Vesicle fusion intermediates obtained from the self-assembly of a cationic platinum(II) complex with sulfonate terminated polystyrenes†
Fang Qu,
Nijuan Liu and
Weifeng Bu*
Key Laboratory of Nonferrous Metals Chemistry and Resources Utilization of Gansu Province, State Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, China. E-mail: buwf@lzu.edu.cn; Fax: +86 9318912582; Tel: +86 931 8915151
First published on 9th December 2013
Abstract
Planar-coil supramolecular block copolymers fabricated from a cationic platinum(II) complex with sulfonate terminated polystyrenes can self-assemble into vesicles, which show further spontaneous fusion, with an hour-scale fusion time, in chloroform–methanol (v/v = 1) mixed solvents. The vesicle fusion intermediates, including docking vesicles, arrow-like protrusions, stalk-like intermediates, hemifusion diaphragms and fusion pores, are clearly imaged by transmission electron microscopy. It is proposed that such a fusion process is triggered by Pt⋯Pt and π–π stacking interactions between the platinum(II) complexes.
Introduction
Membrane fusion is proposed to proceed in sequence through several intermediates including; approaching of two apposed lipid vesicles, merging of the outer proximal leaflets to form a stalk-like intermediate, expanding of the stalk into a hemifusion diaphragm and then fusion pore opening or opening directly from the stalk.1–3 As a result, two initial vesicles become one to facilitate the transport of molecules between and within membranes. Such a fusion process is key within living organisms. However, direct observation of these fusion intermediates, which is very important for the mechanistic insight of the membrane fusion process, remains highly challenging due to the complexity of bio-related membranes and the high speed at which the fusion processes occur, usually within a second-scale fusion time. In this context, various vesicle models are developed to mimic the membrane fusion and thus understand the fusion mechanism.4–17 However, fusion intermediates are rarely captured by transmission electron microscopy (TEM) in these simplified model systems,5,8,9 which again may be due to the really rapid fusion processes.On the other hand, block copolymers, regarded as macromolecular analogues of membrane-forming lipid molecules, can form vesicles with much thicker walls due to having much higher molecular weights.18,19 This, together with the polymeric entanglements, has enabled the formation of kinetically frozen block copolymer vesicles. There are several reports concerning block copolymer based vesicle fusion,20–23 where the fusion time is close to 50 h,22 which is much longer than those of liposomes1–14 and branched polymersomes.15–17 However, the fusion frequencies are rather low. This problem may be mitigated by supramolecular polymer-based vesicles, as we have recently TEM-imaged a series of vesicle fusion intermediates of metallosupramolecular brushes fabricated with a cationic metallosupramolecular polyelectrolyte and sulfonate terminated polystyrenes.24
We prepared three planar-coil supramolecular block copolymers (SBC-1, SBC-2 and SBC-3, Fig. 1) by electrostatic combination of a cationic platinum(II) complex, [Pt(Me2bzimpy)Cl]+Cl− (Me2bzimpy = 2,6-bis(N-methylbenzimidazol-2-yl)pyridine),25,26 with sulfonate terminated polystyrenes (ST-Sn, n = 100, PDI = 1.12; n = 162, PDI = 1.07; n = 301, PDI = 1.05) because; (i) these SBCs can self-assemble into vesicles in chloroform–methanol (v/v = 1, chloroform and methanol are good and poor solvents for polystyrene, respectively) mixed solvents, in which the [Pt(Me2bzimpy)Cl]+ cations are sandwiched by the inner and outer peripheries of ST-Sn (Fig. 1). In comparison with lipid vesicles, the polymeric nature can reduce the fusion speed and thus enable a much longer fusion time for capturing the fusion intermediates by TEM imaging. (ii) The electrostatic interactions of the planar platinum(II) complex with ST-Sn allow the vesicles to be dynamic in nature, compared to those formed by the conventional block copolymers. This, together with the small core thickness of the vesicles, further enhances the probability of fusion events. (iii) The weak Pt⋯Pt and π–π stacking interactions25–29 between [Pt(Me2bzimpy)Cl]+ cations can function as driving forces to trigger the vesicle fusion.
 | ||
| Fig. 1 Structural formulae of SBCs and their self-assembly into vesicles in chloroform–methanol (v/v = 1) mixed solvents. | ||
Experimental section
Materials and instruments
[Pt(Me2bzimpy)Cl]+Cl− was prepared according to a previously published procedure.25 Sulfonic acid terminated poly(styrene)s (ST-Sn, n = 100, PDI = 1.12; n = 162, PDI = 1.07; n = 301, PDI = 1.05) were purchased from Polymer Source Inc. and used without further purification.1H NMR spectra were recorded on a Bruker 400 MHz spectrometer. IR spectra (KBr) were measured with a Nicolet NEXUS 670 spectrometer. UV-vis absorption spectra were recorded using a SHIMADZU UV-2550 spectrophotometer. Luminescence measurements were made on a Hitachi F-7000 spectrofluorimeter with a xenon lamp as the excitation source. Scanning electron microscopy (SEM) measurements were performed on a field emission scanning electron microscope (Hitachi S4800). TEM images were obtained with an FEI Tecnai F30 operating at 300 kV. Dynamic light scattering (DLS) measurements were performed on a Brookhaven BI-200SM spectrometer. Elemental analyses were performed with an Elementar VarioELcube. All measurements were carried out at room temperature.
Preparation and characterization
SBC-1 was prepared as follows: ST-S100 (300 mg, 0.0286 mmol) was dissolved in 20 mL of DMF together with equimolar NaOH. This solution was added dropwise to a solution of [Pt(Me2bzimpy)Cl]+Cl−·2.5H2O (19 mg, 0.292 mmol) in 5 mL water with vigorous stirring. The resulting suspension was further stirred for 3 days. The solid product was collected by filtration, washed with water, and dried in vacuo. SBC-2 and SBC-3 were prepared by a similar method, but with ST-S162 and ST-S301, respectively.For SBC-1, Anal. calcd for [(C8H8)100(CH2)3SO3](C21H17N5ClPt)(H2O)5, 11187.4: C, 88.39; H, 7.50; N, 0.63. Found: C, 88.52; H, 7.25; N, 0.46. UV-vis (CHCl3): λmax = 311, 344, 360, 380 nm (ligand π–π*), 420–510 nm (MLCT), and 543 nm (MMLCT). IR (KBr, cm−1): 3440, 3081, 3059, 3025, 2920, 2848, 194, 1869, 1802, 1744, 1638, 1601, 1538, 1492, 1451, 1369, 1181, 1155, 1068, 1028, 964, 906, 842, 754, 697, 538. 1H NMR (400 MHz, CDCl3, Me4Si) δ 1.41 (2H), 1.83 (1H), 6.57 (2H), and 7.03 (3H) PPM.
For SBC-2, Anal. calcd for [(C8H8)162(CH2)3SO3](C21H17N5ClPt)(H2O)10, 17729.3: C, 89.35; H, 7.61; N, 0.39. Found: C, 89.43; H, 7.577; N, 0.31. UV-vis (CHCl3): λmax = 311, 344, 360, 380 nm (ligand π–π*), 420–510 nm (MLCT), and 543 nm (MMLCT). IR (KBr, cm−1): 3438, 3082, 3059, 3025, 2920, 2849, 1943, 1871, 1802, 1743, 1633, 1601, 1540, 1492, 1451, 1320, 1181, 1154, 1068, 1028, 969, 906, 842, 754, 697, 539. 1H NMR (400 MHz, CDCl3, Me4Si) δ 1.41 (2H), 1.83 (1H), 6.56 (2H), and 7.05 (3H) PPM.
For SBC-3, Anal. calcd for [(C8H8)301(CH2)3SO3](C21H17N5ClPt)(H2O)25, 32464.2: C, 89.90; H, 7.70; N, 0.22. Found: C, 89.58; H, 7.659; N, 0.13. UV-vis (CHCl3): λmax = 311, 344, 360, 380 nm (ligand π–π*), 420–510 nm (MLCT), and 543 nm (MMLCT). IR (KBr, cm−1): 3441, 3082, 3059, 3025, 2920, 2848, 1941, 1871, 1802, 1742, 1636, 1601, 1539, 1492, 1451, 1328, 1181, 1154, 1068, 1028, 964, 906, 841, 753, 696, 538. 1H NMR (400 MHz, CDCl3, Me4Si) δ 1.41 (2H), 1.83 (1H), 6.57 (2H), and 7.05 (3H) PPM.
SBCs cannot be dissolved directly in a mixture of chloroform and methanol which contains 50% methanol. To obtain the vesicular aggregates, SBCs were dissolved first in chloroform and then methanol was added. The final concentration of SBCs in the solvent mixture was controlled at 0.5 mg mL−1.
