Enantioselective 1,4-addition of kojic acid derivatives to β-nitroolefins catalyzed by a cinchonine derived sugar thiourea†
B. V. Subba Reddy*a,
S. Madhusudana Reddya,
Manisha Swaina,
Srikanth Dudemb,
Shasi V. Kalivendib and
C. Suresh Reddyc
aNatural Product Chemistry, Indian Institute of Chemical Technology, Uppal Road, Tarnaka, Hyderabad, 500607, India. E-mail: basireddy@iict.res.in; Fax: +91 40 27160512
bCentre for Chemical Biology, Indian Institute of Chemical Technology, Uppal Road, Tarnaka, Hyderabad, 500607, India
cDepartment of Chemistry, Sri Venkateswra University, Tirupati, India
First published on 21st January 2014
Abstract
A highly enantioselective Michael addition reaction of kojic acid derivatives to β-nitroolefins has been accomplished using a cinchonine derived sugar thiourea. The reaction provides the corresponding Michael adducts in excellent yields with a high degree of enantioselectivity (up to 99% ee) in short reaction time with low catalyst loading. The Michael adducts are found to exhibit promising cytotoxicity against various cancer cell lines.
Introduction
The Michael addition is one of the most elegant approaches for the construction of carbon\carbon bonds under mild conditions in an atom economic fashion.1 In particular, asymmetric Michael reaction2 of nitroalkenes is very attractive to produce the enantiopure nitroalkanes which are important building blocks for the synthesis of chiral amines.3 Recently, cinchona derived thiourea catalysts have received special attention in the field of organocatalysis.4 Among them, sugar–cinchona hybrid thiourea catalysts5 have proved to be highly efficient for the enantioselective Michael reaction.Kojic acid is a fungal metabolite and is known to exhibit a broad spectrum of biological activities such as antimicrobial,6 cosmetics,7 pesticide/insecticide,8 antitumor,9 antidiabetic,10 and anticonvulsant activity.11 Consequently, a large number of kojic acid derivatives have been prepared to evaluate their efficiency in biological systems.12 In addition, kojic acid has also been used for the synthesis of biologically active natural products.13 In fact, kojic acid acts as a highly reactive nucleophile in 1,4-addition reactions.14 On the other hand, asymmetric Michael addition of kojic acid derivatives to nitroalkenes provides a direct access to enantiomerically enriched nitro compounds15 which are key intermediates for β-peptides.16
Inspired by recent advancements in thiourea catalysis we herein report the asymmetric Michael addition of kojic acid derivatives to β-nitrostyrenes to produce the Michael adducts in high yields with good to excellent enantioselectivity using a novel series of cinchona derived sugar thiourea catalysts.
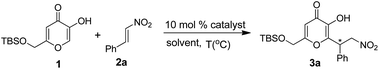
Initially, we investigated the catalytic efficiency of thiourea catalysts I–XII (Fig. 1) was evaluated in the Michael addition of kojic acid TBS ether (1) to β-nitrostyrene (2a). Remarkably, the reaction proceeds at low concentration of the catalyst II with enhanced reaction rates compared to previously reported catalysts.15
Cinchona thioureas (I–XII) containing different chiral diamine skeletons were chosen as the catalysts (Fig. 1). Accordingly, treatment of kojic acid TBS ether (1) with β-nitrostyrene (2a) in the presence of catalysts I and VII in CH2Cl2 at 30 °C afforded the desired product 3a reasonably in good yield with moderate ee (Table 1, entries a and s). Replacement of the catalyst I and VII with IV and X gave the product 3a in low ee with opposite asymmetric induction (Table 1, entries p and v). The above reaction was further carried out with other catalysts II, V, VIII and XI to evaluate their efficiency. But to our surprise, the catalysts II and VIII gave the Michael adduct 3a in excellent yields (85 and 80%) with good ee 75 and 71% respectively (Table 1, entries b and t).
