Deep hydrogenation of coal tar over a Ni/ZSM-5 catalyst†
Shi-Chao Qia,
Lu Zhanga,
Xian-Yong Wei*a,
Jun-ichiro Hayashib,
Zhi-Min Zonga and
Lu-Lu Guoa
aKey Laboratory of Coal Processing and Efficient Utilization, Ministry of Education, China University of Mining & Technology, Xuzhou 221116, P. R. China. E-mail: wei_xianyong@163.com
bDivision of Advanced Device Material, Institute for Materials Chemistry and Engineering, Kyushu University, 6-1, Kasuga Koen, Kasuga, Fukuoka 816-8580, Japan
First published on 14th February 2014
Abstract
We have developed a Ni/ZSM-5 catalyst and utilised it to completely hydrogenate a series of condensed arenes, including naphthalene, anthracene and phenanthrene, under relatively mild conditions. The yields of decalin, perhydroanthracene and perhydrophenanthrene reached 100%, 98.8% and 25.8%, respectively. By analyzing the isomer distribution of the perhydroarenes, we proposed a mechanism of biatomic hydrogen transfer, which was further proven via the hydrogenation of 9,10-diphenylanthracene. Through hydrotreatment over the Ni/ZSM-5 catalyst, both high-temperature coal tar rich in condensed arenes and low-temperature coal tar mixing arenes with alkanes were greatly upgraded. The majority of arenes in the coal tars were deeply and even completely hydrogenated, which may open up a route for further processing and application of coal tar as clean fuels.
The risk of petroleum shortage has stimulated the development of coal to oil technologies,1–3 but the high concentration of condensed arenes (CAs) in the liquids resulting from these technologies limits their application as clean fuels.4–7 Deep hydrogenation of CAs has thus been a major technical subject.4,8,9 However, only few reports were issued on the catalytic hydrogenation of CAs to cyclanes in high yields under relatively mild conditions.8–10 For instance, less than 10% of phenanthrene was hydrogenated to perhydrophenanthrene (PHP) at 250 °C over a Pt–Pd/γ-Al2O3 catalyst.11 Tang et al.12 completely converted naphthalene over a Pd/Al-MCM-41 catalyst, with the main product being tetralin rather than decalin. In addition, quite a few studies on the kinetics of arene hydrogenation over metallic catalysts have been reported13–15 and there are only few articles about the specific pathways for hydrogen transfer to aromatic rings, which is a crucial subject for organic synthesis, oil processing and coal hydroliquefaction.16–18 Coal tar, the viscous liquid from coal carbonization, can be classified into high-temperature coal tar (HTCT) and low-temperature coal tar (LTCT). The annual output of coal tar has been higher than 20 million tons around the world. At present, using coal tar as a fuel faces a huge challenge because of the presence of CAs, especially in HTCT.19 On the other hand, it is so difficult to upgrade coal tar that there are rarely ideal deep hydrogenation results, even though the hydrogenation of coal tar has been extensively investigated. Kusy et al.20 used Co–Mo/Al2O3 to hydrogenate a coal tar, but there were only few saturates in the product. It can be predicted that hydrogenated HTCT (HHTCT) and hydrogenated LTCT (HLTCT) can be used as clean fuels once they are successfully saturated.21,22
In previous work, we developed highly active Ni/ZSM-5.23 Here, the Ni/ZSM-5 is applied to catalyze the hydrogenation of CAs (i.e. naphthalene, anthracene, phenanthrene and 9,10-diphenylanthracene (DPA)), HTCT and LTCT. All the CAs are deeply hydrogenated and both HTCT and LTCT are greatly upgraded; moreover, biatomic hydrogen transfer (BAHT) is put forward as the pathway for CA hydrogenation over nickel.
The Ni/ZSM-5 was prepared according to the method reported.23 Cyclohexane (20 mL), Ni/ZSM-5 (0.5 g) and a substrate (CA, 5 mmol, or the soluble fraction of coal tar in cyclohexane, 0.5 g) were fed into an autoclave. After replacing air in the autoclave and pressurizing with hydrogen to 5.0 MPa at room temperature, the autoclave was heated to an indicated temperature within 20 min and maintained at this temperature for a prescribed period of time with rapid agitation (250 rpm). The total pressure inside the autoclave was maintained at higher than 6.0 MPa via timely H2 supplementation during the reaction. The reaction mixture was taken out of the autoclave and filtered after cooling the autoclave in a water bath. The filtrate was analyzed with a Hewlett-Packard 6890/5973 GC/MS.