Results and discussion
Both the infrared (Fig. S1†) and 1H NMR spectra (Fig. S2†) of SBCs demonstrated typical features of ST-Sn. The UV-vis spectra of the chloroform solutions of SBCs showed characteristic ligand π–π* (311, 344, 360, 380 nm) and metal-to-ligand charge-transfer (MLCT, 420–510 nm) transitions (Fig. S3a–c†) of the [Pt(Me2bzimpy)Cl]+ cation. In addition, the shoulder band near 543 nm was typical of a metal–metal-to-ligand charge-transfer (MMLCT) transition originating from an intermolecular association through Pt⋯Pt and π–π stacking interactions.25,26 Upon excitation at 420 nm, the chloroform solutions showed an emission band at a λmax of 618 nm together with a shoulder near 565 nm (Fig. S3d–f†). The former was accordingly assigned to a 3MMLCT excited state, while the latter should originate from a triplet metal-perturbed intraligand parentage of Me2bzimpy (3IL). Finally, elemental analyses revealed that the molar ratios of [Pt(Me2bzimpy)Cl]+ with ST-Sn were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in SBCs.
1 in SBCs.
The chloroform–methanol (v/v = 1) solutions of SBCs were first subjected to dynamic light scattering (DLS) measurements (Fig. 2). When the solution of SBC-1 was allowed to age for 0.5 h, the DLS plot revealed an average hydrodynamic diameter (Dh) of 350 ± 50 nm, which increased to 450 ± 30 nm at an aging time of 2.5 h. Similarly, the Dh of SBC-2 increased from 245 ± 15 to 350 ± 25 nm when the aging time increased from 0.5 to 7.5 h. In comparison, the Dh of SBC-3 remained almost constant at 115 ± 10 nm even at an aging time of 24 h. The additional increase in the aging time to 36 h led to two modes at 76 and 260 nm in the DLS plot. These DLS signals were attributed to the formation of supramolecular aggregates of SBCs in the solvent mixtures. Their sizes increased with the aging time for the solutions.
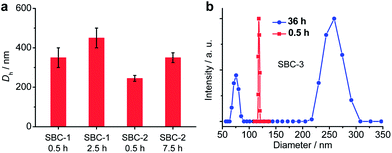 | ||
| Fig. 2 The hydrodynamic diameters of SBC-1, SBC-2 (a), and SBC-3 (b) measured from DLS in the chloroform–methanol mixed solvents (v/v = 1, 0.5 mg mL−1). | ||
To achieve internal insight of the self-assembled structures, the solutions aged for 1 h were cast onto carbon-coated copper grids for TEM observations. Unilamellar vesicles of SBC-1 were observed with a higher transmission in the center than around the peripheral ring (Fig. 3). The vesicle size varied considerably from ca. 200 to 430 nm but with a uniform ring thickness of ca. 1.5 nm. The latter value was really comparable with the molecular size of the [Pt(Me2bzimpy)Cl]+ cation. The gray ST-S100 corona had an average thickness of 36 ± 17 nm, even larger than the fully stretched length of the ST-S100 chain (100 × 0.25 = 25 nm). Importantly, the vesicle fusion process was directly observed. Vesicles were docked through substantial contact of the ST-S100 coronas (Fig. 3a). The arrow-like ring protrusions were captured clearly (Fig. 3b) and further connections led to stalk-like intermediates (Fig. 3c). The thicknesses of both the protrusion and the stalk were determined to be ca. 3 nm, which is twice as large as that of the dark ring (1.5 nm). The stalk nanostructure was then cleaved from the middle to form a small fusion pore (Fig. 3d). The hemifusion diaphragms were also captured by this TEM imaging (Fig. 3e–i). In addition, peanut-like and oblate vesicles were observed clearly (Fig. 3j and k), which should be formed from the stalk or hemifusion diaphragm intermediates. The corresponding SEM image showed that the vesicle fusion was ubiquitous (Fig. S4a†), which was completely consistent with the TEM observations. Combining the TEM and SEM images, we therefore determined the vesicle fusion frequency to be 93% by counting 500 vesicles (Fig. 4 and S5a†). Further ageing the solution for 2.5 h yielded vesicles with diameters of 450–620 nm, where no fusion event was detected (Fig. 3l). Such large vesicular aggregates were further confirmed by SEM imaging (Fig. S4b†).
 | ||
| Fig. 3 TEM images of SBC-1 as drop-cast from 0.5 mg mL−1 chloroform–methanol (v/v = 1) solution aged for 1 h (a–k) and 2.5 h (l) onto carbon-coated copper grids. | ||
 | ||
| Fig. 4 Statistic analyses of the fusion frequencies of SBC-1, SBC-2, and SBC-3 vesicles drop-cast from 0.5 mg mL−1 chloroform–methanol (v/v = 1) solution aged for 1 h (with 500 vesicles). | ||
Similarly, SBC-2 formed unilamellar vesicles with diameters of 60–270 nm (Fig. S6†), where the ring and corona thicknesses were estimated to be 1.5 and 17 ± 5 nm, respectively. The latter was larger than the unperturbed end-to-end distance (R0 = 0.456 × n0.595 = 9.4 nm)30 of the ST-S162 chain, but smaller than its fully extended length (40.5 nm). Various vesicle fusion intermediates were also imaged, including docking vesicles, arrow-like protrusions, stalk-like intermediates, hemifusion diaphragms and fusion pores (Fig. S6†). The corresponding fusion probability was calculated to be 20% (Fig. 4 and S5b†), much smaller than that of SBC-1. The reduced fusion events were due to a much larger polymerization degree in SBC-2 than in SBC-1. Furthermore, SBC-3 also formed unilamellar vesicles but with a much smaller diameter of 54 ± 8 nm and a thinner corona of 6 ± 4 nm (Fig. 5). Unexpectedly, the corona thickness was much smaller than the unperturbed end-to-end distance (14 nm)30 of the ST-S301 chain. In this case, docking vesicles, arrow-like protrusions, stalk-like intermediates and fusion pores were captured and no hemifusion diaphragm was detected (Fig. 5). This absent hemifusion diaphragm should be assigned to the much larger polymerization degree in SBC-3. The fusion frequency was estimated to be ca. 6% (Fig. 4, S5c and S5d†), which was again consistent with the larger polymerization degree in SBC-3. Due to a similar reason, a fusion process with more than three vesicles was generally observed for SBC-1 and SBC-2 (Fig. 3 and S6†), but only occasionally occurred for SBC-3 (Fig. 5). Both the TEM and SEM images (Fig. S4†) showed that the fusion events of SBC-2 and SBC-3 stopped after 8 and 36 h, respectively, which were longer than that of SBC-1 (2.5 h). Such polymerization degree-dependent vesicle fusion processes were completely consistent with the DLS results listed above. It should be noted that the vesicle and corona sizes decreased with an increase in the molecular weight of SBCs.
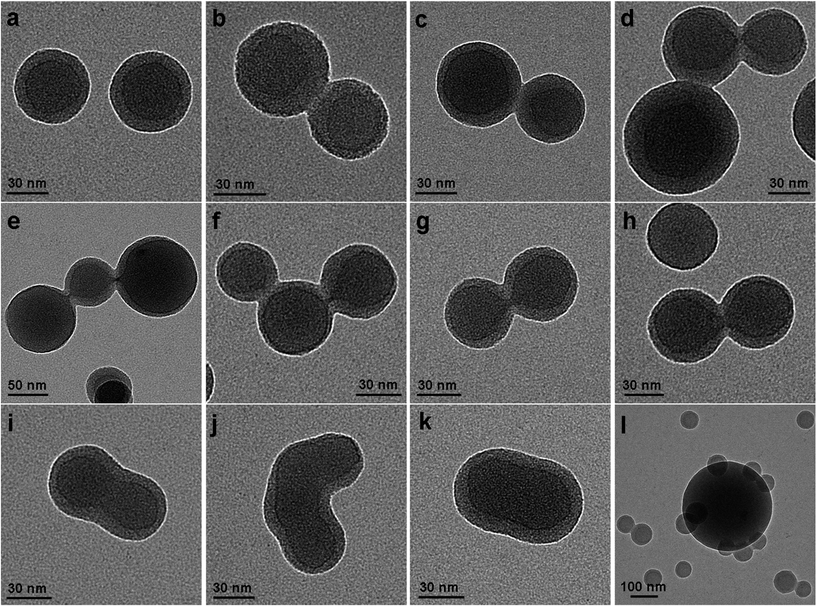 | ||
| Fig. 5 TEM images of SBC-3 as drop-cast from a 0.5 mg mL−1 chloroform–methanol (v/v = 1) solution aged for 1 h (a–k) and 36 h (l) onto carbon-coated copper grids. | ||
To confirm the formation of vesicles, nanobjects should be incorporated inside the lumen. SVP-6 composed of poly(styrene-b-4-vinylpyridinium methyl iodide) (S480-b-V57) with 8 [PW12O40]3− anions can form spherical micelles with an S480 corona and a hybrid core filled with [PW12O40]3− anions, iodides, and nitrates binding to V57 blocks via Coulombic forces in chloroform.31–33 SBC-3 and SVP-6 were first mixed in chloroform, and then methanol was directly added at a volume ratio of 50%, where their concentrations were controlled at 0.5 and 0.33 mg mL−1, respectively. This solution was aged for 1 h and then drop-cast onto a carbon-coated copper grid for TEM observations. The TEM image showed spherical nanoparticles with three host–guest forms: (1) a single dark core was encapsulated by a dark ring together with two alternating gray polystyrene domains (Fig. 6a); (2) 2–8 dark cores appeared in the dark ring (Fig. 6b–g); (3) in addition, multiple dark cores were clearly observed in the dark rings with total sizes ranging from 150–650 nm (Fig. 6h and i). In these host–guest nanostructures, the core diameter, corona and ring thicknesses were determined to be 23 ± 5, 7 ± 3 and 1.5 nm, respectively. The former was completely consistent with that of the micelles of SVP-6 obtained from a chloroform–methanol mixture with a methanol volume ratio of 50%,32,33 while the latter two values agree well with those of the vesicles formed by SBC-3 in the same solvent mixtures as mentioned above. These results suggest that the micelles formed by SVP-6 were incorporated into the inside lumens of the vesicles formed by SBC-3, which confirmed the formation of vesicles of SBCs.
 | ||
| Fig. 6 TEM images of the mixture of SBC-3 (0.5 mg mL−1) and SVP-6 (0.33 mg mL−1) as drop-cast from a chloroform–methanol (v/v = 1) solution, aged for 1 h, onto a carbon-coated copper grid. | ||
According to previous theoretical studies for linear polymers,34,35 repulsive interactions dominate and polymers are swollen in good solvents. A worsening solvent quality leads to stronger van der Waals attraction forces within an isolated linear chain and between linear chains. The casting films of SBC-1, SBC-2 and SBC-3 were prepared from their chloroform–methanol (v/v = 1) solutions, where the vesicles formed. Their UV-vis spectra revealed MMLCT absorption bands at 540–600 nm (Fig. S7†), indicative of the presence of Pt⋯Pt and π–π stacking interactions.25–29 The casting films of both SBC-1 and SBC-2 showed 3MMLCT luminescence bands at 633 nm (Fig. 7). Of difference was that the 3MMLCT luminescence band of the casting film of SBC-3 appeared at 622 nm together with a metal-perturbed 3IL shoulder at 568 nm. The luminescence band of the vesicle of SBC-3 was slightly shifted to shorter wavelengths than those of both SBC-1 and SBC-2, suggesting somewhat longer Pt⋯Pt and π–π separations. These collective features, together with our previous experimental studies on metallosupramolecular brushes24 and polyoxometalate-based supramolecular star polymers33,36 in solvents of variable quality, suggest that the driving forces for vesicle formation and fusion should be due to a delicate balance of the intra- and inter-chain van der Waals attraction forces, repulsive effects and Pt⋯Pt and π–π stacking interactions among SBCs in the chloroform–methanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvents. The unusual chain shrinkage in the vesicle of SBC-3 may be attributed to the much stronger intra- and inter-chain van der Waals attractions than in SBC-1 and SBC-2 as a result of a much larger molecular weight in the former case, which further resulted in the absence of hemifusion diaphragm intermediates and a rather small vesicle size with respect to SBC-1.
1) mixed solvents. The unusual chain shrinkage in the vesicle of SBC-3 may be attributed to the much stronger intra- and inter-chain van der Waals attractions than in SBC-1 and SBC-2 as a result of a much larger molecular weight in the former case, which further resulted in the absence of hemifusion diaphragm intermediates and a rather small vesicle size with respect to SBC-1.
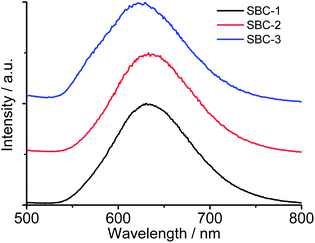 | ||
| Fig. 7 Normalized emission spectra of the casting films of SBC-1, SBC-2, and SBC-3 obtained from their solutions aged for 1 h. | ||
The proximity model suggests that without any fusogenic groups or other biological factors, even small perturbations spontaneously lead to vesicle fusion.37–40 Therefore, the strong van der Waals attractions brought the vesicles into reasonably close contact with the ST-Sn chains. The arrow-like protrusions should originate from small perturbations of Pt⋯Pt and π–π stacking interactions between SBCs. The growth of the protrusions continued to generate a stalk-like intermediate, which was successively expanded into a fusion pore. In addition, the hemifusion diaphragms were observed in the vesicles of SBC-1 and SBC-2, which should be due to their much smaller polymerization degrees and thus much larger contact areas between the platinum(II) cation rings of adjacent vesicles than in SBC-3.24 In the present case, no evidence showed that the hemifusion diaphragm was obtained by expanding the stalk-like intermediate as proposed previously in the case of lipid membranes.1–4 The hemifusion diaphragms could form directly from the docking vesicles, which were further expanded into fusion pores as addressed previously.2 The complete fusion into larger vesicles and thus the decrease in the vesicle curvature were consistent with the favorable Pt⋯Pt and π–π stacking interactions between [Pt(Me2bzimpy)Cl]+ cations.25–29
Conclusions
In summary, we have prepared luminescence planar-coil SBCs by electrostatic combination of a cationic platinum(II) complex with sulfonate terminated polystyrenes. The resulting SBCs can self-assemble into vesicles, which further show a spontaneous fusion with an hour-scale fusion time in chloroform–methanol (v/v = 1) mixed solvents. With this much longer fusion process, a series of vesicle fusion intermediates including docking vesicles, arrow-like protrusions, stalk-like intermediates, hemifusion diaphragms and fusion pores, were clearly captured by TEM imaging. It should be highlighted that the fusion processes of SBCs are proposed to be driven by the weak Pt⋯Pt and π–π stacking interactions between [Pt(Me2bzimpy)Cl]+ cations in the vesicles. This is in sharp contrast to fusion processes induced by hydrogen bonding,4–10 metal ion coordination,11,12 chemical reaction,13,14 and host–guest recognition.16,17Acknowledgements
This work is supported by the NSFC (51173073 and 20931003), the Program for New Century Excellent Talents in University (NCET-10-0462), the Fundamental Research Funds for the Central Universities (lzujbky-2012-k14 and lzujbky-2013-238) and the Open Project of State Key Laboratory of Supramolecular Structure and Materials of Jilin University (sklssm201314).Notes and references
- J. A. McNew, Chem. Rev., 2008, 108, 1669 CrossRef CAS PubMed.
- H. R. Marsden, I. Tomatsu and A. Kros, Chem. Soc. Rev., 2011, 40, 1572 RSC.
- J. Voskuhl and B. J. Ravoo, Chem. Soc. Rev., 2009, 38, 495 RSC.
- F. M. Menger and H. Zhang, J. Am. Chem. Soc., 2006, 128, 1414 CrossRef CAS PubMed.
- M. Ma, A. Paredes and D. Bong, J. Am. Chem. Soc., 2008, 130, 14456 CrossRef CAS PubMed.
- F. Nomura, T. Inaba, S. Ishikawa, M. Nagata, S. Takahashi, H. Hotani and K. Takiguchi, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 3420 CrossRef CAS PubMed.
- G. Stengel, R. Zahn and F. Höök, J. Am. Chem. Soc., 2007, 129, 9584 CrossRef CAS PubMed.
- Y. Gong, M. Ma, Y. Luo and D. Bong, J. Am. Chem. Soc., 2008, 130, 6196 CrossRef CAS PubMed.
- J. M. Hernandez, A. Stein, E. Behrmann, D. Riedel, A. Cypionka, Z. Farsi, P. J. Walla, S. Raunser and R. Jahn, Science, 2012, 336, 1581 CrossRef CAS PubMed.
- I. Tomatsu, H. R. Marsden, M. Rabe, F. Versluis, T. Zheng, H. Zope and A. Kros, J. Mater. Chem., 2011, 21, 18927 RSC.
- A. Richard, V. Marchi-Artzner, M.-N. Lalloz, M. J. Brienne, F. Artzner, T. Gulik-Krzywicki, M.-A. Guedeau-Boudeville and J. M. Lehn, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 15279 CrossRef CAS PubMed.
- T. Tanaka and M. Yamazaki, Langmuir, 2004, 20, 5160 CrossRef CAS.
- F. Loosli, D. A. Doval, D. Grassi, P.-L. Zaffalon, F. Favarger and A. Zumbuehl, Chem. Commun., 2012, 48, 1604 RSC.
- I. Budin and N. K. Devaraj, J. Am. Chem. Soc., 2012, 134, 751 CrossRef CAS PubMed.
- Y. Zhou and D. Yan, J. Am. Chem. Soc., 2005, 127, 10468 CrossRef CAS PubMed.
- H. Jin, Y. Liu, Y. Zheng, W. Huang, Y. Zhou and D. Yan, Langmuir, 2012, 28, 2066 CrossRef CAS PubMed.
- H. Jin, Y. Zheng, Y. Liu, H. Cheng, Y. Zhou and D. Yan, Angew. Chem., Int. Ed., 2011, 50, 10352 CrossRef CAS PubMed.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967 CrossRef CAS PubMed.
- S. Jain and F. S. Bates, Macromolecules, 2004, 37, 1511–1523 CrossRef CAS.
- L. Luo and A. Eisenberg, Langmuir, 2001, 17, 6804–6811 CrossRef CAS.
- M. Maskos and J. R. Harris, Macromol. Rapid Commun., 2001, 22, 271 CrossRef CAS.
- D. M. Vriezema, J. Hoogboom, K. Velonia, K. Takazawa, P. C. M. Christianen, J. C. Maan, A. E. Rowan and R. J. M. Nolte, Angew. Chem., Int. Ed., 2003, 42, 772 CrossRef CAS PubMed.
- A. A. Choucair, A. H. Kycia and A. Eisenberg, Langmuir, 2003, 19, 1001 CrossRef CAS.
- L. He, S. Bi, H. Wang, B. Ma, W. Liu and W. Bu, Langmuir, 2012, 28, 14164 CrossRef CAS PubMed.
- L. J. Grove, J. M. Rennekamp, H. Jude and W. B. Connick, J. Am. Chem. Soc., 2004, 126, 1594 CrossRef CAS PubMed.
- N. Liu, B. Wang, W. Liu and W. Bu, Chem. Commun., 2011, 47, 9336 RSC.
- I. Eryazici, C. N. Moorefield and G. R. Newkome, Chem. Rev., 2008, 108, 1834–1895 CrossRef CAS PubMed.
- K. M. C. Wong and V. W.-W. Yam, Acc. Chem. Res., 2011, 44, 424 CrossRef CAS PubMed.
- X. Zhang, B. Li, Z.-H. Chen and Z.-N. Chen, J. Mater. Chem., 2012, 22, 11427 RSC.
- S. Förster, M. Zisenis, E. Wenz and M. Antonietti, J. Chem. Phys., 1996, 104, 9956–9970 CrossRef PubMed.
- W. Bu, S. Uchida and N. Mizuno, Angew. Chem., Int. Ed., 2009, 48, 8281 CrossRef CAS PubMed.
- Q. Zhang, Y. Liao, L. He and W. Bu, Langmuir, 2013, 29, 4181 CrossRef CAS PubMed.
- Q. Zhang, Y. Liao and W. Bu, Langmuir, 2013, 29, 10630 CrossRef CAS PubMed.
- V. Krakoviack, J.-P. Hansen and A. A. Louis, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 67, 041801 CrossRef CAS.
- A. Narros, A. J. Moreno and C. N. Likos, Macromolecules, 2013, 46, 3654 CrossRef CAS PubMed.
- Q. Zhang, L. He, H. Wang, C. Zhang, W. Liu and W. Bu, Chem. Commun., 2012, 48, 7067 RSC.
- J. Lee and B. R. Lentz, Biochemistry, 1997, 36, 421 CrossRef CAS PubMed.
- J. Lee and B. R. Lentz, Biochemistry, 1997, 36, 6251 CrossRef CAS PubMed.
- G. Cevc and H. Richardsen, Adv. Drug Delivery Rev., 1999, 38, 207 CrossRef CAS.
- S. J. Marrink and A. E. Mark, J. Am. Chem. Soc., 2003, 125, 11144 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Spectral data, SEM and TEM images of SBCs. See DOI: 10.1039/c3ra45574b |
| This journal is © The Royal Society of Chemistry 2014 |
