An efficient selenophene-containing conjugated copolymer for organic solar cells†
Jiamin Caoa,
Shan Chena,
Zhe Qib,
Zuo Xiaoa,
Jizheng Wang*b and
Liming Ding*a
aNational Center for Nanoscience and Technology, Beijing 100190, China. E-mail: opv.china@yahoo.com
bInstitute of Chemistry, Beijing 100190, China. E-mail: jizheng@iccas.ac.cn
First published on 13th December 2013
Abstract
A D–A polymer, PSeTPTI, was developed by copolymerizing a pentacyclic acceptor unit TPTI with a selenophene unit. PSeTPTI possesses a narrow optical bandgap, a low-lying HOMO energy level, and a high hole mobility of 0.26 cm2 V−1 s−1. PSeTPTI/PC71BM solar cells demonstrate a PCE of 6.04%, which is the highest efficiency for the conjugated copolymers using selenophene as the donor unit.
Polymer solar cells (PSCs) with great commercialization potential show unique advantages, such as solution processing, lightweight, and flexibility.1 To date, power conversion efficiencies (PCEs) of single and tandem PSCs have reached 9.2%2 and 10.6%,3 respectively. D–A conjugated copolymers have been widely used to obtain efficient solar cells. An outstanding D–A copolymer should possess: (1) low bandgap for harvesting more photons; (2) low-lying HOMO level for high open-circuit voltage (Voc); (3) high hole mobility for high photocurrent and fill factor (FF); (4) good solubility for facile solution processing and favourable nano-morphology. Polycyclic aromatic donor and acceptor building blocks function very well in PSCs since their extended conjugation can effectively reduce bandgap, enhance light absorbance, and improve charge carrier mobility.4 Recently, we developed a highly efficient D–A polymer PThTPTI using a pentacyclic aromatic lactam unit, thieno[2′,3′:5,6]pyrido[3,4-g]thieno[3,2-c]isoquinoline-5,11(4H,10H)-dione (TPTI), as the acceptor unit. PThTPTI/PC71BM solar cells afforded a PCE of 7.8%.5 PThTPTI is among the best donor materials. However, PThTPTI exhibited relatively narrow absorption spectra with a moderate optical bandgap of 1.86 eV, which limited the absorption in long-wavelength region. One approach to broaden absorption spectra and reduce bandgap is to replace thiophene unit with selenophene unit. Compared with thiophene, the reduced aromaticity of selenophene increases the ground state quinoid resonance of the polymer, and selenophene-containing polymers possess enhanced planarity, extended conjugation length, and reduced bandgaps.6 Besides, selenium is more polarizable than sulfur, Se⋯Se interactions favor interchain charge hopping and enhance hole mobility.7 Selenophene was used in D–A copolymers as a donor unit,8 a π-bridge,9 or part of the donor unit or acceptor unit.10 In this work, we synthesized PSeTPTI based on TPTI acceptor unit and selenophene donor unit (Scheme 1). PSeTPTI exhibited good thermal stability, broad absorption, low optical bandgap, and high hole mobility of 0.26 cm2 V−1 s−1. PSeTPTI/PC71BM solar cells afforded a PCE of 6.04%. To the best of our knowledge, this is the highest efficiency for D–A copolymers using selenophene as the donor unit (Table 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) solar cells under AM 1.5G illumination (100 mW cm−2)
1, w/w) solar cells under AM 1.5G illumination (100 mW cm−2)
| DIO [vol%] | Voc [V] | Jsc [mA cm−2] | FF [%] | PCE [%] |
|---|---|---|---|---|
| 3 | 0.85 | 11.10 | 64 | 6.04 |
| Without | 0.89 | 1.95 | 64 | 1.12 |
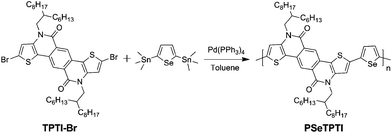
The synthesis of compound TPTI-Br is described in the literature.5 Target polymer PSeTPTI was synthesized through Stille reaction of TPTI-Br and 2,5-bis(trimethylstannyl)selenophene in toluene at 115 °C. The crude product was purified by Soxhlet extraction using methanol, hexane, and chloroform in sequence. The chloroform fraction was added into methanol to obtain PSeTPTI as dark blue solid in 97% yield. The chemical structure of PSeTPTI was confirmed by 1H NMR and elemental analysis. PSeTPTI is highly soluble in common organic solvents, such as toluene, chloroform, chlorobenzene, and o-dichlorobenzene. From gel permeation chromatography (GPC) against polystyrene standards in THF eluent, the number-average molecular weight (Mn) and the polydispersity index (PDI) of the polymer are 34.9 kDa and 3.51, respectively. PSeTPTI shows good thermal stability with a decomposition temperature Td of 417 °C (5% wt loss) (Fig. S1†).
As shown in Fig. 1, PSeTPTI exhibits two absorption peaks at 548 and 602 nm in chloroform solution. The film absorption peak at 638 nm originates from interchain π–π stacking. The absorption onset for PSeTPTI film is 701 nm, corresponding to an optical bandgap of 1.77 eV, which is narrower than that of its analogue PThTPTI (1.86 eV).6,9b The electrochemical property of PSeTPTI film was studied by cyclic voltammetry (CV) and all potentials were calibrated against Fc/Fc+ redox couple. As shown in Fig. S2†, the oxidation onset potential (Eonox) and the reduction onset potential (Eonred) of PSeTPTI are 0.61 and −1.92 V, respectively. According to the empirical formulae: HOMO = −(Eonox + 4.8) eV, LUMO = −(Eonred + 4.8) eV,11 the HOMO and LUMO energy levels of the polymer are calculated to be −5.41 and −2.88 eV, respectively. Compared with PThTPTI, PSeTPTI possesses similar HOMO energy level and lower LUMO energy level.10b Voc is proportional to the difference between the HOMO energy level of donor materials and the LUMO energy level of acceptor materials.12 Deep HOMO energy level should render PSeTPTI high Voc and good stability against oxidization.9b The electrochemical bandgap of PSeTPTI is 2.53 eV, 0.76 eV higher than the optical bandgap, which results from the interface barrier between polymer films and electrode surface.13
Solar cells with a structure of ITO/PEDOT:PSS/PSeTPTI:PC71BM/Ca/Al were fabricated to evaluate the photovoltaic performance of PSeTPTI. After optimization, solar cells with a D–A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w), an active layer thickness of 140 nm, and 3 vol% 1,8-diiodooctane (DIO) gave the best result (see ESI†). The best device afforded a PCE of 6.04%, with a Voc of 0.85 V, a Jsc of 11.10 mA cm−2, and a FF of 64%. DIO addition greatly increases photocurrent by reducing domain size and increasing D–A interfaces in the active layer.14 External quantum efficiency (EQE) spectra indicate that the device with 3 vol% DIO exhibits much higher EQE values than the device without DIO. The integrated currents from EQE curves for the devices without and with DIO are 1.87 and 10.61 mA cm−2, respectively, which consist with the Jsc values from J–V measurements (Fig. 2).
1 (w/w), an active layer thickness of 140 nm, and 3 vol% 1,8-diiodooctane (DIO) gave the best result (see ESI†). The best device afforded a PCE of 6.04%, with a Voc of 0.85 V, a Jsc of 11.10 mA cm−2, and a FF of 64%. DIO addition greatly increases photocurrent by reducing domain size and increasing D–A interfaces in the active layer.14 External quantum efficiency (EQE) spectra indicate that the device with 3 vol% DIO exhibits much higher EQE values than the device without DIO. The integrated currents from EQE curves for the devices without and with DIO are 1.87 and 10.61 mA cm−2, respectively, which consist with the Jsc values from J–V measurements (Fig. 2).
Bottom-gate and bottom-contact organic field-effect transistors (OFETs) were fabricated to explore the charge-transporting property of PSeTPTI film. As shown in Fig. 3, the devices show p-type behavior with typical transfer and output curves. PSeTPTI-based OFET exhibited a high hole mobility of 0.26 cm2 V−1 s−1 with a large on/off current ratio of 1.89 × 108 and a low threshold voltage of −6.51 V. In the annealing temperature range of 20–160 °C, the polymer showed similar mobility (Fig. S3†). The high mobility of PSeTPTI accounts for its good Jsc and FF in solar cells. X-ray diffraction (XRD) pattern of PSeTPTI films shows two diffraction peaks at 2θ = 4.46° and 25.12°, respectively (Fig. S4†). The primary peak at 4.46° corresponds to an interlayer d-spacing of 19.80 Å, and the peak at 25.12° corresponds to a d-spacing of 3.54 Å for π–π stacking. The lamellar structures existing in PSeTPTI film lead to its high mobility.
 | ||
| Fig. 3 (a) Transfer and (b) output curves for PSeTPTI-based OFETs. Device dimension: channel length (L) = 50 μm; channel width (W) = 1400 μm. | ||
In summary, a D–A conjugated polymer, PSeTPTI, based on a pentacyclic acceptor unit, TPTI, and selenophene donor unit, was developed. The optical bandgap of PThTPTI was decreased successfully by replacing thiophene unit with selenophene unit. PSeTPTI possesses good solubility, excellent thermal stability, narrow optical bandgap, low-lying HOMO energy level, and high mobility. PSeTPTI/PC71BM solar cells demonstrate a PCE of 6.04%, which is the highest efficiency for the D–A copolymers using selenophene as the donor unit.
Acknowledgements
This work was supported by the “100 Talents Program” of Chinese Academy of Sciences.Notes and references
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 593 CrossRef CAS.
- J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C.-C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef PubMed.
- (a) J.-S. Wu, Y.-J. Cheng, M. Dubosc, C.-H. Hsieh, C.-Y. Chang and C.-S. Hsu, Chem. Commun., 2010, 46, 3259 RSC; (b) R. S. Ashraf, B. C. Schroeder, H. A. Bronstein, Z. Huang, S. Thomas, R. J. Kline, C. J. Brabec, P. Rannou, T. D. Anthopoulos, J. R. Durrant and I. McCulloch, Adv. Mater., 2013, 25, 2029 CrossRef CAS PubMed.
- J. Cao, Q. Liao, X. Du, J. Chen, Z. Xiao, Q. Zuo and L. Ding, Energy Environ. Sci., 2013, 6, 3224 CAS.
- A. Patra and M. Bendikov, J. Mater. Chem., 2010, 20, 422 RSC.
- I. Kang, T. K. An, J. Hong, H.-J. Yun, R. Kim, D. S. Chung, C. E. Park, Y.-H. Kim and S.-K. Kwon, Adv. Mater., 2013, 25, 524 CrossRef CAS PubMed.
- (a) D. H. Wang, A. Pron, M. Leclerc and A. J. Heeger, Adv. Funct. Mater., 2013, 23, 1297 CrossRef CAS; (b) W.-H. Lee, S. K. Son, K. Kim, S. K. Lee, W. S. Shin, S.-J. Moon and I.-N. Kang, Macromolecules, 2012, 45, 1303 CrossRef CAS.
- (a) L. Dou, W.-H. Chang, J. Gao, C.-C. Chen, J. You and Y. Yang, Adv. Mater., 2013, 25, 825 CrossRef CAS PubMed; (b) B. Kim, H. R. Yeom, M. H. Yun, J. Y. Kim and C. Yang, Macromolecules, 2012, 45, 8658 CrossRef CAS; (c) J. Huang, Y. Zhao, W. He, H. Jia, Z. Lu, B. Jiang, C. Zhan, Q. Pei, Y. Liu and J. Yao, Polym. Chem., 2012, 3, 2832 RSC; (d) M. Shahid, R. S. Ashraf, Z. Huang, A. J. Kronemeijer, T. McCarthy-Ward, I. McCulloch, J. R. Durrant, H. Sirringhaus and M. Heeney, J. Mater. Chem., 2012, 22, 12817 RSC.
- (a) H. A. Saadeh, L. Lu, F. He, J. E. Bullock, W. Wang, B. Carsten and L. Yu, ACS Macro Lett., 2012, 1, 361 CrossRef CAS; (b) J. J. Intemann, K. Yao, H.-L. Yip, Y.-X. Xu, Y.-X. Li, P.-W. Liang, F.-Z. Ding, X. Li and A. K.-Y. Jen, Chem. Mater., 2013, 25, 3188 CrossRef CAS.
- (a) Y. Liang, Y. Wu, D. Feng, S.-T. Tsai, H.-J. Son, G. Li and L. Yu, J. Am. Chem. Soc., 2009, 131, 56 CrossRef CAS PubMed; (b) Z. Xiao, G. Ye, Y. Liu, S. Chen, Q. Peng, Q. Zuo and L. Ding, Angew. Chem., Int. Ed., 2012, 51, 9038 CrossRef CAS PubMed.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789 CrossRef CAS.
- (a) D. A. M. Egbe, L. H. Nguyen, H. Hoppe, D. Mühlbacher and N. S. Sariciftci, Macromol. Rapid Commun., 2005, 26, 1389 CrossRef CAS; (b) J. Cao, W. Zhang, Z. Xiao, L. Liao, W. Zhu, Q. Zuo and L. Ding, Macromolecules, 2012, 45, 1710 CrossRef CAS.
- S. H. Park, A. Roy, S. Beaupré, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Nat. Photonics, 2009, 3, 297 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details including synthesis, measurements, and instruments. See DOI: 10.1039/c3ra47098a |
| This journal is © The Royal Society of Chemistry 2014 |