Photocatalytic inactivation of particle-associated Escherichia coli using UV/TiO2
Shilin Yua,
Tao Lin*ab and
Wei Chen*ab
aCollege of Environment, Hohai University, Nanjing 210098, PR China. E-mail: cw5826@hhu.edu.cn (W.C.); hit_lintao@163.com (T.L.)
bMinistry of Education Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes, Hohai University, Nanjing 210098, PR China
First published on 7th July 2014
Abstract
We investigated the photocatalytic inactivation of free Escherichia coli and E. coli attached to carbon particles using granulated activated carbon filter effluent as a water sample. The inactivation rates of both free and attached E. coli exposed to UV/TiO2 were higher than that using UV irradiation alone. However, attached E. coli was barely inactivated because of the protection of particles, which acted as hotspots for protection against inactivation of attached E. coli in the effluent. During inactivation, a lower UV irradiation intensity favored inactivation by UV/TiO2 for a given UV irradiation dose (irradiation intensity over time), because a longer irradiation period favored higher disinfection efficiency. Particles with a diameter >8 μm were predominantly responsible for protecting attached E. coli, and a higher particle concentration was associated with lower inactivation. The reactivation of E. coli after UV/TiO2 disinfection was lower than that after UV irradiation alone, and the reactivation rate decreased with increasing UV irradiation dose.
1. Introduction
Increasing numbers of particles are found in the effluent from drinking-water-treatment processes, especially those using granulated activated carbon (GAC) filters,1,2 as a result of particle accumulation during filtration. These particles pose a threat to water quality by acting as potential hotspots for bacterial attachment and providing protection for attached bacteria against disinfection.3 Furthermore, the natural organic matter adsorbed to particles may provide nutrients for the reproduction of attached bacteria,4 ultimately reducing the efficacy of the disinfection process. Therefore, the inactivation of pathogens attached to particles has been regarded as a key process for attenuating the concentration of viable pathogens in potable water.5,6 For decades, chlorination and chloramines have been widely used in drinking-water disinfection. However, insufficient inactivation of attached or resistant organisms, accompanied by the formation of disinfection by-products such as trihalomethanes (THMs), represent significant drawbacks of such conventional disinfection techniques.7 UV light can also be used as a disinfectant, but there are concerns that organisms may survive disinfection by being shielded from the UV light by particles. Methods that can inactivate attached bacteria whilst generating fewer byproducts are thus urgently needed in the field of drinking-water disinfection.Advanced oxidation processes (AOPs), including TiO2 photocatalysis, UV/O3 and UV/H2O2, are emerging processes for bacterial inactivation, among which TiO2 has attracted the most attention.8–11 The generated reactive oxygen species (ROS), excited by UV irradiation on TiO2, could easily inactivate the bacteria by strongly oxidizing the bacterial cell membrane.12 Consequently, combined with the UV irradiation, photocatalytic disinfection by UV/TiO2 is thus regarded as an emerging alternative or complement to conventional water-disinfection methods.13 However, previous studies have focused on free bacteria as the inactivation targets and there has been little research into the photocatalytic inactivation of attached bacteria.14,15 Free bacteria account for most of the bacterial population and are easily inactivated compared with attached bacteria, resulting in a relatively high bacterial disinfection rate and masking the poor inactivation of particle-associated bacteria.16,17
Recent reports of photocatalytic disinfection experiments have focused on using the TiO2 catalyst in suspension because slurry-type application has a relatively high disinfection efficiency associated with the presence of more surface active sites for photonic activation.18,19 However, post-separation of the catalyst represents another challenge because of the small scale of the catalysts and the potential for them to cause new pollution in the treated water.20
In this study, we therefore investigated the inactivation performance of UV/TiO2 immobilized on glass plates in relation to particle-associated bacteria. We investigated the effects of light intensity, which has been reported to be a major factor influencing inactivation performance,21,22 and of particle concentration and particle size on bacterial reactivation. GAC filter effluent from drinking-water-treatment processes was used as water samples and Escherichia coli was used as the target organism because of its wide use as a fecal indicator and its resistance to the bactericidal effects of solar irradiation.23 The results of this study provide important information regarding the control of bacteria, especially particle-associated bacteria, in treated water from drinking-water-treatment plants.
2. Materials and methods
2.1. Water samples and particle characterization
Water samples were taken from the effluent of a pilot GAC facility in Nanjing. In the treatment processes, a GAC filter (glass column) was set after sand filtration (Fig. 1). The parameters of the GAC column and the water-quality indexes of the effluent are listed in Table 1. The size distributions of the effluent particles from the GAC filter were divided into four ranges: 2–5 μm, 5–8 μm, 8–10 μm and >10 μm. Water samples (50 mL) were measured using a particle-calculating instrument (WHB1-IBR-B1, Interbasic Resources, USA). The particle distribution in water samples was shown in Fig. 2.| Flow rate (m h−1) | Diameter (mm) | Depth (m) | GAC (type) | Effluent bacterial (colony-forming units per mL) | Effluent particles (counts per mL) |
|---|---|---|---|---|---|
| 5–6 | 100 | 1.1 | Coal | 800–900 | 1800–2200 |
2.2. Bacterial culture and inoculation
A broth culture (100 mL) of E. coli K-12 (ATCC 10798) strain was inoculated in 100 mL of Luria–Bertani nutrient broth (Sigma Aldrich, USA) and incubated on a rotary shaker (KS501, IKA, Germany) at 37 °C overnight. One milliliter of the E. coli culture was then transferred into 100 mL fresh LB broth and incubated on the rotary shaker at 37 °C for more than 4 h. To prepare the reaction suspensions, 50 mL of the E. coli solution (at a concentration around 108 colony-forming units per mL) was centrifuged at 4000×g at ambient temperature for 10 min. The supernatant was discarded and the pellet was re-suspended and then transferred to a GAC effluent water sample.24 Prior to transfer of the dissolved pellet, the GAC effluent was autoclaved for 24 h at 121 °C to eliminate disturbance from other bacteria in the sample. After transfer, the inoculated water sample was transferred to an autoclaved glass beaker (autoclaved at 121 °C for 15 min) and fixed to a rotary shaker at 37 °C overnight to guarantee the effective attachment of E. coli to particles in the water sample. The particle concentration of the inoculated water sample was about 2 × 103 counts per mL.2.3. Photocatalytic disinfection system
Photocatalytic disinfection experiments were carried out in 2 L square glass reactors filled with 1 L of water sample (Fig. 3). The prepared TiO2 nanoparticles (P25 Degussa, USA) were immobilized on the internal glass walls of the reactor using the established method of dip-coating.25 The inoculated water sample was continuously stirred in the reactor with a magnetic stirrer (Style 85-2, Changzhou Guohua Equipment Co., Ltd, China). A UV lamp (T12, Yizheng Karmal Lighting Electronics Co., Ltd, China) placed in a hermetic cylinder (13 cm in diameter) and emitting radiation at 200–420 nm was used as the irradiation source. UV irradiation doses of 0–20 mJ cm−2 were generated by using UV lamps with different powers. The inactivation efficacies of three irradiation intensities (0.01 mW cm−2, 0.05 mW cm−2 and 0.10 mW cm−2, recorded using a digital light intensity meter; LUYOR-340, Shanghai Luyang Equipment Co., Ltd, China) were investigated. Experiments with UV irradiation alone were carried out in the same reactor, without immobilized TiO2.2.4. Preparation for particle analysis
To investigate effects of particle size on inactivation, autoclaving gauze filters with different pore sizes (Millipore, USA) were used to separate the particles in the GAC effluent (autoclaved for 24 h at 121 °C) into four size distributions: 2–5 μm, 5–8 μm, 8–10 μm and >10 μm. The filters were then cut in half aseptically and placed in a vessel containing 50 mL sterile water. Each vessel was shaken vigorously for 2 h to dislodge particles from the filter. The gauze was subsequently removed and the autoclaved GAC filtrate without particles was added to the vessel to give a particle concentration of about 2 × 103 counts per mL, determined using a particle-calculating instrument. This was the normal particle concentration in GAC effluent. The above bacterial inoculation process was used to obtain particle-associated E. coli.To investigate the particle-concentration effect, water samples with particle sizes of 5–8 μm were used as a target. In addition to a concentration of about 2 × 103 counts per mL, concentrations of 1 × 103 and 5 × 102 counts per mL were achieved by serial dilution. Samples were prepared at 37 °C under sterile conditions.
2.5. Separation of particle-associated E. coli from water samples
Separate autoclaving gauze filter was used to trap activated carbon fines from the GAC effluent, which was prepared by previously described methods.26 The concrete steps are as follows: a separate autoclaving gauze filter was used to trap particles from the water sample, which had previously been prepared as described above. One liter of water sample was filtered through the gauze filter, which was then aseptically cut in half and placed in a vessel containing 300 mL of cold, sterile, reagent-grade water. Each vessel was shaken vigorously to dislodge particles from the filter. The gauze was removed and the water samples were chlorinated with 2.0 mg L−1 sodium hypochlorite for 30 min at 4.0 °C (pH 6.5–7.0) in the dark.27 This chlorination procedure effectively eliminated unattached bacteria but had less influence on particle-associated bacteria.28 A homogenization technique was then used to desorb microorganisms from the particles, as described previously.262.6. Reactivation of E. coli
The reactivation of inactivated bacteria was investigated using visible light irradiation for 2 h at 30 °C.29 After inactivation, the treated water samples were placed in sterilized tube, which were then covered with a cap to avoided pollution by bacteria in the air. The closed tube was then irradiated by visible light. Reactivated E. coli showed little change and reached a stable state after 2 h under light in our preliminary experiments, and samples were therefore withdrawn from the closed tubes after 2 h for bacterial counts. The reactivation rate was calculated as follows: reactivation rate = (Nt − N0)/N0, where N0 and Nt represent the initial number of E. coli and the number at the sampling time during the reactivation processes, respectively.2.7. Bacterial count
E. coli were counted according to Method 1604.30 The inactivation efficiency was calculated using the following equation: inactivation efficiency = lg(N0/Nt), where N0 and Nt represent the initial number of E. coli and the number at the sampling time, respectively.2.8. Statistical analysis
Each sample was measured in triplicate. Results were reported as the mean of at least three measurements. Statistical analysis was performed using Origin 8.0 (OriginLab Co., USA). Values are expressed as mean ± standard deviation (SD). All data from different exposure sites were checked for normality (p < 0.05).3. Results and discussion
3.1. Photocatalytic inactivation of free and attached E. coli
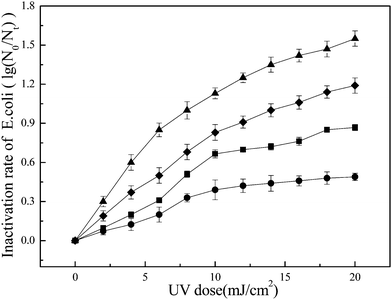
The inactivation rates of free and attached E. coli following exposure to UV/TiO2 and UV irradiation alone respectively are shown in Fig. 4. The inactivation rate increased with increasing UV dose. When the UV dose reached 20 mJ cm−2, the inactivation rate of free E. coli was near 1.2 lg, compared with >1.5 lg for UV/TiO2, indicating that UV/TiO2 caused greater inactivation of free E. coli. Regarding attached E. coli, the inactivation rate after exposure to UV alone was about 0.5 lg, compared with >0.8 lg with UV/TiO2. The inactivation rates of both free and attached E. coli exposed to UV/TiO2 increased faster at the beginning of exposure, compared with UV irradiation alone.During the process of water treatment, large numbers of tiny particles occur in GAC effluent.31 Some organic compounds that are absorbed by the GAC also remain in the effluent, and new granular substances are subsequently generated by the combination of these particles and organic compounds.32 The new particles gradually increase in size and develop extensive structures and micropores, which provide a suitable substrate for bacteria.33 The shielding effect of the particles may hinder the effects of UV irradiation on attached bacteria, while the particles, as well as absorbed organic compounds, may adsorb UV light and thus reduce the dose of UV irradiation reaching the E. coli.
During inactivation by UV/TiO2, activated electrons excite H2O and/or O2 molecules on the TiO2 membrane surface to form ROS such as hydroxyl radicals under UV irradiation. The ROS, which have a strong oxidizing capability, may increase the inactivation efficiency compared with UV irradiation alone. This result is in agreement with the experiments performed by Rincón and Pulgarin.24 During the process of inactivation, ROS may first contact E. coli and then oxidize its cell wall within under 5 min, followed by progressive damage to the cytoplasmic membrane and intracellular components.34 Experiments performed by Huang et al. demonstrated that treatment of free E. coli with TiO2 and light resulted in an immediate increase in the permeability of the bacterial cell wall to small molecules such as o-nitrophenyl-β-D-galactopyranoside, and the leakage of large molecules such as β-D-galactosidase, which correlate well with the loss of cell viability.35 Finally, significant disruption of cell permeability and the decomposition of the cell wall cause bacterial death,36 leading to the increased inactivation efficiency of UV/TiO2 against free E. coli compared with UV irradiation alone. UV/TiO2 disinfection inactivates E. coli via two mechanisms; UV radiation, and ROS produced by photocatalysis.37 In addition, the generated ROS can effectively oxidize the organic compounds absorbed on the particle surface, which mitigate the absorption of UV light and compete for available disinfectant including ROS and UV irradiation. In summary, the two direct pathway, namely UV and ROS attacking the bacteria, and an additional indirect pathway, namely attack of ROS on organic compounds, together contribute to photocatalytic inactivation towards attached E. coli, which was more efficiently than UV irradiation alone.
3.2. Photo-inactivation kinetic analysis
The kinetics of E. coli inactivation by UV typically follow the following first-order kinetic reaction:38| N0/Nt = ekt | (1) |
Lin et al. (2012) indicated that this equation was also applicable to UV dose by replacing t (reaction time) with d (UV dose with the unit of mJ cm−2) for photo-inactivation by UV/TiO2.39 In these experiments, eqn (1) was therefore changed to:
| N0/Nt = ek’d | (2) |
Plotting the data in Fig. 4 in the form of eqn (2) allowed the inactivation rate constants to be calculated from the slopes of the straight lines (Table 2). As can be seen, the k' value of the process via UV alone was smaller than that via UV/TiO2, reflecting the improved inactivation performance. As reflected by the lower R2 values, these results also indicate that generally-used first-order kinetics cannot comprehensively represent the inactivation of attached E. coli by UV/TiO2, because of the disturbance of particles during inactivation. UV/TiO2 disinfection involves a combination of physical and biochemical factors that affect the disinfection process. Xu et al. proposed nonlinear kinetics for particle-associated bacteria subjected to chloramine disinfection.40 During the inactivation of free E. coli via UV/TiO2, three main factors should be considered: the initial E. coli concentration, UV irradiation, and the generation of ROS. However, in the case of attached E. coli, the situation is complicated by the effects of the particles. These particles may act as hotspots, providing shelter for attached E. coli and preventing direct contact between attached bacteria and the generated ROS or direct UV irradiation. Secondly, the particles may scatter the UV irradiation, thus reducing the chance of light-harvesting for bacterial inactivation and catalytic activity. Thirdly, the organic compounds adsorbed by the particles may adsorb UV irradiation and thus compete for ROS with the attached bacteria. The effects of ROS transmission into the particles should also be considered. The various particle-associated parameters thus make it difficult to establish a simple and precise coliform-kill model for the inactivation of particle-associated bacteria. Further studies are needed to develop such a model.
| Treatment | Rate constant of free E. coli (R2 value) | Rate constant of attached E. coli (R2 value) |
|---|---|---|
| UV irradiation UV/TiO2 | 0.0637 (0.947) 0.0734 (0.921) | 0.0256 (0.819) 0.0457 (0.798) |
3.3. Effects of light intensity on inactivation of free and attached E. coli via UV/TiO2
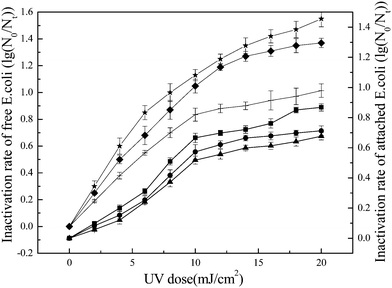
Three different UV irradiation intensities (0.01 mW cm−2, 0.05 mW cm−2 and 0.10 mW cm−2) were used to study the effects of light intensity on inactivation of E. coli by UV/TiO2. When the UV dose reached 20 mJ cm−2, the inactivation rate of free E. coli reached 1.5–1.6 lg at a light intensity of 0.05 mW cm−2 (Fig. 5). The inactivation rate for UV/TiO2 at a light intensity of 0.05 mW cm−2 was higher than the rate at 0.01 or 0.1 mW cm−2, which is in agreement with the results reported by Zhang and Liu.41 There are three steps in the reaction involving ROS: generation of the ROS, contact between ROS and the targets, and finally oxidation of the targets by ROS.42 The processes of ROS generation and oxidation are very fast, and the contact between ROS and the targeted E. coli is thus the key step in determining inactivation efficiency. The UV dose was determined by multiplying the irradiation time and light intensity. However, light intensity was the chosen constant parameter during treatment in the present study, while the accumulated UV dose was achieved by the prolonged irradiation time. Consequently, the chosen lower light intensity would result in a longer irradiation time for the same UV dose. Such longer irradiation times may increase the probability of contact between E. coli and ROS, ultimately improving inactivation performance.| Dose = I × t | (3) |
During photocatalytic inactivation, the generated electron–hole pair is key to determining the ROS production.43 However, photons and hole generations are accompanied by recombination, which is unfavorable in terms of the generation of ROS. Normally, the recombination rate is usually negatively related with the quantum yield.44 However, the higher light intensity would result in a lower the quantum yield, which could lead to increase in recombination rate.45 In conclusion, for the same UV dose, the higher light intensity, accompanied by shorter contact time between ROS and E. coli and the faster photons–hole recombination rate, disfavored the photocatalytic inactivation; when the light intensity is reduced, more photons may be lost because of the weak irradiation intensity.46 If so, UV irradiation intensity is an important factor affecting bacterial inactivation by UV/TiO2.
3.4. Effects of particle concentration and size on photocatalytic inactivation
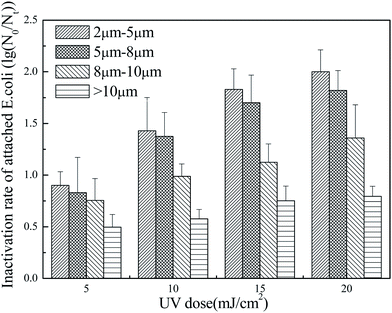
As can be seen, the inactivation rate of attached E. coli decreased with increasing particle size. Normally, larger particles have more complicated inner structures,49 which may provide more interspaces for the attachment of E. coli. The complicated structure may also affect the transport of ROS,41 which occurs over a short time because of the rapid recombination of electrons and holes;50 the ROS may disappear before coming into contact with E. coli, thus reducing the inactivation rate. Additionally, larger particles may have a stronger scattering effect on the UV irradiation, which would further disfavor photocatalytic inactivation.51 In these experiments, particles of 8–10 μm were associated with a relatively low inactivation rate and hindered photocatalytic inactivation; however, particles larger than 10 μm had an even greater inhibitory effect. In summary, the presence of particles larger than 8 μm should be controlled in GAC effluent.
3.5. Analysis of reactivation after photocatalytic inactivation
 | ||
| Fig. 8 Reactivation of attached E. coli inactivated by UV/TiO2 and UV irradiation alone at ambient temperature at a particle concentration of 2 × 103 counts per mL and a particle size of 5–8 μm. | ||
Photocatalytic inactivation by UV/TiO2 normally involves a sequence of cumulative attacks by ROS on the cell membrane-wall system.55 Previous studies defined two distinct phases during the destruction caused by photocatalytic inactivation.56 During the first phase, the cell membrane is the primary target of ROS, which destroy the external membrane permeability leading to the peroxidation of lipids that usually guard against ROS damage.57 In this phase, bacteria are less seriously affected and are able to repair themselves. During the second phase, once the cell membrane-wall system has been seriously damaged, ROS begin to attack the internal cellular components such as intracellular Coenzyme A, DNA, and RNA, making the cells unable to regain activity under visible light.58 However, intracellular peroxidation cause by UV irradiation alone is less than that caused by ROS,59 and the reactivation rate of attached E. coli after UV irradiation is therefore higher than after UV/TiO2. Additionally, Gelover et al. concluded that bacterial inactivation by UV/TiO2 significantly inhibited the reactivation of E. coli.60 Compared with UV irradiation, ROS generated by UV/TiO2 thus play a major role in controlling E. coli reactivation.
4. Conclusions
The results of this study showed that the inactivation of E. coli was enhanced by exposure to UV/TiO2 compared with UV alone. Attached E. coli were more difficult to inactivate than free E. coli because of the protective effects of the particles. Higher UV irradiation intensities were associated with poorer inactivation than lower intensities for the same UV dose. Compared with UV irradiation alone, E. coli inactivated by UV/TiO2 were less likely to regain activity. Furthermore, irradiation intensity is an important factor controlling the reactivation rate.Acknowledgements
Financial support was received from the National Natural Science Foundation of China (Project 51378173) and the Qing Lan Project.References
- S. Velten, D. R. U. Knappe, J. Traber, H. P. Kaiser, U. V. Gunten, M. Boller and S. Meylan, Water Res., 2011, 45, 3951–3959 CrossRef CAS PubMed.
- T. Lin, W. Chen and L. L. Wang, J. Environ. Sci., 2010, 22, 681–688 CrossRef CAS.
- S. E. Duirk and R. L. Valentine, Environ. Sci. Technol., 2007, 41, 7047–7053 CrossRef CAS.
- S. Velten, M. Boller, O. Koster, J. Helbing, H. U. Weilenmann and F. Hammes, Water Res., 2011, 45, 6347–6354 CrossRef CAS PubMed.
- J. Aristegui, J. M. Gasol, C. M. Duarte and G. J. Herndl, Limnol. Oceanogr., 2009, 54, 1501–1529 CrossRef CAS.
- A. Lapoussière, C. Michel, M. Starr, M. Gosselin and M. Poulin, J. Mar. Syst., 2011, 88, 434–445 CrossRef PubMed.
- J. P. Dietrich, H. Basagaoglu, F. J. Loge and T. R. Ginn, Water Res., 2003, 37, 139–149 CrossRef CAS.
- A. G. Rincón, C. Pulgarin, N. Adler and P. Peringer, J. Photochem. Photobiol., A, 2011, 139, 233–241 CrossRef.
- P. S. M. Dunlop, J. A. Byrne, N. Manga and B. R. Eggins, J. Photochem. Photobiol., A, 2002, 148, 355–363 CrossRef CAS.
- W. J. Jiang, J. A. Jones, D. D. Dionysiou and K. E. OShea, J. Photochem. Photobiol., A, 2013, 262, 7–13 CrossRef CAS PubMed.
- S. Zheng, W. J. Jiang, Y. Cai, D. D. Dionysiou and K. E. OShea, Catal. Today, 2014, 224, 83–88 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CAS.
- C. Drosou, A. Coz, N. P. Xekoukoulotakis, A. Moya, Y. Vergara and D. Mantzavinos, J. Chem. Technol. Biotechnol., 2010, 85, 1049–1053 CrossRef CAS.
- L. Rizzo, D. Sannino, V. Vaiano, O. Sacco, A. Scarpa and D. Pietrogiacomi, Appl. Catal., B, 2014, 144, 369–378 CrossRef CAS PubMed.
- N. Lydakis-Simantiris, D. Riga, E. Katsivela, D. Mantzavinos and N. P. Xekoukoulotakis, Desalination, 2010, 250, 351–355 CrossRef CAS PubMed.
- T. J. Zhang, Biomass Chem. Eng., 2009, 43, 54–59 CAS.
- M. R. Templeton, R. C. Andrews and R. Hofmann, Water Res., 2005, 39, 3487–3500 CrossRef CAS PubMed.
- J. M. Wang, C. Li, H. Zhuang and J. H. Zhang, Food Control, 2013, 34, 372–377 CrossRef CAS PubMed.
- R. L. Pozzo, J. L. Giombi, M. A. Baltanas and A. E. Cassano, Catal. Today, 2000, 62, 175–187 CrossRef CAS.
- G. C. C. Yang and C. J. Li, Sep. Purif. Technol., 2007, 58, 159–165 CrossRef CAS PubMed.
- E. O. Gómez, B. E. García, M. M. B. Martín, P. F. Ibanea and J. A. S. Perez, Catal. Today, 2013, 209, 195–200 CrossRef PubMed.
- O. Baghriche, S. Rtimi, C. Pulgarin, R. Sanjines and J. Kiwi, J. Photochem. Photobiol., A, 2013, 251, 50–56 CrossRef CAS PubMed.
- L. W. Gill and O. A. McLoughlin, J. Sol. Energy Eng., 2007, 129, 111–118 CrossRef CAS.
- A. G. Rincón and C. Pulgarin, Catal. Today, 2005, 101, 331–344 CrossRef PubMed.
- B. H. Moon, Y. M. Sung and C. H. Han, Energy Procedia, 2013, 34, 589–596 CrossRef CAS PubMed.
- A. K. Camper, M. W. LeChevallie and S. C. Broadaway, J. Microbiol. Methods, 1985, 3, 187–198 CrossRef.
- A. K. Camper, M. W. LeChevallie and S. C. Broadaway, Appl. Environ. Microbiol., 1986, 52, 434–438 CAS.
- M. W. Lechevallier, T. S. Hassenauer, A. K. Camper and G. A. McFeters, Appl. Environ. Microbiol., 1984, 48, 918–923 CAS.
- M. T. Guo, H. Y. Hu and W. J. Liu, Environ. Sci., 2008, 29, 1644–1648 Search PubMed.
- USEPA, Method 1604: total coliforms and Escherichia coli in Water by Membrane Filtration Using a Simultaneous Detection Technique (MI Medium), EPA 821-R-02-024, Office of Water, Washington, DC, 2002.
- L. L. Wang, W. Chen and T. Lin, Water Sci. Eng., 2008, 1, 102–111 Search PubMed.
- M. Schorer and M. Eisele, Water, Air, Soil Pollut., 1997, 99, 651–659 CAS.
- W. Chen, T. Lin and L. L. Wang, Front. Environ. Sci. Eng. China, 2007, 1, 1–6 Search PubMed.
- J. J. Wang, X. Liu, T. W. Ng, J. W. Xiao, A. T. Chow and P. K. Wong, Water Res., 2013, 47, 2701–2709 CrossRef CAS PubMed.
- Z. Huang, P. C. Maness, D. M. Blake, E. J. Wolfrum, S. L. Smolinski and W. A. Jacoby, Bactericidal mode of titanium dioxide photocatalysis, J. Photochem. Photobiol., A, 2000, 130, 163–170 CrossRef CAS.
- T. Saito, T. Iwase, J. Horis and T. Morioka, J. Photochem. Photobiol., B, 1992, 14, 369–379 CrossRef CAS.
- N. H. Zhang, K. F. Hu and B. H. Shan, Chem. Eng. J., 2014, 243, 7–13 CrossRef CAS PubMed.
- H. Mamanea, H. Shemer and K. G. Linden, J. Hazard. Mater., 2007, 146, 479–486 CrossRef PubMed.
- C. H. Lin, R. F. Yu, W. P. Cheng and C. R. Liu, J. Hazard. Mater., 2012, 209–210, 348–354 CrossRef CAS PubMed.
- H. Xu, W. Chen, C. Wang and L. Zhao, Fresenius Environ. Bull., 2011, 20, 2840–2846 CAS.
- Y. J. Zhang and W. J. Liu, China Water Wastewater, 2005, 21, 1–4 CAS.
- M. R. Templeton, R. C. Andrews and R. Hofmann, Water Res., 2005, 39, 3487–3500 CrossRef CAS PubMed.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- D. F. Ollis, E. Pelizzetti and N. Serpone, Environ. Sci. Technol., 1991, 25, 1522–1529 CrossRef CAS.
- Y. Meng, X. Huang, Y. X. Wu, P. Liang, H. Shi and Y. Qian, Environ. Sci., 2001, 22, 56–59 CAS.
- Z. Bohrerova and K. G. Linden, Water Environ. Res., 2006, 78, 565–571 CrossRef CAS.
- R. E. Cantwell and R. Hofmann, Water Res., 2008, 42, 2729–2735 CrossRef CAS PubMed.
- W. Chen, P. Dai, T. Lin, B. Luo and J. Huazhong, J. Univ. Sci. Technol., Nat. Sci. Ed., 2009, 37, 117–120 CAS.
- J. Ndounla, S. Kenfack, J. Wéthé and C. Pulgarin, Appl. Catal., B, 2014, 148, 144–153 CrossRef PubMed.
- L. Rizzo, A. D. Sala, A. Fiorentino and G. L. Puma, Water Res., 2014, 53, 145–152 CrossRef CAS PubMed.
- H. Mamanea and K. G. Linden, J. Environ. Eng., 2006, 132, 596–606 CrossRef.
- Y. Zhou, H. F. Zhang and H. M. Parikh, et al., Atmos. Environ., 2011, 45, 3882–3890 CrossRef CAS PubMed.
- K. Kollu and B. Ȍrmeci, Water Res., 2012, 46, 750–760 CrossRef CAS.
- P. Xiong and J. Y. Hu, Water Res., 2013, 47, 4547–4555 CrossRef CAS.
- V. S. Desai and M. Kowshik, Res. J. Microbiol., 2009, 4, 97–103 CrossRef CAS.
- A. G. Rincón and C. Pulgarin, Sol. Energy, 2004, 77, 635–648 CrossRef.
- A. G. Rincón and C. Pulgarin, Catal. Today, 2007, 124, 204–214 CrossRef.
- P. Pitsikas, M. A. Francis and A. J. Rainbow, J. Photochem. Photobiol., B, 2005, 81, 89–97 CrossRef CAS PubMed.
- S. Gelover, L. A. Gómez, K. Reyes and M. T. Leal, Water Res., 2006, 40, 3274–3280 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |