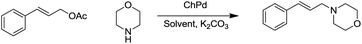
N-Allylation of amines with allyl acetates using chitosan-immobilized palladium†
R. B. Nasir Baig,
Buchi R. Vaddula,
Michael A. Gonzalez and
Rajender S. Varma*
Sustainable Technology Division, National Risk Management Research Laboratory, U. S. Environmental Protection Agency, 26 West Martin Luther King Drive, MS 443, Cincinnati, Ohio 45268, USA. E-mail: Varma.Rajender@epa.gov; Fax: +1 513-569-7677; Tel: +1 513-487-2701
First published on 20th January 2014
Abstract
A simple procedure for N-allylation of amines with allyl acetates has been developed using a biodegradable and easily recyclable heterogeneous chitosan-supported palladium catalyst. The general methodology, applicable to a wide range of substrates, has sustainable features that include a ligand-free reaction with simple workup, recycling and reusability of the catalyst.
Introduction
N-Allylamines are important structural motifs in organic chemistry and are found in a wide range of biologically active natural products.1 They are important intermediates in natural product synthesis and have been successfully used for the total synthesis of many alkaloids such as rosephilin, strychnine, (+)-γ-lycorane, and other natural products.2 The efficient synthesis of N-allylamines via N-allylation of amines is an important organic transformation because of their importance in natural products and amino acid synthesis.3 Palladium catalyzed Tsuji–Trost type N-allylation of allylic compounds namely allylic alcohols, allylic halides, allylic carbonates and allylic acetates, is considered as one of the most important method for the synthesis of N-allylamines.4 The N-allylation reaction proceeds via the intermediate formation of η3-allyl-Pd complex with allylic precursors such as allyl acetates followed by coupling with amines to give N-allylamines.5Non-renewable petrochemical feedstocks are considered the major source of polymer supports.6 Due to environmental consciousness, there is a growing thrust for alternative, ecofriendly, and biodegradable polymer support to be used for recyclable heterogeneous catalysis.7 The studies on the application of bio-derived polymers as heterogeneous supports in catalysis proved that these compounds can serve as efficient and cheap alternatives.7 Naturally abundant chitosan may prove to be an ideal support in the development of recyclable catalysts as chitosan-supported catalysts have been used for many reactions.8,9 In continuation of our effort toward the development of sustainable methods for organic synthesis,10,11 herein, we report a simple and efficient method for the N-allylation of amines with allyl acetates using chitosan–palladium catalyst.
Results and discussion
In view of our earlier success in the development of heterogeneous supported catalysis using palladium,12 we decided to explore the application of allyl acetates using the benign support, chitosan. The objective is to develop a general and ligand-free protocol for the N-allylation reactions by using a chitosan–Pd catalyst. The first step in the accomplishment of this goal was the synthesis of catalyst by immobilization of palladium Pd(0) over chitosan which was accomplished by reducing PdCl2 in an aqueous suspension of chitosan at pH 9 (adjusted using 25% ammonia, basic media) by using sodium borohydride at room temperature to afford the chitosan–Pd catalyst (Scheme 1).The chitosan supported Pd material was separated using a centrifuge and dried under vacuum at 50 °C for 12 hours. The catalyst was characterized by SEM (Fig. 1a) and X-ray diffraction XRD (Fig. 1b); the signals pertaining to Pd metal were not detected in XRD, possibly due to its low percentage. However, the presence of Pd-metal has been confirmed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analysis and the weight percentage of Pd was found to be 5.19%.
The chitosan–Pd catalyst was examined in the N-allylation of amines with allylic acetates in the presence of K2CO3 as base. In our first attempt, a mixture of morpholine, cinnamyl acetate, K2CO3, and the Pd catalyst in water was heated at 100 °C for 8 h. The desired product was not detected; instead cinnamyl acetate was hydrolyzed to cinnamyl alcohol. In order to optimize the reaction conditions, a series of experiments were performed (Table 1).
Among the different conditions explored to optimize the reaction, heating at 120 °C in DMF for 12 h provided the best results (Table 1, entry 7). The active role of palladium in the catalytic cycle of the reaction was established by control experiments; no product formation occurred in the absence of catalyst (Table 1, entry 8). The same reaction, when carried out separately in the presence of chitosan failed as well, but the chitosan–Pd catalyst gave good product yields thus confirming the essential role of palladium as the catalyst. Using these optimized conditions, the reaction was studied for its substrate scope with different amines and allylic acetates (Table 2).
| Entry | Amine | Substrate | Product | Yield |
|---|---|---|---|---|
| a Reaction conditions: 1 mmol of allyl acetate, 1.2 mmol of amine, 2 mmol of K2CO3, 30 mg of ChPd catalyst, 4 mL of DMF, 120 °C, over night. | ||||
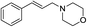
| 1 |  |
 |
 |
88% |
| 2 |  |
 |
 |
84% |
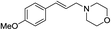
| 3 |  |
 |
 |
89% |
| 4 | 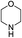 |
 |
 |
92% |
| 5 | 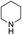 |
 |
 |
91% |
| 6 |  |
 |
 |
89% |
| 7 |  |
 |
 |
87% |
| 8 |  |
 |
 |
91% |
| 9 |  |
 |
 |
87% |
| 10 | 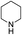 |
 |
 |
85% |
The variation in the structure of allyl acetates has not showed any peculiar effect on yield and product outcome of the reaction (Table 2, entry 1–9). Interestingly, a branched cinnamyl acetate, 1-phenylallyl acetate (Table 2, entry 10) also provided linear trans product (Table 2, entry 10), which confirms the intermediacy of η3-allyl-Pd complex in the reaction. The effect of various nitrogen nucleophiles in progress of the reaction was examined. The reaction of cinnamyl acetate with morpholine, piperidine, benzylamine, (S)-methylbenzylamine, 2-methylpiperidine, and N-methyl piperazine resulted in very good yields (84–92%) of corresponding allyl amines (Table 2, entries 1–10), which indicates that the progress of the reaction is not much influenced by the structural variation of amines.
In order to establish the recyclability of the catalyst, the catalyst was separated by filtration or centrifugation and washed with water and acetone followed by drying in vacuum at 50 °C. The recovered catalyst was used at least five times without losing its activity (ESI, Table 1†). Metal leaching was studied by ICP-AES analysis of the catalyst before and after the reaction cycles. The Pd weight percentage was found to be 5.19% before the reaction and 5.11% after the reaction. The TEM image of the recycled catalyst taken after the reaction did not show significant change in the morphology of the catalyst (ESI, Fig. 1†), which indicates the retention of the catalytic activity after recycling. No significant Pd metal was detected in the reaction solvent after completion of the reaction. This confirms the fact that the chitosan held the Pd metal very tightly thus retaining the integrity of the catalyst, minimizing metal leaching, and facilitating efficient catalyst recycling. This chitosan-supported Pd catalyst has proven its superiority over earlier described cellulose-supported Pd catalyst.7b The chitosan supported catalyst is more active than cellulose-supported Pd catalyst; it shows better recyclability profile and reactivity with lower Pd loading (5.19%) in comparison to loading of 9.8% required for the cellulose Pd catalyst.
Conclusion
A simple and sustainable procedure for the N-allylation of amines has been developed using a biodegradable and recyclable heterogeneous chitosan-supported Pd catalyst. This unprecedented general methodology deploys chitosan directly (unmodified) and is applicable to aliphatic, benzylamines, substituted and unsubstituted allyl acetates. Sustainable features of this protocol include a ligand-free reaction with simple workup, and recycling and reusability of the catalyst.Experimental procedure
A mixture of allyl acetate (1.0 mmol), amine (1.2 mmol), chitosan–Pd (30 mg), and potassium carbonate (2.0 mmol) in anhydrous DMF (4 mL) was heated at 120 °C under N2 atmosphere for 12–15 h. Upon completion of the reaction, as indicated by TLC, the reaction mixture was diluted with water and centrifuged/filtered to separate the catalyst. The decanted liquid was extracted with ethyl acetate (3 × 10 mL); the organic layer was dried over anhydrous sodium sulfate and evaporated under reduced pressure to afford the crude product. The crude product was purified by passing through a short silica gel column (ethyl acetate–hexane) and identification of the pure products by spectroscopic means; 1H and 13C NMR spectra of the representative compounds are provided in the ESI.†Acknowledgements
R.B.N.B. and B.R.V. were supported by the Postgraduate Research Program at the National Risk Management Research Laboratory administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Environmental Protection Agency. We thank Dr M. Nadagouda for recording XRD data and valuable suggestions.Notes and references
- (a) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921–2944 CrossRef CAS PubMed; (b) B. M. Trost, C. Pissot-Soldermann, I. Chen and G. M. Schroeder, J. Am. Chem. Soc., 2004, 126, 4480–4481 CrossRef CAS PubMed.
- (a) S. D. Knight, L. E. Overman and G. Pairaudeau, J. Am. Chem. Soc., 1993, 115, 9293–9294 CrossRef CAS; (b) S. D. Knight, L. E. Overman and G. Pairaudeau, J. Am. Chem. Soc., 1995, 117, 5776–5788 CrossRef CAS; (c) A. Furstner and H. Weintritt, J. Am. Chem. Soc., 1998, 120, 2817–2825 CrossRef; (d) A. Furstner, T. Gastner and H. Weintritt, J. Org. Chem., 1999, 64, 2361–2366 CrossRef; (e) H. Yoshizaki, H. Satoh, Y. Sato, S. Nukui, M. Shibasaki and M. Mori, J. Org. Chem., 1995, 60, 2016–2021 CrossRef CAS.
- (a) B. M. Trost, Angew. Chem., 1989, 101, 1199–1219 (Angew. Chem., Int. Ed. Engl., 1989, 28, 1173–1192) CrossRef CAS; (b) J. F. Bower, R. Jumnah, A. C. Williams and J. M. J. Williams, J. Chem. Soc., Perkin Trans. 1, 1997, 1411–1420 RSC; (c) K. Burgess, L. T. Liu and B. Pal, J. Org. Chem., 1993, 58, 4758–4763 CrossRef CAS.
- (a) D. Polet, A. Alexakis, K. Tissot-Croset, C. Corminboeuf and K. Ditrich, Chem. – Eur. J., 2006, 12, 3596–3609 CrossRef CAS PubMed; (b) C. Welter, O. Koch, G. Lipowsky and G. Helmchen, Chem. Commun., 2004, 896–897 RSC; (c) B. Plietker, Angew. Chem., Int. Ed., 2006, 45, 6053–6056 CrossRef CAS PubMed; (d) M. Kawatsura, F. Ata, T. Hirakawa, S. Hayase and T. Itoh, Tetrahedron Lett., 2008, 49, 4873–4875 CrossRef CAS PubMed.
- (a) J. Tsuji, H. Takahashi and M. Morikawa, Tetrahedron Lett., 1965, 6, 4387–4388 CrossRef; (b) B. M. Trost and D. L. Van Vranken, Chem. Rev., 1996, 96, 395–422 CrossRef CAS PubMed.
- (a) T. V. Khamatnurova and D. E. Bergbreiter, Polym. Chem., 2013, 4, 1617–1624 RSC; (b) S. Iimura, D. Nobutou, K. Manabe and S. Kobayashi, Chem. Commun., 2003, 1644–1645 RSC.
- (a) R. Moucel, K. Perrigaud, J. M. Goupil, P. J. Madec, S. Marinel, E. Guibal, A. C. Gaumont and I. Dez, Adv. Synth. Catal., 2010, 352, 433–439 CrossRef CAS; (b) B. R. Vaddula, A. Saha, R. S. Varma and J. Leazer, Eur. J. Org. Chem., 2012, 6707–6709 CrossRef CAS.
- (a) R. B. Nasir Baig and R. S. Varma, Green Chem., 2013, 15, 1839–1843 RSC; (b) M. Chtchigrovsky, A. Primo, P. Gonzalez, K. Molvinger, M. Robitzer, F. Quignard and F. Taran, Angew. Chem., Int. Ed., 2009, 48, 5916–5920 CrossRef CAS PubMed.
- (a) M. Bradshaw, J. Zou, L. Byrne, K. S. Iyer, S. G. Stewartc and C. L. Raston, Chem. Commun., 2011, 47, 12292–12294 RSC; (b) D. Kuhbeck, G. Saidulu, K. R. Reddy and D. Dıaz, Green Chem., 2012, 14, 378–392 RSC; (c) D. J. Macquarrie and A. Bacheva, Green Chem., 2008, 10, 692–695 RSC.
- (a) R. B. Nasir Baig and R. S. Varma, ACS Sustainable Chem. Eng., 2013, 1, 805–809 CAS; (b) R. B. Nasir Baig and R. S. Varma, Curr. Org. Chem., 2013, 17, 2323–2331 CrossRef; (c) A. Saha, R. B. Nasir Baig, J. Leazer and R. S. Varma, Chem. Commun., 2012, 48, 8889–8891 RSC; (d) R. B. Nasir Baig and R. S. Varma, Chem. Commun., 2012, 48, 5853–5855 RSC; (e) R. B. Nasir Baig, R. N. Chandrakala, V. S. Sudhir and S. Chandrasekaran, J. Org. Chem., 2010, 75, 2910–2921 CrossRef PubMed.
- (a) R. B. Nasir Baig and R. S. Varma, Chem. Commun., 2012, 48, 2582–2584 RSC; (b) R. B. Nasir Baig and R. S. Varma, Green Chem., 2012, 14, 625–632 RSC; (c) R. B. Nasir Baig and R. S. Varma, Chem. Commun., 2012, 48, 6220–6222 RSC; (d) R. B. Nasir Baig and R. S. Varma, Chem. Commun., 2013, 49, 752–770 RSC; (e) R. B. Nasir Baig and R. S. Varma, Curr. Org. Chem., 2013, 17, 2227–2237 CrossRef; (f) R. B. Nasir Baig and R. S. Varma, Green Chem., 2013, 15, 398–417 RSC.
- (a) B. R. Vaddula, A. Saha, J. Leazer and R. S. Varma, Green Chem., 2012, 14, 2133–2136 RSC; (b) A. Saha, J. Leazer and R. S. Varma, Green Chem., 2012, 14, 67–71 RSC; (c) V. Polshettiwar, B. Baruwati and R. S. Varma, Chem. Commun., 2008, 6318–6320 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47899h |
| This journal is © The Royal Society of Chemistry 2014 |