Irradiation induced non-equilibrium phase formation and structural transformation in the Fe–Ti–Nb multilayered films
N.
Li
,
J. H.
Li
and
B. X.
Liu
*
Key Laboratory of Advanced Materials (MOE), School of Materials science and Engineering, Tsinghua University, Beijing 100084, China. E-mail: dmslbx@tsinghua.edu.cn; Fax: +86 10 62771160; Tel: +86 10 62772557
First published on 20th January 2014
Abstract
Six sets of the Fe–Ti–Nb multilayered films were first prepared and then subjected to ion beam irradiation with 180 keV Xe ions. The unique amorphous phases, phase separation induced dual amorphous phase and orientation-preferred BCC–Fe crystalline phase were observed in these Fe–Ti–Nb multilayered films at different irradiation doses. In addition, it is also revealed that the Ti-rich HCP crystalline phase could transform into a metastable FCC phase in the Fe15Ti75Nb10 film. The possible mechanisms of these non-equilibrium phase formation and structural transformations are discussed based on atomic collision theory, geometric crystallography and ab initio calculations.
Introduction
Fe-based amorphous alloys have attracted more and more attention in recent years due to their ultra-high strength, excellent soft magnetic properties, good corrosion resistance, and their rather low cost.1–3 For example, a variety of Fe-based amorphous alloys have been developed by Inoue et al. and other researchers.4 Among these amorphous alloys, Ti-containing Fe-based amorphous alloys are known as one of the potential lightweight engineering materials for industrial applications with high strength and ductility, especially for Fe–Ti–Nb ternary metal system. In Park's research, Fe–Ti–Nb amorphous alloys were obtained to own good compressive mechanical properties, i.e., high yield (1.9–2.2 GPa) and fracture (2.2–2.6 GPa) strength together with large plasticity (6–13%).5 Meanwhile, it has also been found by extensive researches that microstructure and phase transformations have a significant influence on the mechanical properties of amorphous alloys.6,7 Therefore, the present study focuses on the non-equilibrium phase formation and structural transformations of the Fe–Ti–Nb multilayered films upon ion beam irradiation (IBI).Comparing with other non-equilibrium producing techniques such as the liquid melt quenching developed in 1960,8 solid-state reaction and mechanical alloying introduced in 1980s,9–11 the ion beam irradiation has been proven to be very effective in producing metastable amorphous alloys.12 There are three essential features involved in the IBI. First, the individual metal layers elevate the interfacial free energies of the initial multilayered films up to a highly energetic state desired. Second, ion irradiation could be conducted to trigger the interfacial mixing at a low temperature. Third, the irradiation dose could be controlled by adjusting the ion current, thus enable researchers to trace the details of the structural phase transformations taking place in the films. Consequently, IBI has been widely employed to study the non-equilibrium formation and transformation of metal systems.13–15
In order to investigate the related microstructures and phase transformations of the Fe–Ti–Nb ternary metal system, six sets of multilayered films with overall compositions of Fe80Ti12Nb8, Fe65Ti21Nb14, Fe50Ti30Nb20, Fe30Ti42Nb28, Fe15Ti51Nb34 and Fe15Ti75Nb10 were first prepared by electron depositing and then subjected to ion beam irradiation by 180 keV Xe ions upon different doses in the present study.
Experimental procedure
In the experiments, 180 keV Xe ions were used as irradiating ions in IBI. Based on the TRIM calculation,16 the thickness of the Fe–Ti–Nb system should be controlled about 40 nm. Thus, all the Fe–Ti–Nb multilayered films were designed to be around 35 nm. Multilayered films were first prepared by alternatively depositing pure Fe (99.99%), Ti (99.99%) and Nb (99.99%) at a rate of 0.1–0.5 Å s−1 onto newly cleaved NaCl single crystal substrates in an e-gun evaporation system with a vacuum level of 10−6 Pa. The as-deposited films were then subjected to irradiation in an implanter with a vacuum level better than 5 × 10−4 Pa. The doses were in a range from 4 × 1014 Xe+ per cm2 to 7 × 1015 Xe+ per cm2. During irradiation, liquid nitrogen was injected to cool the sample holder and the current density was confined to be 15 μA cm−2. The structures of all the Fe–Ti–Nb multilayered films were examined by bright field image and selected area diffraction (SAD) of transmission electron microscopy (JEM 200 CX) and high-resolution transmission electron microscopy (HRTEM) (TECNAI G2 20). And the X-ray fluorescence (XRF) (XRF-1800) and energy dispersive spectroscopy (EDS) examination were used to ascertain the real compositions of the deposited films, as well as the resultant alloy phases formed.Results and discussion
Formation and phase separation of amorphous phase
In order to present the detailed phase formation and structural phase transformations emerged in the six sets of Fe–Ti–Nb multilayered films, which were irradiated to the dose range from 4 × 1014 to 7 × 1015 Xe+ per cm2, all the experimental results of the samples were summarized in the Table 1. One can see clearly that unique amorphous phases can be formed in the Fe65Ti21Nb14, Fe50Ti30Nb20, Fe30Ti42Nb28 and Fe15Ti51Nb34 multilayered films, yet at different irradiation doses. Meanwhile, in the multilayered films with composition of Fe65Ti21Nb14 and Fe30Ti42Nb28, crystalline phases were formed again with increasing the irradiation dose. However, in the other two films with too much of Fe or Ti, unique amorphous phase was difficult to be obtained.| Ion dose (Xe+ per cm2) | Fe80Ti12Nb8 | Fe65Ti21Nb14 | Fe50Ti30Nb20 | Fe30Ti42Nb28 | Fe15Ti51Nb34 | Fe15Ti75Nb10 |
|---|---|---|---|---|---|---|
| a Fe: Fe-rich BCC phase; Ti: Ti-rich HCP phase; Nb: Nb-rich BCC phase; F: Ti-rich FCC phase; A: amorphous phase. | ||||||
| 0 | Fe + Ti + Nb | Fe + Ti | Fe + Ti + Nb | Fe + Ti + Nb | Fe + Ti + Nb | Fe + Ti + Nb |
| 6 × 1014 | Fe + Ti + Nb + A | Fe + A | Fe + A | Fe + Ti + Nb | Fe + Ti + Nb | Fe + Ti + Nb |
| 1 × 1015 | Fe + A | A | Fe + A | Fe + Ti + Nb | Fe + Ti + Nb | Ti + Nb + F |
| 5 × 1015 | Fe + A | Fe + A | Fe + A | A | Fe + Ti + Nb + A | F |
| 7 × 1015 | Fe + A | Fe + A | A | Fe + A | A | F |
Further, all the formed amorphous phases were examined by HRTEM, and an interesting phenomenon of phase separation was observed in the formed amorphous phase in Fe30Ti42Nb28 multilayered film after irradiated to the dose of 5 × 1015 Xe+ per cm2. Fig. 1 shows the SAD patterns and corresponding bright field image of the amorphous phase by TEM and HRTEM. One can see clearly from the Fig. 1(a) that the SAD pattern is consisted of two halos, which demonstrated the formation of amorphous phase. The bright field image of local region by HRTEM was divided by a groove in the middle into two parts, brighter area in the left and darker area in the right, and the corresponding SAD patterns were shown in the Fig. 1(c) and (d), respectively. From the comparison of the two figures, it can be seen that only one halo was obtained in the two figures and the radiuses in the two figures were also different, suggesting that phase separation may occur in the film. The compositions of the two regions were measured by EDS to be Fe34Ti44Nb22 and Fe18Ti38Nb44, respectively. As the lattice parameter of BCC–Fe is smaller than that of BCC–Nb, it is reasonable that the radius of the halo reflected from Fe-rich amorphous phase is bigger than that of the halo reflected from Nb-rich amorphous phase.
According to the atomic collision theory, a highly energetic state of Fe–Ti–Nb multilayered films was leaded by a sequence of ballistic collisions triggered by IBI. When the collision cascade is terminated, the highly energetic state has to relax to equilibrium. However, as the relaxation period is extremely short, i.e., 10−10 s, atomic rearrangement is hard to be taken place. Consequently, no complicated structured crystalline phase was retained, and only crystalline phase with simple structure, such as BCC, FCC and HCP, or the disordered structure, i.e., amorphous phase, could be preserved. Therefore, the phase formation and transformations are the results of the competitions between simple structural crystalline phases and amorphous phases. Meanwhile, structure and composition fluctuation caused by the ballistic collision may be kept after the extremely short relaxation period, thus phase separation induced dual amorphous phase formed in local regions.
Preferred orientation of BCC–Fe phase
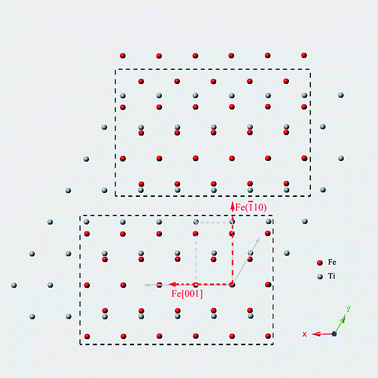
In the Fe50Ti30Nb20 multilayered films, preferred orientations of BCC–Fe phase were observed at as-deposited state and after irradiated to the doses less than 8 × 1014 Xe+ per cm2. Fig. 2 shows the SAD patterns of the Fe50Ti30Nb20 multilayered films at different states. The diffraction rings and symmetrical texture spots in the Fig. 2(a) demonstrated that the resultant phase at as-deposited state was a mixture of Fe-rich BCC phase, Ti-rich HCP phase and Nb-rich BCC phase, with an orientation-preferred phase. The orientation-preferred phase was indexed to be a BCC–Fe phase with lattice parameter of 2.91 Å, which is a little bigger than that of pure BCC–Fe phase. The preferred orientations of BCC–Fe phase were maintained when the irradiation dose was increased to 6 × 1014 Xe+ per cm2, as the Fig. 2(b) shown. However, Fig. 2(c) shows that preferred orientation of BCC–Fe phase disappeared with further increasing the irradiation dose.The formation of preferred orientation of BCC–Fe phase was assumed to be attributable to the direction of atomic vapor during deposition. The preferred orientated BCC–Fe phase maybe resulted from the epitaxial-like growth of the Fe layer on the Ti layer. According to the researches of Bauer and Ramirez,17,18 the epitaxial monolayer or multilayer formed usually with the most densely packed plane paralleled. Thus, mutual epitaxial of the HCP and BCC metals is consisting of stacking sequences of densely packed planes, i.e., (110)BCC and (001)HCP. From purely geometrical considerations, Fig. 3(a) shows the schematic diagram of the orientations (110)Fe deposited on (001)Ti.  and b = aHCP are the nearest-neighbor distance of BCC–Fe phase and HCP–Ti phase. Orientations preferred are those in which the most densely packed rows ([010]HCP) in (001)HCP are paralleled to those ([
and b = aHCP are the nearest-neighbor distance of BCC–Fe phase and HCP–Ti phase. Orientations preferred are those in which the most densely packed rows ([010]HCP) in (001)HCP are paralleled to those ([![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11], [1
11], [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1], [001]) in (110)BCC. The first two are Kurdjumov-Sachs (KS) orientation, and occurs when
1], [001]) in (110)BCC. The first two are Kurdjumov-Sachs (KS) orientation, and occurs when  or
or  . The last one is Nishiyama–Wassermann (NW) orientation occurs when
. The last one is Nishiyama–Wassermann (NW) orientation occurs when  or
or  . Another NW relationship is also preferred when the [112]HCP and the [1
. Another NW relationship is also preferred when the [112]HCP and the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]BCC have the same distance, which is called NW-y configuration occurred when
0]BCC have the same distance, which is called NW-y configuration occurred when 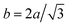 or aHCP/aBCC = 1. In the experiment, the lattice parameter of BCC–Fe phase was expanded from 2.82 Å (pure BCC–Fe) to 2.91 Å, which is almost equal to the aTi = 2.92 Å. Therefore, the formation of orientation-preferred BCC–Fe phase may be attributable to the NW-y configuration, and the Fig. 3(b) shows the possible orientation. In order to have an intuitional analysis, an atomic projection of Fe (110) deposited on Ti (001) by NW-y configuration along [001]Ti was shown in the Fig. 4. It can be seen that good coherent relationship can be achieved with stretching Fe along [
or aHCP/aBCC = 1. In the experiment, the lattice parameter of BCC–Fe phase was expanded from 2.82 Å (pure BCC–Fe) to 2.91 Å, which is almost equal to the aTi = 2.92 Å. Therefore, the formation of orientation-preferred BCC–Fe phase may be attributable to the NW-y configuration, and the Fig. 3(b) shows the possible orientation. In order to have an intuitional analysis, an atomic projection of Fe (110) deposited on Ti (001) by NW-y configuration along [001]Ti was shown in the Fig. 4. It can be seen that good coherent relationship can be achieved with stretching Fe along [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10]Fe or compressing Ti along [112]Ti.
10]Fe or compressing Ti along [112]Ti.
 | ||
| Fig. 3 (a) Schematic diagram of the orientations Fe (110) deposited on Ti (001) preferred and (b) the possible orientation happened in Fe50Ti30Nb20 multilayered films. | ||
Moreover, the mechanisms of thin crystalline films grow near equilibrium maybe the Vomer–Weber (VW), the Stranski–Krastanov (SK) or the Frank–van der Merwe (FM) mode, depending on the relative magnitudes of the surface energies and the interfacial energy.19 In the SK or VW mode, three-dimensional crystals form on the substrate. If possible, nucleation and growth will along unidirectional steps, thus preferred orientation of BCC–Fe crystal phase could be observed. From the geometric crystallography analysis and the film growth mechanism discussion above, the experiment results are reasonable.
Structural transformation from HCP–Ti to FCC–Ti phase
As Table 1 shown, an interesting phase transformation from HCP–Ti to FCC–Ti phase was observed in the Fe15Ti75Nb10 multilayered films with increasing the irradiation dose. Only one FCC–Ti phase was retained in the films after irradiated to the doses more than 5 × 1015 Xe+ per cm2. For comparison, Fig. 5 shows the SAD patterns of the Fe15Ti75Nb10 multilayered films at as-deposited state and after irradiated to the dose of 7 × 1015 Xe+ per cm2. One can see from the diffraction rings in Fig. 5(a) that a mixture of Fe-rich BCC phase, Ti-rich HCP phase and Nb-rich BCC phase was obtained. While, only one Ti-rich FCC phase was observed after the films was irradiated to high dose, as shown in the Fig. 5(b), and the lattice parameter of the FCC–Ti phase was indexed to be 4.11 Å. | ||
| Fig. 5 The SAD patterns of (a) the resultant phases of Fe15Ti75Nb10 multilayered films at as-deposited state and (b) the newly formed FCC–Ti after irradiated to the dose of 7 × 1015 Xe+ per cm2. | ||
The formation of the metastable FCC–Ti phase and such a phase transformation from BCC–Ti to FCC–Ti may be the results of the atomic collision physical process of IBI discussed above. In the thermodynamic aspect, the energy difference between the HCP–Ti and FCC–Ti phases calculated by Miedema et al. is relatively small to be 0.5 kJ mol−1.20 Thus, the transformation from HCP–Ti phase to a metastable FCC–Ti phase is possible by techniques far from equilibrium, e.g. IBI. In the kinetics aspects, the transformation is readily, as both HCP and FCC structures are close-packed with the same atomic packing density and the only difference between them is the stacking order. The shearing mechanism (SM) proposed by Liu et al. is probably one of the possible explain for the transformation.21 The phase is transformed with the sliding of the (0002)HCP plane atoms along the vector direction of 1/3〈1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 00〉. The lattice constant relationship between the two structures can be readily determined to be
00〉. The lattice constant relationship between the two structures can be readily determined to be  . With the relationship, the lattice constants of the FCC–Ti phase can be calculated to be 4.13 Å, which is consistent with 4.11 Å obtained in experiment.
. With the relationship, the lattice constants of the FCC–Ti phase can be calculated to be 4.13 Å, which is consistent with 4.11 Å obtained in experiment.
To further confirm the process of phase transformation in the Fe15Ti75Nb10 multilayered films, ab initio calculation based on Cambridge Sequential Total Energy Package (CASTEP) was carried out. In the present calculations, the exchange and correlation effects are described by the function of Perdew and Wang,22 which employs generalized gradient approximation (GGA).23 The interaction between the valance electrons and ionic cores is represented by ultrasoft pseudopotentials (US-PP). Brillouin zone integrations were performed using and 16 × 16 × 16 Monkhorst–Pack grid,24 which led to 120 irreducible κ points. The lattice parameter of FCC–Ti determined by CASTEP is to be 4.09 Å, which is in good accordance with the experimental observation (aFCC–Ti = 4.11 Å). The results are also consistent with that calculated by Wang et al. using VASP with PAW-GGA potential to be 4.10 Å.25
Conclusions
Upon the appropriate ion beam irradiation, the unique amorphous phases could be obtained in the films with overall compositions of Fe65Ti21Nb14, Fe50Ti30Nb20, Fe30Ti42Nb28 and Fe15Ti51Nb34. Meanwhile, phase separation caused by composition fluctuation was also observed in the Fe30Ti42Nb28 multilayered film and the phase separation further results in the formation of dual amorphous phase. In addition, BCC–Fe phases in the Fe50Ti30Nb20 multilayered films exhibit the preferred orientations at as-deposited state and after irradiated to doses less than 8 × 1014 Xe+ per cm2. It was assumed to be attributable to the unidirectional epitaxial-like growth of atomic vapor during deposition. Besides, for the FCC–Ti phase formed in the Fe15Ti75Nb10 multilayered films at high irradiation doses, the lattice constant is calculated to be compatible with both the SM prediction and ab initio calculation.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (50971072, 51131003), the Ministry of Science and Technology of China (973 Program 2011CB606301, 2012CB825700), and the Administration of Tsinghua University.Notes and references
- K. P. Tai, T. L. Wang and B. X. Liu, Appl. Phys. Lett., 2007, 91, 204101 CrossRef PubMed.
- M. Nabialek, J. Zbroszczyk, J. Olszewski, M. Hasiak, W. Ciurzynska, K. Sobczyk, J. Swierczek, J. Kaleta and A. Lukiewska, J. Magn. Magn. Mater., 2008, 320, E787–E791 CrossRef PubMed.
- Z. L. Long, Y. Shao, F. Xu, H. Q. Wei, Z. C. Zhang, P. Zhang and X. P. Su, Mater. Sci. Eng., B, 2009, 164, 1–5 CrossRef PubMed.
- A. Inoue and J. S. Gook, Mater. Trans., 1995, 36, 1282–1285 CrossRef.
- J. M. Park, J. H. Han, K. B. Kim, N. Mattern, J. Eckert and D. H. Kim, Philos. Mag. Lett., 2009, 89, 623–632 CrossRef.
- Z. Y. Xiao, C. Y. Tang, T. L. Ngai, C. Yang and Y. Y. Li, Phys. B, 2012, 407, 258–262 CrossRef PubMed.
- D. K. Misra, S. W. Sohn, H. Gabrisch, W. T. Kim and D. H. Kim, Intermetallics, 2010, 18, 342–347 CrossRef PubMed.
- W. Klement, R. H. Willens and P. Duwez, Nature, 1960, 187, 869–870 CrossRef.
- B. X. Liu, W. L. Johnson, M. A. Nicolet and S. S. Lau, Appl. Phys. Lett., 1983, 42, 45–47 CrossRef PubMed.
- C. C. Koch, O. B. Cavin, C. G. Mckamey and J. O. Scarbrough, Appl. Phys. Lett., 1983, 43, 1017–1019 CrossRef PubMed.
- R. B. Schwarz and W. L. Johnson, Phys. Rev. Lett., 1983, 51, 415–418 CrossRef.
- M. Nastasi and J. W. Mayer, Mater. Sci. Rep., 1991, 6, 1–51 CrossRef.
- I. P. Jain and G. Agarwal, Surf. Sci. Rep., 2011, 66, 77–172 CrossRef PubMed.
- R. S. Averback and P. Bellon, Fundamental concepts of ion beam processing, Springer-Verlag Berlin, Berlin, 2010, vol. 116, pp. 1–28 Search PubMed.
- C. Lin, G. W. Yang and B. X. Liu, J. Appl. Phys., 2000, 87, 2821–2824 CrossRef PubMed.
- J. F. Ziegler, J. P. Biersack and L. U. Littmark, The stopping and range of ions in solids, Pergamon Press, New York, 1985, ch. 2, pp. 79–82 Search PubMed.
- E. Bauer and J. H. Vandermerwe, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 3657–3671 CrossRef.
- R. Ramirez, A. Rahman and I. K. Schuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1984, 30, 6208–6210 CrossRef.
- H. Brune, Surf. Sci. Rep., 1998, 31, 121–229 CrossRef.
- F. R. De Boer, R. Boom, W. C. M. Mattens, A. R. Miedema and A. K. Niessen, Cohesion in metals: Transition metal alloys, Elsevier, NorthHolland, Amsterdam, 1989, ch. 2, pp. 79–82 Search PubMed.
- B. X. Liu and Z. J. Zhang, J. Mater. Res., 1994, 9, 357–361 CrossRef.
- J. P. Perdew and Y. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 13244–13249 CrossRef.
- J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh and C. Fiolhais, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 6671–6687 CrossRef.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 5188–5192 CrossRef.
- Y. Wang, S. Curtarolo, C. Jiang, R. Arroyave, T. Wang, G. Ceder, L. Q. Chen and Z. K. Liu, Calphad, 2004, 28, 79–90 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |