Synthesis of a DWNTs/PAni composite and its supercapacitive behavior compared to the SWNTs/PAni and MWNTs/PAni composites
Lixiang Liab,
Guangyao Lia and
Baigang An*ab
aSchool of Chemical Engineering, University of Science and Technology Liaoning, 185 Qianshangzhong Road, Anshan 114051, China. E-mail: bgan@ustl.edu.cn; Fax: +86 0412 5929627; Tel: +86 0412 5929171
bInstitute of Materials Electrochemistry Research, University of Science and Technology Liaoning, 185 Qianshangzhong Road, Anshan 114051, China
First published on 10th December 2013
Abstract
Double walled carbon nanotubes (DWNTs) have unique coaxial structures and corresponding physical and chemical properties, making them good candidates for supercapacitor materials. However, there are almost no reports on DWNT based composites for supercapacitor applications. In this study, a DWNTs/PAni composite was synthesized by in situ chemical polymerization of aniline onto DWNTs, and its electrochemical performance as the electrode material in a supercapacitor was studied and compared to single-walled carbon nanotubes (SWNTs)/PAni and multi-walled carbon nanotubes (MWNTs)/PAni composites synthesized by the same method. TEM and SEM observations show that the PAni is uniformly coated on the DWNTs to form DWNTs/PAni bundles with diameters of about 100 nm. Raman characterization further indicates the strong interactions between the DWNT and PAni components in the DWNTs/PAni composite. Electrochemical analyses demonstrate that the DWNTs/PAni composite has not only significantly higher specific capacitance (576 F g−1) but also better cycling stability than the SWNTs/PAni and MWNTs/PAni composites. The excellent electrochemical performance of the DWNTs/PAni composite as the electrode material in a supercapacitor is attributed to the good charge transfer ability between the PAni and DWNT components, the comparatively large specific surface area of the DWNTs/PAni composite and the good stress and load transfer properties of DWNT due to its coaxial structure.
1. Introduction
Supercapacitors, also called electrochemical capacitors, are electrical devices for energy storage, filling the gap between batteries and conventional capacitors due to their high specific power and good specific capacity. The capacitive behavior of supercapacitors can be classified into two categories based on the energy storage mechanism: (1) electric double-layer capacitance (EDLC) that arises from pure electrostatic attraction between the ions and the charged surface of the electrode, such as the capacitance contributed by porous carbon materials,1 and (2) pseudocapacitance that derives from a Faradic reaction of the electrolyte ions with the electro-active materials of the electrode, such as the capacitance supplied by electronically conducting polymers (ECPs)2 or transition metal oxides.3 Recently, more attention has been paid to supercapacitors combining EDLC and pseudocapacitance to optimize electrochemical performance, such as supercapacitors based on heteroatom doped porous carbons4,5 and composites of ECPs or transition metal oxides with carbon.6–10Carbon nanotubes (CNTs) have hollow fiber-like structures, good conductivity, moderate specific surface area (SSA), and ultrahigh mechanical strength, hence they are considered good candidate materials for supercapacitors based on EDLC. However, pure CNTs generally supply a specific capacity of several tens of F g−1 due to their undeveloped pore texture. On the other hand, ECPs can introduce a large pseudocapacitance of up to several hundreds of F g−1 through a Faradic reaction based on the state exchange of p-doped and n-doped ECPs interacting with the electrolyte ions. Unfortunately, the volumetric shrinkage and swelling of ECPs during the charge–discharge cyclic process causes breaking and cracking of the electrode, which results in a poor cycling stability and impairs the supercapacitive performance of the ECP electrode.
Interestingly, composites of CNTs with ECPs exhibit much larger specific capacitance than ECPs, and show improved cycling stability due to a synergistic effect between the composite components. This synergy has been well demonstrated in many reports by using composites of multi-walled CNTs (MWNTs) or single-walled CNTs (SWNTs) with polyaniline (PAni) or polypyrroles (PPy) as the electrode materials in supercapacitors.11–15 The enhanced supercapacitive performance of the composites can be attributed to the relatively high SSA, unique mesoporous structure, excellent mechanical strength and good electronic properties of the CNT component, which correspondingly enlarges the active surface of the ECPs, improves ion transport between the electrode and electrolytes, alleviates stress caused by the volume change of the ECPs during the charge–discharge process, and enhances the charge transport ability of the electrode. Therefore, the structure and texture of CNTs play a key role in the supercapacitive performance of the composites. However, most studies on CNT/ECP composites for supercapacitors have been focused on either MWNT or SWNT based composites. To the best of our knowledge, there are almost no reports on composites of double-walled CNTs (DWNTs) with ECPs and their supercapacitive behavior, although DWNTs are important members of the CNT family, which have better mechanical, thermal, electronic and structural stability properties than those of SWNTs or MWNTs, owing to their coaxial structure,16–18 and large scale syntheses of DWNTs have been achieved.19,20
Herein, we report the synthesis of a DWNTs/PAni composite and its electrochemical performance as the electrode material in a supercapacitor. The results show that the DWNTs/PAni composite has better conductivity, stronger charge transfer ability between the DWNT and PAni components, and higher SSA than the SWNTs/PAni and MWNTs/PAni composites, hence as a supercapacitor electrode material, the DWNTs/PAni composite exhibits significantly higher specific capacitance than the SWNTs/PAni and MWNTs/PAni composites. Meanwhile, the DWNTs/PAni composite also exhibits excellent cycling stability due to the fact that the unique coaxial structure of DWNTs can more efficiently alleviate stress due to the volume change of PAni during the charge–discharge process.
2. Experimental
DWNTs and SWNTs were synthesized by a hydrogen arc discharge process.20,21 MWNTs were synthesized by a floating catalyst method.22 Before the syntheses of the CNTs/PAni composites, the CNTs were purified using the method reported by Hou et al.23 All the CNTs/PAni composites were synthesized by an in situ chemical polymerization method. The purified CNTs were dispersed in 200 mL of 1.5 M hydrochloric acid solution containing 3 mL of aniline monomer by ultra-sonication. 1.8 g of ammonium persulphate oxidant was added dropwise into the mixed suspension at 0–5 °C for 4 h with magnetic stirring. The composites obtained were filtered and rinsed repeatedly with distilled water and then ethanol until colorless, followed by drying under vacuum at 85 °C for 24 h.The morphology and structure of the samples were characterized by scanning electron microscopy (SEM, JSM 6301F), high-resolution transmission electron microscopy (HRTEM, JOEL 2010, 200 kV), micro-Raman spectroscopy (Jobin Yvon HR800 with an excitation laser energy of 1.96 eV) and Fourier transform infrared spectroscopy (Nicolet 5700 spectrometer with a resolution of 2 cm−1). N2 adsorption–desorption isotherms of the samples were measured at 77 K using a volumetric adsorption analyzer (Micromeritics, ASAP2010) and electron microscopy (TEM, JEOL JEM-1400). The SSA of the samples was determined according to the Brunauer–Emmett–Teller (BET) method. The pore size distribution (PSD) plot was recorded from the adsorption branch of the isotherm based on the Barrett–Joyner–Halenda (BJH) model.
For the preparation of supercapacitor electrodes, the CNTs/PAni composite was mixed with an acetylene black conducting agent and a PTFE binder in the ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and was then spread onto nickel foam, followed by pressing at 0.34 GPa. Electrochemical analyses of the samples were performed with a Solartron 1286/1287 electrochemical system using a three electrode system, in which a saturated calomel electrode (SCE) and a platinum plate were used as the reference electrode and counter electrode, respectively. The capacity and cycling ability of the supercapacitor were measured using a unit cell of two electrodes. The unit cell was charged at a voltage of 1.0 V and discharged at a constant current density of 100 mA g−1 using an Arbin BT-2000 battery test system. The electrolyte used for the electrochemical tests was 1 M H2SO4.
