One-step synthesis of surface passivated carbon microspheres for enhanced photoluminescence and their application in multifunctional magnetic-fluorescent imaging
Tian Liu†
a,
Xunwei Liu†c,
Yanjie Yaoa,
Juan Zhoub,
Jun Zhu*b,
Gang Sun*c and
Dannong He*ab
aSchool of Materials Science and Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, P. R. China. E-mail: hdn_nercn@163.com
bNational Engineering Research Center for Nanotechnology, 28 East Jiang Chuan Road, Shanghai 200241, P. R. China. E-mail: yzjzhu@163.com
cDepartment of Medical Imaging, Jinan Military General Hospital, 25 Shifan Road, Jinan, Shandong 250031, P. R. China. E-mail: cjr.sungang@vip.163.com
First published on 18th February 2015
Abstract
Multimodality molecular imaging has recently attracted much attention, because it can take advantage of individual imaging modalities by fusing together information from several molecular imaging techniques. Herein, we report a bifunctional contrast agent connecting MR and luminescent imaging. The bifunctional contrast agent, carbon@Gd-DTPA microspheres, arise from carbon microspheres, which are synthesized on a large scale through a Na3cit-assisted solution route. The T1-agent, Gd-DTPA, is then conjugated to the carbon microspheres through N-ethyl-N9-[3-(dimethylamino)propyl]carbodiimide hydrochloride/N-hydroxysuccinimide (EDC/NHS) coupling chemistry using surface absorbed Na3cit molecules as an intermedium. Meanwhile, a formation mechanism of the carbon microspheres has been suggested. Furthermore, we also prove that the application of the carbon@Gd-DTPA bifunctional contrast agents for MR imaging and luminescent imaging can be established successfully. These results show that the primary Na3cit molecules have been confirmed to serve triplicate roles as an oriented agent to produce carbon microspheres, an intermedium to conjugate Gd-DTPA and surface passivation agents to improve photoluminescence.
Introduction
Gd-DTPA, named Magnevist commercially, is one of the most common clinically applied T1-weighted contrast agents due to its high magnetic resonance (MR) imaging efficiency. Recently, some functional contrast agents based on Gd-DTPA have been researched in order to overcome some Gd-DTPA limitations or design some multifunctional nanoprobes.1–4 Specially, some multimodal imaging or diagnose and therapy nanoprobes have been developed. For example, A targeted dual T1- and T2-contrast agent based on Fe3O4@SiO2(Gd-DTPA)–RGD nanoparticles was prepared and applied on MR imaging of other biological systems with high accuracy.5 Roux et al. prepared gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging.6 Li et al. reported a multimodality molecular imaging based nanoparticle for near-infrared to near-infrared upconversion luminescence, X-ray computed tomography and T1-enhanced magnetic resonance tri-modality in vivo imaging.7 Moreover, our group has made an effort to prepare multifunctional nanoprobes with MR and luminescent imaging based on quantum dot or upconverting nanoparticle, which is highly desirable due to their ability to be detected at two different modes, optical and T1-weighted magnetical imaging.8,9 However, given recent advances in nanoprobes, the research on the design and synthesis of the multifunctional composites based on Gd-DTPA with high luminescent efficiency and biocompatibility still remains a great challenge.To these days, the carbon based nanostructure is one of the most important group IV nanomaterials due to the environmentally benign element and relatively low cytotoxicity, and their surfaces can be functionalized with hydroxyl, carboxyl, and amino groups to make them water-soluble and be easier to crosslink with biomolecules. These different structured or shaped carbon nanomaterials, such as nanotubes, nanowires, nanospheres, porous carbon and hollow carbon spheres, have been synthesized and extensively applied.10–15 Specially, the emergence of photoluminescent carbon-based nanomaterials has presented exciting opportunities in the search for benign “nanolanterns”, which are highly desired in bioimaging, disease detection, and drug delivery because they are superior in chemical inertness, biocompatibility and potentially toxicity compared to fluorescent semiconductor nanocrystals.16–19 Actually, carbon is hardly considered as a luminescent material, but carbon nanoparticles could be made to brightly luminesce.20 Furthermore, intense research has focused on the preparation of carbon nanostructures with high photoluminescent efficiency through changing their structure or composition.21–24 Among them, the surface passivation of carbon nanoparticles is proved an effective strategy for enhancing multicolor photoluminescence. For example, a series of surface passivation agents has been used to improve the fluorescence of the carbon nanoparticles and their mechanism has been suggested by Liu et al.23,24
In the present work, we are aiming to synthesize carbon materials, which are used as a luminescent imaging agent. Moreover, the commercially applied T1 agent, Gd-DTPA, is grafted into the carbon material to develop a bifunctional contrast agent connecting MR and luminescent imaging. To prepare such the contrast agent, we have designed carbon microspheres via a Na3cit-assisted solution route. Furthermore, the surface absorbed Na3cit molecules used as an intermedium to conjugate Gd-DTPA to the carbon nanospheres through N-ethyl-N9-[3-(dimethylamino)propyl]carbodiimide hydrochloride/N-hydroxysuccinimide (EDC/NHS) coupling chemistry. Finally, a microsphere-like bifunctional contrast agent, carbon@Gd-DTPA, is obtained. To our surprise, the primary Na3cit molecules have been confirmed to serve triplicate roles as an oriented agent to produce carbon microsphere, an intermedium to conjugate Gd-DTPA and surface passivation agents to improve photoluminescence. The bifunctional contrast agents obtained in this study are potential candidates for biomedical engineering in the future.
Experimental section
Materials
Glucose, sodium citrate (Na3cit, C6H5Na3O7·2H2O), triethylamine (TEA), N-hydroxysuccinimide (NHS), dimethylsulfoxide (DMSO) and diethylenetriamine pentaacetic acid (DTPA) were analytical grade. All the chemicals above were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). N-Ethyl-N′-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDC, 98%) were purchased from ALDRICH.Synthesis of carbon microspheres
In a typical procedure to prepare carbon microspheres, 1.35 g glucose and 0.1 g Na3cit were dissolved into 15 mL distilled water. After being stirred at room temperature for 30 min, the solution was transferred into a Teflon-lined stainless steel autoclave with a capacity of 20 mL, and then it was heated at 160 °C for 6 h. Then the autoclave was cooled to room temperature naturally, and the samples were collected followed by washing with deionized water for several times, and drying at 60 °C in vacuum.Other samples were prepared under the similar reaction condition by changing Na3cit mass. The detailed reaction condition and corresponding results are summarized in Table 1.
| Sample | Na3cit mass | Morphology | ID/IG in Raman spectra |
|---|---|---|---|
| 1 | 0 g | Nanospheres with 400 nm in diameter | — |
| 2 | 0.1 g | Microspheres with 1.5 μm in diameter | 0.660 |
| 3 | 0.3 g | Microspheres with 4 μm in diameter | 0.645 |
| 4 | 0.5 g | Microspheres with 5 μm in diameter | 0.618 |
| 5 | Sample 2 modified by Gd-DTPA | Microspheres about 1.5 μm with a smooth surface | — |
Surface conjugation of Gd-DTPA
500 mg DTPA was dissolved into 50 mL dry DMSO and then activated orderly by reaction with 2000 mg TEA, 300 mg NHS and 300 mg EDC in the presence of nitrogen. The reaction mixture was kept stirring for 24 h at room temperature. Subsequently, 50 mg sample 2 was dispersed in 50 mL water through ultrasonication, and the solution was dropped into the above reaction system. 80 mg NHS and 80 mg EDC were quickly added into the reaction mixture, and then the mixture was stirred for 24 h at room temperature. The resulting products were collected after centrifuging and washing with ethanol for three times. Then, the products were sonicated again in distilled water to form a homogenous solution. Afterwards, 150 mg GdCl3·6H2O was added into the above solution and stirred for 24 h at room temperature. Finally, the Gd-DTPA modified carbon microspheres (labelled by carbon@Gd-DTPA) were obtained by centrifuging, washing and drying at 60 °C in sequence.Characterization
The morphology and energy dispersive X-ray (EDX) analysis of the samples were recorded on field-emission scanning electron microscope (FESEM, Hitachi, S-4800). Fourier transform infrared spectroscopy (FT-IR) and Raman spectroscopy were recorded on a Nicolet 6700 instrument and Renishaw inVia Raman microscope, respectively. Photoluminescence spectra were recorded using a Hitachi fluorescence spectrophotometer (F-7000).Measurement of MRI signals
All MRI scans were carried out with a 3.0 T whole body system (3.0 T Intera Achieva, Philips Medical Systems, Best, The Netherlands) with brain Crossed Coil. A dosage of 0, 0.1, 0.2, 0.4, 0.6 and 0.7 mg mL−1 obtained microspheres in deionized water were placed in a series of 45 mL tubes for T1-weighted MR imaging, using a standard spin echo sequence. The sequence parameters were TR/TE, 4.6/2.4 ms; flip angle, 12°; FOV, 26 cm; matrix, 320 × 256; effective slice thickness, 0.6 mm. The 3D MRIs were then reconstructed from the original data set at each time point using maximum-intensity projection (MIP).Confocal imaging of cells
Confocal imaging of cells was performed using a Leica laser scanning confocal microscope. HCT 116 cells (1 × 106 cells per mL) were incubated with carbon@Gd-DTPA for 2 h for confocal imaging, fixed with 4% paraformaldehyde for 30 min and stained by DAPI for 8 min. All cells were washed twice with cell culture medium before confocal imaging. Imaging of carbon@Gd-DTPA was carried out at 488 nm laser excitation, with its emission collected from 500 to 600 nm.Cell viability experiments
L929 cells (0.5 × 104 cells per well) in log phase were incubated into 96-well flat-bottomed plates (Costar, Charlotte, NC). The cells were incubated for 24 h at 37 °C under 5% CO2. The obtained microspheres at concentrations of 0, 0.001, 0.005, 0.01, 0.1 and 1 mg mL−1 in DMEM (Gibco, USA) were added to the wells of the treatment group, respectively. The cells were incubated for 12 h at 37 °C under 5% CO2. Then, all cells were removed from the medium and 5 mg mL−1 MTT (20 μL in a well) in PBS solution was added to each well and incubated at 37 °C for 4 h. Formazan extraction was performed with DMSO, followed by reading the optical density value at 492 nm using a plate reader to measure the amount of cell proliferation. The following formula was used to calculate the viability of cell growth: viability (%) = (mean of absorbance value of treatment group/mean absorbance value of control) × 100%. The results were expressed as an average over three nominally identical measurement.Results and discussion
Morphology and structure of the carbon microspheres
The morphology of the obtained products is characterized by field-emission scanning electron microscope (FESEM). As shown in Fig. 1a, the sample 1 is well-dispersed and uniform in size with an average diameter of about 400 nm. The result is similar to the reported literature without additives.14 However, the diameter of the obtained products is increased to about 1.5 μm if the mass of Na3cit is 0.1 g (sample 2, Fig. 1b), which implies that the carbon microspheres are produced. On further increasing the mass of Na3cit, the size of carbon microspheres is further enlarged (sample 3, Fig. 1c). When the mass of Na3cit is 0.5 g, nearly monodispersed carbon microspheres of about 5 μm in diameter can be obtained on a large scale (sample 4, Fig. 1d). The results show that Na3cit plays an important role in the fabrication of carbon microspheres. | ||
| Fig. 1 SEM images of obtained carbon architecture with different mass of Na3cit: (a) 0, (b) 0.1 g, (c) 0.3 g and (d) 0.5 g. | ||
To evaluate the effect of Na3cit on the structure of carbon microspheres, FT-IR spectra of the products were performed. As shown in Fig. 2, the bands at 1640 cm−1 of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations are observed in the absence of Na3cit (curve 1), which implies that the carbonization process from cross-linking by intermolecular dehydration has been induced in our experiment. Moreover, the other obvious bands in the range of 1000–1200 cm−1 are also found, resulting from C–OH stretching vibrations and –OH bending vibrations. The results reveal the existence of resulted conjugate structures and residual hydroxy groups. However, when Na3cit is introduced (curves 2–4), the band at 3300 cm−1 of –OH stretching vibrations is strengthened and widen, but the bands at 1000–1200 cm−1 are weaken, which implies that the Na3cit molecules could take part in the carbonization process. As we known, there are three COO− groups and –OH group in the Na3cit molecules, so besides intramolecular dehydration of glucose, there is intermolecular dehydration between Na3cit and glucose molecules. Thus, the obtained carbon microspheres would be modified by Na3cit molecules, which leads to the decrease of residual hydroxy groups and the increase of residual carboxyl groups, and further the association of carboxyl groups and hydroxy groups by hydrogen bonding. Therefore, the band at 3300 cm−1 could be strengthened and widen due to the association of carboxyl groups and hydroxy group by hydrogen bond, but the bands at 1000–1200 cm−1 could be weaken due to the decrease of residual hydroxy groups. Meanwhile, the new bands at about 1700, 1600 and 1380 cm−1 appear and their intensities are gradually improved with the addition of Na3cit. Generally, the bands at about 1600 and 1400 cm−1 are attributed to characteristic asymmetric and symmetric stretching vibrations of COO−, respectively, and the band at about 1700 cm−1 is attributed to characteristic COOH stretching vibrations. Therefore, the occurrence of the band at 1600 and 1380 cm−1 further proves that the carbon microspheres have been modified by Na3cit. Moreover, their intensity is increased and the band at 1600 cm−1 is shifted to lower wavenumber, which shows the strong interaction of residual carboxyl groups with the increasing of Na3cit mass. Additionally, the occurrence of the band at 1700 cm−1 also proves the strong hydrogen bonding interaction between carboxyl groups and hydroxy groups. Therefore, the above results reveal that Na3cit has an important effect on the morphology and structure of the carbon microspheres.
C stretching vibrations are observed in the absence of Na3cit (curve 1), which implies that the carbonization process from cross-linking by intermolecular dehydration has been induced in our experiment. Moreover, the other obvious bands in the range of 1000–1200 cm−1 are also found, resulting from C–OH stretching vibrations and –OH bending vibrations. The results reveal the existence of resulted conjugate structures and residual hydroxy groups. However, when Na3cit is introduced (curves 2–4), the band at 3300 cm−1 of –OH stretching vibrations is strengthened and widen, but the bands at 1000–1200 cm−1 are weaken, which implies that the Na3cit molecules could take part in the carbonization process. As we known, there are three COO− groups and –OH group in the Na3cit molecules, so besides intramolecular dehydration of glucose, there is intermolecular dehydration between Na3cit and glucose molecules. Thus, the obtained carbon microspheres would be modified by Na3cit molecules, which leads to the decrease of residual hydroxy groups and the increase of residual carboxyl groups, and further the association of carboxyl groups and hydroxy groups by hydrogen bonding. Therefore, the band at 3300 cm−1 could be strengthened and widen due to the association of carboxyl groups and hydroxy group by hydrogen bond, but the bands at 1000–1200 cm−1 could be weaken due to the decrease of residual hydroxy groups. Meanwhile, the new bands at about 1700, 1600 and 1380 cm−1 appear and their intensities are gradually improved with the addition of Na3cit. Generally, the bands at about 1600 and 1400 cm−1 are attributed to characteristic asymmetric and symmetric stretching vibrations of COO−, respectively, and the band at about 1700 cm−1 is attributed to characteristic COOH stretching vibrations. Therefore, the occurrence of the band at 1600 and 1380 cm−1 further proves that the carbon microspheres have been modified by Na3cit. Moreover, their intensity is increased and the band at 1600 cm−1 is shifted to lower wavenumber, which shows the strong interaction of residual carboxyl groups with the increasing of Na3cit mass. Additionally, the occurrence of the band at 1700 cm−1 also proves the strong hydrogen bonding interaction between carboxyl groups and hydroxy groups. Therefore, the above results reveal that Na3cit has an important effect on the morphology and structure of the carbon microspheres.
 | ||
| Fig. 2 FT-IR spectra of obtained carbon architecture with different mass of Na3cit: (1) 0, (2) 0.1 g, (3) 0.3 g and (4) 0.5 g. | ||
To further shed light on Na3cit effect, Raman spectra is presented. Fig. 3 shows the Raman spectra of the obtained products. There are no obvious peaks observed in the absence of Na3cit (curve 1). However, with the addition of Na3cit, the spectra exhibit two broad and strongly peaks at 1380 and 1580 cm−1, respectively, which shows that Na3cit is helpful for the crystalline graphite.25 Furthermore, the peak at 1380 cm−1 is usually associated with the vibrations of carbon atoms with dangling bonds for the in-plane terminations of disordered graphite, and it is labeled as the D-band. The peak at 1580 cm−1 (G-band) (corresponding to the E2g mode) is closely related to the vibration in all sp2 bonded carbon atoms in a 2-dimensional hexagonal lattice, such as in a graphene layer. The relative integrated intensity ratio of the D- and G-bands (ID/IG) reveals the graphite degree.26,27 Therefore, the Raman spectra of sample 2–4 are integrated and their values of ID/IG are calculated. As shown in Table 1, with the increasing the Na3cit mass, the values of ID/IG decrease, which further proves that Na3cit can contribute to the crystalline graphite of the carbon microspheres.
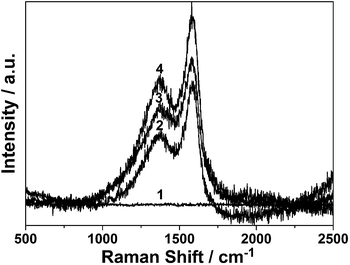 | ||
| Fig. 3 Raman spectra of obtained carbon architecture with different mass of Na3cit: (1) 0, (2) 0.1 g, (3) 0.3 g and (4) 0.5 g. | ||
Formation mechanism of the carbon microspheres
Based on the above results, the formation mechanism of carbon microspheres modified by Na3cit can be suggested to be the following: as shown in Fig. 4, similar to the reported process,14 the growth model for carbon microspheres includes the polymerization (step 1) and carbonization (step 2). For example, in absence of Na3cit, glucose molecules could be dehydrated each other. Thus, some aromatic compounds and oligosaccharides could be obtained in the polymerization step (step 1a). Subsequently, the carbonization may arise (step 2a), in which the intermolecular dehydration of linear or branchlike oligosaccharides results in the polysaccharides. Finally, these aromatic compounds and polysaccharides would produce the carbonized core and the hydrophilic surface of carbon spheres, respectively. However, according to our FT-IR results, besides the intermolecular dehydration of glucose, the intermolecular dehydration of glucose and Na3cit also could be happened under hydrothermal conditions with the addition of Na3cit. Thus, the hydroxy group of the oligosaccharides could be replaced by Na3cit molecule (step 1b), which could further produce the polysaccharides modified by Na3cit molecule (step 2b).Furthermore, these polysaccharides result in the hydrophilic surface of carbon spheres. And the increase of Na3cit mass could lead to more and more Na3cit molecules anchoring on the surface of carbon spheres. In other words, Na3cit molecules, used as a kind of passivation agent, take part in the formation of carbon spheres. Additionally, due to many carboxyl groups in Na3cit molecules, a few of hydrogen bonds on the hydrophilic surface of carbon spheres could be formed, which possibly enlarges the interspaces between polysaccharides chains. Therefore, the size of carbon spheres could be enhanced, and the carbon microspheres would be formed with increasing the Na3cit mass. Thus, we suggest that the Na3cit molecules play a role in the formation of carbon microspheres during the hydrothermal process as an oriented agent and a surface passivation agent.
To explore the effect of Na3cit on the surface passivation of the as-prepared carbon microspheres, we carried out a detailed photoluminescent (PL) study with different excitation wavelengths at 400 nm and 800 nm. Both in Fig. 5a and b, two PL peaks can be found without addition of Na3cit, such as 460 nm and 520 nm for 400 nm excitation (Fig. 5a) and 460 nm and 540 nm for 800 nm excitation (Fig. 5b), which is similar to the reported literature.28 Further observation finds that their intensity is gradually enhanced and their peaks are blue-shifted with the increasing of Na3cit, which shows that Na3cit has an important effect on PL properties of carbon spheres. According to the previous literature, the surface passivation processes and basic medium can contribute to the fluorescence enhancement and the increase in distribution of carbon nanoparticles emitting at shorter wavelengths, respectively.23,28 Therefore, it is reasonable that the pH increase with the Na3cit addition during hydrothermal process and the surface passivation effect of Na3cit are accomplished simultaneously when Na3cit is introduced into present reaction, which contributes to the fluorescence change.
 | ||
| Fig. 5 The photoluminescence spectra of obtained carbon architecture with excitation wavelengths at (a) 400 nm and (b) 800 nm with different mass of Na3cit: (1) 0, (2) 0.1 g, (3) 0.3 g and (4) 0.5 g. | ||
Surface conjugation of Gd-DTPA on the carbon microspheres
Citric acid or sodium citrate can always be used to treat the nanomaterial, which enables easy bioconjugation of nanomaterials with biomolecules in the biomedical applications through EDC/NHS coupling chemistry.29 In the present experiment, sodium citrate, used as a ligand, has been absorbed on the surface of carbon microspheres naturally in the preparation process. Thus, the Gd-DTPA conjugated carbon microspheres can be easily synthesized by conjugating activated DTPA through EDC/NHS coupling chemistry. The sample 5 was fabricated from the sample 2 by conjugating DTPA and complexing with Gd3+, and its SEM image is shown in Fig. 6a. The sample 5 exhibited uniform microspheres about 1.5 μm, which is similar to that of sample 1. However, closer observations found that the surface becomes smoother, which probably contributes to the coat by organic compound. Moreover, as shown in Fig. 6b, the EDX analysis of sample 5 confirmed the presence of the Gd elements, whose atom percentage is 1.73%. These results indicate that Gd3+ ions are successfully loaded on the carbon microspheres through conjugating with DTPA molecules. Therefore, the multifunctional magnetic-fluorescent carbon microspheres (carbon@Gd-DTPA) are obtained successfully. | ||
| Fig. 6 (a) SEM image and (b) energy dispersive X-ray analysis of carbon@Gd-DTPA microspheres (sample 5). | ||
The quantum yield (QY) of obtained carbon@Gd-DTPA microspheres were measured according to a reported literature,30 in which the rhodamine 6G ethanol solutions (QY = 95%) was chose as a standard. As shown in Fig. 7, the QY of carbon microspheres before and after loading Gd-DTPA is measured and calculated to be 0.9% and 3.4%, respectively. Additionally, the inset shows that the room-temperature PL spectra of carbon microspheres before and after loading Gd-DTPA, which reveals that carbon microspheres are successfully modified by Gd-DTPA. Compared with the values of sample 2, the intensity of sample 5 is dramatically increased, which could attribute to larger conjugated system after being modified by Gd-DTPA.
The luminescence and magnetic resonance imaging of carbon@Gd-DTPA were further investigated to evaluate their multifunction. Confocal imaging of HCT 116 cells was performed to evaluate the fluorescent imaging of living cells. As shown in Fig. 8a, the confocal image reveals the result of HCT116 cells incubated with carbon@Gd-DTPA microspheres for 2 h. Blue and green fluorescence emissions are recorded as HCT116 cells and carbon@Gd-DTPA microspheres under the excitation of 488 nm lasers, respectively. The result reveals that the carbon@Gd-DTPA microspheres have excellent fluorescent imaging and are partly internalized into cells through endocytosis, which demonstrates this newly developed carbon@Gd-DTPA microspheres can be used in cell tracer applications. On the other hand, MRI signals of carbon@Gd-DTPA microspheres were measured in vitro. Different mass concentrations of carbon@Gd-DTPA microspheres in the centrifuge tubes, as well as pure water for the background signal, were measured for their T1 relaxation time by a 3T MR imaging scanner. T1-weighted maps in Fig. 8b show that the T1-weighted MR imaging signal intensity is continuously enhanced, resulting in brighter images with increasing the mass. The above results further reveal that carbon@Gd-DTPA is successfully fabricated with luminescence and MR properties.
 | ||
| Fig. 8 (a) Confocal microscopy images of HCT 116 cells labeled with carbon@Gd-DTPA microspheres (sample 5), and (b) T1-weighted maps of various contents of carbon@Gd-DTPA microspheres (sample 5). | ||
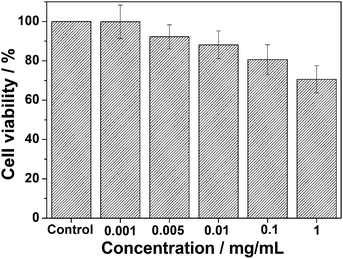
To examine the feasibility of the using of the obtained carbon@Gd-DTPA microspheres in biomedical applications, their cytotoxicity was investigated. As shown in Fig. 9, the effect of varying concentrations (0.001–1 mg mL−1) of carbon@Gd-DTPA microspheres on the viability of L929 cells after exposure for 12 h is revealed. The cellular viabilities decrease with an increasing carbon@Gd-DTPA microspheres concentration, and about 70% cell viabilities are maintained even up to a relatively high dose of 1 mg mL−1. Therefore, the MTT assay results demonstrate that the obtained microspheres have a low cytotoxicity.
Conclusions
Carbon microspheres have been successfully synthesized on a large scale via a Na3cit-assisted solution route. The growth process has been examined by controlling the role of Na3cit has been evaluated by changing the mass of Na3cit. A formation mechanism of the carbon microspheres has been suggested, which shows that Na3cit molecules serve both an oriented agent to produce carbon microsphere and surface passivation agents to improve photoluminescence. Furthermore, the surface absorbed Na3cit molecules are used as an intermedium to conjugate Gd-DTPA to the carbon microspheres through EDC/NHS coupling chemistry. Thus, the bifunctional contrast agent, carbon@Gd-DTPA microspheres, connecting MR imaging and luminescent imaging, are formed, in which carbon microspheres enable the possibility of fluorescent imaging and the grafted Gd-DTPA is highly efficient for T1-weighted MR imaging.Acknowledgements
The work is supported by Shanghai Rising-Star Program (13QB1402200).References
- M. Botta and L. Tei, Eur. J. Inorg. Chem., 2012, 12, 1945 CrossRef.
- X. J. Li, Y. F. Qian, T. Liu, X. L. Hu, G. Y. Zhang, Y. Z. You and S. Y. Liu, Biomaterials, 2011, 32, 6595 CrossRef CAS PubMed.
- H. W. Zhang, L. Q. Wang, Q. F. Xiang, Q. Zhong, L. M. Chen, C. X. Xu, X. H. Xiang, B. Xu, F. Meng, Y. Q. Wan and D. Y. B. Deng, Biomaterials, 2014, 35, 356 CrossRef CAS PubMed.
- Z. L. Cheng, D. L. J. Thorek and A. Tsourkas, Angew. Chem., Int. Ed., 2010, 49, 346 CrossRef CAS PubMed.
- H. Yang, Y. M. Zhuang, Y. Sun, A. T. Dai, X. Y. Shi, D. M. Wu, F. Y. Li, H. Hu and S. P. Yang, Biomaterials, 2011, 32, 4584 CrossRef CAS PubMed.
- C. Alric, J. Taleb, G. L. Duc, C. Mandon, C. Billotey, A. L. Meur-Herland, T. Brochard, F. Vocanson, M. Janier, P. Perriat, S. Roux and O. Tillement, J. Am. Chem. Soc., 2008, 130, 5908 CrossRef CAS PubMed.
- A. Xia, M. Chen, Y. Gao, D. M. Wu, W. Feng and F. Y. Li, Biomaterials, 2012, 33, 5394 CrossRef CAS PubMed.
- Q. Lu, D. X. Wei, J. J. Cheng, J. R. Xu and J. Zhu, J. Solid State Chem., 2012, 192, 75 CrossRef CAS PubMed.
- J. Zhu, J. Zhou, D. X. Wei and S. Y. Liu, CrystEngComm, 2013, 15, 6221 RSC.
- Y. C. Choi and W. Choi, Carbon, 2005, 43, 2737 CrossRef CAS PubMed.
- X. Y. Wang, H. Q. Wang, Q. F. Dai, Q. Y. Li and J. H. Yang, Colloids Surf., A, 2009, 346, 213 CrossRef CAS PubMed.
- D. S. Yuan, T. X. Zhou, S. L. Zhou, W. J. Zou, S. S. Mo and N. N. Xia, Electrochem. Commun., 2011, 13, 242 CrossRef CAS PubMed.
- Y. Li, J. F. Chen, Q. Xu, L. H. He and Z. M. Chen, J. Phys. Chem. C, 2009, 113, 10085 CAS.
- X. M. Sun and Y. D. Li, Angew. Chem., Int. Ed., 2004, 43, 597 CrossRef PubMed.
- M. H. Lan, J. F. Zhang, Y. S. Chui, H. Wang, Q. D. Yang, X. Y. Zhu, H. X. Wei, W. M. Liu, J. C. Ge, P. F. Wang, X. F. Chen, C. S. Lee and W. J. Zhang, J. Mater. Chem. B, 2015, 3, 127 RSC.
- L. Cao, X. Wang, M. J. Meziani, F. S. Lu, H. F. Wang, P. J. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S. Y. Xie and Y. P. Sun, J. Am. Chem. Soc., 2007, 129, 11318 CrossRef CAS PubMed.
- B. S. Harrison and A. Atala, Biomaterials, 2007, 28, 344 CrossRef CAS PubMed.
- K. Welsher, Z. Liu, D. Daranciang and H. Dai, Nano Lett., 2008, 8, 586 CrossRef CAS PubMed.
- P. W. Barone, S. Baik, D. A. Heller and M. S. Stroano, Nat. Mater., 2005, 4, 86 CrossRef CAS PubMed.
- J. Y. Fan and P. K. Chu, Small, 2010, 6, 2080 CrossRef CAS PubMed.
- R. L. Liu, D. Q. Wu, S. H. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598 CrossRef CAS PubMed.
- J. E. Riggs, Z. X. Guo, D. L. Carroll and Y. P. Sun, J. Am. Chem. Soc., 2000, 122, 5879 CrossRef CAS.
- X. Y. Zhai, P. Zhang, C. J. Liu, T. Bai, W. C. Li, L. M. Daic and W. G. Liu, Chem. Commun., 2012, 48, 7955 RSC.
- C. J. Liu, P. Zhang, F. Tian, W. C. Li, F. Li and W. G. Liu, J. Mater. Chem., 2011, 21, 13163 RSC.
- A. Ilie, C. Durkan, W. I. Milne and M. E. Welland, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 045412 CrossRef.
- C. D. Sheng, Fuel, 2007, 86, 2316 CrossRef CAS PubMed.
- A. Sadezky, H. Muckenhuber, H. Grothe, R. Niessner and U. Pöschl, Carbon, 2005, 43, 1731 CrossRef CAS PubMed.
- X. D. He, H. T. Li, Y. Liu, H. Huang, Z. H. Kang and S. T. Lee, Colloids Surf., B, 2011, 87, 326 CrossRef CAS PubMed.
- C. C. Mi, J. P. Zhang, H. Y. Gao, X. L. Wu, M. Wang, Y. F. Wu, Y. Q. Di, Z. R. Xu, C. B. Mao and S. K. Xu, Nanoscale, 2010, 2, 1141 RSC.
- J. N. Demasa and G. A. Crosby, J. Phys. Chem., 1971, 75, 991 CrossRef.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |