution of surface energy to the overall free energy budget. Further, sorption of trace Cr(III) to surface defects could have a leveraged effect on dissolution kinetics. This has implications for interpreting the increased thermal stability of so called “Cr-doped” boehmite17 as well protocols designed to lower the Al load in HLW streams at the Hanford site.Experimental section
Chemicals and materials
All chemicals were reagent grade or better. Gibbsite, Al(OH)3(C-333) was obtained from Almatis, USA. Aluminium nitrate nonahydrate (Al(NO3)3·9H2O; 98%) and chromium nitrate nonahydrate (Cr(NO3)3·9H2O; 99%) were obtained from Sigma-Aldrich, USA. NaOH was obtained from Fischer Scientific, USA. DI water (18 MΩ) was used as a base for all solutions.Solid phase characterization
The details of solid phase characterization techniques used in this work which include X-ray diffraction (XRD), time-of-flight (TOF) neutron diffraction, Fourier transform infra-red (FTIR), Raman, excitation and emission spectroscopies, X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), scanning electron microscopy (SEM) and computational calculations are detailed in the ESI.†Computational approach
To test the viability of Cr incorporation into the boehmite structure, atomic-scale models were developed representing a 4 × 1 × 4 supercell of boehmite (256 atoms) using the structure proposed by Corbato et al.20 with Cmc21 space group symmetry. Additional symmetries were evaluated (ESI, Table S2†), with P21/b symmetry being applied to surface energy and slab incorporation energies. To complement Cr-doping levels in experimentally synthesized samples, one Al(III) center in the model was replaced by a Cr(III) center amounting to 1.38 atomic weight% of Cr(III) substitution. Models with pairs of Cr(III) replacing Al(III) (i.e., 2.76 atomic weight% of Cr(III)) were also evaluated to determine the effect of Cr-loading on incorporation energy. Incorporation energies for the charge-balanced substitution were calculated using the quantum-mechanical code, CRYSTAL14, at the unrestricted Hartree–Fock (UHF) level of theory.31 All-electron basis sets for Cr, Al, O, and H were chosen based on previous success modeling aluminum oxyhydroxides32 and chromium oxides.33,34 Here, basis set performance was evaluated based on comparing the relaxed lattice parameters of complementary M2O3 structures (where M = Al(III) or Cr(III); ESI, Table S3†). Additionally, surface energy calculations were performed on commonly expressed, low-index surfaces of boehmite in order to determine their relative reactivity (eqn (S1), ESI†). Then, incorporation energies involving pairs of Cr(III) were performed on the most stable and more reactive terminations of boehmite to evaluate whether Cr(III) prefers bulk-like or (near-) surface environments when associating with this phase (eqn (S2), ESI†). Current calculations reflect single-point energies (i.e., no structural relaxation) at 0 K; however, additional structural relaxations and hydration energy corrections can be applied to link the calculations to more experimentally-relevant conditions.35,36Acknowledgements
This work was supported by the Laboratory Directed Research and Development (LDRD), Nuclear Processing Science Initiative (NPSI). Pacific Northwest National Laboratory (PNNL) is a multi-program national laboratory operated for the U.S. Department of Energy (DOE) by Battelle Memorial Institute under Contract DE-AC06-76RLO 1830. Part of this research was performed at EMSL, a national scientific user facility at PNNL managed by the Department of Energy's Office of Biological and Environmental Research. A portion of the research used resources at the Spallation Neutron Source (SNS), a DOE Office of Science User Facility operated by the Oak Ridge National Laboratory. Calculations were performed using PNNL Institutional Computing resources at Pacific Northwest National Laboratory.References
- Y. X. Zhang, X. B. Zhou, Z. L. Liu, B. Li, Q. C. Liu and X. H. Li, Monodispersed hierarchical gamma-AlOOH/Fe(OH)(3) micro/nanoflowers for efficient removal of heavy metal ions from water, RSC Adv., 2016, 6, 6695–6701 RSC.
- T. Kim, H. B. Li, J. B. Lian, H. H. Jin, J. M. Ma, X. C. Duan, G. Yao and W. J. Zheng, Ionic liquid-assisted hydrothermal synthesis of gamma-Al2O3 hierarchical nanostructures, Cryst. Res. Technol., 2010, 45, 767–770 CrossRef CAS.