| Entry | Catalysta | Solvent | T (°C) | Yield (%)b | ee (%)c |
|---|---|---|---|---|---|
| a Reaction was carried out with 1 (0.2 mmol), 2a (0.22 mmol) in the presence of 10 mol% of organocatalyst.b Isolated yields.c Determined by chiral HPLC.d 5 mol% catalyst used. | |||||
| a | I | CH2Cl2 | 30 | 75 | 68 |
| b | II | CH2Cl2 | 30 | 85 | 75 |
| c | II | PhCH3 | 30 | 89 | 78 |
| d | II | Xylene | 30 | 90 | 85 |
| e | II | CH3CN | 30 | 91 | 79 |
| f | II | EtOH | 30 | 94 | 85 |
| g | II | THF | 30 | 93 | 78 |
| h | II | MeOH | 30 | 96 | 89 |
| i | II | Et2O | 30 | 71 | 67 |
| j | II | i-PrOH | 30 | 97 | 91 |
| k | II | i-PrOH | −10 | 96 | 97 |
| l | II | i-PrOH | 0 | 97 | 98 |
| m | II | i-PrOH | 5 | 98 | 99 |
| n | II | i-PrOH | 5 | 98 | 99d |
| o | III | CH2Cl2 | 30 | 71 | 55 |
| p | IV | CH2Cl2 | 30 | 74 | −56 |
| q | V | CH2Cl2 | 30 | 84 | −55 |
| r | VI | CH2Cl2 | 30 | 76 | −45 |
| s | VII | CH2Cl2 | 30 | 76 | 67 |
| t | VIII | CH2Cl2 | 30 | 80 | 71 |
| u | IX | CH2Cl2 | 30 | 78 | 54 |
| v | X | CH2Cl2 | 30 | 72 | −52 |
| w | XI | CH2Cl2 | 30 | 79 | −56 |
| x | XII | CH2Cl2 | 30 | 76 | −54 |
However, the catalysts V and XI gave the product 3a in similar yields but with low ee (Table 1, entries q and w). The efficiency of other cinchona bifunctional thiourea catalysts such as III, VI, IX and XII was also tested in the above reaction. None of them gave the 3a with satisfactory yields and enantioselectivity (Table 1, entries o, r, u and x).
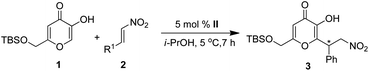
After numerous experiments, the catalyst II was found to be superior in terms of yield and enantioselectivity (75% ee, Table 1, entry b) than other catalysts. With the best catalyst in hand, we next screened the effect of other parameters like solvent, temperature and catalyst loading. Of various solvents tested, the best results were achieved in i-PrOH, whereas the moderate enantioselectivity was observed in xylene, EtOH and MeOH. However, poor yields and low enantioselectivity were obtained in toluene, CH3CN, THF and Et2O. Therefore, i-PrOH was chosen as the optimal solvent for this reaction (91% ee). Indeed, the temperature was found to have a significant influence on the enantioselectivity. For example, lowering the reaction temperature to −10 °C, the product 3a was obtained in 96% yield with 97% ee (Table 1, entry k) after a long reaction time. By increasing the reaction temperature to 5 °C, the reaction was complete in short time with a gradual increase of yield and ee (Table 1, entry l and m). By decreasing the amount of the catalyst from 10 to 5 mol% did not alter the yield and enantioselectivity (98% of yield and 99% of ee, Table 1, entry n). Therefore, no significant effect of the catalyst loading either on yield or enantioselectivity was observed. Thus, the optimal reaction conditions for the Michael addition were determined to be 0.1 mmol of the kojic acid TBS ether (1), 0.11 mmol of β-nitrostyrene (2a), 5 mol% of the catalyst II in 1 mL i-PrOH at 5 °C for 7 h. The scope of the reaction was further investigated under optimized conditions and the results are summarized in Table 2. Notably, several aromatic nitroalkenes afforded the desired products in high to excellent enantioselectivities (Table 2, 90–99%, entries a–s) with (R)-configuration. The absolute configuration of the Michael products was assigned by comparison of the optical rotation with known compounds.15 It was found that nitroolefins (2) either with electron-withdrawing or with electron-donating groups on the phenyl ring gave the products in good to excellent yields (85–99%) with high enantioselectivity (84–99% ee). In addition, heteroaromatic substrates such as 2-furanyl and 2-thienyl gave the Michael adducts with good yields and ee (Table 2, entries r and s). It is noteworthy to mention that aliphatic nitroalkene also furnished the Michael adduct with excellent selectivity (Table 2, entry t, 89% ee). A large number of kojic acid derivatives (3a–3t) were prepared in high yields with excellent enantioselectivities (up to 99% ee).
| Entry | R1 | Producta | Yield (%)b | ee (%)c | Conf. |
|---|---|---|---|---|---|
| a Reaction was carried out with 1 (0.1 mmol), 2 (0.11 mmol), and in 1 mL of i-PrOH.b Isolated yields.c Determined by chiral HPLC. | |||||
| a | C6H5 | 3a | 97 | 99 | R |
| b | 4-FC6H4 | 3b | 98 | 96 | R |
| c | 4-ClC6H4 | 3c | 97 | 94 | R |
| d | 4-BrC6H4 | 3d | 95 | 94 | R |
| e | 4-MeC6H4 | 3e | 90 | 91 | R |
| f | 4-MeOC6H4 | 3f | 95 | 91 | R |
| g | 3-FC6H4 | 3g | 96 | 85 | R |
| h | 3-ClC6H4 | 3h | 95 | 88 | R |
| i | 2-FC6H4 | 3i | 99 | 95 | R |
| j | 2-ClC6H4 | 3j | 98 | 88 | R |
| k | 2-BrC6H4 | 3k | 96 | 91 | R |
| l | 2-MeOC6H4 | 3l | 97 | 88 | R |
| m | 2-Naphthyl | 3m | 93 | 91 | R |
| n | 2-BnOC6H4 | 3n | 92 | 84 | R |
| o | 2,4-Cl2C6H3 | 3o | 95 | 87 | R |
| p | 3,4-Cl2C6H3 | 3p | 94 | 91 | R |
| q | 3,5-(Me)2C6H3 | 3q | 90 | 95 | R |
| r | 2-Furanyl | 3r | 94 | 95 | R |
| s | 2-Thienyl | 3s | 85 | 92 | R |
| t | Butyl | 3t | 90 | 89 | S |
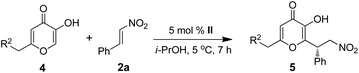
Encouraged by the above results, we turned our attention to evaluate the synthetic potential of catalyst II in Michael reaction of 4a and 4b to β-nitrostyrene 2a. Interestingly, the reaction proceeded effectively under present conditions to afford the respective Michael products 5a and 5b in good yields (96–95%) with excellent enantioselectivity (95–97%) (Table 3, entries a and b).
Based on the above results, a possible ternary complex of the catalyst II was proposed in Fig. 2. According to the results reported in Table 2, it was believed that nitroolefin could be effectively activated by thiourea moiety of the catalyst, while the tertiary amine deprotonates the enolic OH to generate the kojic acid enolate. In a ternary complex, both nitroolefin and kojic acid coordinate with thiourea, tertiary amine and carbohydrate of the catalyst II respectively through hydrogen bonding. The nucleophilic attack of the enolate on β-nitrostyrene from the Re-face leading to the formation of (R)-enantiomer as a major product, which is consistent with the observed results. The attack of enolate to the Si-face of the nitroolefin is restricted by cinchonine and carbohydrate moieties of the catalyst. The carbohydrate serves as the chiral auxiliary to enhance the selectivity. As shown in Fig. 2, the complexation of cinchonine, thiourea and carbohydrate moieties of the bifunctional catalyst with kojic acid and nitroolefin plays a significant role in controlling the enantioselectivity of the Michael reaction.
