Chemoselective deprotection and deprotection with concomitant reduction of 1,3-dioxolanes, acetals and ketals using nickel boride†
Abstract
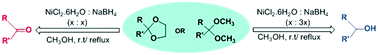
An efficient and mild methodology for the reductive deprotection of 1,3-dioxolanes, acetals and ketals to the corresponding aldehydes/ketones and also deprotection with concomitant reduction to the corresponding alcohols has been achieved in quantitative yields using nickel boride generated in situ from nickel chloride and sodium borohydride in methanol. The reactions are chemoselective as halo, alkoxy and methylenedioxy groups are unaffected.

 Please wait while we load your content...
Please wait while we load your content...