Sweet side chain: a glycopeptide-based alignment medium to measure residual dipolar couplings in neat acetonitrile
Lukas
Laux
 and
Christina M.
Thiele
and
Christina M.
Thiele
 *
*
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Peter-Grünberg-Straße 16, 64287, Darmstadt, Germany. E-mail: cthiele@thielelab.de
First published on 29th August 2025
Abstract
Homopolyglutamates are well-known liquid crystalline polymers (LCPs) with a semi-flexible main chain. The polymeric backbone forms an α-helical secondary structure stabilized through intramolecular hydrogen bonds, making these rigid-rod-type polymers macroscopic mesogens. One important application of these homopolyglutamates is their use as lyotropic liquid crystalline (LLC) alignment media, which allows the determination of highly important anisotropic NMR observables like residual dipolar couplings (RDCs) for the structure elucidation of organic compounds. Herein, we report a novel polyglutamate bearing a per-acetylated glucose side chain, introduced by a postpolymerization modification approach via click-chemistry. The modified polymer exhibits unique properties concerning solvent compatibility, including the formation of stable LLC-phases in neat acetonitrile, which is a first of its kind for LLC-based alignment media.
1. Introduction
The unique properties of liquid crystals enable several applications of such materials in optoelectronics,1–3 such as sensors,4 biomedicine,5 and many more.6 Liquid crystals combine the characteristics of fluids and crystals and are built from shape-anisotropic mesogens. The formation of such liquid crystalline phases is not only limited to mesogenic small molecules. Many polymers can also form stable mesophases if the mesogenic elements are (incorporated into) the polymer's backbone or side chain.7,8 Thus, liquid crystalline polymers (LCPs) are categorized as main-chain or side-chain LCP according to the presence of mesogenic units in the backbone or side chain. Helmut Ringsdorf, inter alia, was a pioneer in the field of liquid crystalline side chain polymers and a main contributor to early research and innovations on LCPs.9–11 A polymer does not necessarily need to contain mesogenic units in its backbone, also the polymer itself can function as a main-chain LCP. Several types of such polymers, e.g. polyacetylenes,12 polyisocyanides,13 and (some) homopolypeptides,14 are known to form α-helical secondary structures through intramolecular hydrogen bonds, making them mesogenic rigid-rod-type polymers. The concepts of helical polymers and side chain LCPs can also be combined by adding mesogenic motives into the side chain of helical polymers to further enhance their (liquid crystalline) properties.15–17Moreover, these helical polymers play a pivotal role in the NMR-based structure elucidation of organic compounds as their liquid crystalline behavior enables their use as alignment media,18–21 with polyglutamates being one of the best-investigated classes of polymeric alignment media.16,17,22–26 In appropriate solvents, rod-like polypeptide mesogens like poly-γ-benzyl-L-glutamate (PBLG) exhibit positional long-range order, resulting in lyotropic liquid crystalline phases (LLC-phases) of cholesteric nature outside a magnetic field, which show characteristic patterns in polarized optical microscopy.27 When brought into magnetic fields (e.g. of an NMR spectrometer) the mesogens orient parallel to the magnetic field due to their positive diamagnetic susceptibility anisotropy.28,29 The formation of the then nematic phase can be concluded from the 2H-NMR spectrum of the deuterated solvent. The concentration at which LLCs are formed is system-specific and called the critical concentration ccrit, and depends i.e. on solvent and chain length.30–32 This order induced can partially be transferred to an organic compound dissolved in the LLC, hindering its free rotation and tumbling motion.33,34 In this weakly aligned state, anisotropic NMR observables like residual dipolar couplings (RDCs) can be observed. These RDCs are global NMR parameters offering distance and angular information and thus emerged as powerful tools to elucidate configurations and conformations of organic compounds and natural products.34–42
Although polypeptide-based alignment media offer advantages like fast sample preparation and excellent spectral quality, they usually lack compatibility with polar organic solvents like DMSO, methanol, or acetonitrile. These solvents tend to interfere with the polymeric backbone through competing hydrogen bonds and thus break down the LLC-phases.43–45 As many organic compounds and especially natural products require such polar solvents, the design of novel alignment media, providing compatibility with these solvents, is of particular interest. Other types of alignment media can meet these demands, for instance, self-assembling oligopeptides,46,47 graphene oxide,48,49 or stretched and compressed polymeric gels.50–52 The alignment of such polymeric gels does not require the formation of a stable secondary structure, making them insensitive to hydrogen bond-breaking organic solvents. However, these polymeric gels usually do not enable enantiodifferentiation of chiral compounds, a well-known feature of polypeptide-based alignment media.22,23 Thus, we aim to create polyglutamates featuring the excellent properties of polypeptide-based alignment media, and additionally exhibit compatibility with neat polar organic solvents.
We recently introduced the alkyne-terminated polyglutamate PPOBLG 1 (see Fig. 1) as a highly versatile alignment medium, exhibiting compatibility with a wide range of organic solvents, covering non-polar and polar solvents like DMSO.53 To further increase the scope of accessible solvents, we aimed to develop a novel polyglutamate with a polar side chain to increase solubility in highly polar solvents like methanol or acetonitrile and to stabilize the α-helical secondary structure in these solvents. Especially acetonitrile is of special interest as a solvent as – to the best of our knowledge – no LLC-based alignment medium is reported to be compatible with neat acetonitrile. Besides graphene oxide, which forms stable LLC-phases in water/acetonitrile mixtures,48 only few cross-linked polymeric gels are known to swell in neat acetonitrile.50,52,54 Thus, creating an LLC-based alignment medium compatible with neat acetonitrile would be an important step toward the widespread use of RDCs in the structure elucidation of organic compounds and natural products.
A feasible strategy to increase the hydrophilicity of a polypeptide's side chain is the modification with glycosides,55,56 but the incorporation of such motives is challenging. The usual synthesis protocol for homopolypeptides is based on the ring-opening polymerization (ROP) of corresponding N-carboxy anhydrides (NCAs) with bases or nucleophiles.57–59 Several examples of the ROP of glycosylated NCAs are reported, and the resulting polymers can adopt α-helical conformations.60–63 However, no polymerization of glutamate-based glycosylated NCAs is present in the literature. Additionally, the synthesis of these NCAs requires multiple steps and elaborate purification of the NCA.60,64,65 Furthermore, only limited molecular weights are obtained in some cases, which is attributed to insufficient purity of the NCAs.64,66 Such high molecular weights are necessary for the application as an alignment medium to decrease the critical concentration and thus the degree of order.31 Also, the glycoside moieties are often polymerized with protecting groups,60,62,63,67 whose removal is especially challenging for polyglutamates, which are polyesters, and thus could also decompose through cleavage of ester protection groups of the glycoside (e.g. acetyl groups). To overcome these challenges and to obtain high molecular weight glycopeptides, another synthesis strategy is chosen herein. This alternative method to incorporate a glycoside moiety is the postpolymerization modification using click-chemistry (see Fig. 1). For this concept, PPOBLG 1 is the ideal parent polymer as the terminal alkyne group enables the polymer analogous modification via the copper-catalyzed azide–alkyne cycloaddition (CuAAC).68,69 The CuAAC reaction tolerates most functional groups and was already successfully applied in the modification of alkyne-terminated polyglutamates with glycosides with (nearly) quantitative conversion of the alkyne group.70–72
Indeed, we have successfully modified PPOBLG 1 with a per-acetylated D-glucose and a D-glucose moiety. The application of both polymers obtained, poly-β-D-acetylglucosyl-triazoloxybenzyl-L-glutamate (PAcG-TOBLG) 2 and poly-β-D-glucosyl-triazoloxybenzyl-L-glutamate (PG-TOBLG) 3, as alignment media is presented herein with PAcG-TOBLG 2 being the first LLC-based alignment medium exhibiting compatibility with neat acetonitrile.
2. Results and discussion
2.1. Synthesis, characterization and solvent compatibility
Our first approach for introducing a polar side chain to PPOBLG 1 has been the postpolymerization modification with an azide-bearing glucose derivative. PPOBLG 1 is synthesized as described previously,53 and the glucose azide is obtained following literature-known procedures (see SI, Section S1.3 for detailed synthesis protocols and characterization). The following polymer analogous modification using the CuAAC reaction is successfully applied, resulting in the glucose-modified PG-TOBLG 3. Notably, polymer 3 forms stable LLC-phases in neat DMSO, DMF, and pyridine (see SI, Section S1.4.2), making PG-TOBLG 3 one of, to this point, only two polypeptides compatible with neat DMSO. However, the modification with a glucose motive does not increase the polymer's solubility in polar organic solvents and surprisingly even decreased the number of accessible solvents compared to PPOBLG 1. To accomplish our goal of unlocking compatibility with more polar organic solvents, we have turned our attention toward the per-acetylated glucose derivative. We have presumed that protection of the hydroxy groups of the glucose side chain should weaken intermolecular hydrogen bonds as the side chain functions as a hydrogen acceptor but not as a donor in this case. The four carbonyl groups should additionally increase the polarity of the side chain compared to PPOBLG 1. Performing the postpolymerization modification via the CuAAC reaction with the per-acetylated glucose derivative yields the modified PAcG-TOBLG 2. Strikingly, PAcG-TOBLG 2 shows the same (isotropic) solubility as the parent polymer PPOBLG 1, including DMSO, but, additionally, solubility in neat acetonitrile. Encouraged by these results, we have tested if PAcG-TOBLG 2 also forms stable LLC-phases in these solvents.First, investigations on the polymer's secondary structure have been conducted. As shown in Fig. 2(I), two characteristic negative bands at ca. 220 nm are obtained in a circular dichroism (CD) spectrum in acetonitrile. This is typical for a right-handed α-helical structure and matches the expectation for an L-configured polyglutamate.73,74 A stable, form anisotropic secondary structure is crucial for the formation of LLC-phases. Thus, PAcG-TOBLG 2 is a promising candidate as a new alignment medium. We have prepared concentrated solutions of polymer 2 in seven deuterated solvents (CD3CN, CDCl3, THF-d8, DMSO-d6, DMF-d7, acetone-d6, and pyridine-d5) and measured 2H-NMR spectra to validate the formation of an LLC-phase. In the case of anisotropy, a quadrupolar splitting of the solvent's deuterium signal is expected. Impressively, we observe a quadrupolar splitting for all tested deuterated solvents (Fig. 2(II)). For DMSO and THF, two doublet of a doublet fine-splittings are obtained, which are due to the prochiral nature of both solvents and the homochiral nature of PAcG-TOBLG 2. The enantiotopic CD2/CD3 groups become diastereotopic through interaction with a chiral alignment medium, which is also a strong indication of excellent enantiodifferentiating properties of the alignment medium.22,23,75 The broad solvent compatibility makes PAcG-TOBLG 2 an even more versatile alignment medium than the parent polymer PPOBLG 1,53 as the excellent solvent compatibility is retained while also providing compatibility with neat acetonitrile. This furthermore makes PAcG-TOBLG 2 the first LLC-based alignment medium to be compatible with neat acetonitrile and, together with PG-TOBLG 3, the first alignment media to be designed via postpolymerization modification.
 | ||
| Fig. 2 (I) Structure of PAcG-TOBLG 2 and the corresponding circular dichroism (CD) spectrum in acetonitrile (1 mg mL−1 in a 1 mm quartz cuvette). The characteristic two negative bands at ca. 220 nm are associated with a right-handed α-helical secondary structure.73,74 (II) 2H NMR spectra (700 MHz, 300 K) of seven deuterated solvents in LLC-phases of PAcG-TOBLG 2. The quadrupolar splitting of the deuterium signal proves the formation of a stable LLC-phase in all solvents. A flame-sealed capillary with a different deuterated solvent is added to all samples to facilitate easier locking and shimming (marked with an asterisk). | ||
2.2. Orienting properties and structure elucidation of natural products
To further investigate the suitability of the synthesized glycopeptides as alignment media and to compare their orienting properties, we have prepared LLC-phases with the well-known model compound (−)-isopinocampheol21,45,76–79(−)-4 in DMSO-d6. Moreover, we compare the orienting properties with the parent polymer PPOBLG 1 to evaluate if the postpolymerization-modification approach not only allows for the targeted modification of polymer and LLC properties but also creates different orientations of the analyte investigated.We have measured 1H–13C-perfect-CLIP HSQCs80 and F1-coupled HSQCs81,82 to extract the isotropic scalar 1JC–H couplings and anisotropic total 1TC–H couplings, from which the experimental 1DC–H RDCs are determined. As shown in Fig. 3, the experimental RDCs in the three LLC-phases are all within the same order of magnitude and are thus obtained in a similar alignment strength. However, we do not observe a linear trend concerning the size of the experimental RDCs, which would indicate scaling. Also, the sign of the RDCs varies randomly, which is exemplarily highlighted with dashed boxes in Fig. 3. These findings are strong indications for different orientations of the analyte (−)-IPC (−)-4 in the studied alignment media. This is underlined by the 5D β-angle83 between the orientation tensors of (−)-4 in these alignment media. The 5D β-angle is commonly used to quantify the differences in orientation of two orientation tensors and is defined in a range of 0°–180°, except if enantiodifferentiation is analyzed. In this case, the maximum β-angle is restricted to 90° due to the symmetry of both the compound and the alignment medium.18,84
 | ||
| Fig. 3 Comparison of the 1DC–H RDCs of (−)-IPC (−)-4 in LLC-phases of PPOBLG 1 (blue), PAcG-TOBLG 2 (blue-green), and PG-TOBLG 3 (mint) in DMSO-d6. The RDCs do not follow a linear trend concerning size and sign (highlighted for the couplings C1–H1, C3–H3, C5–H5, and C7–H7a with dashed boxes). This is, together with the high 5D β-angles (see below), a strong indication for different orientations of the compound (−)-4 in all these alignment media. The couplings of (−)-IPC (−)-4 in an LLC-phase of PPOBLG 1 in DMSO-d6 were those from a previous investigation.53 | ||
We obtain β-angles of 50.3° (PAcG-TOBLG 2vs. PPOBLG 1), 76.7° (PG-TOBLG 3vs. PPOBLG 1), and 30.1° (PAcG-TOBLG 2vs. PG-TOBLG 3). The high β-angles between the modified polymer 2 and 3 and the parent polymer PPOBLG 1 confirm the indication of a random variation of size and sign of the experimental RDCs and thus strongly imply a different orientation of (−)-IPC (−)-4 through the side chain modification. Moreover, the β-angle of 30.1° when comparing the orientation tensors of (−)-4 in LLC-phases of PAcG-TOBLG 2 and PG-TOBLG 3 indicates also a different orientation of (−)-IPC (−)-4 when different motives are used for the side chain modification via CuAAC. Therefore, the postpolymerization modification approach not only enables the targeted modification of polymer and LLC properties but could also be valuable in the de novo structure elucidation, as this method requires several linearly independent orientations of a compound of interest.85–88
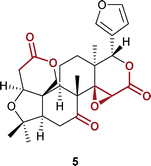
To test if PAcG-TOBLG 2 also exhibits compatibility with different functional groups, we have prepared LLC-phases with various natural products and pharmaceutically relevant compounds (see Table 1). The LLC-phases with all compounds have been analyzed in CD3CN to highlight the suitability of PAcG-TOBLG 2 as the first LLC-based acetonitrile-compatible alignment medium. The experimental RDCs are evaluated with the software RDC@hotFCHT89,90via singular value decomposition (SVD),91 resulting in a set of back-calculated RDCs for given structural proposals and quality factors like the Cornilescu Q factor92 that describe the quality of the fit. The results of this procedure are summarized in Table 1. High spectral quality is obtained for all investigated analytes, as exemplarily presented for the compound limonin 5 in Fig. 4.
Limonin 5 is a tetracyclic triterpenoid that is common in many plants and fruits and is tested as a drug candidate for several pharmacological applications, including anti-tumor, anti-inflammatory, and anti-viral activity and many others.93 The compound is built of a sterane framework and contains two lactones and one epoxide group. (−)-Cytisine 6 is a natural alkaloid bearing a secondary amine and a tertiary amide functionality. The compound is the most prominent radioligand for nicotinic acetylcholine receptors and thus an archetype of a class of compounds for the treatment of neurodegenerative disorders. Furthermore, it functions as a smoking cessation agent.94 (−)-Galantamine 7 is also a natural alkaloid with inhibitory effects on the acetylcholinesterase, which makes it a potential drug candidate for the treatment of Alzheimer's disease.95 Galantamine 7 contains different functional groups, including a tertiary amine, a hydroxy group, and an aromatic ring. Moreover, the analyte has some conformational flexibility, making fitting experimental RDCs more challenging. However, as recently shown by Rettig et al.,96 the main conformer of galantamine 7 is populated with >94% in chloroform. The RDC analysis with a single structure model may be sufficient in such a case, as also presented previously.53 As summarized in Table 1, we obtain low Q factors <0.1 and a high consistency of the experimental and back-calculated RDCs (R2 ≥ 0.990) for each compound, which underlines the excellent suitability of PAcG-TOBLG 2 as an alignment medium. Thus, we demonstrate the compatibility of the polymer with several functional groups, including amines, amides, carbonyls, hydroxy groups, and aromatics.
To furthermore demonstrate the unambiguous elucidation of relative configurations and the correct assignment of diastereotopic protons using the newly presented glycopeptide-based alignment media, we perform the SVD analysis via RDC@hotFCHT with a DFT-optimized C11 epimer of galantamine 7. Additionally, we permute the assignment of the diastereotopic protons H12, H13, H14, and H16. As presented in Fig. 5, the correct diastereoisomer is unambiguously identified by the lowest Q-factor, indicating the best agreement with the experimental RDC data. The wrong structure models show orders of magnitudes worse agreement than the best-fitting model of the correct structure.
2.3. Enantiodifferentiation of organic compounds
As already suggested by the 2H spectra of THF-d8 and DMSO-d6 in an LLC-Phase of PAcG-TOBLG 2, the polymer has enantiodifferentiating properties, which is a well-known and valuable feature of helically chiral polymeric alignment media.22,23,75 To investigate these for PAcG-TOBLG 2, we have prepared LLC-phases with both enantiomers of IPC ((−)-IPC (−)-4 and (+)-IPC (+)-4) in CD3CN. The fitting procedure using RDC@hotFCHT89,90 yields the orientation tensors of both enantiomers, which are compared via the 5D β-angle, ranging from 0°–90°.18,84 We obtain a β-angle of 69° in acetonitrile (see Table 1), which is a comparatively high value and within the same magnitude as for PPOBLG 1.53Moreover, we demonstrate the enantiodifferentiation of a racemic mixture by analyzing (±)-epichlorohydrin 8 in an LLC-phase of PAcG-TOBLG 2 in CD3CN. As presented in Fig. 6 (left), two sets of signals are obtained in an F1-coupled J-scaled BIRD-HSQC,81,82 highlighting the distinguishability of a racemic mixture using the homochiral alignment medium PAcG-TOBLG 2. Furthermore, we investigate the enantiodifferentiation of axially chiral compounds97 by preparing a scalemic mixture of (R)-BINOL (R)-9 and (S)-BINOL (S)-9 in a ratio of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. Surprisingly, we do not observe a quadrupolar splitting of the CD3CN deuterium signal in the sample of this scalemic mixture in PAcG-TOBLG 2 at a mass concentration of 24.23 wt% of polymer. As the splitting is very small for CD3CN anyway, this can be either interpreted as the splitting being too small to be detected or the addition of the enantiomers of BINOL 9 interfering with the formation of a completely anisotropic LLC-phase. However, it is still possible to differentiate both enantiomers (R)-9 and (S)-9 by the difference in their chemical shift in a corresponding 13C spectrum (see Fig. 6, right). Two well-separated sets of signals are obtained, which are assigned to either (R)-BINOL (R)-9 or (S)-BINOL (S)-9 according to their intensity.
1.5. Surprisingly, we do not observe a quadrupolar splitting of the CD3CN deuterium signal in the sample of this scalemic mixture in PAcG-TOBLG 2 at a mass concentration of 24.23 wt% of polymer. As the splitting is very small for CD3CN anyway, this can be either interpreted as the splitting being too small to be detected or the addition of the enantiomers of BINOL 9 interfering with the formation of a completely anisotropic LLC-phase. However, it is still possible to differentiate both enantiomers (R)-9 and (S)-9 by the difference in their chemical shift in a corresponding 13C spectrum (see Fig. 6, right). Two well-separated sets of signals are obtained, which are assigned to either (R)-BINOL (R)-9 or (S)-BINOL (S)-9 according to their intensity.
These results demonstrate that PAcG-TOBLG 2 provides excellent enantiodifferentiating properties as the enantiomers of homochiral and axially chiral organic compounds are differentiated via different couplings and different chemical shifts. Also, a high enantiodifferentiation is quantified via the 5D β-angle using the enantiomers of IPC 4 as model compounds.
3. Conclusions
In summary, we have presented the glycoside-modified polyglutamates PAcG-TOBLG 2 and PG-TOBLG 3 as the first alignment media to be designed via a postpolymerization-modification approach utilizing CuAAC with PPOBLG 1 as the parent polymer. Both polymers form stable LLC-phases in neat DMSO, which was, to this point, a unique feature of PPOBLG 1 among the polypeptide-based alignment media. Using the model compound (−)-IPC (−)-4, we demonstrate that different orientations of the analyte are induced through the side chain modification of PPOBLG 1 and additionally when comparing the protected and deprotected glycoside moiety. Especially PAcG-TOBLG 2 exhibits outstanding properties as the broad solvent compatibility of PPOBLG 1 is retained completely, and compatibility with neat acetonitrile is furthermore achieved. This makes PAcG-TOBLG 2 the first LLC-based alignment medium to be compatible with neat acetonitrile. We demonstrate the polymer to be a potent alignment medium compatible with different functional groups and different classes of natural products. The elucidation of relative configurations and the assignment of diastereotopic protons is unambiguously possibly using the novel alignment medium as demonstrated for (−)-galantamine 7. Furthermore, PAcG-TOBLG 2 exhibits excellent enantiodifferentiating properties as homochiral and axially chiral organic compounds are differentiated via their residual dipolar couplings and/or their chemical shifts, and high β-angles can be obtained when comparing the orientation tensors of enantiomers. We believe that unlocking compatibility with the highly important NMR solvent acetonitrile and the unique versatility of PAcG-TOBLG 2 is another step towards the routine usage of RDCs as a tool in the structure elucidation of organic compounds.Author contributions
Lukas Laux: conceptualization, investigation, data curation, formal analysis, validation, visualization, and writing (lead). Christina M. Thiele: project administration, supervision, and resources (lead); conceptualization and writing (supporting).Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article is available in the SI. Raw data for this article, are available at Zenodo at https://doi.org/10.5281/zenodo.16628414. Supplementary information: Detailed synthesis protocols, compound characterization including NMR, MS, IR, SEC, CD, and [α] data, detailed sample preparation, RDC analysis input and output data, and structure coordinates. See DOI: https://doi.org/10.1039/d5tc02303cAcknowledgements
The authors thank M.Sc. Justus Bienert for synthetic assistance and Dr Lukas Kaltschnee for his support in performing the NMR experiments necessary for the assignment of the polymer signals. Furthermore, the authors acknowledge support by the mass spectrometry core facility team of the Chemistry Department (TU Darmstadt) for measurements of the ESI/APCI spectra and the German Research Foundation (DFG) through grant no. INST 163/444-1 FUGG (QTOF MS).Notes and references
- M. Schadt, Annu. Rev. Mater. Sci., 1997, 27, 305–379 CrossRef CAS
.
- B. R. Kaafarani, Chem. Mater., 2011, 23, 378–396 CrossRef CAS
.
- B. T. Hogan, E. Kovalska, M. F. Craciun and A. Baldycheva, J. Mater. Chem. C, 2017, 5, 11185–11195 RSC
.
- R. J. Carlton, J. T. Hunter, D. S. Miller, R. Abbasi, P. C. Mushenheim, L. N. Tan and N. L. Abbott, Liq. Cryst. Rev., 2013, 1, 29–51 CrossRef CAS PubMed
.
- S. J. Woltman, G. D. Jay and G. P. Crawford, Nat. Mater., 2007, 6, 929–938 CrossRef CAS PubMed
.
- H. K. Bisoyi and Q. Li, Chem. Rev., 2022, 122, 4887–4926 CrossRef CAS PubMed
.
- H. Ringsdorf and A. Schneller, Br. Polym. J., 1981, 13, 43–46 CrossRef CAS
.
- H. Finkelmann, Angew. Chem., Int. Ed. Engl., 1987, 26, 816–824 CrossRef
.
- H. Finkelmann, H. Ringsdorf and J. H. Wendorff, Makromol. Chem., 1978, 179, 273–276 CrossRef CAS
.
- H. Finkelmann, J. Koldehoff and H. Ringsdorf, Angew. Chem., Int. Ed. Engl., 1978, 17, 935–936 CrossRef
.
- J. H. Wendorff, H. Finkelmann and H. Ringsdorf, J. Polym. Sci., Polym. Symposia, 1978, 63, 245–261 CrossRef CAS
.
- J. Liu, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2009, 109, 5799–5867 CrossRef CAS PubMed
.
- M. M. Green, N. C. Peterson, T. Sato, A. Teramoto, R. Cook and S. Lifson, Science, 1995, 268, 1860–1866 CrossRef CAS PubMed
.
- C. Robinson, Trans. Faraday Soc., 1956, 52, 571–592 RSC
.
- K. Akagi, H. Goto, Y. Kadokura, H. Shirakawa, S.-Y. Oh and K. Araya, Synth. Met., 1995, 69, 13–16 CrossRef CAS
.
- S. Jeziorowski and C. M. Thiele, Chem. – Eur. J., 2018, 24, 15631–15637 CrossRef CAS PubMed
.
- D. S. Schirra, S. Jeziorowski, M. Lehmann and C. M. Thiele, Macromolecules, 2022, 55, 3430–3436 CrossRef CAS
.
- N. Meyer, A. Krupp, V. Schmidts, C. M. Thiele and M. Reggelin, Angew. Chem., Int. Ed., 2012, 51, 8334–8338 CrossRef CAS PubMed
.
- M. Dama and S. Berger, Tetrahedron Lett., 2012, 53, 6439–6442 CrossRef CAS
.
- M. Dama and S. Berger, Org. Lett., 2012, 14, 241–243 CrossRef CAS PubMed
.
- S. Wesp, K. Wolf, S. Immel and M. Reggelin, ChemPlusChem, 2022, 87, e202100507 CrossRef CAS PubMed
.
- A. Meddour, I. Canet, A. Loewenstein, J. M. Pechine and J. Courtieu, J. Am. Chem. Soc., 1994, 116, 9652–9656 CrossRef CAS
.
- I. Canet, A. Meddour, J. Courtieu, J. L. Canet and J. Salaun, J. Am. Chem. Soc., 1994, 116, 2155–2156 CrossRef CAS
.
- C. Aroulanda, M. Sarfati, J. Courtieu and P. Lesot, Enantiomer, 2001, 6, 281–287 CAS
.
- C. M. Thiele, J. Org. Chem., 2004, 69, 7403–7413 CrossRef CAS PubMed
.
- A. Marx, V. Schmidts and C. M. Thiele, Magn. Reson. Chem., 2009, 47, 734–740 CrossRef CAS PubMed
.
- D. B. DuPré and R. W. Duke, J. Chem. Phys., 1975, 63, 143–148 CrossRef
.
- S. Sobajima, J. Phys. Soc. Jpn., 1967, 23, 1070–1078 CrossRef CAS
.
- M. Panar and W. D. Phillips, J. Am. Chem. Soc., 1968, 90, 3880–3882 CrossRef CAS PubMed
.
- L. Onsager, Ann. N. Y. Acad. Sci., 1949, 51, 627–659 CrossRef CAS
.
- A. Marx and C. Thiele, Chem. – Eur. J., 2009, 15, 254–260 CrossRef CAS PubMed
.
- P. Lesot, C. Aroulanda, P. Berdagué, A. Meddour, D. Merlet, J. Farjon, N. Giraud and O. Lafon, Prog. Nucl. Magn. Reson. Spectrosc., 2020, 116, 85–154 CrossRef CAS PubMed
.
- C. M. Thiele, Concepts Magn. Reson., Part A, 2007, 30A, 65–80 CrossRef CAS
.
- C. M. Thiele, Eur. J. Org. Chem., 2008, 5673–5685 CrossRef CAS
.
- C. M. Thiele and S. Berger, Org. Lett., 2003, 5, 705–708 CrossRef CAS PubMed
.
- C. Aroulanda, V. Boucard, F. Guibé, J. Courtieu and D. Merlet, Chem. – Eur. J., 2003, 9, 4536–4539 CrossRef CAS
.
- L. Verdier, P. Sakhaii, M. Zweckstetter and C. Griesinger, J. Magn. Reson., 2003, 163, 353–359 CrossRef CAS PubMed
.
- P. Lesot and J. Courtieu, Prog. Nucl. Magn. Reson. Spectrosc., 2009, 55, 128–159 CrossRef CAS
.
- R. R. Gil, Angew. Chem., Int. Ed., 2011, 50, 7222–7224 CrossRef CAS PubMed
.
- V. Schmidts, Magn. Reson. Chem., 2017, 55, 54–60 CrossRef CAS PubMed
.
-
J. Rettig, M. Brauser and C. M. Thiele, in Residual Dipolar Couplings, ed. L. Yao and B. Vogeli, Royal Society of Chemistry, 2024, pp. 252–279 Search PubMed
.
-
Y. Liu, G. E. Martin, G.-W. Li, X. Lei and R. T. Williamson, in Residual Dipolar Couplings, ed. L. Yao and B. Vogeli, Royal Society of Chemistry, 2024, pp. 306–369 Search PubMed
.
- M. Goodman, A. S. Verdini, C. Toniolo, W. D. Phillips and F. A. Bovey, Proc. Natl. Acad. Sci. U. S. A., 1969, 64, 444–450 CrossRef CAS PubMed
.
- M. Jackson and H. H. Mantsch, Biochim. Biophys. Acta, 1991, 1078, 231–235 CrossRef CAS PubMed
.
- A. Marx, B. Böttcher and C. M. Thiele, Chem. – Eur. J., 2010, 16, 1656–1663 CrossRef CAS PubMed
.
- X. Lei, F. Qiu, H. Sun, L. Bai, W. Wang, W. Xiang and H. Xiao, Angew. Chem., Int. Ed., 2017, 56, 12857–12861 CrossRef CAS PubMed
.
- S. Qin, Y. Jiang, H. Sun, H. Liu, A. Zhang and X. Lei, Angew. Chem., Int. Ed., 2020, 59, 17097–17103 CrossRef CAS PubMed
.
- X. Lei, Z. Xu, H. Sun, S. Wang, C. Griesinger, L. Peng, C. Gao and R. X. Tan, J. Am. Chem. Soc., 2014, 136, 11280–11283 CrossRef CAS PubMed
.
- W. Zong, G. Li, J. Cao, X. Lei, M. Hu, H. Sun, C. Griesinger and R. X. Tan, Angew. Chem., Int. Ed., 2016, 55, 3690–3693 CrossRef CAS PubMed
.
- J. C. Freudenberger, S. Knör, K. Kobzar, D. Heckmann, T. Paululat, H. Kessler and B. Luy, Angew. Chem., Int. Ed., 2005, 44, 423–426 CrossRef CAS PubMed
.
- P. Haberz, J. Farjon and C. Griesinger, Angew. Chem., Int. Ed., 2005, 44, 427–429 CrossRef CAS PubMed
.
- C. Merle, G. Kummerlöwe, J. C. Freudenberger, F. Halbach, W. Stöwer, C. L. V. Gostomski, J. Höpfner, T. Beskers, M. Wilhelm and B. Luy, Angew. Chem., Int. Ed., 2013, 52, 10309–10312 CrossRef CAS PubMed
.
- L. Laux, M. Alcaraz-Janßen and C. M. Thiele, J. Am. Chem. Soc., 2025 DOI:10.1021/jacs.5c09152
.
- R. R. Gil, C. Gayathri, N. V. Tsarevsky and K. Matyjaszewski, J. Org. Chem., 2008, 73, 840–848 CrossRef CAS PubMed
.
- J. R. Kramer and T. J. Deming, Polym. Chem., 2014, 5, 671–682 RSC
.
- K.-S. Krannig and H. Schlaad, Soft Matter, 2014, 10, 4228–4235 RSC
.
- R. B. Woodward and C. H. Schramm, J. Am. Chem. Soc., 1947, 69, 1551–1552 CrossRef CAS PubMed
.
- T. J. Deming, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 3011–3018 CrossRef CAS
.
- N. Hadjichristidis, H. Iatrou, M. Pitsikalis and G. Sakellariou, Chem. Rev., 2009, 109, 5528–5578 CrossRef CAS PubMed
.
- J. R. Kramer and T. J. Deming, J. Am. Chem. Soc., 2010, 132, 15068–15071 CrossRef CAS PubMed
.
- T. Stöhr, A.-R. Blaudszun, U. Steinfeld and G. Wenz, Polym. Chem., 2011, 2, 2239–2248 RSC
.
- J. R. Kramer and T. J. Deming, J. Am. Chem. Soc., 2012, 134, 4112–4115 CrossRef CAS PubMed
.
- D. Pati, A. Y. Shaikh, S. Das, P. K. Nareddy, M. J. Swamy, S. Hotha and S. S. Gupta, Biomacromolecules, 2012, 13, 1287–1295 CrossRef CAS PubMed
.
- E. Rüde and M. Meyer-Delius, Carbohydr. Res., 1968, 8, 219–232 CrossRef
.
- J. R. Kramer and T. J. Deming, Biomacromolecules, 2010, 11, 3668–3672 CrossRef CAS PubMed
.
- M. I. Gibson, G. J. Hunt and N. R. Cameron, Org. Biomol. Chem., 2007, 5, 2756–2757 RSC
.
- A. Y. Shaikh, S. Das, D. Pati, V. Dhaware, S. Sen Gupta and S. Hotha, Biomacromolecules, 2014, 15, 3679–3686 CrossRef CAS PubMed
.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS PubMed
.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed
.
- C. Xiao, C. Zhao, P. He, Z. Tang, X. Chen and X. Jing, Macromol. Rapid Commun., 2010, 31, 991–997 CrossRef CAS PubMed
.
- V. Dhaware, A. Y. Shaikh, M. Kar, S. Hotha and S. Sen Gupta, Langmuir, 2013, 29, 5659–5667 CrossRef CAS PubMed
.
- K. Ren, C. He, C. Xiao, G. Li and X. Chen, Biomaterials, 2015, 51, 238–249 CrossRef CAS PubMed
.
- T. Ooi, R. A. Scott, G. Vanderkooi and H. A. Scheraga, J. Chem. Phys., 1967, 46, 4410–4426 CrossRef CAS PubMed
.
- J. F. Yan, G. Vanderkooi and H. A. Scheraga, J. Chem. Phys., 1968, 49, 2713–2726 CrossRef CAS PubMed
.
- C. Aroulanda and P. Lesot, Chirality, 2022, 34, 182–244 CrossRef CAS PubMed
.
- S. Hansmann, T. Larem (née Montag) and C. M. Thiele, Eur. J. Org. Chem., 2016, 1324–1329 CrossRef CAS
.
- L. Arnold, A. Marx, C. M. Thiele and M. Reggelin, Chem. – Eur. J., 2010, 16, 10342–10346 CrossRef CAS PubMed
.
- A. Krupp and M. Reggelin, Magn. Reson. Chem., 2012, 50, 45–52 CrossRef PubMed
.
- G.-W. Li, X.-J. Wang, S.-H. Shi, L.-T. Liu, J.-Q. Li, H. Sun, Z.-Q. Wu and X. Lei, Anal. Chem., 2023, 95, 18850–18858 CrossRef CAS PubMed
.
- L. Castañar, E. Sistaré, A. Virgili, R. T. Williamson and T. Parella, Magn. Reson. Chem., 2015, 53, 115–119 CrossRef PubMed
.
- K. Fehér, S. Berger and K. E. Kövér, J. Magn. Reson., 2003, 163, 340–346 CrossRef PubMed
.
- C. M. Thiele and W. Bermel, J. Magn. Reson., 2012, 216, 134–143 CrossRef CAS PubMed
.
- J. Sass, F. Cordier, A. Hoffmann, M. Rogowski, A. Cousin, J. G. Omichinski, H. Löwen and S. Grzesiek, J. Am. Chem. Soc., 1999, 121, 2047–2055 CrossRef CAS
.
- M. Schwab, V. Schmidts and C. M. Thiele, Chem. – Eur. J., 2018, 24, 14373–14377 CrossRef CAS PubMed
.
- J. Meiler, J. J. Prompers, W. Peti, C. Griesinger and R. Brüschweiler, J. Am. Chem. Soc., 2001, 123, 6098–6107 CrossRef CAS PubMed
.
- J. R. Tolman, J. Am. Chem. Soc., 2002, 124, 12020–12030 CrossRef CAS PubMed
.
- F. A. Roth, V. Schmidts and C. M. Thiele, J. Org. Chem., 2021, 86, 15387–15402 CrossRef CAS PubMed
.
- F. A. Roth, V. Schmidts, J. Rettig and C. M. Thiele, Phys. Chem. Chem. Phys., 2022, 24, 281–286 RSC
.
- R. Berger, C. Fischer and M. Klessinger, J. Phys. Chem. A, 1998, 102, 7157–7167 CrossRef CAS
.
- V. Schmidts, PhD thesis, Technische Universität Darmstadt, 2013.
- J. A. Losonczi, M. Andrec, M. W. F. Fischer and J. H. Prestegard, J. Magn. Reson., 1999, 138, 334–342 CrossRef CAS PubMed
.
- G. Cornilescu, J. L. Marquardt, M. Ottiger and A. Bax, J. Am. Chem. Soc., 1998, 120, 6836–6837 CrossRef CAS
.
- S. Fan, C. Zhang, T. Luo, J. Wang, Y. Tang, Z. Chen and L. Yu, Molecules, 2019, 24, 3679 CrossRef CAS PubMed
.
- J. Rouden, M.-C. Lasne, J. Blanchet and J. Baudoux, Chem. Rev., 2014, 114, 712–778 CrossRef CAS PubMed
.
- C. M. Halpin, C. Reilly and J. J. Walsh, J. Chem. Educ., 2010, 87, 1242–1243 CrossRef CAS
.
- J. Rettig, M. Gölz and C. M. Thiele, Magn. Reson. Chem., 2025, 63, 406–416 CrossRef CAS PubMed
.
- P. Lesot, O. Lafon, H. B. Kagan and C.-A. Fan, Chem. Commun., 2006, 389–391 RSC
.
| This journal is © The Royal Society of Chemistry 2025 |