Direct interfacial growth of MnO2 nanoparticles on carbon nanofiber surfaces for high-performance asymmetric supercapacitors†
Chang-Feng Zhao‡
,
Ke Lu‡ and
Houyi Ma*
Key Laboratory for Colloid and Interface Chemistry of State Education Ministry, School of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, China. E-mail: hyma@sdu.edu.cn; Fax: +86-531-88564464; Tel: +86-531-88364959
First published on 31st October 2016
Abstract
Facile interfacial growth of MnO2 nanoparticles on carbon nanofibers (MnO2/CNF) and the application of this material as a high-performance positive electrode material in asymmetric supercapacitors are presented. Rational combination of MnO2/CNF positive and CNF negative electrode materials enabled asymmetric devices with extended operating voltage windows of 1.8 V, and much higher power densities (4434 W kg−1) and energy densities (36 W h kg−1) compared with similar devices being reported. The assembled novel hybrid aqueous asymmetric capacitor shows excellent cycling stability with 90.9% capacity retention for 1000 cycles. The asymmetric supercapacitors were based on low cost and environmental friendly materials and have great potential for practical devices.
1. Introduction
Owing to their high power density and good long-term stability, supercapacitors (SCs) have gained commercial success and been successfully adopted as energy sources in several portable electronic devices.1–3 High-energy/power-density as well as recyclability improvements are still required for further large-scale applications.4 As such, much effort is still needed to explore novel electrode materials with better electrochemical performance. Based on their energy storage mechanisms, SCs can be categorized as electric double layer capacitors (EDLCs) and pseudocapacitors.5–7 Over the past decade, carbonaceous materials (e.g., graphene, porous carbon, carbon nanofiber) with high surface area and high electrical conductivity have received more attention and have been widely used as standard electrode materials for EDLCs in commercial SCs and/or rechargeable batteries.5,6 Whereas various transition metal oxides/hydroxides and/or conducting polymers have been investigated as pseudocapacitor electrode materials,8–17 transition metal oxides (e.g., MnO2, Fe3O4, and V2O5) with selected three dimensional (3D) hierarchically nanostructured frameworks are highly attractive pseudocapacitive materials because of their specific capacitance compared to EDLCs, and are therefore under intensive investigation.18–22Among these pseudocapacitive transition metal oxides, MnO2 in particular has been considered as one of the most scalable alternatives due to its natural abundance, high theoretical specific capacitance, relatively low cost, and environmental friendliness.18 A key limitation to the use of MnO2 active materials is its limited electronic conductivity (10−5 to 10−6 S cm−1) and poor rate capability, which impede its practical application in energy storage.23,24 To this end, binary and/or ternary nanostructured electrodes made by integrating metal oxide particles with carbon hosts via simple procedures with high electrochemical performance need to be developed.4,8 In MnO2/carbon nanofiber (MnO2/CNF) binary nanocomposites, the carbon nanofiber (CNF) can not only contribute to the maximum accessible surface area/overall capacity through the EDLC mechanism but can also provide effective conductive networks for electrical transport by bridging MnO2 particles.8 So, surface growth/incorporation of MnO2 particles on CNF surfaces can overcome these limitations and improve electrochemical activity, and can therefore serve as high-performance candidates for SCs. As for practical applications, however, the energy densities, operating voltages, and capacitances of SCs still need to be improved significantly. Hitherto, asymmetric supercapacitors combining MnO2-based positive electrodes with carbon-based negative electrodes have been constructed to maximise specific capacitance and enlarge the operating voltage window.8
Directed interfacial grown of MnO2 nanoparticles on highly conductive CNF surfaces was obtained through a straightforward one-step hydrothermal processing technique.25 The conductivity of the pseudocapacitive MnO2 was effectively enhanced and its electrochemical performance was accordingly maximised. In view of the interconnected CNF’s superior electrical transport properties, direct/continuous surface growth of MnO2 nanoparticles enables excellent supercapacitive performance, which is obviously desirable for high-performance supercapacitors. An advanced asymmetric supercapacitor with MnO2/CNF positive and CNF negative electrodes in a mild aqueous electrolyte (0.5 M Na2SO4) was fabricated. The asymmetric supercapacitor demonstrated excellent cycle stability in a large voltage window of 1.8 V, high energy density (36 W h kg−1), and much higher power density (4434 W kg−1) compared with similar devices being reported. Benefiting from these features, the as-fabricated hybrid supercapacitor can serve as a promising candidate for next-generation energy storage systems.
2. Experimental section
Materials preparation
All the reagents are analytical grade and were used without further purification. The aqueous solution was freshly prepared with high purity water generated by a GenPure UV-TOC/UF ultra-pure water system (TKA, Niederelbert, Germany).CNFs were synthesized according to the hydrothermal Te@C nanocable template process.26,27 Te nanowires were prepared using a hydrothermal method reported by Yu’s group.27,28 Typically, the as-obtained Te wires were dispersed in 24 mL water with vigorous magnetic stirring, and then 1.5 g of glucose was added to the mixture to form a clear solution. The final solution was transferred into a Teflon-lined stainless steel autoclave and reacted at 200 °C for 10 h. The products were collected by centrifugation and were then washed with deionized water and absolute ethanol three times. After removal of the core of the product with 30 mL of an aqueous solution containing 2 mL of hydrochloric acid (36.5 wt%), 5 mL of H2O2 (30 wt%), and 20 mL of double-distilled water (HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2
H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) at room temperature for 12 h, the obtained black powder was further calcined at 600 °C for 2 h in an N2 flow, yielding pure CNFs.
20, v/v) at room temperature for 12 h, the obtained black powder was further calcined at 600 °C for 2 h in an N2 flow, yielding pure CNFs.
MnO2/CNF nanocomposites were prepared using a simple in situ hydrothermal method.25 In a typical synthesis, 0.5 g of SDBS and 0.2 g of the CNFs were dissolved in 40 mL of water with 0.005 M MnSO4 and 0.005 M KMnO4, and the mixture was stirred for 1 h. The mixture was then transferred into a Teflon-lined stainless steel autoclave and reacted at 160 °C for 14 h. The products were collected by centrifugation, washed with deionized water three times, and were dried overnight at 60 °C. For comparison, α-MnO2 nanowires were also synthesized according to a hydrothermal method which has been previously reported.29
Materials characterization
The morphologies of the samples were investigated using a Hitachi X650 scanning electron microscope (SEM) and a JEOL JEM-1011 transmission electron microscope (TEM). X-ray powder diffraction (XRD) patterns were recorded using a Bruker D8 advanced X-ray diffractometer equipped with a Cu Kα (λ = 1.54178 Å) radiation source. X-ray photoelectron spectra (XPS) were recorded on an ESCALAB 250 X-ray photoelectron spectrometer.Electrochemical characterization
The working electrodes consisted of the active materials, acetylene black (AB), and poly(tetrafluoroethylene) (PTFE) with a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and were pasted onto a stainless steel mesh. The as-prepared electrodes were dried at 80 °C for 10 h in a vacuum. Electrochemical measurements were performed using three-electrode cells and a 0.5 M Na2SO4 aqueous solution as the electrolyte, in which the saturated calomel reference electrode (SCE) and a platinum sheet were used as the reference and the counter electrodes, respectively. The weight ratio of the MnO2/CNF electrode and the CNF electrode was 0.66 (m+/m− = C−ΔV−/C+ΔV+) to balance the charge at each electrode. For the MnO2/CNF//CNF hybrid supercapacitor,4 1 mg of MnO2/CNF as the positive electrode was paired with 1.5 mg of CNF as the negative electrode in a 0.5 M Na2SO4 solution. Galvanostatic charge–discharge measurements were performed between 0 and 1.8 V at different current densities. EIS measurements were carried out with a potential amplitude of 5 mV over a frequency range of 100 kHz to 100 mHz. The gravimetric specific capacitance as measured by the galvanostatic charge–discharge method can be calculated according to the equation C = IΔt/mΔV, where C (F g−1) is specific capacitance, I (mA) designates the constant discharge current, and ΔV (V) is the voltage change during the discharge. Δt (s) is the discharge time. In the three-electrode system, m (mg) is the mass loading of active material in a single electrode. In a two-electrode cell, m (mg) represents the total mass loading of active materials on both electrodes. The energy density E (W h kg−1) and power density P (W kg−1) of the asymmetric supercapacitors were obtained from the equations E = 0.5CΔV2/3.6, and P = 3600E/Δt, where C (F g−1) is the specific capacitance of the asymmetric devices, ΔV (V) is the discharge voltage range and Δt (s) is the discharge time.
5 and were pasted onto a stainless steel mesh. The as-prepared electrodes were dried at 80 °C for 10 h in a vacuum. Electrochemical measurements were performed using three-electrode cells and a 0.5 M Na2SO4 aqueous solution as the electrolyte, in which the saturated calomel reference electrode (SCE) and a platinum sheet were used as the reference and the counter electrodes, respectively. The weight ratio of the MnO2/CNF electrode and the CNF electrode was 0.66 (m+/m− = C−ΔV−/C+ΔV+) to balance the charge at each electrode. For the MnO2/CNF//CNF hybrid supercapacitor,4 1 mg of MnO2/CNF as the positive electrode was paired with 1.5 mg of CNF as the negative electrode in a 0.5 M Na2SO4 solution. Galvanostatic charge–discharge measurements were performed between 0 and 1.8 V at different current densities. EIS measurements were carried out with a potential amplitude of 5 mV over a frequency range of 100 kHz to 100 mHz. The gravimetric specific capacitance as measured by the galvanostatic charge–discharge method can be calculated according to the equation C = IΔt/mΔV, where C (F g−1) is specific capacitance, I (mA) designates the constant discharge current, and ΔV (V) is the voltage change during the discharge. Δt (s) is the discharge time. In the three-electrode system, m (mg) is the mass loading of active material in a single electrode. In a two-electrode cell, m (mg) represents the total mass loading of active materials on both electrodes. The energy density E (W h kg−1) and power density P (W kg−1) of the asymmetric supercapacitors were obtained from the equations E = 0.5CΔV2/3.6, and P = 3600E/Δt, where C (F g−1) is the specific capacitance of the asymmetric devices, ΔV (V) is the discharge voltage range and Δt (s) is the discharge time.
3. Results and discussion
X-ray diffraction analysis, scanning/transmission electron microscopy and X-ray photoelectron spectroscopy were performed to characterize the as-synthesized MnO2/CNF positive and CNF negative materials. Representative morphological micrographs of the prepared positive and negative materials are shown in Fig. 1. As mentioned in the experimental section, the CNF materials were synthesized through a template-removal (Te/C nanocables) process. After chemical etching and high-temperature calcination in a nitrogen atmosphere, uniform carbon nanofibers with an average diameter of 40 nm (Fig. 1a) were observed. As seen, these carbonaceous nanotubes featured an outer porous layer and an inner hollow cavity (Fig. 1b). These unique porous structural features of the conductive carbon host not only facilitate the diffusion of the electrolyte, but also effectively alleviate the volume change of the active material (MnO2) during redox reactions. Fig. 1c shows a typical SEM image of the MnO2/CNF nanocomposite. The MnO2 particles possessed amorphous shapes and the composites aggregated and formed multi-layered structures with micron sizes. The continuous interconnected layered networks of MnO2/CNF further favour rapid transport of charge carriers throughout the material.22 Fig. 1d shows a typical TEM image of the MnO2/CNF composite. It can be seen clearly that MnO2 nanoparticles were coated uniformly on the surface of CNF and generated interconnected networks which could bridge each other when used as electrode materials. The lattice spacing of 0.69 nm between adjacent lattice planes in the image corresponds to the distance between two (110) crystal planes (Fig. 1d, inset, HRTEM). So, the CNF could act as a conductive agent and also construct a conductive network, enhancing the electrical conductivity of the nanocomposites and effectively promoting the interconnection/interactions between CNF and MnO2 nanoparticles, thus improving the electrochemical performance as a whole.30 The results above firmly confirm the successful formation of the MnO2/CNF nanocomposites. α-MnO2 nanowires were also prepared for comparison purposes. The lengths of the α-MnO2 nanowires ranged between 500 nm and several micrometers, and the diameters ranged between 20 and 100 nm (Fig. 1e and f).25As shown in Fig. 2a, the broad (002) peak of the CNF matrix ranged from 20° to 30°, suggesting that the carbon host was amorphous in character. What is more, compared to the typical XRD patterns of the resultant MnO2/CNF and MnO2 samples, in addition to the diffraction peaks of the carbon substrates, all of the other peaks can be indexed to the tetragonal phase of α-MnO2 (JCPDS No. 44-0141).25,29 This indicates that the MnO2 nanoparticles had a relatively high crystallinity after combination/interfacial growth. Highly crystalline MnO2 can be formed, as confirmed by XRD, TEM and HRTEM. The XPS patterns of the MnO2/CNF sample (Fig. 2b) provide further insights into the elements’ oxidation states. The binding energy in the case of Mn 2p peaks are 642.0 and 653.8 eV, and demonstrate that the Mn element in the MnO2/CNF sample was present in the chemical state of Mn(VI).18,22 This corresponds well with the structure information obtained from S/TEM and XRD.
 | ||
| Fig. 2 (a) Powder X-ray diffraction spectra of the MnO2/CNF, CNF and MnO2 samples. (b) High-resolution Mn 2p and C 1s (inset) of the MnO2/CNF sample. | ||
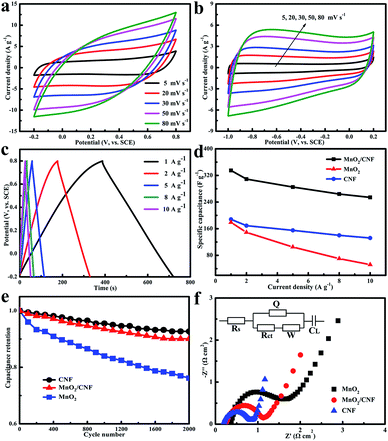
To assess the feasibility of using MnO2/CNF and CNFs for construction of asymmetric supercapacitor devices, the electrochemical properties of MnO2/CNF binary composites, α-MnO2, and CNFs were studied separately in 0.5 M Na2SO4 using CV, galvanostatic charge–discharge, and EIS techniques. Cyclic voltammetry (CV) was initially employed to estimate their electrochemical properties in the three-electrode cell in Na2SO4 (−0.2 to 0.8 V vs. SCE). Fig. 3a and b illustrates the CV curves of the as-synthesized MnO2/CNF positive electrode and the CNF negative electrode at different scan rates. Accordingly, all of the current densities have been normalized based on the mass of electroactive material. For the MnO2/CNF composites (Fig. 3a), the shape of the CV curves gradually transforms from ideal rectangular to oval with increasing scan rate, which may be caused by the increased internal resistance of the electrode, the limited conductivity of the aqueous electrolytes, and the slow diffusion kinetics of Na+.31–33 Additionally, the area of the curve is commonly used to calculate the value of the specific capacitance. CV curves of the CNF materials (Fig. 3b, −1.0 to 0.2 V vs. SCE) exhibit a rectangular shape under different scan rates without obvious distortion, suggesting a good electrical double-layer capacitive (EDLC) nature with good ion transport in the carbon electrode.4,34 Although influenced by charge-transfer resistance, the changes of current with the applied potential did not give straight lines that parallel the horizontal axis for both positive and negative electrodes. Typical charge/discharge linear curves of MnO2/CNF materials collected at different current rates are shown in Fig. 3c, indicating the good electrochemical reversibility. Furthermore, the non-obvious iR drop for these discharge curves further proves its high electrical conductivity and/or lower internal resistance.34 The calculated specific capacitance of the composite electrodes is 335, 309, 285, 264, 254 F g−1 at 1, 2, 5, 8, 10 A g−1, respectively, and is summarized in Fig. 3d. According to TG analysis (ESI, Fig. S1†), the amounts of the carbon component in MnO2/CNF composites are estimated to be 18%. So, the specific capacitance contributed by MnO2 for the MnO2/CNF composites were about 89.9% and 90.1% at current densities of 1 A g−1 and 5 A g−1. As can be found, the porous CNF matrix not only contributes to the overall specific capacitance values but also maintains high capacitance values even at high current densities. In contrast, the discharge capacitance obtained by the pure α-MnO2 electrode is much smaller (Fig. 3d). The specific capacitances calculated from CV curves are also taken into account to verify the result from the charge-discharge curves. According to the CV measurements, the capacitances are 438, 329, 290, 210, and 159 F g−1 at 5, 20, 30, 50, and 80 mV s−1, respectively. So, we can conclude that the high capacitive current responses of MnO2-containing composite electrodes mainly result from the efficient redox reaction of MnO2 within the effective interconnected conductive framework provided by the CNF hosts.4 It was found that the pure CNF negative electrode has quite good specific capacitances, which are 188, 169, 155, 140, 132 F g−1 at 1, 2, 5, 8, 10 A g−1, respectively, and in the potential range between −1.0 and 0.2 V vs. SCE. Furthermore, the cyclic stability of electrodes made with MnO2/CNF and CNFs were evaluated by a 2000 cycle galvanostatic charge–discharge test conducted at 8 A g−1. Both positive and negative electrodes retain more than 90% (91.4% retention for the positive electrode and 93.5% retention for the negative electrode) of the initial capacitance after 2000 cycles at 8 A g−1 (Fig. 3e). However, as can be seen, the cycling performance of the pure α-MnO2 electrode is very limited, which further convinces us of the necessity and effectiveness of combining it with carbon scaffolds. EIS, an effective tool to analyze the internal resistance and the electron transfer rate at the electrode–electrolyte interface, was performed to explore related electrodes. Fig. 3f shows typical Nyquist plots for different electrodes. As noted, all of them had a straight line at low frequencies and a semi-circle at high frequencies. The circuit used to fit the impedance spectrum is listed in the inset. The values of the charge-transfer resistance for MnO2/CNF, MnO2 and the CNFs were determined to be 0.62, 1.3, and 0.50 Ω cm2, respectively. The lower charge-transfer resistance values imply the easy penetration of the electrolyte within the working electrode and the maximized utilization of the electrode materials. Overall, the cycling performance and capacity of this binary material is comparable or even superior to that of similar materials (see Table S1†). Taking the above-mentioned electrochemical results into consideration, the MnO2/CNF and CNF materials can be selected as positive and negative electrodes to construct asymmetric supercapacitors.
Asymmetric supercapacitors using the MnO2/CNF as positive and CNF as negative materials were assembled. The mass ratio of the positive to the negative was optimized at 0.66 on the basis of charge balance theory. Due to the facile kinetics on both the positive (battery-type material) and negative (capacitor-type material) electrodes, this class of device ensures high power and high energy density.34 CV measurements were first performed to explore the stable potential window of the as-prepared asymmetric supercapacitor. The operating potential was investigated as shown in Fig. 4a. When the cell voltage reaches 1.8 V, there is no bump which may caused by some irreversible side-reactions. Consequently, the cell voltage of the asymmetric supercapacitors was selected as 0–1.8 V. As shown in Fig. 4a, each CV curve keeps a similar shape without apparent distortion under a series of scan rates (5 to 80 mV s−1), indicating that the as-fabricated asymmetric supercapacitors show good capacitive behavior with quasi-rectangular shapes. This large potential window, along with the fast charge/discharge properties, can meet the demand of practical applications. Representative galvanostatic charge/discharge curves for the asymmetric prototypes at different current densities are shown in Fig. 4b. Both the MnO2/CNF composite and the CNFs contribute to the whole capacitance, and no noticeable change was perceived for the shapes of the CV and discharge curves, which illustrates the fast charge–discharge performance of the asymmetric devices. Specific capacitance values (based on the total mass of positive and negative active materials), calculated based on the discharge curves, are summarized in Fig. 4c. As shown in Fig. 4c, the calculated specific capacitance of the asymmetric devices was 80 F g−1 at a current density of 0.5 A g−1. The specific capacitance decreases slightly due to the voltage drop with increased current density but is maintained at 52 F g−1 even at 5 A g−1, demonstrating the excellent rate capability.Both the pseudocapacitance of the MnO2/CNF nanocomposite and the EDLC of the CNFs are beneficial for high-performance energy storage devices. Additionally, the long-term cycling stability also should be taken into consideration. The asymmetric supercapacitors were further examined using a long-term charge–discharge test at a scan rate of 2 A g−1 (Fig. 4d). The capacitance retention of the device is maintained at 90.2% of its original specific capacitance after 1000 cycles (88% after 5000 cycles at a high current rate of 5 A g−1, Fig. S2†). The EIS of the asymmetric supercapacitor device was also tested, and is shown in Fig. 4e. The small charge-transfer resistance (Rct, 2.3 Ω cm2) is also beneficial for high performance devices. The Ragone plot (energy density vs. power density) was further employed to show the electrochemical performance of MnO2/CNF//CNF asymmetric supercapacitors. The plot reveals a significant increase in the supercapacitor performance when pairing different electrodes to construct a hybrid supercapacitor. The calculated energy density (normalized to the whole active weight) of our asymmetric supercapacitor is 36 W h kg−1 at a power density of 450 W kg−1, which is higher than or comparable to previously reported similar hybrid supercapacitors. The maximum power density is 4433 W kg−1, comparable to commercially available supercapacitors. This tentative test suggests that these devices possess better energy and power densities, and are very promising candidates for high performance energy storage systems or portable electronic devices.
4. Conclusions
In summary, a novel and durable aqueous asymmetric supercapacitor based on MnO2/CNF pseudocapacitance electrodes and CNF electrical double-layer capacitive materials has been successfully fabricated via a facile scalable method. The newly designed hybrid supercapacitor demonstrated a large potential window of 1.8 V and a high energy density of 36 W h kg−1 (power density, 4433 W kg−1). MnO2-based composites with high electrical conductivity are very attractive for use in energy storage applications because of their facile synthesis, natural abundance, operation in aqueous electrolytes and good electrochemical performance. Together, these encouraging characteristics give our asymmetric supercapacitors great potential for practical applications in future energy storage systems.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21373129).Notes and references
- Y. Hou, Y. W. Cheng, T. Hobson and J. Liu, Nano Lett., 2010, 10, 2727–2733 CrossRef CAS PubMed.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- K. Lu, D. Li, X. Gao, H. Dai, N. Wang and H. Ma, J. Mater. Chem. A, 2015, 3, 16013–16019 CAS.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, J. Phys. Chem. C, 2010, 114, 658–663 CAS.
- J. Mu, B. Chen, Z. Guo, M. Zhang, Z. Zhang, P. Zhang, C. Shao and Y. Liu, Nanoscale, 2011, 3, 5034–5040 RSC.
- T. Cottineau, M. Toupin, T. Delahaye, T. Brousse and D. Belanger, Appl. Phys. A: Mater. Sci. Process., 2006, 82, 599–606 CrossRef CAS.
- Y. Cheng, S. Lu, H. Zhang, C. V. Varanasi and J. Liu, Nano Lett., 2012, 12, 4206–4211 CrossRef CAS PubMed.
- M. Toupin, T. Brousse and D. Bélanger, Chem. Mater., 2004, 16, 3184–3190 CrossRef CAS.
- D. W. Wang, F. Li and H. M. Cheng, J. Power Sources, 2008, 185, 1563–1568 CrossRef CAS.
- C. P. Fonseca, J. E. Benedetti and S. Neves, J. Power Sources, 2006, 158, 789–794 CrossRef CAS.
- H. Wang, Q. Hao, X. Yang, L. Lu and X. Wang, Electrochem. Commun., 2009, 11, 1158–1161 CrossRef CAS.
- Y. Chen, X. Zhang, D. Zhang, P. Yu and Y. Ma, Carbon, 2011, 49, 573–580 CrossRef CAS.
- J. Xiao, S. Yang, L. Wan, F. Xiao and S. Wang, J. Power Sources, 2014, 245, 1027–1034 CrossRef CAS.
- J. Zhu, S. Tang, H. Xie, Y. Dai and X. Meng, ACS Appl. Mater. Interfaces, 2014, 6, 17637–17646 CAS.
- Y. Sun, Z. Fang, C. Wang, K. R. R. M. Ariyawansha, A. Zhou and H. Duan, Nanoscale, 2015, 7, 7790–7801 RSC.
- Y. Sun, Y. Cheng, K. He, A. Zhou and H. Duan, RSC Adv., 2015, 5, 10178–10186 RSC.
- K. Lu, Z. Hu, Z. Xiang, J. Ma, B. Song, J. Zhang and H. Ma, Angew. Chem., Int. Ed., 2016, 55, 10448–10452 CrossRef CAS PubMed.
- X. Du, C. Wang, M. Chen, Y. Jiao and J. Wang, J. Phys. Chem. C, 2009, 113, 2643–2646 CAS.
- C. Yang, J. J. Wu and Y. L. Hou, Chem. Commun., 2011, 47, 5130–5141 RSC.
- J. Liang, Y. Huang, J. Oh, M. Kozlov, D. Sui, S. Fang, R. H. Baughman, Y. Ma and Y. Chen, Adv. Funct. Mater., 2011, 21, 3778–3784 CrossRef CAS.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS PubMed.
- X. Zhao, L. L. Zhang, S. Murali, M. D. Stoller, Q. H. Zhang, Y. W. Zhu and R. R. Ruoff, ACS Nano, 2012, 6, 5404–5412 CrossRef CAS PubMed.
- J. Zhang and X. S. Zhao, J. Phys. Chem. C, 2012, 116, 5420–5426 CAS.
- F. Teng, S. Santhanagopalan and D. D. Meng, Solid State Sci., 2010, 12, 1677–1682 CrossRef CAS.
- K. Mi, Y. Jiang, J. Feng, Y. Qian and S. Xiong, Adv. Funct. Mater., 2016, 26, 1571–1579 CrossRef CAS.
- L. F. Chen, X. D. Zhang, H. W. Liang, M. Kong, Q. F. Guan, P. Chen, Z. Y. Wu and S. H. Yu, ACS Nano, 2012, 6, 7092–7102 CrossRef CAS PubMed.
- H. W. Liang, Q. F. Guan, L. F. Chen, Z. Zhu, W. J. Zhang and S. H. Yu, Angew. Chem., Int. Ed., 2012, 51, 5101–5105 CrossRef CAS PubMed.
- S. Liang, F. Teng, B. Gaugeu, R. Zong and Y. Zhu, J. Phys. Chem. C, 2008, 112, 5307–5315 CAS.
- Y.-C. Hsieh, K.-T. Lee, Y.-P. Lin, N.-L. Wu and S. W. Donne, J. Power Sources, 2008, 177, 660–664 CrossRef CAS.
- V. Subramanian, H. Zhu, R. Vajtai, P. M. Ajayan and B. Wei, J. Phys. Chem. B, 2005, 109, 20207–20214 CrossRef CAS PubMed.
- V. Subramanian, H. Zhu and B. Wei, J. Power Sources, 2006, 159, 361–364 CrossRef CAS.
- K.-W. Nam, M. G. Kim and K.-B. Kim, J. Phys. Chem. C, 2007, 111, 749–758 CAS.
- K. Lu, B. Song, X. Gao, H. Dai, J. Zhang and H. Ma, J. Power Sources, 2016, 303, 347–353 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23195k |
| ‡ C. F. Zhao and K. Lu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |