Structure formation process, heat-insulation property and cyclic compression–resilience performance of mullite fibres/whiskers frameworks†
Shan Liu,
Jiachen Liu,
Haiyan Du,
Feng Hou*,
Sue Ren and
Haitao Geng
Key Laboratory of Advanced Ceramics and Machining Technology of Ministry of Education, School of Materials Science, Engineering of Tianjin University, Tianjin 300072, China. E-mail: houf@tju.edu.cn; Fax: +86-22-27402102
First published on 24th January 2014
Abstract
Mullite fibres/whiskers frameworks (MF/MW frameworks) were prepared by mixed-slurry-filtration and heat-treating. Hierarchical fibres/whiskers structure formed in the frameworks through fluorine-catalyzed gas-phase reaction. Topaz rods as a transient product first formed in the frameworks, and then gradually transformed into mullite whiskers. Intersected mullite whiskers served as lap-jointing points of the fibres. The volume density, apparent porosity and thermal conductivity of the samples heat-treated at 1100–1500 °C were 0.402–0.541 g cm−3, 81.2–85% and 0.1191–0.1647 W m−1 k−1, respectively. Cyclic compression–resilience performance (maximum stress = 0.4 MPa) of the samples was tested at room temperature. The compression ratio, permanent deformation ratio and resilience ratio of the samples in the first circle compression–resilience test were 2.031–3.833%, 0.125–0.042% and 93.85–98.28%, respectively. The excellent thermal/mechanical properties indicated that the MF/MW frameworks were suitable for being applied as high temperature heat-insulation/sealing materials.
1. Introduction
Heat-insulation/sealing materials have been widely used in aerospace, industry or civil fields.1 The requisite characteristics of them should be lightweight, high heat-resistance, low thermal conductivity, proper strength, and some other good mechanical performances. The heat-insulation/sealing components were usually applied under certain thermal/mechanical stress which was produced by the adjacent components' expansion at high temperature, therefore, compression–resilience was a significant mechanical performance for ensuring the tight connection between the heat-insulation/sealing components and the adjacent components.2Mullite had been extensively used in high-temperature field, due to its high mechanical strength, low thermal conductivity, high temperature stability, good thermal shock resistance and creep resistance, etc.3–7 Short mullite fibres, which possessed all the properties of mullite, were always introduced in the ceramics as toughening phase,8 or to form frameworks as the main structure of the materials.9,10 Short mullite fibres' frameworks used for heat-insulation/sealing had been reported in some literatures. X. Dong et al.11 reported a kind of bird's nest-structural fibrous mullite ceramic which was prepared through infiltration process. In their samples, the cross-linked mullite fibres were bonded by silica–boron binder. Besides, Z.G. Hou et al.2 had prepared analogous fibrous mullite ceramic through TBA (tert-butyl alcohol) based gel-casting method, and the fibres were bonded by SiO2-sintered compact in their material. However, for these two kinds of materials, the binders in the ceramics had disadvantageous effects on keeping the heat-resistance of the whole composite material. Moreover, the lap-jointing points of the fibres were immovable “soldered dots” which would produce inner stress in thermal/mechanical cyclic circumstance, and this would result in a relatively low thermal shock resistance and low fatigue resistance. Therefore, modifying the lap-jointing points of the fibres was extremely necessary for improving the thermal/mechanical properties of these fibrous ceramics and made them more usable and durable at high temperature.
In our former work,12 mullite fibres/whiskers frameworks (MF/MW frameworks) applied for heat-insulation/sealing had been prepared through mixed-slurry filtration and heat-treating method. In the frameworks, hierarchical fibres/whiskers structure formed through fluorine-catalyzed gas-phase reaction. Lap-jointing points of the fibres were served by intersected mullite whiskers. These unfixed lap-jointing points were benefit for obtaining good thermal/mechanical properties. In this paper, we focused on the details of the phase transition and structure formation of the MF/MW frameworks. Besides, the heat-insulation and cyclic compression–resilience properties of the MF/MW frameworks formed at different temperatures were also carefully discussed.
