Sub-20 nm sandwich-structured NaGdF4:Yb/Tm@NaLuF4:Yb/Tm@NaYF4 nanocrystals for in vivo upconversion luminescence/computed tomography imaging†
Ji-Wei Shena,
Jianing Wangb,
Deling Kongb and
Xiu-Ping Yan*a
aState Key Laboratory of Medicinal Chemical Biology (Nankai University), Tianjin Collaborative Innovation Center of Chemical Science and Engineering, and Research Center for Analytical Sciences, College of Chemistry, Nankai University, 94 Weijin Road, Tianjin 300071, China. E-mail: xpyan@nankai.edu.cn; Fax: +86-22-23506075; Tel: +86-22-23504605
bState Key Laboratory of Medicinal Chemical Biology (Nankai University), College of Life Sciences, Nankai University, 94 Weijin Road, Tianjin 300071, China
First published on 10th December 2013
Abstract
Sub-20 nm sandwich-structured NaGdF4:Yb/Tm@NaLuF4:Yb/Tm@NaYF4 nanocrystals were fabricated for in vivo upconversion luminescence and computed tomography imaging.
Versatile rare-earth upconversion nanocrystals (UCNCs) have become a new generation of promising in vivo multimodal bioimaging agents.1 Upconversion luminescence (UCL)/computed tomography (CT) dual-modal imaging enables one to acquire more abundant and accurate imaging information than each modality alone by combining high sensitivity of UCL with high spatial resolution of CT.2 Small-sized UCNCs with intense near-infrared (NIR) UCL are favourable for in vivo small animal imaging because efficient clearance characteristics of smaller NCs from the body allow the possibility of using higher dosage of contrast agents and intense NIR UCL renders high penetration depth in bioimaging application.3
Yb/Tm (Er) co-doped NaGdF4 NCs (∼25 nm) have been used for multimodal imaging.4 NaGdF4 NCs could be tuned to ultra-small (∼5 nm) with excellent paramagnetic behaviour.5 However, ultra-small NaGdF4 UCNCs are not suitable for in vivo small animal UCL imaging because they are apt to suffer from serious surface quenching effects. Core/shell NaGdF4:Yb/Er@NaGdF4 structure or sandwich-structured NaGdF4:Er@NaGdF4:Ho@NaGdF4 enable to improve the UCL efficiency,6 but Gd3+ ions can still transfer energy from upconversion activator to surface quenchers.7 The UCL efficiency of UCNCs could thus not be fully developed by using NaGdF4 host matrix solely. Hexagonal (β) phase NaLuF4 is promising as efficient UCL host matrix and CT contrast agent,8 but direct synthesis of small-sized NaLuF4 based UCNCs with uniform shape is difficult due to its narrow β-phase transformation temperature range.9 As a result, few reports are available on the preparation of small-sized β-phase NaLuF4 based UCNCs.10 For the above reasons, facile methods for preparing small-sized UCNCs are highly desirable to fully take the advantages of NaLuF4 in UCL/CT and ultra-small characteristic of NaGdF4.
Here we show the preparation of sub-20 nm sandwich-structured NaGdF4:Yb/Tm@NaLuF4:Yb/Tm@NaYF4 (GdLuY) NCs for in vivo UCL/CT imaging. As shown in Scheme 1, ultra-small NaGdF4:Yb/Tm NCs are used as UCL center as well as core NCs to achieve epitaxial growth of NaLuF4:Yb/Tm NCs. Tm3+ ions, which are used as the upconversion activator for their transition from 3H4 to 3H6, can exhibit practically useful NIR UCL at 800 nm in the “optical transmission window” of biological tissues under continuous-wave excitation at 980 nm.1 The NaLuF4:Yb/Tm NCs not only provide the second UCL center for cooperative enhancement of NIR UCL, but also supply a potential functional CT contrast layer. Furthermore, the optically inert NaYF4 shell layer7 is firstly used to improve the UCL intensity of the NaLuF4:Yb/Tm NCs.
The oleic acid capped sandwich-structured GdLuY NCs were prepared by a modified solvothermal method in combination with seed-mediated synthesis protocol.5,11 Ultra-small NaGdF4:Yb/Tm NCs (∼5 nm) were obtained by controlling the crystal nucleation period (Fig. 1A).5a The ultra-small NaGdF4:Yb/Tm core and seed-mediated synthesis protocol allow the growth of a thin layer of NaLuF4:Yb/Tm (∼3.5 nm) and NaYF4 (∼2 nm) to ensure the generation of small-sized NaGdF4:Yb/Tm@NaLuF4:Yb/Tm (∼12 nm) and GdLuY (∼16 nm) NCs (Fig. 1B and C). The d-spacing values of 0.52, 0.52 and 0.30 nm for the NaGdF4:Yb/Tm, NaGdF4:Yb/Tm@NaLuF4:Yb/Tm and GdLuY revealed by high-resolution transmission electron microscopy (HRTEM) correspond to the (100), (100) and (110) planes of β-phase NaGdF4, NaLuF4 and NaYF4, respectively (Fig. 1A–C, insets). The β-phase crystal structure was further confirmed by fast Fourier transform (FFT) of the HRTEM image (Fig. 1D) and X-ray powder diffraction (XRD) pattern (Fig. 1E). The existence of lanthanide dopants in the GdLuY NCs was supported by energy-dispersive X-ray analysis (EDXA) (Fig. S1, ESI†), while the sandwich structure of the GdLuY NCs was ascertained by X-ray photoelectron spectroscopy (XPS) (Fig. S2, ESI†). The above results demonstrate the sandwich structure of the as-prepared GdLuY NCs.
The double UCL centers, NaGdF4:Yb/Tm and NaLuF4:Yb/Tm, along with the optically inert shell layer of NaYF4 resulted in cooperative UCL enhancement effects (Fig. 2A). The shell coating of NaLuF4:Yb/Tm gave a 3.1-fold enhancement of the UCL intensity at 800 nm for the NaGdF4:Yb/Tm NCs. A further shell layer of NaYF4 provided an impressive 36-fold enhancement of UCL intensity at 800 nm for NaGdF4:Yb/Tm@NaLuF4:Yb/Tm NCs. The shell layer of NaYF4 on the present double UCL centered NaGdF4:Yb/Tm@NaLuF4:Yb/Tm NCs gave much higher UCL enhancement than the shell layer of NaGdF4 on the single UCL centered NaGdF4:Yb/Tm NCs (ca. 10-fold).5a The above results demonstrate the significance of the optically inert shell layer for improving the UCL intensity of small-sized UCNCs via inhibiting energy transfer from upconversion activator to surface quenchers.
To further demonstrate the validity of the double UCL centers for cooperative UCL enhancement by the sandwich-structured design, NaGdF4@NaLuF4:Yb/Tm@NaYF4 NCs with single UCL center were also synthesized in parallel. The GdLuY NCs with double UCL centers gave a 5.5-fold UCL intensity of NaGdF4@NaLuF4:Yb/Tm@NaYF4 NCs with single UCL center at 800 nm, demonstrating a cooperative UCL enhancement effect of the double UCL centers in the present sandwich-structured design. NaYF4 is one of the most efficient UCL host matrix.12 The GdLuY NCs with smaller size (∼16 nm) even showed 1.5-fold UCL intensity of NaYF4:Yb/Tm NCs with larger size (∼25 nm) (Fig. S3, ESI†) at 800 nm. In addition, the as-prepared GdLuY NCs exhibited much higher absorbance than NaYF4:Yb/Tm NCs in the NIR region (∼980 nm) (Fig. 2B). The above results suggest that higher UCL efficiency of the as-prepared GdLuY NCs resulted from their stronger NIR absorption ability.
The hydrophobic oleic acid capped GdLuY NCs were transferred into aqueous phase with biomimetic DSPE-PEG 2000 for further biological evaluation and application. The DSPE-PEG 2000 functionalization was supported by Fourier transform infrared spectra (Fig. S4, ESI†) and TGA curves (Fig. S5, ESI†). The DSPE-PEG 2000 capped GdLuY (DPGLY) NCs not only showed excellent dispersion in water, but also intense NIR UCL and bright blue UCL (Fig. S6, ESI†).
The cytotoxicity of the DPGLY NCs was investigated to evaluate the potential for biomedical application. The cytotoxicity of DPGLY NCs against mouse embryonic fibroblast (NIH 3T3) cells was evaluated when the cells were exposed to 0–800 μg mL−1 DPGLY NCs for 24 h with a standard MTT assay. The NIH 3T3 cell lines had a cell viability of 102% after 24 h treatment with DPGLY NCs up to 800 μg mL−1 (Fig. 3; ESI†), demonstrating low cytotoxicity of the DPGLY NCs.
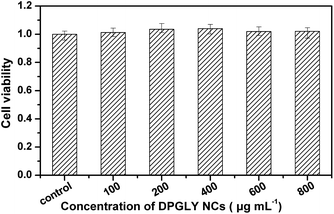 | ||
| Fig. 3 Cell viability in the presence of different concentrations of DPGLY NCs determined by MTT assay (n = 3). | ||
To demonstrate the capacity of the as-prepared DPGLY NCs for small animal UCL imaging in vivo, DPGLY NCs (100 μL, 3 mg mL−1) were intravenously administered to a normal nude mouse, followed by collecting UCL images (Fig. 4, ESI†). A strong UCL signal of the DPGLY NCs was observed in the mouse at 0.5 h post-injection. In vivo imaging showed high contrast between the UCL signal and the background, indicating the DPGLY NCs can serve as an ideal in vivo UCL bioimaging nanoprobe.
To show the potential of the DPGLY NCs, mainly composed of rare-earth elements with larger atomic number (Lu, Yb and Gd), for CT application, X-ray CT of a phantom was performed at various concentrations of the DPGLY NCs. The images of X-ray CT of a phantom become brighter and the Hounsfield unit values increased with the mass concentration of the DPGLY NCs from 0 to 30 mg mL−1 (Fig. 5A). The above results demonstrate the as-prepared GdLuY NCs can serve as a promising CT contrast agent.
The CT contrast effect of the X-ray CT of a phantom encouraged us to apply the GdLuY NCs for CT contrast experiments in vivo. Aqueous solution of the DPGLY NCs (500 μL, 30 mg mL−1) was administered to a Kunming mouse via tail vein injection under isoflurane anesthesia, followed by collecting CT images (Fig. 5B and C). Although the dosage of the DPGLY NCs was only 15 mg, the CT signal enhancement was significant in the liver and spleen of the mouse at 2 h post-injection. The positive contrast effect renders the DPGLY NCs potential as in vivo contrast agent for CT imaging.
Conclusions
In conclusion, we have reported the sub-20 nm (∼16 nm) sandwich-structured multifunctional nanoprobe for dual-modal UCL/CT bioimaging in vivo. The active shell layer of NaLuF4:Yb/Tm (∼3.5 nm) on the active and ultra-small NaGdF4:Yb/Tm (∼5 nm) NCs not only provide the second UCL center, but also serve as CT contrast agent. The merits of the double UCL centers of the as-prepared sandwich-structured multifunctional nanoprobe for improving the UCL efficiency have been fully uncovered by further coating of an optically inert NaYF4 shell layer. The as-prepared sandwich-structured multifunctional nanoprobe show promising for in vivo dual-modal UCL/CT imaging.Acknowledgements
This work was supported by the National Basic Research Program of China (Grant 2011CB707703), the National Natural Science Foundation of China (Grants 21275079, 20935001), the Fundamental Research Funds for the Central Universities.References
- J. Zhou, Z. Liu and F. Y. Li, Chem. Soc. Rev., 2012, 41, 1323 RSC.
- (a) D. E. Lee, H. Koo, I. C. Sun, J. H. Ryu, K. Kim and I. C. Kwon, Chem. Soc. Rev., 2012, 41, 2656 RSC; (b) H. Y. Xing, X. P. Zheng, Q. G. Ren, W. B. Bu, W. Q. Ge, Q. F. Xiao, S. J. Zhang, C. Y. Wei, H. Y. Qu, Z. Wang, Y. Q. Hua, L. P. Zhou, W. J. Peng, K. L. Zhao and J. L. Shi, Sci. Rep., 2013, 3, 1751 CAS.
- (a) Y. Zhang, J. D. Lin, V. Vijayaragavan, K. K. Bhakoo and T. T. Y. Tan, Chem. Commun., 2012, 48, 10322 RSC; (b) T. S. Yang, Y. Sun, Q. Liu, W. Feng, P. Y. Yang and F. Y. Li, Biomaterials, 2012, 33, 3733 CrossRef CAS PubMed; (c) G. Y. Chen, T. Y. Ohulchanskyy, R. Kumar, H. Ågren and P. N. Prasad, ACS Nano, 2010, 4, 3163 CrossRef CAS PubMed.
- (a) J. Zhou, Y. Sun, X. X. Du, L. Q. Xiong, H. Hu and F. Y. Li, Biomaterials, 2010, 31, 3287 CrossRef CAS PubMed; (b) C. Y. Liu, Z. Y. Gao, J. F. Zeng, Y. Hou, F. Fang, Y. L. Li, R. R. Qiao, L. Shen, H. Lei, W. S. Yang and M. Y. Gao, ACS Nano, 2013, 7, 7227 CrossRef CAS PubMed.
- (a) N. J. J. Johnson, W. Oakden, G. J. Stanisz, R. Scott Prosser and F. C. J. M. van Veggel, Chem. Mater., 2011, 23, 3714 CrossRef CAS; (b) Y. Hou, R. R. Qiao, F. Fang, X. X. Wang, C. Y. Dong, K. Liu, C. Y. Liu, Z. F. Liu, H. Lei, F. Wang and M. Y. Gao, ACS Nano, 2013, 7, 330 CrossRef CAS PubMed.
- (a) F. Wang, J. A. Wang and X. G. Liu, Angew. Chem., Int. Ed., 2010, 49, 7456 CrossRef CAS PubMed; (b) D. Q. Chen, L. Lei, A. P. Yang, Z. X. Wang and Y. S. Wang, Chem. Commun., 2012, 48, 5898 RSC; (c) F. Y. Liu, X. X. He, L. Liu, H. P. You, H. M. Zhang and Z. X. Wang, Biomaterials, 2013, 34, 5218 CrossRef CAS PubMed.
- Q. Q. Su, S. Y. Han, X. J. Xie, H. M. Zhu, H. Y. Chen, C. K. Chen, R. S. Liu, X. Y. Chen, F. Wang and X. G. Liu, J. Am. Chem. Soc., 2012, 134, 20849 CrossRef CAS PubMed.
- A. Xia, M. Chen, Y. Gao, D. M. Wu, W. Feng and F. Y. Li, Biomaterials, 2012, 33, 5394 CrossRef CAS PubMed.
- J. Wang, H. W. Song, W. Xu, B. Dong, S. Xu, B. T. Chen, W. Yu and S. Zhang, Nanoscale, 2013, 5, 3412 RSC.
- (a) Q. Liu, Y. Sun, T. S. Yang, W. Feng, C. G. Li and F. Y. Li, J. Am. Chem. Soc., 2011, 133, 17122 CrossRef CAS PubMed; (b) Y. Sun, J. J. Peng, W. Feng and F. Y. Li, Theranostics, 2013, 3, 346 CrossRef CAS PubMed; (c) J. J. Peng, Y. Sun, L. Z. Zhao, Y. Q. Wu, W. Feng, Y. H. Gao and F. Y. Li, Biomaterials, 2013, 34, 9535 CrossRef CAS PubMed.
- (a) Z. Q. Li, Y. Zhang and S. Jiang, Adv. Mater., 2008, 20, 4765 CrossRef CAS; (b) K. A. Abel, J. C. Boyer and F. C. J. M. van Veggel, J. Am. Chem. Soc., 2009, 131, 14644 CrossRef CAS PubMed; (c) F. Wang, R. R. Deng, J. Wang, Q. X. Wang, Y. Han, H. M. Zhu, X. Y. Chen and X. G. Liu, Nat. Mater., 2011, 10, 968 CrossRef CAS PubMed.
- H. Hu, L. Q. Xiong, J. Zhou, F. Y. Li, T. Y. Cao and C. H. Huang, Chem. – Eur. J., 2009, 15, 3577 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional figures and tables. See DOI: 10.1039/c3ra46885b |
| This journal is © The Royal Society of Chemistry 2014 |





