Facile preparation of a composite of TiO2 nanosheets and polyaniline and its gas sensing properties†
Yang Li*,
Huitao Ban,
Huijie Zhao and
Mujie Yang
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Cyrus Tang Centre for Sensor Materials and Applications, Zhejiang University, Hangzhou 310027, China. E-mail: liyang@zju.edu.cn
First published on 11th December 2015
Abstract
Gas sensors based on a composite of TiO2 nanosheets and polyaniline (PANI) were fabricated by the deposition of water-dispersible PANI on interdigitated gold electrodes decorated with TiO2 nanosheets via dip coating. Specifically, the TiO2 nanosheets were in situ grown on the electrodes by a simple hydrothermal treatment of the electrospun nanofibers of poly(methyl methacrylate) containing a tetrabutyl titanium precursor at a temperature as low as 135 °C in the presence of only water. The method circumvents high temperature calcination and is environmentally friendly. The structure and morphology of the TiO2 nanosheets and their nanocomposites with PANI were characterized by Fourier-transform infrared spectroscopy, X-ray diffraction spectroscopy, scanning electron microscopy, and high resolution transmission electron microscopy. The nanocomposite exhibited highly sensitive (detection limit as low as ∼45 ppb), selective and repeatable electrical responses towards NH3 at room temperature, which is better than those of the separate components and demonstrates an obvious synergetic effect. The enhanced sensing properties of the nanocomposite are proposed to relate to the high specific surface area and the p/n heterojunction between TiO2 nanosheets and PANI.
1. Introduction
TiO2 is one of the most extensively investigated metal oxide semiconductors with attractive electronic, electrochemical and photochemical properties, and has found wide applications in photocatalysts, lithium ion batteries, solar cells and sensors.1–4 In recent years, nanostructured TiO2 and its hierarchical hybrids with inorganic or organic materials have received much attention due to their significantly improved properties compared with the corresponding bulk counterparts.5,6Among the various methods to prepare nanoscaled TiO2 and its composites, electrospinning (ES) and hydrothermal synthesis (HS) show great promise. ES is a versatile and low cost method for the facile fabrication of one dimensional (1D) nanostructured polymers or the inorganic materials under the high voltage electrical field with the aid of Coulomb force. There have been a number of reports on the preparation of one dimensional TiO2 or its hybrids with other inorganic materials by ES.7–14 On the other hand, HS is a well known approach for the simple preparation of inorganic nanomaterials with the prominent advantages of low operation temperature and environmental friendliness, and controllable morphology.15,16 Moreover, there have been reports on the preparation of TiO2 of special crystal structure or morphology, or the hybrids with other metal oxide semiconductors by combination of ES and HS.9,17–20 However, TiO2 nanomaterials obtained by ES usually involved high temperature calcination, whereas the nanostructured TiO2 or its hybrids evolved from HS needs further separation and purification process.9 Therefore, it is difficult to realize direct growth of nanostructured TiO2 and/or its hybrids on the substrate to develop functional devices for practical applications, which is more prominent when organic materials or polymers are employed as the substrate for the fabrication of flexible devices. Apparently, it is desirable to develop a simple method for the in situ growth of nanostructured TiO2 on the substrate via a low temperature procedure to allow the direct preparation of TiO2 based device and the use of flexible substrate.
TiO2 based gas sensors showed the advantages of easy preparation, good stability and high sensitivity.19–22 Nonetheless, the high operation temperature becomes an obstacle for the wide applications. Forming composites with conducting polymers, including polyaniline (PANI), polypyrrole (PPy), etc., is proved to be an effective method to address the problem.23–27 In particular, the composite of nanostructured TiO2 and PANI received most attention, due to the prominent merits of PANI, such as low cost, reasonable stability and wide range of conductivity modulation by interactions with a large spectrum of gases. The morphology and nanostructure of the sensing materials are known to have a great effect on the sensing performance. Nowadays, most of the nanocomposite of TiO2 and PANI are prepared by the solution polymerization of aniline in the presence of TiO2 nanoparticles. Recently, Gong et al. fabricated the composite of TiO2 microfiber and PANI nanograin by in situ polymerization of aniline on the surface of calcinated electrospun TiO2 microfiber, which exhibited ultrahigh sensitivity to NH3.23 Wang and coworkers developed the composite nanofibers of TiO2–PANI/polyamide 6 (PA6) by sputtering TiO2 nanoparticles on the surface of PA6 covered with PANI formed by solution polymerization, which showed relatively low sensitivity towards NH3 (relative resistance change of ∼400% towards 50 ppm of NH3).24 We also fabricated the composite of PANI with TiO2 nanoparticles by electrostatic self-assembly, and revealed its fast response, and good reversibility and repeatability towards NH3 of low concentrations.25 However, to our best knowledge, there have been no reports on two dimensional (2D) nanostructured TiO2 with PANI for the applications as gas sensors. It is proposed that the inorganic semiconductors with 1D structure could facilitate directional charge motion, and reduce the contact resistance, thus enhance the sensing behaviors.22 Similarly, it is expected that the hybrids of 2D nanosheets of TiO2 with PANI might exhibit distinct sensing properties.
Very recently, we reported the preparation of nanostructured SnO2 and its composites with PPy by combination of ES and HS for application in gas sensors,28 which represents new efforts for the facile in situ preparation of inorganic metal oxides and their composites on various substrates. As an extension of the work, in this paper, we developed a new route involving the hydrothermal treatment of electrospun poly(methyl methacrylate) (PMMA) nanofibers at a low temperature of 135 °C for 8 h to obtain 2D TiO2 nanosheets directly grown on the substrate. Subsequent coating with water-dispersible PANI resulted in a nanocomposite of TiO2 nanosheets with PANI. The as-prepared nanocomposite showed ohmic contact with underlying substrate, which is beneficial for the enhancement of the sensing properties.22 Gas sensors based on the 2D nanocomposite of TiO2/PANI exhibited very high sensitivity towards NH3 at room temperature (relative resistance change of 3730% towards 10.7 ppm of NH3, the detection limit: ∼45 ppb). Moreover, the nanocomposite revealed good selectivity. The composite of 2D TiO2 nanosheets and PANI showed better sensing properties than the constituent component, revealing an obvious synergetic effect. Furthermore, the sensing mechanism of the nanocomposite has been explored.
It is obvious that the new method combines the advantages of ES and HS, and could easily realize in situ preparation of nanostructured inorganic metal oxides on various substrates (including flexible organic substrates since no high temperature calcination is involved). Moreover, different nanostructures (including 2D nanosheets) are expected to be in situ obtained by modulating the composition of the electrospun precursors and HS conditions. Therefore, it could find wide applications in the preparation of advanced optoelectronic and electrochemical devices based on nanostructured semiconductors and their nanocomposites.
2. Experimental
2.1 Materials
Aniline (Sinopharm Chemical Regent Co., Ltd.) was purified by distillation under reduced pressure and stored in a refrigerator prior to use. Poly(methyl methacrylate) (PMMA) (MW: 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were supplied by Alfa Aesar. Tetrabutyl titanium (TBT) was purchased from J & K Chemical Technology. Ethanol (EtOH), methanol (MeOH), tetrahydrofuran (THF), acetone, ether, n-hexane, dichloromethane, and glacial acetic acid (HAc) were all supplied by Sinopharm Chemical Regent Co., Ltd. Poly(styrene sulfonic acid) (PSSA, Mw: 75
000) were supplied by Alfa Aesar. Tetrabutyl titanium (TBT) was purchased from J & K Chemical Technology. Ethanol (EtOH), methanol (MeOH), tetrahydrofuran (THF), acetone, ether, n-hexane, dichloromethane, and glacial acetic acid (HAc) were all supplied by Sinopharm Chemical Regent Co., Ltd. Poly(styrene sulfonic acid) (PSSA, Mw: 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 30 wt% water solution) were obtained from Alfa Aesar. Ammonium persulfate (APS) (analytical pure grade) was purchased from Shantou Xilong Chem. Co. All the chemicals were of analytical grade and used as received unless noted otherwise.
000, 30 wt% water solution) were obtained from Alfa Aesar. Ammonium persulfate (APS) (analytical pure grade) was purchased from Shantou Xilong Chem. Co. All the chemicals were of analytical grade and used as received unless noted otherwise.
2.2 Fabrication of TiO2 nanosheets and gas sensors based on TiO2/PANI nanocomposites
TiO2 nanosheets were grown on the substrates by the hydrothermal treatment of the electrospun nanofibers containing TBT. In a generic procedure, 0.64 g of PMMA was dissolved in the mixed solution of 1 mL of ethanol and 4 mL of dichloromethane by vigorous stirring to get the solution A. Meanwhile, 1 mL of TBT was blended with the mixture of ethanol (1 mL) and HAc (1 mL) to obtain the solution B. The mixture of solutions A and B was used as the ES solution, which was loaded into a plastic syringe with a pinhead whose internal diameter was 0.7 mm. The pinhead was connected to a high voltage supply (DW-P303-1ACF0, Tianjin Dongwen High Voltage Power Supply Plant). The operating voltage applied for ES was ∼6.3 kV, and the flow rate of the ES solution was controlled at 1.5 mL h−1 by a syringe pump (WZ-50C6, Smith Medical Instrument (Zhejiang) Co. Ltd.). A grounded aluminum foil was situated 15 cm from the tip of the pinhead. The electrospun nanofibers were collected onto glass or interdigitated gold electrodes placed on the Al foil using a device as shown in our previous work and dried in air.29 The gold electrodes (size: 6 mm × 5 mm × 0.5 mm) possessed a ceramic substrate, where an interdigitated array of gold tracks had been previously evaporated and photolithographically defined (both width and gaps of the gold tracks were 40 μm).The resulting interdigitated gold electrodes were transferred into a Teflon-lined stainless-steel autoclave with moderate amount of deionized water and maintained at 135 °C for 8 h. The autoclave naturally cooled down to room temperature and the interdigitated gold electrodes were taken out and dried in air to obtain the sensors based on TiO2 nanosheets.
