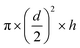Facile, tunable, and SERS-enhanced HEPES gold nanostars†
S. Saverota,
X. Gengb,
W. Lengc,
P. J. Vikeslandcd,
T. Z. Grove‡
*b and
L. R. Bickford‡*de
aDepartment of Biological Systems Engineering, Virginia Tech, Seitz Hall, Blacksburg, VA 24061, USA
bDepartment of Chemistry, Virginia Tech, Hall, Blacksburg, VA 24061, USA. E-mail: tijana.grove@vt.edu; Tel: +1-540-231-7011
cDepartment of Civil and Environmental Engineering, Virginia Tech, Durham Hall, Blacksburg, VA 24061, USA
dVirginia Tech Center for Sustainable Nanotechnology, Virginia Tech, Kelly Hall, Blacksburg, VA 24061, USA. E-mail: bickford@vt.edu; Tel: +1-540-231-7896
eDepartment of Biomedical Engineering and Mechanics, Virginia Tech, Kelly Hall, Blacksburg, VA 24061, USA
First published on 16th March 2016
Abstract
Gold nanoparticles have shown promise as effective tools in biomedicine for drug delivery, imaging, and photo thermal therapy. We present the preparation of gold nanostars (AuNSs) through a two-step approach utilizing a common Good's buffer, HEPES, as a weak reducing agent. AuNSs with tunable branch-lengths up to 30 nm in length were produced in a two-step synthesis. We have investigated the effect of the ionic strength on the AuNS growth and branch length. AuNSs were found to be near-infrared responsive and provide an augmented SERS signal, with an enhancement factor, defined as the magnitude of SERS signal amplification, of approximately 107. The unique branched morphology of the AuNSs confers optical properties advantageous for in vivo biomedical applications.
1. Introduction
Gold nanoparticles are studied for their localized surface plasmon resonance (LSPR), and the collective resonant electron oscillations that may be excited by exposure to an electromagnetic field.1 LSPR is tunable through the nanoparticles' size, shape, and composition. LSPR in the near-infrared (NIR) region, where the absorption spectra of biomolecules reaches a minimum2,3 is advantageous for in vivo imaging because of a deeper tissue penetration and a better signal to noise ratio.4–7Noble metal nanostructures demonstrate a phenomenon known as surface-enhanced Raman scattering (SERS) that results in an enhanced Raman scattering signal for surface associated molecules by several orders of magnitude.8–10 This capability is due to the excitation of the surface plasmons of nanoparticles and an increased polarizability of the target molecules under incident radiation as new chemical bonds are formed with the metallic surface.8–11 As a result, much attention has been placed into tuning nanoparticles' shape-dependent optical properties and creating “hot spots” (areas with strongly enhanced electromagnetic fields) typical at junctions and sharp tips.9,11,12
Gold star-shaped nanoparticles, nanostars (AuNSs), have received attention as capable platforms for NIR absorption and SERS applications due to their many branches and sharp tips.3,4,11,12 Particles with a greater number of longer branches produce enhanced peak resonance.3,13,14 Many studies have been conducted utilizing AuNSs' unique properties, notably Yuan et al. fabricated strong NIR resonant SERS AuNS probes and Gao et al. designed multifunctional AuNS based nanocomposites for NIR, SERS and magnetic resonance techniques.15,16
AuNSs are commonly synthesized in a buffer composed of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) through one-pot methods.3,4,12,17–19 HEPES buffer is a zwitterionic organic buffer agent, utilized in cell culture, desired for its maximal solubility in water, low permeability to cell membranes, and minimal salt and temperature effects.4 Based on the original published procedure, HEPES-mediated AuNSs were reported to have LSPR peak at 688 nm.4 Although the entire IR region consists of wavelengths between 700 and 2500 nm,2,20,21 for biomedical purposes we are interested in the biological NIR window. This window extends between 650 nm and 900–950 nm dependent upon tissue type,5–7 a range in which water and hemoglobin have their lowest absorption. Thus, it stands to reason that reported HEPES-mediated AuNSs are minimally NIR sensitive.
To serve as effective biomedical tools, nanoparticles must be well-characterized.17,22 One-pot reactions, valued for simplicity and use of weak reducing agents, do not offer sufficient precision in controlling branch growth, thus yielding effectively highly polydisperse samples. It is critical to optimize methods to yield suspensions with low polydispersity to maintain performance and predictability in nanoparticle use.17,22 Seed-mediated approaches reported to date have overcome aforementioned inconsistencies of one-pot reactions. However, they often require several surfactants such as cetrimonium bromide, dimethylformamide, polyvinyl pyrrolidone, some of which are known to be cytotoxic, that need to be removed prior to application.3,4,14,23,24 Therefore, there is a need for a robust biocompatible method for AuNS synthesis. Towards this goal, we report a tunable and green synthesis of NIR-sensitive and SERS-functional AuNSs. Both nanoparticle size and branch length can be controlled in this simple, two-step approach.
Herein, we describe a two-step synthesis of AuNSs that resembles seed-mediated approaches while making no effort to separate gold seeds and the growth solution. In this two-step procedure, gold nuclei are produced at low precursor concentrations after which concentration of gold ions is increased through addition of the fresh precursor to promote growth on existing particles. We investigate the effect of solution ionic strength and consider halide effects on AuNS formation and stability. Lastly, we investigate the SERS properties of these AuNSs using the conventional Raman reporter, 4-mercaptobenzoic acid (4-MBA).
2. Experimental
2.1 Gold nanostar synthesis
A 2 mM hydrogen tetrachloroaurate trihydrate (HAuCl4·3H2O, Sigma Aldrich) stock solution was prepared in Milli-Q water in amber bottles and allowed to age for 3 days prior to synthesis. HEPES buffer was prepared fresh with Milli-Q water at a final concentration of 150 mM and the pH adjusted to 7.4 through the addition of 1 M sodium hydroxide (NaOH) solution. In a typical experiment, 666 μL of HEPES was mixed with 333 μL of 0.15 mM HAuCl4 solution, with no stirring at room temperature (25 °C) and was allowed to react for 80 minutes. At this time, 833 μL of 0.15 mM HAuCl4 was added under the same conditions to the reaction and allowed to react overnight. The process was scalable up to ten times volume. Resulting suspensions were centrifuged at 4000 rpm for 15 min to remove excess water. The one-step procedure is identical to that previously described, with modified amount of HAuCl4 added to constitute the total volume (1.166 mL) and allowed to react overnight. In ionic strength studies, 6 M solutions of NaCl and NaNO3 in Milli-Q water were added to prepared HEPES buffer at different concentrations (5, 10, 25, 50 mM) prior to synthesis under similar two-step conditions.2.2 Nanoparticle characterization
UV/vis spectrophotometry analysis was performed using a Cary 60 UV/vis spectrophotometer (Agilent). Transmission electron microscopy (TEM) analysis was performed on a Philips EM420 at an accelerating voltage of 120 kV. TEM samples were prepared by applying 10 μL of nanoparticle suspension (0.5 mg mL−1) onto 200 mesh carbon-coated Cu grids (Ted Pella), followed by drying overnight prior to imaging. The particle-size distribution was estimated by measuring the size of approximately 100 nanoparticles in different grid regions using ImageJ software. Crystal structure of AuNSs were examined using JEOL 2100 field thermionic emission high resolution (HR-TEM) equipped with a silicon drifted detector-based EDS system, where 300 mesh ultrathin grids (EM Science) were used as sample supports.2.3 Colloidal stability
Nanoparticles were suspended in Milli-Q water at an OD of 0.3. Colloidal stability was monitored weekly, over 3 weeks, using a UV/vis spectrophotometer. Sample absorbance spectra were normalized to the initial spectra, post synthesis.2.4 Conjugation of 4-MBA to AuNSs
AuNSs were conjugated to 4-mercaptobenzoic acid (4-MBA) according to a modified protocol adapted from Li et al.11 Briefly, 20 μL of 10 mM 4-MBA dissolved in ethanol was added to a solution of the above mentioned AuNSs and the resultant solution was agitated for five hours (Rotoflex). The solution was then centrifuged at 9000 rpm for 30 min to remove excess 4-MBA and re-suspended in Milli-Q water. Although SERS signal form as prepared NSs was greatly enhanced, it was impossible to quantify the enhancement factor (EF). Since AuNSs are anisotropic, it is difficult to accurately estimate number and density of 4-MBA molecules attached to the NS surface. Assembling NSs into SERS substrates circumvents that uncertainty and we went ahead with that strategy to measure EF.2.5 SERS measurement
The purified AuNS colloidal suspension was drop-casted onto aluminium foil and dried overnight to form a thin layer of AuNS substrate. Then, 10 μL of 0.1 mM 4-MBA ethanol solution was applied onto the as-prepared AuNS substrate and dried overnight before use. SERS spectra were collected with a WITec alpha 500R using a 785 nm excitation source. Backscattered light was collected using 10× and 100× objectives at integration times ranging from 50 ms to 1 s. The signal was dispersed using a 300 groove per mm grating and the dispersed light was collected by a Peltier cooled charge coupled device (CCD). We assumed that a monolayer of 4-MBA was attached onto the surface of the AuNS substrate.The pure 4-MBA control spectrum was obtained through the drop casting of 4-MBA in ethanol in similar manner described above. All reported SERS data is an average of 100 collected spectra over a 2000 × 500 μm scan area of the substrate. The enhancement factor (EF) was calculated using the following equation: EF = (ISERS/Ibulk) × (NBulk/NSERS), where the peak at 1590 cm−1 was selected for the EF evaluation.11,25 The terms ISERS and IBulk are the intensities of the same Raman band in the SERS and bulk Raman spectra, respectively.11 We further define the terms NBulk and NSERS as: 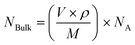 and
and 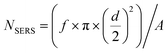 , respectively. Further experimental parameters can be found in Table 1.
, respectively. Further experimental parameters can be found in Table 1.
3. Results and discussion
Fig. 1a shows the typical absorption spectra of AuNSs synthesized through one-step or two-step method. Both spectra feature an absorption peak near 530 nm and a NIR absorption peak above 740 nm. The two-step method resulted in red-shifted NIR peaks between 760 and 815 nm whereas one-step methods yielded particles with a range between 720–780 nm from multiple trials. Particle size and aspect ratio are known to govern the optical properties of nanoparticles.3,4,26,27 Gold nanoparticles with elongated features present a longitudinal plasmon resonance that red-shifts relative to the LSPR of spherical gold nanoparticles (520 nm).1,4,26 TEM analysis of the recovered AuNSs show that the two-step approach produced particles with a greater number of branches of increased length compared to the one-step synthesis (Fig. 1b). The two-step method produced AuNSs with average branch length of 20.7 nm ± 7.7 nm compared to the one-step with average branches of 14.4 nm ± 4.5 nm (Fig. S1†). However, TEM analysis considers a limited sample size and can misrepresent particle homogeneity. To address this, the quality of NS preparation and procedure repeatability was evaluated from LSPR peaks. From DLS measurements, an average estimated hydrodynamic diameter of AuNSs is 61 nm (Fig. S2†) consistent with the measurement of AuNS from tip to tip in TEM images.We next investigated the mechanism of AuNS formation. Gold seeds were prepared through a first addition of HAuCl4 to prepared HEPES buffer. The second addition was introduced after 80 minutes of reaction time, after which typical AuNS structures were formed over the course of the day as observed in TEM images (Fig. S3b†). We surmise that upon the second addition of precursor the gold seed to gold precursor ratio is achieved that favours deposition upon already present particles. After one-day, AuNSs exhibited characteristic long branches. A kinetic scan of the typical reaction over one-day is shown in Fig. S3a.† We hypothesize that low concentrations and a low gold seed to gold precursor ratios deter the production of additional nuclei, favouring deposition upon existing nuclei and growth of anisotropic nanoparticles. Operating within the confines of the slow growth rate, AuNSs were synthesized without the need of additional surfactants or coordinating ligands. Colloidal stability of AuNSs in D.I. water was analysed over three weeks by observing changes in LSPR peaks, as these are more informative measures of physical changes to particles.28 AuNSs are strikingly stable experiencing only a minor (18%) loss in absorbance intensity (Fig. S4a†) over the three-week period while maintaining branched morphology without any significant agglomeration and change in branch length (Fig. S4b†). Similarly, incubation of as-prepared AuNSs in 1× PBS over six hours resulted in a loss of only 6% absorbance intensity (Fig. S5†). It is important to note that surface of these NSs has not been altered and that for any biological application PEG or targeting ligand would be added to the surface through standard coupling strategies. Nevertheless, presented data indicate that AuNSs are fairly stable in solution without any surface functionalization.
In the fast Fourier transform (FFT) patterns from HR-TEM images two symmetrical diffraction spots are observed (Fig. 2a), consistent with the single crystalline structure of AuNSs. The lattice spacing between the (111) planes is about 0.236 nm, which is similar to that of bulk gold.29 The inverse FFT image in Fig. 2b is equivalent to the image in the Fig. 2a suggesting that the entire AuNS, including the four tips and the central region of the NS, are contributing to the FFT pattern. Identical FFT patterns for different tips can be seen in Fig. S6.†
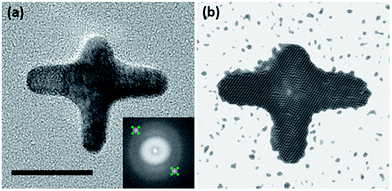 | ||
| Fig. 2 (a) HR-TEM image of representative AuNS (inset = FFT diffraction pattern). (b) HR-TEM image of the inverse FFT, from corresponding diffraction pattern (scale bar = 20 nm). | ||
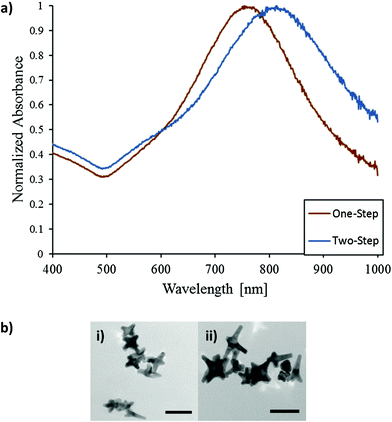
The effects of the ionic strength on nanoparticle stability and aggregation are well described and understood.30–33 In particular, the addition of salt is a particle independent method to affect the repulsive forces between charged particles.31 Notably, increases in ionic strength through salt addition have been shown to increase particles' hydrodynamic diameter as well as, increase aggregation, and decrease stability.30,32,33 Herein, we investigate the effects of varying ionic strengths through salt additions during AuNS synthesis. We observed a modest shift in particle LSPR with increasing concentrations of NaNO3 (Fig. 3a). Additionally, for the highest concentration tested, AuNS branch length increased as seen in Fig. 3a. Against our chemical intuition, the increase in the ionic strength does not give rise to significant aggregation.
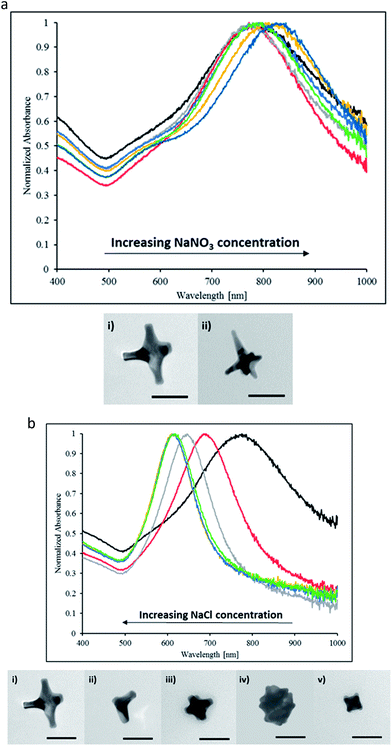
To pinpoint the effector ion, we conducted a similar study with sodium chloride (NaCl) salt (Fig. 3b). Fig. 3b shows the result of NaCl addition to reaction mixture. Increasing concentrations of NaCl elicited blue-shifting in particle LSPR as well as reduced peak intensities. Particles formed in the presence of high concentrations of NaCl have a NIR peak near 615 nm. TEM images show the length of AuNSs arms could be modulated by the concentration of NaCl, indicative that sodium or chloride ions had an effect in precursor deposition on gold seeds (Fig. 3b). Comparing results from NaNO3 and NaCl additions in the same ionic strength range, we conclude that the chloride ions dominate the morphological evolution of nanoparticles rather than the effect of ionic strength. This is consistent with publisher reports that the addition of halides such as chloride and bromide may significant slow the growth kinetics which in turn hinders the elongation of branches in the <111> direction.13 Thus in the case of NaCl addition, the preferential binding of chloride ions onto the gold surfaces inhibits tip growth resulting in the blue-shifting of the NIR peak and LSPR consistent with less anisotropic morphologies. At the same time, although sodium and nitrate ions affect the ionic strength, they do not impart any specific ion effect and thus may only impose minor influence onto the morphology of AuNSs.
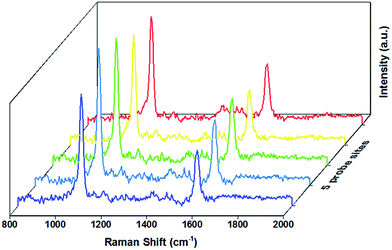
The SERS performance of the AuNSs was examined through measurement of the Raman spectra of 4-MBA deposited onto self-assembled AuNS substrate (Fig. 4). For comparison, the Raman spectrum of pure 4-MBA is shown in Fig. S7.† The EF value is estimated to 107. Details of calculation are presented in Section 2.5. We attribute this enhancement to the well-defined tips of AuNSs as observed in TEM images. The value of EF is likely underestimated because of the incomplete surface coverage. Nevertheless, SERS signal of 4-MBA in several locations on the substrate is highly reproducible indicating the homogenous self-assembly of AuNSs and 4-MBA deposition.
4. Conclusions
We demonstrate the facile, green synthesis of AuNSs for SERS applications. The absorption peak of AuNSs can be tuned between 615 nm and 815 nm by simply adjusting reaction conditions. The HR-TEM analysis confirms the formation of monocrystalline particles with multiple branches up to 30 nm in length. We investigated the effect of ionic strength upon AuNS growth, noting the undesirable effects of chloride ions. AuNSs were found to exhibit enhanced SERS performance, with an enhancement factor of approximately 9.8 × 106. By virtue of their exhibited sensitivity to both NIR and SERS, these prepared AuNSs could be well suited as biosensors, reporters or targeted biomedical treatments. Further studies to evaluate performance of these NSs in SERS imaging and photo thermal therapy are undergoing in our laboratories.Acknowledgements
The authors would like to thank Chris Winkler from the Nanoparticle Characterization and Fabrication Laboratory (NCFL) for assistance with nanoparticle characterization and imaging.Notes and references
- V. Juve, F. Cardinal, A. Lombardi, A. Crut, P. Maioli, J. Perez-Juste, L. Liz-Marzan, N. D. Fatti and F. Vallee, Nano Lett., 2013, 13, 2234–2240 CrossRef CAS PubMed.
- W. Cai, T. Gao, H. Hong and J. Sun, Nanotechnology, 2008, 1, 17–32 CAS.
- V. Ng, R. Berti, F. Lesage and A. Kakkar, J. Mater. Chem. B, 2013, 1, 9–25 RSC.
- J. Xie, J. Y. Lee and D. Wang, Chem. Mater., 2007, 19, 2823–2830 CrossRef CAS.
- R. Weissleder, Nat. Biotechnol., 2001, 19, 327–331 CrossRef PubMed.
- P. Jain, I. El-Sayed and M. El-Sayed, Nano Today, 2007, 2, 18–29 CrossRef.
- A. Smith, M. Mancini and S. Nie, Nat. Nanotechnol., 2009, 4, 710–711 CrossRef CAS PubMed.
- M. Moskovita, in Surface-Enhanced Raman Scattering: Physics and Applications, ed. K. Kneipp, M. Moskovits and H. Kneipp, Springer, 2006 Search PubMed.
- K. Kwon, K. Y. Lee, Y. W. Lee, M. Kim, J. Heo, S. J. Ahn and S. W. Han, J. Phys. Chem. C, 2007, 111, 1161–1165 CAS.
- C. Talley, L. Jusinski, C. Hollars, S. Lane and T. Huser, Anal. Chem., 2004, 76, 7064–7068 CrossRef CAS PubMed.
- J. Li, J. Zhou, T. Jiang, B. Wang, M. Gu, L. Petti and P. Mormile, Phys. Chem. Chem. Phys., 2014, 16, 25601–25608 RSC.
- J. Xie, Q. Zhang, J. Y. Lee and D. Wang, ACS Nano, 2008, 2, 2473–2480 CrossRef CAS PubMed.
- S. Lohse, N. Burrows, L. Scarabelli, L. Liz-Marzan and C. Murphy, Chem. Mater., 2014, 26, 34–43 CrossRef CAS.
- H. Yuan, C. Khoury, H. Hwang, C. Wilson, G. Grant and T. Vo-Dinh, Nanotechnology, 2012, 23, 1–9 Search PubMed.
- H. Yuan, Y. Liu, A. M. Fales, Y. L. Li, J. Liu and T. Vo-Dinh, Anal. Chem., 2013, 85, 208–212 CrossRef CAS PubMed.
- Y. Gao, Y. Li, J. Chen, S. Zhu, X. Liu, L. Zhou, P. Shi, D. Niu, J. Gu and J. Shi, Biomaterials, 2015, 60, 31–41 CrossRef CAS PubMed.
- D. Dam, K. Culver, I. Kandela, R. Lee, K. Chandra, H. Lee, C. Mantis, A. Ugolkov, A. Mazar and T. Odom, Nanomedicine, 2014, 11, 671–679 Search PubMed.
- D. Dam, J. Lee, P. Sisco, D. Co, M. Zhang, M. Wasielewski and T. Odom, ACS Nano, 2012, 6, 3318–3326 CrossRef CAS PubMed.
- C. Rong, W. Jiliang, L. Hui, C. Gang, L. Zhong and C. Chi-Ming, Rare Met., 2010, 29, 180–186 CrossRef.
- S. Palantavita, R. Tang, G. P. Sudlow, W. J. Akers, S. Achilefu and I. Sokolov, J. Mater. Chem. B, 2014, 2, 3107–3114 RSC.
- A. Manickavasagan, Imaging with Electromagnetic Spectrum: Applications in Food and Agriculture, Springer, 2014 Search PubMed.
- M. K. Clancy, in Cancer Theranostics, ed. X. Chen and S. Wong, Academic Press, Oxford, 2014, pp. 439–456 Search PubMed.
- C. Khoury and T. Vo-Dinh, J. Phys. Chem. C, 2008, 112, 18849–18859 CAS.
- P. C. Ray, H. Yu and P. Fu, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2009, 27, 1–35 CrossRef CAS PubMed.
- B. Xue, D. Wang, J. Zuo, X. Kong, Y. Zhang, X. Liu, L. Tu, Y. Chang, C. Li, F. Wu, Q. Zeng, H. Zhao, H. Zhaoc and H. Zhang, Nanoscale, 2015, 7, 8048–8057 RSC.
- B. Nikoobakht and M. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS.
- C. Nehl, H. Liao and J. Hafner, Nano Lett., 2006, 6, 683–688 CrossRef CAS PubMed.
- T. Ray, B. Lettiere, J. Rutte and S. Pennathur, Langmuir, 2015, 31, 3577–3586 CrossRef CAS PubMed.
- S. Khanal, G. Casillas, N. Bhattarai, J. J. Velázquez-Salazar, U. Santiago, A. Ponce, S. Mejía-Rosales and M. José-Yacamán, Langmuir, 2013, 29, 9231–9239 CrossRef CAS PubMed.
- S. Lin and M. Wiesner, Langmuir, 2012, 28, 11032–11041 CrossRef CAS PubMed.
- C. Burns, W. U. Spendel, S. Puckett and G. E. Pacey, Talanta, 2006, 69, 873–876 CrossRef CAS PubMed.
- A. E. Badawy, T. Luxton, R. Silva, K. Scheckel, M. Suidan and T. Tolaymat, Environ. Sci. Technol., 2010, 44, 1260–1266 CrossRef PubMed.
- R. Pamies, J. H. Cifre, V. F. Espin, M. Collado-Gonzalez, F. D. Banos and J. G. D. L. Torre, J. Nanopart. Res., 2014, 16, 2376 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00450d |
| ‡ Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |