Colloidosomes from poly(N-vinyl-2-pyrrolidone)-coated poly(N-isopropylacrylamide-co-acrylic acid) microgels via UV crosslinking†
Abstract
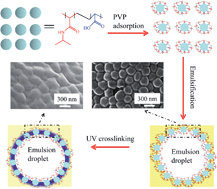
We first report the synthesis of colloidosomes via UV crosslinking of PVP-coated microgels. The photo-crosslinkable poly(N-vinyl-2-pyrrolidone) (PVP) was coated as a layer on the surface of poly(N-isopropylacrylamide-co-acrylic acid) (P(NIPAM-co-AAc)) microgels at pH = 3.5. A toluene-in-water Mickering emulsion was further fabricated based on the resulting PVP/P(NIPAM-co-AAc) microgels and then exposed to UV irradiation leading to the formation of colloidosomes. Since only the microgels on the surface of emulsion droplets could be crosslinked by UV irradiation, the yield and the mean size of the colloidosomes can be controlled by the amount of toluene used in the preparation. The proposed method presents a new platform to fabricate colloidosomes since photo-responsive comonomers or/and chemical crosslinkers are not essential in the system. The colloidosomes can be used as a vehicle for both hydrophilic and hydrophobic dyes. The release of the encapsulated dyes can be controlled by temperature.

 Please wait while we load your content...
Please wait while we load your content...