Novel functional cisplatin carrier based on carbon nanotubes–quercetin nanohybrid induces synergistic anticancer activity against neuroblastoma in vitro†
Orazio Vittorio‡
ab,
Miriam Brandl‡a,
Giuseppe Cirillo‡*cd,
Umile Gianfranco Spizzirric,
Nevio Piccic,
Maria Kavallarisabe,
Francesca Iemmac and
Silke Hampeld
aChildren's Cancer Institute Australia, Lowy Cancer Research Centre, University of New South Wales, NSW, Australia
bAustralian Centre for NanoMedicine, University of New South Wales, NSW, Australia
cDepartment of Pharmacy, Health and Nutritional Sciences, University of Calabria, Rende, CS, Italy. E-mail: giuseppe.cirillo@unical.it; Fax: +39 0984 493011; Tel: +39 0984 493011
dLeibniz Institute for Solid State and Materials Research Dresden, Dresden, Germany
eARC Centre of Excellence in Convergent Bio-Nano Science and Technology, Australia
First published on 1st July 2014
Abstract
The synergistic effects of a three-functional hybrid material composed of methacrylic acid (MAA), quercetin (Q) and carbon nanotubes (CNT), with cisplatin (CP) was evaluated in human neuroblastoma cells. A three-functional hybrid material suitable for CP combination therapy was synthesized through the free radical-induced reaction between methacrylic acid, quercetin and carbon nanotubes. Two-functional materials were prepared and fully characterised by coupling CNT and MAA, as well as MAA and Q. A Folin–Ciocalteu assay was used to assess the functionalisation degree expressed as mg of Q per gram of materials and we found to be 2.33 for CNT_PMAA_Q and 2.01 for PMAA_Q. The anticancer activity of CNT_PMAA_Q was shown in human neuroblastoma cells using the Alamar blue cell proliferation assay. Successively, cells were treated with a combination of CP and the nanocomposite showing strong synergistic anticancer effects in neuroblastoma cells. These studies showed that nanoparticle formulations incorporating quercetin and carbon nanotubes are good candidates for CP synergistic treatment against neuroblastoma.
Introduction
Neuroblastoma is the third most common type of childhood cancer after leukemia and brain tumours. It is a neuroendocrine tumour, arising from any neural crest element of the sympathetic nervous system.1 It most frequently originates in one of the adrenal glands, but can also develop in nerve tissues in the neck, chest, abdomen, or pelvis. Current therapy for patients with high-risk tumours predominantly consists of surgery and a cocktail of high dose chemotherapy. Despite this aggressive treatment approach, five-year survival for advanced stage neuroblastoma remains dismal.2 Among the anticancer drugs used for the treatment of neuroblastoma, one of the most common is cis-diamminedichloroplatinum(II) (cisplatin, CP), the first platinum based molecule introduced in clinical chemotherapy.3 Like other chemotherapy agents, CP has a number of toxic effects in normal healthy cells, leading to nausea, bone-marrow suppression, and nephrotoxicity,3 and improved delivery methods are required. Many efforts have been put on the development of more efficient therapeutic strategies, mainly involving the surgical removal of the tumour mass, or the combination use of radio- and/or chemo-therapies.4 However, these therapies can cause serious damage to healthy cells, highlighting the need for the development of more effective and less cytotoxic treatments.5To overcome the drawbacks of current chemotherapy,6 engineered drug nanocarriers have been developed,7 ranging from polymer- to lipid-based nanoparticles, carbon nanostructures and hybrid materials.8 Among the different nanostructures, carbon nanotubes have been widely explored by virtue of their superior chemical, optical, electrical, thermal, mechanical, and biological properties.9 CNT drug transporting capabilities, together with the ability to pierce through the cell membrane,8 makes these carbon nanostructures promising candidates for reducing the systemic toxicity often associated with conventionally administrated chemotherapeutics.9 CNT are able to resolve some clinical drawbacks of CP therapies,10 since they enhance CP anticancer efficiency by adhering to the tumour cells, thus increasing the local (near-cell) concentration of the drug.11 Two mechanisms were proposed to drive this effect: the encapsulation of CP (or CP pro-drugs) inside the CNT inner surface exploiting the capillary force,12 or the chemical functionalisation of the CNT outer surface with the drug.13 Furthermore, by simply adding CNT and CP, an enhanced drug efficacy was recorded.14
It should be highlighted that pristine CNT cannot be employed within biological systems due to serious toxicity concerns.15,16 Thus, several different functionalisation approaches have been developed to produce more effective drug delivery carriers.17 Amongst others, the preparation of nanohybrids composed of CNT and polymers was recently proposed, which offers the possibility to combine the biocompatibility of polymers and the efficient cellular uptake of CNT.18 A direct upgrade of this concept is the development of hybrid CNT–polymer therapeutic systems, in which the therapeutic agent is covalently linked to the polymeric backbone, in order to avoid the random bioavailability of low molecular weight drugs and consequently increase the drug's accumulation at the tumour site.4,9
We previously developed an innovative and efficient strategy to couple the favourable properties of a natural anticancer compound, namely the flavonoid quercetin (Q), the biocompatibility properties of methacrylate polymers (polymethacrylic acid, PMAA), and the cell interaction ability of CNT. The resulting nanocomposite materials, coded CNT_PMAA_Q, showed enhanced anticancer activity of the flavonoid on HeLa cancer cells and, more interestingly, less toxicity on non-malignant cells.19
In this paper, we investigated the ability of CNT_PMAA_Q to act as a functional carrier for the delivery of CP. The aim of the study is the development of a functional drug delivery system, in which the biological effect are related to both the loaded drug and the carrier itself, able to drastically minimise side effects in normal healthy cells. The effects of CP, nanocarrier, and their combination were extensively investigated in an in vitro model of neuroblastoma using IMR-32 cells.
Experimental
Synthesis of CNT, CNT_PMAA_Q, CNT_PMAA, and PMAA_Q
The synthesis of the materials used in this study was operated as follows (all chemicals purchased by Sigma-Aldrich, St. Louis, MO, USA):- CNT were synthesized by aerosol assisted chemical vapour deposition method as described.16 Ferrocene was selected as metal–organic catalyst precursor, cyclohexane as carbon source, and 100 sccm Ar as carrier gas flow, with the excitation frequency operating at 850 kHz. A thermal treatment at 450 °C in air for 1 h and a hydrochloric acid treatment were employed to remove the amorphous carbon and the catalyst particles from the as-grown material.
- CNT_PMAA_Q was synthesized as reported.19 Briefly, 0.02 g of CNT were dispersed in 10 ml of N,N-dimethylformamide (DMF) containing 10 mM ascorbic acid and 7 mM H2O2. The mixture was stirred at 25 °C for 2 h under atmospheric air. Subsequently, 0.2 g of methacrylic acid (MAA) and 0.5 g of Q were added and the mixture was maintained under stirring for further 18 h. The obtained nanocomposite was filtered, washed with 200 ml of water, 2-propanol, ethanol, and finally dried under vacuum overnight at room temperature.
- CNT_PMAA was prepared and purified under the same operating conditions used for CNT_PMAA_Q without adding Q, as described.20
- PMAA_Q was prepared under the same conditions used for CNT_PMAA_Q, without adding CNT as reported.21 The conjugate was purified by pouring the reaction mixture into acetone, followed by filtration of the precipitated polymer by sintered glass filter funnel (Pyrex, Ø 30 mm; porosity 3). Three dissolution/precipitation cycles (methanol–acetone) were operated and the obtained powder was eventually dried under vacuum overnight at room temperature.
HPLC analysis of the filtrates was employed to assess the absence of un-reacted species and thus the complete purification of the synthesized materials. The liquid chromatography consisted of a Jasco PU-2089 Plus liquid chromatography apparatus equipped with a Rheodyne 7725i injector (fitted with a 20 μl loop), a Jasco UV-2075 HPLC detector and Jasco-Borwin integrator (Jasco Europe s.r.l., Milan, Italy). A reverse-phase C18 Hibar column, 250 mm × 4 μm, particle size = 5 μm, pore size = 120 Å (Merck, Darmstadt, Germany), was employed. In accordance with literature data,19 the mobile phase adopted for the detection of Q was a 1% (v/v) aqueous solution of formic acid–acetonitrile–2-propanol (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) at a flow rate of 0.2 ml min−1.
8) at a flow rate of 0.2 ml min−1.
Characterisation procedures
FT-IR spectra were recorded as pellets in KBr in the range 4000–400 cm−1 using a Jasco FT-IR 4200 spectrophotometer with a resolution 1.0 cm−1 (Jasco Europe, Milan, Italy). UV spectra were recorded with a V-530 Jasco UV-Vis spectrophotometer using 1.0 cm quartz cells (Jasco Europe, Milan, Italy). The scanning electron microscope consists of a NOVA NanoSEM 200 (0–30 kV), and the transmission electron microscope were of a HRTEM/Tecnai F30 (300 kV) (FEI Company, Hillsboro, OR, USA). For morphological analyses, the samples were grounded in an agate mortar and deposited onto self-adhesive, conducting carbon tape (Plano GmbH, Wetzlar, Germany) for SEM analysis, while they were pressed between two small slides of aluminium foil on a Cu TEM grid (200 mesh, Plano GmbH, Wetzlar, Germany) for TEM. The Raman experiments were performed in a Raman Fourier transform spectrometer IFS 100, with a wavelength of 633 nm and a laser power of 8 mW by preparing the samples on an aluminium foil.The size and distribution of CNT_PMAA_Q were determined by dynamic light scattering (DLS) analysis using a 90 Plus Particle Size Analyzer (Brookhaven Instruments Corp, USA) at 25.0 ± 0.1 °C by measuring the autocorrelation function at 90°. The laser was operating at 658 nm.22 Zeta potential was determined with the laser Doppler electrophoretic mobility measurements using the Zetasizer 2000 (Malvern Instruments Ltd., Malvern, U.K.), at 25.0 ± 0.1 °C. The polydispersity index (PDI) and the zeta-potential were used for evaluating the dispersion properties. All the samples (1 mg ml−1 in distilled water) were analysed at different time intervals after the preparation (0.5, 24, 38, and 72 h). The same measurements were performed after CP loading.
Folin–Ciocalteu assay was selected to evaluate the amount of Q equivalent (mg) per gram of sample (all chemicals by Sigma-Aldrich).19 Briefly, in separate experiments, 10.0 mg PMAA_Q and CNT_PMAA_Q were dispersed in 6.0 ml distilled water and 1.0 ml Folin–Ciocalteu reagent. The mixtures were stirred thoroughly and, after 3 min, 3 ml Na2CO3 (2%, w/w) were added. After 2 h incubation with intermittent shaking, absorbance of filtered mixture was measured at 760 nm and crosschecked using the blank materials, PMAA and CNT_PMAA, respectively, under the same reaction conditions. Q equivalents were calculated through the equation of the calibration curve of the free Q, recorded by employing five different standard solutions of Q. 0.5 ml amount of each solution was added to the Folin–Ciocalteu system to raise the final concentrations of 8.0, 16.0, 24.0, 32.0, and 40.0 μM. After 2 h, the absorbance of the solutions was measured to record the calibration curve and the correlation coefficient (R2), slope and intercept of the regression equation obtained were calculated by the method of least squares.
In vitro drug release studies
In separate experiments, variable amount of CP was mixed with 1 mg ml−1 PMAA_Q, CNT_PMAA, and CNT_PMAA_Q and stirred at RT for 24 h. Thereafter, the products were collected and dried under vacuum at RT. Loaded nanocomposites (10 mg) were dispersed into 3.0 ml distilled water, and this solution was put into a dialysis tube (Mw cut-off 6000 g mol−1). The dialysis tube was put into a 50 ml Falcon tube with 37 ml phosphate-buffered saline (PBS 0.01 M. pH 7.4, 37 °C). At predetermined time intervals, two milliliters of the sample solution was taken for measurement of drug release, and the solution was supplemented with 2 ml fresh release medium (PBS). The release amount of CP was measured with the UV spectrophotometer at 310 nm. For comparison, 2 mg free CP was added to 5 ml PBS and magnetically stirred for 6 h. After that, the solution was introduced into a dialysis tube and a release test performed.23Cell culture
Human neuroblastoma cells (IMR-32) were cultured in DMEM medium supplemented with 10% FBS, 1% L-glutamate, and 1% penicillin–streptomycin. Cells were grown as a monolayer in a humidified atmosphere at 37 °C and in 5% CO2. All chemicals were purchased by Sigma-Aldrich.Cell growth inhibition assays
PMAA_CNT, PMAA_Q, and PMAA_CNT_Q were prepared in DMSO (stock solution 3.3 mM) and stored at RT. Clinical grade CP (1.0 mM) (Pfizer, US) was acquired from the local pharmacy and stored at RT. CP was loaded onto carriers by mixing suitable aliquots of the counterparts in water to raise the selected concentrations ratio (w/w). Samples were subsequently dried and re-dissolved in DMSO before use. Treatment effects on IMR-32 cell growth were measured on the basis of the metabolic activity of cells using Alamar Blue assays and spectrophotometric analysis. Briefly, cells were plated in clear transparent 96-well plates at an optimised cell density of 15 × 103 cells per well 48 h prior to treatment. Cells were then treated with either single compounds or their CNT combinations, and effects on cell growth assessed 72 h later. DMSO 0.1% was indicated as a further control as it was used as a carrier for compounds.Assessment of combination effects
The effect of combination treatment was quantitatively measured using the combination index (CI), which is based on dose–effect curves and median–effect equation and calculated according to eqn (1):
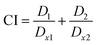 | (1) |
D1 and D2 denote doses of compound 1 and compound 2 required to reach x% effect as single treatment, while Dx1 and Dx2 denote the doses required of the compounds in combination to have a similar effect.24
Combination effects were examined for being antagonistic (CI > 1.1), nearly additive (0.9 < CI < 1.1), or synergistic (CI < 0.9). CIs were computed using the software CalcuSyn version 2.1 (Biosoft, Cambridge, UK).25
Statistical analysis
Three experiments were carried out in sextuplicate. Values are expressed as means ± standard error of the mean (SEM). Statistical significance was assessed by one-way analysis of variance followed by post-hoc comparison test (Tukey's test). Significance was set at p < 0.01.Results and discussion
Preparation and characterisation of CNT-PMAA_Q
The functional drug carrier was synthesized as described in the literature.19 The radical insertion of Q into growing polymethacrylic acid chains simultaneously linked to the CNT outer surface was raised by a radical reaction operating in mild conditions. Similarly, control materials made of polymeric and CNT components without Q, as well as of polymer and flavonoid without CNT have been prepared according to our previous publications.20,21 The inclusion of PMAA allowed us to develop hybrid composites (CNT_PMAA_Q and CNT_PMAA) and functional bio-conjugate (PMAA_Q). These hybrids composite were able to address a key requirement of any nanostructure for biological applications, water affinity. The synthesis, the purification and characterisation of all the compounds were optimised in our previous works.19–21The present study aims to extend the initial development on these hybrid nanomaterials and it is focused on selecting the best conditions to maximise the PMAA and Q content within the materials.
The physic-chemical characterisation was obtained by means of FT-IR to assess the presence of the Q and/or PMAA functionalities onto the samples, while UV-Vis and Raman analyses were used to assess the covalent coupling between PMAA and Q, and between PMAA and CNT, respectively (Fig. 1).
In the FT-IR spectrum of CNT_PMAA_Q, the presence of the flavonoid was evident by the band at 1523 cm−1 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching within the aromatic rings), while the peaks at 1725 and 1240 cm−1 (C
C stretching within the aromatic rings), while the peaks at 1725 and 1240 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O stretching of the carboxyl group of MAA residues) highlights the presence of the polymeric material (ESI†).
O and C–O stretching of the carboxyl group of MAA residues) highlights the presence of the polymeric material (ESI†).
The covalent introduction of the flavonoid into PMAA resulted in a bathochromic shift of the UV-Vis absorption peak of its aromatic portion, while the polymer linkage to the CNT outer surface was highlighted by the perturbation of the local order around the binding site resulting in a modification in the relative ratio between the D, G and D′ bands placed at 1310, 1577 and 1609 cm−1. A combined SEM/TEM analysis of both CNT_PMAA and CNT_PMAA_Q samples showed their surface morphology, characterised by a strong polymeric network connecting individual CNT filaments (Fig. 2).
Dynamic light-scattering (DLS) measurements provide information on the hydrodynamic diameter (Z-average) of CNT.26 Pristine CNT showed aggregates of various sizes and an average hydrodynamic diameter of around 1.8 μm, with a PDI of 0.312. The presence of big aggregates in the micrometre range, indicated a poor dispersivity as a result of the hydrophobic behaviour of the CNT outer surface. On the other side, the hydrodynamic diameter of CNT_PMAA_Q is 0.315 μm (PDI 0.05), since the MAA residues are hydrophilic and prevented the aggregation of CNT. These values are indicative of a better dispersion of CNT_PMAA_Q compared to pristine CNTs.22
Finally, by the Folin–Ciocalteu test, the functionalisation degree was determined and expressed as Q equivalent (mg) per gram of material. The results highlighted functionalisation degrees of 2.01 and 2.33 mg per gram of PMAA_Q and CNT_PMAA_Q, respectively. The higher value recorded in the sample containing CNT was related to the ability of CNT to undergo free radical reaction, thus increasing the number of possible binding sites available for Q.
Evaluation of anticancer activity
Neuroblastoma is often poorly responsive to therapy and combination therapy including CP is the standard chemotherapy for advanced-stage neuroblastoma with high initial platinum responsiveness.27 Unfortunately, due to the toxic side effects of combination chemotherapy patients often suffer lifelong health issues.The aim of this work is to evaluate the possibility of using the CNT_PMAA_Q composite as functional drug carriers for efficient CP delivery in neuroblastoma cells.
Initially, the release profile of CP from the nanohybrids was evaluated. Zeta potential measurements were used to evaluate the interaction of CP with the nanocomposite. CNT_PMAA_Q shows a negative zeta potential of −37.34 mV that decreases to −19.21 mV upon CP loading. According to the literature,23 the carboxylic moieties of MAA residues are involved in the CP coordination, and this result in a reduction of the negative charge and thus in a decreased zeta-potential.
More information can be obtained by the analysis of the CP release profile of the loaded materials at a CP and Q equivalent concentrations of 1.0 and 16.0 μM, respectively (Fig. 3).
 | ||
Fig. 3 CP dissolution ( ) and release profiles from CNT_PMAA_Q ( ) and release profiles from CNT_PMAA_Q ( ) and PMAA_Q ( ) and PMAA_Q ( ) in PBS at pH 7.4 and 37 °C. ) in PBS at pH 7.4 and 37 °C. | ||
The release profile of CP from the nanocomposite is mainly dependent on the presence of the –COO− groups on the nanocomposite, and thus the release is more sustained over time and it is completed in 180 h. Interestingly, the CP release patterns from PMAA_Q and CNT_PMAA are not significantly different, since the interaction between the drug and the composite occurs on the material surface, which is covered by the polymeric moieties.23
After this preliminary characterisation we evaluated the effect of only CNT_PMAA and PMAA_Q to investigate the biological activity of all the composite components.
Cell growth data showed that the CNTs coated with PMAA alone resulted in the formation of a compound that did not cause cell inhibition within the tested concentration range (Fig. 4A).
This is in accordance with the biocompatibility properties of PMAA, which are conferred to CNT after their functionalisation.19
In contrast the conjugation of Q to PMAA led to the formation of an effective anticancer polymeric compound, showing growth inhibition at a range of concentrations that started at concentrations higher than 16 μM, with a marked growth inhibition of 60% at 64 μM (Q equivalent as assessed by Folin–Ciocalteu Assay, Fig. 4A). This confirmed that the covalent conjugation to the polymeric backbone preserves the anticancer activity of the flavonoid, which was also found not to be significantly modified compared to the free Q. This is in agreement with our previous data on HeLa cancer cells.21 We then tested the effect of CNT_PMAA_Q on IMR-32 cell growth. Of particularly relevance, the biological activity of CNT_PMAA_Q exceeded that monitored for the PMAA_Q conjugate at equal concentrations of Q.
As a result of CNT' ability to strongly interact with cell membranes, promoting the internalisation process and thus the anticancer activity, CNT_PMAA_Q induced increased growth inhibition in IMR-32 cells compared to PMAA_Q (Fig. 4A). This reduction in cell growth became obvious at 4 μM of Q, and it was reduced to lower than 40% at 16 μM. This represented a significant enhancement of the CNT_PMAA_Q activity of almost 4-fold compared to the PMAA_Q. This was also reflected in the IC50 value, which was four times lower for CNT_PMAA_Q (12.35 μM) compared to PMAA_Q (54.11 μM).
Fig. 4B depicts the effect of CP on IMR-32 cell growth as a function of concentration at the identical incubation time used for the nano-modified compounds. The IC50 value for free CP was found to be 0.88 μM, and cell growth was reduced by 100% at 5 μM. After loading of CP onto the different synthesized carriers (CNT_PMAA, PMAA_Q, and CNT_PMAA_Q) we evaluated the neuroblastoma cells growth inhibition by using different concentrations of linked Q and CP in combination (Fig. 4C, D and E).
When loaded onto CNT_PMAA, CP was found to retain its biological properties, but a significant reduction of the efficiency was recorded, probably as a consequence of a strong interaction between higher concentrations of the carrier and the drug. This interaction consequently led to less available CP to reduce IMR-32 cell growth (Fig. 4C).
When loaded on PMAA_Q, the CP dose–response profile shows higher similarity to that of the free drug (Fig. 4D), even if also in this case some reduction in its activity was observed at higher concentrations of the carrier. Therefore, the strong interaction at high concentrations of the carriers, PMAA_Q and PMAA_CNT, with CP resulted in decreased potency.
Interestingly, a completely different behaviour was observed when CNT_PMAA_Q was evaluated as carrier for CP (Fig. 4E). We observed a strong reduction of IMR-32 cell growth compared to single compound treatments for the greater part of dose pairs tested.
For example, doses of 0.75 and 1 μM CP in combination with 16 μM PMAA_CNT_Q killed 100% of IMR-32 cells. To reach the same effect with CP alone its concentration would have required a 5-fold increase.
Quantitative assessment using the CI theorem25 verified that the growth inhibition induced by CP loaded onto PMAA_CNT_Q treatment was predominantly nearly additive or synergistic (11 out of 16 dose pairs tested) (Table 1). The reduction of anticancer activity was limited to 0.1 μM CP or 2 μM PMAA_CNT_Q concentrations, at which single compounds, as well as their combination, hardly affect cell growth.
| PMAA_CNT_Q (μM) | ||||
|---|---|---|---|---|
| Cisplatin (μM) | 2.0 | 4.0 | 8.0 | 16.0 |
| 0.1 | 0.98 | 1.65 | 1.23 | 1.70 |
| 0.5 | 1.20 | 1.09 | 0.92 | 0.81 |
| 0.75 | 1.10 | 1.06 | 0.82 | 0.61 |
| 1.0 | 1.05 | 1.09 | 0.14 | 0.14 |
| CI > 1.10 | Antagonism |
| 0.90 < CI < 1.10 | Nearly additivity |
| CI < 0.90 | Synergy |
By comparing the data of the in vitro release studies with those of the in vitro anticancer activity, it can be concluded that the synergistic activity is ascribable to the simultaneous biological effect of Q and CP, while the CNT component enhances the cell interaction and thus the local concentration of the drug, without significantly affecting the drug performance. This effect is of significance when considering potential clinical applications of the proposed carrier system, since it is possible to use a CP concentration 5 times lower to reach the same biological effect, with significant implication from a toxicological point of view.
Conclusions
An innovative drug carrier for CP was developed by the synthesis of hybrid materials composed of CNT, PMAA and Q that collectively act as cell membrane transporter, biocompatible element and biologically active moiety validated in an in vitro model of neuroblastoma. We demonstrated that the introduction of the flavonoid Q into the hybrid material CNT_PMAA, generating the CNT_PMAA_Q, resulted in an efficient carrier for CP enhancing its anticancer effect, which was reflected in a significant reduction of neuroblastoma cell growth in vitro. This greatly increased the potential application of the composite materials, since it strongly increased the anticancer activity of CP compared to the drug alone and therefore allows for a reduction of CP concentrations, which could result in reduced clinical side effects often observed with this drug.Acknowledgements
This work was financially supported by Regional Operative Program Calabria ESF 2007/2013 – IV Axis Human Capital – Operative Objective M2 – Action D.5 (G.C.), Vice Chancellor's Fellowship of the University of New South Wales (O.V.) and the NHMRC Senior Research Fellowship Award (M.K.).Notes and references
- K. M. Smith, A. Datti, M. Fujitani, N. Grinshtein, L. Zhang, O. Morozova, K. M. Blakely, S. A. Rotenberg, L. M. Hansford, F. D. Miller, H. Yeger, M. S. Irwin, J. Moffat, M. A. Marra, S. Baruchel, J. L. Wrana and D. R. Kaplan, EMBO Mol. Med., 2010, 2, 371 CrossRef CAS PubMed.
- S. Don, N. M. Verrills, T. Y. E. Liaw, M. L. M. Liu, M. D. Norris, M. Haber and M. Kavallaris, Mol. Cancer Ther., 2004, 3, 1137 CAS.
- Cancer chemotherapy handbook, ed. R. T. Dorr and D. D. von Hoff, Appleton & Lange, 1994 Search PubMed.
- S. Prakash, M. Malhotra, W. Shao, C. Tomaro-Duchesneau and S. Abbasi, Adv. Drug Delivery Rev., 2011, 63, 1340 CrossRef CAS PubMed.
- R. Sinha, G. J. Kim, S. Nie and D. M. Shin, Mol. Cancer Ther., 2006, 5, 1909 CrossRef CAS PubMed; R. Tietze, S. Lyer, S. Dürr and C. Alexiou, Nanomedicine, 2012, 7, 447 CrossRef PubMed; L. S. Jabr-Milane, L. E. van Vlerken, S. Yadav and M. M. Amiji, Cancer Treat. Rev., 2008, 34, 592 CrossRef PubMed.
- A. J. Thorley and T. D. Tetley, Pharmacol. Ther., 2013, 140, 176 CrossRef CAS PubMed; P. Kesharwani, R. Ghanghoria and N. K. Jain, Drug Discovery Today, 2012, 17, 1023 CrossRef PubMed.
- J. Li, S. Q. Yap, S. L. Yoong, T. R. Nayak, G. W. Chandra, W. H. Ang, T. Panczyk, S. Ramaprabhu, S. K. Vashist, F.-S. Sheu, A. Tan and G. Pastorin, Carbon, 2012, 50, 1625 CrossRef CAS PubMed; A. Bianco, R. Sainz, S. Li, H. Dumortier, L. Lacerda, K. Kostarelos, S. Giordani and M. Prato, in Medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes series: carbon materials: chemistry and physics, ed. F. Cataldo and T. Da Ros, Springer Science + Business Media B.V., Berlin, 2008, pp. 23–50 CrossRef PubMed; S. K. Vashist, D. Zheng, G. Pastorin, K. Al-Rubeaan, J. H. T. Luong and F.-S. Sheu, Carbon, 2011, 49, 4077 CrossRef PubMed; U. G. Spizzirri, S. Hampel, G. Cirillo, F. P. Nicoletta, A. Hassan, O. Vittorio, N. Picci and F. Iemma, Int. J. Pharm., 2013, 448, 115 CrossRef PubMed; V. Raffa, L. Gherardini, O. Vittorio, G. Bardi, A. Ziaei, T. Pizzorusso, C. Riggio, S. Nitodas, T. Karachalios, K. T. Al-Jamal, K. Kostarelos, M. Costa and A. Cuschieri, Nanomedicine, 2011, 6, 1709 CrossRef PubMed.
- P. Avouris, Phys. World, 2007, 20, 40 Search PubMed; M. F. Serag, N. Kaji, C. Gaillard, Y. Okamoto, K. Terasaka, M. Jabasini, M. Tokeshi, H. Mizukami, A. Bianco and Y. Baba, ACS Nano, 2011, 5, 493 CrossRef CAS PubMed; C. Fabbro, H. Ali-Boucetta, T. Da Ros, K. Kostarelos, A. Bianco and M. Prato, Chem. Commun., 2012, 48, 3911 RSC.
- S.-R. Ji, C. Liu, B. Zhang, F. Yang, J. Xu, J. Long, C. Jin, D.-L. Fu, Q.-X. Ni and X.-J. Yu, Biochim. Biophys. Acta, 2012, 1806, 29 Search PubMed.
- T. A. Hilder and J. M. Hill, Curr. Appl. Phys., 2008, 8, 258 Search PubMed; C. Sanchez-Cano and M. J. Hannon, Dalton Trans., 2009, 48, 10702 RSC.
- K. Ajima, T. Murakami, Y. Mizoguchi, K. Tsuchida, T. Ichihashi, S. Iijima and M. Yudasaka, ACS Nano, 2008, 2, 2057 CrossRef CAS PubMed.
- C. Tripisciano, K. Kraemer, A. Taylor and E. Borowiak-Palen, Chem. Phys. Lett., 2009, 478, 200 CrossRef CAS PubMed; S. L. Yoong, B. S. Wong, Q. L. Zhou, C. F. Chin, J. Li, T. Venkatesan, H. K. Ho, V. Yu, W. H. Ang and G. Pastorin, Biomaterials, 2014, 35, 748 CrossRef PubMed; D. M. Webster, P. Sundaram and M. E. Byrne, Eur. J. Pharm. Biopharm., 2013, 84, 1 CrossRef PubMed; C. Tripisciano, S. Costa, R. J. Kalenczuk and E. Borowiak-Palen, Eur. Phys. J. B, 2010, 75, 141 CrossRef; T. Panczyk, A. Jagusiak, G. Pastorin, W. H. Ang and J. Narkiewicz-Michalek, J. Phys. Chem. C, 2013, 117, 17327 Search PubMed; K. Ajima, M. Yudasaka, T. Murakami, A. Maigné, K. Shiba and S. Iijima, Mol. Pharmaceutics, 2005, 2, 475 CrossRef PubMed.
- C. Tripisciano and E. Borowiak-Palen, Phys. Status Solidi B, 2008, 245, 1979 CrossRef CAS; M. Adeli, F. Hakimpoor, M. Ashiri, R. Kabiri and M. Bavadi, Soft Matter, 2011, 7, 4062 RSC; R. P. Feazell, N. Nakayama-Ratchford, H. Dai and S. J. Lippard, J. Am. Chem. Soc., 2007, 129, 8438 CrossRef PubMed; S. Dhar, Z. Liu, J. Thomale, H. Dai and S. J. Lippard, J. Am. Chem. Soc., 2008, 130, 11467 CrossRef PubMed.
- J. Ringel, K. Erdmann, S. Hampel, K. Kraemer, D. Maier, M. Arlt, D. Kunze, M. P. Wirth and S. Fuessel, J. Biomed. Nanotechnol., 2014, 10, 463 CrossRef CAS PubMed.
- O. Vittorio, V. Raffa and A. Cuschieri, Nanomedicine: Nanotechnology, Biology and Medicine, 2009, 5, 424 CrossRef CAS PubMed.
- G. Cirillo, S. Hampel, R. Klingeler, F. Puoci, F. Iemma, M. Curcio, O. I. Parisi, U. G. Spizzirri, N. Picci, A. Leonhardt, M. Ritschel and B. Büchner, J. Pharm. Pharmacol., 2011, 63, 179 CrossRef CAS PubMed.
- Y. Zhang, Y. Bai and B. Yan, Drug Discovery Today, 2010, 15, 428 CrossRef CAS PubMed; G. Pastorin, Pharm. Res., 2009, 26, 746 CrossRef PubMed.
- G. Cirillo, O. Vittorio, S. Hampel, U. G. Spizzirri, N. Picci and F. Iemma, Int. J. Pharm., 2013, 446, 176 CrossRef CAS PubMed.
- G. Cirillo, O. Vittorio, S. Hampel, F. Iemma, P. Parchi, M. Cecchini, F. Puoci and N. Picci, Eur. J. Pharm. Sci., 2013, 49, 359 CrossRef CAS PubMed.
- G. Cirillo, T. Caruso, S. Hampel, D. Haase, F. Puoci, M. Ritschel, A. Leonhardt, M. Curcio, F. Iemma, V. Khavrus, M. Grobosch and N. Picci, Colloid Polym. Sci., 2013, 291, 699 CAS.
- F. Puoci, C. Morelli, G. Cirillo, M. Curcio, O. I. Parisi, P. Maris, D. Sisci and N. Picci, Anticancer Res., 2012, 32, 2843 CAS.
- H.-C. Tsai, J.-Y. Lin, F. Maryani, C.-C. Huang and T. Imae, Int. J. Nanomed., 2013, 8, 4427 CrossRef PubMed.
- K. D. Lee, Y.-I. Jeong, D. H. Kim, G.-T. Lim and K.-C. Choi, Int. J. Nanomed., 2013, 8, 2835 Search PubMed.
- T.-C. Chou and P. Talalay, Adv. Enzyme Regul., 1984, 22, 27 CrossRef CAS.
- T.-C. Chou, G. M. Otter and F. M. Sirotnak, Cancer Chemother. Pharmacol., 1996, 37, 222 CrossRef CAS.
- T. Arake, K. Shikinaka, T. Sugioka, H. Hashimoto, Y. Sumida and Y. Kaneko, Polymer, 2013, 54, 5643 CrossRef CAS PubMed.
- A. D. Pearson, C. R. Pinkerton, I. J. Lewis, J. Imeson, C. Ellershaw and D. Machin, Lancet Oncol., 2008, 9, 247 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03331k |
| ‡ Authors equally contributed. |
| This journal is © The Royal Society of Chemistry 2014 |