Water-dispersible PANI, which was obtained by chemical oxidation polymerization with PSSA as the dopant ([PSSA]: 0.068 mol L−1; [APS]: 0.06 mol L−1; molar ratio of aniline to structural unit of PSSA: 1/1) using our previous method,25 was prepared as an aqueous solution (10 mg mL−1). As-prepared gas sensors based on TiO2 nanosheets were dipped into the PANI solution using an automatic dip-coating machine, followed by drying in air to give gas sensors based on TiO2/PANI nanocomposite.
For comparison, blank electrodes were dipped into the PANI solution using an automatic dip-coating machine, followed by drying to give gas sensors based on PANI.
2.3 Measurements
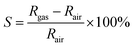
The morphologies of the electrospun nanofibers, TiO2 nanosheets and TiO2/PANI nanocomposites were investigated using a field-emission scanning electron microscope (FE-SEM, s-4800, Hitachi, accelerating voltage of 3 kV). Lattice spacing, fast Fourier transform (FFT) images and Energy Dispersive X-ray Spectrum (EDX) were obtained with a high resolution transmission electron microscope (HRTEM, JEM-2100F, JEOL, accelerating voltage of 200 kV). Fourier transform infrared (FT-IR) spectra were recorded on a Bruker Vector 22 infrared spectrometer (KBr pellets). Thermal gravimetric analysis (TGA) was carried out in air with a DSCQ200 (TA Instruments). X-ray powder diffraction (XRD) patterns were collected on a PAN analytical X'Pert PRO using CuKα (λ = 0.15418 nm) radiation with a 2θ scanning range of 20–80°. Current–voltage (I–V) characteristics were determined using a CHI660D electrochemical workstation (Chenhua instrument Co. Ltd., Shanghai).Gas sensing properties of PANI and TiO2/PANI nanocomposites were examined by measuring their real-time electrical responses to NH3 at room temperature (∼20 °C) with an Agilent 34972A LXI Data Acquisition/Switch Unit (Agilent technologies Co. Ltd). Different concentrations (0.5–10.7 ppm) of NH3 were obtained by diluting standard NH3 (NH3 in air, Hangzhou New Century Gas Co.) with dry synthetic air using computer-driven digital mass flow controllers (MFC CS200A, Beijing Sevenstar Electronics Co., Ltd.). Both standard NH3 and diluting synthetic air were completely dry and the humidity in the test chamber was very low. The total gas flow rate was maintained at 1 L min−1, and the volume of the test chamber was 350 mL. The response magnitude is defined as
 | (1) |
3. Results and discussion
3.1 Characterizations of TiO2 nanosheets and the composites with PANI
As discussed above, the preparation of nanostructured TiO2 or the hybrids by ES or the combination of ES and HS usually involves the high temperature calcination to obtain TiO2 nanocrystals. Li et al. reported that the preparation of TiO2 nanowires via the calcination of electrospun poly(vinyl pyrrolidone) nanowire containing TBT.30 Moreover, Sun et al. employed thin films composed of anatase TiO2 nanowire, which was prepared from the calcination of electrospun nanowires containing TBT, as the template to guide the growth of rutile TiO2 nanorods in subsequent HS procedure and obtained the hierarchical nanostructured anatase/rutile TiO2 composite film.9In this work, we used a different strategy for the preparation of the TiO2/PANI nanocomposites as shown in Scheme 1. Electrospun PMMA nanowires, in which TBT was dispersed and used as the precursor of TiO2, were deposited on the substrate and used as the template. Subsequent hydrothermal treatment of the nanofibers in the presence of water at a low temperature of 135 °C for 8 h resulted in the conversion to TiO2 nanosheets directly on the substrate. Apparently, our method is free from the high temperature calcination process, and realizes the in situ growth of 2D TiO2 nanosheets on the substrates. Such advantages could facilitate the preparation of gas sensors, which is more apparent when organic or polymer substrates are used in the development of flexible devices.
Fig. 1 depicts the XRD patterns of electrospun nanofibers, as-prepared TiO2 nanosheets, PANI and the TiO2/PANI nanocomposites. A broad diffraction peak at 2θ = 29.3° is observed in the XRD pattern of the electrospun nanofibers of PMMA and the precursor Ti salts, which can be ascribed to PMMA.31 In the XRD pattern of PANI, the weak and broad peak centred at 2θ = 25.3° could be attributed to crystallinity of PANI in the form of emeraldine salt.32,33 Moreover, as-prepared TiO2 nanosheets exhibits a tetragonal anatase crystal structure (JCPDS 65-5714), and the sample of TiO2/PANI nanocomposite reveals the same diffraction peaks as the TiO2 nanosheets.
 | ||
| Fig. 1 XRD patterns of (a) electrospun nanofibers, (b) PANI, (c) as-prepared TiO2 nanosheets and (d) TiO2/PANI nanocomposites. | ||
The morphology of the electrospun nanofibers and resulting TiO2 nanosheets were investigated by FE-SEM, as presented in Fig. 2. It is found that smooth nanofibers of PMMA containing TBT with diameters of ∼500 nm deposited on the substrate during the ES process (Fig. 2a and b). By contrast, the hydrothermally treated nanofibers present completely different morphology. The shape of the nanofibers was largely maintained. However, the nanofibers, which were composed of small intersectionally arranged TiO2 nanosheets, exhibited highly rugged and porous structure. Additionally, the substrate originally unoccupied by the electrospun nanofibers was covered with densely arranged TiO2 nanosheets (Fig. 2c–f). It is proposed that the titanium salt in the electrospun PMMA nanofiber template diffused towards the substrate uncovered by the nanofibers, and realized the in situ conversion to the TiO2 nanosheets during the hydrothermal treatment.
 | ||
| Fig. 2 SEM micrographs of electrospun nanofibers (a and b) before and (c–f) after the hydrothermal treatment. | ||
Fig. 3 presents the morphology of PANI, the surface and cross-section view of the TiO2/PANI nanocomposite. It is seen that PANI presents a compact film. By contrast, the TiO2/PANI nanocomposite, which was obtained by depositing PANI layer on the as-prepared TiO2 nanosheets, exhibits a distinct morphology. Apparently, both the nanofibers and the substrate were generally covered by a layer of PANI, and some small cracks were observed. The thickness of the PANI coating is ∼1 μm as estimated from the cross-section view of the nanocomposite.
 | ||
| Fig. 3 SEM micrographs of (a) PANI, (b and c) surface and (d) cross-section view of TiO2/PANI nanocomposite. | ||
The morphology and crystalline structure of as-prepared TiO2 nanosheets have been further explored by HRTEM as illustrated in Fig. 4. It is revealed that the lattice spacing of the nanocrystals is 0.36 nm, attributed to the d-spacing of the (101) plane of tetragonal anatase TiO2.34 The corresponding fast Fourier transform (FFT) patterns of the nanosheets (Fig. 4, inset) display sharp homocentric rings composed of bright diffraction spots which is indexed for anatase TiO2. Furthermore, the results of the EDX measurements revealed the strong peaks for Ti and O, indicating the presence of TiO2 (Fig. S1, please see the ESI†). Obviously, the TEM observation confirms the tetragonal anatase TiO2 crystal structure of the sample, and is in good agreement with the XRD analysis.
Fig. 5 presents the FT-IR spectra of electrospun nanofibers, PANI, as-prepared TiO2 nanosheets and the TiO2/PANI nanocomposite. In the spectrum of the electrospun nanofibers, the bands at 2951, 1732, 1446 and 1151 cm−1 could be ascribed to the CH stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, CH3 stretching and –O–CH3 stretching vibrations of PMMA, respectively.35 The bands at about 1610 cm−1 and 1438 cm−1 in the spectrum of PANI are assigned to the C
O stretching, CH3 stretching and –O–CH3 stretching vibrations of PMMA, respectively.35 The bands at about 1610 cm−1 and 1438 cm−1 in the spectrum of PANI are assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching modes of the quinoid and benzoid rings, respectively.24,36,37 Moreover, the vibration band of the protonated imine of PANI appears at 1151 cm−1, which suggests that the PANI is in the form of the conducting emeraldine salt.38 Furthermore, in the spectrum of the TiO2 nanosheets prepared by the hydrothermal treatment of the electrospun nanofibers, the broad bands originating from the Ti–O vibration are observed between 500–800 cm−1 in addition to the characteristic bands of PMMA.39 The characteristic absorptions of both PANI and TiO2 are observed in the spectrum of the nanocomposite, indicative of the successful preparation of composite of TiO2 and conducting PANI.
C stretching modes of the quinoid and benzoid rings, respectively.24,36,37 Moreover, the vibration band of the protonated imine of PANI appears at 1151 cm−1, which suggests that the PANI is in the form of the conducting emeraldine salt.38 Furthermore, in the spectrum of the TiO2 nanosheets prepared by the hydrothermal treatment of the electrospun nanofibers, the broad bands originating from the Ti–O vibration are observed between 500–800 cm−1 in addition to the characteristic bands of PMMA.39 The characteristic absorptions of both PANI and TiO2 are observed in the spectrum of the nanocomposite, indicative of the successful preparation of composite of TiO2 and conducting PANI.
 | ||
| Fig. 5 FT-IR spectra of PANI, electrospun nanofibers, as-prepared TiO2 nanosheets and TiO2/PANI nanocomposites. | ||
Kim et al. proposed that the electrospun nanofibers directly deposited on the substrate might exhibit poor contact with the substrate, while the method of hot-pressing could effectively improve the contact between the nanofibers and underlying electrodes. However, the pristine nanostructures could not be maintained.22 In this work, the TiO2 nanosheets were in situ formed on the substrate, and the contact with the underlying electrode is expected to be improved, which is beneficial for the enhancement in the sensor sensitivity. We measured the current–voltage (I–V) curves of the nanocomposite sensor at room temperature in air and NH3 of different concentrations and the results are shown in Fig. S2 (please see the ESI†). The current varies linearly with the current in the range of −1 V to 1 V irrespective of the atmosphere, indicating an ohmic contact. Apparently, the sensor prepared with the new method exhibits good adhesion and contact between the sensing materials and underlying electrode. Therefore, the sensor is anticipated to show higher sensitivity.
3.2 Gas sensing properties of the TiO2/PANI nanocomposites
NH3 is a typical pollutant in air, and its detection has aroused great concern.33 Although TiO2 could exhibit good sensitivity to NH3 at elevated temperature, the sensor based on the TiO2 nanosheets so prepared exhibits too high resistance at room temperature, and is insensitive to NH3 without heating, which is in accordance with the literature report.40By contrast, TiO2/PANI nanocomposite based sensor exhibited appreciable resistance change upon contact with NH3 at room temperature, and the dynamic responses and calibration curves are illustrated in Fig. 6. It is seen that the nanocomposite exhibited S of 3730% towards NH3 of 10.7 ppm. By contrast, PANI based sensor revealed much lower S of 1770% upon contact with 10.7 ppm of NH3 (Fig. S3, please see the ESI†). Apparently, the composite showed much improved sensing properties towards low concentration of NH3 compared with the sensor based on PANI or TiO2, demonstrating an obvious synergetic effect. Moreover, the dynamic response of the composite towards NH3 of increasing concentrations (Fig. 6a) revealed its good reversibility upon refreshed with dry air. The detection limit (DL) of the composite is estimated to be ∼45 ppb as judged from its calibration curve (Fig. 6b) using the equation: DL = 3 × rmsd/sensitivity.32 The repeatability of the nanocomposite in the detection of NH3 has been examined and the results are presented in Fig. 7. It is observed that the composite sensor showed repeatable responses during alternate exposure to 5 ppm of NH3 and air for three cycles, indicating its good repeatability.
 | ||
| Fig. 6 (a) Dynamic responses of TiO2/PANI nanocomposite towards NH3 at room temperature; (b) calibration curves of the TiO2/PANI nanocomposite. | ||
 | ||
| Fig. 7 Dynamic responses of TiO2/PANI nanocomposite at room temperature during alternate exposures to dry air and NH3 of 5 ppm in cycle tests. | ||
The electrical responses of the nanocomposite towards a number of organic vapors were also measured and compared with the response towards NH3. The results demonstrated in Fig. 8 clearly revealed that the nanocomposite exhibited high response magnitude of ∼3770% towards 10.7 ppm of NH3. By contrast, the nanocomposite revealed resistance decrease upon contact with a number of organic vapors at room temperature. Specifically, the composite presented small negative responses (S < 10%) towards high concentration of polar or non-polar organic vapors (5000 ppm) including MeOH, EtOH, ether, acetone, THF, and n-hexane.
 | ||
| Fig. 8 Response magnitude of TiO2/PANI nanocomposite towards different gases at room temperature. The concentration is 5000 ppm for all gases except NH3 ([NH3] = 10.7 ppm). | ||
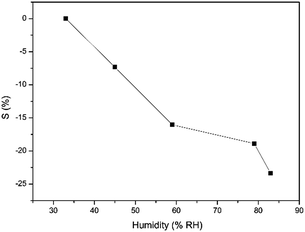
Apart from the organic vapors, humidity is reported to have an effect on the response of the gas sensors.41,42 Therefore, we also investigated the humidity response of the nanocomposite sensor, and the results are presented in Fig. 9. The resistance of the nanocomposite sensor decreased with the increase of humidity, exhibiting negative responses. However, the response magnitude is no more than 25% from 33% RH to 84% RH, which is quite low when compared with its ultrahigh response to NH3. It is seen clearly that the nanocomposite shows very good selectivity towards NH3 at room temperature.
Table 1 summarizes the NH3 sensing properties of TiO2/PANI nanocomposites reported in literatures. It is seen that the sensitivity of the nanocomposite sensor prepared in this work is comparable or even higher than most of the reported composite sensor. Moreover, our composite sensor is featured with very high selectivity with negligible interference from the high concentration of vapors of common organic solvents.
3.3 Gas sensing mechanism of the TiO2/PANI nanocomposites
There have been a number of reports on the sensing mechanism of TiO2/PANI nanocomposite based sensors. Typically, Gong et al. prepared the composite of n-type TiO2 microfiber and p-type PANI nanograin, and proposed that the established p/n depletion layer and the resistance change of bulk PANI nanoparticles could work as a current switch to realize ultrahigh response towards very low concentration of NH3.23 Tai et al. postulated that the p/n junction between TiO2 and PANI, and the interparticle electron migration from TiO2 to PANI at the junction reduced the activation energy and enthalpy of the physical adsorption of NH3, leading to higher sensitivity. Moreover, the well matching of the energy bandgap between TiO2 and PANI for charge transfer was also proposed to enhance the gas sensing properties.43–45In our work, the 2D TiO2 nanosheets were in situ grown on the substrate, and then covered with a layer of PANI. Good contact with the underlying electrode as proved by the ohmic contact is beneficial for the enhancement of the sensitivity. Moreover, the nanostructure of the composite leads to high specific surface area, provides more reaction sites, and facilitates the adsorption, desorption and diffusion of NH3. Therefore, the gas sensing properties could be improved. Additionally, different from the reported composite in which TiO2 nanoparticles were mainly included in the PANI matrix, or the 1D TiO2 microfiber were covered with PANI nanograin, our composite exhibits the structure of 2D nanosheets surrounded by the PANI coating, which could lead to substantial reduction in the contact resistance and promote the charge transfer when compared with the composite of TiO2 nanoparticles buried inside PANI matrix. Consequently, the sensing behaviors of our nanocomposites could be improved as shown in Table 1. Furthermore, the p/n junctions established at the interface between TiO2 nanosheets and PANI coating results in a depletion layer. Upon contact with NH3, PANI could be dedoped due to the interactions between electron rich N atom of NH3 and the holes in PANI, resulting in increased resistance. Meanwhile, the nanostructure could allow the diffusion of NH3 into the place near the interface of PANI and TiO2, and lead to the broadening of the depletion layer. As a result, the resistance change is augmented, leading to higher sensitivity.
The nanocomposite in this work did not exhibit very fast response and recovery, similar to the reports by Mikhaylov et al.33 It is proposed that the covered PANI layer is not very thin, especially when compared with the layer-by-layer assembled PANI/TiO2 thin film.25 Consequently, the adsorption on and desorption from the nanocomposites of NH3 is hindered. Furthermore, the concentration of NH3 is very low (0.5 to 10 ppm), and more time is needed to achieve the equilibrium in the sensing chamber.33
The high selectivity of our nanocomposite sensor towards NH3 is impressive. Generally, the adsorption of vapors of common organic solvents on PANI could lead to the slight expansion of the sensing film, and hinder the charger transfer process. Therefore, small increase in the resistance is expected.47,48 However, the nanocomposite presented slightly decreased resistance in contact with the organic vapors such as MeOH, EtOH, ether, acetone, THF. It is proposed that the organic vapors exhibited weak reducing ability, and the interactions of high concentrations of the organic vapors with TiO2/PANI could also modify the charge hopping rate of the nanocomposite and increase the conductivity.49 The net effect is that nanocomposite reveals slightly decreased conductivity, as observed in Fig. 8. More detailed investigations are needed to better understand the selective response of the nanocomposite.
4. Conclusions
TiO2 nanosheets were readily grown on the substrate via the hydrothermal treatment of electrospun nanofibers containing titanium salt. Specifically, the preparation process avoids high temperature calcination of nanofibers and the use of additives for the hydrothermal synthesis. The coverage of water-dispersible PANI on the surface of TiO2 nanosheets resulted in the nanocomposite based gas sensors. The nanocomposite sensor exhibited desirable sensing properties towards low concentration of NH3 (0.5–10.7 ppm) at room temperature, including very high response magnitude (3770% towards 10.7 ppm of NH3) and ultralow detection limit (∼45 ppb), good repeatability and high selectivity. The attractive gas sensing properties of the nanocomposite is ascribed to its high specific surface area, good contact between the composite and underlying electrode, and establishment of p/n junction at the interface of 2D TiO2 nanosheets and PANI.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Contract no. 51273174 and 51073134).Notes and references
- S. Kitano, A. Tanaka, K. Hashimoto and H. Kominami, Phys. Chem. Chem. Phys., 2014, 16, 12554–12559 RSC
.
- R. Mo, Z. Lei, K. Sun and D. Rooney, Adv. Mater., 2014, 26, 2084–2088 CrossRef CAS PubMed
.
- H.-S. Kim and N.-G. Park, J. Phys. Chem. Lett., 2014, 5, 2927–2934 CrossRef CAS PubMed
.
- S. H. Nimkar, S. P. Agrawal and S. B. Kondawar, Procedia Mater. Sci., 2015, 10, 572–579 CrossRef CAS
.
- S.-Z. Chu, S. Inoue, K. Wada, D. Li, H. Haneda and S. Awatsu, J. Phys. Chem. B, 2003, 107, 6586–6589 CrossRef CAS PubMed
.
- L. Zhang and M. Wan, J. Phys. Chem. B, 2003, 107, 6748–6753 CrossRef CAS
.
- Z. Liu, D. D. Sun, P. Guo and J. O. Leckie, Nano Lett., 2007, 7, 1081–1085 CrossRef CAS PubMed
.
- A. Kumar, R. Jose, K. Fujihara, J. Wang and S. Ramakrishna, Chem. Mater., 2007, 19, 6536–6542 CrossRef CAS
.
- C. Sun, N. Wang, S. Zhou, X. Hu, S. Zhou and P. Chen, Chem. Commun., 2008, 3293–3295 RSC
.
- W. S. Lee, Y.-S. Park and Y.-K. Cho, ACS Appl. Mater. Interfaces, 2014, 6, 12189–12195 CAS
.
- X. Liu, J. Fang, M. Gao, H. Wang, W. Yang and T. Lin, Mater. Chem. Phys., 2015, 151, 330–336 CrossRef CAS
.
- A. Yousef, M. El-Halwany, N. A. Barakat, M. N. Al-Maghrabi and H. Y. Kim, J. Ind. Eng. Chem., 2015, 26, 251–258 CrossRef CAS
.
- M. Motlak, N. A. Barakat, M. S. Akhtar, A. G. El-Deen, M. Obaid, C. S. Kim, K. A. Khalil and A. A. Almajid, Chem. Eng. J., 2015, 268, 153–161 CrossRef CAS
.
- H.-G. Lee, A.-I. Gopalan, G. Sai-Anand, B.-C. Lee, S.-W. Kang and K.-P. Lee, Mater. Chem. Phys., 2015, 154, 125–136 CrossRef CAS
.
- P. Zhang, S. Yin and T. Sato, Appl. Catal., B, 2009, 89, 118–122 CrossRef CAS
.
- H. Xu, H. Wang, Y. Zhang, W. He, M. Zhu, B. Wang and H. Yan, Ceram. Int., 2004, 30, 93–97 CrossRef CAS
.
- A. Yousef, N. A. Barakat, M. H. El-Newehy, M. Ahmed and H. Y. Kim, Colloids Surf., A, 2015, 470, 194–201 CrossRef CAS
.
- F. Zhang, Z. Cheng, L. Kang, L. Cui, W. Liu, G. Hou, H. Yang and X. Xu, RSC Adv., 2014, 4, 63520–63525 RSC
.
- J. Deng, L. Wang, Z. Lou and T. Zhang, J. Mater. Chem. A, 2014, 2, 9030–9034 CAS
.
- Z. Lou, F. Li, J. Deng, L. Wang and T. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 12310–12316 CAS
.
- F. Zhang, Z. Cheng, L. Kang, L. Y. Cui, W. Liu, G. Hou, H. Yang and X. Xu, RSC Adv., 2014, 4, 63520–63525 RSC
.
- I.-D. Kim, A. Rothschild, B. H. Lee, D. Y. Kim, S. M. Jo and H. L. Tuller, Nano Lett., 2006, 6, 2009–2013 CrossRef CAS PubMed
.
- J. Gong, Y. Li, Z. Hu, Z. Zhou and Y. Deng, J. Phys. Chem. C, 2010, 114, 9970–9974 CAS
.
- Q. Wang, X. Dong, Z. Pang, Y. Du, X. Xia, Q. Wei and F. Huang, Sensors, 2012, 12, 17046–17057 CrossRef CAS PubMed
.
- Q. Lin, Y. Li and M. Yang, Synth. Met., 2012, 162, 2242–2249 CrossRef CAS
.
- T. Jiang, Z. Wang, Z. Li, W. Wang, X. Xu, X. Liu, J. Wang and C. Wang, J. Mater. Chem. C, 2013, 1, 3017–3025 RSC
.
- Y. Wang, W. Jia, T. Strout, A. Schempf, H. Zhang, B. Li, J. Cui and Y. Lei, Electroanalysis, 2009, 21, 1432–1438 CrossRef CAS
.
- Y. Li, H. Ban and M. Yang, Sens. Actuators, B, 2016, 224, 449–457 CrossRef CAS
.
- Q. Lin, Y. Li and M. Yang, Sens. Actuators, B, 2012, 171, 309–314 CrossRef
.
- Z. Li, H. Zhang, W. Zheng, W. Wang, H. Huang, C. Wang, A. G. MacDiarmid and Y. Wei, J. Am. Chem. Soc., 2008, 130, 5036–5037 CrossRef CAS PubMed
.
- R. Baskaran, S. Selvasekarapandian, N. Kuwata, J. Kawamura and T. Hattori, Solid State Ionics, 2006, 177, 2679–2682 Search PubMed
.
- Z. Ye, Y. Jiang, H. Tai, N. Guo, G. Xie and Z. Yuan, J. Mater. Sci.: Mater. Electron., 2015, 26, 833–841 CrossRef CAS
.
- S. Mikhaylov, N. Ogurtsov, Y. Noskov, N. Redon, P. Coddeville, J.-L. Wojkiewicz and A. Pud, RSC Adv., 2015, 5, 20218–20226 RSC
.
- X. Li, Y. Chen, H. Yao, X. Zhou, J. Yang, H. Huang, Y.-W. Mai and L. Zhou, RSC Adv., 2014, 4, 39906–39911 RSC
.
- S. Ramesh, K. H. Leen, K. Kumutha and A. Arof, Spectrochim. Acta, Part A, 2007, 66, 1237–1242 CrossRef CAS PubMed
.
- G. Li, H. Peng, Y. Wang, Y. Qin, Z. Cui and Z. Zhang, Macromol. Rapid Commun., 2004, 25, 1611–1614 CrossRef CAS
.
- Y. Yu, B. Che, Z. Si, L. Li, W. Chen and G. Xue, Synth. Met., 2005, 150, 271–277 CrossRef CAS
.
- S. Yang, W. Chen and K. You, Synth. Met., 1997, 84, 77–78 CrossRef CAS
.
- Z. Pang, J. Fu, L. Luo, F. Huang and Q. Wei, Colloids Surf., A, 2014, 461, 113–118 CrossRef CAS
.
- S. Pawar, M. Chougule, S. Patil, B. Raut, P. Godse, S. Sen and V. Patil, IEEE Sens. J., 2011, 11, 3417–3423 CrossRef CAS
.
- N. Hu, Y. Wang, J. Chai, R. Gao, Z. Yang, E. S.-W. Kong and Y. Zhang, Sens. Actuators, B, 2012, 163, 107–114 CrossRef CAS
.
- L. Wang, H. Huang, S. Xiao, D. Cai, Y. Liu, B. Liu, D. Wang, C. Wang, H. Li and Y. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 14131–14140 CAS
.
- H. Tai, Y. Jiang, G. Xie and J. Yu, J. Mater. Sci. Technol., 2010, 26, 605–613 CAS
.
- H. Tai, Y. Jiang, G. Xie, J. Yu, X. Chen and Z. Ying, Sens. Actuators, B, 2008, 129, 319–326 CrossRef CAS
.
- H. Tai, Y. Jiang, G. Xie, J. Yu and X. Chen, Sens. Actuators, B, 2007, 125, 644–650 CrossRef CAS
.
- S. Pawar, S. Patil, M. Chougule, B. Raut, P. Godase, R. Mulik, S. Sen and V. Patil, IEEE Sens. J., 2011, 11, 2980–2985 CrossRef CAS
.
- C. Murugan, E. Subramanian and D. P. Padiyan, Synth. Met., 2014, 192, 106–112 CrossRef CAS
.
- S. Antwi-Boampong and J. J. BelBruno, Sens. Actuators, B, 2013, 182, 300–306 CrossRef CAS
.
- M. Mabrook, C. Pearson and M. Petty, Sens. Actuators, B, 2006, 115, 547–551 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures concerning the sensing properties of the gas sensor based on PANI, and the EDX pattern of as-prepared TiO2 nanosheets. See DOI: 10.1039/c5ra20879c |
| This journal is © The Royal Society of Chemistry 2015 |