- M. Ireland, X. Wang, T. Radomirovic, P. Smith and F. Jones, Investigating the impact of anatase on the dissolution of boehmite, Hydrometallurgy, 2014, 147, 246–254 CrossRef.
- K. Wefers, Nomenclature, preparation, and properties of aluminium oxide hydroxides, and trihydroxides, in Alumina Chemicals: Science and Technology Handbook, ed. L. D. Hart, The American Ceramic Society, Westerville, OH, 1990, pp. 13–22 Search PubMed.
- G. Martino, P. Courty, C. Marcilly, K. Kochloefl and J. H. Lunsford, Energy-Related Catalysis: Sections 3.1–3.7.5, VCH Verlag Gesellschaft, Weinheim, Germany, 1997 Search PubMed.
- M. Trueba and S. P. Trasatti, Gamma-Alumina as a support for catalysts: A review of fundamental aspects, Eur. J. Inorg. Chem., 2005, 3393–3403 CrossRef CAS.
- S. E. Fendorf, Surface-Reactions of Chromium in Soils and Waters, Geoderma, 1995, 67, 55–71 CrossRef CAS.
- G. E. Brown and G. A. Parks, Sorption of trace elements on mineral surfaces: Modern perspectives from spectroscopic studies, and comments on sorption in the marine environment, Int. Geol. Rev., 2001, 43, 963–1073 CrossRef.
- R. E. Gephart, A short history of waste management at the Hanford Site, Phys. Chem. Earth, 2010, 35, 298–306 CrossRef.
- J. G. Reynolds, G. A. Cooke, D. L. Herting and R. W. Warrant, Salt Mineralogy of Hanford High-Level Nuclear Waste Staged for Treatment, Ind. Eng. Chem. Res., 2013, 52, 9741–9751 CrossRef CAS.
- D. L. Herting, J. G. Reynolds and W. B. Barton, Conversion of Coarse Gibbsite Remaining in Hanford Nuclear Waste Tank Heels to Solid Sodium Aluminate [NaAl(OH)(4) center dot 1.5H(2)O], Ind. Eng. Chem. Res., 2014, 53, 13833–13842 CrossRef CAS.
- J. G. Reynolds, G. A. Cooke, D. L. Herting and R. W. Warrant, Evidence for dawsonite in Hanford high-level nuclear waste tanks, J. Hazard. Mater., 2012, 209, 186–192 CrossRef PubMed.
- J. G. Reynolds, J. K. McCoskey and D. L. Herting, Gibbsite Solubility in Hanford Nuclear Waste Approached from above and below Saturation, Ind. Eng. Chem. Res., 2016, 55, 5465–5473 CrossRef CAS.
- J. D. Vienna, P. Hrma, J. V. Crum and M. Mika, Liquidus temperature-composition model for multi-component glasses in the Fe, Cr, Ni, and Mn spinel primary phase field, J. Non-Cryst. Solids, 2001, 292, 1–24 CrossRef CAS.
- S. K. Fiskum, E. C. Buck, R. C. Daniel, K. E. Draper, M. K. Edwards, T. L. Hubler, L. K. Jagoda, E. D. Jenson, A. E. Kozelisky, G. J. Lumetta, P. J. MacFarlan, B. K. McNamara, R. A. Peterson, S. I. Sinkov, L. A. Snow and R. G. Swoboda, Characterization and Leach Testing for REDOX Sludge and S-Saltcake Actual Waste Sample Composites, Richland, 2008 Search PubMed.
- B. M. Rapko and J. D. Vienna, Selective Leaching of Chromium from Hanford Tank Sludge 241-U-108, Richland, WA, 2002 Search PubMed.
- J. Yang, R. L. Frost and Y. Yuan, Synthesis and characterization of chromium doped boehmite nanofibres, Thermochim. Acta, 2009, 483, 29–35 CrossRef CAS.
- R. L. Russell and R. A. Peterson, Boehmite Dissolution Model Based on Simulant Data, Ind. Eng. Chem. Res., 2010, 49, 4542–4545 CrossRef CAS.
- Powder Diffraction File Inorganic and Organic Data Book, ed. S. Kabekkodu, International Center for Diffraction Data, Newtown Square, PA, USA, 2015, vol. 60 Search PubMed.
- C. E. Corbato, R. T. Tettenhorst and G. G. Christoph, Structure Refinement of Deuterated Boehmite, Clays Clay Miner., 1985, 33, 71–75 CAS.
- J. Zhang, S. Y. Wei, J. Lin, J. J. Luo, S. J. Liu, H. S. Song, E. Elawad, X. Ding, J. M. Gao, S. R. Qi and C. C. Tang, Template-free preparation of bunches of aligned boehmite nanowires, J. Phys. Chem. B, 2006, 110, 21680–21683 CrossRef CAS PubMed.
- H. D. Ruan, R. L. Frost and J. T. Kloprogge, Comparison of Raman spectra in characterizing gibbsite, bayerite, diaspore and boehmite, J. Raman Spectrosc., 2001, 32, 745–750 CrossRef CAS.
- J. T. Kloprogge, L. V. Duong, B. J. Wood and R. L. Frost, XPS study of the major minerals in bauxite: Gibbsite, bayerite and (pseudo-)boehmite, J. Colloid Interface Sci., 2006, 296, 572–576 CrossRef CAS PubMed.
- P. Marcus and J. M. Grimal, The Anodic-Dissolution and Passivation of Ni-Cr-Fe Alloys Studied by Esca, Corros. Sci., 1992, 33, 805–814 CrossRef CAS.
- S. Shoval, M. Gaft and G. Panczer, Luminescence of Cr3+ in natural and calcined diaspore, J. Therm. Anal. Calorim., 2003, 71, 699–706 CrossRef CAS.
- L. Xia and R. L. McCreery, Chemistry of a chromate conversion coating on aluminum alloy AA2024-T3 probed by vibrational spectroscopy, J. Electrochem. Soc., 1998, 145, 3083–3089 CrossRef CAS.
- J. D. Ramsey and R. L. McCreery, Raman microscopy of chromate interactions with corroding aluminum alloy 2024-T3, Corros. Sci., 2004, 46, 1729–1739 CrossRef CAS.
- R. D. Shannon, Revised Effective Ionic-Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- A. Navrotsky, Physics and Chemistry of Earth Materials, Cambridge University Press, New York, NY, 1994 Search PubMed.
- X. Y. Chen, H. S. Huh and S. W. Lee, Hydrothermal synthesis of boehmite (gamma-AlOOH) nanoplatelets and nanowires: pH-controlled morphologies, Nanotechnology, 2007, 18, 285608 CrossRef.
- R. Dovesi, R. Orlando, A. Erba, C. M. Zicovich-Wilson, B. Civalleri, S. Casassa, L. Maschio, M. Ferrabone, M. De La Pierre, P. D'Arco, Y. Noel, M. Causa, M. Rerat and B. Kirtman, CRYSTAL14: A Program for the Ab Initio Investigation of Crystalline Solids, Int. J. Quantum Chem., 2014, 114, 1287–1317 CrossRef CAS.
- R. Demichelis, Y. Noel, P. Ugliengo, C. M. Zicovich-Wilson and R. Dovesi, Physico-Chemical Features of Aluminum Hydroxides As Modeled with the Hybrid B3LYP Functional and Localized Basis Functions, J. Phys. Chem. C, 2011, 115, 13107–13134 CAS.
- M. Catti, G. Sandrone, G. Valerio and R. Dovesi, Electronic, magnetic and crystal structure of Cr2O3 by theoretical methods, J. Phys. Chem. Solids, 1996, 57, 1735–1741 CrossRef CAS.
- E. Ruiz, M. Llunell and P. Alemany, Calculation of exchange coupling constants in solid state transition metal compounds using localized atomic orbital basis sets, J. Solid State Chem., 2003, 176, 400–411 CrossRef CAS.
- F. N. Smith, C. D. Taylor, W. Um and A. A. Kruger, Technetium Incorporation into Goethite (alpha-FeOOH): An Atomic-Scale Investigation, Environ. Sci. Technol., 2015, 49, 13699–13707 CrossRef CAS PubMed.
- F. N. Smith, W. Um, C. D. Taylor, D. S. Kim, M. J. Schweiger and A. A. Kruger, Computational Investigation of Technetium(IV) Incorporation into Inverse Spinels: Magnetite (Fe3O4) and Trevorite (NiFe2O4), Environ. Sci. Technol., 2016, 50, 5216–5224 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20234a |
| This journal is © The Royal Society of Chemistry 2016 |