Superhydrophobic fibers from cigarette filters for oil spill cleanup†
Junfei Ou*ab,
Beibei Wanab,
Fajun Wangab,
Mingshan Xueab,
Huiming Wuc and
Wen Liab
aSchool of Materials Science and Engineering, Nanchang Hangkong University, Nanchang 330063, P. R. China. E-mail: oujunfei_1982@163.com; Fax: +86-971-6453210; Tel: +86-971-6453210
bKey Laboratory for Microstructural Control of Metallic Materials of Jiangxi Province, Nanchang Hangkong University, Nanchang 330063, P. R. China
cCollege of Chemistry & Chemical Engineering, Hubei University, Wuhan 430062, P. R. China
First published on 26th April 2016
Abstract
Cigarette butts make up the largest contribution to solid waste worldwide. A promising way to control the environmental impacts of cigarette butts is to convert the filter wastes to desired products. Herein, by immersing cigarette filters sequentially into an aqueous solution of NaOH and an ethanolic solution of hexadecyltrimethoxysilane, the filters became converted into superhydrophobic/superoleophilic fibers, with measured water and kerosene contact angles of 156.9° ± 3.1° and approximately 0°, respectively. Due to this ability to exclude water and absorb kerosene, the so-obtained fibers were used to clean up a staged spill of kerosene on the surface of water. The cleanup efficiency of these fibers was measured to be as high as approximately 96%, and did not decrease after 10 cycles of use. The moisture content in the kerosene collected from the surface of the water was unexpectedly lower than the moisture content in the kerosene before it was poured onto the water surface.
1. Introduction
Cigarette butts constitute the most common type of solid waste worldwide. It is estimated that as many as 4.5 trillion cigarette butts are discarded into the environment every year.1,2 The primary environmental impacts of the discarded cigarette butts are the non-biodegradability of the filters and the toxicity of the extract.3 Most cigarette filters are made of cellulose acetate, which is not biodegradable.3–5 The non-biodegradability of the filters increases landfill demands, adds costs to waste-disposal programs, and creates environmental blight in public spaces. Moreover, a cigarette butt soaked in a liter of water for four days has been reported to kill both the topsmelt and the freshwater fathead minnow fish species. Given the toxicity of just one butt, a few trillion of them can potentially do a lot of environmental damage.6–10A promising way to control the environmental impacts of cigarette butts is to convert the filter wastes to desired products. For example, in the research by Zhao et al., the used cigarette butts were applied as corrosion inhibitors for N80 steel at 90 °C in hydrochloric acid. It was found that the cigarette-derived cocktail could reduce the corrosion of N80 steel by between 90 and 94 percent.11 In the research by Yi et al., the used cigarette filters were transformed into a high-performing carbon-based material using a simple one-step process, which at the same time offered a green solution to meeting the energy demands of society.12 Herein, we aim to convert used cigarette filters to superhydrophobic fibers (SFs), which can be used for cleanup of oil spills on water. Pollution from oil spills is another environmental disaster, and is increasing in frequency with the increasing levels of oil production and transport.13–15 Since Jiang et al. reported separating water and oil by using a Teflon-coated mesh film,16 there have been many studies on separating water and oil and on cleaning up oil spills by using superhydrophobic materials, including zero-dimensional particles, one-dimensional fibers, two-dimensional meshes, and three-dimensional sponges.17–21 Compared with the traditional technologies for oil spill cleanup, such as oil containment booms, skimmers, dispersants, bioremediation, and in situ burning,22–24 the technologies based on recyclable superhydrophobic materials do not cause secondary pollution.
To fabricate SFs from cigarette filters, the acetate fibers from used cigarette filters were roughened by carrying out a hydrolysis of the butts in an aqueous NaOH solution and subsequent passivation by hexadecyltrimethoxysilane (schematically shown in Fig. 1a). The so-obtained SFs absorbed kerosene and excluded water, and were found to be superhydrophobic and superoleophilic. Taking advantage of these properties of the SFs, a cylinder with tiny holes was filled with the SFs and used for collecting kerosene “spilled” on water (Fig. S1†). The collection process was observed to be continuous and the collection rate was high (above 96%). Moreover, compared with the raw kerosene poured onto the water, the moisture content of the collected kerosene was found to be even lower. With these features, such SFs made from cigarette filters may help alleviate the adverse effects of cigarette butts on the environment and at the same time provide a new method for cleaning up oil spills.
 | ||
| Fig. 1 Schematic illustration of the preparation of the superhydrophobic fibers (a) and the mechanism for step (i) (b) as well as step (ii) (c). | ||
2. Experimental section
2.1 Fabrication of SFs from cigarette filters
Cigarette filters collected from used Baisha cigarette butts (Hunan Tobacco Industry Co., Ltd, China) were torn into tiny bundles and immersed into 25 mL ethanol to remove the adsorbates under ultrasonication for 1 h. Then, they were immersed into an aqueous solution of NaOH (1 mol L−1) for t min (t = 5, 10) at room temperature, followed by successive ultrasonication in ultrapure water and ethanol for 10 min each. Finally, the cigarette filters were immersed into the ethanolic solution of hexadecyltrimethoxysilane (HDTMS, obtained from Aladdin, 85%, with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) for 3 h, followed by heating at 110 °C for 0.5 h. For convenience, the acetate fibers (AF) hydrolyzed in NaOH for t min were denoted as AF-NaOH(t); after further passivation by HDTMS, the samples were denoted as AF-NaOH(t)-HDTMS. A control sample denoted as AF-HDTMS was also fabricated according to the above-mentioned procedures but omitting the hydrolysis in NaOH. Ultrapure water with a resistivity above 18.0 MΩ cm was used.
1000) for 3 h, followed by heating at 110 °C for 0.5 h. For convenience, the acetate fibers (AF) hydrolyzed in NaOH for t min were denoted as AF-NaOH(t); after further passivation by HDTMS, the samples were denoted as AF-NaOH(t)-HDTMS. A control sample denoted as AF-HDTMS was also fabricated according to the above-mentioned procedures but omitting the hydrolysis in NaOH. Ultrapure water with a resistivity above 18.0 MΩ cm was used.
2.2 Characterizations
An optical contact angle meter (Easydrop, Krüss, Germany) with a computer-controlled liquid dispensing system and a motorized tilting stage was used to measure the contact angle at room temperature (25 °C) with ultrapure water (7 μL) and kerosene (7 μL) as probe liquids. The average contact angle values were obtained by measuring the sample at five different positions of the substrate. The surface morphologies were observed by using a field emission scanning electron microscope (FE-SEM, Nova NanoSEM, FEI, USA) under a vacuum environment, and the samples were sputter-coated with a thin gold film prior to FE-SEM observation. The surface chemistry of the samples was analyzed by using an X-ray photoelectron spectrometer (XPS, Physical Electronics, PHI-5702, USA). The measurements were taken using monochromated Al-Kα irradiation and the chamber pressure was about 3 × 10−8 Torr under the test conditions. The binding energy of adventitious carbon (C 1s: 284.8 eV) was used as a basic reference.The set up for the oil spill cleanup is shown in Fig. S1.† Specifically, a cylinder with tiny holes was filled with the so-obtained superhydrophobic fibers, and was then connected to a Büchne flask with a soft pipe and with a pump. The moisture content in the collected oil (20 mL) was measured using an oil moisture meter (GY106, Wuhan Guoyi Company, China).
3. Results and discussion
3.1. Fabrication and characterization of the SFs
It is well known that surface superhydrophobicity results from rough surface structures and low surface energy. To obtain the proper rough surface structures, the cigarette butt fibers were immersed into an aqueous solution of NaOH. In such an alkaline solution, ester groups are expected to be converted to hydroxyl groups (C–OH), which would facilitate the subsequent surface passivation by HDTMS (Fig. 1c).To verify the hydrolysis and subsequent surface passivation, the fibers were characterized by XPS (Fig. 2). For the pristine and hydrolyzed fibers, C 1s and O 1s peaks appeared in the survey spectrum. The C 1s core level spectrum for the pristine fiber showed three main components with binding energy levels at 289.0 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 286.6 eV (C–O), and 284.8 eV (C–C/C–H). After being hydrolyzed in aqueous NaOH for 10 min, the peak at 289.0 eV decreased considerably due to deacetylation. Moreover, due to the deacetylation, the amount of –CH3 decreased and the peak at 284.8 eV (C–C/C–H) weakened. After being passivated by HDTMS, new peaks originating from the element Si emerged in the survey spectrum (Fig. 2a). Due to the long carbon chain in HDTMS, the peak at 284.8 eV (C–C/C–H) in the C 1s core level spectrum of the superhydrophobic fibers became much stronger. The fibers were also characterized by FT-IR, and the results are shown in Fig. 3. An obvious difference between these FT-IR curves was the difference in transmittance for the C–H stretching vibrations (νC–H). Specifically, after being hydrolyzed by NaOH, the intensities of the νC–H peaks decreased due to the deacetylation (Fig. 3b). After being passivated by HDTMS, the intensities of the νC–H peaks then increased due to the abundance of C–H bonds in the HDTMS molecules. Moreover, after being passivated by HDTMS, new peaks at 1067 cm−1 and 1026 cm−1 emerged (Fig. 3c). The appearance of these new peaks was probably due to the formation of Si–O–C bonds between the hydrolyzed fiber and the HDTMS. These analyses demonstrate that the fibers were hydrolyzed and passivated by HDTMS successfully.
O), 286.6 eV (C–O), and 284.8 eV (C–C/C–H). After being hydrolyzed in aqueous NaOH for 10 min, the peak at 289.0 eV decreased considerably due to deacetylation. Moreover, due to the deacetylation, the amount of –CH3 decreased and the peak at 284.8 eV (C–C/C–H) weakened. After being passivated by HDTMS, new peaks originating from the element Si emerged in the survey spectrum (Fig. 2a). Due to the long carbon chain in HDTMS, the peak at 284.8 eV (C–C/C–H) in the C 1s core level spectrum of the superhydrophobic fibers became much stronger. The fibers were also characterized by FT-IR, and the results are shown in Fig. 3. An obvious difference between these FT-IR curves was the difference in transmittance for the C–H stretching vibrations (νC–H). Specifically, after being hydrolyzed by NaOH, the intensities of the νC–H peaks decreased due to the deacetylation (Fig. 3b). After being passivated by HDTMS, the intensities of the νC–H peaks then increased due to the abundance of C–H bonds in the HDTMS molecules. Moreover, after being passivated by HDTMS, new peaks at 1067 cm−1 and 1026 cm−1 emerged (Fig. 3c). The appearance of these new peaks was probably due to the formation of Si–O–C bonds between the hydrolyzed fiber and the HDTMS. These analyses demonstrate that the fibers were hydrolyzed and passivated by HDTMS successfully.
 | ||
| Fig. 2 Survey (a) and C1s (b) X-ray photoelectron spectra of various samples. For the hydrolyzed fiber and the superhydrophobic fiber, the hydrolyzing time in the NaOH aqueous solution was 10 min. | ||
The surface microstructures of the fibers were characterized by using FE-SEM. The cigarette butt was observed to be composed of smooth and intertwined fibers with an average diameter of about 30 μm (Fig. 4a and b). After hydrolysis for 5 min, microwrinkles and microburrs were observed on the surface of the fibers (Fig. 4c and d). When the hydrolysis time was increased to 10 min, more microburrs were generated and the microwrinkles became more obvious (Fig. 4e and f). In other words, as the hydrolysis time was increased, the fibers become rougher. The roughening of the fiber was due to its having lost weight as a result of being deacetylated. A similar example was reported by Yamashita et al.,25 who showed that a cellulose acetate film immersed in 0.5 M aqueous NaOH for 24 h lost its original shape due to deacetylation, with a resulting weight loss of 40–50%. In our current work, the solid (fiber)/liquid (NaOH solution) interface was expected to be much larger than the one in the reported work by Yamashita et al.25 due to the cellulose acetate being in the fiber form. In such cases, the deacetylation and decomposition would be expected to be much faster. When immersed in the NaOH solution for 60 min, the fiber shape was obviously broken (Fig. S2†). While such deacetylation on the molecular scale is the simple displacement of ester groups with hydroxyl groups, the deacetylation on the macroscopic scale would cause decomposition and weight loss.
 | ||
| Fig. 4 Surface microstructures for the pristine fiber (a and b) and the hydrolyzed fiber (c–f). For (c) and (d), the hydrolyzing time was 5 min; for (e) and (f), the hydrolyzing time was 10 min. | ||
After being treated by the above-described multi-step process, the surface wettability of the so-obtained fibers was characterized. These fibers were made to adhere to a glass substrate, and then water and kerosene as probe liquids were dripped onto the fibers and the water contact angles (WCAs) were measured (Table 1). Acetate fibers, and hence the AF, AF-NaOH(5), and AF-NaOH(10) samples, have many polar side groups such as acetyl and hydroxyl groups, and the intrinsic WCAs (θi in eqn (1)) of these samples were expected to be lower than 90°.26 Wenzel found that the apparent WCA (θa in eqn (1)) decreases as surface roughness factor (r in eqn (1)) is increased.27
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θa = r θa = r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θi θi
| (1) |
Thus, due to the hydrophilic nature and the intertwined rough surface structures, the so-dripped water droplets were absorbed quickly due to the capillary effect, and the apparent WCA was approx. 0°.28 Once modified by HDTMS molecules, the outermost groups became –CH3 groups, which are non-polar and have low surface energy. In this case, the intrinsic WCA (θi in eqn (1)) exceeded 90°; so the AF-NaOH-HDTMS samples became (super)hydrophobic. The WCA for the AF-NaOH-HDTMS sample depended greatly on the hydrolyzing time in the aqueous NaOH solution. Specifically, as the hydrolyzing time was increased from 5 to 10 min, the fibers became rougher (Fig. 4, as discussed earlier), and the WCA increased from 148.6 ± 2.8° to 156.9 ± 3.1°. For all these samples, even the AF-NaOH(10)-HDTMS SFs, the kerosene spread out quickly once dripped on them.
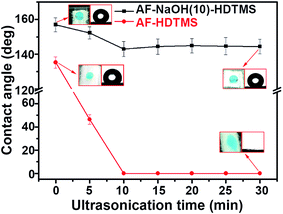
The durability of the SFs was simply estimated by carrying out an ultrasonication treatment. Specifically, the SFs and the control AF-HDTMS sample were ultrasonically cleaned in ethanol for a certain period of time and subsequently dried at 60 °C for 3 h. The WCAs for these fibers were measured (Fig. 5). After ultrasonication for 30 min, the WCAs for the SFs were still as high as 144.4 ± 3.9°. However, the WCA for the control AF-HDTMS sample, after ultrasonication for 10 min, decreased sharply from 135.3° ± 4.1° to 0°. We ascribed this obvious difference in durability to the different interfacial bonding. Specifically, for the SFs, hydroxyl groups were generated on the hydrolyzed fibers, which served as active sites for inducing the anchoring of HDTMS (Fig. 1c). For the control sample, the pristine fibers were expected to be wrapped by HDTMS via physisorption since no active groups such as hydroxyls were present on their surface.
3.2. Oil spill cleanup
Due to their special wetting behaviors towards water and kerosene, viz., absorbing kerosene and excluding water, the SFs were used for oil spill cleanup experiments. The installation and process for such an oil spill cleanup are shown in Fig. S1.† Specifically, a cylinder with tiny holes was filled with the SFs (Fig. S1-a†), and was placed on the surface of the water and connected to a Büchne flask with a soft pipe (Fig. S1-b†). Kerosene (20 mL) dyed by Sudan black that was “spilled” on the water became absorbed and was pumped to the flask slowly. The test was repeated five times and it was found that, in 182 ± 34 s, the kerosene was cleaned up, and kerosene residues floating on the water were difficult to see (Fig. S1-c†). The cleanup efficiency (k) for kerosene was calculated by applying the equation| k = vc/v0 |
4. Conclusions
In summary, cellulose acetate fibers from cigarette butts were converted into superhydrophobic/superoleophilic fibers by hydrolyzing the cigarette butts in an aqueous solution of NaOH and subsequently passivating them in an ethanolic solution of hexadecyltrimethoxysilane. Due to their special wetting behaviors towards water and kerosene, viz., absorbing kerosene and excluding water, the so-obtained fibers (SFs) were used for oil spill cleanup experiments. The cleanup efficiency for a staged kerosene spill was above 96% for 10 cycles of SF use, and the moisture content in the collected kerosene was even lower than that in the raw kerosene poured onto the water. Due to these features of the SFs in oil spill cleanup (viz., the high cleanup efficiency, the low moisture content in the collected kerosene, and the reusability), the SFs may provide a potential way to alleviate the adverse effects of cigarette butts and clean up oil spills simultaneously.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51563018), the xxx Basic Scientific Research Plan (A0520110xxx).References
- A. L. Roder Green, A. Putschew and T. Nehls, Littered cigarette butts as a source of nicotine in urban waters, J. Hydrol., 2014, 519, 3466–3474 CrossRef CAS.
- D. J. Bootha, P. Gribbenb and K. Parkinsona, Impact of cigarette butt leachate on tidepool snails, Mar. Pollut. Bull., 2015, 95, 362–364 CrossRef PubMed.
- W. Lee and C. C. Lee, Developmental toxicity of cigarette butts – An underdeveloped issue, Ecotoxicol. Environ. Saf., 2015, 113, 362–368 CrossRef CAS PubMed.
- S. Polarz, B. Smarsly and J. H. Schattka, Hierachical porous carbon structures from cellulose acetate fibers, Chem. Mater., 2002, 14, 2940–2945 CrossRef CAS.
- S. M. Soltani, S. K. Yazdi, S. Hosseini and M. K. Gargari, Effect of nitric acid modification on porous characteristics of mesoporous char synthesized from the pyrolysis of used cigarette filters, J. Environ. Chem. Eng., 2014, 2, 1301–1308 CrossRef CAS.
- J. Moerman and G. Potts, Analysis of metals leached from smoked cigarette litter, Tobac Contr, 2011, 20, 30–35 CrossRef PubMed.
- E. T. Novotny, S. N. Hardin, L. R. Hovda, D. J. Novotny, M. K. McLean and S. Shan, Tobacco and cigarette butt consumption in humans and animals, Tobac Contr, 2011, 20, 17–20 CrossRef PubMed.
- Y. S. Kazemi, S. S. Masoudi and S. Hossein, An investigation into the optimum carbonization conditions for the production of porous carbon from a solid waste, Adv. Mater. Res., 2012, 587, 88–92 CrossRef.
- M. Czayka and M. Fisch, Effects of electron beam irradiation of cellulose acetate cigarette filters, Radiat. Phys. Chem., 2012, 81, 874–878 CrossRef CAS.
- H. J. Shin, H. O. Sohn, J. H. Han, C. H. Park, H. S. Lee, D. W. Lee, K. J. Hwang and H. C. Hyun, Effect of cigarette filters on the chemical composition and in vitro biological activity of cigarette mainstream smoke, Food Chem. Toxicol., 2009, 47, 192–197 CrossRef CAS PubMed.
- J. Zhao, N. S. Zhang, C. T. Qu, X. M. Wu, J. T. Zhang and X. Zhang, Cigarette Butts and Their Application in Corrosion Inhibition for N80 Steel at 90 °C in a Hydrochloric Acid Solution, Ind. Eng. Chem. Res., 2010, 49, 3986–3991 CrossRef CAS.
- M. Lee, G. Kim, H. D. Song, S. Park and J. Yi, Preparation of energy storage material derived from a used cigarette filter for a supercapacitor electrode, Nanotechnology, 2014, 25, 345–601 Search PubMed.
- M. Lee and J. Y. Jung, Pollution risk assessment of oil spill accidents in Garorim Bay of Korea, Mar. Pollut. Bull., 2015, 100, 297–303 CrossRef CAS PubMed.
- J. Li, L. Yan, H. Y. Li, W. J. Li, F. Zha and Z. Q. Lei, Underwater superoleophobic palygorskite coated mesh for the efficient oil/water separation, J. Mater. Chem. A, 2015, 3, 14696–14702 CAS.
- D. D. Lan, B. Liang, C. G. Bao, M. H. Ma, Y. Xu and C. Y. Yu, Marine oil spill risk mapping for accidental pollution and its application in a coastal city, Mar. Pollut. Bull., 2015, 96, 220–225 CrossRef CAS PubMed.
- L. Feng, Z. G. Zhang, Z. H. Mai, Y. M. Ma, B. Q. Liu, L. Jiang and D. B. Zhu, A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water, Angew. Chem., Int. Ed., 2004, 43, 2012–2014 CrossRef CAS PubMed.
- Z. P. Zhou and X. F. Wu, Electrospinning superhydrophobic–superoleophilic fibrous PVDF membranes for high-efficiency water–oil separation, Mater. Lett., 2015, 160, 423–427 CrossRef CAS.
- Y. X. Jin, P. Jiang, Q. P. Ke, F. H. Cheng, Y. Zhu and Y. X. Zhang, Superhydrophobic and superoleophilic polydimethylsiloxane-coated cotton for oil–water separation process: An evidence of the relationship between its loading capacity and oil absorption ability, J. Hazard. Mater., 2015, 300, 175–181 CrossRef CAS PubMed.
- B. Ge, X. T. Zhu, Y. Li, X. H. Men, P. L. Li and Z. Z. Zhang, Versatile fabrication of magnetic superhydrophobic foams and application for oil–water separation, Colloids Surf., A, 2015, 482, 687–692 CrossRef CAS.
- F. Liu, M. L. Ma, D. L. Zang, Z. X. Gao and C. Y. Wang, Fabrication of superhydrophobic/superoleophilic cotton for application in the field of water/oil separation, Carbohydr. Polym., 2014, 103, 480–487 CrossRef CAS PubMed.
- D. L. Zang, F. Liu, M. Zhang, X. G. Niu, Z. X. Gao and C. Y. Wang, Superhydrophobic coating on fiberglass cloth for selective removal of oil from water, Chem. Eng. J., 2015, 262, 210–216 CrossRef CAS.
- J. Saththasivam, K. Loganathan and S. Sarp, An overview of oilewater separation using gas flotation systems, Chemosphere, 2016, 144, 671–680 CrossRef CAS PubMed.
- M. Seddighi and S. M. Hejazi, Water–oil separation performance of technical textiles used for marine pollution disasters, Mar. Pollut. Bull., 2015, 96, 286–293 CrossRef CAS PubMed.
- C. Liu, B. B. Chen, J. Yang and C. S. Li, One-step fabrication of superhydrophobic and superoleophilic cigarette filters for oil–water separation, J. Adhes. Sci. Technol., 2015, 29, 2399–2407 CrossRef CAS.
- Y. Yamashita and T. Endo, Deterioration behavior of cellulose acetate films in acidic or basic aqueous solutions, J. Appl. Polym. Sci., 2004, 91, 3354–3361 CrossRef CAS.
- T. Mohan, R. Kargl, K. E. Tradt, M. R. Kulterer, M. Braćić, S. Hribernik, K. Stana-Kleinschek and V. Ribitsch, Antifouling coating of cellulose acetate thin films with polysaccharide multilayers, Carbohydr. Polym., 2015, 116, 149–158 CrossRef CAS PubMed.
- R. N. Wenzel, Resistance of solid surfaces to wetting by water, Ind. Eng. Chem., 1936, 28, 988–994 CrossRef CAS.
- M. Y. Li, T. T. Deng, S. X. Liu, F. X. Zhang and G. X. Zhang, Superhydrophilic surface modification of fabric via coating with nano-TiO2 by UV and alkaline treatment, Appl. Surf. Sci., 2014, 297, 147–152 CrossRef CAS.
- J. Li, L. Yan, X. H. Tang, H. Feng, D. C. Hu and F. Zha, Robust superhydrophobic fabric bag filled with PU sponges used for vacuum-assisted continuous and ultrafast absorption and collection of oils from water, Adv. Mater. Interfaces, 2016, 3, 1500770 Search PubMed.
- K. Rohrbach, Y. Y. Li, H. L. Zhu, Z. Liu, J. Q. Dai, J. Andreasen and L. B. Hu, A cellulose based hydrophilic, oleophobic hydrated filter for water/oil separation, Chem. Commun., 2014, 50, 13296–13299 RSC.
- D. L. Zang, F. Liu, M. Zhang, X. G. Niu, Z. G. Gao and C. Y. Wang, Superhydrophobic coating on fiberglass cloth for selective removal of oil from water, Chem. Eng. J., 2015, 262, 210–216 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01303a |
| This journal is © The Royal Society of Chemistry 2016 |