Carboxymethylpullulan promoted Cu2O-catalyzed Huisgen-click reaction†
Weiwei Zhang,
Baoqi Ren,
Yuqin Jiang* and
Zhiguo Hu*
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, P. R. China. E-mail: jiangyuqin@htu.cn; zghu@htu.cn
First published on 13th January 2015
Abstract
A combination of carboxymethylpullulan (CMP) and Cu2O has been developed as a highly efficient catalytic system for the Huisgen-click reaction. Our results indicate that the acidic CMP could be used for 6 cycles without decreasing the activating efficiency for Cu2O.
Introduction
By now click chemistry has emerged as a prominent organic transformation. The copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction is one of the most popular reactions within the click chemistry concept since being discovered by the groups of Sharpless1 and Meldal2 independently. The CuAAC reaction has attracted much attention and has been applied in the synthesis of pharmaceuticals, agrochemicals, dyes, corrosion inhibitors, biochemicals, polymers and functional materials due to its reliability, specificity and biocompatibility.3A plethora of copper catalytic systems have been utilized for CuAAC reaction including Cu(I) species,4 Cu(II) salts/reducing agents2,5 and metallic copper.6 Compared with other Cu(I) species, Cu2O is the most readily available and practical catalyst. Efforts were made to enhance the catalytic efficiency of Cu2O7–11 because the directly used Cu2O powder in the CuAAC reaction showed very poor catalytic efficiency.12 To date, the catalytic capability of Cu2O in CuAAC reactions has not been thoroughly exploited because of lacking appropriate reaction conditions. Very recently, inspired by the reported PhCOOH/Cu2O catalytic system by Hu et al.,8 we have reported Cu(OAc)2·H2O/NH2NH2·H2O as a highly efficient catalytic system for the CuAAC reaction, which in situ generates Cu2O-NPs and HOAc in water at room temperature.13 Our results indicate that the in situ generated HOAc plays an important role in the CuAAC reaction. While not only the PhCOOH/Cu2O catalytic system, but also the Cu(OAc)2·H2O/NH2NH2·H2O catalytic system has drawbacks due to the unrecoverable and unreusable properties of the used small molecular organic acid in the processes, which both show better activating efficiency for Cu2O.
Until now, polymers, as important supports, have been employed for immobilization of copper species for CuAAC reaction and resulted in easy removal, recovery and reusability of the copper catalyst.14 To solving the recyclability problem of small organic acid mentioned above, a water-soluble polymer linked with such kind of small molecular organic acid could be considered, which usually should not dissolve in common organic solvents, such as ethyl acetate, CHCl3, CH2Cl2. Therefore, after the 1,2,3-triazoles precipitated from the reaction mixture or extracted by water-insoluble organic solvents, the water-soluble polymers could still remain in the water phase for reusing. Carboxymethylpullulan (CMP), which has been widely studied in polymer chemistry,15 is a water-soluble, nontoxic derivative of pullulan with carboxylic acid groups linked to the main chain. The main aim of this work is to investigate the activated efficiency and the recyclability ability of CMP in the Cu2O-catalyzed CuAAC reaction. To the best of our knowledge, there is still no report of CMP as the activator for Cu2O on the CuAAC reaction. The work described in this paper selected Cu2O/CMP as a catalyst system for the CuAAC reaction in water and demonstrated that the CMP showed better recyclability ability for Cu2O than the reported PhCOOH and HOAc (Scheme 1).
Results and discussion
CMP was prepared by reaction of alkali-pullulan with sodium chloroacetate in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 isopropanol–water mixture according to the reported procedure.15a The Cu2O was prepared according our previous literature.13
1 isopropanol–water mixture according to the reported procedure.15a The Cu2O was prepared according our previous literature.13
The reaction between propargyl phenyl ether and azide benzyl was selected as the model reaction (Scheme 2) to test the catalytic efficiency of the Cu2O/CMP catalyst system. The reaction conditions were settled as follows: propargyl phenyl ether (1.0 mmol), azide benzyl (1.0 mmol), Cu2O (2 mol%), CMP (10 mol%), and water (2 mL). The influence of the amount of CMP on the isolated yields of the model reaction was investigated.
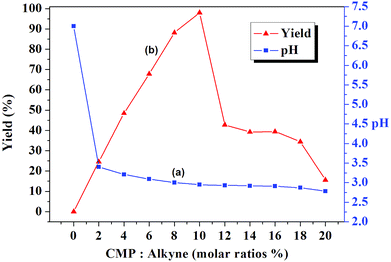
Initially, the pH values of different amount of CMP in 2 mL water were measured and the pH value of the used water is 7.00. As shown in Fig. 1, the pH value of the CMP solution greatly affected the isolated yield of the model reaction. When the amounts of the CMP (based on the alkyne) increased from 2 mol% to 20 mol%, the pH value decreased from 3.40 to 2.78 (line a), which is easy to understand that the more amount of acid the lower the pH value. The yields increased significantly over the concentration range of 2 mol% to 10 mol% (yield from 24.6% to 98.0%) and decreased over the concentration range of 10 mol% to 20 mol% (yield from 98.0% to 15.6%). The yield over the concentration range of 2 mol% to 10 mol% increased with the decreased of the pH values, which is consistent with our previous results that is the lower the pH value the faster the reaction rate.13 As we all known that Cu2O is almost insoluble in water, while in acidic water it becomes soluble and the Cu(I) ions are released, which is ready to disproportionate or be oxidized to copper(II). The lower the pH values, the faster the release rate. The CMP has two important roles in the Huisgen-click reaction. One is providing the needed protons, which promote the release of Cu(I) ions. The other role of CMP is acting as a stabling agent to prevent the disproportionation or oxidization of the released of Cu(I) ions. The in situ released Cu(I) in the reaction mixture catalyzed the Huisgen-click reaction between the added organic alkynes and azides. The more Cu(I) ions, the faster the reaction rate. While the contrary results obtained from the yields over the concentration range of 10 mol% to 20 mol%, which decreased from 98.0% to 15.6%. One reason is ascribed to the higher viscosity of the aqueous CMP solutions at the high concentration. Another reason is ascribed to the strong copper chelation ability of CMP. For proving this, special experiments were designed and carried out. To the round bottom flask, Cu2O (2 mol%), CMP (10 mol%), and water (2 mL) were added. After stirred for 6 min, propargyl phenyl ether (1.0 mmol) and azide benzyl (1.0 mmol) were added and stirred for another 20 min. After working up, only 40% isolated yield was obtained. It is worthy to be mentioned that when Cu2O (2 mol%), CMP (20 mol%), and water (2 mL) were put together and stirred for 6 min, coagulation was observed. The same experimental result was obtained when it was carried out under a nitrogen atmosphere. The above experimental results confirmed the copper(I) chelation ability of CMP. Therefore the optimized reaction was settled as follows: Cu2O (2 mol%), CMP (10 mol%), and water (2 mL) and the Cu2O (2 mol%) should be the last one to be added.
Secondly, the affection of the Cu2O-loading on the model reaction was investigated. The model reaction was carried out in water at room temperature in 20 min in presence of 10 mol% CMP. As shown in Table 1, it could be seen that the larger the catalyst loading, the faster the reaction rate (entry 1 to 6). The model reaction could be finished in 20 min by increasing the catalyst loading above 2 mol%. When decreasing the catalyst loading to 1 mol%, it would take 2 h to complete the reaction. Further reducing the catalyst loading to 0.5 mol% and 0.1 mol%, it would take 10 h and 17 h to finish the model reaction respectively. Unfortunately, when the catalyst loading was reduced to 0.05 mol%, very poor yield (8%) was obtained even after reacting for 24 h. For improving the reaction rate of the model reaction at lower catalyst loading, heating is considered as a simple and direct way.16 Very encouraged, the model reaction could complete in 20 min when catalyzed by 0.5 mol% Cu2O at 80 °C (entry 9). At the same condition mentioned above, the reaction could complete smoothly in 20 min even the amount of CMP reducing to 5 mol%. It is worth to mention that only 15% yield is obtained when catalyzed by 10 mol% Cu2O in absence of CMP (entry 8), which indicated the importance of the CMP. The results above encouraged us to study the effect of the reaction temperature on the model reaction with the relatively lower loading of Cu2O and CMP.
| Entry | Cu2O (mol%) | Timesa (min) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: propargyl phenyl ether (1 mmol), azide benzyl (1 mmol), CMP (10 mol%), H2O (2 mL), rt.b Isolated yields.c In absence of CMP.d Reaction temperature is at 80 °C.e CMP (5 mol%), H2O (2 mL), reaction temperature is at 80 °C. | |||
| 1 | 10 | 20 | 98/15c |
| 2 | 5 | 20 | 98 |
| 3 | 2 | 20 | 98 |
| 4 | 1 | 20 | 24 |
| 5 | 0.5 | 20 | 16 |
| 6 | 0.1 | 20 | 5 |
| 7 | 0.05 | 24 h | 8 |
| 8 | 0.05d | 24 h | 15 |
| 9 | 0.5d | 20 | 98 |
| 10 | 0.5e | 20 | 98 |
For investigating the effect of reaction temperature on the model reaction, the reaction conditions were settled as follows: propargyl phenyl ether (1.0 mmol), azide benzyl (1.0 mmol), Cu2O (0.5 mol%), CMP (5 mol%), and water (2 mL). The model reaction was carried out in 20 min at different temperature among the temperature range from 30 °C to 100 °C. As shown in Fig. 2, the higher the reaction temperature, the faster the reaction rate from 30 °C to 60 °C. When the temperature is above 60 °C, the yield obtained in 20 min is almost the same. So the optimized reaction condition is as follows: propargyl phenyl ether (1.0 mmol), azide benzyl (1.0 mmol), Cu2O (0.5 mol%), CMP (5 mol%) and water (2 mL) at 60 °C.
There is an interesting thing happened during working up the model reaction. When the reaction was extracted by ethyl acetate, a yellow organic phase and an almost colorless water phase were obtained (Fig. 4). It could be imaged that the in situ formed copper(I) ion might be coordinated with the obtained triazoles.17 The bright yellow organic phase could maintain for several weeks without changes. When the propargyl phenyl ether was replaced by phenylacetylene, the extracting phenomenon was the same, even using CH2Cl2 or chloroform as the extracting solvents. It was very interesting that when the pure obtained triazoles from the model reaction were stirred with CMP and Cu2O for 20 min and the mixture were extracted by ethyl acetate, the organic phase was colorless. This indicated that chelation of triazoles with copper(I) happened in the Huisgen-click reaction cycles, which should be responsible for the leaching of Cu(I) from the catalytic system. When the yellow organic phase was washed by aqueous ammonia, it became colorless and the water phase became blue. The results mentioned above proving that the copper almost transferred to the organic phase, thus the catalysis copper could not be recycled. Therefore the recyclability of the CMP was investigated. The model reactions were carried out at 60 °C in a relatively large scale: propargyl phenyl ether (10 mmol), azide benzyl (10 mmol), Cu2O (0.5 mol%), CMP (5 mol%), and water (20 mL). After the reaction mixture extracting by ethyl acetate, the remaining water phase was added the same amount of starting alkyne, azide and Cu2O. As shown in Fig. 3, after the model reaction carried out for 6 cycles, the yield showed slowly decreasing. In the ninth cycle, the yield decreased sharply to 50% and the pH values of the remaining water phase is 4.36. It could be imaged that the hydrogen ions were slowly consumed by Cu2O. When the Cu2O was replaced by CuOH (1 mol%), the same trend of pH and catalytic efficiency changes could be obtained. It was very interesting that after the remaining water phase was acidified by HCl to 2.82, the catalytic efficiency of CMP was recovered. The remaining water phase could be reused for another 6 times without notable yields changes. It is worth to mention that when the pure water in the absence of CMP was acidified by HCl to 2.82, the obtained yield is only 25.6% even if the reaction temperature is at 60 °C. It should attribute to the stable ability of CMP for copper(I) ion and the importance of CMP for Cu2O-AAC reaction.
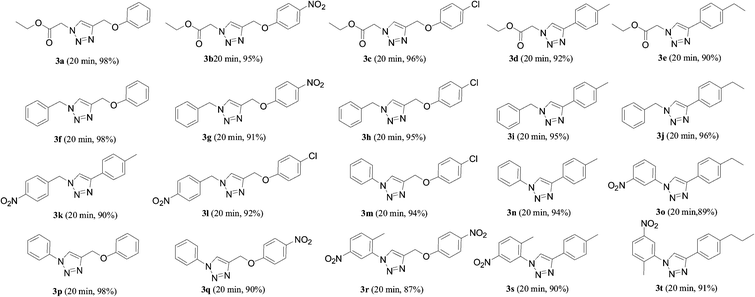
A wide range of diversely substituted terminal alkynes and azides were carried out under the optimized conditions in water at 60 °C. As shown in Table 2, the reactions work well not only with alkyl azides, but also with aryl azides. All reactions were highly regioselective towards the 1,4-disubstituted triazoles within 20 min.
The proposed mechanism (Scheme 3) for the reaction will be the same as established mechanism shown in earlier report.18 The CMP played important roles in the reaction:8,19–21 (a) CMP breaks the crystal structure of Cu2O to allow the formation of copper(I) species-highly efficient catalysts for CuAAC reaction assisted by the its acidity; (b) CMP− as a ligand coordinated Cu(I) of monomer to promote the formation of alkynyl-copper(I) intermediate (3) (step 2); (c) it had active effect on cycloaddition (step 3) and protonation of C–Cu bond (step 4); (d) the CMP comes into another cycle.
Conclusions
In summary, we presented a novel and high yield Cu2O/CMP catalyst system in water with a relatively short reaction time. Such a method will be interesting as the remaining water phase containing CMP could be reused for several runs and could be recovered by simply acidified by hydrochloride acid.Acknowledgements
This work was supported financially by the Natural Science Foundation of China (no. 21172058), Scientific Research Foundation for Doctors (no. 01036500508), Youth Foundation (2012QK11) of Henan Normal University, Foundation of Henan Educational Committee (no. 14A350005).Notes and references
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef PubMed.
- (a) M. Whiting, J. Muldoon, Y.-C. Lin, S. M. Silverman, W. Lindstrom, A. J. Olson, H. C. Kolb, M. G. Finn, K. B. Sharpless, J. H. Elder and V. V. Fokin, Angew. Chem., Int. Ed., 2006, 45, 1435 CrossRef CAS PubMed; (b) H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128 CrossRef CAS; (c) M. J. Giffin, H. Heaslet, A. Brik, Y. C. Lin, G. Cauvi, C.-H. Wong, D. E. McRee, J. H. Elder, C. D. Stout and B. E. Torbett, J. Med. Chem., 2008, 51, 6263 CrossRef CAS PubMed; (d) H. Rajaram, M. K. Palanivelu, T. V. Arumugam, V. M. Rao, P. Nicholas Shaw, R. P. McGeary and B. P. Ross, Bioorg. Med. Chem. Lett., 2014, 24, 4523 CrossRef CAS PubMed; (e) H. Nandivada, X. W. Jiang and J. Lahann, Adv. Mater., 2007, 19, 2197 CrossRef CAS; (f) C. F. Ye, G. L. Gard, R. W. Winter, R. G. Syvret, B. Twamley and J. M. Shreeve, Org. Lett., 2007, 9, 3841 CrossRef CAS PubMed; (g) P. Wu, A. K. Feldman, A. K. Nugent, C. J. Hawker, A. Scheel, B. Voit, J. Pyun, J. M. J. Frechet, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2004, 43, 3928 CrossRef CAS PubMed; (h) E.-H. Ryu and Y. Zhao, Org. Lett., 2005, 7, 1035 CrossRef CAS PubMed; (i) G. K. Such, J. F. Quinn, A. Quinn, E. Tjipto and F. Caruso, J. Am. Chem. Soc., 2006, 128, 9318 CrossRef CAS PubMed; (j) C. H. Wong and S. C. Zimmerman, Chem. Commun., 2013, 49, 1679 RSC; (k) W. P. Zhu, M. J. Zhong, W. W. Li, H. C. Dong and K. Matyjaszewski, Macromolecules, 2011, 44, 1920 CrossRef CAS; (l) Z. J. Zheng, F. Ye, L. S. Zheng, K. F. Yang, G. Q. Lai and L. W. Xu, Chem.–Eur. J., 2012, 18, 14094 CrossRef CAS PubMed; (m) X. B. Yao, Y. Gong, R. Mamuti, W. W. Xing, H. Zheng, X. Y. Tang and Y. Wang, RSC Adv., 2014, 4, 30492 RSC; (n) J. L. Huang, C. J. Li and D. G. Gray, RSC Adv., 2014, 4, 6965 RSC.
- (a) S. Díez-González and S. P. Nolan, Angew. Chem., Int. Ed., 2008, 47, 8881 CrossRef PubMed; (b) S. Díez-González, E. D. Stevens and S. P. Nolan, Chem. Commun., 2008, 39, 4747 RSC; (c) B. W. T. Gruijters, M. A. C. Broeren, F. L. Delft, R. P. Sijbesma, P. H. H. Hermkens and F. P. J. T. Rutjes, Org. Lett., 2006, 8, 3163 CrossRef CAS PubMed; (d) S. Özçubukçu, E. Ozkal, C. Jimeno and M. A. Pericàs, Org. Lett., 2009, 11, 4680 CrossRef PubMed; (e) J. García-Álvarez, J. Díez and J. Gimeno, Green Chem., 2010, 12, 2127 RSC; (f) L. Li, P. S. Lopes, V. Rosa, C. A. Figueira, M. A. Lemos, M. T. Duarte, T. Aviles and P. T. Gomes, Dalton Trans., 2012, 5144 RSC; (g) S. Lal, J. McNally, A. J. P. White and S. Díez-González, Organometallics, 2011, 30, 6225 CrossRef CAS.
- (a) K. B. Sharpless, V. V. Fokin, L. G. Green and V. V. Rostovtsev, Angew. Chem., Int. Ed., 2002, 114, 2708 CrossRef; (b) F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210 CrossRef CAS PubMed.
- (a) L. D. Pachón, J. H. Maarseveen and G. Rothenberg, Adv. Synth. Catal., 2005, 347, 811 CrossRef; (b) G. Molteni, C. L. Bianchi, G. Marinoni, N. Santo and A. Ponti, New J. Chem., 2006, 30, 1137 RSC; (c) I. S. Park, M. S. Kwon, Y. Kim, J. S. Lee and J. Park, Org. Lett., 2008, 10, 497 CrossRef CAS PubMed.
- Z. Zhang, C. Dong, C. Yang, D. Hu, J. Long, L. Wang, H. Li, Y. Chen and D. Kong, Adv. Synth. Catal., 2010, 352, 1600 CrossRef CAS.
- C. Shao, R. Zhu, S. Luo, Q. Zhang, X. Wang and Y. Hu, Tetrahedron Lett., 2011, 52, 3782 CrossRef CAS PubMed.
- H. López-Ruiz, J. E. D. L. Cerda-Pedro, S. Rojas-Lima, I. Pérez-Pérez, B. V. Rodríguez-Sánchez, R. Santillan and O. Coreño, ARKIVOC, 2013, 139 Search PubMed.
- M. N. S. Rad, S. Behrouz, A. Movahedian, M. M. Doroodmand, Y. Ghasemi, S. Rasoul-Amini, A.-R. A. Gandomani and R. Rezaie, Helv. Chim. Acta, 2013, 96, 688 CrossRef CAS.
- K. Wang, X. Bi, S. Xing, P. Liao, Z. Fang, X. Meng, Q. Zhang, Q. Liu and Y. Ji, Green Chem., 2011, 13, 562 RSC.
- (a) F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Eur. J. Org. Chem., 2010, 2010, 1875 CrossRef; (b) Please see ESI† of the following report: E. J. Yoo, M. Ahlquist, S. H. Kim, I. Bae, V. V. Fokin, K. B. Sharpless and S. Chang, Angew. Chem., Int. Ed., 2007, 46, 1730 CrossRef CAS PubMed; (c) D. Wang, N. Li, M. Zhao, W. Shi, C. Ma and B. Chen, Green Chem., 2010, 12, 2120 RSC.
- Y. Q. Jiang, D. Y. Kong, J. L. Zhao, Q. H. Qi, W. Li and G. Q. Xu, RSC Adv., 2014, 4, 1010 RSC.
- (a) C. Girard, E. Önen, M. Aufort, S. Beauvière, E. Samson and J. Herscovici, Org. Lett., 2006, 8, 1689 CrossRef CAS PubMed; (b) A. Sarkar, T. Mukherjee and S. Kapoor, J. Phys. Chem. C, 2008, 112, 3334 CrossRef CAS; (c) S. Roy, T. Chatterjee and Sk. M. Islarm, Green Chem., 2013, 15, 2532 RSC; (d) R. B. Nasir Baig and R. S. Varma, Green Chem., 2013, 15, 1839 RSC; (e) T. R. Chan and V. V. Fokin, QSAR Comb. Sci., 2007, 11–12, 1274 CrossRef; (f) M. Chtchigrovsky, A. Primo, P. Gonzalez, K. Molvinger, M. Robitzer, F. Quignard and F. Taran, Angew. Chem., Int. Ed., 2009, 48, 5916 CrossRef CAS PubMed; (g) M. N. Tahir, R. Qamar, A. Adnan, E. Cho and S. Jung, Tetrahedron Lett., 2013, 54, 3268 CrossRef CAS PubMed; (h) L. Bonami, W. van Camp, D. van Rijckegen and F. E. Du Prez, Macromol. Rapid Commun., 2009, 30, 34 CrossRef CAS PubMed; (i) K. R. Reddy, K. Rajgopal and M. L. Kantam, Catal. Lett., 2007, 114, 36 CrossRef CAS; (j) U. Sirion, Y. J. Bae, B. S. Lee and D. Y. Chi, Synlett, 2008, 2326 CAS.
- (a) I. Bataille, J. Huguet, G. Muller, G. Mocanu and A. Carpov, Int. J. Biol. Macromol., 1997, 20, 179 CrossRef CAS; (b) G. Ali, C. Rihouey, D. Le Cerf and L. Picton, Carbohydr. Polym., 2013, 93, 109 CrossRef CAS PubMed; (c) G. Mocanu, Z. Souguir, L. Picton and D. Le Cerf, Carbohydr. Polym., 2012, 89, 578 CrossRef CAS PubMed; (d) M. Legros, P. Cardinael, V. Dulong, L. Picton and D. Le Cerf, Polym. J., 2008, 40, 1132 CrossRef CAS; (e) K. Horie, M. Sakagami, K. Masuda, M. Notoya, H. Hamana, T. Yoshikawa and K. Hirano, Biol. Pharm. Bull., 2004, 27, 1275 CrossRef CAS.
- Y. Q. Jiang, D. Y. Kong, J. L. Zhao, W. W. Zhang, W. J. Xu, W. Li and G. Q. Xu, Tetrahedron Lett., 2014, 55, 2410 CrossRef CAS PubMed.
- (a) D. Mendoza-Espinosa, G. E. Negrón-Silva, D. Ángeles-Beltrán, A. Álvarez-Hernández, O. R. Suárez-Castillo and R. Santillán, Dalton Trans., 2014, 7069 RSC; (b) D. Schweinfurth, K. I. Hardcastle and U. H. F. Bunz, Chem. Commun., 2008, 2203 RSC; (c) C. Deraedt, N. Pinaud and D. Astruc, J. Am. Chem. Soc., 2014, 136, 12092 CrossRef CAS PubMed.
- F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210 CrossRef CAS PubMed.
- N. Balcioglu, I. Uraz (Unalan), C. Bozkurt and F. Sevin, Polyhedron, 1997, 16, 327 CrossRef.
- C. Shao, X. Wang, J. Xu, J. Zhao, Q. Zhang and Y. Hu, J. Org. Chem., 2010, 75, 7002 CrossRef CAS PubMed.
- C. Shao, G. Cheng, D. Su, J. Xu, X. Wang and Y. Hu, Adv. Synth. Catal., 2010, 352, 1587 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14813d |
| This journal is © The Royal Society of Chemistry 2015 |