2. Experiments
2.1 Preparation of the MF/MW frameworks
Mixed-slurry-filtration method was employed for preparing the green bodies of the MF/MW frameworks. The flow chart of the whole preparation process was showed in Fig. 1. First, a kind of fresh Si-sol was prepared by hydrolyzing the TEOS (tetraethoxysilane, analytically pure) with HCl (analytically pure) as catalyst. The detailed procedure of the preparation of Si-sol had been reported in other literature.13 To obtain Si-sol with proper viscosity, 60 °C-water-heating was applied for 30 min. Polycrystalline short mullite fibres (commercially purchased, with diameter of 15–40 μm and length of 1–2 mm) and raw material powders [AlF3 and SiO2, analytically pure, blended by high-energy ball-milling at the speed of 700 r min−1 for 3 h] were mixed with the Si-sol by seriously stirring. Thus, mixed fibres-powders-sol slurry was formed. The mass ratio of mullite fibres and raw material powders was 2/1. Then the mixed-slurry was poured into a Buchner funnel (with 2 layers of intermediate speed filter paper) and was filtrated by the vacuum pump to prepare a green body. In this stage, most Si-sol of the slurry was extracted out into the filter flask. Only small part of the Si-sol remained in the green body as a binder. The green bodies were dried at 60 °C in an oven after being demoulded from the Buchner funnel. Then, the green bodies were heat-treated at 600–1500 °C for 2 h, in airtight alumina crucibles.2.2 Analysis and test methods
X-ray diffraction (XRD, Rigaku D/Max 2500v/PC) was employed for detecting the phase composition of the MF/MW frameworks. Scanning electron microscope (SEM, Nanosem430 from FEI) was used to observe the microstructure of the MF/MW frameworks. Transmission electron microscope (TEM, Tecnai G2 F20 from FEI) were applied to detect the mullite whiskers' growth situation. The volume densities and the apparent porosities of the frameworks were tested and calculated by the Archimedes' method. Hot-wire thermal conductivity meter (XIATECH TC3000) was used to determine the thermal conductivities of the frameworks, at room temperature. The cyclic compression–resilience performance was tested by electronic universal testing machine (CSS-44001), at room temperature.3. Results and discussion
3.1 Crystalline phase transition of the MF/MW frameworks
Fig. 2 showed the XRD patterns of the green body and the samples that formed at 600–1500 °C. From Fig. 2a, three main phases existed in the green body: mullite phase (Al6Si2O13, syn), quartz (SiO2, syn) and rosenbergite (AlF3·3H2O, syn). It indicated that AlF3 first chemically combined with H2O to form rosenbergite in the green body. The rosenbergite dehydrated to form Aluminum Fluoride (AlF3) at 600 °C (Fig. 2b). When the heat-treating temperature went up to 700 °C, no obvious change was found in the XRD pattern (Fig. 2c). Topaz [Al2SiO4(F,OH)2] as transient phase appeared at 800 °C. Meanwhile, some tiny diffraction peak of moganite phase (SiO2) was detected at this temperature (Fig. 2d). At 900–1000 °C, the topaz phase gradually disappeared (Fig. 2e and f). Trace of tridymite–O phase (SiO2) was detected when the heat-treating temperature was 1000 °C and 1100 °C (Fig. 2f and g). At 1200–1300 °C (Fig. 2h), small part of tridymite–O transformed into cristobalite (SiO2). Then at 1400 °C (Fig. 2j), mullite became the only main crystalline phase. Besides, small amount of tridymite–O and cristobalite still existed in the sample. At 1500 °C, no evident change of phase composition was detected in the XRD pattern (Fig. 2k).According to Fig. 2, phase transition showed a clearly gas-phase reaction process in the samples. The complex reaction equations of the whole process were inferred as follow, which was carefully analyzed according to the XRD patterns in our work (Fig. 2) and some other related literatures.14–18
| AlF3 + H2O → AlOF + 2HF | (1) |
| SiO2 + 4HF → SiF4 + 2H2O | (2) |
| 2AlOF + SiF4 + (4 − x)H2O → Al2SiO4Fx(OH)2−x(topaz, x = 0 ∼ 1) + (6 − x)HF | (3) |
| 3Al2SiO4Fx(OH)2−x + (4 − 3x)HF → Al6Si2O13 + SiF4 + (5 − 3x)H2O | (4) |
At the first stage (∼800 °C), all of the AlF3 reacted with H2O and produced gaseous AlOF14 and catalysis atmosphere HF15,16 [reaction (1)]. A part of SiO2 transformed into gaseous SiF4 under the catalysis of HF14 [reaction (2)]. The two gaseous products, AlOF and SiF4, rapidly reached their saturated vapour pressures in the airtight crucible and then reacted to form transient phase topaz17 [reaction (3)]. Plentiful of HF gas was also released in this stage. Then, topaz transformed into mullite at higher temperature (900–1000 °C)18 [reaction (4)]. In the whole reaction process, the equilibrium of reaction (2) gradually shifted to the positive direction due to the continuous consumption of the SiF4. At 1100–1400 °C, the generation rate of topaz [reaction (3)] was almost equal to its transformation rate [reaction (4)]. In other words, once the topaz formed, it would swiftly transformed into mullite. Hence, the topaz phase could not be detected in Fig. 2i–j. The gas-phase reaction was almost finished at 1500 °C.
3.2 Structure forming process of the MF/MW frameworks
Fig. 3a was the SEM image of the natural cross-section of the green body of MF/MW framework. The short mullite fibres lap-jointed with each other and formed the main structure of the green body. The raw material powders accumulated among the short mullite fibres. Small amount of Si-sol remained in the green body, acted as a dependable binder that kept the green body in a stable and regular shape after the drying treatment. The microstructure of the sample heat-treated at 800 °C was showed in Fig. 3b. At this temperature, short rod-like topaz grains (according to the XRD pattern in Fig. 2d) appeared in the framework. A part of topaz grains in situ formed in the pores of the framework or grew densely on the fibres. At 900 °C (Fig. 3c), the topaz grains disappeared, and a mass of spinous mullite whiskers were observed growing on the fibres or in situ forming in the pores of the framework. When the heating-temperature was heightened to 1000 °C, some of the in situ formed mullite whiskers disappeared.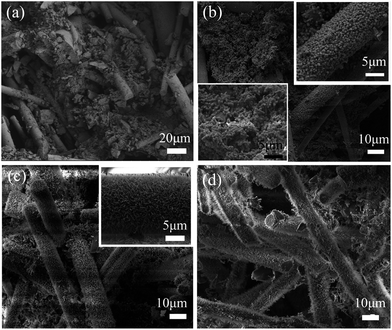 | ||
| Fig. 3 SEM images of the green body (a) and the MF/MW frameworks that were heat-treated at 800 °C (b), 900 °C (c) and 1000 °C (d). | ||
Fig. 4 showed the microstructure of the MF/MW frameworks that formed at 1100–1500 °C, and the TEM images of the mullite whiskers were showed in Fig. S1.† At 1100 °C, the framework structure became more clear. The mullite whiskers in the framework had relatively small size, and the diameter of them was about 0.15–0.25 μm (Fig. S1a†). As the heating temperature rising (1100–1400 °C, Fig. 4a–d), the aspect ratio of the mullite whiskers enlarged obviously, and the frameworks were observed to be more denser. When the heating temperature was 1400 °C, the mullite whiskers' aspect ratio increased significantly, and the diameter of them was approximately 0.1–0.3 μm (Fig. S1b†). However, for the MF/MW framework forming at 1500 °C (Fig. 4e), the aspect ratio of the mullite whiskers became decreased obviously and the whiskers' diameter was about 0.25–0.4 μm (Fig. S1c†). The decreased aspect ratio indicating coarsening process of the mullite whiskers. It had been reported that, at a higher heating temperature, the growth rate along every growth orientation tended to be the same, and this ultimately led to coarsening of the mullite whiskers.19 Moreover, in the frameworks, intersected mullite whiskers served as lap-jointing points of the mullite fibres, which was showed in Fig. 4f as example.
In the MF/MW frameworks, the mullite fibres served as curved substrates for the mullite whiskers' growth, and the fibres' surfaces that were full of small mullite grains acted as the seeding layers.12 The schematic diagram of MF/MW framework's forming procedure was showed in Fig. 5. Firstly, AlF3 and SiO2 as raw material transformed into gaseous AlOF and SiF4, and these two products deposited on the fibres' surfaces and reacted to form topaz rod-like grains. Meanwhile, a part of topaz in-situ formed at the pores of the framework. Then the topaz which grew on the fibres transformed into mullite whiskers, and also the in situ topaz grains transformed into in situ mullite whiskers at the pores of the framework. As the heating temperature rising and under the catalysis of HF, the in situ mullite whiskers decomposed into AlOF and SiF4 again [reaction (5)], and these two products subsequently deposited and reacted on the mullite whiskers which had grown on the fibres, according to the evaporation-condensation mechanism. Therefore, the hierarchical mullite fibres/whiskers structure was formed. At the lap-jointing points of the fibres, mullite whiskers intersected with each other to keep the whole framework stable.
| Al6Si2O13 + 14HF → 6AlOF4 + 7H2O | (5) |
3.3 Volume density, apparent porosity and thermal conductivity of the MF/MW frameworks
Fig. 6 showed the volume density, apparent porosity and thermal conductivity curves of the MF/MW frameworks which formed at 1100–1500 °C. The volume density and apparent porosity of the frameworks were 0.402–0.541 g cm−3 and 81.2–85.0%, respectively. As the heating temperature heightened, the volume density increased while the apparent porosity decreased in some extent. The samples had extremely low thermal conductivity of 0.1191–0.1647 W m−1 k−1, which was slightly increased as the heating-temperature rising. There were three main reasons for explaining the low thermal conductivity of the frameworks. First, mullite as the main composition offered high heat-resisting property and low thermal conductivity to the frameworks. Second, the hierarchical fibres/whiskers structure had long heat transfer path which was benefit for decreasing the thermal conductivity.20 And third, the high apparent porosity could also lead to low thermal conductivity and high heat-insulating performance. | ||
| Fig. 6 Volume density (triangle symbol), apparent porosity (circle symbol) and thermal conductivity (square symbol) curves of the MF/MW frameworks that formed at 1100–1500 °C. | ||
3.4 Cyclic compression–resilience performance of the MF/MW frameworks
The cyclic compression–resilience performance (the maximum compression stress was 0.4 MPa) of the samples tested at RT were showed in Fig. S2.† The details of the compression–resilience process of the MF/MW frameworks had been reported in our former work,12 and also they were generally described in the ESI† of this paper. For the samples' first circle compression–resilience curve, the compression ratio (total deformation ratio after compression), the permanent deformation ratio (after compression–resilience) and the resilience ratio of the frameworks were 2.031–3.833%, 0.125–0.042% and 93.85–98.28%, respectively, calculated according to Fig. S2,† and these results were showed in Fig. 7.According to Fig. 7, the compression ratio, permanent deformation ratio and resilience ratio of the 1100 °C-sample' first circle compression–resilience test were 2.031%, 0.125% and 93.85%, separately. The mullite whiskers had relatively small scale (Fig. 4a), which made the whiskers' deeper intersection and sliding more easily to be realized, and the temporary deformation forming in these two stages was smaller. Besides, the weaker whiskers were damaged in the compression process, and this resulted in higher permanent deformation ratio and lower resilience ratio. For the 1200 °C-sample, the compression ratio, permanent deformation ratio and resilience ratio of the sample's first circle compression–resilience test were 2.188%, 0.120% and 94.5%, respectively (Fig. 7). In the 1200 °C-sample, the mullite whiskers had larger scale (Fig. 4b). In the compression process, deeper intersection of the whiskers could result in a larger deformation, which directly led to increased compression ratio. Moreover, the lower permanent deformation ratio and higher resilience ratio of the 1200 °C-sample was on account of the increased strength of the intersected mullite whiskers. For the 1300 °C-sample, the compression ratio, permanent deformation ratio and resilience ratio of the sample's first circle compression–resilience test were 3.833%, 0.096% and 97.50%, respectively (Fig. 7). The mullite whiskers grew more larger in this sample, and this resulted in higher compression deformation which formed through the mullite whiskers' deeper intersection. Also, the decreased permanent deformation and increased resilience ratio were due to the heightened strength of the intersected mullite whiskers. For the 1400 °C-sample, the compression ratio, permanent deformation ratio and resilience ratio of the sample's first circle compression–resilience test were 3.250%, 0.076% and 97.82%, respectively (Fig. 7). The mullite whiskers in this sample became more larger and denser, and also larger resistance was produced between the whiskers. Therefore, the deeper intersection was relatively difficult to the whiskers, and this led to decreased compression ratio of the sample. Besides, the decreased permanent deformation ratio and increased resilience ratio also indicated the increased stiffness of the sample. For the 1500 °C-sample, the compression ratio, permanent deformation ratio and resilience ratio were 2.438%, 0.042% and 98.28%, respectively (Fig. 7). The decreased compression ratio, lower permanent deformation ratio and higher resilience ratio were both owing to the increased density and stiffness of the sample. In the samples, the permanent deformation was mainly due to the structure damage of the mullite whiskers, such as obscission or collapse which formed in the compression–resilience process. The example of the mullite whiskers' structure damage was showed in Fig. 8. After one circle compression–resilience test, the samples still had stable structure which could bear a new cycle compression–resilience test under the same conditions. For each sample, the consecutive compression–resilience curves were almost parallel with each other (Fig. S2†), indicating that the MF/MW frameworks could be cycle used under proper conditions (including proper maximum compression stress, proper loading rate, etc).
 | ||
| Fig. 8 SEM images, example of the structural damage of mullite whiskers after being employed in the compression–resilience test. | ||
4. Conclusions
In this work, mullite fibres/whiskers frameworks (MF/MW frameworks) were prepared through mixed-slurry-filtration and heat-treating method. The MF/MW frameworks had hierarchical fibres/whiskers structure which formed through fluorine-catalyzed gas-phase reaction. Topaz as transient phase firstly formed at 800 °C in the frameworks and then gradually transformed into mullite at 900–1500 °C. In the structure formation process, mullite fibres served as the substrates for the mullite whiskers' growth. In the frameworks, intersected mullite whiskers acted as unfixed lap-jointing points of the fibres. The mullite whiskers' growth situation and the frameworks' microstructure were directly affected by the heat-treating temperature. The MF/MW frameworks were lightweight and had high apparent porosity. Besides, the unique fibres/whiskers structure had long heat transfer path, and this was benefit for obtaining low thermal conductivity. Cyclic compression–resilience of the frameworks under maximum stress of 0.4 MPa was realized indicating that the frameworks could be cycle used under proper conditions. The MF/MW frameworks owning excellent thermal/mechanical properties were deemed to be promising candidates for heat-insulation/sealing.Acknowledgements
The authors would like to acknowledge the National Natural Science Foundation of China (Project no. 51172156 and Project No.51272171) for financial support.References
- J. J. Haslam and F. F. Lange, J. Am. Ceram. Soc., 2006, 89, 2043–2050 CrossRef CAS.
- Z. G. Hou, H. Y. Du, J. C. Liu, R. H. Hao, X. Dong and M. X. Liu, J. Eur. Ceram. Soc., 2013, 33, 717–725 CrossRef CAS PubMed.
- L. S. Cividanes, T. M. B. Campos, L. A. Rodrigues, D. D. Burnelli and G. P. Thim, J. Sol-Gel Sci. Technol., 2010, 55, 111–125 CrossRef CAS.
- K. Q. Li, T. D. Shimizu and K. Igarashi, J. Am. Ceram. Soc., 2001, 84, 497–503 CrossRef CAS.
- R. Torrecillas, J. M. Calderón, J. S. Moya, M. J. Reece, C. K. L. Davies, C. Olagnon and G. Fantozzi, J. Eur. Ceram. Soc., 1999, 19, 2519–2527 CrossRef CAS.
- M. G. Holmquist, T. C. Radsick, O. H. Sudre and F. F. Lange, Composites, Part A, 2003, 34, 163–170 CrossRef.
- C. Kaya, E. G. Butler, A. Selcuk, A. R. Boccaccini and M. H. Lewis, J. Eur. Ceram. Soc., 2002, 22, 2333–2342 CrossRef CAS.
- A. Demir, J. Eur. Ceram. Soc., 2012, 32, 1405–1411 CrossRef CAS PubMed.
- M. K. Naskar, K. Basu and M. Chatterjee, Ceram. Int., 2009, 35, 3073–3079 CrossRef CAS PubMed.
- A. Dey, M. Chatterjee, M. K. Naskar and K. Basu, Mater. Lett., 2003, 57, 2919–2926 CrossRef CAS.
- X. Dong, J. C. Liu, R. H. Hao, A. R. Guo, Z. G. Hou and M. X. Liu, J. Eur. Ceram. Soc., 2013, 33, 3477–3481 CrossRef CAS PubMed.
- S. Liu, J. C. Liu, H. Y. Du, F. Hou, S. E. Ren and H. T. Geng, Ceram. Int., 2013 DOI:10.1016/j.ceramint.2013.10.154.
- H. F. Hou and Y. Q. Chen, J. Sol-Gel Sci. Technol., 2007, 43, 53–57 CrossRef CAS PubMed.
- K. Okada and N. Otsuka, J. Mater. Sci. Lett., 1989, 8, 1052–1054 CrossRef CAS.
- K. Okada and N. Ōtuska, J. Am. Ceram. Soc., 1991, 74, 2414–2418 CrossRef CAS.
- H. J. Choi and J. G. Lee, J. Am. Ceram. Soc., 2002, 85, 481–483 CrossRef CAS.
- A. J. Pyzik and C. G. Li, Int. J. Appl. Ceram. Technol., 2005, 2, 440–451 CrossRef CAS.
- A. J. Pyzik, C. S. Todd and C. Han, J. Eur. Ceram. Soc., 2008, 28, 383–391 CrossRef CAS PubMed.
- W. H. Jiang, Y. F. Peng, J. M. Liu, G. Feng, X. Y. Tan and Y. Yu, J. Inorg. Mater., 2010, 25, 532–536 CrossRef CAS.
- Y. H. Dong, C. A. Wang, J. Zhou and Z. L. Hong, J. Eur. Ceram. Soc., 2012, 32, 2213–2218 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47394e |
| This journal is © The Royal Society of Chemistry 2014 |





