Preparation, characterization, and chemical-induced hydrophobicity of thermostable amine-modified graphene oxide†
Jing Wanga,
Hong-Zhang Geng *a,
Zhi-Jia Luoa,
Songting Zhangb,
Jinqiu Zhangb,
Juncheng Liu*a,
Hai-Jie Yanga,
Sujun Mab,
Baoquan Sunb,
Yan Wanga,
Shi-Xun Daa and
Yun-Qiao Fua
*a,
Zhi-Jia Luoa,
Songting Zhangb,
Jinqiu Zhangb,
Juncheng Liu*a,
Hai-Jie Yanga,
Sujun Mab,
Baoquan Sunb,
Yan Wanga,
Shi-Xun Daa and
Yun-Qiao Fua
aState Key Laboratory of Separation Membranes and Membrane Processes, School of Material Science and Engineering, Tianjin Polytechnic University, Tianjin, 300387, China. E-mail: genghz@tjpu.edu.cn; jchliu@tjpu.edu.cn; Fax: +86 22 83955055; Tel: +86 22 83955812
bResearch Institute of Petroleum Engineering, SINOPEC Shengli Oilfield Company, Dongying, Shandong 257000, China
First published on 1st December 2015
Abstract
In this study, a hydrophobic and thermostable amine-modified graphene oxide (MGO) material was fabricated using two different methods. p-Toluidine was covalently introduced onto the surface of graphene oxide (GO) sheets using direct and indirect amidation processes to obtain the amine-modified GO (MGO-1 and MGO-2). Successful grafting of p-toluidine at the graphitic framework of graphene oxide was confirmed using infrared spectroscopy and ultraviolet-visible spectroscopy. X-ray photoelectron spectroscopy showed the simultaneous reduction and modification. The reduced weight loss, increased initial decomposition temperature and enhanced residue formation indicate significant improvement of the thermal stability of MGO compared to GO. In addition, MGO-2 has greater thermostability and hydrophobic properties than MGO-1, with a water/air contact angle of ∼126.4°. The hydrophobic and thermostable MGO material could be applied to separate organic contaminants from water, which has great potential in environmentally friendly, low-cost advanced liquid–liquid separation or water treatment technologies.
Introduction
Materials researchers have shown exponentially increased interest in graphene since its discovery in 2004 by Geim et al.1 because of its remarkable physical, chemical and biological characteristics.2–5 Many potential applications6–8 such as nanocomposites, actuator and batteries have been actively pursued. Liu et al. studied the divalent metal (Cu, Ca or Mg) ion-coordinated oxidized carbon nanotube/graphene network, which can be widely used in transparent conductive films9 and nanocomposites.10 Usually, graphene is chemical stable and has poor solubility in some solvents, so it is difficult to be surface-modified. Graphene oxide (GO), graphene and reduced GO are indispensable members of a large family of graphene nanomaterials.11 GO is a layered material, which is produced by the controlled oxidation of graphite.12 GO is usually prepared using the Hummers–Offeman method with strong concentrated oxidizing acids (HNO3 and H2SO4) and strong oxidizing salts (KClO3 or KMnO4).13–15 Earlier reports show that the generally accepted structure of GO sheets consists of epoxy and hydroxy groups in its basal plane and carboxyl groups on the edges of the basal planes.12,16 Among the carboxyl groups, the carboxyl group on the edge has the highest reactivity and undergoes various types of reactions with some attached molecules.17 Numerous investigations have been conducted on covalent or noncovalent modification of the carboxyl functional group in the GO layers. Bourlinos et al.18 and Cao et al.19 described the functionalization of graphene oxide with amine-containing species (the amine groups originating from aliphatic amine and long chain alkyl amine, respectively). Yu et al.15 synthesized functionalized GO by covalently bonding the carboxyl groups on GO nanosheets and 2-amino-4,6-didodecylamino-1,3,5-triazine (ADDT) and achieved GO–ADDT with enhanced thermal stability. In general, the noncovalent modification of GO involves hydrophobic effects, π-stacking interactions, van der Waals forces and electrostatic interactions.20 Yang et al. prepared a GO-based carrier, where a hydrophobic drug molecule (DOX) is strongly stacked onto GO sheets because of the effect of π-stacking interactions between the aromatic domains of the graphene plane and the DOX.21 Moreover, non-covalent modification is also common method, Liu et al.22 developed a simple method to produce high-concentration solutions of graphene sheets noncovalently modified poly(styrene-co-butadiene-co-styrene) (SBS), which has an excellent electrical conductivity with a good percolation threshold.Recently, the control of surface wettability has aroused great interest because of numerous applications,2,23 such as corrosive-resistant coating and drug delivery. There are various reports on the hydrophilic modification of GO sheets; such functionalization has been confirmed to be possible by Dai et al., who explored the properties of hydrophilic PEGylated GO for cell imaging.24 Amine-terminated were grafted onto GO flakes via carbodiimide activation chemistry through the formation of amide bonds. The same group also designed a drug delivery system for water-insoluble cancer drugs based on the PEGylation of GO nanosheets.2 They synthesized and functionalized nanoscale GO (NGO) sheets (<50 nm) using branched, highly hydrophilic and biocompatible polyethylene glycol. In another study, GO was functionalized based on the reaction of allylamine with carboxyl groups of the GO sheets, which can be used as additives in polymer-based composites and other fields.25 Additionally, ever-increasing research is currently devoted to study the hydrophobic surface of chemically modified GO materials. Moreover, the modification of hydrophobic GO has many potential applications,13,15,26 which range from miniaturized reactors and nanofillers to water treatment technology to separation organic contaminants from water using an environmentally friendly method. These attributes are achievable by appropriate chemical modification (covalent or noncovalent) of GO. From this viewpoint, Xue et al.13 used polyhedral oligomeric silsesquioxane (POSS) to modify GO sheets and simultaneously achieved superhydrophobic GO powders, which provided a versatile platform to construct marbles. Furthermore, Wang et al.25 reported the preparation of hydrophobic GO nanosheets via functionalizing with phenylisocynate through a solvothermal synthesis process, which effectively improved the dispersion of GO nanosheets in organic solvents. From the aforementioned literature reviews, the surface modification of GO sheets is clearly important in tuning their characteristic to produce new applied materials.
From a large variety of previous studies, two main strategies have been proposed to produce amine-modified GO (MGO) sheets. Many active acyl chloride groups were attached onto GO sheets by the treatment with thionyl chloride and subsequent substitution by other grafting molecules.14,27 Alternatively, coupling reactions were performed using several catalysts (e.g., carbodiimide compounds and 4-dimethylaminopyridine).5,17,24,28 Although numerous studies describe the two synthetic routes, the differences of both modified GO materials in terms of thermal stability and hydrophobicity have not been mentioned. Additionally, we also have a question: is a small molecule suitable modifier for significantly improving the wettability and thermostability of graphene oxide?
In this paper, we outline two processes for synthesizing hydrophobic and thermostable MGO (MGO-1 and MGO-2) using covalently grafting small molecule p-toluidine: (i) a direct amidation reaction between the amine groups and carboxyl groups of GO sheets in the presence of dicyclohexylcarbodiimide (DCC, as a catalyst) and (ii) an indirect amide formation between the amine groups of p-toluidine and the carboxyl groups of GO flakes by preparing acylated GO and simultaneously achieving the hydrophobic and thermostable amine-modified GO. Additionally, we conclude that a small, only including one benzene and simple groups (methyl and amino), can change the surface behaviors of GO. Fig. 1 presents the schematics of the synthesis routes of MGO. We are planning studies in our laboratory to evaluate their practical application.
Experimental
Experimental
GO was synthesized in a strong oxidizing reaction according to the modified Hummers method by our group.29 Thionyl chloride (SOCl2, >99.5%) and DCC (99%) were purchased from Tianjin Fengchuan Chemical Reagent Science And Technology Co., Ltd. and Tianjin Guangfu Fine Chemical Research Institute, respectively. Other chemical reagents, which included p-toluidine (99%), tetrahydrofuran (THF, 99%), and N,N-dimethylformamide (DMF, 99.5%) were analytically pure and purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. Anhydrous DMF was obtained using 4A molecular sieves from Tianjin Kemiou Chemical Reagent Co. Ltd. for 24 hours. Unless specifically noted, all other chemicals were used as received without further purification.Synthesis process of MGO-1
The obtained GO (0.1 g) was dispersed in a mixture of 120 mL DMF, 0.25 g p-toluidine, and 0.5 g DCC (as a catalyst). After a brief ultrasonication (30 min, 150 W, HQ300-VDE), the mixture was refluxed at 120 °C for 24 h. At the end of this reaction, the resultant solution was filtrated with a PTFE membrane filter (0.22 μm) and washed several times with ethanol and deionized water. After the filtration, the product was dried at 80 °C in a vacuum oven. The resultant was called MGO-1.Synthesis process of MGO-2
First, 0.1 g of GO was dispersed in 45 mL of SOCl2 in the presence of 2 mL anhydrous DMF, and this mixture was subjected to 30 min ultrasonic treatment with proper intensity (150 W, T < 25 °C). Then, the mixture was refluxed with magnetic stirring at 70 °C for 24 h. After this reaction was completed, the solution was repeatedly rinsed with anhydrous THF to remove the unreacted SOCl2 using vacuum filtration. Then, the product was refluxed in 1 mg mL−1 p-toluidine (250 mg) of DMF solution at 120 °C for 48 h. The resultant was filtered through a PTFE membrane with a 0.22 μm pore size and washed several times with excess ethanol and deionized water. The remaining solid was dried at 80 °C in vacuum overnight, and the final product was named MGO-2.Characterization
To detect the changes produced by the functional groups on the surface of the GO, Fourier transform infrared (FT-IR) spectroscopic characterizations of GO sheets, MGO-1 and MGO-2 were performed on a spectrometer (TENSOR37) in the range of 400–4000 cm−1 at a resolution of 2.0 cm−1. X-ray photoelectron spectroscopy (XPS) analysis was used to detect the element variation of the GO before and after modification. The thermogravimetric analysis (TGA) was performed on a STA409PC thermogravimetric analyzer (NETZSCH) with a heating rate of 10 °C min−1. The morphologies of GO, MGO-1, and MGO-2 were observed using a transmission electron microscope (TEM, H-7650). Ultraviolet-visible (UV-Vis) spectroscopy was measured with a UV-1901 spectrometer. The contact angle was measured using a contact angle analysis device (DSA100, KRUSS) at five different points. A scanning electron microscope (SEM, S-4800) was used to observe the rough surfaces of MGO-1 and MGO-2 films. The well-established finding that heating a DMF solution of GO results to the reduction and alteration of the GO sheets in the absence of any additional reagents has been taken into careful consideration. So the blank samples were also prepared and compared with the modified-graphene oxide materials (see experimental procedures in the ESI†).Results and discussion
Amine-modified GOs were imaged using TEM to differentiate two grafting paths in the morphologies. Fig. 2 displays the TEM images of GOs before and after the modification. The wrinkled GO sheets before modification (Fig. 2a) typically have a relatively smooth surface without impurities. The morphology of MGO-1 after modification via the first grafting path (Fig. 2b) significantly changes, and it is more crumpled, creased and scrolled30 than pristine GO. In contrast with the first approach, when p-toluidine was covalently grafted onto the surface of GO flakes using the other method, more broken aggregations and ragged defects can be observed in the TEM image (Fig. 2c). The smashing of the GO platelets, which occurs during the chemical reaction, may be relative to various active sites on the GO edges of the basal planes and the proximity of the active sites. These reactions result in inadequate space to pile up the phenyl groups on the edges of the GO sheets. Accordingly, the steric restrictions can structurally break down the GO sheets. A representative TEM image of GO in DMF presented in Fig. S1a† shows the similar morphology as pristine GO, while GO in p-toluidine of DMF showed randomly aggregated sheets loosely connected to one another and fewer folds than MGO (see Fig. S1 in ESI†). In general, the above TEM images demonstrate that new molecules were successfully introduced on the surface of GO. | ||
| Fig. 2 TEM images of (a) GO, (b) MGO-1, and (c) MGO-2 sheets that were deposited on a standard TEM grid. | ||
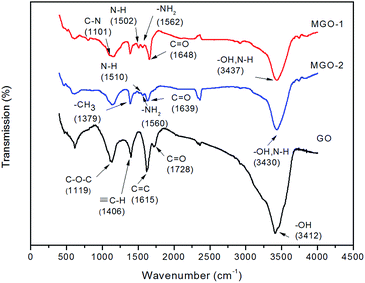
The changes in these processes of converting GO to MGO by grafting amine onto graphene were investigated using FT-IR spectroscopy as shown in Fig. 3. The curve of GO shows a broad absorption band at 3412 cm−1, which is attributed to the –OH groups. The characteristic absorption peaks at 1119, 1615 and 1728 cm−1 are typical stretching vibrations of C–O–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C
C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (–COOH),29 respectively. After the reaction with p-toluidine, the characteristic bands of –C–O–C–, –OH, and –COOH of GO decreased (both for two methods), implying the degree of reduction. The presence of the grafting molecule in MGO-1 and MGO-2 can also be confirmed using IR characterization. The characteristic absorption peaks at 3437, 1648, 1562 and 1502 cm−1 for MGO-1 and 3430, 1639, 1560 and 1510 cm−1 for MGO-2 are related to –OH (non-hydrogen bonded N–H), C
O (–COOH),29 respectively. After the reaction with p-toluidine, the characteristic bands of –C–O–C–, –OH, and –COOH of GO decreased (both for two methods), implying the degree of reduction. The presence of the grafting molecule in MGO-1 and MGO-2 can also be confirmed using IR characterization. The characteristic absorption peaks at 3437, 1648, 1562 and 1502 cm−1 for MGO-1 and 3430, 1639, 1560 and 1510 cm−1 for MGO-2 are related to –OH (non-hydrogen bonded N–H), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (–CONH–), –NH2, and N–H stretching,24,28,31 respectively, which reveals the attachment of the p-toluidine molecule to GO sheets through experiment protocols. The absorption peak at 1101 cm−1 of MGO-1 is assigned to the C–N stretching vibration, whereas that at 1379 cm−1 of MGO-2 represents the –CH3 bonding, which reveals the presence of the p-toluidine molecule.14,32 There is no obvious peaks in the spectrum of GO in DMF (Fig. S2 in ESI†).
O (–CONH–), –NH2, and N–H stretching,24,28,31 respectively, which reveals the attachment of the p-toluidine molecule to GO sheets through experiment protocols. The absorption peak at 1101 cm−1 of MGO-1 is assigned to the C–N stretching vibration, whereas that at 1379 cm−1 of MGO-2 represents the –CH3 bonding, which reveals the presence of the p-toluidine molecule.14,32 There is no obvious peaks in the spectrum of GO in DMF (Fig. S2 in ESI†).
UV-Vis spectroscopy was used to identify the existence of p-toluidine in amine-modified GO as a complementary analysis to confirm the FT-IR results of the samples. The UV-Vis spectra of GO, MGO-1 and MGO-2 in ethanol at a concentration of 0.05 mg mL−1 and the p-toluidine dilute ethanol solution as a comparison matter are illustrated in Fig. 4. The typical characteristics with a peak at 230 nm corresponds to the p–p* transitions of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in GO.4,24 The synthesized hydrophobic GOs by grafting p-toluidine contain non-polar benzene groups on the edges of the GO sheets. Consequently, the amount of non-polar benzene groups can make MGO sheets insoluble in deionized water. Furthermore, the absorption peaks at 235 nm and 291 nm of p-toluidine are also shown in Fig. 4. The peak absorption of MGO at ∼245 nm exhibits a slight red-shift compared with GO in the UV-Vis range, which suggests that the electronic conjugation restores within the graphene sheets because of the reduction of GO upon amine modification.33 Furthermore, the shifted peak of p-toluidine after reacting with GO from 271 nm to ∼330 nm can be attributed to the ground-state electron donor–acceptor interaction between the two components.21 The chemical process changes the chemical functional groups on the sheets, which may be responsible for this shift.24
C bonds in GO.4,24 The synthesized hydrophobic GOs by grafting p-toluidine contain non-polar benzene groups on the edges of the GO sheets. Consequently, the amount of non-polar benzene groups can make MGO sheets insoluble in deionized water. Furthermore, the absorption peaks at 235 nm and 291 nm of p-toluidine are also shown in Fig. 4. The peak absorption of MGO at ∼245 nm exhibits a slight red-shift compared with GO in the UV-Vis range, which suggests that the electronic conjugation restores within the graphene sheets because of the reduction of GO upon amine modification.33 Furthermore, the shifted peak of p-toluidine after reacting with GO from 271 nm to ∼330 nm can be attributed to the ground-state electron donor–acceptor interaction between the two components.21 The chemical process changes the chemical functional groups on the sheets, which may be responsible for this shift.24
The modification and reduction of graphene oxide sheets were characterized by XPS. The analysis of the chemical environment is of great importance as it illustrates the graphene derivatives. A significant decrease of the signals of the oxygen-containing groups at 286–291 eV, which corresponds to C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, indicated that the toluidine-modified graphene oxide was partial reduced (Fig. 5a). Typically, the C1s region of GO can be fitted using five components as presented in Fig. 5b. The binding energies at 285.0 eV, 286.3 eV, 287.2 eV, 288.2 eV, and 289.2 eV are assigned to C–C in graphite, C–OH, –C–O–C–, C
O groups, indicated that the toluidine-modified graphene oxide was partial reduced (Fig. 5a). Typically, the C1s region of GO can be fitted using five components as presented in Fig. 5b. The binding energies at 285.0 eV, 286.3 eV, 287.2 eV, 288.2 eV, and 289.2 eV are assigned to C–C in graphite, C–OH, –C–O–C–, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –O–C
O and –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. However, in the XPS spectra of MGO, new peaks appeared at 285.4 eV and 287.8 eV, due to C–N and N–C
O, respectively. However, in the XPS spectra of MGO, new peaks appeared at 285.4 eV and 287.8 eV, due to C–N and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, demonstrating the reaction of GO with p-toluidine (Fig. 5c and d). By the calculation of functional groups contents, the grafting degree of MGO-2 (1 p-toluidine molecule in every 7 graphene carbon atoms) is higher than MGO-1 (1 p-toluidine molecule in every 17 graphene carbon atoms), so the MGO-2 has greater thermostable and hydrophobic properties than MGO-1 (detailed calculation process in ESI†). Meanwhile, the corresponding N1s core level spectra also demonstrate this conclusion (see Fig. S3 in ESI†).
O, demonstrating the reaction of GO with p-toluidine (Fig. 5c and d). By the calculation of functional groups contents, the grafting degree of MGO-2 (1 p-toluidine molecule in every 7 graphene carbon atoms) is higher than MGO-1 (1 p-toluidine molecule in every 17 graphene carbon atoms), so the MGO-2 has greater thermostable and hydrophobic properties than MGO-1 (detailed calculation process in ESI†). Meanwhile, the corresponding N1s core level spectra also demonstrate this conclusion (see Fig. S3 in ESI†).
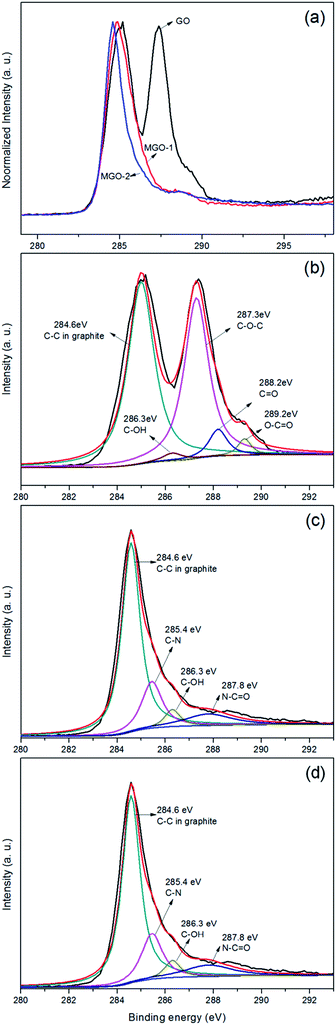 | ||
| Fig. 5 (a) XPS spectra of GO, MGO-1, and MGO-2, C1s region of (b) GO, (c) MGO-1, and (d) MGO-2. The N1s regions of p-toluidine-modified GO (see Fig. S3†). | ||
The thermostability of MGO materials was reflected in the enhanced thermal stability in both air and N2 atmosphere (Fig. 6). There is a slight weight loss for GO at approximately 100 °C in air (Fig. 6a) because of the removal of adsorbed water. GO also has a severe mass loss of 28 wt% at approximately 200 °C because of the loss of those labile oxygen-containing groups, which produce CO2, CO and steam.14 The final mass loss of GO is 40 wt% at 450 °C, which is associated with the decomposition of the stable functional groups and further carbonization of the graphitic substrate.13 However, MGO display different behaviors. For them, there is no weight loss below 100 °C mainly because of their hydrophobicity. Complete mass loss was observed for MGO-1 and MGO-2 in the range of 100–600 °C, which is 64 and 35 wt%, respectively. Additionally, the MGO materials also have higher thermal stability than pure p-toluidine in air. Even in N2 atmosphere (Fig. 6b), compared to the total mass loss of 63 wt% of GO, the total mass loss of MGO-2 is only 21 wt%. As an organic small molecule, p-toluidine shows a low thermal stability that was almost exhausted when the temperature was increased to 125 °C, whereas GO maintained continuous decomposition until the temperature reached 510 °C. Below 400 °C, MGO exhibits enhanced thermal stability. However, at higher temperature (>400 °C), MGO-1 showed a little larger mass loss than GO. The higher weight loss for MGO-1 is due to the decomposition of unsteadily bonded p-toludine.34 There is not similar phenomenon in MGO-2, it demonstrates that MGO-2 has more enhanced thermal stability. Nonetheless, both MGO-1 and MGO-2 exhibit less mass loss than GO in the temperature range of 25–600 °C. Based on these results, MGO materials clearly have more enhanced thermal stability than GO. The enhanced thermal stability of MGO can be attributed to the chemically bonded GO and p-toluidine. Comparing the two amine-modified GO materials, MGO-2 shows a higher residue mass of 68 wt% in air. The TG profiles in N2 (Fig. 6b) have similar results. Furthermore, as additional evidence, MGO-2 has a higher initial temperature than GO and MGO-1, which indicates that the second approach for amine modification is more favorable to improve the deoxygenation of GO sheets.
The presence of the aromatic segments of p-toluidine makes the hydrophilic GO hydrophobic, which is proven by the nondispersion of MGO in water. The hydrophobicity of MGO-1 and MGO-2 are intuitively observed using simple photographs (Fig. 7). The dispersibility of GO, MGO-1 and MGO-2 in deionized water was semi-qualitatively determined by mixing 2 mg of GO, MGO-1 and MGO-2 in 20 mL of deionized water, followed by sonicating with a sonicator at a power of 300 W for 1 h and resting for 24 h without treatment. Compared with the pristine GO, which shows easy dispersibility in deionized water, the synthesized MGO-1 and MGO-2 do not disperse at all in water, and only few fragments suspend in water. In contrast to MGO-2, MGO-1 entirely floats on water after a brief ultrasonic treatment because it has fewer flakes. Moreover, the bottom views show that the precipitates are clearly observed for the dispersed system compared to pristine GO in the bottom of the vial after 24 h. The dispersibility of MGO strongly depends on the structure and amounts of grafted organic moieties. In consequence, the conversion from the carboxylic groups (–COOH) to amide groups (–CONH–) is responsible for the much lower dispersibility of the amine-modified GO.
In this study, it is found that p-toluidine-modified GO tends to be hydrophobic. The resultants that were obtained using two grafting routes have notably different characteristics on the surface morphology of films. Fig. 8a was obtained from the GO aqueous solution after it was directly dried, whereas Fig. 8b and c were obtained after filtering the MGO-1 and MGO-2 ethanol solution through a PTFE membrane with pore size of 0.2 μm, respectively. Fig. 8d–f show the water droplet and air/water contact angles of GO, MGO-1, and MGO-2, respectively. It is reasonable to conclude that the increased contact angle from 58.7° for GO (Fig. 8d) to 118.2° for MGO-1 (Fig. 8e) and 126.4° for MGO-2 (Fig. 8f) is attributed to the surface chemistry change and the attachment of small molecules.
 | ||
| Fig. 8 SEM images of (a) GO, (b) MGO-1, and (c) MGO-2 films and (d), (e) and (f) their corresponding water droplet and air/water contact angles. | ||
It is noteworthy that the amine-modified GO has an increased water contact angle, and this feature is governed by both chemical composition and surface structure (or surface roughness).35–37 Regarding the rough surface that was treated with p-toluidine, the contact angle measurements indicate that the increased water contact angle value is significantly enhanced by the surface roughness. The SEM images (Fig. 8b and c) confirm the formation of a rough surface of the filtrated film of hydrophobic MGO-1 and MGO-2, whereas pristine GO (Fig. 8a) exhibits a relatively flat, smooth and compact surface.32,37 The formation of notably rough surfaces of the amine-grafted GO samples results from the solution filtration process and modification with p-toluidine. Generally, the flat, smooth and compact surface of GO is destroyed by grafting new groups during the intensely chemical process. Obviously, MGO-2 shows a more hydrophobic property because of the formation of a rougher surface by covalently bonding onto the GO sheets. However, no significant differences in contact angle values between pristine GO and GO in DMF at 120 °C for 24 h, 12 h and 48 h, respectively (see Fig. S4 in ESI†). The grafting of p-toluidine was responsible for the significantly improved hydrophobicity.
Conclusions
In summary, hydrophobic and thermostable amine-modified GOs were successfully synthesized using two grafting paths. p-Toluidine was covalently attached onto the surface of GO sheets using the two approaches, which were supported with FT-IR and UV-Vis spectra. The simple photographs of dispersibility before and after the modification confirm the preparation of hydrophobic MGO materials, which are notably consistent with the data from the contact angle tests. Moreover, the X-ray photoelectron spectroscopy showed the simultaneous reduction and modification. The reduced weight loss rate, increased onset decomposition temperature and greater residue formation in TGA testing indicate that MGO has a significantly better thermal stability than GO. In particular, compared with the two different grafting approaches, MGO-2 gains more thermostable and hydrophobic properties, and the experimental results validate the conclusion.Acknowledgements
Financial support from the Key Scientific and Technological Project of Sinopec Group (P14105), National Natural Science Foundation of China (11344007 & 51352002), and Natural Science Foundation of Tianjin, China (12JCZDJC27300 & 15JCZDJC37900) is gratefully acknowledged.References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- Z. Liu, J. T. Robinson, X. Sun and H. J. Dai, J. Am. Chem. Soc., 2008, 130, 10876 CrossRef CAS PubMed.
- W. Wei and X. Qu, Small, 2012, 8, 2138 CrossRef CAS PubMed.
- S. K. Singh, M. K. Singh, P. P. Kulkarni, V. K. Sonkar, J. A. Grácio and D. Dash, ACS Nano, 2012, 6, 2731 CrossRef CAS PubMed.
- S. Sahoo, P. Bhattacharya, G. Hatui, D. Ghosh and C. K. Das, J. Appl. Polym. Sci., 2013, 128, 1476 CAS.
- K. L. Xu, G. M. Chen and D. Qiu, Chem.–Asian J., 2015, 10, 1225 CrossRef CAS PubMed.
- Y. Zhao, L. Song, Z. P. Zhang and L. T. Qu, Energy Environ. Sci., 2013, 6, 3520 CAS.
- Y. T. Liu, X. D. Zhu, Z. Q. Duan and X. M. Xie, Chem. Commun., 2013, 49, 10305 RSC.
- Y. T. Liu, Q. P. Feng, X. M. Xie and X. Y. Ye, Carbon, 2011, 49, 3371–3375 CrossRef CAS.
- Y. T. Liu, M. Dang, X. M. Xie, Z. F. Wang and X. Y. Ye, J. Mater. Chem., 2011, 21, 18723–18729 RSC.
- A. Tiwari and S. K. Shukla, Advanced Carbon Materials and Technology, Scrivener Publishing LLC, 2014 Search PubMed.
- S. Stankovich, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Carbon, 2006, 44, 3342 CrossRef CAS.
- Y. Xue, Y. Liu, F. Lu, J. Qu, H. Chen and L. Dai, J. Phys. Chem. Lett., 2012, 3, 1607 CrossRef CAS PubMed.
- A. M. Shanmugharaj, J. H. Yoon, W. J. Yang and S. H. Ryu, J. Colloid Interface Sci., 2013, 401, 148 CrossRef CAS PubMed.
- X. Z. Tang, W. Li, Z. Z. Yu, M. A. Rafiee, J. Rafiee, F. Yavari and N. Koratoar, Carbon, 2011, 49, 1258 CrossRef CAS.
- H. Bao, Y. Pan, Y. Ping, N. G. Sahoo, T. Wu, L. Li and L. H. Gan, Small, 2011, 7, 1569 CrossRef CAS PubMed.
- M. Quintana, E. Vazquez and M. Prato, Acc. Chem. Res., 2012, 46, 138 CrossRef.
- A. B. Bourlinos, D. Gournis, D. Petridis, T. Szabo, A. Szeri and I. Dekany, Langmuir, 2003, 19, 6050 CrossRef CAS.
- Y. Cao, J. Feng and P. Wu, Carbon, 2010, 48, 1683 CrossRef CAS.
- V. Georgakilas, Functionalization of Graphene, Wiley-VCH Verlag GmbH & Co. KGaA, 2014 Search PubMed.
- X. Yang, X. Zhang, Z. Liu, Y. Ma, Y. Huang and Y. Chen, J. Phys. Chem. C, 2008, 112, 17554 CAS.
- Y. T. Liu, X. M. Xie and X. Y. Ye, Carbon, 2011, 49, 3529 CrossRef CAS.
- X. Yao, Y. Song and L. Jiang, Adv. Mater., 2011, 23, 719 CrossRef CAS PubMed.
- X. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. Dai, Nano Res., 2008, 1, 203 CrossRef CAS PubMed.
- G. Wang, B. Wang, J. Park, J. Yang, X. Shen and J. Yao, Carbon, 2009, 47, 68 CrossRef CAS.
- B. Duan, H. Gao, M. He and L. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 19933 CAS.
- S. Niyogi, E. Bekyarova, M. E. Itkis, J. L. McWilliams, M. A. Hamon and R. C. Haddon, J. Am. Chem. Soc., 2006, 128, 7720 CrossRef CAS PubMed.
- J. Shen, Y. Hu, C. Li, C. Qin and M. Ye, Small, 2009, 5, 82 CrossRef CAS PubMed.
- Z. J. Luo, H. Z. Geng, S. T. Zhang, B. T. Du, X. Zhang, J. Wang, Z. G. Lu, S. J. Ma, J. Q. Zhang, H. J. Yang and J. Wang, Mater. Sci. Forum, 2015, 809, 243 Search PubMed.
- W. Yu, H. Xie, X. Wang and X. Wang, Nanoscale Res. Lett., 2011, 6, 47 Search PubMed.
- Y. Lin, J. Jin and M. Song, J. Mater. Chem., 2011, 21, 3455 RSC.
- H. L. Ma, Y. Zhang, Q. H. Hu, D. Yan, Z. Z. Yu and M. Zhai, J. Mater. Chem., 2012, 22, 5914 RSC.
- H. L. Ma, H. B. Zhang, Q. H. Hu, W. J. Li, Z. G. Jiang, Z. Z. Yu and A. Dasari, ACS Appl. Mater. Interfaces, 2012, 4, 1948 CAS.
- Z. Lin, Y. Liu and C. Wong, Langmuir, 2010, 26, 16110 CrossRef CAS PubMed.
- T. Sun, G. Wang, L. Feng, B. Liu, Y. Ma, L. Jiang and D. Zhu, Angew. Chem., Int. Ed., 2004, 43, 357 CrossRef CAS.
- H. Liu, Y. Ding, Z. Ao, Y. Zhou, S. Wang and L. Jiang, Small, 2015, 11, 1782 CrossRef CAS PubMed.
- K. Liu and L. Jiang, Nanoscale, 2011, 3, 825 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19166a |
| This journal is © The Royal Society of Chemistry 2015 |