5 and was then spread onto nickel foam, followed by pressing at 0.34 GPa. Electrochemical analyses of the samples were performed with a Solartron 1286/1287 electrochemical system using a three electrode system, in which a saturated calomel electrode (SCE) and a platinum plate were used as the reference electrode and counter electrode, respectively. The capacity and cycling ability of the supercapacitor were measured using a unit cell of two electrodes. The unit cell was charged at a voltage of 1.0 V and discharged at a constant current density of 100 mA g−1 using an Arbin BT-2000 battery test system. The electrolyte used for the electrochemical tests was 1 M H2SO4.
3. Results and discussion
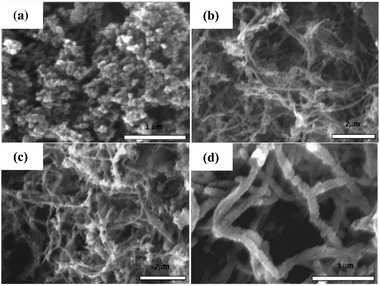
Fig. 1 shows the TEM images of DWNTs and the DWNTs/PAni composite. The DWNTs have a diameter of about 2–3 nm. It is observed that in situ polymerization of aniline results in PAni coated on the DWNTs to form a DWNTs/PAni composite with a diameter of about 20 nm. The SEM images of pure PAni and the CNTs/PAni composites are shown in Fig. 2. Pure PAni has a morphology consisting of accumulated particles, but the composites display a fiber-like morphology, indicating that the aniline monomers are uniformly polymerized on the surface of the CNTs. It can be seen from Fig. 2 that the DWNTs/PAni composite appears to be a fiber with a diameter of about 100 nm, since van der Waals' forces cause the DWNTs/PAni composite to form big bundles. The morphology of SWNTs/PAni is similar to that of DWNTs/PAni, which also consists of composite fibers with a diameter of about 100 nm. However, the MWNTs/PAni composite has a much larger diameter (140–200 nm).Raman spectroscopy is an effective technique to characterize the structures of CNTs and their composites. Fig. 3 shows the Raman spectra of DWNTs, pure PAni and the DWNTs/PAni composite. For the DWNTs, the D-band (1320 cm−1) is assigned to the presence of structural defects or disorder in the carbon systems, and the G-band (1588 cm−1) is attributed to the stretching vibration of any pair of sp2 sites inside the graphitic pattern. The DWNTs exhibit a much sharper and stronger G-band (1588 cm−1) than their D-band (1320 cm−1), indicating their highly graphitized structure. Pure PAni shows the following main bands: (1) C–H bending of the quinoid rings at 1161 cm−1, (2) C–N˙+ stretching at 1334 cm−1, (3) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration at 1462 cm−1, and (4) C
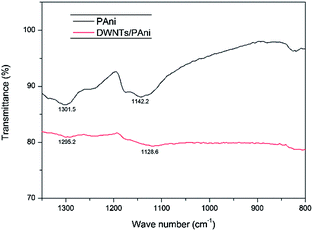
N stretching vibration at 1462 cm−1, and (4) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the quinoid rings at 1595 cm−1.24 DWNTs/PAni displays a Raman spectrum composed of two constituents, further confirming that the PAni has been successfully coated on the DWNTs by in situ chemical polymerization. It is worth noting that the G-band of the DWNTs shifts significantly from 1588 cm−1 to 1573 cm−1, which is indicative of strong π–π interactions between the more planar PAni conformation and the hexagonal surface lattice of the CNTs. Furthermore, the FTIR spectra of PAni and the DWNTs/PAni composite (Fig. 4) show that the bands around 1301 cm−1 (C–N stretching vibration) and 1142 cm−1 (C–H in-plane bending vibration) of PAni shift to 1295 cm−1 and 1128 cm−1 respectively for the DWNTs/PAni composite, which again indicates the interaction of PAni with the DWNTs. Such strong interactions favor a charge transfer process between the two components,25,26 which can combine with the highly graphitized DWNTs to enhance the electronic conductivity of DWNTs/PAni as an electrode material.
C stretching of the quinoid rings at 1595 cm−1.24 DWNTs/PAni displays a Raman spectrum composed of two constituents, further confirming that the PAni has been successfully coated on the DWNTs by in situ chemical polymerization. It is worth noting that the G-band of the DWNTs shifts significantly from 1588 cm−1 to 1573 cm−1, which is indicative of strong π–π interactions between the more planar PAni conformation and the hexagonal surface lattice of the CNTs. Furthermore, the FTIR spectra of PAni and the DWNTs/PAni composite (Fig. 4) show that the bands around 1301 cm−1 (C–N stretching vibration) and 1142 cm−1 (C–H in-plane bending vibration) of PAni shift to 1295 cm−1 and 1128 cm−1 respectively for the DWNTs/PAni composite, which again indicates the interaction of PAni with the DWNTs. Such strong interactions favor a charge transfer process between the two components,25,26 which can combine with the highly graphitized DWNTs to enhance the electronic conductivity of DWNTs/PAni as an electrode material.
N2 adsorption–desorption isotherms and pore size distributions (PSD) of DWNTs, pure PAni and the DWNTs/PAni composite are shown in Fig. 5. The isotherm of the DWNTs is type IV, which implies that the pore structures are dominated by mesopores. However, the DWNTs/PAni composite has a much lower adsorption volume than that of the DWNTs due to a large reduction in the number of micropores and mesopores in the composite caused by the process of in situ polymerization of aniline onto the DWNTs. The PSD curves show that the DWNTs/PAni composite still retains the mesoporous features of the DWNTs, but its pore volume becomes much lower. For the sake of comparison, the PSDs of SWNTs/PAni and MWNTs/PAni composites were also analyzed, and the results together with the PSD of the DWNTs/PAni composite are shown in Fig. 5b. The PSD of the SWNTs/PAni composite is very similar to that of DWNTs/PAni, but the DWNTs/PAni composite has a few more small mesopores (2–5 nm) suitable for ion storage and migration than the SWNTs/PAni composite. The MWNTs/PAni composite contains no small mesopores and has a much lower pore volume than DWNTs/PAni and SWNTs/PAni. The BET SSAs of the CNTs before and after PAni coating are listed in Table 1. The SSAs of the CNTs/PAni composites are much lower than those of the CNTs before PAni coating. The DWNTs/PAni composite has the highest SSA among the composites.
 | ||
| Fig. 5 (a) N2 adsorption–desorption isotherms of DWNTs, PAni and DWNTs/PAni; (b) pore size distributions of DWNTs/PAni, SWNTs/PAni and MWNTs/PAni. | ||
| Item | DWNTs | SWNTs | MWNTs |
|---|---|---|---|
| BET SSA of CNTs (m2 g−1) | 177 | 113 | 40 |
| BET SSA of CNTs/PAni (m2 g−1) | 75 | 65 | 30 |
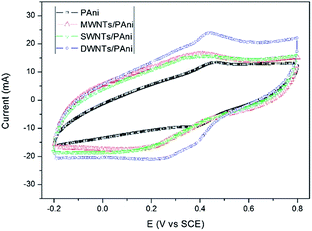
Cyclic voltammetry (CV) is a useful technique to analyze the electrochemical behavior of supercapacitor electrode materials. For comparison, the CV of the DWNTs/PAni composite together with the CVs of SWNTs/PAni, MWNTs/PAni and pure PAni are shown in Fig. 6. The CVs of the CNTs/PAni composites present an improved quasi-rectangular shape compared to that of pure PAni, and a couple of peaks arising from oxidation and reduction of PAni can also be seen for pure PAni and the CNTs/PAni composites, suggesting that the CNTs/PAni composites supply both EDLC and pseudocapacitance. It is notable that the DWNTs/PAni composite presents the largest response current among all the samples, indicating its highest specific capacitance. Furthermore, it is noteworthy that the peak potentials of both the SWNTs/PAni and MWNTs/PAni composites are significantly shifted in comparison to that of pure PAni. However, the redox potential of the DWNTs/PAni composite is almost the same as that of pure PAni despite DWNTs/PAni having the highest peak current among all the samples, which is indicative of good charge transfer ability between the DWNTs and PAni due to the strong interactions between these two components. Such interactions are further confirmed by the CVs of DWNTs/PAni (shown in Fig. 7) measured at different scan rates. The redox potential and CV shapes of DWNTs/PAni remain unchanged at different scan rates. This good synergistic effect of charge transfer between the two components can reduce energy loss during charge transfer and storage, and thus improve the capacitive performance of the DWNTs/PAni composite as the electrode material in supercapacitors.
Electrochemical impedance spectroscopy (EIS) has been widely used to evaluate electrode conductivity. The EIS of CNTs/PAni and PAni electrodes are shown in Fig. 8. The intercept of the high-frequency semicircle with the real axis in the EIS reflects the internal resistance of the electrode, hence indicating the conductivity of the electrode materials. According to the EIS, the internal resistance is about 0.13, 0.55, 0.62 and 2.1 Ohm for the DWNTs/PAni, MWNTs/PAni, SWNTs/PAni and pure PAni electrodes respectively, which demonstrates that all the composites have much better conductivity than pure PAni. Especially, it can be noted that DWNTs/PAni has the best conductivity among the composites, which is most probably ascribed to the better charge transfer ability between the DWNTs and PAni resulting from their strong interaction as mentioned above. On the other hand, it has been revealed that efficient charge transfer occurs from the outer to the inner tubes of DWNTs18 and that DWNTs exhibit metallic behavior due to the intershell interactions,27 which may also be important factors in the good conductivity of DWNTs/PAni.
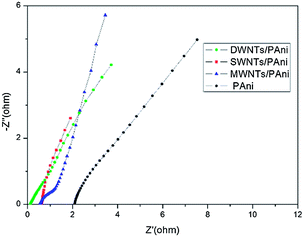 | ||
| Fig. 8 Electrochemical impedance spectra of pure PAni and the composites of PAni with DWNTs, SWNTs and MWNTs. | ||
The charge–discharge curves of the samples measured using a two-electrode system at the discharge current of 100 mA g−1, as shown in Fig. 9, were used to calculate the specific capacitance of the samples. The results show that the specific capacitance is 576, 390, 344 and 226 F g−1 for the composites of DWNTs/PAni, SWNTs/PAni, MWNTs/PAni and pure PAni, respectively. The capacitances of the composites are much higher than those of the corresponding CNTs (55, 18 and 36 F g−1 for DWNTs, SWNTs and MWNTs respectively). Note that the DWNTs/PAni composite exhibits a much higher capacitance than the other composites and pure PAni. It has been widely accepted that the enhanced capacitance of CNTs/PAni composites is ascribed to the enlarged interaction interface between the electrolyte ions and the surface of the composite and the improved conductivity of the composite by CNTs. Compared to the other CNTs/PAni composites, DWNTs/PAni has not only a larger SSA for ion access but also a better conductivity for charge transfer, as mentioned above. In addition, the good charge transfer ability between the DWNT and PAni components of DWNTs/PAni can more efficiently utilize the pseudocapacitance introduced by PAni. All of these contribute to the stronger charge storage ability of the DWNTs/PAni composite. The energy and power densities of cells based on the different materials were also calculated and are listed in Table 2. It can be seen that the energy density of DWNTs/PAni is the highest among all the composites; in particular the power density of DWNTs/PAni is almost five times those of the other composites. The large energy and power density of DWNTs/PAni could be attributed to its high specific capacitance combined with its low internal resistance.
| Item | DWNTs/PAni | SWNTs/PAni | MWNTs/PAni |
|---|---|---|---|
| Energy density (kW h kg−1) | 30.2 | 18.6 | 10.8 |
| Power density (kW kg−1) | 27 | 5.6 | 5.0 |
Moreover, as the electrode material in a supercapacitor, DWNTs/PAni has an excellent cycling stability. As shown in Fig. 10, after 1000 cycles the capacity retention of the DWNTs/PAni electrode is 87%, which is significantly higher than those of SWNTs/PAni (81%), MWNTs/PAni (71%) and pure PAni (43%). It is known that CNTs can act as the backbone of PAni to alleviate the stress resulting from the shrinkage and swelling of PAni during the charge–discharge process and thus improve the cyclability of CNTs/PAni composites as supercapacitor electrode materials.6,7 Compared to SWNTs, DWNTs can efficiently transfer stress or load through interactions of the coaxial inner and outer tubes.28 MWNTs generally contain many defects that aggravate their mechanical performance, and the stress transfer efficiency decreases correspondingly with the increasing number of MWCNT layers.29 Therefore, from a structural point of view, DWNTs have a stronger ability to alleviate stress derived from the volume change of PAni than SWNTs and MWNTs, which combines with the strong interactions between the PAni layer and the DWNTs and the good conductivity of DWNTs/PAni electrodes, making the DWNTs/PAni composite exhibit much better cyclability than the SWNTs/PAni and MWNTs/PAni composites.
4. Conclusions
By in situ chemical polymerization of aniline onto DWNTs, a DWNTs/PAni composite with the PAni uniformly coated on the DWNTs has been successfully synthesized. Raman analysis indicates the strong interactions between the PAni and DWNT components in the composite. Compared to the SWNTs/PAni and the MWNTs/PAni composites synthesized by the same method, the DWNTs/PAni composite has better conductivity, stronger charge transfer ability between the PAni and DWNTs, and relatively high specific surface area, hence as the electrode material in a supercapacitor the DWNTs/PAni composite supplies a specific capacitance as high as 576 F g−1, which is significantly larger than the capacitance of the SWNTs/PAni composite (390 F g−1) and the MWNTs/PAni composite (344 F g−1). Moreover, due to the coaxial structure of DWNTs that can more efficiently transfer the stress and load due to the volume changes in PAni during the charge–discharge process, the DWNTs/PAni composite exhibits better cycling stability than the SWNTs/PAni and MWNTs/PAni composites; after 1000 charge–discharge cycles the capacity retention of the DWNTs/PAni electrode is 87%.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (51102126), the Foundation of the Ministry of Education of China for Returned Scholars (2011508), the Growth Plan for Distinguished Young Scholars in Colleges and Universities of Liaoning, China (LJQ2011024 and LJQ2012026), and the Education Department Foundation of Liaoning, China (L2010197).Notes and references
- Y. Zhai, Y. Dou, D. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828 CrossRef CAS PubMed.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1 CrossRef CAS PubMed.
- W. Deng, X. Ji, Q. Chen and C. E. Banks, RSC Adv., 2011, 1, 1171 RSC.
- W. Shen and W. Fan, J. Mater. Chem. A, 2013, 1, 999 CAS.
- B. G. An, S. F. Xu, L. X. Li, J. Tao, F. Huang and X. Geng, J. Mater. Chem. A, 2013, 1, 7222 CAS.
- E. Frackowiak, V. Khomenko, K. Jurewicz, K. Lota and F. Béguin, J. Power Sources, 2006, 153, 413 CrossRef CAS PubMed.
- S. Bose, T. Kuila, A. K. Mishra, R. Rajasekar, N. H. Kim and J. H. Lee, J. Mater. Chem., 2012, 22, 767 RSC.
- H. Lin, L. Li, J. Ren, Z. Cai, L. Qiu, Z. Yang and H. Peng, Sci. Rep., 2013, 3, 1353 Search PubMed.
- K. Zhang, L. L. Zhang, X. S. Zhao and J. Wu, Chem. Mater., 2010, 22, 1392 CrossRef CAS.
- Y. M. Shulga, S. A. Baskakov, V. V. Abalyaeva, O. N. Efimov, N. Y. Shulga and A. Michtchenko, J. Power Sources, 2013, 224, 195 CrossRef CAS PubMed.
- Y. Zhou, Z.-Y. Qin, L. Li, Y. Zhang, Y.-L. Wei, L.-F. Wang and M.-F. Zhu, Electrochim. Acta, 2010, 55, 3904 CrossRef CAS PubMed.
- M. Sun, G. Wang, X. Li, Q. Cheng and C. Li, Ind. Eng. Chem. Res., 2012, 51, 3981 CrossRef CAS.
- T. Abdiryim, A. Ubul, R. Jamal and A. Rahman, Materials, 2012, 5, 1219 CrossRef CAS.
- X. Lu, H. Dou, C. Yuan, S. Yang, L. Hao and F. Zhang, J. Power Sources, 2012, 197, 319 CrossRef CAS PubMed.
- T. Wang, A. Kiebele, J. Ma, S. Mhaisalkar and G. Gruner, J. Electrochem. Soc., 2011, 158, A1 CrossRef CAS PubMed.
- C. Shen, A. H. Brozena and Y. Wang, Nanoscale, 2011, 3, 503 RSC.
- L. Chen and S. Kumar, J. Appl. Phys., 2011, 110, 074305 CrossRef PubMed.
- R. Pfeiffer, F. Simon, H. Kuzmany, V. N. Popov, V. Zólyomi and J. Kürti, Phys. Status Solidi B, 2006, 243, 3268 CrossRef CAS.
- M. Endo, H. Muramatsu, T. Hayashi, Y. A. Kim, M. Terrones and M. S. Dresselhaus, Nature, 2005, 433, 476 CrossRef CAS PubMed.
- L. Li, F. Li, C. Liu and H.-M. Cheng, Carbon, 2005, 43, 623 CrossRef CAS PubMed.
- C. Liu, H. T. Cong, F. Li, P. H. Tan, H. M. Cheng, K. Lu and B. L. Zhou, Carbon, 1999, 37, 1865 CrossRef CAS.
- Y. Y. Fan, F. Li, H. M. Cheng, G. Su, Y. D. Yu and Z. H. Shen, J. Mater. Res., 1998, 13, 2342 CrossRef CAS.
- P. X. Hou, S. Bai, Q. H. Yang, C. Liu and H. M. Cheng, Carbon, 2002, 40, 81 CrossRef CAS.
- R. V. Salvatierra, L. G. Moura, M. M. Oliveira, M. A. Pimenta and A. J. G. Zarbin, J. Raman Spectrosc., 2012, 43, 1094 CrossRef CAS.
- R. Sainz, A. M. Benito, M. T. Martinez, J. F. Galindo, J. Stores, A. M. Baro, B. Corraze, O. Chauvet and W. K. Maser, Adv. Mater., 2005, 17, 278 CrossRef CAS.
- M. Kalbac, L. Kavan and L. Dunsch, Compos. Sci. Technol., 2009, 69, 1553 CrossRef CAS PubMed.
- Y. Tison, C. E. Giusca, J. Sloan, S. Ravi and P. Silva, ACS Nano, 2008, 2, 2113 CrossRef CAS PubMed.
- T.-C. Lu and J.-L. Tsai, Composites, Part B, 2013, 44, 394 CrossRef CAS PubMed.
- L. Zalamea, H. Kim and R. B. Pipes, Compos. Sci. Technol., 2007, 67, 3425 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |