The gelation process and protein absorption property of injectable SA-CMBC hydrogel used for procoagulant material
Qun Yu,
Yudong Zheng*,
Ning Yan,
Yajie Xie,
Kun Qiao and
Rui Jin
School of Materials Science and Engineering, University of Science and Technology, Beijing, Beijing 100083, PR China. E-mail: zhengyudong@mater.ustb.edu.cn; Fax: +86-010-62332336; Tel: +86-010-62330802,
First published on 24th November 2015
Abstract
Carboxymethylated bacterial cellulose (CMBC) was composited with sodium alginate (SA) to obtain SA-CMBC hydrogel, cross-linked by calcium ion, which was generated from the hydrolyzing of calcium carbonate with the addition of glucono-delta-lactone (GDL). The addition of CMBC could regulate the properties of SA-CMBC hydrogel, such as gelation time, mechanical property, protein absorption and procoagulant property. The addition of CMBC shortened the gelation time, the shortest of which reached to 4.9 min, and increased the mechanical property, the best of which reached to 0.118 MPa. The hydrogel with 25% weight fraction of CMBC showed the best protein absorption property. The procoagulant activity of the SA-CMBC hydrogel was investigated by activated partial thromboplastin time (APTT) and prothrombin time (PT), and hydrogel with 20% weight fraction of CMBC showed the best procoagulant property.
Introduction
Injectable hydrogel has attracted more and more attention in recent years. Hydrogel can be injected into specific positions with minimal trauma; drugs, bioactive molecules and cells or stem cells can be delivered to human body by simply mixing with hydrogel before injection. Hydrogel could conform into specific shapes in correspondence with the irregular positions where it was injected. Due to the advantages mentioned above, different kinds of injectable hydrogels could be used in various medical fields including drug controlled release,1,2 tissue engineering scaffold,3 tissue repair4,5 and intraoperative hemostasis.6 In addition, by controlling the gelation process and other properties of hydrogel, the injectable hydrogel could be used in wound dressing, tissue repair and intraoperative hemostasis fields.Sodium alginate (SA), a well-known natural polysaccharide that carries a negative charge, has several favourable properties, including biocompatibility, non-toxicity and ease of gelation.7 Due to these properties, SA has numerous applications in biomedical fields such as wound dressing,8,9 cell encapsulation,10,11 drug delivery12,13 and tissue engineering.14 However, its applications are limited by the low mechanical performance and uncontrolled crosslinking speed of pure alginate hydrogel. To solve this problem, different components, such as chitosan,15 hyaluronate,16 calcium silicate,17 bioactive glass,18 have been composited with SA to improve its performance.
Bacterial cellulose (BC), a pure cellulose nanofiber produced by bacteria, is remarkable for its high mechanical strength and significant physical properties. Its high water content and purity enable the material biocompatible for multiple medical applications.19 Furthermore, research on different derivatives of BC, such as acetylation,20 sulfation,21 phosphorylation22 and succinylation,23 has been directed toward special applications in the field of biomaterials. Carboxymethylated bacterial cellulose (CMBC) is a derivative of cellulose, which is obtained by reacting cellulose with sodium monochloroacetate in the presence of NaOH. CMBC has a wide range of applications, such as sewage purification,24 drug release,25 wound dressing,26 etc. Referring to the previous study in our laboratory, we have confirmed the protein absorption property of CMBC membrane.27 Our group has also composited CMBC with SA and discussed the basic performances of the hydrogel preliminarily, like chemical structure, swelling property and mechanical property,28 whereas there is still no research reported about the potential biomedical applications of this composite hydrogel, such as its use in protein absorption and procoagulant property.
In this study, we focused on exploring the possibilities of SA-CMBC hydrogel as an injectable protein absorption and procoagulant material, and we focused specifically on the influence of CMBC on the properties of injectable hydrogel. The hydrogel was injected before its crosslinking, at which time the fluidity of the hydrogel was suitable for injection. CMBC, acting as a new crosslinking agent, could adjust the gelation process, mechanical property and other properties of hydrogels. The components of the system were varied so that the gelation process of hydrogel could be adjusted to satisfy its injectable property. The protein absorption of hydrogel was investigated using ultraviolet spectrophotometry, Fourier Transform Infrared Spectroscopy (FTIR) and scanning electron microscopy (SEM). Furthermore, the procoagulant activity of the SA-CMBC hydrogel was investigated by classical coagulation assays, activated partial thromboplastin time (APTT) and prothrombin time (PT).
Materials and methods
Materials
The BC used in this study was supplied by the Hainan Yida Food Co, Ltd. BC was a gel-like cellulose membrane formed by A. xylinum AGR 60. As a pre-treatment to remove the bacterial cell debris, BC membranes were immersed in 0.1 mol L−1 sodium hydroxide solution for 60 min in a water bath at 90 °C, and then thoroughly washed to neutral by de-ionized water. The bovine serum albumin (BSA) for the adsorption experiments was used as received from Wako Pure Chemical Industries, Osaka, Japan. APTT and PT assay reagents were provided by Tianjin MD Pacific Technology Co, Ltd. The rats supplying the blood were bought from the Laboratory Animal Center of Chinese PLA General Hospital. All other reagents were reagent grade and used as received.Preparation of the SA/CMBC hydrogel
CMBC was prepared from BC membranes according to our recent report.28 After pre-treatment, BC membranes were broken up into fine fibers using a high-speed disperser. BC fibers were soaked in 10% sodium hydroxide solution, in which the BC/NaOH mass ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and the proportion of water and ethanol was 3
9 and the proportion of water and ethanol was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The BC fibers were then stirred at low speed at room temperature for 1 hour. Sodium chloroacetate (the molar weight of which was equal to NaOH) was then dissolved in a suitable amount of deionized water, and the solution was added to the mixture and stored in a water bath at 90 °C for 4 hours. Finally, the entire mixture was centrifuged and neutralized with 10% HCl, and then thoroughly washed with deionized water to generate CMBC.
4. The BC fibers were then stirred at low speed at room temperature for 1 hour. Sodium chloroacetate (the molar weight of which was equal to NaOH) was then dissolved in a suitable amount of deionized water, and the solution was added to the mixture and stored in a water bath at 90 °C for 4 hours. Finally, the entire mixture was centrifuged and neutralized with 10% HCl, and then thoroughly washed with deionized water to generate CMBC.
Sodium alginate was slowly added to deionized water to get a ratio of 2% (w/v) and the mixture was stirred using a magnetic stirrer. After complete dissolution of alginate, CMBC was added (using different weight fractions of CMBC from 10% to 30%) and stirring of the samples was continued. Then CaCO3 (using various f-value, molar ratios of calcium ion and carboxyl) and glucono-delta-lactone (GDL, using various n-value, molar ratios of GDL and calcium ion) were added and stirred until evenly mixed. The solutions were allowed to stand for 2 hours to allow it to fully form a gel. The samples with different weight fraction of CMBC were named SA-CMBC10, SA-CMBC15, SA-CMBC20, SA-CMBC25 and SA-CMBC30, respectively.
Physical properties
To measure the gelation time, 5 mL sol sample was put into the test tube and sloped every 30 seconds until the sample stopped flowing. The gelation time was then recorded.The hydrogel samples were kept still for 24 hours to fully gel. Then the compressive tests were performed by using a TA.XT Plus Texture Analyzer, equipped with two flat-surface compression stages. Before this test, the samples were cut into cuboids of 2 cm length, 2 cm width and 1 cm height, and the sample size was measured using a standard caliper. The strain ramp rate was maintained at 1 mm per second for all of the tests.
Chemical structure information was recorded by FTIR (NICOLET 750) with the frequency ranging from 4000 cm−1 to 450 cm−1 and the resolution of 4 cm−1. Before test, the hydrogels were freeze-dried using a freeze dryer (Labconco Corporation, USA).
Adsorption of BSA on the hydrogel
0.2 g BSA was dissolved in 100 mL PBS (pH = 3) solution in order to generate 2 mg mL−1 BSA solution. The samples, which were cut into cuboids of 2 cm length, 2 cm width and 1 cm height, were immersed in BSA solution and placed in a constant temperature humidity chamber of 37 °C and 60% humidity. At regular intervals, a certain volume of BSA solution was taken out and the ultraviolet absorption peak at 276 nm was measured. The standard curve of BSA with different concentrations was established in advance.The hydrogels before and after protein absorption were freeze-dried and were tested by FTIR, respectively. The morphologies and structures of hydrogels before and after protein absorption were examined by SEM (Apollo 300, 10 kV). The samples were freeze-dried and coated with gold in a sputter coater under nitrogen atmosphere.
Procoagulant properties
The coagulant activity of the SA-CMBC hydrogel was investigated by the classical coagulation assays APTT and PT. Before the tests, different weight fractions of samples were prepared. Through the analysis of the value of APTT and PT, we could see the effect of weight fraction differences on coagulation activity. The assays were carried out according to the instructions of the manufactures.The blood was collected from the jugular vein of rats. Then 0.109 M (3.2%) trisodium citrate was mixed well with whole blood in proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, and the mixture was centrifuged at 4 °C and 3000 rpm for 15 min. The upper plasma was taken out with a plastic pipette and used within 2 hours.
9, and the mixture was centrifuged at 4 °C and 3000 rpm for 15 min. The upper plasma was taken out with a plastic pipette and used within 2 hours.
APTT assay summaries were as follows: 0.1 mL citrated normal rat plasma was added onto the surface of the hydrogel and incubated for 3 min at 37 °C, then 0.1 mL APTT assay reagent, that was pre-incubated for 3 min at 37 °C, was added and incubated for 5 min at 37 °C. After that, 0.1 mL 0.025 mol L−1 CaCl2 solution, which pre-incubated for 3 min at 37 °C, was added and the clotting time was recorded. For the PT assay, 0.1 mL citrated normal rat plasma was added onto the surface of the hydrogel and incubated for 3 min at 37 °C. Then 0.2 mL PT assay reagent, that was pre-incubated for 3 min at 37 °C, was added and the clotting time was recorded.
Statistical analysis
All results were expressed as mean ± standard deviation (SD) and statistical analysis of was performed by one-way analysis of variance (ANOVA). P < 0.05 was considered as statistically significant.Results and discussion
Gelation process
The gelation time was measured using a tilt test as shown in Fig. 1A and B (A showed longer gelation time than B). With the passage of time, the cross-linking degrees of samples gradually increased whereas the mobility decreased continually until a steady gel was generated. Fig. 1C and D exhibited a gelation time reduced with a increase of f-value and n-value. With the increase of f-value, the total amount of calcium in the system raised; with the enhancement of n-value, GDL can be hydrolyzed to generate more gluconic acid, which decomposed calcium carbonate into more calcium ion in the same period. In view of the above-mentioned reasons, increasing of f-value and n-value could promote the cross-linking of sodium alginate and then shorten the gelation time. Fig. 1E shows the change of gelation time over the weight fraction of CMBC. The gelation time shortened with the increase of CMBC weight fraction at first, and the gelation time instead decreased when the weight fraction of CMBC continued increasing, which is explained in Fig. 2. | ||
| Fig. 1 (A and B) Image of gelation time measurement using tilt test; gelation time with different (C) f-value; (D) n-value; (E) weight fraction of CMBC. | ||
 | ||
| Fig. 2 Cross-linking mechanisms of (A) SA with no CMBC; (B) SA with moderate CMBC; (C) SA with excessive CMBC. | ||
G-cells in alginate can combine with calcium ion to form a stable “egg-shell” structure,29 making SA into a steady gel (Fig. 2A). With the addition of CMBC, calcium ions together with the carboxyl groups of CMBC could form an unstable flat grid structure,30 which enhances the stability of the gel (Fig. 2B). Also, the CMBC fibers were fixed inside the hydrogel to enhance the stability. Therefore, the gelation time reduced with increase of the weight fraction of CMBC. However, when the weight fraction of CMBC was excessive, the reduction of SA, which was the principal part of cross-linking, would make the gel system unstable (Fig. 2C), causing the gelation time to increase.
Chemical structure
The infrared spectroscopy curves of SA, CMBC and SA-CMBC are shown in Fig. 3A. The SA-CMBC composites exhibited characteristic absorption bands at 3370 cm−1 (–OH stretching), 1595 cm−1 and 1412 cm−1 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and 1060 cm−1 (C–O–C stretching). There was no appearance or disappearance of any peaks associated with SA and CMBC, which proved that no bonding appeared between SA and CMBC within the composite hydrogel and that no chemical reaction occurred between SA and CMBC. Fig. 3B shows the infrared spectroscopy curves of gels with different weight fractions of CMBC. From the figure, we can see that with the increase of weight fraction of CMBC, the absorption peak value of hydroxyl in 3370 cm−1, carboxyl in 1412 cm−1 and 1595 cm−1, and ether bond in 1060 cm−1 all improved. These changes of absorption peak were attributed to intermolecular interactions between the hydroxyl groups of CMBC and carboxyl groups of SA.
O stretching) and 1060 cm−1 (C–O–C stretching). There was no appearance or disappearance of any peaks associated with SA and CMBC, which proved that no bonding appeared between SA and CMBC within the composite hydrogel and that no chemical reaction occurred between SA and CMBC. Fig. 3B shows the infrared spectroscopy curves of gels with different weight fractions of CMBC. From the figure, we can see that with the increase of weight fraction of CMBC, the absorption peak value of hydroxyl in 3370 cm−1, carboxyl in 1412 cm−1 and 1595 cm−1, and ether bond in 1060 cm−1 all improved. These changes of absorption peak were attributed to intermolecular interactions between the hydroxyl groups of CMBC and carboxyl groups of SA.
 | ||
| Fig. 3 (A) Infrared spectroscopy curves of SA, CMBC and SA-CMBC; (B) infrared spectroscopy curves of gels with different weight fractions of CMBC. | ||
Mechanical property
Tables 1 and 2 illustrate the changes of maximum load over f-value and n-value when the gel system bears pressure stress. We can see that maximum load rose with an increase of f-value and n-value at first, but it decreased while the f-value and n-value continued to increase. At the stage of load increasing, the system could generate more calcium ion with an increase of f-value and n-value, which could promote the cross-linking density of the gel and then increase the maximum load. However, when the f-value and n-value were too large, the reaction rate of calcium carbonate and gluconic acid hydrolyzed from GDL became so fast that the coproduct, carbon dioxide, could not get away from the system and the residual carbon dioxide destroyed the gel structure, which leads to the reduction of gel strength.| f-Value | 2 | 4 | 6 | 8 | 10 |
| Stress/kPa | 15.55 | 23.27 | 29.16 | 31.19 | 27.88 |
| f-Value | 2 | 4 | 6 | 8 | 10 |
| Stress/kPa | 15.55 | 23.27 | 29.16 | 31.19 | 27.88 |
Fig. 4A displays stress–strain curves of gels with different weight fractions of CMBC. The two curves in Fig. 4B show the mechanical properties of gels with different weight fraction of CMBC, which were the changes of maximum stress and fracture strain, respectively. The maximum stress and fracture strain increased with the addition of CMBC at first, and then decreased from a certain degree. With the addition of CMBC, its fibrous structure enhanced the strength of the gel, which increased the load that the gel could bear. While the weight fraction of CMBC was more than 20%, the decrease of SA would make the gel system unstable and reduce the gel strength. This kind of change could be explained by the gelation mechanism shown in Fig. 2.
 | ||
| Fig. 4 (A) Stress–strain curve of series of SA/CMBC composites; (B) mechanical property with different weight fractions of CMBC. | ||
Protein absorption
The SA-CMBC25 gel (the weight fraction of CMBC in the gel was 25%) was placed in BSA solution of 2 mg mL−1 and then the ultraviolet absorption spectrum of the BSA solution was measured at regular intervals. The absorption quantity of BSA on different hydrogels over time is shown in Fig. 5A. With the passage of time, the absorption quantity of BSA gradually increased, and the absorption rate reduced to zero after 40 hours, which meant the absorption of protein tended to be saturated. Fig. 5B shows the final absorption quantity of BSA on hydrogels with different weight fractions. With the increase of CMBC weight fraction, the hydrogel absorbed more protein, and then the absorbing capacity instead dropped down when the weight fraction of CMBC added up to a certain degree. This outcome was due to the decrease of gelation degree and the unstability of the hydrogel structure. Compared with our previous study,27 the maximum protein absorption capacity of CMBC membrane was about 20%, while that of SA-CMBC hydrogel reached to 80%. This outcome was because of the porous structure of the SA-CMBC hydrogel, which allowed more protein entering inner material and improved protein absorption capacity. | ||
| Fig. 5 (A) Absorption quantity of BSA on different hydrogels over time; (B) final absorption quantity of BSA on different hydrogels. | ||
The isoelectric point of BSA was 4.5–6.0. When the pH of the solution was below the isoelectric point of BSA, BSA was positively charged, as at pH above the isoelectric point, BSA was negatively charged. The carboxyl in CMBC could be dissociated to be negatively charged in solution, so that at pH below the isoelectric point, BSA could be absorbed onto the hydrogel due to electrostatic interaction (Fig. 6). With the increase of fraction weight of CMBC in the composite hydrogel, the absorption capacity became higher.
Fig. 7A and C show surface morphology images (×5000) of hydrogels before and after absorbing protein, and Fig. 7D is a partially enlarged detail (×10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) of Fig. 7C. It can be seen that in contrast with the material before absorbing protein, particles with a few hundred nanometers size could be observed on the surface of material, which was considered as gathered protein absorbed on the material surface. The infrared spectroscopy curves in Fig. 7B further approve the change of hydrogels before and after absorbing protein. Apparently, the characteristic absorption peak of amide I at 1655 cm−1, which is a characteristic absorption peak for protein, demonstrated the absorption of protein on the hydrogel. The cellular functionality in terms of adhesion, proliferation and differentiation depends on protein adsorption on the scaffold, which indicated the potential applications of injectable SA-CMBC hydrogel on tissue repair.
000) of Fig. 7C. It can be seen that in contrast with the material before absorbing protein, particles with a few hundred nanometers size could be observed on the surface of material, which was considered as gathered protein absorbed on the material surface. The infrared spectroscopy curves in Fig. 7B further approve the change of hydrogels before and after absorbing protein. Apparently, the characteristic absorption peak of amide I at 1655 cm−1, which is a characteristic absorption peak for protein, demonstrated the absorption of protein on the hydrogel. The cellular functionality in terms of adhesion, proliferation and differentiation depends on protein adsorption on the scaffold, which indicated the potential applications of injectable SA-CMBC hydrogel on tissue repair.
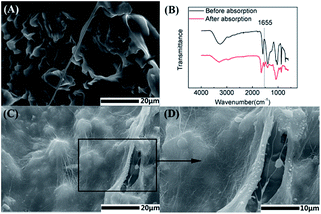 | ||
| Fig. 7 Surface morphology images (A) before and (C and D) after protein absorption; (B) FTIR spectrum of hydrogels. | ||
Procoagulant properties
The APTT assay measures the coagulation factors in the intrinsic pathway, and the PT assay measures the activity of the extrinsic pathway.31 Fig. 8A and B show the results of APTT and PT tests, in which “Blank” was the control group and S1–S6 were hydrogels with different weight fractions of CMBC. The blank control group shows APTT as 24 s, while in test groups, the hydrogels show APTT as less than 16 s. Moreover, the blank control group shows PT as 14 s, while in the test groups, the hydrogels show PT as less than 9 s. In general, the normal range of APTT and PT is 22–28 s and 10–14 s, respectively.32 In fact, the clotting time of the plasma added with SA-CMBC hydrogel was conspicuously decreased. This outcome showed that the SA-CMBC hydrogel had a certain degree of procoagulant activities. We can see from the comparison between test groups that the sample of S4, in which the weight fraction of CMBC was 20%, showed superior promoting action in the blood coagulation process. With obvious procoagulant activity, the hydrogel could be a potential material for hemostasis.Conclusions
In this study, bacterial cellulose was carboxymethylated to generate CMBC, which was composited with SA to obtain SA-CMBC hydrogel. CMBC displayed obvious influence on properties of SA-CMBC hydrogel-gelation time, protein absorption and procoagulant properties. f-Value of 8 and n-value of 0.5 gave rise to the best gelation process, which had shorter gelation time and better mechanical property. With different weight fractions of CMBC, the hydrogels showed different levels of promoting effects on protein absorption, and the hydrogel presents the best protein absorption property with 25% weight fraction of CMBC. The procoagulant property of SA-CMBC hydrogel was best when the weight fraction of CMBC was 20%. Therefore, SA-CMBC hydrogel might be a desirable injectable protein absorption and procoagulant material that could be used in wound dressing, intraoperative hemostasis or other biomedical fields.Acknowledgements
The authors are grateful for the support of the National Natural Science Foundation of China (Grant no. 51273021 and 51473019).References
- K. Li, L. Yu, X. Liu, C. Chen, Q. Chen and J. Ding, Biomaterials, 2013, 34, 2834–2842 CrossRef CAS PubMed.
- Z. Lin, W. Gao, H. Hu, K. Ma, B. He, W. Dai, X. Wang, J. Wang, X. Zhang and Q. Zhang, J. Controlled Release, 2014, 174, 161–170 CrossRef CAS PubMed.
- T. D. Johnson and K. L. Christman, Expert Opin. Drug Delivery, 2013, 10, 59–72 CrossRef CAS PubMed.
- J. E. Frith, A. R. Cameron, D. J. Menzies, P. Ghosh, D. L. Whitehead, S. Gronthos, A. C. Zannettino and J. J. Cooper-White, Biomaterials, 2013, 34, 9430–9440 CrossRef CAS PubMed.
- J. Wu, Q. Ding, A. Dutta, Y. Wang, Y. H. Huang, H. Weng, L. Tang and Y. Hong, Acta Biomater., 2015, 16, 49–59 CrossRef CAS PubMed.
- R. Wang, B. Zhou, W. Liu, X.-H. Feng, S. Li, D.-F. Yu, J.-C. Chang, B. Chi and H. Xu, J. Biomater. Appl., 2015, 29, 1167–1179 CrossRef CAS PubMed.
- C. H. Goh, P. W. S. Heng and L. W. Chan, Carbohydr. Polym., 2012, 88, 1–12 CrossRef CAS.
- J. L. Shamshina, G. Gurau, L. E. Block, L. K. Hansen, C. Dingee, A. Walters and R. D. Rogers, J. Mater. Chem. B, 2014, 2, 3924 RSC.
- K. Y. Lee and D. J. Mooney, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- I. Ghidoni, T. Chlapanidas, M. Bucco, F. Crovato, M. Marazzi, D. Vigo, M. L. Torre and M. Faustini, Cytotechnology, 2008, 58, 49–56 CrossRef CAS PubMed.
- S. Utech, R. Prodanovic, A. S. Mao, R. Ostafe, D. J. Mooney and D. A. Weitz, Adv. Healthcare Mater., 2015, 4, 1628–1633 CrossRef CAS PubMed.
- J. A. Chikar, J. L. Hendricks, S. M. Richardson-Burns, Y. Raphael, B. E. Pfingst and D. C. Martin, Biomaterials, 2012, 33, 1982–1990 CrossRef CAS.
- W.-P. Voo, B.-B. Lee, A. Idris, A. Islam, B.-T. Tey and E.-S. Chan, RSC Adv., 2015, 5, 36687–36695 RSC.
- Y. M. Kolambkar, K. M. Dupont, J. D. Boerckel, N. Huebsch, D. J. Mooney, D. W. Hutmacher and R. E. Guldberg, Biomaterials, 2011, 32, 65–74 CrossRef CAS PubMed.
- L. Lacerda, A. L. Parize, V. Favere, M. C. Laranjeira and H. K. Stulzer, Mater. Sci. Eng., C, 2014, 39, 161–167 CrossRef CAS.
- D. S. Morais, M. A. Rodrigues, T. I. Silva, M. A. Lopes, M. Santos, J. D. Santos and C. M. Botelho, Carbohydr. Polym., 2013, 95, 134–142 CrossRef CAS.
- Y. Han, Q. Zeng, H. Li and J. Chang, Acta Biomater., 2013, 9, 9107–9117 CrossRef CAS PubMed.
- Y. Luo, C. Wu, A. Lode and M. Gelinsky, Biofabrication, 2013, 5, 015005 CrossRef PubMed.
- N. Petersen and P. Gatenholm, Appl. Microbiol. Biotechnol., 2011, 91, 1277–1286 CrossRef CAS PubMed.
- L. C. Tomé, R. J. B. Pinto, E. Trovatti, C. S. R. Freire, A. J. D. Silvestre, C. P. Neto and A. Gandini, Green Chem., 2011, 13, 419 RSC.
- Z. Qin, L. Ji, X. Yin, L. Zhu, Q. Lin and J. Qin, Carbohydr. Polym., 2014, 101, 947–953 CrossRef CAS PubMed.
- T. Oshima, S. Taguchi, K. Ohe and Y. Baba, Carbohydr. Polym., 2011, 83, 953–958 CrossRef CAS.
- X. Yin, C. Yu, X. Zhang, J. Yang, Q. Lin, J. Wang and Q. Zhu, Polym. Bull., 2010, 67, 401–412 CrossRef.
- S. Chen, Y. Zou, Z. Yan, W. Shen, S. Shi, X. Zhang and H. Wang, J. Hazard. Mater., 2009, 161, 1355–1359 CrossRef CAS PubMed.
- X. Shi, Y. Zheng, G. Wang, Q. Lin and J. Fan, RSC Adv., 2014, 4, 47056–47065 RSC.
- J. W. Yu, X. L. Liu, C. S. Liu and D. P. Sun, Mater. Sci. Forum, 2011, 685, 322–326 CrossRef CAS.
- Q. Lin, Y. Zheng, G. Wang, X. Shi, T. Zhang, J. Yu and J. Sun, Int. J. Biol. Macromol., 2015, 73, 264–269 CrossRef CAS PubMed.
- Q. Lin, Y. Zheng, L. Ren, J. Wu, H. Wang, J. An and W. Fan, J. Appl. Polym. Sci., 2014, 131, 3948–3957 Search PubMed.
- G. T. Grant, E. R. Morris, D. A. Rees, P. J. Smith and D. Thom, FEBS Lett., 1973, 32, 195–198 CrossRef CAS.
- M. Nara, H. Torii and M. Tasumi, J. Phys. Chem., 1996, 100, 19812–19817 CrossRef CAS.
- K. Matsubara, Y. Matsuura, A. Bacic, M.-L. Liao, K. Hori and K. Miyazawa, Int. J. Biol. Macromol., 2001, 28, 395–399 CrossRef CAS PubMed.
- L. Fan, X. Zhou, P. Wu, W. Xie, H. Zheng, W. Tan, S. Liu and Q. Li, Int. J. Biol. Macromol., 2014, 66, 245–253 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |