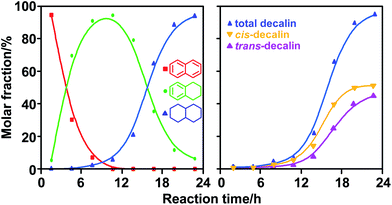
Naphthalene hydrogenation over Ni/ZSM-5 at 180 °C produced tetralin and decalin (Fig. 1, left). The highest yield of tetralin was obtained after 10 h, then the molar fraction of tetralin decreases rapidly. Correspondingly, a rapid increase in the molar fraction of decalin is also observed. Deep hydrogenation of naphthalene over Ni/ZSM-5 produced trans- and cis-decalin simultaneously (Fig. 1, right). The concentration of cis-decalin is higher than that of trans-decalin throughout because the BAHT over the metallic catalyst is favorable.8,24 The trans-isomer is generally more thermodynamically stable than the cis-isomer and the molar ratio of trans-/cis-isomer rises slowly from 0.33 to 0.87 with reaction time, indicating the configuration inversion from cis-decalin to trans-decalin.
Perhydroanthracenes (PHAs) and perhydrophenanthrenes (PHPs) were obtained in high yields over Ni/ZSM-5 at 160 °C for 24 h. As shown in Table 1, PHAs almost makes up the whole composition of the products. Deep hydrogenation of phenanthrene is difficult due to its steric configuration.1–4 However, the yield of PHP also reached 25.8 mol%, and the total yield of octahydrophenanthrenes (OHPs) reached 62.9 mol%. As shown in Fig. S1 to S7,† all hydroanthracenes and hydrophenanthrenes are confirmed via GC/MS analysis. According to the isomer distribution of the PHAs (Table 2), four configurational isomers manifest the diverse pathways of complete hydrogenation and the cis-isomers share the highest yields. The isomer distribution of PHPs is similar to yields. Moreover, the yields of (4aR,8aS,9aS,10aR)-PHA and PHPs are higher than that of (4aR,8aR,9aS,10aS)-PHA. These facts imply that the intermediates with smaller steric hindrance are more easily adsorbed on the nickel surface for the subsequent hydrogenation step than those with bigger steric hindrance and that the predominant hydrogenation pathway of arenes on the nickel surface is BAHT.
Monatomic hydrogen transfer to CAs favors the generation of thermodynamically stable trans-isomers, whereas Ni/ZSM-5, as a metallic catalyst, mainly catalyzes BAHT to aromatic rings.25,26 H2 adsorbed on the Ni surface is parallelly added to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and saturates it, which favors the production of cis-isomers. During naphthalene hydrogenation, trans-decalin can be produced via BAHT to the 1- and 8a-positions from one side of the molecular plane and subsequent BAHT to the 4- and 4a-positions from another side (Scheme 1, Path III). Cis-decalin would be generated if 4- and 4a-positions were hydrogenated via BAHT from the same side as 8a-H (Scheme 1, Path II). Clearly, sym-octalin can be hydrogenated only to cis-decalin via BAHT (Scheme 1, Path I). Moreover, because of the reversible processes of arene hydrogenation,8,9 the cis-isomer can transform into the trans-isomer via the inverse process of Path II cooperating with Path III, which explains the increase in the molar ratio of trans-/cis-decalin with prolonging the reaction (Fig. 1, right).
C and saturates it, which favors the production of cis-isomers. During naphthalene hydrogenation, trans-decalin can be produced via BAHT to the 1- and 8a-positions from one side of the molecular plane and subsequent BAHT to the 4- and 4a-positions from another side (Scheme 1, Path III). Cis-decalin would be generated if 4- and 4a-positions were hydrogenated via BAHT from the same side as 8a-H (Scheme 1, Path II). Clearly, sym-octalin can be hydrogenated only to cis-decalin via BAHT (Scheme 1, Path I). Moreover, because of the reversible processes of arene hydrogenation,8,9 the cis-isomer can transform into the trans-isomer via the inverse process of Path II cooperating with Path III, which explains the increase in the molar ratio of trans-/cis-decalin with prolonging the reaction (Fig. 1, right).
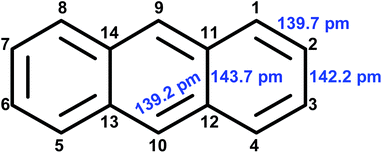
In order to certify BAHT by an easy and effective method, DPA was partially hydrogenated over Ni/ZSM-5 at 150 °C for 5 h. As shown in Fig. 2, DPA was completely converted but not fully saturated due to the controlled reaction conditions. All the products from DPA hydrogenation contain benzene ring in the center. According to the theory of superdelocalizability,27 the 9- and 10-positions on anthracene ring are most readily hydrogenated. However, as shown in Fig. 3, the bond length between the 9- and 10-positions of gaseous anthracene is ca. 300 pm and the lattice spacing of Ni (111), which is the main catalytic lattice plane of Ni for arene hydrogenation, is only ca. 250 pm.28–30 According to the multiplet theory of catalysis,31,32 it is impossible for the Ni adsorbing and cracking H2 to be added to the two carbon atoms at the 9- and 10-positions. Such impossibility results in the difficulty in hydrogenating the central benzene ring in DPA via BAHT.
 | ||
| Fig. 2 Total chromatogram of partially hydrogenated DPA (150 °C, 5 h) and mass spectra of the products. | ||
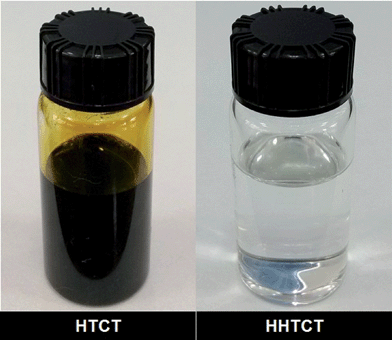
As demonstrated in Figs 4 and 5, the dark brown liquids HTCT and LTCT were hydrogenated to the colorless and transparent liquids HHTCT and HLTCT, respectively, over fresh Ni/ZSM-5 three times at 160 °C (each for 15 h). This implies that most of the unsaturated species in both HTCT and LTCT were hydrogenated. As shown in Fig. S8 and Table S1,† HTCT is rich in a variety of CAs, such as naphthalene (25.484 wt%), phenanthrene (12.850 wt%), fluoranthene (7.019 wt%) and fluorene (6.266 wt%). Furthermore, there are also a few heteroatomic compounds (HACs) detected in HTCT, such as dibenzofuran (3.088 wt%), benzothiophene (1.057 wt%) and quinoline (0.548 wt%), which would passivate and poison the catalyst. The catalyst was therefore renewed during the hydrogenation process. Neglecting the impact on the deactivation of the catalyst by the HACs HTCT is still difficult to be hydrogenated because the blended CAs in HTCT severely restrain mutual reactivity toward hydrogenation owing to the competitive adsorption on the catalyst used.33–37 However, as shown in Fig. S9 and Table S2,† most of the arenes were converted to cyclanes, such as decalin (20.896 wt%), hexadecahydrofluoranthene (8.143 wt%) and dodecahydrofluorene (5.288 wt%), and a few CAs were deeply hydrogenated to hydroarenes (HAs) containing single benzene ring, such as decahydropyrene (3.203 wt%) and dodecahydrochrysene (1.933 wt%). Hydrogenating the remaining benzene ring is extremely difficult due to the severe steric hindrance caused by the cyclane ring on the benzene ring,8,9,23 which is in agreement with the results of phenanthrene hydrogenation (Table 1). Moreover, compared with HTCT, there are no obvious HACs detected in HHTCT because heteroatoms could be deprived from HTCT via reaction with metallic species.38–40 Indeed, the nickel sites located on ZSM-5, which are poisoned by adsorbed sulfide, may be partially recovered by spillover hydrogen from the acidic support.41,42
As shown in Fig. S10 and Table S3,† LTCT contains a series of CAs like HTCT, but their molecular weights are apparently smaller than those in HTCT. In addition, there are a series of alkanes which make up most of LTCT with small amounts of alkenes, arenes and HACs. As exhibited in Fig. S11 and Table S4,† all the alkenes and most of the arenes were saturated by Ni/ZSM-5 catalyzed hydrogenation and only very small amounts of HAs and HACs appear in HHLTCT. These facts indicate that the Ni/ZSM-5 also plays an important role in upgrading LTCT. As displayed in Table 3, carbon contents in both HTCT and LTCT was obviously reduced by the catalytic hydrogenation and the ratio of C/H of HTCT dropped more obviously than that of LTCT because more arenes are saturated in HTCT than in LTCT. Meanwhile, the heteroatoms in HTCT and N and S in LTCT were completely removed and oxygen content in LTCT was significantly reduced by the catalytic hydrogenation. These facts are in agreement with the results of GC/MS analysis (Fig. S8 to S11†).
| Sample | Content (wt%) | ||||
|---|---|---|---|---|---|
| C | H | O | N | S | |
| HTCT | 93.14 | 5.81 | 0.44 | 0.15 | 0.46 |
| HHTCT | 87.83 | 12.17 | 0 | 0 | 0 |
| LTCT | 87.68 | 11.07 | 1.25 | 0 | 0 |
| HLTCT | 85.77 | 14.00 | 0.22 | 0 | 0 |
In summary, the Ni/ZSM-5 we developed displayed a very high activity for the hydrogenation of CAs, including naphthalene, anthracene and phenanthrene. The CAs were completely converted to afford high yields of cyclanes under relatively mild conditions. On the nickel surface, CAs were hydrogenated via a BAHT mechanism, which favors the generation of cis-cyclanes, which was further proved via the hydrogenation of DPA. HTCT and LTCT were well upgraded over the catalyst and the majority of the CAs in both HTCT and LTCT were completely saturated to cyclanes, which may open up a route for further processing and application of coal tar as clean fuels.
Acknowledgements
This work was supported by National Basic Research Program of China (Grant 2011CB201302), National Natural Science Foundation of China (Grants 20936007, 51221462 and 51074153), the Key Project of Coal Joint Fund from National Natural Science Foundation of China and Shenhua Group Corporation Limited (Grant 51134021), the Strategic Chinese-Japanese Joint Research Program (Grant 2013DFG60060), the Fundamental Research Fund for the Doctoral Program of Higher Education (Grant 20120095110006) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- I. P. Fisher, F. Souhrada and H. J. Woods, J. Am. Chem. Soc., Div. Pet. Chem., 1982, 27, 838–848 CAS.
- J. P. Longwell, E. S. Rubin and J. Wilson, Prog. Energy Combust. Sci., 1995, 21, 269–360 CrossRef CAS.
- F. N. Cayan, M. Zhi and S. R. Pakalapati, J. Power Sources, 2008, 185, 595–602 CrossRef CAS PubMed.
- M. Breysse, M. G. Djega and S. Pessayre, Catal. Today, 2003, 4, 129–138 CrossRef.
- H. Schulz, W. Böhringer and P. Waller, Catal. Today, 1999, 49, 87–97 CrossRef CAS.
- A. J. Suchanek, Oil Gas J., 1990, 88, 109 CAS.
- X. Ma, K. Sakanishi and T. Isoda, Energy Fuels, 1995, 9, 33–37 CrossRef CAS.
- A. Stanislaus and B. H. Cooper, Catal. Rev., 1994, 36, 75–123 CAS.
- B. H. Cooper and B. Donnis, Appl. Catal., A, 1996, 137, 203–223 CrossRef CAS.
- M. Nuzzi and B. Marcandalli, Fuel Process. Technol., 2003, 80, 35–45 CrossRef CAS.
- W. Qian, Y. Yoda and Y. Hirai, Appl. Catal., A, 1999, 184, 81–88 CrossRef CAS.
- T. Tang, C. Yin and L. Wang, J. Catal., 2008, 257, 125–133 CrossRef CAS PubMed.
- J. W. Thybaut, M. Saeys and G. B. Marin, Chem. Eng. J., 2002, 90, 117–129 CrossRef CAS.
- S. Smeds, T. Salmi and D. Y. Murzin, Appl. Catal., A, 1999, 185, 131–136 CrossRef CAS.
- M. Saeys, M. F. Reyniers and J. W. Thybaut, J. Catal., 2005, 236, 129–138 CrossRef CAS PubMed.
- M. I. Chowdhry, P. N. Horton and M. B. Hursthouse, Tetrahedron Lett., 2009, 50, 3400–3403 CrossRef CAS PubMed.
- A. Corma, M. Faraldos and A. Martínez, J. Catal., 1990, 122, 230–239 CrossRef CAS.
- M. L. Poutsma, Energy Fuels, 1990, 4, 113–131 CrossRef CAS.
- M. M. Podorozhanskii, Coke Chem., 1969, 35 Search PubMed.
- J. Kusy, L. Andel and M. Safarova, Fuel, 2012, 101, 38–44 CrossRef CAS PubMed.
- J. Yan, C. S. Lv, A. H. Liu and J. W. Da, Petrochem. Technol., 2006, 35, 33–36 CAS.
- Q. K. Li and S. J. Wang, Fuel Chem. Process., 2013, 44, 4–6 Search PubMed.
- S. C. Qi, X. Y. Wei and Z. M. Zong, ChemCatChem, 2013, 5, 3543–3547 CrossRef CAS PubMed.
- S. C. Qi, X. Y. Wei, Z. M. Zong and Y. K. Wang, RSC Adv., 2013, 3, 14219–14232 RSC.
- X. Y. Wei, E. Ogata and E. Niki, Chem. Lett., 1991, 2199–2202 CrossRef CAS.
- X. Y. Wei, Z. H. Ni, Z. M. Zong, S. L. Zhou, Y. C. Xiong and X. H. Wang, Energy Fuels, 2003, 17, 652–657 CrossRef CAS.
- Y. M. Ma, X. Y. Wei, X. Zhou and K. Y. Cai, Energy Fuels, 2009, 23, 638–645 CrossRef CAS.
- S. N. Ketker, M. Kelley and M. Fink, J. Mol. Struct., 1981, 77, 127–138 CrossRef.
- Z. Y. Hou, J. Gao and J. Z. Guo, J. Catal., 2007, 250, 331–341 CrossRef CAS PubMed.
- J. Gao, Z. Y. Hou and X. S. Liu, Int. J. Hydrogen Energy, 2009, 34, 3734–3742 CrossRef CAS PubMed.
- A. A. Balandin, Adv. Catal., 1969, 19, 1–210 CAS.
- J. K. Nørskov, T. Bligaard and A. Logadottir, J. Catal., 2002, 209, 275–278 CrossRef.
- J. Wang, L. Huang and Q. Li, Appl. Catal., A, 1998, 175, 191–199 CrossRef CAS.
- J. Wang, H. Chen and Q. Li, React. Kinet. Catal. Lett., 2000, 69, 277–284 CrossRef CAS.
- T. C. Huang and B. C. Kang, Ind. Eng. Chem. Res., 1995, 34, 1140–1148 CrossRef CAS.
- K. Ito, Y. Kogasaka and H. Kurokawa, Fuel Process. Technol., 2002, 79, 77–80 CrossRef CAS.
- S. Jongpatiwut, Z. Li and D. E. Resasco, Appl. Catal., A, 2004, 262, 241–253 CrossRef CAS PubMed.
- V. Meille, E. Schulz, M. Lemaire and M. Vrinat, J. Catal., 1997, 170, 29–36 CrossRef CAS.
- C. Zhao, S. Kasakov, J. He and J. A. Lercher, J. Catal., 2012, 296, 12–23 CrossRef CAS PubMed.
- J. C. García-Martínez and C. O. Castillo-Araiza, Chem. Eng. J., 2012, 210, 53–62 CrossRef PubMed.
- C. Song and X. L. Ma, Appl. Catal., B, 2003, 41, 207–238 CrossRef CAS.
- C. Song, CHEMTECH, 1999, 29, 26–30 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47701k |
| This journal is © The Royal Society of Chemistry 2014 |