Intriguingly tuning the fluorescence of AIEgen using responsive polyelectrolyte microspheres†
Xiaolin Guan*a,
Donghai Zhanga,
Tianming Jiaa,
Yang Zhanga,
Li Menga,
Qijun Jina,
Hengchang Maa,
Dedai Lua,
Shoujun Laib and
Ziqiang Lei*a
aKey Laboratory of Eco-Environment-Related Polymer Materials Ministry of Education, Key Laboratory of Polymer Materials Ministry of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu 730070, P. R. China. E-mail: guanxiaolin@nwnu.edu.cn
bSchool of Chemical Engineering, Lanzhou University of Arts and Science, Lanzhou, Gansu 730070, P. R. China
First published on 28th October 2016
Abstract
In this study, we present a practical approach to tune the fluorescence of AIEgen (the luminogens exhibiting AIE attributes) based on counterion-sensitive polyelectrolyte microspheres. The tunable fluorescence is induced by counterion-driven interactions in polyelectrolyte microspheres by a simple exchange of the counterions. The effects of different types of opposite counterions on the fluorescent properties of a new polyelectrolyte tetraphenylethene-graft-poly[2-(methacryloyloxy)-ethyltrimethylammonium chloride] (TPE–PMETAC), which was synthesized by Atom Transfer Radical Polymerization (ATRP) using a TPE derivative containing four arms as an initiator, were systematically investigated. For cationic microspheres with quaternary ammonium groups, the fluorescence intensity progressively increased according to the counterion series Cl− < ClO4− < PF6− < TFSI−, which is in accord with the ability of exchanged hydrophilic Cl− by hydrophobic anions in the order of ClO4− < PF6− < TFSI−. The mechanism of tuning fluorescence was determined by dynamic light scattering (DLS), zeta-potentials and scanning electron microscopy (SEM). We proved that the size of the microspheres and electrostatic repulsive forces between the microspheres were decreased by the addition of counterions due to the hydrophobic-induced collapse of the surfaces of the microspheres. As a result, the obvious increase in the fluorescence of AIEgen was obtained based on the aggregation of microspheres.
Introduction
In recent years, efficient luminescence materials in the aggregate or solid state have been vigorously investigated due to their vast potential applications in the areas of photoelectricity1–3 and biological science.4–9 In general, luminescence is often weakened or quenched in the aggregate state because of the aggregation-caused quenching (ACQ) effect,10 which is harmful for luminescent materials used in the aggregate state, particularly in light-emitting electrochemical cells and organic light-emitting diodes (OLEDs).11 Fortunately, Tang's group discovered a unique phenomenon of aggregation-induced emission (AIE).12 The AIE fluorogen is almost nonemissive or very weakly emissive in solutions but becomes highly fluorescent in the aggregate state. Moreover, research on AIE-active materials has attracted considerable attention for various applications in the field of OLEDs, luminescence probes and bioluminescence imaging.13 Tetraphenylethene (TPE) consisting of a central olefin stator and surrounding four peripheral aromatic rotors (phenyl rings) is a typical AIEgen. The isolated TPE molecules in a dilute solution are almost nonemissive because of the aromatic rotors, through single-bond axes, rotate around the olefinic stator and consume the excited state energy. In the aggregate state, the synergistic effects of the highly twisted molecular conformation and the restricted intramolecular rotation (RIR) caused the AIE phenomenon of TPE.14 Zhu and co-workers15 directly confirmed the RIR hypothesis by decorated phenyl rings in TPE with multiple methyl groups at the ortho-positions. Wang et al.16 reported a novel AIE probe (TPE–triazole–CD) for the specific detection of Cd2+. The coordination complexes are formed with the Cd2+ addition, which caused the ligands of TPE–triazole–CD to have less rotational freedom and emission of the TPE–triazole–CD.Based on the principles of the RIR, AIE polymers have been explored because the molecular rotations are more easily restricted in polymer systems.17–22 Compared with small molecule materials, polymer materials can tune the polymer structure and functionalities.23 Once the unique properties of a polymer are combined with AIE characteristics, an AIE polymer will possess numerous fascinating functionalities and will give a fluorescence response towards an outside stimulus such as temperature, light, chemical analytes, and biological molecules.24 Moreover, amphiphilic AIE polymers can self-assembled to nanoparticles, resulting in stability, enhanced emission and biocompatibility, which give them potential applications for fluorescent sensors and imaging.25 Ma et al.17 successfully synthesized a temperature sensitive AIE polymer (TPE–PNIPAm) with desirable LCST at 37.5 °C, which was used for fluorescent imaging in A549 human lung adenocarcinoma cells and tracing of stained cells for as long as 10 passages. Taniguchi et al.18 constructed AIE elastomers based on TPE–CL and H-terminated PDMS, which was responsive to organic solvents and temperature. An exciting area of research is to develop numerous AIE polymers that combine AIE characteristic with the excellent properties of a polymer.
Due to the many ionic groups in the chain or side chain, the polyelectrolyte has good stability, hydrophilicity and charge in water, which has made it applicable to numerous fields such as lubrication, colloids, biological interactions, adhesives and coatings.26–30 Poly[2-(methacryloyloxy)-ethyltrimethylammonium chloride] (PMETAC), a typical cationic polyelectrolyte, was widely researched because of its response to a variety of outside stimuli such as pH, salt concentration, and counterions.31–37 Its responsive behaviors is caused by the swelling-collapse transition of polymer chains due to the shrinkage of polymer chains from fully stretched conformation to tiled conformation. Huck et al.34 generated a responsive colloidal system based on silica nanoparticles coated with PMETAC brushes, and the perchlorate-induced particle aggregation was confirmed by studying the changes in the polymer chain length, brush conformation and brush density. Wei et al.35 achieved tuning the friction from superior lubrication to ultrahigh friction by responsive polyelectrolyte brushes.
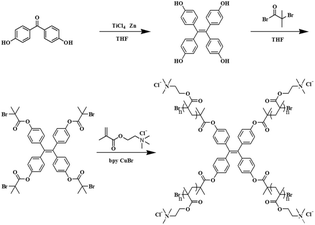
It is uncertain if conformational changes in polyelectrolyte microspheres be translated to fluorescence control. This is important in applications of polyelectrolyte microspheres in order to control their dispersion, aggregation, and assembly. The hydrophobic-induced collapse behavior of PMETAC makes it possible to achieve fluorescence control by combining the excellent properties of PMETAC with AIE characteristics. In this context, we successfully synthesized tetraphenylethene-graft-poly[2-(methacryloyloxy)-ethyltrimethylammonium chloride] (TPE–PMETAC) by ATRP under mild conditions (Scheme 1), which tends to form a core–shell structure of microspheres in an aqueous solution with TPE as the core and PMETAC as the shell. The purpose of this study was to control the shrinkage of the polymer chains, to change the aggregation of microspheres, and to achieve stimuli sensitive AIE properties. We studied the responsive behavior of microspheres using different counterions of perchlorate ion (ClO4−), hexafluorophosphate ion (PF6−) and bis-(trifluoromethane) ion (TFSI−). These ions interacted strongly with the quaternary ammonium moieties of the polymer through ion-pairing interactions that led to the “hydrophobic-induced collapse” of the surfaces of the microspheres. The effects of the counterions on the fluorescence, size and electrostatic exclusion forces of the microspheres were investigated in detail. To the best of our knowledge, this is the first report on tuning the fluorescence of AIEgen using responsive polyelectrolyte microspheres by simply incorporating different counterions. Our results provide a completely new idea of controlling the fluorescence properties of AIEgen by a simple method.
Experimental
Materials and methods
4,4′-Dihydroxybenzophenone (98%), titanium tetrachloride (TiCl4, 99%), zinc (Zn, AR), tetrahydrofuran (THF, AR), 2-bromo-2-methylpropionyl bromide (BIBB, 98%), methacryloxyethyltrimethyl ammonium chloride (METAC, 80% aqueous solution), copper(I) bromide (CuBr, 99%), 2,2-bipyridyl (bpy, 99%), sodium perchlorate (ClO4−, AR), ammonium hexafluorophosphate (PF6−, AR), and bis(trifluoromethanesulfonimide) lithium salt (TFSI−, AR) were purchased from Energy Chemical. Tetrahydrofuran was purified by distillation. Copper(I) bromide was purified by reflux in acetic acid.1H NMR and 13C NMR spectroscopic measurements were performed on a MERCURY spectrometer. MS measurements were carried out in full scan mode in a positive electrospray ionization mass spectrometer. FTIR spectra were obtained by a Nicolet AVATAR 360 FT-IR infrared spectrometer. The GPC measurements were performed by GPCV2000 gel permeation chromatography (Waters, America). The fluorescence measurements were carried out on a F97 Pro fluorometer at room temperature using a monochromatic Xe lamp as the excitation source. The excitation and emission slits were both set at 5 nm. UV-Vis spectroscopic measurements were carried out on a Shimadzu Model 3100 UV-Vis spectrophotometer in the wavelength range from 200 to 800 nm at room temperature. The hydrodynamic particle diameter and the zeta potential of the microspheres were determined by a Zetasizer Nano ZS dynamic light scattering (DLS) system. SEM measurements were performed on a ZEISS ULTRA PLUS scanning electron microscope and the sample was deposited on silicon wafers.
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluent. TPE-OH was obtained as a slight yellow powder of 30% yield (3.1 g). 1H NMR (600 MHz, d-DMSO), δ (ppm): 9.18 (s, 4H), 6.68 (d, J = 6.9 Hz, 8H), 6.45 (d, J = 8.0 Hz, 8H). 13C NMR (151 MHz, d-DMSO), δ (ppm): 155.78, 138.12, 135.49, 132.37, 114.91. ESI-MS calcd for C26H20O4: m/z 396.14. Found m/z 396.09.
1, v/v) as the eluent. TPE-OH was obtained as a slight yellow powder of 30% yield (3.1 g). 1H NMR (600 MHz, d-DMSO), δ (ppm): 9.18 (s, 4H), 6.68 (d, J = 6.9 Hz, 8H), 6.45 (d, J = 8.0 Hz, 8H). 13C NMR (151 MHz, d-DMSO), δ (ppm): 155.78, 138.12, 135.49, 132.37, 114.91. ESI-MS calcd for C26H20O4: m/z 396.14. Found m/z 396.09.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) as the eluent to obtain a white powder of 65% yield (6.5 g). 1H NMR (600 MHz, CDCl3), δ (ppm): 7.05 (d, J = 8.6 Hz, 8H), 6.92 (d, J = 8.7 Hz, 8H), 2.04 (s, 24H). 13C NMR (151 MHz, CDCl3), δ (ppm): 170.00, 149.43, 140.89, 139.64, 132.35, 120.58, 55.32, 30.61. ESI-MS calcd for C42H40Br4O8: m/z 992.38. Found m/z: 1014.93 [M + Na+].
5, v/v) as the eluent to obtain a white powder of 65% yield (6.5 g). 1H NMR (600 MHz, CDCl3), δ (ppm): 7.05 (d, J = 8.6 Hz, 8H), 6.92 (d, J = 8.7 Hz, 8H), 2.04 (s, 24H). 13C NMR (151 MHz, CDCl3), δ (ppm): 170.00, 149.43, 140.89, 139.64, 132.35, 120.58, 55.32, 30.61. ESI-MS calcd for C42H40Br4O8: m/z 992.38. Found m/z: 1014.93 [M + Na+].![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v), after adding 2,2-bipyridyl (0.19 g, 1 mmol), copper(I) bromide (0.048 g, 0.33 mmol) and TPE-BMP (0.05 g, 0.05 mmol) at room temperature under argon with stirring for 6 h. The reaction mixture was diluted with 50 mL ethanol and the resulting white flocculent precipitate was collected by centrifugation and purified by water and ethanol (1
2, v/v), after adding 2,2-bipyridyl (0.19 g, 1 mmol), copper(I) bromide (0.048 g, 0.33 mmol) and TPE-BMP (0.05 g, 0.05 mmol) at room temperature under argon with stirring for 6 h. The reaction mixture was diluted with 50 mL ethanol and the resulting white flocculent precipitate was collected by centrifugation and purified by water and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) in three repeated cycles to give the product (3.2 g). 1H NMR (600 MHz, D2O) δ (ppm): 7.15 (d, Ar–H), 6.85 (d, Ar–H), 4.41 (s, –O–CH2–), 3.73 (s, –CH2–N+), 3.19 (d, J = 10.5 Hz, –N+–CH3), 1.92 (s, –CH2–), 1.30–0.34 (m, –CH3). IR (KBr): 3020 cm−1 (w, Ar–H), 2928 cm−1 (s, –CH2–), 1728 cm−1 (s,–C
3 v/v) in three repeated cycles to give the product (3.2 g). 1H NMR (600 MHz, D2O) δ (ppm): 7.15 (d, Ar–H), 6.85 (d, Ar–H), 4.41 (s, –O–CH2–), 3.73 (s, –CH2–N+), 3.19 (d, J = 10.5 Hz, –N+–CH3), 1.92 (s, –CH2–), 1.30–0.34 (m, –CH3). IR (KBr): 3020 cm−1 (w, Ar–H), 2928 cm−1 (s, –CH2–), 1728 cm−1 (s,–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1634 cm−1 (m,–C
O), 1634 cm−1 (m,–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 1479 cm−1 (m, –CH3), 1267 cm−1 (w, C–N+), 1153 cm−1 (s, –C–O). The number-average molecular weight (Mn) was 2.7 × 104 with a polydispersity index (PDI) of 1.224.
C–), 1479 cm−1 (m, –CH3), 1267 cm−1 (w, C–N+), 1153 cm−1 (s, –C–O). The number-average molecular weight (Mn) was 2.7 × 104 with a polydispersity index (PDI) of 1.224.Results and discussion
Characterization of TPE–PMETAC
Tetraphenylethylene-2-bromo-2-methylpropionate (TPE-BMP) containing four arms was used as the initiator for synthesizing the AIE polymer of TPE–PMETAC by the ATRP method due to its great advantage in the controllable synthesis of polymers.38 The 1H NMR spectrum of TPE–PMETAC in D2O, as shown in Fig. 1, successfully confirmed the structure of the polymer. The peak marked as a indicates the quaternary ammonium group in the PMETAC segments, and peak marked as b and c are ascribed to the methylene groups, which were connected to the nitrogen atom in the quaternary ammonium group. The peak marked as d and e represent methylene and methyl groups in the skeleton of the PMETAC block, respectively. The peak marked as g and f refer to the aromatic protons of the benzyl group. The FTIR spectra of TPE–PMETAC (Fig. S1†) exhibited the characteristic peaks of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration (1728 cm−1), C–H bending vibration (1479 cm−1) and C–N+ stretching band (1267 cm−1). The number-average and weight-average molecular weights of the TPE–PMETAC determined by gel permeation chromatography (GPC) were 2.7 × 104 and 3.3 × 104 with a polydispersity index (PDI) of 1.224 (Fig. S2 and Table S1†). Furthermore, the structures of TPE-OH and TPE-BMP were confirmed by 1H NMR, 13C NMR and mass spectrometry (ESI, Fig. S3–S8†).
O stretching vibration (1728 cm−1), C–H bending vibration (1479 cm−1) and C–N+ stretching band (1267 cm−1). The number-average and weight-average molecular weights of the TPE–PMETAC determined by gel permeation chromatography (GPC) were 2.7 × 104 and 3.3 × 104 with a polydispersity index (PDI) of 1.224 (Fig. S2 and Table S1†). Furthermore, the structures of TPE-OH and TPE-BMP were confirmed by 1H NMR, 13C NMR and mass spectrometry (ESI, Fig. S3–S8†).
AIE properties of TPE–PMETAC
To confirm the AIE property of the TPE–PMETAC, one experiment was planned to change the concentration of the polymer. The fluorescence spectra of TPE–PMETAC at different concentrations are shown in Fig. 2. At low concentrations from 0.005 to 0.1 mg mL−1, there were rather low emission signals being recorded when the dilute aqueous solutions of TPE–PMETAC were excited at 340 nm, and no visible photoluminescence was observed. Moreover, both the absorption intensity at 320 nm and the fluorescence intensity at 430 nm of TPE–PMETAC intensely increased as the concentration increased from 0.1 to 5 mg mL−1 (Fig. 2 and S9a†), and the emission of TPE–PMETAC can be observed with the naked eye at high concentration (Fig. 2b). Therefore, the abovementioned results indicate that the AIE feature of TPE–PMETAC is due to the real collapse and aggregation of polymer chains at high concentrations.Another experiment was performed to add THF into the TPE–PMETAC aqueous solution. THF was chosen because it is a typical nonsolvent for TPE–PMETAC wherein the polymer chains must aggregate in the aqueous mixtures with excess THF. Fluorescence spectra of TPE–PMETAC in the water/THF system are shown in Fig. 3. As show in Fig. 3, the fluorescence intensities of TPE–PMETAC increased in a nonlinear fashion as the fraction of the THF volume increased, which is similar to the aggregation-induced enhanced emission (AIEE).39 Moreover, TPE–PMETAC gave a bright blue emission with maxima at ∼465 nm in the 2/98 water/THF mixture, and the sizes of the TPE–PMETAC microspheres reduced as the fraction of the THF volume increased (Fig. S10†). The experiment result also indicates the AIE feature of emission with maxima at ∼465 nm in the 2/98 water/THF mixture. The experimental result also indicates the AIE feature of the TPE–PMETAC.
Tuning the fluorescence of TPE–PMETAC by counterions
Tuning of the fluorescence of TPE–PMETAC was investigated by adding three counterions, ClO4−, PF6− and TFSI− into 1 mg mL−1 of TPE–PMETAC aqueous solution (Fig. 4). All the fluorescence intensities increased in a nonlinear fashion and the fluorescence emission wavelengths slightly red-shifted as the concentrations of the three counterions increased (Fig. 4a–c). It can be noted that the fluorescence property of the TPE–PMETAC is closely related to the aggregation of microspheres, which mainly resulted from the interaction between the quaternary ammonium groups (QA+) and the counterions. In fact, the QA+–Cl− ion pair of TPE–PMETAC dissociates in water, which caused the electrostatic repulsion force of the positive charges on the polymer chains and the polymer chains showed a fully stretched conformation in an aqueous solution.40 After counterions were added, a collapse transition exclusion of polymer chains was caused by the exchange from hydrophilic Cl− of the surfaces of the microspheres to hydrophobic counterions, such as ClO4−, PF6− and TFSI−, which would lead to the tiled collapsed conformation with the reducing of the electrostatic exclusion force and size of microspheres. As a result, the aggregation of the microspheres was closer once the counterions were added into the system. The schematic of the aggregation mode is described in Fig. 5. The same phenomenon was also found in the changes in the UV-Vis spectra of TPE–PMETAC as the concentrations of the three counterions increased, respectively (Fig. S9†). These results show that the fluorescence emission of TPE–PMETAC in an aqueous solution can be tuned by adding different counterions. The fluorescence intensities of 1 mg mL−1 of the TPE–PMETAC aqueous solution rapidly increased after adding ClO4−, PF6− and TFSI− with the concentrations of 7.5 × 10−3 mol L−1, 5 × 10−3 mol L−1 and 2.5 × 10−3 mol L−1, respectively. Moreover, it is clear that the fluorescence of the TPE–PMETAC aqueous solution increased with the addition of hydrophobic anions of ClO4− < PF6− < TFSI− at the same concentration of 5 × 10−3 mol L−1 (Fig. 4d). This is in agreement with the well-known Hofmeister series41 wherein hydrophilic Cl− is exchanged by hydrophobic anions with ClO4− < PF6− < TFSI−. It's worth noting that white solid would appear once the concentration of TFSI− exceeded 5 × 10−3 mol L−1. So, the highest concentration of TFSI− added into the system was controlled below 5 × 10−3 mol L−1. In comparison to hydrated Cl−, the anions ClO4−, PF6− and TFSI− are barely hydrated, large and highly polarizable species; therefore, they can interact strongly with the quaternary ammonium pendant groups of TPE–PMETAC through ion-pairing interactions and hydration of microspheres. The impact of three counterions was further studied according to the changing of the electrostatic exclusion force and size of microspheres. | ||
| Fig. 5 Schematic of the possible mechanism of aggregation of TPE–PMETAC in pure water with the addition of counterions. | ||
Tuning size of TPE–PMETAC microspheres by counterions
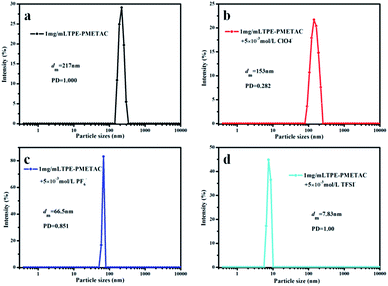
The zeta-potential of TPE–PMETAC indicated the existence of electrostatic exclusion forces between each microsphere. According to the classical Derjaguin–Landau–Verwey–Overbeek (DLVO) theory, repulsive electrostatic forces are one of the factors for the stability of microspheres in polar solutions.42 It is the energy barrier resulting from the electrostatic repulsive forces that prevents microspheres from approaching each other. As the concentration of counterions increased, the zeta-potential of the microspheres reduced close to 0 at a counterion concentration of 10−2 mol L−1 (Fig. 6), which confirmed the electrostatic screening effect by the addition of counterions. It is interesting that the fluorescence and zeta-potential considerably changed with the same concentration of counterions added, which strongly suggests that microsphere aggregation occurs when electrostatic repulsive forces decreased to a critical point when the concentration of counterions increased.On the other hand, the size of microspheres was studied by dynamic light scattering (DLS) and scanning electron microscopy (SEM). When the concentration of counterions increased, the sizes of the microspheres decreased (Fig. S11†). Fig. 7 shows the size of 1 mg mL−1 of TPE–PMETAC in an aqueous solution and after the addition of 5 × 10−3 mol L−1 counterions. When the Cl− anions were exchanged with ClO4−, PF6−, and TFSI−, the sizes of the microspheres reduced from 217 nm to 153 nm and 66.5 nm to 7.93 nm, respectively. The experiments of scanning electron microscopy (SEM) more clearly confirmed that the aggregation of particles occurs with the addition of counterions, as shown in Fig. 8. Compared with the 1 mg mL−1 TPE–PMETAC aqueous solution, the size of the microspheres reduced and the microspheres are closer together in the order of ClO4− < PF6− < TFSI−. Therefore, we could conclude that tuning the fluorescence of TPE–PMETAC by counterions is based on the decreased size of the microspheres and electrostatic repulsive forces between each microsphere.
Conclusions
We developed a method for synthesis of TPE-based counterions-sensitive PMETAC. The obtained microspheres based on TPE–PMETAC respond to counterions by hydrophobic-induced collapse of polymer chains due to the decreasing of the size of microspheres and electrostatic repulsive forces between each microsphere, which caused the aggregation of microspheres. The fluorescence of TPE–PMETAC can be tuned by a simple counterions exchange according to the order of ClO4− < PF6− < TFSI−. The results showed that the fluorescence of this type of AIE-based polymer bearing polyelectrolyte can be tuned by control aggregation of microspheres and they have the potential application as a probe. Moreover, the research about the tunable fluorescence based on counterions would be helpful to further understand the process of AIE.Acknowledgements
Funding was partially provided by the National Natural Science Foundation of China (51363019) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (20136203120002).Notes and references
- Z. Zhao, J. W. Y. Lam and B. Tang, J. Mater. Chem., 2012, 22, 23726–23740 RSC.
- M. Zhu and C. Yang, Chem. Soc. Rev., 2013, 42, 4963–4976 RSC.
- J. Huang, X. Yang, J. Wang, C. Zhong, L. Wang, J. Qin and Z. Li, J. Mater. Chem., 2012, 22, 2478–2484 RSC.
- D. Ding, K. Li, B. Liu and B. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed.
- Z. Wang, T. Yong, J. Wan, Z. Li, H. Zhao, Y. Zhao, L. Gan, X. Yang, H. Xu and C. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 3420–3425 CAS.
- Y. Chen, L. Zhou, Y. Pang, W. Huang, F. Qiu, X. Jiang, X. Zhu, D. Yan and Q. Chen, Bioconjugate Chem., 2011, 22, 1162–1170 CrossRef PubMed.
- T. Kato, A. Kawaguchi, K. Nagata and K. Hatanaka, Biochem. Biophys. Res. Commun., 2010, 394, 200–204 CrossRef CAS PubMed.
- Z. Song, Y. Hong, R. T. K. Kwok, J. W. Y. Lam, B. Liu and B. Tang, J. Mater. Chem. B, 2014, 2, 1717–1723 RSC.
- Z. Zhu, L. Xu, H. Li, X. Zhou, J. Qin and C. Yang, Chem. Commun., 2014, 50, 7060–7062 RSC.
- J. B. Birks, Photophysics of Aromatic Molecules, Wiley, New York, 1970 Search PubMed.
- H. Xiang, J. Cheng, X. Ma, X. Zhou and J. J. Chruma, Chem. Soc. Rev., 2013, 42, 6128–6185 RSC.
- J. Luo, Z. Xie, J. Y. W. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Tang, Chem. Commun., 2001, 18, 1740–1741 RSC.
- X. Ma, R. Sun, J. Cheng, J. Liu, F. Gou, H. Xiang and X. Zhou, J. Chem. Educ., 2016, 93, 345–350 CrossRef CAS.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. Y. W. Lam and B. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- G. Zhang, Z. Chen, M. P. Aldred, Z. Hu, T. Chen, Z. Huang, X. Meng and M. Zhu, Chem. Commun., 2014, 50, 12058–12060 RSC.
- L. Zhang, W. Hu, L. Yu and Y. Wang, Chem. Commun., 2015, 51, 4298–4301 RSC.
- H. Ma, C. Qi, C. Cheng, Z. Yang, H. Cao, Z. Yang, J. Tong, X. Yao and Z. Lei, ACS Appl. Mater. Interfaces, 2016, 8, 8341–8348 CAS.
- R. Taniguchi, T. Yamada, K. Sada and K. Kokado, Macromolecules, 2014, 47, 6382–6388 CrossRef CAS.
- S. Tian, G. Liu, X. Wang, T. Wu, J. Yang, X. Ye, G. Zhang, J. Hu and S. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 3693–3702 CAS.
- M. Tian, J. Wang, E. Zhang, J. Li, C. Duan and F. Yao, Langmuir, 2013, 29, 8076–8085 CrossRef CAS PubMed.
- H. Li, H. Wu, E. Zhao, J. Li, J. Sun, A. Qin and B. Tang, Macromolecules, 2013, 46, 3907–3914 CrossRef CAS.
- Y. He, S. Shi, N. Liu, Y. Ding, J. Yin and Z. Wu, Macromolecules, 2016, 49, 48–58 CrossRef CAS.
- J. Liu, J. W. Y. Lam and B. Tang, Chem. Rev., 2009, 109, 5799–5867 CrossRef CAS PubMed.
- R. Hu, J. W. Y. Lam and B. Tang, Macromol. Chem. Phys., 2013, 214, 175–187 CrossRef CAS.
- R. Hu, N. L. C. Leung and B. Tang, Chem. Soc. Rev., 2014, 43, 4494–4562 RSC.
- E. B. Zhulina and M. Rubinstein, Macromolecules, 2014, 47, 5825–5838 CrossRef CAS PubMed.
- C. Schneider, A. Jusufi, R. Farina, F. Li, P. Pincus, M. Tirrell and M. Ballauff, Langmuir, 2008, 24, 10612–10615 CrossRef CAS PubMed.
- J. M. Y. Carrillo and A. V. Dobrynin, Macromolecules, 2010, 43, 2589–2604 CrossRef CAS.
- S. Kumar, Y. L. Dory, M. Lepage and Y. Zhao, Macromolecules, 2011, 44, 7385–7393 CrossRef CAS.
- L. Zhang and J. Sun, Macromolecules, 2010, 43, 2413–2420 CrossRef CAS.
- H. S. Lim, S. G. Lee, D. H. Lee, D. Y. Lee, S. Lee and K. Cho, Adv. Mater., 2008, 20, 4438–4441 CrossRef CAS.
- K. Y. Tan, J. E. Gautrot and W. T. S. Huck, Langmuir, 2011, 27, 1251–1259 CrossRef CAS PubMed.
- O. Azzaroni, S. Moya, T. Farhan, A. A. Brown and W. T. S. Huck, Macromolecules, 2005, 38, 10192–10199 CrossRef CAS.
- O. Azzaroni, A. A. Brown and W. T. S. Huck, Adv. Mater., 2007, 19, 151–154 CrossRef CAS.
- Q. Wei, M. Cai, F. Zhou and W. Liu, Macromolecules, 2013, 46, 9368–9379 CrossRef CAS.
- R. Zhang, S. Ma, Q. Wei, Q. Ye, B. Yu, J. V. D. Gucht and F. Zhou, Macromolecules, 2015, 48, 6186–6196 CrossRef CAS.
- E. Spruijt, M. A. C. Stuart and J. V. D. Gucht, Macromolecules, 2010, 43, 1543–1550 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS PubMed.
- H. Deng, R. Hu, E. Zhao, C. Y. K. Chan, J. Y. W. Lam and B. Tang, Macromolecules, 2014, 47, 4920–4929 CrossRef CAS.
- T. Farhan, O. Azzaroni and W. T. S. Huck, Soft Matter, 2005, 1, 66–68 RSC.
- W. Kunz, J. Henle and B. W. Ninham, Curr. Opin. Colloid Interface Sci., 2004, 9, 19–37 CrossRef CAS.
- G. M. Dougherty, K. A. Rose, J. B. H. Tok, S. S. Pannu, F. Y. S. Chuang, M. Y. Sha, G. Chakarova and S. G. Penn, Electrophoresis, 2008, 29, 1131–1139 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: GPC, FTIR, 1H NMR, 13C NMR, UV-Vis and DLS data. See DOI: 10.1039/c6ra23380e |
| This journal is © The Royal Society of Chemistry 2016 |