In vitro anticancer activity
The Michael adducts were evaluated for their anti-proliferative activity against four different human tumor cell lines; HeLa (human epithelial cervical cancer), A549 (human lung carcinoma epithelial), DU145 (human prostate carcinoma epithelial), and MCF-7 (human breast adenocarcinoma). The inhibitory concentration (IC50) values are summarized in Table 4.| Compounda | HeLa | A549 | DU145 | MCF7 |
|---|---|---|---|---|
| a Cell lines were treated with different concentration of compounds for 48 h as described in experimental section.b IC50 values are indicated as a mean value of three independent experiments. | ||||
| 3a | 53 ± 2.1 | 38 ± 2.1 | 31 ± 1.7 | 60 ± 2.4 |
| 3b | >100 | 47 ± 1.5 | 40 ± 2.6 | 56 ± 2.8 |
| 3c (racemic) | 37 ± 2.3 | 38 ± 2.7 | 51 ± 2.9 | 34 ± 2.4 |
| 3c | 11 ± 1.5 | 18 ± 1.7 | 16 ± 0.8 | 18 ± 1.1 |
| 3d | 9.2 ± 1.1 | 8.3 ± 1.1 | 13 ± 1.4 | 24 ± 1.6 |
| 3e | 15 ± 1.2 | 29 ± 1.8 | 51 ± 2.7 | 53 ± 2.8 |
| 3f | 27 ± 1.7 | 38 ± 2.7 | >100 | >100 |
| 3g | 34 ± 1.8 | 84 ± 3.4 | 36 ± 1.9 | 44 ± 2.5 |
| 3h | 10 ± 0.9 | 9.1 ± 0.8 | 14 ± 1.2 | 35 ± 2.4 |
| 3i | 51 ± 2.6 | 37 ± 2.8 | >100 | >100 |
| 3j | 92 ± 3.3 | 36 ± 2.4 | 34 ± 2.6 | 48 ± 2.4 |
| 3k | 31 ± 2.8 | 50 ± 2.8 | 63 ± 2.7 | 35 ± 2.1 |
| 3l | 22 ± 2.1 | 55 ± 2.7 | 38 ± 2.8 | 57 ± 2.6 |
| 3m | 56 ± 2.4 | >100 | 45 ± 2.6 | 47 ± 2.6 |
| 3n | 75 ± 2.9 | 37 ± 2.4 | 33 ± 2.4 | 38 ± 2.8 |
| 3o | 63 ± 3.7 | 32 ± 1.9 | >100 | 54 ± 2.4 |
| 3p | 33 ± 2.5 | >100 | 3.5 ± 1.8 | 37 ± 1.9 |
| 3q | 24 ± 1.7 | 78 ± 2.8 | 33 ± 2.2 | 26 ± 1.4 |
| 3r | 27 ± 1.8 | 40 ± 2.8 | 46 ± 2.1 | 58 ± 2.7 |
| 3s | 58 ± 3.1 | >100 | 37 ± 2.8 | 87 ± 2.8 |
| 3t | 82 ± 2.9 | 43 ± 1.8 | 24 ± 2.1 | 65 ± 3.2 |
| 5a | 28 ± 2.3 | >100 | 41 ± 2.6 | 47 ± 1.6 |
| 5b | 22 ± 1.1 | 55 ± 2.8 | 36 ± 2.7 | >100 |
As evident from Table 4, the test compounds showed moderate to good cytotoxicity against four different cancer cell lines. The chiral compound 3c was highly active than its racemic form. The study of % viability vs concentration of chiral and racemic products of 3c is shown in Fig. 3. Compound 3c was active against A549, DU145, MCF7 cell lines at 18 μM, 16 μM, and 18 μM respectively whereas 3c was active against HeLa cell lines at 11 μM. Furthermore, compound 3d showed good activity against HeLa, A549 and DU145 cell lines at 9 μM, 8 μM, and 13 μM respectively while compound 3h was also active against A549, HeLa and Du145 cell lines at 9 μM, 10 μM and 14 μM respectively.
Conclusion
In conclusion, a novel bifunctional cinchona–sugar based thiourea catalyst has proved to be a highly efficient organocatalyst for the asymmetric Michael addition of kojic acid derivatives to β-nitroolefins. Our approach has significantly improved the reaction rate to 7 h using a new quinine–sugar–thiourea catalyst, whereas the reported reaction took four days for the completion using cyclohexane-derived thiourea catalyst. High enantioselectivity (99%), low catalyst loading (5 mol%), excellent yields, short reaction times, broad substrate scope and mild reaction conditions are the salient features of this methodology. This is the first report on the evaluation of anti-cancer properties of the Michael adducts, which are found to exhibit promising anti-cancer activity. Further application of sugar thiourea catalysts for asymmetric synthesis is under progress in our laboratory.Experimental
General. All reactions were carried out under nitrogen atmosphere. Commercial reagents were used as received, unless otherwise stated. 1H NMR spectra were recorded on 300 MHz or 500 MHz spectrometer using CDCl3 and DMSO-d6 as a solvent. 13C NMR were recorded on 75 MHz and 125 MHz spectrometer using CDCl3 and DMSO-d6. TMS was used as an internal reference for 1H NMR analysis. All the compounds were purified by column chromatography on silica gel (60–120 mesh) using hexane–ethyl acetate mixture as eluent. Mass analysis was carried out using ESI mass spectrometer. Infrared (IR) spectra were recorded on FT-IR spectrometer. Optical rotations were measured using Rudolph (Digipol 781 M6U) polarimeter. The ee values were determined by chiral high-performance liquid chromatography (HPLC) using Daicel Chiracel OJ-H.Materials and methods
Cell culture and maintenance
All cell lines used in this study were purchased from the American Type Culture Collection (ATCC). A549m (human lung carcinoma epithelial) and HeLa (human epithelial cervical cancer) were grown in Dulbecco's modified Eagle's medium (DMEM) containing non-essential amino acids and 10% FBS. MCF-7 (human breast adenocarcinoma) and DU145 (human prostate carcinoma epithelial) cells were cultured in Eagle's minimal essential medium (MEM) containing non-essential amino acids, 1 mM sodium pyruvate, and 10% FBS. All cell lines maintained in humidified atmosphere of 5% CO2 at 37 °C. Cells were trypsinized when sub confluent from T75 flasks per 90 mm dishes and seeded in 96 well plate at a concentration of 1 × 104 cells mL−1 in complete medium, treated with compounds at desired concentrations and harvested as required.17Cell proliferation assay using MTT
The assay is a quantitative colorimetric method for the determination of cell survival and proliferation. Metabolically active cells reduce pale yellow tetrazolium salt (MTT) to a dark blue water-insoluble formazan, which is quantified after solubilisation with DMSO. The absorbance of the solubilized formazan directly correlates with a number of viable cells. Cells were plated at a density of 1 × 104 cells in 200 μL of medium per well of 96-well plate. The 96-well microtiter plate was incubated for 24 h prior to addition of the experimental compounds. Cells were treated at different concentrations (1, 10 and 25 μM) of the test compounds for 48 hours. The assay was terminated with the addition of MTT (5%, 10 μL) and incubated for 60 min at 37 °C. The supernatant was aspirated and plates were air dried. MTT-formazon crystals were dissolved in 100 μL DMSO. The optical density (O.D) was measured at 560 nm using TECAN multimode reader. The growth percentage of each treated well of 96 well plate was calculated based on test wells relative to control wells. The cell growth inhibition of compounds was analyzed by generating dose response curves as a plot of the percentage of surviving cells versus drug concentration. Anticancer activity of the cancer cells to the test compounds was articulated in terms of IC50 value, which defines as a concentration of compound resulting in the reduction of absorbance to 50% with respect to controls.18Acknowledgements
S. M. S. R. and M. S thanks to Council of Scientific and Industrial Research, Government of India for the award of a fellowship. Funding from the CSIR project, SMiLE is gratefully acknowledged.Notes and references
- P. Perlmutter, Conjugate addition reactions in organic synthesis, Pergamon: Oxford, UK, 1992 Search PubMed.
- For selected reviews of asymmetric Michael additions see: (a) B.-L. Li, Y.-F. Wang, S.-P. Luo, A.-G. Zhong, Z.-B. Li, X.-H. Du and D.-Q. Xu, Eur. J. Org. Chem., 2010, 656 CrossRef; (b) J.-R. Chen, Y.-J. Cao, Y.-Q. Zhou, F. Tan, L. Fu, X.-Y. Zhu and W.-J. Xiao, Org. Biomol. Chem., 2010, 8, 1275 RSC; (c) X.-Y. Cao, J.-C. Zheng, Y.-X. Li, Z.-C. Shu, X.-L. Sun, B.-Q. Wang and Y. Tang, Tetrahedron, 2010, 66, 9703 CrossRef CAS PubMed; (d) C. Yu, Y. Zhang, A. Song, Y. Ji and W. Wang, Chem. – Eur. J., 2011, 17, 770 CrossRef CAS PubMed; (e) Y. Zhang and W. Wang, Catal. Sci. Technol., 2012, 2, 42 RSC.
- (a) O. N. Garcia and R. Alonso, Org. Biomol. Chem., 2013, 11, 512 RSC; (b) H. Ishikawa, M. Honma and Y. Hayashi, Angew. Chem., Int. Ed., 2011, 50, 2824 CrossRef CAS PubMed.
- (a) B. Vakulya, S. Varga, A. Csampai and T. Soos, Org. Lett., 2005, 7, 1967 CrossRef CAS PubMed; (b) B. Vakulya, S. Varga and T. Soós, J. Org. Chem., 2008, 73, 3475 CrossRef CAS PubMed; (c) S. J. Connon, Chem. Commun., 2008, 2499 RSC; (d) C. Curti, G. Rassu, V. Zambrano, L. Pinna, G. Pelosi, A. Sartori, L. Battistini, F. Zanardi and G. Casiraghi, Angew. Chem., Int. Ed., 2012, 51, 6200 CrossRef CAS PubMed.
- (a) S. Kong, W. Fan, G. Wu and Z. Miao, Angew. Chem., Int. Ed., 2012, 51, 8864 CrossRef CAS PubMed; (b) B. V. S. Reddy, S. M. Reddy and M. Swain, RSC Adv., 2013, 3, 930 RSC.
- (a) J. Brtko, L. Rondahl, M. Ficková, D. Hudecová, V. Eybl and M. Uher, Cent. Eur. J. Public Health, 2004, 12, 8 Search PubMed; (b) M. D. Aytemir, D. D. Erol, R. C. Hider and M. Özalp, Turk. J. Chem., 2003, 27, 757 Search PubMed; (c) M. D. Aytemir, R. C. Hider, D. D. Erol, M. Özalp and M. Ekizoglu, Turk. J. Chem., 2003, 27, 445 CAS; (d) A. Fassihi, D. Abedi, L. Saghaie, R. Sabet, H. Fazeli and G. Bostaki, Eur. J. Med. Chem., 2009, 44, 2145 CrossRef CAS PubMed.
- (a) G. A. Burdock, M. Soni and I. G. Carabin, Regul. Toxicol. Pharmacol., 2001, 33, 80 CrossRef CAS PubMed; (b) J. M. Noh, S. Y. Kwak, D. H. Kim and Y. S. Lee, Biopolymers, 2007, 88, 300 CrossRef CAS PubMed.
- J. Alverson, J. Invertebr. Pathol., 2003, 83, 60 CrossRef CAS.
- (a) Y. Higa, M. Kawawbe, K. Nabae, Y. Toda, S. Kitamoto and T. Hara, J. Toxicol. Sci., 2007, 32, 143 CrossRef CAS; (b) M. Yamato, K. Hashigaki, Y. Yasumoto, J. Sakai, R. F. Luduena and A. Banerjee, J. Med. Chem., 1987, 30, 1897 CrossRef CAS.
- X. Xiong and M. C. Pirrung, Org. Lett., 2008, 10, 1151 CrossRef CAS PubMed.
- M. D. Aytemir, E. Septioglu and Ü. Çalıs, Arzneimittelforschung, 2010, 60, 22 CAS.
- (a) V. G. Yuen, P. Caravan, L. Gelmini, N. Glover, J. H. Mcneill, I. A. Setyawati, Y. Zhouand and C. J. Orvig, J. Inorg. Biochem., 1997, 73, 109 CrossRef; (b) Y. Ohyamaand and Y. Mishima, Fragrance J., 1990, 6, 53 Search PubMed; (c) J. S. Chen, C. I. Wei, R. S. Rolle, W. S. Otwell, M. O. Balaban and M. R. Marshall, J. Agric. Food Chem., 1991, 39, 1396 CrossRef CAS; (d) H. Mitani, I. Koshiishi, T. Sumita and T. Imanari, Eur. J. Pharmacol., 2001, 411, 169 CrossRef CAS; (e) H. S. Rho, S. M. Ahn, D. S. Yoo, M. K. Kim, D. H. Cho and J. Y. Cho, Bioorg. Med. Chem. Lett., 2010, 20, 6569 CrossRef CAS PubMed; (f) S. Y. Kwak, J. M. Noh, S. H. Park, J. W. Byun, H. R. Choi, K. C. Rark and Y. S. Lee, Bioorg. Med. Chem. Lett., 2010, 20, 738 CrossRef CAS PubMed; (g) A. Shahrisa and Z. Ghasemi, Chem. Heterocycl. Compd., 2010, 46, 37 CrossRef; (h) B. V. S. Reddy, M. R. Reddy, G. Narasimhulu and J. S. Yadav, Tetrahedron Lett., 2010, 51, 5677 CrossRef CAS PubMed; (i) J. H. Kasser, W. Kandioller, C. G. Hartinger, A. A. Nazarov, V. B. Arion, P. J. Dyson and B. K. Keppler, J. Organomet. Chem., 2010, 695, 875 CrossRef CAS PubMed.
- (a) P. A. Wender, N. D. Angelo, V. I. Elitzin, M. Ernst, E. E. J. Ugueto, J. A. Kowalski, S. McKendry, M. Rehfeuter and R. Sun, Org. Lett., 2007, 9, 1829 CrossRef CAS PubMed; (b) P. A. Wender and J. L. Mascarenas, Tetrahedron Lett., 1992, 33, 2115 CrossRef CAS; (c) P. A. Wender and J. L. Mascarenas, J. Org. Chem., 1991, 56, 6267 CrossRef CAS; (d) P. A. Wender and F. E. McDonald, J. Am. Chem. Soc., 1990, 112, 4956 CrossRef CAS.
- (a) A. A. Shestopalov, L. A. Rodinovskaya, A. M. Shestopalov and V. P. Litvinov, Russ. Chem. Bull., 2004, 53, 724 CrossRef CAS; (b) M.-Z. Piao and K. Imafuku, Tetrahedron Lett., 1997, 38, 5301 CrossRef CAS.
- J. Wang, Q. Zhang, H. Zhang, Y. Feng, W. Yuana and X. Zhang, Org. Biomol. Chem., 2012, 10, 2950 CAS.
- (a) R. P. Cheng, S. H. Gellman and W. F. DeGrado, Chem. Rev., 2001, 101, 3219 CrossRef CAS PubMed; (b) D. Seebach, A. K. Beck, S. Capone, G. Deniau, U. Groselj and E. Zass, Synthesis, 2009, 1 CrossRef CAS PubMed; (c) D. Seebach and J. Gardiner, Acc. Chem. Res., 2008, 41, 1366 CrossRef CAS PubMed; (d) G. Lelais and D. Seebach, Biopolymers, 2004, 76, 206 CrossRef CAS PubMed.
- M. A. Reddy, N. Jain, D. Yada, C. Kishore, J. R. Vangala, P. R. Surendra, A. Addlagatta, S. V. Kalivendi and B. Sreedhar, J. Med. Chem., 2011, 54, 6751 CrossRef CAS PubMed.
- A. S. Kumar, M. A. Reddy, N. Jain, C. Kishor, T. R. Murthy, D. Ramesh, B. Supriya, A. Addlagatta, S. V. Kalivendi and B. Sreedhar, Eur. J. Med. Chem., 2013, 60, 305 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectrum of products. Detailed procedures and spectroscopic data for novel catalysts. Copies of 1H NMR, 13C NMR and HPLC data of novel compounds and catalysts prepared are available. See DOI: 10.1039/c3ra47423b |
| This journal is © The Royal Society of Chemistry 2014 |