Tailoring strained oxanorbornane headgroups to dimensionally controlled nanostructures through hydrogen bonding†
Nivarthi Ramesha,
M. Ganesanb,
Nirod Kumar Sarangia,
K. M. Muraleedharanb and
Archita Patnaik*a
aDepartment of Chemistry, Indian Institute of Technology Madras, Chennai 600 036, India. E-mail: archita59@yahoo.com; Tel: +91-44-22574217
bDepartment of Chemistry, Indian Institute of Technology Madras, Chennai 600 036, India. E-mail: mkm@iitm.ac.in; Tel: +91-44 2257 4233
First published on 2nd December 2013
Abstract
An amphiphilic oxanorbornane skeletal frame has been efficiently organized to direct the formation of dimensionally controllable, geometry-specific nanostructures as diverse as 0-D surface/reverse micelles, 1-D nanofibers, 2-D rectangular/square sheets, and 3-D flowers. The racemate with its precise stereo-projection of the –OH groups and the ring oxygen could be used for Li+ ion sensing and aided in the formation of pre-micellar aggregates of conventional surfactants.
1. Introduction
Self-assembly schemes are often inspired by the wealth of biological examples present in nature.1 To exactly mimic the in vitro reassembly (of proteins and lipids) to that of the in vivo counterparts, a thermodynamic equilibrium defined by external conditions such as ionic strength, temperature and pH2 is mandatory. During the mimicking process, the kinetics involved in the self assembly processes might typically be very slow or very fast depending on the growth phase, making it difficult to observe the processes under equilibrium. As a result, deciphering the molecular and supramolecular pathways towards complex self-assembly is exceedingly complicated and they have rarely been elucidated by the observation of stable intermediates. Enticed by this challenge, an oxanorbornane based sugar-like amphiphilic scaffold is designed that has a furanose ring and is endowed with multiple hydroxyl groups. These sugars are thermo-dynamically disfavoured compared to pyranose sugars.3 Bacterial polysaccharides and glycoconjugates contain saccharides in the furanose conformation and moreover, furanose sugars are an essential part of extremophiles which live in harsh environments and can endure high salinity, high temperatures and organic solvents. In this context, self assembly of polyhydroxyoxanorbornanes is explored at the air/water interface and in the bulk leading to complex architectures with experimental access to each metastable state simulating the above environments.2. Experimental
Synthesis of the target molecules started with the cycloaddition of O-benzyl protected furfuryl alcohol with maleic anhydride. Using this method we were able to prepare a compound with one head group and two lipophilic chains as shown in Scheme 1 (ESI†). To achieve this, the anhydride (±)-3 was first reduced using lithium aluminium hydride to obtain the diol (±)-4 and then this was acylated with palmitoyl chloride. Treatment of the resulting diesters (±)-5 with OsO4 led to the formation of the cis-dihydroxylated product (±)-6, which upon hydrogenolysis in the presence of H2–Pd/C afforded the amphiphile (±)-7 in a 71% yield.Monolayer isotherm measurements
The amphiphile monolayers were spread on the surface of ultrapure water (Millipore-Academic) of resistivity 18.2 MΩ cm, from chloroform (Uvasol, Merck India) solutions. The pH of the subphase was altered by adding hydrochloric acid (for acidic pH) or NaOH (for basic pH). Soluble metal chlorides (1 mM) were used for creating saline conditions. Subsequently, surface pressure–area isotherms were obtained using a computer controlled double barrier Langmuir trough, KSV 5000 (Finland) after a thorough clean with alternating rounds of HPLC grade chloroform and methanol (spectroscopic grade, SRL fine chemicals). For isotherm measurements, 100 μL of 1 mM 1a solution was spread using a Hamilton syringe and the surface pressure was measured by the Wilhelmy plate method with a platinum sensor of 0.1 mN m−1 accuracy. After an equilibration period of 20 min with solvent evaporation, isotherms were acquired from the compressed Langmuir films at an optimal speed of 5 mm min−1. Then, Langmuir–Blodgett films on hydrophilized Si(100) substrates were prepared by transferring the monolayers at a desired surface pressure using the vertical dipping method. Hydrophilization was carried out by etching the substrates with hot piranha solution (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 conc. H2SO4–H2O2) at 70 °C, followed by rinsing with Millipore water.
1 conc. H2SO4–H2O2) at 70 °C, followed by rinsing with Millipore water.
Infrared reflection–absorption spectroscopy
Real-time spectral measurements were performed on a Bruker Optic GmbH (Germany) Vertex 70 spectrometer equipped with a mercury cadmium telluride (MCT) detector (spectral resolution 0.1 cm−1), and a home-built optical attachment placed on a vibration isolated Newport I-2000 table (Fountain Valley, CA). The spectrometer was equipped with an external variable-angle reflectance accessory XA 511 coupled to a custom designed Langmuir trough (R & K, Germany). The IR beam was focused onto the water surface with an off-axis parabolic mirror with a focal length of 120 mm, and the desired angle of incidence was obtained when the computer driven stepper motors rotated the mirrors. The angle of incidence was varied between 25 and 65° from the surface normal, the latter being the maximum grazing incidence obtainable. Alternate s- and p-polarized spectra were acquired with a resolution of 4 cm−1 by co-addition of 2048 (for s-polarization) and 4096 (for p-polarization) scans. The entire experimental setup was enclosed and purged with dry N2 gas. The spectra resulting from this experimental setup were reflectance–absorbance (RA) spectra, where RA = −log(R/R0), with R being the IR reflectivity of the surface of interest and R0 being the IR reflectivity of the surface background.Atomic force microscopy
Sensors fabricated from the layer-by-layer LB transferred films as well as from the solvent-cast films were analyzed with an atomic force microscope (XE-100, Park Systems, Korea) in noncontact and contact modes. The cantilevers (SHOCONA-SS and ACTA, AppNano) were fitted with monolithic, single crystal silicon tips with radii of curvatures <5 nm. Probes used in noncontact mode imaging had a 300 kHz resonance frequency with a spring constant 42 mN m−1. Images were analyzed using XEI image analysis software. To compensate sample tilt, all of the images were subjected to first order plane fitting.Fabrication of 1a/SAM modified Au electrodes
Gold electrodes (2 mm in diameter) were polished with 0.3 μm alpha alumina powder & 0.05 μm gamma alumina powder on a felt pad for at least 15 min, then rinsed several times with ethanol and water, and finally sonicated in triple-distilled water and absolute alcohol for 10 min, successively. The polished electrodes were further immersed in a 0.5 M H2SO4 solution and cleansed electrochemically by applying consecutive cycles of negative potential between −0.4 V and −1.2 V until the obtained voltammograms no longer changed and a characteristic voltammogram of a clean Au electrode was obtained. The clean electrodes were then immersed in ethanol solution containing 2.0 mM C12H25SH for a period of time ranging from 30 min to 5 h at room temperature to form the SAM (self-assembled monolayer) on the surface. The SAM modified Au electrodes were then thoroughly rinsed with ethanol and dried under a stream of N2. The electrodes were finally soaked in a 1 mM 1a solution in CH3OH–CHCl3 mixture (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and stored in triple-distilled water.
1) and stored in triple-distilled water.
Electrochemical measurements
A CHI 660B Electrochemical Workstation (CH Instruments, US) was used for all of the electrochemical measurements. A conventional three-electrode setup was employed, with the chemically modified gold electrode as the working electrode, a platinum-wire counter electrode, and a Ag/AgCl electrode as the reference electrode. All of the solutions were prepared in Tris buffer (pH 7.4) with a 1 mM Fe(CN)64−/3− internal standard in 0.1 M KCl electrolyte. Electrochemical impedance measurements for the fabricated sensors were performed using the CHI 660B Electrochemical Workstation with E° = 240 mV. Impedance measurements were performed in a 0.1 M KCl solution containing equal concentrations of oxidized and reduced forms of the Fe(CN)6 3−/4− redox couple. The frequency range used was 100 kHz to 0.01 Hz with an ac amplitude of 5 mV.Computational methods
Geometry optimization of the individual molecules of interest and the association complexes was done using density functional calculations at the B3LYP/6-31+G(d,p) and B3LYP/6-31G(d) levels of theory respectively, as included in the Gaussian 09 set of programs. All of the optimizations for dimers were carried out with tight convergence criteria without any symmetry constraints. Geometry optimization was followed by frequency calculations for all models to calculate the harmonic vibration modes of the molecule and also to assure the global minimum of the stationary points. To probe the dynamics of lithium ion sensing, we employed an ab initio molecular dynamics (AIMD) approach with Atom-centered Density Matrix Propagation (ADMP) as included in the Gaussian 09 set of programs. The dynamics have been performed at the B3LYP/6-31G(d) level with a minimum trajectory of 300 fs with converged SCF (self-consistent field) results at each point. The fictitious electron mass (EMass) was set to the default 0.1 amu, and the temperature was set to 298 K, similar to the temperature used to acquire IRRAS data, in order to extract the dynamical behavior of the clusters. A velocity scaling thermostat was used to maintain the temperature during the 300 fs equilibration run. The time step employed in each simulation was 0.25 fs. As no thermal and zero-point corrections were applied towards tracing the trajectory, all discussions have been limited to relative self-consistent field energies.Surface tension measurements
The critical micellar concentration (CMC) of 1b was determined from surface tension measurements using the Wilhelmy method (KSV, Finland) with a platinum plate sensor. The temperature was controlled with a Julabo thermo controller with an accuracy of ±0.5 °C. Surface tension measurements were done at equilibrium at the set temperature until concordance was obtained from three consecutive measurements for each concentration. For binary surfactant systems involving 1b, data were acquired by adding a solution of 1b dissolved in HFIP (hexafluoro-2-propanol) to water and subsequently stirring and purging with dry nitrogen gas to remove the HFIP.3. Results and discussion
The designed amphiphiles and 2-D molecular organization
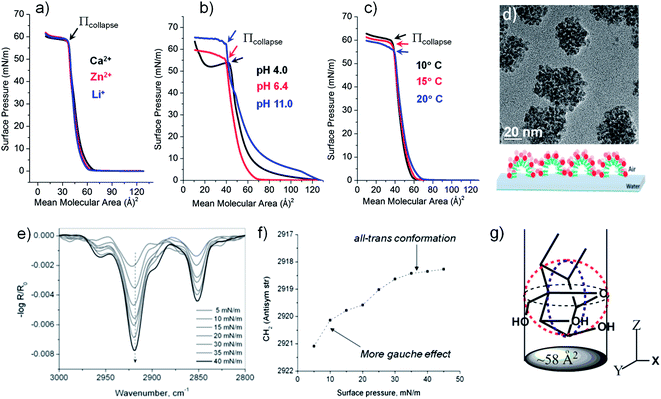
Twin-tailed amphiphiles 1a and 1b (Fig. 1a) were designed for the current study, and 1c and 1d (Fig. 1a) were used as controls; their synthetic details are given in Scheme 1 of ESI.† The skeletal framework of these molecules is a highly strained system4 (bridgehead bond angle, 96.58°; Fig. 1b and S1†), which has in fact previously provided means to determine the distance dependence of intramolecular charge transfer.5 In addition to the usual torsional strain arising due to repulsion between the bond pair of electrons (repulsion due to the eclipsed conformation of the C5–O3 bond with respect to C6–O2), the bridging of carbons C1 and C4 of the boat involved in the construction of the oxanorbornane skeletal framework introduces appreciable angle strain. The strain is physically reflected as a high heat of combustion. The single-crystal X-ray diffraction derived geometrical parameters of a benzyl protected oxanorbornane6 skeletal frame are given in Fig. 1b. Until and unless H-bonded functionality is embedded into the oxanorbornane skeleton, disordered crystals are expected. In this context, the air/water interface which restricts the facial and dual/alternate H-bonded interactions between the secondary hydroxyl groups (Fig. 1c), provides an excellent platform to study the disordered packing of these strained systems. 1a (1 mM in CHCl3) spread at the air/water interface shows an apparent molecular area of 58 Å2 per molecule (at pH 6.4) with a collapse pressure of 56 mN m−1 confirming the ability of this molecule to form stable monolayers (Fig. 2b). The stability of a monolayer at the air/water (A/W) interface is often inferred from its collapse surface pressure (Table 1). In Langmuir isotherms it is indicated as the point at which the surface pressure plateaus sharply. It can be seen in Fig. 2a–c that metal ion variation in the subphase did not affect the collapse surface pressures whereas basic pH and low temperatures gave rise to high collapse surface pressures indicating the high stability of monolayers under the respective environmental conditions. A molecular area of 58 Å2 is in good agreement with the size of 1a in the cylindrical conformation (Fig. 2g). In the condensed phase, films transferred at 40 mN m−1 showed discontinuous 0-D-surface micelles of sizes in the range 20–50 nm when visualized with HR-TEM (Fig. 2d). The surface micellar orderliness is confirmed by the presence of a diffraction pattern when an electron beam is encountered (Fig. S2†). Real-time Infrared Reflection-Absorption spectral (IRRAS) monitoring (Fig. 2e) of the νa(CH2) stretching confirms an all-trans conformation of the hydrophobic alkyl chains where surface micelles are observed (Fig. 2f). This rules out gauche-confirmation directed disorderliness in the surface micelles and in turn strongly supports the origin of the crystallinity in surface micelles of 1a to be solely induced by alkyl chains and not by the strained oxanorbornane rings. Earlier reports focused on surface micelles made from strongly polar surfactants7 and a semi-fluorinated non-polar alkane,8 C8F17C16H33. The feasibility of depositing them over a wide area opens avenues in the applications of surface decoration/template technologies. Both acidic and basic pH subphases (pH 4.0 & pH 11.0) were found to have an expanding effect with high lift-off surface areas (Fig. 2b), whereas low temperatures (Fig. 2c) and metal ion doped subphases (Fig. 2a) have a condensing effect on the monolayers, with Li+ affecting them the most. The mean molecular areas at the condensed phases and collapse surface pressures are given for all of the simulated environments in Table 1.3-D molecular organization directed via diverse H-bonding
To test the H-bond effects on the packing efficiency of oxanorbornane skeletons, 2-D restrictions at the air/water interface (A/W) were lifted by allowing the molecule to organize in bulk CHCl3. Freshly prepared 1a solutions (0.5 mM to 1 mM) gave rise to reverse micelles of 5.5 nm in size and were observed using HR-TEM (Fig. 3a). Time-lapsed mechanistic studies, by incubating pirahna treated Si(100) wafers in CHCl3 solution, showed directionally elongated nucleation centers comprising 1-D nanofibers (Fig. 3b and d) with a thickness of 5.5 nm (Fig. 3e and A3†) after a day, which subsequently evolved into a 3-D micro-flower geometry analogous to Ostwald ripening. In a binary system of solid solutions or liquid sols, Ostwald ripening is a thermodynamically driven spontaneous process involving growth of larger particles at the expense of smaller ones. For 1a, the large surface area of the 0-D reverse micelles along with the driven facial, dual and alternate H-bonds favor 1-D nanofibers at the expense of reverse micelles. The subsequent transformation of the 1-D nanofibers to 3-D microflowers involves only vertical directional growth. From the force microscopic images it can be observed that the diameter of a 3-D microflower equals the length of a single precursor nanofiber. Ostwald ripening is reported in amphiphilic azobenzene based π-systems where 0-D nanodot transformation to pseudo 1-D nanorods occurred under UV light, inducing the essential requirement of a binary system in the form of cis–trans isomers.9 At the solid/liquid interface, Ostwald ripening is reported in two-dimensional crystals of oligothiophenes.10 The kinetically stable 1-D nanofibers transforming into thermodynamically favoured 3-D microflowers (Fig. 3b and f) involves minimization of the surface free energy of the system within a given volume. In striking contrast, when the two secondary OH groups are chemically protected (ketal, Fig. S3a†) preventing alternative zigzag H-bonds, 1-D nanofibers could not be observed in CHCl3, suggesting that their assembly involves the strained oxanorbornane skeleton and is H-bond driven. Deprotecting the secondary hydroxyl groups and protecting the primary hydroxyl with a benzyl moiety (Fig. S3b†) gave rise to a structural hierarchy similar to 1a in CHCl3 (Fig. S4†). This confirms that 1-D nanofiber formation is dictated by the two secondary hydroxyl groups only.The alternative zigzag H-bond network (Fig. 4a) was further disrupted by embedding11 1a into dodecanethiol SAM on the Au(111) surface (Fig. 4b) via hydrophobic interactions (Fig. S5†). Interdigitated bilayers of ∼2.8 nm thickness (Fig. 4c) thus formed in the binary solutions of CHCl3 and CH3OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) as a result of constricting the translational degrees of freedom of the alkyl chains, anticipated to act as templates for 1a. The bilayer deposited Au electrodes when incubated in 1 mM 1a (1
4, v/v) as a result of constricting the translational degrees of freedom of the alkyl chains, anticipated to act as templates for 1a. The bilayer deposited Au electrodes when incubated in 1 mM 1a (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v CHCl3–CH3OH) revealed a discrete collection of square nano-sheets with almost similar average lengths and widths irrespective of incubation times. A typical square nano-sheet embedded with a 2-D rectangular sheet could be imaged through non-contact mode AFM, as shown in Fig. 4d. The latter are expected to be precursors to the square nano-sheets, evident from their estimated thicknesses in the line profiles (Fig. S6†) in which the head groups are linked together via H-bonds (Fig. 4e). The two facial hydroxyl groups of embedded 1a enables the freely dispersed 1a to bind through its hydroxyl groups and this growth process propagates along the direction of the H-bond leading to a 3-D network of single-crystalline grains in which several bilayers stack on top of themselves via H-bonds. Fig. 4e dictates the most judicious mechanistic route for sheet formation, where the oxanorbornane heads behave exclusively as single proton donors and acceptors. Additionally, experiments carried out on ITO substrates yielded similar square nano-sheets (Fig. S7†). The shape and sharp edges suggest these sheets to be single-crystalline nano-sheets. Single-crystalline square nano-sheets were earlier reported for porphyrins by adopting a re-precipitation method.12 Fei Wang et al. reported rectangular 2-D nano-architectures of zinc hydroxydodecylsulfate which were single-crystalline and a product of solution-based synthesis.13 The possibility of using SAM surfaces to stabilize thermodynamically metastable structures offers a powerful tool in the development of oriented crystals using bottom-up approach processes.
1 v/v CHCl3–CH3OH) revealed a discrete collection of square nano-sheets with almost similar average lengths and widths irrespective of incubation times. A typical square nano-sheet embedded with a 2-D rectangular sheet could be imaged through non-contact mode AFM, as shown in Fig. 4d. The latter are expected to be precursors to the square nano-sheets, evident from their estimated thicknesses in the line profiles (Fig. S6†) in which the head groups are linked together via H-bonds (Fig. 4e). The two facial hydroxyl groups of embedded 1a enables the freely dispersed 1a to bind through its hydroxyl groups and this growth process propagates along the direction of the H-bond leading to a 3-D network of single-crystalline grains in which several bilayers stack on top of themselves via H-bonds. Fig. 4e dictates the most judicious mechanistic route for sheet formation, where the oxanorbornane heads behave exclusively as single proton donors and acceptors. Additionally, experiments carried out on ITO substrates yielded similar square nano-sheets (Fig. S7†). The shape and sharp edges suggest these sheets to be single-crystalline nano-sheets. Single-crystalline square nano-sheets were earlier reported for porphyrins by adopting a re-precipitation method.12 Fei Wang et al. reported rectangular 2-D nano-architectures of zinc hydroxydodecylsulfate which were single-crystalline and a product of solution-based synthesis.13 The possibility of using SAM surfaces to stabilize thermodynamically metastable structures offers a powerful tool in the development of oriented crystals using bottom-up approach processes.
Li+ ion sensing with organized norbornanes – (a) an electrochemical perspective
In view of Li+ ions’ superior ability to condense 1a monolayers, over Ca2+ and Zn2+ ions (Fig. 2a), Li+ sensing using embedded 1a was undertaken electrochemically through impedance spectroscopy in the presence of a Fe(CN)64−/3− redox probe. Lithium salts have been extensively used for treating neurological and nervous disorders.14 In lithium therapy, the slow penetration of lithium ions through the blood brain barrier and other biomembranes owing to its strong hydration, poses a problem. To cater to medical needs, research is mainly focused on increasing the lipophilicity and transport selectivity of the lithium ion carrier in case a competitive interference exists in the form of other alkali metal ions, such as sodium and potassium. Further, sensing lithium in aqueous media using hydroxyl group containing sensors is also difficult as water poses as a potential and competitive binder. Fast electrode reactions at the bare Au surface gave rise to the linear part of the Warburg impedance in Fig. 4f, while SAM/Au and 1a/SAM/Au modified electrodes exhibited blocking (Fig. S8†) of the electron transfer to the redox probe with high Rct (charge transfer resistance) values of 292 kΩ and 275 kΩ respectively ((b) and (c) in Fig. 4f). Sensitive to the binding characteristics, increasing the concentration of Li+ led to a decrease in Rct from 241 kΩ at 0.5 mM to 90 kΩ at 8 mM Li+ concentration (Fig. 4g). Increasingly positive 1a/SAM/Au surfaces, due to Li+ binding, increased local concentrations of the negatively charged redox probe, where electrostatic attractions facilitated electron transfer to/from Fe(CN)64−/3− which caused an increase in the current in the CV profiles (Fig. S9†). The same 1a/SAM/Au electrode responded weakly to Ca2+ and Zn2+ ions (Fig. S10†).Li+ ion sensing with organized norbornanes – (b) a molecular dynamics perspective
An ideal platform for an interfacial 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with Li+ ions (Fig. 5a) was thus created as a strong function of (i) the racemic nature of 1a coupled with precise stereo-projection of the –OH groups and the ring oxygen and (ii) the low dielectric constant15 of the bilayer 1a/SAM/Au phase. Li+ ionophores reported prior to the current work necessitated pre-requisites of high rigidity and structural pre-organization for binding without any conformational change.16 Accommodating Li+ ions introduced extra strain through deformation of the already strained oxanorbornane frame and as a consequence 1a had to adopt a less-than-stable conformation. This deformation energy (Table 2) was calculated to be positive and higher for the bidentate complex (12.96 kcal mol−1) than for the tridentate one (11.83 kcal mol−1). The binding of Li+ to 1a is dominated by stabilization interactions mainly arising due to the electrostatic attraction between the ligand and the metal ions. The extent to which these stabilization interactions outweigh the unfavourable interactions due to the deformation energy depends on the number of atoms participating in the complex formation. For the bidentate complex, the ratio of Estabilization/Edeformation is much lower (6.615) when compared to the tridentate complex (7.32). DFT calculations further indicate that the tridentate complex has a higher stabilization energy and lower deformation energy suggesting a higher probability of its formation compared to the bidentate complex, and involves the Li+ interacting with three oxygen atoms (two facial and one ring) simultaneously. Interestingly, ab initio molecular dynamics calculations using Atom-centered Density Matrix Propagation17 (ADMP) indicated time evolutionary transformation (Fig. 5b, c and e and S11†) of the bidentate complex to a tridentate complex, where new O–Li+ bond formation occurs at the crucial ethereal bridged oxygen (Fig. 5b). The distances are well under 2.0–2.5 Å, which is similar to natural carbohydrate–Ca2+ complexes.18
1 complex with Li+ ions (Fig. 5a) was thus created as a strong function of (i) the racemic nature of 1a coupled with precise stereo-projection of the –OH groups and the ring oxygen and (ii) the low dielectric constant15 of the bilayer 1a/SAM/Au phase. Li+ ionophores reported prior to the current work necessitated pre-requisites of high rigidity and structural pre-organization for binding without any conformational change.16 Accommodating Li+ ions introduced extra strain through deformation of the already strained oxanorbornane frame and as a consequence 1a had to adopt a less-than-stable conformation. This deformation energy (Table 2) was calculated to be positive and higher for the bidentate complex (12.96 kcal mol−1) than for the tridentate one (11.83 kcal mol−1). The binding of Li+ to 1a is dominated by stabilization interactions mainly arising due to the electrostatic attraction between the ligand and the metal ions. The extent to which these stabilization interactions outweigh the unfavourable interactions due to the deformation energy depends on the number of atoms participating in the complex formation. For the bidentate complex, the ratio of Estabilization/Edeformation is much lower (6.615) when compared to the tridentate complex (7.32). DFT calculations further indicate that the tridentate complex has a higher stabilization energy and lower deformation energy suggesting a higher probability of its formation compared to the bidentate complex, and involves the Li+ interacting with three oxygen atoms (two facial and one ring) simultaneously. Interestingly, ab initio molecular dynamics calculations using Atom-centered Density Matrix Propagation17 (ADMP) indicated time evolutionary transformation (Fig. 5b, c and e and S11†) of the bidentate complex to a tridentate complex, where new O–Li+ bond formation occurs at the crucial ethereal bridged oxygen (Fig. 5b). The distances are well under 2.0–2.5 Å, which is similar to natural carbohydrate–Ca2+ complexes.18
| 1a–Li+ complex | ΔHbindinga (kcal mol−1) | Edeformationb (kcal mol−1) | Estabilizationc (kcal mol−1) |
|---|---|---|---|
| a Enthalpy of binding, ΔH is defined as: (E+Li + E1a) − (E+Li–1a) + (ZPE1a − ZPEE+Li–1a). The total electronic energy, E is calculated at the B3LYP/6-31+G(d,p) level of theory and ZPE, the zero point energy is calculated at the HF/6-31+G(d,p) level. The vibrational frequencies were scaled by a factor of 0.8929.b Deformation energy is defined as the energy difference between 1a in its complexed form compared to 1a adopting the most stable conformation in its uncomplexed form.c Stabilization energy is defined as the summation of the enthalpy of binding and deformation energy. | |||
| Bidentate complex | 72.7973 | 12.9639 | 85.7612 |
| Tridentate complex | 74.5312 | 11.8346 | 86.6693 |
We next turned our attention to 1b (Fig. 1a) which can interact with CTAB (cetyltrimethylammonium bromide) and SDS (sodium dodecyl sulfate) in the aqueous phase through C–H⋯O and H-bond interactions, respectively. Prior to this, control experiments executed with pure CTAB and SDS (Fig. S12†) in separate sets under varying conditions of salinity gave critical micellar concentrations that agreed well with previously published results.19 The so-called “synergetic effect”,20 identified by a decrease in surface tension, was observed (Fig. 6a and b) when water was replaced by 0.5 mM 1b aqueous solution. The initial decrease in the surface tension started much earlier when compared to the titrations of pure CTAB and SDS indicating that 1b enhances the formation of pre-micellar aggregation of both CTAB and SDS. The enhancement is particularly strong in the case of 1b/CTAB due to three C–H⋯O interactions. In the case of 1b/SDS, due to a single hydrogen bond, the CMC values did not show a drastic reduction in the critical micellar concentration values. To further quantitatively estimate the interactions involved between 1b and the conventional surfactants, topology analysis21 was used. The relationship between the bond energy EH-bond and the potential energy density V(r) at the corresponding Bond Critical Points (BCP) could be described as, EH-bond = V(r)/2. Using this formula22 the interaction energy was evaluated. Csp3–H is a weak proton donor compared to Csp–H or Csp2–H. Therefore, in the case of the 1b/CTAB complex, positively charged nitrogen activates the Csp3–H bond and gives it sufficient strength to act as a proton donor in the formation of weak C–H⋯O interactions. The computed Csp3–H⋯O interaction energies (Fig. 6c) and H⋯O distances at the B3LYP/6-31G(d) level of theory are in good agreement with the acceptable23 range of values. Similarly, the hydrogen bond strength in 1b/SDS was estimated to be −46.97 kJ mol−1 (Fig. 6d). The plots in Fig. 6a and b show sharp break points corresponding to the CMC of the mixtures. Increasing the salinity decreased electrostatic repulsion between the ionic head groups in the pre-micellar aggregates and as a consequence the CMC values further decreased.
4. Conclusions
In summary, methodical access to a wide range of geometrical assemblies encompassing all of the dimensions of a strained structural oxanorbornane framework at the A/W interface as well as in the bulk was demonstrated. A Li+ ion sensor was designed by precisely embedding alkyl chains into conventional SAMs. The polyhydroxyl groups of the oxanorbornane framework could be conveniently used to enhance the pre-micellar aggregate formation in conventional surfactants.Acknowledgements
The authors thank the Department of Science and Technology, New Delhi, India (Grant no. SR/S2 CMP-57/2006). NR and NKS acknowledge the Senior Research Fellowship from IIT Madras. The Computer Centre, IIT Madras is gratefully acknowledged for providing the computational facilities. Financial support of this work by the Department of Science and Technology, INDIA (Grants SR/NM/NS-1034/2012) is also gratefully acknowledged.References
- (a) J. Aizenberg, J. C. Weaver, M. S. Thanawala, V. C. Sundar, D. E. Morse and P. Fratzl, Science, 2005, 309, 275–278 CrossRef CAS PubMed; (b) M. Hildebrand, Chem. Rev., 2008, 108, 4855–4874 CrossRef CAS PubMed.
- (a) B. Ozbas, J. Kretsinger, K. Rajagopal, J. P. Schneider and D. J. Pochan, Macromolecules, 2004, 37, 7331–7337 CrossRef CAS; (b) D. J. Pochan, J. P. Schneider, J. Kretsinger, B. Ozbas, K. Rajagopal and L. Haines, J. Am. Chem. Soc., 2003, 125, 11802–11803 CrossRef CAS PubMed; (c) A. Aggeli, M. Bell, L. M. Carrick, C. W. G. Fishwick, R. Harding, P. J. Mawer, S. E. Radford, A. E. Strong and N. Boden, J. Am. Chem. Soc., 2003, 125, 9619–9628 CrossRef CAS PubMed.
- M. Fukuda, Methods in Enzymology, Glycomics, Elsevier, 2010, vol. 478, 1st edn Search PubMed.
- (a) M. G. Newton, N. S. Pantaleo, S. Kirbawy and N. L. Allinger, J. Am. Chem. Soc., 1978, 100, 2176–2180 CrossRef CAS; (b) M. Tamres, S. Searles and J. M. Goodenow, J. Am. Chem. Soc., 1964, 86, 3934–3936 CrossRef CAS.
- H. Oevering, M. N. Paddon-Row, M. Heppener, A. M. Oliver, E. Cotsaris, J. W. Verhoeven and N. S. Hush, J. Am. Chem. Soc., 1987, 109, 3258–3269 CrossRef CAS.
- M. Ganesan and K. M. Muraleedharan, RSC Adv., 2012, 2, 4048 RSC.
- T. Kato, M. Kameyama, M. Ehara and K.-i. Iimura, Langmuir, 1998, 14, 1786–1798 CrossRef CAS.
- M. Maaloum, P. Muller and M. P. Krafft, Angew. Chem., Int. Ed., 2002, 41, 4331–4334 CrossRef CAS.
- S. Mahesh, A. Gopal, R. Thirumalai and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 7227 CrossRef CAS PubMed.
- R. Heinz, A. Stabel, F. C. De Schryver and J. P. Rabe, J. Phys. Chem., 1995, 99, 505 CrossRef CAS.
- J. Zhu, Y. Qin and Y. Zhang, Anal. Chem., 2009, 82, 436–440 CrossRef PubMed.
- Z. Wang, Z. Li, C. J. Medforth and J. A. Shelnutt, J. Am. Chem. Soc., 2007, 129, 2440–2441 CrossRef CAS PubMed.
- F. Wang, J. E. Jakes, D. Geng and X. Wang, ACS Nano, 2013, 7, 6007–6016 CrossRef CAS PubMed.
- N. E. Rosenthal and F. K. Goodwin, Annu. Rev. Med., 1982, 33, 555 CrossRef CAS PubMed.
- H. Tamagawa, M. Sakurai, Y. Inoue, K. Ariga and T. Kunitake, J. Phys. Chem. B, 1997, 101, 4817–4825 CrossRef CAS.
- (a) L. A. Paquette and J. Tae, J. Am. Chem. Soc., 2001, 123, 4974–4984 CrossRef CAS PubMed; (b) J. Tae, R. D. Rogers and L. A. Paquette, Org. Lett., 2000, 2, 139–142 CrossRef CAS PubMed.
- S. S. Iyengar, H. B. Schlegel, J. M. Millam, G. A. Voth, G. E. Scuseria and M. J. Frisch, J. Chem. Phys., 2001, 115, 10291–10302 CrossRef CAS PubMed.
- C. H. S. Wong, F. M. Siu, N. L. Ma and C. W. Tsang, J. Mol. Struct.: THEOCHEM, 2001, 536, 227–234 CrossRef CAS.
- H. Nakahara, O. Shibata and Y. Moroi, J. Phys. Chem. B, 2011, 115, 9077–9086 CrossRef CAS PubMed.
- L. Shedlovsky, J. Ross and C. W. Jacob, J. Colloid Sci., 1949, 4, 25 CrossRef CAS.
- B. Silvi and A. Savin, Nature, 1994, 371, 683–686 CrossRef CAS.
- E. Espinosa, E. Molins and C. Lecomte, Chem. Phys. Lett., 1998, 285, 170–173 CrossRef CAS.
- (a) U. Koch and P. L. A. Popelier, J. Phys. Chem., 1995, 99, 9747–9754 CrossRef CAS; (b) T. Steiner and W. Saenger, J. Am. Chem. Soc., 1992, 114, 10146–10154 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, electrochemical experiments, DFT calculations, surface tension measurements, additional AFM, SEM & TEM images. See DOI: 10.1039/c3ra45708g |
| This journal is © The Royal Society of Chemistry 2014 |